Abstract
Extensive research has established the presence of resting-state brain functional damage in psychosis. However, the genetic mechanisms of such disease phenotype are yet to be unveiled. We investigated resting-state brain functional alterations in patients with drug-naive first-episode psychosis (DFP) by performing a neuroimaging meta-analysis of 8 original studies comprising 500 patients and 469 controls. Combined with the Allen Human Brain Atlas, we further conducted transcriptome-neuroimaging spatial correlations to identify genes whose expression levels were linked to brain functional alterations in DFP, followed by a range of gene functional characteristic analyses. Meta-analysis revealed a mixture of increased and decreased brain function in widespread areas including the default-mode, visual, motor, striatal, and cerebellar systems in DFP. Moreover, these brain functional alterations were spatially associated with the expression of 1662 genes, which were enriched for molecular functions, cellular components, and biological processes of the cerebral cortex, as well as psychiatric disorders including schizophrenia. Specific expression analyses demonstrated that these genes were specifically expressed in the brain tissue, in cortical neurons and immune cells, and during nearly all developmental periods. Concurrently, the genes could construct a protein-protein interaction network supported by hub genes and were linked to multiple behavioral domains including emotion, attention, perception, and motor. Our findings provide empirical evidence for the notion that brain functional damage in DFP involves a complex interaction of polygenes with various functional characteristics.
Subject terms: Schizophrenia, Biomarkers
Introduction
Schizophrenia, a severe and chronic psychiatric condition with a lifetime prevalence of about 1%1, is characterized by hallucinations, delusions and cognitive deficits. It is generally accepted that many of the clinical manifestations thought to be characteristic of psychosis may be attributable to abnormal brain function2,3. Given the presence of spontaneous neural activity along with intrinsic metabolic and perfusion patterns in the brain, considerable effort in the past decade has been dedicated to examine resting-state brain function in health and disease4–7. In this context, resting-state brain function has been frequently measured by amplitude of low-frequency fluctuations (ALFF) and regional homogeneity (ReHo) derived from functional magnetic resonance imaging (fMRI)8–10 as well as by cerebral blood flow (CBF) derived from arterial spin labeling (ASL) or positron emission tomography (PET)11,12. Use of these resting-state brain functional measures facilitates generalization of findings in the domain of psychiatry, given that collection of these data is now commonplace in semi-standardized ways and is less prone to the confounding effects of variation in task compliance. By leveraging these approaches, extensive research has established the presence of resting-state functional alterations in psychosis7,13–18, but inconsistency in the location and nature of effects makes it difficult to unify this research. This is largely due to the fact that most prior studies have focused on antipsychotic-medicated and chronic psychosis patients, which may introduce various confounds of antipsychotic medication and illness duration.
Benefiting from factoring out the confounds of antipsychotics and illness chronicity, investigations of resting-state brain functional alterations in patients with drug-naive first-episode psychosis (DFP) have attracted intense interest from researchers19–26. However, the reliability of these previous studies has been challenged by the concerns of small sample sizes, clinical heterogeneity, and flawed correction for multiple comparisons, which jointly work to inflate false positive rates. Despite the limitations, these studies set the stage for neuroimaging meta-analysis, a useful method that is capable of enlarging sample size and increasing statistical power by a more in-depth synthesis of findings in the literature. With the use of neuroimaging meta-analysis, investigators have enjoyed significant success in unraveling reliable and reproducible brain structural and functional changes in DFP27–30.
Psychosis is a highly heritable disorder with heritability estimated at up to 79%31,32. Although a large-scale genome-wide association study (GWAS) has identified many risk genetic loci in association with schizophrenia33, relatively little is known about the exact genetic mechanisms underlying certain disease phenotypes, such as brain functional damage. The integration of brain-wide gene expression data such as the Allen Human Brain Atlas (AHBA)34 and brain imaging data has given rise to the nascent field of neuroimaging transcriptomics35–38. In this framework, spatial correlations between brain transcriptome and disease neuroimaging phenotypes are a topic of active investigation, which may aid in the identification of genes whose expression profiles are associated with brain structural and functional change patterns in psychiatric conditions39–47. By means of this powerful method, several earlier studies have discovered several sets of genes in relation to abnormal brain structural properties in psychosis, such as structural covariance network deficiency39, white matter dysconnectivity40, morphometric similarity abnormality41, and gray matter volume changes42. Nevertheless, the genetic mechanisms of brain functional alterations in DFP are yet to be unveiled.
To this end, a comprehensive neuroimaging meta-analysis of ALFF, ReHo, and CBF was initially performed to investigate resting-state brain functional alterations in DFP. In conjunction with the AHBA, transcription-neuroimaging spatial correlation analyses were then conducted to detect genes whose expression levels were linked to brain functional alterations in DFP. Finally, we carried out a series of post hoc analyses (i.e., specific expression, functional enrichment, and protein-protein interaction analyses) for the identified genes to examine their functional characteristics. A schematic summary of the analysis pipeline is shown in Fig. 1.
Fig. 1. A schematic summary of the analysis pipeline.
DFP patients with drug-naive first-episode psychosis.
Methods
Literature search and selection
The literature search strategy was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines48. A systematic search was performed independently by two authors (Q.L. and X.X.) using the PubMed and Web of Science databases to identify studies examining differences in resting-state brain function between DFP and healthy controls (HC), published before February 2021. Considering that brain functional measures involved ReHo, ALFF, and CBF, we used various combinations of the following keywords during the search: “schizophr*”, “psychosis”, “psychotic disorders”, “first episode”, “first episodes”, “1ST-EPISODE”, “FEP”, “FES”, “free”, “naive”, “never medicated”, “ReHo”, “regional homogeneity”, “local connectivity”, “local functional connectivity”, “ALFF”, “fALFF”, “LFF”, “LFO”, “low-frequency fluctuation”, “low-frequency fluctuations”, “amplitude of low-frequency fluctuation”, “amplitude of low-frequency fluctuations”, “amplitude of low-frequency oscillation”, “amplitude of low-frequency oscillations”, “CBF”, “brain blood flow”, “cerebral blood flow”, “rCBF”, and “rest*”. We also screened the reference lists of the identified studies, review articles, and meta-analyses to search for additional qualified studies.
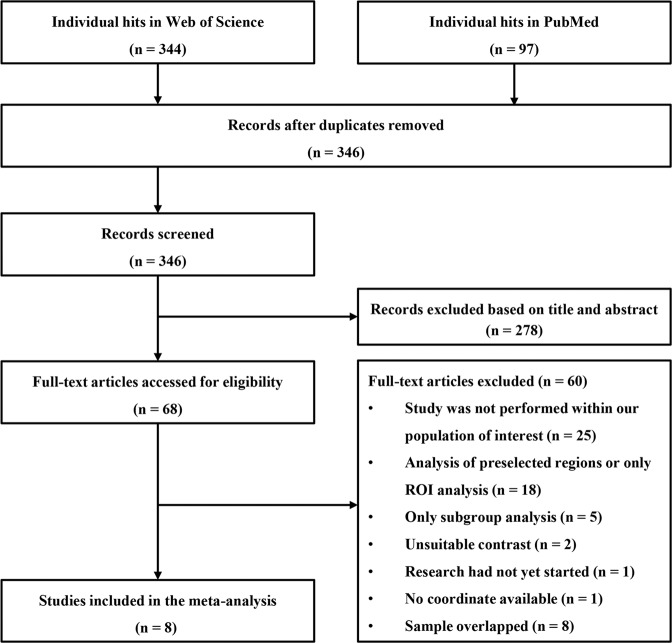
Studies were included if they met the following criteria: (1) studies examined resting-state brain functional differences between DFP and HC in a voxel-wise manner; (2) group comparisons were conducted at the whole-brain level; (3) statistical results were reported in a standard anatomical reference space (Montreal Neurological Institute [MNI] or Talairach space). In cases where coordinates were not reported in published papers, corresponding authors were contacted for details. Studies were excluded based on the following criteria: (1) voxel-wise comparisons were carried out within regions of interest; (2) studies involved animal experiments; (3) data could not be obtained from published papers or after contacting the authors; (4) participants were single genders. Notably, we also excluded studies whose data overlapped with those of other studies (e.g., from the same institution or authors), and the studies with the most results or larger effect sizes were included in our meta-analysis. A flowchart of the detailed study selection process is shown in Fig. 2. This meta-analysis was registered on PROSPERO (https://www.crd.york.ac.uk/PROSPERO/, NO: CRD42021252171).
Fig. 2. Flowchart of the study selection process.
ROI region of interest.
Neuroimaging meta-analysis
Voxel-wise meta-analysis of brain functional alterations in DFP was conducted using Seed-based d Mapping with Permutation of Subject Images (SDM-PSI) software package (version 6.21 for Windows) in a standard process (https://www.sdmproject.com). In contrast to the traditional activation likelihood estimation (ALE) method, SDM-PSI enables investigators to combine both peak coordinates and statistical parametric maps and uses standard effect size and variance-based meta-analytic calculations. The detailed analysis steps have been described in the SDM-PSI tutorial and previous publications49,50. First, we extracted peak coordinates and corresponding effect sizes (e.g., t-values) of clusters showing statistically significant differences in brain function between DFP and HC from each study. Coordinates in different standard anatomical reference spaces were converted from Talairach to MNI coordinates by using icbm2tal tools51 and z- or P-values were converted to t-values by using SDM online conversion utilities (https://www.sdmproject.com/utilities/?show=Statistics). Then, a standard MNI map of brain functional differences between DFP and HC was separately created for each study using a Gaussian kernel of 20 mm full width at half maximum (FWHM). Next, these maps were combined in a standard random-effects model considering sample size, intra-study variability and between-study heterogeneity, yielding a final map of brain functional differences (z map) for all included studies. Given the relatively small number of studies, we adopted a threshold of uncorrected voxel-level P = 0.005 and cluster extent = 20 voxels to optimally balance sensitivity and specificity52.
A range of supplementary analyses were pursued to test the reliability and robustness of our meta-analysis results. First, I2 statistic was calculated to describe which percentage of the variability between studies might be due to between-study heterogeneity, with I2 = 25%, 50%, and 75% indicating low, medium, and high heterogeneity, respectively53,54. Second, potential publication bias was assessed by funnel plots where two tests for small-study effect and excess significance were performed55,56. Finally, to test the repeatability of the results, we carried out a jackknife sensitivity analysis by repeating meta-analyses K times iteratively (K is the number of included studies), leaving out one study each time57,58. The results that remain significant in all or most of the combinations of studies are considered to be highly repeatable.
Brain gene expression data processing
Brain gene expression data were obtained from the AHBA dataset (http://www.brainmap.org), which was derived from six human postmortem donors (Supplementary Table S1). The original expression data of more than 20,000 genes at 3702 spatially distinct brain tissue samples were processed using a newly proposed pipeline35. A schematic of a workflow for processing brain gene expression data is shown in Supplementary Fig. S1. Specifically, the probe-to-gene annotations were first updated based on the latest available information from the National Center for Biotechnology Information (NCBI) using the Re-Annotator package59. With intensity-based filtering, we excluded probes that did not exceed the background noise in at least 50% of samples across all donors. Since multiple probes were used to measure the expression level of a single gene, we further used the RNA-seq data as a reference to select probes. After excluding genes that did not overlap between RNA-seq and microarray datasets, we computed the correlations between microarray and RNA-seq expression measures for the remaining genes. After ruling out probes with low correlations (r < 0.2), a representative probe was selected for a gene based on its highest correlation with the RNA-seq data. Here, only the tissue samples in the left cerebral cortex were included. For one, all six donors had expression data in the left hemisphere, whereas only two donors had samples in the right hemisphere. For another, the inclusion of subcortical samples might introduce potential biases given the substantial divergence in gene expression between cortical and subcortical regions34. To account for potential between-sample differences and donor-specific effects in gene expression, we conducted both within-sample cross-gene and within-gene cross-sample normalization with the scaled robust sigmoid normalization method. Differential stability (DS) is a measure of consistent regional variation across donor brains. Earlier work has demonstrated that genes with high DS show more consistent spatial expression patterns between donors and are enriched for brain-related biological functions60. Since gene expression conservation across subjects is a prerequisite for the transcriptome-neuroimaging spatial correlations, only genes with relatively more conserved expression patterns were selected for analysis. To achieve this goal, we ranked the genes by their DS values and chose the genes with the top 50% highest DS for the main analysis. Furthermore, to evaluate the effect of different DS threshold selections, we conducted sensitivity analyses by using two other DS cutoff thresholds (top 40% and 60%). After these processing procedures, we obtained normalized expression data of 5013 genes for 1280 tissue samples. Because our neuroimaging meta-analysis was performed within a gray matter mask provided by the SDM-PSI, we further restricted our analyses to the samples within this mask, resulting in a final sample × gene matrix of 894 × 5013. The numbers of remaining probes and genes at each processing step are shown in Supplementary Fig. S2.
Transcription-neuroimaging association analysis
To derive the DFP-related functional alteration of a given brain tissue sample, we defined a 3-mm radius sphere centered at the MNI coordinate of this sample and extracted the average z value of voxels within the sphere from the meta-analysis z map. Then, gene-wise cross-sample Pearson’s correlations between gene expression and z values were performed to determine genes whose expression levels were correlated with brain functional changes in DFP. Multiple comparisons were corrected using the Benjamini and Hochberg method for false discovery rate (FDR-BH) (q < 0.05).
To further test whether the number of the identified genes was significantly greater than the random level, a permutation test was pursued to establish the significance of our results. As transcriptional data are spatially autocorrelated, i.e., nearby anatomical regions tend to have more similar patterns of gene expression than spatially distant regions, the standard non-parametric null (i.e., randomly shuffling the sample labels) is strongly violated by the spatial autocorrelation of brain maps, yielding increased family-wise error rates61. To address this issue, we used a spatially constrained null model proposed by Burt et al.62 to conduct the permutation test. This method is implemented in an open-access, Python-based software package, BrainSMASH: Brain Surrogate Maps with Autocorrelated Spatial Heterogeneity (https://github.com/murraylab/brainsmash). It can simulate volumetric surrogate brain maps that preserve the spatial autocorrelation using three-dimensional Euclidean distance between regions. To correct the spatial autocorrelation in transcriptional data, we used this method to generate spatial autocorrelation-preserving surrogate maps for each gene. These surrogate maps were used to re-identify genes related to brain functional changes in DFP using exactly the same method as described above. Next, we repeated this procedure 5000 times and recorded the number of genes identified in each test to build a null distribution. Finally, we compared the number of genes identified using the real data with this null distribution to determine whether our results were different from random.
Gene enrichment analysis
A series of enrichment analyses were performed for the identified genes associated with brain functional alterations in DFP. First, functional annotation was carried out with the use of the ToppGene portal (https://toppgene.cchmc.org/)63. Gene ontology (GO) was adopted to assess biological functions including molecular functions, cellular components and biological processes. The disease and pathway databases were used to assess the related diseases and biological pathways. Second, online tissue-specific expression analysis (TSEA) (http://genetics.wustl.edu/jdlab/tsea/) and cell type-specific expression analysis (CSEA) (http://genetics.wustl.edu/jdlab/csea-tool-2/)64 tools were utilized to conduct tissue, cell type, and temporal-specific expression analyses, with the aim of determining the specific tissues, cortical cell types, and developmental stages in which these genes were overrepresented. A specificity index probability (pSI) was used to index how genes are more enriched in specific terms relative to others65 and four pSI thresholds (0.05, 0.01, 0.001, and 0.0001) were employed in this analysis. Finally, we examined the overlap between genes associated with brain functional alterations in DFP found in the present study and schizophrenia-associated genes in the MalaCards database (https://www.malacards.org/)66. For the aforementioned enrichment analyses, Fisher’s exact tests were utilized to assess their significance. Multiple testing was corrected using the FDR-BH (q < 0.05).
Protein-protein interaction analysis
Protein-protein interaction (PPI) analysis was performed by STRING v11.0 (https://string-db.org/) to determine whether the genes associated with brain functional alterations in DFP could construct a PPI network. All PPI pairs with a confidence interaction score > 0.9 were extracted. Genes with the top 10% highest degree values (i.e., the number of edges connected to a gene) were defined as hub genes. In addition, the Human Brain Transcriptome database (http://hbatlas.org/) was employed to characterize the spatial-temporal expression trajectory of hub genes with the highest degree values.
Behavioral relevance analysis
To capture the behavioral relevance of the genes related to brain functional alterations in DFP, we tested the associations between gene expression and behavioral domains via the Neurosynth (https://neurosynth.org/), a well-validated and publicly available platform for meta-analysis of neuroimaging literature67. The Neurosynth database contains activation maps of 1335 behavioral terms that describe nearly all aspects of human behavior. For each term, cross-sample correlation analyses were performed between its activation values and gene expression measures, resulting in a set of correlation coefficients corresponding to the genes. A positive correlation coefficient indicates that a brain region with higher gene expression tends to show greater neural activation, while a negative correlation coefficient means that a brain region with lower gene expression tends to show greater neural activation. Thus, both positive and negative correlation coefficients indicate that a gene contributes to a behavioral term. To avoid biases due to offset, we averaged the absolute values of these correlation coefficients (|r | mean) to index the extent to which this set of genes was linked to each behavioral term. Finally, the behavioral terms were ordered based on their |r | mean and those with the highest |r | mean were selected to capture the behavioral relevance of the genes related to brain functional alterations in DFP. Here, a threshold of |r | mean > 0.2 was chosen for ease of interpretability.
Results
Resting-state brain functional alterations in DFP
After a comprehensive literature search and selection, our meta-analysis included 8 studies comprising 500 DFP and 469 HC. Demographic, clinical, and imaging characteristics of the included studies are shown in Table 1. Voxel-wise neuroimaging meta-analysis revealed widespread brain functional differences between DFP and HC (Fig. 3 and Table 2). In comparison with HC, DFP showed increased function in the left cerebellar hemisphere, left putamen, right calcarine sulcus, and right caudate, as well as decreased function in the bilateral posterior cingulate gyrus, bilateral precuneus, right cuneus, right precentral gyrus, and right inferior parietal lobule. Moreover, heterogeneity analysis (I2 < 25%) (Supplementary Table S2), publication bias analysis including funnel plots with small-study effect and excess significance tests (P > 0.05) (Supplementary Fig. S3 and Supplementary Table S3), and jackknife sensitivity analyses (75–100% consistency) (Supplementary Tables S4 and S5) suggested the reliability and robustness of our meta-analysis results.
Table 1.
Demographic, clinical, and imaging characteristics of the included studies.
| Demographic characteristics | Clinical characteristics (patients only) | Imaging characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number/female | Age, yr | PANSS score | ||||||||||
| Study | DFP | HC | DFP | HC | Drug status | Illness duration, mo | Total | Negative | Positive | Scanner | Software | Measures |
| Li et al.19 | 72/48 | 79/46 | 14.7 ± 1.78 | 14.3 ± 2.17 | Drug naive | < 12 | 68.9 ± 18.4 | 16.3 ± 7.53 | 15.8 ± 5.19 | 3.0 T | DPABI | ALFF |
| Yan et al.20 | 69/19 | 74/29 | 24.22 ± 7.08 | 26.27 ± 6.97 | Drug naive | 13.74 ± 11.76 | 84.19 ± 8.25 | 17.58 ± 4.10 | 24.42 ± 3.88 | 3.0 T | DPARSF | ReHo |
| Zhao et al.21 | 44/13 | 26/9 | 23.7 ± 5.3 | 22.6 ± 3.7 | Drug naive | 12 ± 9.2 | 102 ± 16.7 | 24.7 ± 7.5 | 15.3 ± 3.3 | 3.0 T | DPARSF | ReHo |
| Wu et al.22 | 32/16 | 32/11 | 30.94 ± 8.25 | 31.37 ± 7.84 | Drug naive | 12 ± 9.2 | 77.38 ± 5.17 | 20.59 ± 3.46 | 20.00 ± 4.32 | 3.0 T | SPM8 | fALFF |
| Liu et al.23 | 48/NA | 31/NA | 15.79 ± 1.64 | 15.42 ± 1.52 | Drug naive | 5.35 ± 6.12 | 75.10 ± 9.88 | 17.92 ± 6.95 | 21.50 ± 5.01 | 3.0 T | SPM8 | ReHo |
| Lei et al.24 | 124/63 | 102/52 | NA | NA | Drug naive | NA | NA | NA | NA | 3.0 T | DPARSF | ALFF |
| Ren et al.25 | 100/59 | 100/59 | 24.30 ± 7.45 | 24.39 ± 7.58 | Drug naive | 6.25 ± 11.04 | 97.88 ± 17.75 | 18.84 ± 7.70 | 25.11 ± 5.97 | 3.0 T | DPARSF | ALFF |
| Scheef et al.26 | 11/3 | 25/13 | 32 ± 5 | 30 ± 6 | Drug naive or free | NA | 43.1 ± 8.5 | 21.4 ± 7.9 | 20.2 ± 2.9 | 3.0 T | SPM2 | CBF |
DFP patients with drug-naive first-episode psychosis, HC healthy controls, PANSS Positive and Negative Syndrome Scale, NA not available, ALFF amplitude of low-frequency fluctuations, fALFF fractional amplitude of low-frequency fluctuations, ReHo regional homogeneity, CBF cerebral blood flow.
Fig. 3. Brain regions with functional differences between DFP and HC.

DFP patients with drug-naive first-episode psychosis, HC healthy controls, L left, R right.
Table 2.
Brain regions showing functional differences between DFP and HC identified by voxel-wise meta-analysis.
| Regions | Cluster size (voxels) | Peak MNI coordinate | SDM-z | P value |
|---|---|---|---|---|
| DFP > HC | ||||
| Left cerebellar hemisphere | 187 | −18, −58, −20 | 3.20 | 6.93 × 10−4 |
| Left putamen | 158 | −20, 10, −4 | 4.24 | 1.11 × 10−5 |
| Right calcarine sulcus | 184 | 14, −94, 2 | 3.43 | 3.02 × 10−4 |
| Right caudate | 24 | 8, 8, 6 | 3.17 | 7.63 × 10−4 |
| Right caudate | 46 | 16, 14, 16 | 3.23 | 6.11 × 10−4 |
| DFP < HC | ||||
| Bilateral posterior cingulate gyrus | 280 | 4, −58, 14 | −4.13 | 1.81 × 10−5 |
| Bilateral precuneus | 227 | 2, −46, 36 | −3.97 | 3.56 × 10−5 |
| Right cuneus | 94 | 10, −76, 40 | −3.48 | 2.47 × 10−4 |
| Right precentral gyrus | 187 | 54, −10, 38 | −3.75 | 8.82 × 10−5 |
| Right inferior parietal lobule | 101 | 50, −52, 44 | −3.68 | 1.18 × 10−4 |
DFP patients with drug-naive first-episode psychosis, HC healthy controls, MNI Montreal Neurological Institute, SDM seed-based d mapping.
Genes associated with brain functional alterations in DFP
By leveraging transcription-neuroimaging spatial correlation analysis, we found that expression measures of 1662 genes were significantly linked to brain functional alterations in DFP (FDR-BH correction, q < 0.05) (Supplementary File 1). The spatially-constrained permutation test demonstrated that none out of 5000 permutations yielded more genes than those identified using the real data (P < 0.0002), indicating that our results were different from random. Moreover, we observed significant overlaps between the genes in the main analyses and those identified using two other DS cutoff thresholds of 40% (overlap ratio: 96.61%) and 60% (overlap ratio: 92.33%) (Supplementary Table S6 and Supplementary File 2).
Gene enrichment results
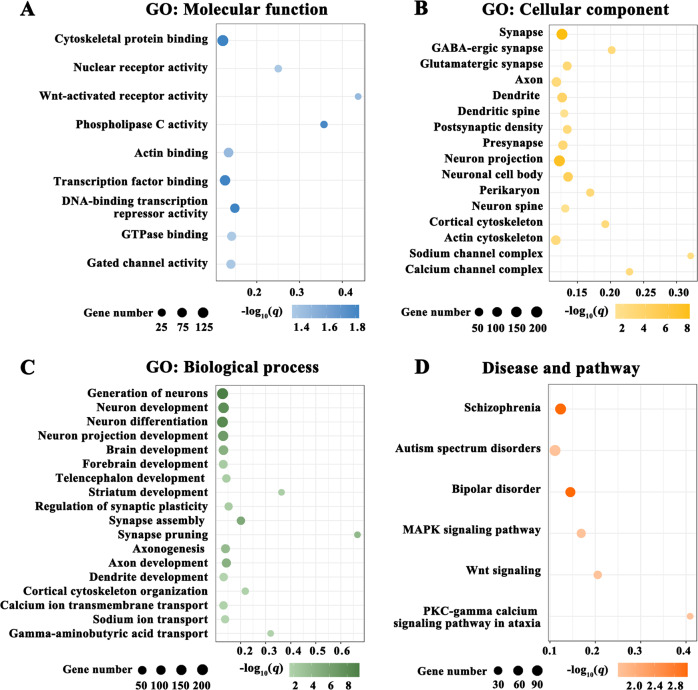
To characterize the biological functions, diseases, and pathways of the genes associated with brain functional alterations in DFP, we performed functional enrichment analyses using the ToppGene portal. The enrichment results are listed in Supplementary File 3 and are illustrated in Fig. 4. In terms of GO, the 1662 genes were mainly enriched for molecular functions (e.g., cytoskeletal protein binding, actin binding, nuclear receptor activity, and gated channel activity) (Fig. 4A), cellular components (e.g., synapse, glutamatergic synapse, axon, dendrite, neuron, and ion channel) (Fig. 4B), and biological processes (e.g., neuron development, neuron differentiation, synapse assembly, synapse pruning, cytoskeleton organization, and ion transport) (Fig. 4C) of the cerebral cortex. With respect to diseases, the identified genes were enriched for several psychiatric disorders including schizophrenia, autism spectrum disorders, and bipolar disorder (Fig. 4D). As to pathways, these genes were enriched for MAPK signaling pathway, Wnt signaling, and PKC-gamma calcium signaling pathway in ataxia (Fig. 4D).
Fig. 4. Functional enrichment of the genes associated with brain functional alterations in DFP.
A GO: Molecular function. B GO: Cellular component. C GO: Biological process. D Disease and pathway. The x axis shows rich factor and the y axis shows items. The globule size represents the number of genes overlapping with those belonging to each item, and the globule color represents statistical significance. The rich factor refers to the ratio of the number of significant genes annotated to the item to the number of all genes annotated to the item. GO gene ontology, DFP patients with drug-naive first-episode psychosis.
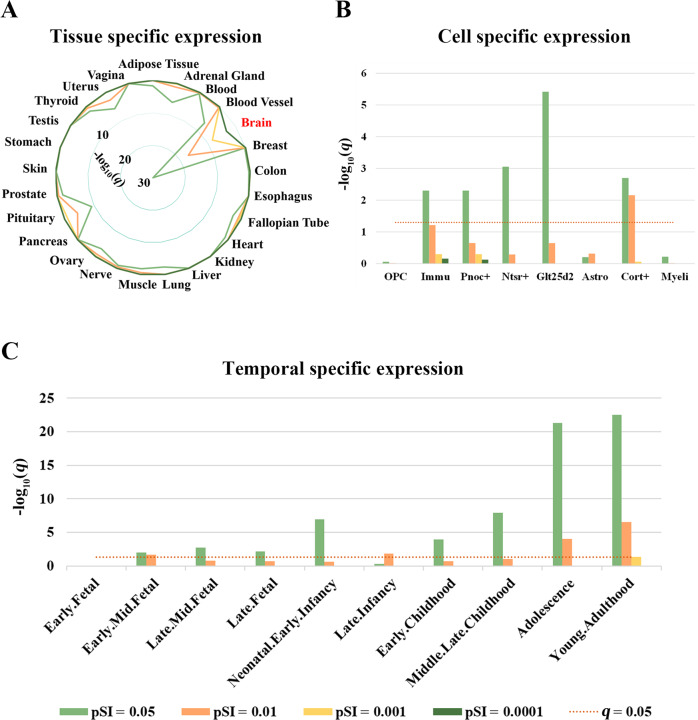
Tissue-specific expression analysis demonstrated that the 1662 genes were specifically expressed in the brain tissue (Fig. 5A and Supplementary File 4, sheet “Tissues”). Regarding cortical cell types, these genes were specifically expressed in multiple types of neurons such as Pnoc + , Ntsr + , Glt25d2, and Cort+ neurons as well as immune cells (Fig. 5B and Supplementary File 4, sheet “Cell types”). Temporal-specific expression analysis revealed that these genes were preferentially expressed during nearly all developmental periods with exception of the early fetal stage (Fig. 5C and Supplementary File 4, sheet “Developmental stages”).
Fig. 5. Specific expression of the genes associated with brain functional alterations in DFP.
A Tissue-specific expression. B Cell-specific expression. C Temporal-specific expression. OPC oligodendrocyte progenitor cells, Immu immune cells, Pnoc + prepronociceptin-expressing neurons, Ntsr + corticothalamic neurons, Glt25d2 corticopontine neurons, Astro Astrocytes, Cort + corticosterone-expressing neurons, Myeli myelinating oligodendrocytes, pSI specificity index probability, DFP patients with drug-naive first-episode psychosis.
Fisher’s exact test revealed that the genes related to brain functional alterations in DFP found in the present study significantly overlapped with the 400 schizophrenia-related genes from the MalaCards database (52 overlap genes, odds ratio = 1.64, P = 0.001), indicating some degree of psychosis specificity of our findings.
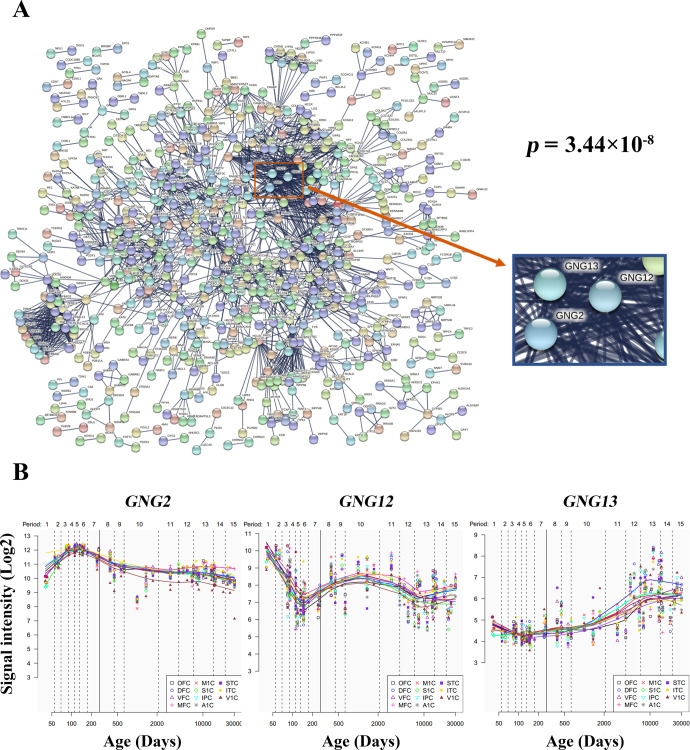
PPI network and hub genes
PPI analysis revealed that 689 genes from the 1662 genes could construct an interconnected PPI network (Fig. 6A). This network consisted of 2151 edges, which was significantly higher than expected (P = 3.44 × 10−8). In total, 69 genes with the top 10% highest degree values were defined as hub genes (Supplementary File 5). We also delineated the spatial-temporal expression trajectory of three hub genes with the highest degree values, i.e., G protein subunit gamma 2 (GNG2, degree value = 52), G protein subunit gamma 12 (GNG12, degree value = 51) and G protein subunit gamma 13 (GNG13, degree value = 49) (Fig. 6B).
Fig. 6. PPI network and hub genes.
A A PPI network with 689 genes and 2151 edges. The P value denotes the statistical significance of how likely the proteins encoded by the input genes are connected to construct a network. B Spatial and temporal expression curves of three hub genes with the highest degree values (i.e., GNG2, GNG12, and GNG13). GNG G protein subunit gamma, PPI protein-protein interaction.
Associations with behavioral terms
By linking gene expression with behavioral domains via the Neurosynth, we found that the genes associated with brain functional alterations in DFP were correlated with multiple behavioral terms including “visual”, “lingual”, “attention”, “emotion”, “perceptual”, “motion”, and “fear” (|r | mean > 0.2) (Fig. 7 and Supplementary File 6).
Fig. 7. Behavioral relevance of the genes associated with brain functional alterations in DFP.

Font sizes of the behavioral terms represent the correlations (|r | mean) between their activation values and gene expression measures. Blue terms are those with the highest correlations (|r | mean > 0.2). DFP patients with drug-naive first-episode psychosis.
Discussion
This study opens new perspectives by being the first to investigate the genetic mechanisms underlying resting-state brain functional alterations in DFP using a combination of neuroimaging meta-analysis and transcriptome-neuroimaging spatial correlation. Meta-analysis revealed a mixture of increased and decreased resting-state brain function in widespread areas including the default-mode (posterior cingulate gyrus, precuneus, and inferior parietal lobule), visual (calcarine sulcus and cuneus), motor (precentral gyrus), striatal (putamen and caudate), and cerebellar systems in DFP. Moreover, these brain functional alterations were spatially associated with the expression of 1662 genes, which were enriched for molecular functions, cellular components, and biological processes of the cerebral cortex, as well as psychiatric disorders including schizophrenia. Specific expression analyses demonstrated that these genes were specifically expressed in the brain tissue, in cortical neurons and immune cells, and during nearly all developmental periods. Concurrently, the genes associated with brain functional alterations could construct a PPI network supported by hub genes. In addition, these genes were linked to multiple behavioral domains including emotion, attention, perception, and motor. Collectively, these findings endorse the notion that brain functional damage in psychosis involves a complex interaction of polygenes with various functional characteristics.
Our meta-analysis demonstrated that widespread brain functional abnormalities were present in DFP. Decreased brain function was observed in posterior cingulate gyrus, precuneus, and inferior parietal lobule, which serve as key components of the default-mode network (DMN). DMN is involved in the processing of various high-level cognitive functions, such as self-related cognition and memory68,69. In psychosis patients, DMN dysfunction may relate to overly intensive self-reference and memory impairments70. Hyperactivity of the striatum appears to be consistent findings across psychosis studies, thereby providing biomarkers for this disorder and targets for its treatment71 given that the striatum harbors the largest density of dopamine D2 receptors72. The calcarine sulcus and cuneus belong to the primary visual cortex, which is involved in visual processing and associated with visual hallucinations73–75. The precentral gyrus is a critical part of the mirror neuron system and its functional damage is believed to play an important role in social cognition impairment in schizophrenia76,77. Our observation of increased function in the cerebellum supports the concept that disrupted synchronization in the cerebellar prefrontal circuitry underlies negative symptoms and possibly also cognitive dysfunction in psychosis78. Notably, since our meta-analysis focused on DFP and was not contaminated by confounding factors such as medication use and/or long illness duration, the present findings may provide a more refined picture of resting-state brain functional alterations specific to psychosis.
Further transcriptome-neuroimaging spatial correlation analyses revealed that the brain functional alterations in DFP were related to expression of 1662 genes, which were enriched for molecular functions, cellular components, and biological processes of the cerebral cortex. Indeed, damaging mutations in genes that are critical to synaptic structure, function, and plasticity play a prominent role in the pathophysiology of schizophrenia79. For instance, disruptions in the synaptic structural organization are critical to the development of psychosis. The postsynaptic density is a vital component of dendritic spines, with marked thickening in postsynaptic membrane of excitatory synapses and a lack in the symmetric inhibitory synapses80,81. The postsynaptic density has been strongly implicated in psychosis at both genetic and protein levels82–85. In addition, remodeling of dendritic trees and dendritic spines has been observed as a result of environmental and genetic influences acting both early and later in life in schizophrenia86. Alterations in dendritic morphology, such as reduction in the size of dendritic arborization, have been described in postmortem samples from patients with schizophrenia87. There is ample evidence that DFP is associated with elevations in glutamatergic metabolites across several brain regions, which may reflect increases in glutamatergic synaptic activity88. Several more recent magnetic resonance spectroscopy studies have documented glutamate alterations in the salience network89 and hippocampus90 in DFP. These previous findings are consistent with the current observation of significant gene enrichment for glutamatergic synapse. The mature neuron is composed of multiple subdomains, each with a distinctive cytoskeletal organization91. Actinin plays an important role in the regulation of cytoskeletal remodeling and the inactivation of the N-methyl-D-aspartate receptor (NMDAR) for glutamate, which have been implicated in psychosis by influencing brain development and plasticity processes92,93. Moreover, reelin is found to stabilize the actin cytoskeleton by inducing cofilin phosphorylation; decreased reelin expression in the mature brain causes destabilization of neurons and their processes, leading to aberrant plasticity and aberrant wiring of brain circuitry, which may be related to psychosis94. Ion channels are vital for neuronal functions, triggering nerve impulses, and neurotransmitter release95. Voltage-gated sodium channels are broadly distributed in the central nervous system and are linked to neuronal excitability, with their dysfunction being implicated in psychosis pathogenesis96,97. Furthermore, calcium signaling has been noted to be of particular relevance in the etiology of schizophrenia98–100. GWAS have indicated that genetic variations associated with the calcium signaling pathway can increase the risk of developing schizophrenia101. Aside from a better understanding of the molecular basis of brain functional alterations in psychosis, these findings might hold clinical value and translational potential. It is largely known that all drugs currently licensed to treat psychosis are dopamine D2/3 blockers102. Although helpful for reducing psychotic symptoms, these agents are not curative and generally do not address the neurocognitive and social difficulties inherent to the disorder103. Our data indicate that novel interventions designed to remediate disruptions in synaptic structural organization potentially offer more specific therapeutic benefits.
These genes were enriched for psychiatric disorders including schizophrenia, autism spectrum disorders, and bipolar disorder, in favor of the idea that these conditions with several common clinical features may share some similar genetic basis. The enriched pathways include MAPK signaling pathway, Wnt signaling and PKC-gamma calcium signaling pathway in ataxia. Previous studies have documented that MAPK signaling alterations could result from a protein dephosphorylation defect in vivo and might be involved in the pathology of psychosis104,105. Zhang et al. reported that PKC γ-mediated phosphorylation signal was impaired in Hint1-deficient neuron, which might contribute to the pathogenesis of psychosis106. Furthermore, there is empirical evidence for the crucial role of Wnt signaling pathway in neurodevelopment and in regulating the function and structure of the adult nervous system. Prior research shows that psychosis is characterized by abnormal Wnt gene expression and plasma protein levels, suggesting that drugs targeting the Wnt pathway may have a role in the treatment of this disorder107.
Specific expression analysis demonstrated that the genes associated with brain functional alterations in DFP were specifically expressed in the brain tissue and neurons, which may enhance our confidence in interpreting our findings. Besides, these genes showed specific expression in immune cells, in accordance with the previous finding that cognitive deficits in psychosis may be associated with altered expression of molecules that regulate immune cell trafficking108. Critically, these genes were found to be specifically expressed during nearly all developmental periods, which is coherent with the “2-hit” hypothesis of schizophrenia109. In that model, genetic or environmental factors disrupt early brain development (i.e., “first hit” during pre- and perinatal periods). These early disruptions produce long-term vulnerability to a “second hit” during the periods of adolescence and young adulthood, which may ultimately give rise to full-blown psychosis110–112.
The genes associated with brain functional alterations could construct a PPI network supported by hub genes, e.g., G protein-related genes. Many neurotransmitters involved in schizophrenia act through metabotropic G protein-coupled receptors (GPCRs). Moreover, the GPCRs for serotonin, dopamine, and glutamate have been traditionally recognized as molecular targets for antipsychotics103. These receptors mediate slow synaptic transmission by modulating intracellular signal transduction and induction of gene expression to exert antipsychotic action113,114. By correlating gene expression with behavioral terms via the Neurosynth, we found that the genes associated with brain functional alterations in DFP were linked to multiple behavioral terms including emotion, attention, perception, and motor. Echoing this finding, extensive literature has provided strong evidence that psychosis patients present with deficits in these behavioral domains115–122.
This study has several limitations that should be considered. First, the meta-analysis z map cannot represent the exact nature of resting-state brain functional differences between DFP and HC across the whole brain, as only peak coordinates and corresponding effect sizes of significant clusters in the previous studies were utilized. Our preliminary results warrant further validation by conducting analysis in a large cohort of DFP. Second, a relatively liberal statistical threshold (uncorrected voxel-level P = 0.005 and cluster extent = 20 voxels) was set for neuroimaging meta-analysis, considering the small number of included studies and the more recent work demonstrating that small P values may not yield robust findings123. Third, the gene expression data were derived from the AHBA postmortem brains, while the neuroimaging data were obtained from living brains of DFP. This concern may be mitigated by focusing on genes with more conservative expression patterns, but this may obscure correlations with genes exhibiting variable expression patterns across individuals. Finally, given limited gene expression data in the right hemisphere and considerable differences in gene expression between cortical and subcortical regions, our study only considered the tissue samples in the left cerebral cortex, which may introduce potential biases.
In summary, our neuroimaging meta-analysis demonstrated that DFP manifested a mix of increased and decreased resting-state brain function in widespread areas including the default mode, visual, motor, striatal, and cerebellar systems. Combined with the brain-wide transcriptome, we found that these brain functional alterations were spatially associated with the expression of 1662 genes, which showed a rich range of functional characteristics. These findings may shed light on the genetic mechanisms underlying resting-state brain functional alterations in psychosis.
Supplementary information
Acknowledgements
The study was supported by the National Natural Science Foundation of China (grant number: 82071905), the Outstanding Youth Support Project of Anhui Province Universities (grant number: gxyqZD2022026), the Scientific Research Key Project of Anhui Province Universities (grant number: 2022AH051135), the Scientific Research Foundation of Anhui Medical University (grant number: 2022xkj143), and the Anhui University Collaborative Innovation Project (grant number: GXXT-2021-065). We thank the Allen Institute for Brain Science founders and staff who supplied the brain expression data. We also thank the subjects who contributed to this study.
Author contributions
Y.Y. and J.Z. designed the study. Q.L., X.X., J.Z., and H.C. acquired the data, which Q.L., X.X., Y.Q., H.C., W.Z., and J.Z. analyzed. Q.L. and X.X. wrote the article, which Y.Y. and J.Z. reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Data availability
The Allen Human Brain Atlas is available at https://human.brain-map.org/static/download. The MalaCards database is available at https://www.malacards.org/. The Neurosynth database is available at https://neurosynth.org/. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
Code availability
All relevant analysis scripts are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Qian Li, Xiaotao Xu.
Contributor Information
Jiajia Zhu, Email: zhujiajiagraduate@163.com.
Yongqiang Yu, Email: cjr.yuyongqiang@vip.163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41537-023-00338-z.
References
- 1.Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet. 2022;399:473–486. doi: 10.1016/S0140-6736(21)01730-X. [DOI] [PubMed] [Google Scholar]
- 2.Alnaes D, et al. Brain heterogeneity in schizophrenia and its association with polygenic risk. JAMA Psychiatry. 2019;76:739–748. doi: 10.1001/jamapsychiatry.2019.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moser DA, et al. Multivariate associations among behavioral, clinical, and multimodal imaging phenotypes in patients with psychosis. JAMA Psychiatry. 2018;75:386–395. doi: 10.1001/jamapsychiatry.2017.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raichle ME. The restless brain: how intrinsic activity organizes brain function. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015;370:20140172. doi: 10.1098/rstb.2014.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sripada C, et al. Prediction of neurocognition in youth from resting state fMRI. Mol. Psychiatry. 2020;25:3413–3421. doi: 10.1038/s41380-019-0481-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gohel S, et al. Frequency specific resting state functional abnormalities in psychosis. Hum. Brain Mapp. 2018;39:4509–4518. doi: 10.1002/hbm.24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu J, et al. Altered resting-state cerebral blood flow and its connectivity in schizophrenia. J. Psychiatr. Res. 2015;63:28–35. doi: 10.1016/j.jpsychires.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Yang H, et al. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage. 2007;36:144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 9.Zou QH, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J. Neurosci. Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 11.Haller S, et al. Arterial spin labeling perfusion of the brain: emerging clinical applications. Radiology. 2016;281:337–356. doi: 10.1148/radiol.2016150789. [DOI] [PubMed] [Google Scholar]
- 12.Hooker JM, Carson RE. Human positron emission tomography neuroimaging. Annu. Rev. Biomed. Eng. 2019;21:551–581. doi: 10.1146/annurev-bioeng-062117-121056. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Zhuo C, Qin W, Zhu J, Yu C. Altered spontaneous brain activity in schizophrenia: a meta-analysis and a large-sample study. Biomed. Res. Int. 2015;2015:204628. doi: 10.1155/2015/204628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lottman KK, et al. Four-way multimodal fusion of 7 T imaging data using an mCCA+jICA model in first-episode schizophrenia. Hum. Brain Mapp. 2018;39:1475–1488. doi: 10.1002/hbm.23906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sui J, et al. In search of multimodal neuroimaging biomarkers of cognitive deficits in schizophrenia. Biol. Psychiatry. 2015;78:794–804. doi: 10.1016/j.biopsych.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwabuchi SJ, Palaniyappan L. Abnormalities in the effective connectivity of visuothalamic circuitry in schizophrenia. Psychol. Med. 2017;47:1300–1310. doi: 10.1017/S0033291716003469. [DOI] [PubMed] [Google Scholar]
- 17.Zhu J, et al. Altered coupling between resting-state cerebral blood flow and functional connectivity in schizophrenia. Schizophr. Bull. 2017;43:1363–1374. doi: 10.1093/schbul/sbx051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu J, Zhu DM, Qian Y, Li X, Yu Y. Altered spatial and temporal concordance among intrinsic brain activity measures in schizophrenia. J. Psychiatr. Res. 2018;106:91–98. doi: 10.1016/j.jpsychires.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Li Q, et al. Dynamic alterations of amplitude of low-frequency fluctuations in patients with drug-naive first-episode early onset schizophrenia. Front. Neurosci. 2020;14:901. doi: 10.3389/fnins.2020.00901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan W, et al. Relationships between abnormal neural activities and cognitive impairments in patients with drug-naive first-episode schizophrenia. BMC Psychiatry. 2020;20:283. doi: 10.1186/s12888-020-02692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao X, et al. Abnormalities of regional homogeneity and its correlation with clinical symptoms in Naive patients with first-episode schizophrenia. Brain Imaging Behav. 2019;13:503–513. doi: 10.1007/s11682-018-9882-4. [DOI] [PubMed] [Google Scholar]
- 22.Wu R, et al. Reduced brain activity in the right putamen as an early predictor for treatment response in drug-naive, first-episode schizophrenia. Front. Psychiatry. 2019;10:741. doi: 10.3389/fpsyt.2019.00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, et al. Abnormal neural activity as a potential biomarker for drug-naive first-episode adolescent-onset schizophrenia with coherence regional homogeneity and support vector machine analyses. Schizophr. Res. 2018;192:408–415. doi: 10.1016/j.schres.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 24.Lei W, et al. Sex-specific patterns of aberrant brain function in first-episode treatment-naive patients with schizophrenia. Int. J. Mol. Sci. 2015;16:16125–16143. doi: 10.3390/ijms160716125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren W, et al. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am. J. Psychiatry. 2013;170:1308–1316. doi: 10.1176/appi.ajp.2013.12091148. [DOI] [PubMed] [Google Scholar]
- 26.Scheef L, et al. Resting-state perfusion in nonmedicated schizophrenic patients: a continuous arterial spin-labeling 3.0-T MR study. Radiology. 2010;256:253–260. doi: 10.1148/radiol.10091224. [DOI] [PubMed] [Google Scholar]
- 27.Brugger SP, Howes OD. Heterogeneity and homogeneity of regional brain structure in schizophrenia: a meta-analysis. JAMA Psychiatry. 2017;74:1104–1111. doi: 10.1001/jamapsychiatry.2017.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan RC, Di X, McAlonan GM, Gong QY. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr. Bull. 2011;37:177–188. doi: 10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding Y, et al. Cerebellar structural and functional abnormalities in first-episode and drug-naive patients with schizophrenia: a meta-analysis. Psychiatry Res. Neuroimaging. 2019;283:24–33. doi: 10.1016/j.pscychresns.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Gong J, et al. Abnormalities of intrinsic regional brain activity in first-episode and chronic schizophrenia: a meta-analysis of resting-state functional MRI. J. Psychiatry Neurosci. 2020;45:55–68. doi: 10.1503/jpn.180245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilker R, et al. Heritability of schizophrenia and schizophrenia spectrum based on the nationwide danish twin register. Biol. Psychiatry. 2018;83:492–498. doi: 10.1016/j.biopsych.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 32.McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia—an overview. JAMA Psychiatry. 2020;77:201–210. doi: 10.1001/jamapsychiatry.2019.3360. [DOI] [PubMed] [Google Scholar]
- 33.Schizophrenia Working Group of the Psychiatric Genomics, C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawrylycz MJ, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnatkeviciute A, Fulcher BD, Fornito A. A practical guide to linking brain-wide gene expression and neuroimaging data. Neuroimage. 2019;189:353–367. doi: 10.1016/j.neuroimage.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Fornito A, Arnatkeviciute A, Fulcher BD. Bridging the gap between connectome and transcriptome. Trends Cogn. Sci. 2019;23:34–50. doi: 10.1016/j.tics.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, C. et al. Genetic architecture underlying differential resting-state functional connectivity of subregions within the human visual cortex. Cereb. Cortex32, 2063–2078 (2021). [DOI] [PubMed]
- 38.Liu S, et al. Frequency-dependent genetic modulation of neuronal oscillations: a combined transcriptome and resting-state functional MRI study. Cereb. Cortex. 2022;32:5132–5144. doi: 10.1093/cercor/bhac003. [DOI] [PubMed] [Google Scholar]
- 39.Liu F, Tian H, Li J, Li S, Zhuo C. Altered voxel-wise gray matter structural brain networks in schizophrenia: Association with brain genetic expression pattern. Brain Imaging Behav. 2019;13:493–502. doi: 10.1007/s11682-018-9880-6. [DOI] [PubMed] [Google Scholar]
- 40.Romme IA, de Reus MA, Ophoff RA, Kahn RS, van den Heuvel MP. Connectome disconnectivity and cortical gene expression in patients with schizophrenia. Biol. Psychiatry. 2017;81:495–502. doi: 10.1016/j.biopsych.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Morgan SE, et al. Cortical patterning of abnormal morphometric similarity in psychosis is associated with brain expression of schizophrenia-related genes. Proc. Natl Acad. Sci. USA. 2019;116:9604–9609. doi: 10.1073/pnas.1820754116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji Y, et al. Genes associated with gray matter volume alterations in schizophrenia. Neuroimage. 2021;225:117526. doi: 10.1016/j.neuroimage.2020.117526. [DOI] [PubMed] [Google Scholar]
- 43.Xue, K. et al. Local dynamic spontaneous brain activity changes in first-episode, treatment-naive patients with major depressive disorder and their associated gene expression profiles. Psychol. Med.52, 2052–2061 (2020). [DOI] [PubMed]
- 44.Xie Y, et al. Brain mRNA expression associated with cortical volume alterations in autism spectrum disorder. Cell Rep. 2020;32:108137. doi: 10.1016/j.celrep.2020.108137. [DOI] [PubMed] [Google Scholar]
- 45.Li J, et al. Cortical structural differences in major depressive disorder correlate with cell type-specific transcriptional signatures. Nat. Commun. 2021;12:1647. doi: 10.1038/s41467-021-21943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson KM, et al. Convergent molecular, cellular, and cortical neuroimaging signatures of major depressive disorder. Proc. Natl Acad. Sci. USA. 2020;117:25138–25149. doi: 10.1073/pnas.2008004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Althubaity N, et al. Choroid plexus enlargement is associated with neuroinflammation and reduction of blood brain barrier permeability in depression. Neuroimage Clin. 2022;33:102926. doi: 10.1016/j.nicl.2021.102926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Albajes-Eizagirre A, Solanes A, Vieta E, Radua J. Voxel-based meta-analysis via permutation of subject images (PSI): theory and implementation for SDM. Neuroimage. 2019;186:174–184. doi: 10.1016/j.neuroimage.2018.10.077. [DOI] [PubMed] [Google Scholar]
- 50.Albajes-Eizagirre, A. et al. Meta-analysis of voxel-based neuroimaging studies using seed-based d mapping with permutation of subject images (SDM-PSI). J. Vis. Exp.153, e59841 (2019). [DOI] [PubMed]
- 51.Lancaster JL, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radua J, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatry. 2012;27:605–611. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 54.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 55.Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320:1574–1577. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stanley TD, Doucouliagos H, Ioannidis JPA, Carter EC. Detecting publication selection bias through excess statistical significance. Res. Synth. Methods. 2021;12:776–795. doi: 10.1002/jrsm.1512. [DOI] [PubMed] [Google Scholar]
- 57.Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br. J. Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 58.Su T, et al. Structural and functional brain alterations in anorexia nervosa: a multimodal meta-analysis of neuroimaging studies. Hum. Brain Mapp. 2021;42:5154–5169. doi: 10.1002/hbm.25602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arloth J, Bader DM, Roh S, Altmann A. Re-Annotator: annotation pipeline for microarray probe sequences. PLoS ONE. 2015;10:e0139516. doi: 10.1371/journal.pone.0139516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hawrylycz M, et al. Canonical genetic signatures of the adult human brain. Nat. Neurosci. 2015;18:1832–1844. doi: 10.1038/nn.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Markello RD, Misic B. Comparing spatial null models for brain maps. Neuroimage. 2021;236:118052. doi: 10.1016/j.neuroimage.2021.118052. [DOI] [PubMed] [Google Scholar]
- 62.Burt JB, Helmer M, Shinn M, Anticevic A, Murray JD. Generative modeling of brain maps with spatial autocorrelation. Neuroimage. 2020;220:117038. doi: 10.1016/j.neuroimage.2020.117038. [DOI] [PubMed] [Google Scholar]
- 63.Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009;37:W305–W311. doi: 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dougherty JD, Schmidt EF, Nakajima M, Heintz N. Analytical approaches to RNA profiling data for the identification of genes enriched in specific cells. Nucleic Acids Res. 2010;38:4218–4230. doi: 10.1093/nar/gkq130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu X, Wells AB, O’Brien DR, Nehorai A, Dougherty JD. Cell type-specific expression analysis to identify putative cellular mechanisms for neurogenetic disorders. J. Neurosci. 2014;34:1420–1431. doi: 10.1523/JNEUROSCI.4488-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rappaport N, et al. MalaCards: an amalgamated human disease compendium with diverse clinical and genetic annotation and structured search. Nucleic Acids Res. 2017;45:D877–D887. doi: 10.1093/nar/gkw1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu ML, et al. A review of the functional and anatomical default mode network in schizophrenia. Neurosci. Bull. 2017;33:73–84. doi: 10.1007/s12264-016-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rotarska-Jagiela A, et al. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr. Res. 2010;117:21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 71.Sarpal DK, et al. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 2015;72:5–13. doi: 10.1001/jamapsychiatry.2014.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hall H, et al. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology. 1994;11:245–256. doi: 10.1038/sj.npp.1380111. [DOI] [PubMed] [Google Scholar]
- 73.Cao H, Zhou H, Cannon TD. Functional connectome-wide associations of schizophrenia polygenic risk. Mol. Psychiatry. 2021;26:2553–2561. doi: 10.1038/s41380-020-0699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seymour K, et al. Altered contextual modulation of primary visual cortex responses in schizophrenia. Neuropsychopharmacology. 2013;38:2607–2612. doi: 10.1038/npp.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zmigrod L, Garrison JR, Carr J, Simons JS. The neural mechanisms of hallucinations: a quantitative meta-analysis of neuroimaging studies. Neurosci. Biobehav. Rev. 2016;69:113–123. doi: 10.1016/j.neubiorev.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 76.Jani M, Kasparek T. Emotion recognition and theory of mind in schizophrenia: a meta-analysis of neuroimaging studies. World J. Biol. Psychiatry. 2018;19:S86–S96. doi: 10.1080/15622975.2017.1324176. [DOI] [PubMed] [Google Scholar]
- 77.Watanuki T, et al. Precentral and inferior prefrontal hypoactivation during facial emotion recognition in patients with schizophrenia: a functional near-infrared spectroscopy study. Schizophr. Res. 2016;170:109–114. doi: 10.1016/j.schres.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 78.Cao H, Cannon TD. Cerebellar dysfunction and schizophrenia: from “cognitive dysmetria” to a potential therapeutic target. Am. J. Psychiatry. 2019;176:498–500. doi: 10.1176/appi.ajp.2019.19050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gulsuner S, et al. Genetics of schizophrenia in the South African Xhosa. Science. 2020;367:569–573. doi: 10.1126/science.aay8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim E, Sheng M. The postsynaptic density. Curr. Biol. 2009;19:R723–R724. doi: 10.1016/j.cub.2009.07.047. [DOI] [PubMed] [Google Scholar]
- 81.Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu. Rev. Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- 82.Bayes A, et al. Characterization of the proteome, diseases and evolution of the human postsynaptic density. Nat. Neurosci. 2011;14:19–21. doi: 10.1038/nn.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grant SG. Synaptopathies: diseases of the synaptome. Curr. Opin. Neurobiol. 2012;22:522–529. doi: 10.1016/j.conb.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 84.Kirov G, et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol. Psychiatry. 2012;17:142–153. doi: 10.1038/mp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kristiansen LV, Patel SA, Haroutunian V, Meador-Woodruff JH. Expression of the NR2B-NMDA receptor subunit and its Tbr-1/CINAP regulatory proteins in postmortem brain suggest altered receptor processing in schizophrenia. Synapse. 2010;64:495–502. doi: 10.1002/syn.20754. [DOI] [PubMed] [Google Scholar]
- 86.Copf T. Impairments in dendrite morphogenesis as etiology for neurodevelopmental disorders and implications for therapeutic treatments. Neurosci. Biobehav. Rev. 2016;68:946–978. doi: 10.1016/j.neubiorev.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 87.Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK. Nature of glutamate alterations in schizophrenia: a meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiatry. 2016;73:665–674. doi: 10.1001/jamapsychiatry.2016.0442. [DOI] [PubMed] [Google Scholar]
- 89.Maximo JO, Briend F, Armstrong WP, Kraguljac NV, Lahti AC. Salience network glutamate and brain connectivity in medication-naive first episode patients—a multimodal magnetic resonance spectroscopy and resting state functional connectivity MRI study. Neuroimage Clin. 2021;32:102845. doi: 10.1016/j.nicl.2021.102845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Briend F, et al. Hippocampal glutamate and hippocampus subfield volumes in antipsychotic-naive first episode psychosis subjects and relationships to duration of untreated psychosis. Transl. Psychiatry. 2020;10:137. doi: 10.1038/s41398-020-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nirschl JJ, Ghiretti AE, Holzbaur ELF. The impact of cytoskeletal organization on the local regulation of neuronal transport. Nat. Rev. Neurosci. 2017;18:585–597. doi: 10.1038/nrn.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Michailidis IE, et al. Phosphatidylinositol-4,5-bisphosphate regulates NMDA receptor activity through alpha-actinin. J. Neurosci. 2007;27:5523–5532. doi: 10.1523/JNEUROSCI.4378-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oikonomou KG, Zachou K, Dalekos GN. Alpha-actinin: a multidisciplinary protein with important role in B-cell driven autoimmunity. Autoimmun. Rev. 2011;10:389–396. doi: 10.1016/j.autrev.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 94.Frotscher M. Role for Reelin in stabilizing cortical architecture. Trends Neurosci. 2010;33:407–414. doi: 10.1016/j.tins.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 95.Kumar P, Kumar D, Jha SK, Jha NK, Ambasta RK. Ion channels in neurological disorders. Adv. Protein Chem. Struct. Biol. 2016;103:97–136. doi: 10.1016/bs.apcsb.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 96.Eijkelkamp N, et al. Neurological perspectives on voltage-gated sodium channels. Brain. 2012;135:2585–2612. doi: 10.1093/brain/aws225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rees E, et al. Targeted sequencing of 10,198 samples confirms abnormalities in neuronal activity and implicates voltage-gated sodium channels in schizophrenia pathogenesis. Biol. Psychiatry. 2019;85:554–562. doi: 10.1016/j.biopsych.2018.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Berridge MJ. Calcium signalling and psychiatric disease: bipolar disorder and schizophrenia. Cell Tissue Res. 2014;357:477–492. doi: 10.1007/s00441-014-1806-z. [DOI] [PubMed] [Google Scholar]
- 99.Hertzberg L, Katsel P, Roussos P, Haroutunian V, Domany E. Integration of gene expression and GWAS results supports involvement of calcium signaling in Schizophrenia. Schizophr. Res. 2015;164:92–99. doi: 10.1016/j.schres.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 100.Lidow MS. Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain Res. Brain Res. Rev. 2003;43:70–84. doi: 10.1016/S0165-0173(03)00203-0. [DOI] [PubMed] [Google Scholar]
- 101.Cross-Disorder Group of the Psychiatric Genomics, C. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kaar SJ, Natesan S, McCutcheon R, Howes OD. Antipsychotics: mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology. 2020;172:107704. doi: 10.1016/j.neuropharm.2019.107704. [DOI] [PubMed] [Google Scholar]
- 103.Goff DC. The pharmacologic treatment of schizophrenia-2021. J. Am. Med. Assoc. 2021;325:175–176. doi: 10.1001/jama.2020.19048. [DOI] [PubMed] [Google Scholar]
- 104.Kim MJ, et al. Functional analysis of rare variants found in schizophrenia implicates a critical role for GIT1-PAK3 signaling in neuroplasticity. Mol. Psychiatry. 2017;22:417–429. doi: 10.1038/mp.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kyosseva SV, et al. Mitogen-activated protein kinases in schizophrenia. Biol. Psychiatry. 1999;46:689–696. doi: 10.1016/S0006-3223(99)00104-3. [DOI] [PubMed] [Google Scholar]
- 106.Zhang F, Fang Z, Wang JB. Hint1 knockout results in a compromised activation of protein kinase C gamma in the brain. Brain Res. 2015;1622:196–203. doi: 10.1016/j.brainres.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 107.Hoseth EZ, et al. Exploring the Wnt signaling pathway in schizophrenia and bipolar disorder. Transl. Psychiatry. 2018;8:55. doi: 10.1038/s41398-018-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cai HQ, et al. Altered levels of immune cell adhesion molecules are associated with memory impairment in schizophrenia and healthy controls. Brain Behav. Immunity. 2020;89:200–208. doi: 10.1016/j.bbi.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 109.Maynard TM, Sikich L, Lieberman JA, LaMantia AS. Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr. Bull. 2001;27:457–476. doi: 10.1093/oxfordjournals.schbul.a006887. [DOI] [PubMed] [Google Scholar]
- 110.Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/S0140-6736(94)90569-X. [DOI] [PubMed] [Google Scholar]
- 111.Cannon M, et al. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch. Gen. Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- 112.Erlenmeyer-Kimling L. Neurobehavioral deficits in offspring of schizophrenic parents: liability indicators and predictors of illness. Am. J. Med. Genet. 2000;97:65–71. doi: 10.1002/(SICI)1096-8628(200021)97:1<65::AID-AJMG9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 113.Grundmann M, et al. Lack of beta-arrestin signaling in the absence of active G proteins. Nat. Commun. 2018;9:341. doi: 10.1038/s41467-017-02661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.O’Hayre M, et al. Genetic evidence that beta-arrestins are dispensable for the initiation of beta2-adrenergic receptor signaling to ERK. Sci. Signal. 2017;10:eaal3395. doi: 10.1126/scisignal.aal3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schneider F, et al. Impairment in the specificity of emotion processing in schizophrenia. Am. J. Psychiatry. 2006;163:442–447. doi: 10.1176/appi.ajp.163.3.442. [DOI] [PubMed] [Google Scholar]
- 116.Hall J, et al. Overactivation of fear systems to neutral faces in schizophrenia. Biol. Psychiatry. 2008;64:70–73. doi: 10.1016/j.biopsych.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 117.Kinoshita Y, et al. Fear of negative evaluation is associated with delusional ideation in non-clinical population and patients with schizophrenia. Soc. Psychiatry Psychiatr. Epidemiol. 2011;46:703–710. doi: 10.1007/s00127-010-0237-8. [DOI] [PubMed] [Google Scholar]
- 118.Carment L, et al. Impaired attentional modulation of sensorimotor control and cortical excitability in schizophrenia. Brain. 2019;142:2149–2164. doi: 10.1093/brain/awz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.O’Donnell BF, et al. Selective deficits in visual perception and recognition in schizophrenia. Am. J. Psychiatry. 1996;153:687–692. doi: 10.1176/ajp.153.5.687. [DOI] [PubMed] [Google Scholar]
- 120.Crawford TJ, et al. The perception of real and illusory motion in schizophrenia. Neuropsychologia. 2010;48:3121–3127. doi: 10.1016/j.neuropsychologia.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 121.Kim J, Doop ML, Blake R, Park S. Impaired visual recognition of biological motion in schizophrenia. Schizophr. Res. 2005;77:299–307. doi: 10.1016/j.schres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 122.Chen Y. Abnormal visual motion processing in schizophrenia: a review of research progress. Schizophr. Bull. 2011;37:709–715. doi: 10.1093/schbul/sbr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jia X-Z, et al. Small P values may not yield robust findings: an example using REST-meta-PD. Sci. Bull. 2021;66:2148–2152. doi: 10.1016/j.scib.2021.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Allen Human Brain Atlas is available at https://human.brain-map.org/static/download. The MalaCards database is available at https://www.malacards.org/. The Neurosynth database is available at https://neurosynth.org/. All other data supporting the findings of this study are available from the corresponding author on reasonable request.
All relevant analysis scripts are available from the corresponding author upon request.