Abstract
Objective:
The function of Th17 cells in the neuroinflammatory process in multiple sclerosis (MS) has been previously clarified. It has been suggested that Quercetin can influence MS due to a variety of anti-inflammatory effects. The present study aimed to examine in vitro immunomodulatory aspects of Quercetin Penta Acetate as a modified compound on Th17 cells of MS patients and also to compare its effects with Quercetin.
Materials and Methods:
In this experimental study, peripheral blood mononuclear cell (PBMCs) were isolated and stained with CFSE then, half-maximal inhibitory concentration (IC50) values were determined using different doses and times for Quercetin Penta Acetate, and Methyl Prednisolone Acetate. Th17 cell proliferation was analyzed by flow cytometry and the expression levels of IL-17 and RORc genes were assessed by real-time polymerase chain reaction (PCR) method.
Results:
The results showed that IL-17A gene expression was inhibited by Quercetin Penta Acetate (P=0.0081), but Quercetin Penta Acetate did not have a significant inhibitory effect on Th17 cells proliferation (P= 0.59) and RORc gene expression (P=0.1), compared to Quercetin.
Conclusion:
Taken together, our results showed some immunomodulatory aspects of Quercetin Penta Acetate on Th17 cells are more effective than Quercetin and it could be considered in the treatment of MS.
Keywords: Multiple Sclerosis, Prednisolone, Quercetin, Th17 Cells
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease of the central nervous system (CNS) characterized by neuroinflammation which is followed by demyelination and neurodegeneration. There are over 2.5 million people worldwide who have MS. While all the factors involved in MS have not been completely revealed, gene-environment interaction creates an imbalance in the normal function of the immune system and this is where the inflammation processes initiate. Two key cells of adaptive immunity T helper (Th1), and Th17 are involved in MS (1). The differentiation of Th17 cells depends on the gene expression of retinoic acid-related orphan receptor (RORc).
The Th17 cells have mainly cytokine profile including IL-17, IL-23, IL-22, and IL-21 with IL-17A being the most prominent one. In the IL-17 family, IL-17A and IL-17F are better known for their functional and biological importance (2). IL-17 secretion is induced by TGF-β1, IL-1β, IL-6, and IL-23 in Th17 (3) and the function of Th17 cells in the neuroinflammatory process of the immunopathogenesis experimental autoimmune encephalomyelitis (EAE) model has been clarified (1). The levels of IL-17 in human sera are undetectable but data have reported increases in IL-17mRNA in peripheral blood mononuclear cells (PMBC) obtained from MS patients (4). IL-17-blocking antibodies have been shown to attenuate experimental autoimmune diseases (2), suggesting that the IL-17A plays a crucial role in the development of CNS inflammation in MS patients Also, the Th17 cells contribute to breaking of Th1⁄Th2 axis in an EAE model that is important in the immune pathogenesis of MS (5). The Th17 cells are able to pass blood brain barrier (BBB) more easily compared to the Th1 due to the surface CCR6. Mice lacking CCR6, developed Th17 responses but were highly resistant to the induction of experimental autoimmune (6). In MS, the Th17 cells play their role by proliferation, producing IL-17 and cooperation with other cells.
There is no definitive treatment for MS. Although interferon beta and corticosteroids are commonly used to control the clinical symptoms.
For many years, folk medicines have been important because of their natural basis. Quercetin (3, 3′, 4′, 5, 7-penta hydroxyl flavone) is a flavonoid found in a wide variety of fruits and vegetables, including nuts, grapes, apples, berries, onions, kale, and black tea (7).
Studies have established Quercetin with anti-oxidant (8), anti-inflammatory (9) and anti-infectious (10) properties.
In rats, the administration of Quercetin improves recovery after acute traumatic spinal cord injury in a dose-dependent manner (11) . Quercetin can also reduce apoptotic neuronal cell death induced by microglial stimulation. Combination of Quercetin and the interferon beta (IFN-β) on PBMCs of MS patients have supported positive results on proliferation and gene expression level (12). These findings show that Quercetin can be considered in the treatment of MS because of its immunomodulatory potentials.
Both lipophilic and hydrophilic of Quercetin can be improved by adding groups like acetylation (carbon18) (13, 14). So, changes in the chemical composition of a substance may have some positive or negative effects on its properties.
The effects of Quercetin on Th17 cells have not yet been fully studied. Due to the lipid profile of the BBB region, lipophilicity of the drug is considered as a privilege and can be important along with other antiinflammatory properties (15). Quercetin Penta Acetate was used as a more lipophilic compound with the same natural base.
The aim of the present study was to examine the primary aspects of immunomodulation of Quercetin Penta Acetate as a modified compound on the expression of major differentiating genes, of the Th17 cells of MS patients and the results were compared with Quercetin and Methyl Prednisolone Acetate.
Materials and Methods
Materials
Quercetin (5 mg, Sigma, Germany), Quercetin Penta Acetate (5 mg, Isfahan Pharmaceutical Sciences Research Center, Faculty of Pharmacy, Isfahan University of Medical Sciences, Iran) (the product is still investigational), and Methyl Prednisolone Acetate (5 mg, Kaspean Tamin Pharmaceutical company, Iran) powders, were dissolved in dimethyl sulfoxide (DMSO, Sigma, Germany) with a primary concentration of 10000 µm and then other dilutions were prepared by culture medium RPMI1640 of this base.Subjects
Subjects
In this experimental study, healthy volunteers (n=3) and newly diagnosed MS patients (n=5) participated. All patients were diagnosed based on McDonald criteria and referred by the clinic of MS center Isfahan, Iran (sex and age-matched). MS patients did not have any history of other autoimmune diseases, allergies and, also none of them were treated with anti-inflammatory medications, including steroids therapy or IFN-β at the time of sampling.
They participated in the study at the beginning of remission symptoms that usually was not more than a week. Informed consent form was approved by the Ethical Committees of Isfahan University Medical of Science, Isfahan, Iran (IR.MUI.REC.1396.3.857) and signed by all the participants before enrolling in this study.
All the mandatory laboratory and safety procedures were followed at any experimental work.
Isolation and CFSE stain peripheral blood mononuclear cell
Heparinized venues blood (10 ml) was collected and then PBMCs were isolated by Ficoll gradient density (Biosera, France). PBMCs were removed from the plasma/Ficoll and their viability was checked by trypan blue dye exclusion test.
Briefly, the PBMCs were suspended in phosphate buffered saline (PBS) at the concentration of 10-100×106 cells/ml. Then the cells were stained with 5 µm CFSE (5-(and-6)-carboxy fluorescein diacetate succinimidyl ester prob) (Biolegend, San Diego, CA, USA), according to the kit’s instructions.
IC50 calculation and time response
The CFSE-labeled PBMCs obtained from the previous step were plated in the 24 well plates at a density of 1×106 cells/well, containing RPMI 1640 medium [heatinactivated fetal bovine serum (FBS)] 10% and penicillin/ streptomycin 1%). The cells were stimulated by 0.1 μg/ ml of each soluble anti -CD3 and anti- CD28 monoclonal antibodies (Mabtech, Sweden) and were kept at 37˚C in the humidified atmosphere of 95% air and 5% CO2 . After 24 hours incubation, soluble IL-2 was added with concentration of 100 U/ml (Pepro Tech, UK) and the cells were treated by 1, 10, 100 µm concentrations of Quercetin Penta Acetate for 24, 48, 72 hours and 0.05, 0.5, 5 µm concentrations of Methyl Prednisolone Acetate for 48 hours. The final concentration of DMSO in all experiments did not exceed 1% which is acceptable and was used as a negative control.
To evaluation of the half-maximal inhibitory concentration (IC50) value, CFSE labeled PBMCs were stained with an antibody against theCD4+ (Percp cy5.5, Biolegend, San Diego, CA, USA) and were analyzed using CFSE histogram by a flow cytometry FACS calibur (Becton Dickenson, Bioscience, San Jose, CA, USA). Dose and time with 50% inhibition of proliferation were selected as IC50 value (the doses of 100 µm and 2.5 µm were considered for Quercetin Penta Acetate and Methyl Prednisolone Acetate respectively) and the data were analyzed by the Prism 8.0 and Excel 2015 software (16, 17).
Determination of cytotoxicity effects
To assess cytotoxicity, the PBMCs were cultured with 100 µm Quercetin Penta Acetate, 2 and-5 µm Methyl Prednisolone Acetate and 100 µm Quercetin for 48 hours using the Annexin FITC/PI double staining apoptosis detection kit (BD Biosciences, Waltham, MA). The cells were stained with FITC Annexin V and PI (5 μl) and incubated for 10 minutes at the room temperature in a dark place.
Flow cytometry
The effect of Quercetin, Quercetin Penta Acetate, and Methyl Prednisolone Acetate on the proliferation of CD4+ IL-17+ cells were evaluated in MS patients by CFSE histogram. The CFSE-labeled cells were cultured in 24-well plates and treated with Quercetin (100 µM/ml), Quercetin Penta Acetate (100 µM/ ml), Methyl Prednisolone Acetate (2.5 µM/ml), and DMSO for 48 hours. We added the cells 2 µl/ ml PMA (Phorbol Myristate Acetate)/ionomycin in the presence of brefeldin A (activation cocktail with BFA, Biolegend) for 6 hours, then washed and stained using an intracellular protocol. In brief, the cells were suspended by fixation buffer (Biolegend, San Diego, CA, USA) and washed by permeabilization buffer (Biolegend San Diego, CA, USA) and antibody IL-17 (PE, Biolegend, San Diego, CA, USA).
Real- time polymerase chain reaction
First, total RNA was isolated from the cultured PBMCs using an extraction kit (Yekta tajhiz azma Co. Iran) according to the manufacture instructions. The purity of isolated RNA was determined using a spectrophotometer. The first strand cDNA (Yekta tajhiz azma Co. Iran) was synthesized using the total RNA in the Eppendorf thermal cycler. The expression of RORc and IL-17 mRNAs in were detected in the control and treated groups by quantitative real-time polymerase chain reaction (PCR) using the SYBR Green PCR master mix (BioFact Co. South Korea). The following primers were used by Primer3 site:
RORc-
F: 5ʹ-AGAGATAGAGCACCTGGT-3ʹ
R: 5ʹ-CCACATGGACTTCCTCTG-3´
IL-17A-
F: 5ʹ-GAATCTCCACCGCAATGA-3ʹ
R: 5ʹ-GACACCAGTATCTTCTCCAG-3ʹ.
Finally, the specificity of the primers was evaluated using the Blast NCBI site. The gene levels were normalized to β-act as an internal housekeeping control and the relative expression levels were assessed using the method.
Statistic
Data were analyzed using the SPSS software (V. 22, IBM, Chicago, IL.) and GraphPad Prism 8.0.2 (GraphPad software, San Diego, CA). Data were expressed as mean ± standard error of the mean (SEM). Kolmogorov-Smirnov test was used to detect the normal distribution of data. One-way ANOVA and independent sample t tests were employed for the comparison of significant difference among experimental groups with a normal distribution, while Mann–Whitney and Kruskal–Wallis tests were used to contrast the groups with non-normal distributions. P<0.05 were considered statistically significant.
Results
Evaluation of IC50 value
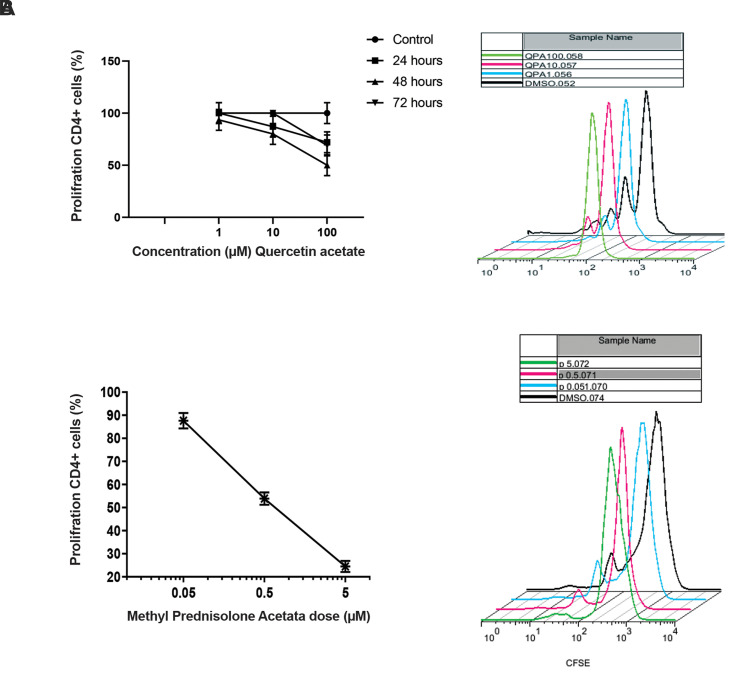
To measure IC50, cultured PBMCs of healthy participants were treated for 24, 48 and 72 hours with different doses of Quercetin Penta Acetate and Methyl Prednisolone Acetate. Quercetin Penta Acetate inhibited proliferation in a dose and time-dependent manner. The IC50 value on cell proliferation was where Quercetin Penta Acetate could inhibit the CD4+ cells proliferation approximately 50% (percentage of proliferation inhibition by CFSE and flow cytometry). As shown Figure 1A, 1 and 10 µm of Quercetin Penta Acetate did not show an effective influence on the cell proliferation after 24, 48, 72 hours of incubation, while 100 µm concentration of Quercetin Penta Acetate had 50% inhibitory effects, IC50 for Methyl Prednisolone Acetate was 2.5 µm concentration after 48 hours culture (Fig .1B).
Fig 1.
IC50 calculation. A. Different doses of Quercetin Penta Acetate for 24, 48, 72 hours and Methyl Prednisolone Acetate for 48 hours. B. The IC50 value CFSE histogram Quercetin Penta Acetate. CFSE histogram Methyl Prednisolone Acetate (0.05, 0.5, 5) for 48 hours in order to determine the dose capable of inhibiting the CD4 cells proliferation by half using flow cytometry. Data were analyzed by flow cytometry histogram of CSFE. Dose/time dependent curve were analyzed by the Prism software for IC50 value and the overly curves were drawn with Flow Jo 7.6.1 software. The data are presented as mean ± SD. Data were analyzed using GraphPad Prism 8.0. One-way ANOVA and independent sample t tests were employed for comparison of significant difference among groups.
The Effect of compounds on cell death in peripheral blood mononuclear cell multiple sclerosis patients
We analyzed PBMCs apoptosis by flow cytometry after Annexin V/PI staining. The data are plotted in two-dimensional dot plots in which PI is represented versus Annexin V-FITC. Different population of cells were considered as Viable cells (PI/FITC-/), apoptotic cells (PI/FITC-/+), late apoptotic cells (PI/FITC +/+) and at least necrotic cells (PI/FITC +/). As shown in Figure 2, while the percentage of necrotic cells and the double positive cells (late apoptosis/necrosis) increased following Quercetin Penta Acetate and Quercetin treatment compared to the control, no obvious difference was observed between Quercetin and Quercetin Penta Acetate.
Fig 2.
The cytotoxicity effect of Q, QPA and MPA. Cultured PBMCs were treated with Q (100 µm), QPA (100 µm) and MPA (2.5 µm). Data indicated double positive cells (late apoptosis/necrosis) increased in QPA and Q compared to controls. MPA; Methyl Prednisolone Acetate, Q; Quercetin, QPA; Quercetin Penta Acetate, PBMCS; Human peripheral blood mononuclear cells, and DMSO; Dimethyl sulfoxide.
The effect of Quercetin Penta Acetate on Th17 cells proliferation in multiple sclerosis patients
In order to test whether Quercetin Penta Acetate can inhibit proliferation of Th17 cells, isolated PBMCs of MS patients were treated at IC50 dose of each compound for 48 hours. We gated CD4+ , IL-17+ cells (Fig .3A, B), the proliferation reduction in the treated Th17 cells with Quercetin Penta Acetate was not remarkable. There was no significant difference among the experimental groups in the proliferation of Th17 cells as shown in (Fig .3C), compared to the control group (P=0.59). The impact of the treatments on the Th17 cells proliferation by CFSE assay (Fig .3D).
Fig 3.
The effects of different treatments on proliferative response Th17 cells in MS patients. CFSE stained PBMCs of MS patients were cultured with 100 µm QPA and Q, 2.5 µm MPA for 48 hours, then the cells were labeled by CD4+ and IL-17 antibody using intracellular staining protocol. Data were pooled from five MS patients and expressed as mean SEM. A. The lymphocyte population were gated using forward and side scatter. B. The population CD4+ (FL3+ Per.cp. cy5.5) IL-17+ (FL2+ PE) were gated to detect proliferation in Th17 cells. C. Comparison between QPA, Q and MPA on Th17 proliferation with DMSO. There was no significant difference between groups with DMSO as control group (P=0.59). D. Different compound on Th17 cells proliferation. One-way ANOVA and independent sample t tests were employed for comparison of significant difference among groups with normal distribution. MS; Multiple sclerosis, PBMCs; Human peripheral blood mononuclear cells, DMSO; Dimethyl sulfoxide, Q; Quercetin, QPA; Quercetin Penta Acetate, and MPA; Methyl Prednisolone Acetate.
Effect of Quercetin Penta Acetate on IL-17A and RORc gene expression in multiple sclerosis patients
The comparison of Quercetin Penta Acetate with Quercetin showed that Quercetin did not have a dramatic effect on IL-17A mRNA level in MS patients. Despite of RORc detection, none of these agents had an effect on the gene expression of RORc in MS patients (P=0.1, Fig .4A). The qPCR results showed (Fig .4B) that IL-17A was reduced significantly by Quercetin Penta Acetate (P=0.0008) and Methyl Prednisolone Acetate (P=0.0081) at the mRNA level compared to treated cells with DMSO (control group).
Fig 4.
The effect of different treatment on gene expression in MS patients. Relative gene expression was measured by RT-q PCR in isolated PBMC from MS patients after co-culturng with QPA (100 µm), Q (100 µm) and MPA (2.5 µm). Data were pooled from five MS patients and expressed as mean SEM. A. To RORc gene expression, there was no significant difference between treated groups and DMSO as a control group (P=0.1). B. IL-17A was reduced significantly by QPA (P=0.0008) and MPA (P=0.0081) at the mRNA level compared to treated cells with DMSO (control group), but effect Q was not significant. One-way ANOVA and independent sample t tests were employed for the comparison of significant difference among groups with normal distribution, while Mann– Whitney and Kruskal–Wallis tests were used to compare the groups with non-normal distribution. P<0.05 were considered statistically significant. ***; P=0.001, **; P=0.001, MS; Multiple sclerosis, RT-q PCR; Quantitative reverse transcription polymerase chain reaction, PBMCs; Human peripheral blood mononuclear cells, DMSO; Dimethyl sulfoxide, Q; Quercetin, QPA; Quercetin Penta Acetate, and MPA; Methyl Prednisolone Acetate.
Discussion
Jadidi-Niaragh et al. (1) have thoroughly described the function of Th17 cells in MS and explained how both Th17 cells and IL-17 have become an interesting therapeutic target in many autoimmune diseases.
Today, a variety of immunomodulatory medicines are used to control MS, which their availability to the CNS can be an important factor in their effectiveness. Many in vitro research have suggested that Quercetin possesses anti-inflammation and immunological functions. Some conjugated metabolites have different behavioral and functional properties (18) , so it is possible these forms of Quercetin might be more efficient and favorable immunomodulatory effects (19). The number of published articles for the effects of Quercetin on Th17 cells and its cytokines are low, thus our article can be considered for this reason.
The second phase of inflammation which leads to the production of pro-inflammatory cytokines occurs when activated cells pass through the BBB and proliferate (20). Quercetin could influence on T cells proliferation and activation by blocking the signaling pathway of IL-12 (21). A Dose-response study revealed that proliferating PBMCs were resistant to some Quercetin metabolites (22). Sternberg et al. (12) presented the first evidence of the beneficial immunomodulatory effect(s) of Quercetin on isolated PBMCs from MS patients, and reported that proliferation PBMCs of in MS patients could be restrained by Quercetin. In the present study, we examined the effect of Quercetin Penta Acetate and Quercetin on the proliferation of the Th17 cells for the first time in MS patients and we showed that proliferation was reduced by proximately 5% in the T cells, however, this decrease was not significant in Th17s. This suggests that the results of the recent study may be because of the effect of Quercetin on other T cell subclasses, not Th17. In line with the previous study, we found that Quercetin and Quercetin Penta Acetate may induce an apoptotic effect that overlaps with the necrotic effect, they did not differ from each other in terms of cytotoxic effect on cells. Although the loss of significant proliferation was consistent with previous studies, the slight reduction of proliferation in PBMCs may depend on cell death and activation of apoptotic proteins (23).
Our goal in this study was not only to investigate the effect of Quercetin on Th17 cells of MS patients but also to investigate whether it can be improved by Quercetin Penta Acetate (24). The lipophilic properties of Quercetin and its improvement by adding carboxyl groups have already been proven, butwhether Quercetin Penta Acetate was more potent than Quercetin in properties such as inhibition of proliferation and gene expression were yet to be explored. Many efforts have already been made to improve drug delivery such as using Nano-carriers, Nano-capsule, or adding lipophilic structures such as acetate (25, 26).
While administration of high-dose intravenous Methyl Prednisolone in MS patients, remarkably reduces the count of Th17 cells after treatment (27), the present study indicated that Methyl Prednisolone Acetate did not have a significant effect on Th17 cells proliferation in IC50 dose.
In glial cells, Quercetin can inhibit LPS-induced mRNA levels of TNF (28). Besides the inhibition of the IL-12- STAT4 signaling pathway, Quercetin also suppressed IL-2 and IFNɣ in T lymphocytes and attenuated the EAE model (21). Since, the inhibitory role of Quercetin on a wide range of pro-inflammatory cytokines such as IL6, IL-1β, and TNF has been proven by targeting many intracellular signaling (kinase and phosphatase) and membrane proteins, it has been suggested that Quercetin has the potential to downregulate the inflammatory process in MS patients. Albegova et al. have reported that Quercetin dihydrate is able to decline the level of IL-17 protein to an undetectable extent (29).
Our study showed that Quercetin Penta Acetate could decline the IL-17A gene expression dramatically, but this reduction was more than Quercetin. Suppressor effects of Methyl Prednisolone on IL-17 have been investigated in previous studies and our results were consistent with those studies (30).
RORc, as a specific transcription factor of IL-17A, has an essential role in the development of Th17 cells and the inhibition of RORCc gene expression has been reported in the PBMCs MS patients treated with a high-dose of intravenous Methylprednisolone (31), but our study did not show the same results. It may be due to the result of data scattering or the sample size.
It is known that besides RORc many transcription factors such as STAT3, IRF4, KLF4, and also AHR receptors and microRNA are involved in the regulation of the IL-17A level, however, the signaling pathways which lead to activation of these transcriptional profiles are poorly understood. Therefore they can be the aim of future studies but it’s known that some of these agents can regulate Th17 development via binding to the IL-17A promoter directly without changing ROR expression (2). Hence Quercetin Penta Acetate may decrease IL-17A expression independent of the RORc pathway. In Th1 cells, Quercetin could decrease IL-2 independent of T-bet (32).
Previously, a similar study was conducted on the metabolite Apigenin and the results showed that its Acetate metabolite were more effective in curb proliferation and gene expression than the basic compound (17).
Despite the anti-inflammatory role of Quercetin in Th cells, its regulatory mechanisms remained unknown. We examined the effect of Quercetin Penta Acetate on phenotype Th17 cells proliferation and IL-17, RORc gene expression and also compared it with its base compound Quercetin and common treatment MS, Methyl Prednisolone Acetate. Our results indicated that in the reduction of proliferation there was no difference but Quercetin Penta Acetate acted more efficiently on inhibition of IL-17 gene expression.
Conclusion
It was shown that some immunomodulatory aspects of Quercetin Penta Acetate on Th17 cells of MS patients are more effective than Quercetin, this compound has the potential to have a chance to participate in clinical trials or as a supplementary along with other MS medications. Since the future challenge in this field is to attain a beneficial bioactive form of Quercetin, further research is needed to understand its other immunological aspects.
Acknowledgements
This work is supported by the Applied Physiology Research Center of Isfahan University of Medical Science [no: 298091]. The authors declare that there is no conflict of interest concerning this study.
Authors’ Contributions
N.E., L.A.; Contributed to experimental design, collection and/or assembly of data, N.E.; Contributed to conception and design, and final approval of the manuscript. M.Gh., M.R., N.K., M.E., M.T., N.E., F.A.; Contributed to conception, design, and final approval of the manuscript. All authors read and approved the final manuscript.
References
- 1.Jadidi-Niaragh F, Mirshafiey A. Th17 cell, the new player of neuroinflammatory process in multiple sclerosis. Scand J Immunol. 2011;74(1):1–13. doi: 10.1111/j.1365-3083.2011.02536.x. [DOI] [PubMed] [Google Scholar]
- 2.Khan D, Ansar Ahmed S. Regulation of IL-17 in autoimmune diseases by transcriptional factors and microRNAs. Front Genet. 2015;6:236–236. doi: 10.3389/fgene.2015.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wienke J, Janssen W, Scholman R, Spits H, van Gijn M, Boes M, et al. A novel human STAT3 mutation presents with autoimmunity involving Th17 hyperactivation. Oncotarget. 2015;6(24):20037–20042. doi: 10.18632/oncotarget.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmadi M, Yousefi M, Abbaspour-Aghdam S, Dolati S, Aghebati- Maleki L, Eghbal-Fard S, et al. Disturbed Th17/Treg balance, cytokines, and miRNAs in peripheral blood of patients with Behcet’s disease. J Cell Physiol. 2019;234(4):3985–3994. doi: 10.1002/jcp.27207. [DOI] [PubMed] [Google Scholar]
- 5.Milo R, Miller A. Revised diagnostic criteria of multiple sclerosis. Autoimmun Rev. 2014;13(4-5):518–524. doi: 10.1016/j.autrev.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Notoya M, Tsukamoto Y, Nishimura H, Woo JT, Nagai K, Lee IS, et al. Quercetin, a flavonoid, inhibits the proliferation, differentiation, and mineralization of osteoblasts in vitro. Eur J Pharmacol. 2004;485(1-3):89–96. doi: 10.1016/j.ejphar.2003.11.058. [DOI] [PubMed] [Google Scholar]
- 7.Kelly GS. Quercetin.Monograph. Altern Med Rev. 2011;16(2):172–194. [PubMed] [Google Scholar]
- 8.Costa LG, Garrick JM, Roquè PJ, Pellacani C. Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid Med Cell Longev. 2016;2016:2986796–2986796. doi: 10.1155/2016/2986796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginwala R, Bhavsar R, Chigbu DI, Jain P, Khan ZK. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants (Basel) 2019;8(2):35–35. doi: 10.3390/antiox8020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biharee A, Sharma A, Kumar A, Jaitak V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia. 2020;146:104720–104720. doi: 10.1016/j.fitote.2020.104720. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Li W, Wang M, Lin C, Li G, Zhou X, et al. Quercetin reduces neural tissue damage and promotes astrocyte activation after spinal cord injury in rats. J Cell Biochem. 2018;119(2):2298–2306. doi: 10.1002/jcb.26392. [DOI] [PubMed] [Google Scholar]
- 12.Sternberg Z, Chadha K, Lieberman A, Hojnacki D, Drake A, Zamboni P, et al. Quercetin and interferon-beta modulate immune response(s) in peripheral blood mononuclear cells isolated from multiple sclerosis patients. J Neuroimmunol. 2008;205(1-2):142–147. doi: 10.1016/j.jneuroim.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Islam MS, Quispe C, Hossain R, Islam MT, Al-Harrasi A, Al-Rawahi A, et al. Neuropharmacological effects of quercetin: a literaturebased review. Front Pharmacol. 2021;12:665031–665031. doi: 10.3389/fphar.2021.665031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grande F, Parisi OI, Mordocco RA, Rocca C, Puoci F, Scrivano L, et al. Quercetin derivatives as novel antihypertensive agents: Synthesis and physiological characterization. Eur J Pharm Sci. 2016;82:161–170. doi: 10.1016/j.ejps.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Khosa A, Saha RN, Singhvi G. In: Nanomaterials for drug delivery and therapy.New York.Elsevier. Grumezescu AM, editor. Elsevier; 2019. Drug delivery to the brain; pp. 461–514. [Google Scholar]
- 16.Scottà C, Fanelli G, Hoong SJ, Romano M, Lamperti EN, Sukthankar M, et al. Impact of immunosuppressive drugs on the therapeutic efficacy of ex vivo expanded human regulatory T cells. Haematologica. 2016;101(1):91–100. doi: 10.3324/haematol.2015.128934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahmati M, Ghannadian SM, Kasiri N, Ahmadi L, Motedayyen H, Shaygannejad V, et al. Modulation of Th17 proliferation and IL-17A gene expression by acetylated form of apigenin in patients with multiple sclerosis. Immunol Invest. 2021;50(2-3):216–229. doi: 10.1080/08820139.2020.1726381. [DOI] [PubMed] [Google Scholar]
- 18.Andrade PB, Grosso C, Valentao P, Bernardo J. Flavonoids in neurodegeneration: limitations and strategies to cross CNS barriers. Curr Med Chem. 2016;23(36):4151–4174. doi: 10.2174/0929867323666160809094934. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, et al. Quercetin, inflammation and immunity. Nutrients. 2016;8(3):167–167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang K, Song F, Fernandez-Escobar A, Luo G, Wang JH, Sun Y. The properties of cytokines in multiple sclerosis: pros and cons. Am J Med Sci. 2018;356(6):552–560. doi: 10.1016/j.amjms.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Muthian G, Bright JJ. Quercetin, a flavonoid phytoestrogen, ameliorates experimental allergic encephalomyelitis by blocking IL-12 signaling through JAK-STAT pathway in T lymphocyte. J Clin Immunol. 2004;24(5):542–552. doi: 10.1023/B:JOCI.0000040925.55682.a5. [DOI] [PubMed] [Google Scholar]
- 22.Cao L, Yang Y, Ye Z, Lin B, Zeng J, Li C, et al. Quercetin3methyl ether suppresses human breast cancer stem cell formation by inhibiting the Notch1 and PI3K/Akt signaling pathways. Int J Mol Med. 2018;42(3):1625–1636. doi: 10.3892/ijmm.2018.3741. [DOI] [PubMed] [Google Scholar]
- 23.Khorsandi L, Orazizadeh M, Niazvand F, Abbaspour MR, Mansouri E, Khodadadi A. Quercetin induces apoptosis and necroptosis in MCF-7 breast cancer cells. Bratisl Lek Listy. 2017;118(2):123–128. doi: 10.4149/BLL_2017_025. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Bai L, Li X, Xiong J, Xu P, Guo C, et al. Transport of active flavonoids, based on cytotoxicity and lipophilicity: an evaluation using the blood-brain barrier cell and Caco-2 cell models. Toxicol In Vitro. 2014;28(3):388–396. doi: 10.1016/j.tiv.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Tosi G, Duskey JT, Kreuter J. Nanoparticles as carriers for drug delivery of macromolecules across the blood-brain barrier. Expert Opin Drug Deliv. 2020;17(1):23–32. doi: 10.1080/17425247.2020.1698544. [DOI] [PubMed] [Google Scholar]
- 26.Md S, Bhattmisra SK, Zeeshan F, Shahzad N, Mujtaba MA, Meka VS, et al. Nano-carrier enabled drug delivery systems for nose to brain targeting for the treatment of neurodegenerative disorders. J Drug Deliv Sci Technol. 2018;43:295–310. [Google Scholar]
- 27.Dos Passos GR, Sato DK, Becker J, Fujihara K. Th17 cells pathways in multiple sclerosis and neuromyelitis optica spectrum disorders: pathophysiological and therapeutic implications. Mediators Inflamm. 2016;2016:5314541–5314541. doi: 10.1155/2016/5314541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siard MH, McMurry KE, Adams AA. Effects of polyphenols including curcuminoids, resveratrol, quercetin, pterostilbene, and hydroxypterostilbene on lymphocyte pro-inflammatory cytokine production of senior horses in vitro. Vet Immunol Immunopathol. 2016;173:50–59. doi: 10.1016/j.vetimm.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Albegova DZ, Kamkina OV, Pavlova SI, Albegova ZhK, Laptev OS, Kozlov IG. Evaluation of the effect of modified bioflavonoid and quercetin dihydrate on cytokine secretion by mitogen-activated mononuclear cells. Bull Exp Biol Med. 2015;159(5):626–628. doi: 10.1007/s10517-015-3031-5. [DOI] [PubMed] [Google Scholar]
- 30.Miljković Z, Momcilović M, Miljković D, Mostarica-Stojković M. Methylprednisolone inhibits IFN-gamma and IL-17 expression and production by cells infiltrating central nervous system in experimental autoimmune encephalomyelitis. J Neuroinflammation. 2009;6:37–37. doi: 10.1186/1742-2094-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu M, Hu X, Wang Y, Peng F, Yang Y, Chen X, et al. Effect of highdose methylprednisolone treatment on Th17 cells in patients with multiple sclerosis in relapse. Acta Neurol Scand. 2009;120(4):235–241. doi: 10.1111/j.1600-0404.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee KM, Kang JH, Yun M, Lee SB. Quercetin inhibits the poly(dA:dT)-induced secretion of IL-18 via down-regulation of the expressions of AIM2 and pro-caspase-1 by inhibiting the JAK2/ STAT1 pathway in IFN-γ-primed human keratinocytes. Biochem Biophys Res Commun. 2018;503(1):116–122. doi: 10.1016/j.bbrc.2018.05.191. [DOI] [PubMed] [Google Scholar]