Abstract
The four class A penicillin-binding proteins (PBPs) of Bacillus subtilis appear to play functionally redundant roles in polymerizing the peptidoglycan (PG) strands of the vegetative-cell and spore walls. The ywhE product was shown to bind penicillin, so the gene and gene product were renamed pbpG and PBP2d, respectively. Construction of mutant strains lacking multiple class A PBPs revealed that, while PBP2d plays no obvious role in vegetative-wall synthesis, it does play a role in spore PG synthesis. A pbpG null mutant produced spore PG structurally similar to that of the wild type; however, electron microscopy revealed that in a significant number of these spores the PG did not completely surround the spore core. In a pbpF pbpG double mutant this spore PG defect was apparent in every spore produced, indicating that these two gene products play partially redundant roles. A normal amount of spore PG was produced in the double mutant, but it was frequently produced in large masses on either side of the forespore. The double-mutant spore PG had structural alterations indicative of improper cortex PG synthesis, including twofold decreases in production of muramic δ-lactam and l-alanine side chains and a slight increase in cross-linking. Sporulation gene expression in the pbpF pbpG double mutant was normal, but the double-mutant spores failed to reach dormancy and subsequently degraded their spore PG. We suggest that these two forespore-synthesized PBPs are required for synthesis of the spore germ cell wall, the first layer of spore PG synthesized on the surface of the inner forespore membrane, and that in the absence of the germ cell wall the cells lack a template needed for proper synthesis of the spore cortex, the outer layers of spore PG, by proteins on the outer forespore membrane.
Peptidoglycan (PG) is the essential structural element that provides shape and stability to most bacterial cells. In the dormant endospore PG is required for the maintenance of spore core dehydration and therefore for spore heat resistance. Both vegetative-cell and spore PGs are composed of glycan strands of repeating N-acetylglucosamine and N-acetyl muramic acid residues cross-linked by peptide side chains (reviewed in reference 2). Polymerization of PG involves the addition of disaccharide pentapeptide subunits onto a growing glycan strand by a glycosyltransferase. The peptide side chains are then utilized by a transpeptidase to cross-link the glycan strands. Side chains that are not utilized for cross-linking are cleaved to tripeptides or tetrapeptides.
Spore PG consists of two layers that can be distinguished structurally and functionally. The germ cell wall is adjacent to the inner forespore membrane and serves as the initial cell wall during spore germination and outgrowth. It is surrounded by the cortex, which comprises the outer 70 to 90% of the spore PG (28) and which is rapidly degraded during spore germination (4, 14). Cortex PG is more loosely cross-linked than vegetative PG, and 50% of the N-acetyl muramic acid residues have had their peptide side chains removed and have been converted to muramic δ-lactam (3, 37, 54, 55). The structure of the germ cell wall appears to be more similar to that of vegetative PG in that most of the peptide side chains are tripeptides and there is little or no muramic δ-lactam (4, 28, 53). Despite the differences between the spore and vegetative PG structure, the mechanisms of PG polymerization in the two situations appear to be similar.
The glycosyltransferase and transpeptidase activities required for PG synthesis are found in the penicillin-binding proteins (PBPs) (15). The PBPs can be placed into three classes based on amino acid sequence similarities (15, 16). Class A high-molecular-weight PBPs are bifunctional PBPs that contain an N-terminal glycosyltransferase domain and a C-terminal transpeptidase domain. Class B high-molecular-weight PBPs are known to have only transpeptidase activity and are, in some cases, required for cell septation and maintenance of cell shape (30, 49, 50, 56). Low-molecular-weight PBPs generally have d,d-carboxypeptidase activity and, in some cases, are involved in regulating the number of cross-links between the glycan strands (36, 39, 46).
Redundancy in the functions of multiple class A PBPs has been previously demonstrated in vegetative cells of Bacillus subtilis (44), Escherichia coli (13, 52, 57), and Streptococcus pneumoniae (19, 34). Sequence analysis of the B. subtilis genome (24) revealed genes encoding four class A PBPs (35, 44). Loss of three (PBP1, PBP2c, and PBP4) of the four slows the vegetative-growth rate, mostly due to the loss of PBP1, and decreases the production of spores 10-fold (44). Recent studies indicated that YwhE, the fourth class A PBP, has no effect on vegetative PG synthesis and demonstrated that ywhE is expressed only in the forespore under the control of ςF and, to a lesser degree, ςG (35). Another class A PBP, PBP2c, is expressed vegetatively but is also induced in the forespore under the control of ςG (42), suggesting potential roles for both PBP2c and YwhE in spore PG synthesis or spore germination. In this communication we present studies examining the phenotypes of double and triple class A PBP mutants lacking ywhE. We demonstrate that loss of both PBP2c and YwhE has no effect on vegetative growth but that this double mutant is unable to complete sporulation.
MATERIALS AND METHODS
Bacterial growth, transformation, and sporulation.
All strains of B. subtilis listed in Table 1 were derivatives of strain 168. Transformation was performed as previously described (1). Transformants were selected and maintained using appropriate antibiotics: chloramphenicol (3 μg/ml), spectinomycin (100 μg/ml), kanamycin (10 μg/ml), tetracycline (10 μg/ml), and erythromycin (0.5 μg/ml) plus lincomycin (12.5 μg/ml; macrolide-lincosamide-streptogramin B resistance). Antibiotics were omitted in cultures grown for determination of growth rates, sporulation efficiencies, and spore PG structure.
TABLE 1.
B. subtilis strains used
| Strain | Genotypea | Transformation
|
Source or reference | |
|---|---|---|---|---|
| Donor(s) | Recipient | |||
| IA626 | hisA82::Tn917 | Bacillus Genetic Stock Center | ||
| AD51 | gerE-lacZ | 11 | ||
| AD52 | cotD-lacZ | 58 | ||
| AD799 | sspB-lacZ | 26 | ||
| DPVB29 | Double ΔpbpD::Cm | pDPC271 | PS832 | This work |
| DPVB30 | ΔpbpD (Spo−) | This work | ||
| DPVB40 | ΔpbpD hisA82::Tn917 (Spo+) | 1A626 | DPVB30 | This work |
| DPVB42 | ΔpbpD (Spo+) | DPVB30 | DPVB40 | This work |
| DPVB45 | ΔpbpG::Kn | pDPV35 | PS832 | This work |
| DPVB46 | ΔpbpD ΔpbpF::Ermr | PS1869 | DPVB42 | This work |
| DPVB49 | ΔpbpD ΔpbpG::KnΔpbpF::Ermr | DPVB45 | DPVB46 | This work |
| DPVB56 | ΔpbpG::KnΔpbpF::Ermr | DPVB45 | PS1869 | This work |
| DPVB57 | ΔpbpD ΔpbpG::Kn | DPVB45 | DPVB42 | This work |
| DPVB61 | ΔponA::Sp ΔpbpG::Kn | PS2062 | DPVB45 | This work |
| DPVB62 | ΔponA::Sp ΔpbpD ΔpbpG::Kn | PS2062 | DPVB57 | This work |
| DPVB63 | ΔponA::Sp ΔpbpG::Kn ΔpbpF::Ermr | PS2062 | DPVB56 | This work |
| DPVB68 | ΔponA::Sp ΔpbpD | PS2062 | DPVB42 | This work |
| DPVB69 | ΔponA::Sp ΔpbpD ΔpbpF::Ermr | PS2062 | DPVB46 | This work |
| DPVB84 | sspB-lacZ | AD799 | PS832 | This work |
| DPVB85 | sspB-lacZ ΔpbpF::Ermr | AD799 | PS1869 | This work |
| DPVB86 | sspB-lacZ ΔpbpG::Kn | AD799 | DPVB45 | This work |
| DPVB89 | sspB-lacZ ΔpbpG::Kn ΔpbpF::Ermr | DPVB84 | DPVB56 | This work |
| DPVB90 | gerE-lacZ | AD51 | PS832 | This work |
| DPVB91 | gerE-lacZ ΔpbpF::Ermr | AD51 | PS1869 | This work |
| DPVB92 | gerE-lacZ ΔpbpG::Kn | AD51 | DPVB45 | This work |
| DPVB93 | gerE-lacZ ΔpbpG::Kn ΔpbpF::Ermr | AD51 | DPVB56 | This work |
| DPVB94 | cotD-lacZ | AD52 | PS832 | This work |
| DPVB95 | cotD-lacZ ΔpbpF::Ermr | AD52 | PS1869 | This work |
| DPVB96 | cotD-lacZ ΔpbpG::Kn | AD52 | DPVB45 | This work |
| DPVB97 | cotD-lacZ ΔpbpG::Kn ΔpbpF::Ermr | AD52 | DPVB56 | This work |
| DPVB141 | ΔpbpG::Kn cwlD::Cm | PS2307 | DPVB45 | This work |
| DPVB142 | ΔpbpF::ErmrcwlD::Cm | PS2307 | PS1869 | This work |
| DPVB143 | ΔpbpG::Kn ΔpbpF::Erm cwlD::Cm | PS2307 | DPVB56 | This work |
| DPVB157 | ΔpbpF::Erm sleB::Sp cwlJ::Tetr | FB111, FB112 | PS1869 | This work |
| DPVB158 | ΔpbpG::Kn sleB::Sp cwlJ::Tetr | FB111, FB112 | DPVB45 | This work |
| DPVB159 | ΔpbpG::Kn ΔpbpF::ErmrsleB::Sp cwlJ::Tetr | FB111, FB112 | DPVB56 | This work |
| FB111 | cwlJ::Tetr | 33 | ||
| FB112 | sleB::Sp | 33 | ||
| FB113 | cwlJ::TetrsleB::Sp | 33 | ||
| PS832 | Prototrophic revertant of strain 168 | Laboratory stock | ||
| PS1869 | ΔpbpF::Ermr | pDPC89 | PS832 | 42 |
| PS2062 | ΔponA::Sp | pDPC197 | PS832 | 41 |
| PS2251 | ΔponA::Sp ΔpbpF::Ermr | PS2062 | PS1869 | 44 |
| PS2307 | cwlD::Cm | ADD1 | PS832 | 45 |
Abbreviations: Ermr resistance to erythromycin and lincomycin; Sp, resistance to spectinomycin; Cm, resistance to chloramphenicol; Kn, resistance to kanamycin; Tetr, resistance to tetracycline.
Growth rates were determined in 2× SG medium (25) at 37°C with shaking. Cultures were allowed to sporulate following nutrient exhaustion for 24 h, at which time spore heat resistance and chloroform resistance were measured as previously described (31). β-Galactosidase activity, glucose dehydrogenase activity, and dipicolinic acid contents of sporulating cultures were assayed as previously described (31). Sporulating cells were prepared for electron microscopy as previously described, except that grids were stained with uranyl acetate as well as Reynolds lead (9). The amount and structure of spore PG produced within cultures were analyzed as previously described (28).
Class A PBP mutant construction.
Plasmid pDPC145 (43) was digested with EcoRI and EcoRV to produce an 800-bp fragment containing the first 147 bp of pbpD plus upstream sequences. This fragment was ligated into EcoRI- and PvuII-digested pDPC179 (43), which contains the last 243 bp of pbpD, to create an in-frame deletion of codons 50 to 543 (out of 624 codons). The plasmid with the deletion, pDPC271, was used to transform PS832 with selection for chloramphenicol resistance. Insertion of pDPC271 into the chromosome via a single crossover results in copies of pbpD on both sides of the vector sequence. Transformants were screened by PCR to identify a strain in which a recombination event caused both copies of pbpD to have the in-frame deletion (ΔpbpD; strain DPVB29) (data not shown). This strain was grown for 50 generations in nonselective liquid media, allowing for recombination of the plasmid out of the chromosome to leave a single ΔpbpD. The culture was plated for single colonies on nonselective media and replica plated to identify a chloramphenicol-sensitive isolate (DPVB30). The single ΔpbpD in this strain was verified using PCR and Southern blot analysis (data not shown). DPVB30 was found to have a Spo− phenotype that was not present in DPVB29 and that was believed to result from a spontaneous mutation in an unrelated locus. DPVB30 was transformed with chromosomal DNA of strain 1A626 with selection for macrolide-lincosamide-streptogramin B resistance. The resulting colonies were screened for cotransformation to a Spo+ phenotype, and one Spo+ isolate was saved as DPVB40. This strain was then transformed with limiting chromosomal DNA from DPVB30 with selection for His+. Most transformants retained the Spo+ phenotype, and one (DPVB42) was verified using PCR to contain ΔpbpD.
A PCR product containing a sequence from 417 bp upstream of the ywhE start codon to 212 bp downstream of the ywhE stop codon (24, 35) was ligated into the pGEM-T vector (Stratagene) to produce pDPV24. This plasmid was digested with PvuII and SalI to obtain a 524-bp fragment containing the first 90 bp of ywhE and with PvuII and SphI to obtain a 485-bp fragment containing the final 251 bp of ywhE. These two fragments were ligated with SphI- and SalI-digested pJH101 to obtain pDPV33, in which bases 91 to 1691 of ywhE are deleted. Plasmid pDG780 (18) was digested with SmaI and HincII to obtain a kanamycin resistance cassette that was inserted into pDPV33 at the PvuII site at the point of the ywhE deletion to create pDPV35. pDPV35 was linearized with ScaI and used to transform PS832, with selection for kanamycin resistance, to create DPVB45 (ΔywhE::Kn). Strains containing multiple mutations were made by transformation using limiting chromosomal DNA and selection with the appropriate antibiotics.
PBP detection.
Penicillin X was synthesized and labeled with 125I as previously described (21, 27). Membranes were prepared from B. subtilis cells at the fourth hour of sporulation in 2× SG medium as previously described (41). Membrane samples containing 40 μg of protein were incubated for 30 min at 30°C with 3 μCi of labeled penicillin X in a total volume of 20 μl of 50 mM Tris-HCl, pH 8.0–1 mM β-mercaptoethanol–0.1 mM phenylmethylsulfonyl fluoride. Proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 7.5% polyacrylamide gel. PBPs were detected and signal intensities were integrated using a STORM 860 phosphorimager and ImageQuant software (Molecular Dynamics).
RESULTS
Construction of class A PBP mutant strains.
Previous genetic analysis of the class A PBPs in B. subtilis utilized some Campbell-type plasmid insertion mutations that have the ability to revert. The appearance of revertants produced significant problems in strains with reduced growth rates due to the loss of multiple class A PBPs. To avoid this problem, we utilized nonrevertible mutations, such as deletions or deletions with antibiotic resistance insertions, in each of the four genes. Each of the mutations removed ≥79% of the genes' coding sequences. Deletion mutations in ponA (41), pbpF (42), and ywhE terminated the coding sequences at or before codon 30 (out of ≥647 codons) so that any resulting protein product would be missing all nine highly conserved motifs found within class A PBPs (16). The in-frame deletion in pbpD removed 79% of the coding sequence, including conserved motifs 1 to 8 (16).
PCR was used to verify the presence of the expected alleles in each mutant strain (data not shown). Southern blot analysis (48) was then used to verify that all of the expected mutations were present as well as the fact that none of the genes were present in a form undetectable by our PCR assay (undetectable due to some undefined nonhomologous recombination event). Two probes were used for each gene. One probe contained a region of the gene outside the deletion to verify the existence of either the wild-type or mutant allele in each strain, and a second probe contained a region interior to the deleted region to verify the complete absence of this region in each mutant. The expected wild-type and mutant genes are present in each of the triple mutants (data not shown).
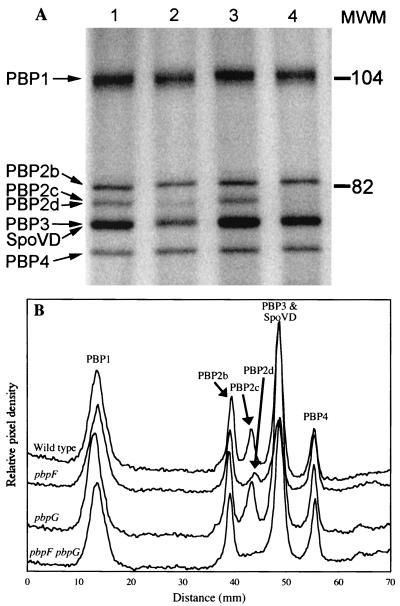
We used radioactively labeled penicillin to visualize the PBPs present in our wild-type and mutant strains. We identified a PBP that appeared to be the product of ywhE in membranes prepared from cells in the fourth hour of sporulation. This PBP migrated on an SDS-PAGE gel in nearly the same place as PBP2c and could only be clearly seen in a pbpF mutant (Fig. 1). This result is consistent with the predicted molecular masses of PBP2c (42) and the ywhE product (35), 79 and 77 kDa, respectively. We refer to ywhE as pbpG and to the gene product as PBP2d from this point on.
FIG. 1.
PBP profiles of wild-type, pbpF mutant, and pbpG mutant strains. Membranes were purified from cultures at the fourth hour of sporulation. (A) Membranes were incubated with 125I-labeled penicillin X, proteins were separated on an SDS–7.5% PAGE gel, and PBPs were detected using a phosphorimager. Lane 1, wild type; lane 2, pbpF; lane 3, pbpG; lane 4, pbpF pbpG. Calibrated molecular mass standards (MWM; in kilodaltons) were Bio-Rad low-range-prestained SDS-PAGE standards. PBP2a decreases dramatically during sporulation (7) and is not visible on this gel. (B) Histogram of PBP band intensities produced by integrating signal strength within columns that covered 90% of each lane's width. PBPs are numbered as previously described (5, 23).
Growth and sporulation of class A PBP mutants.
The growth rates and sporulation efficiencies of ponA, pbpD, and pbpF single- and multiple-mutant strains were consistent with those previously reported (Table 2) (44). Deletion of pbpG, alone and in multiple mutants, had no effect on growth rate (Table 2) (35). The sporulation efficiency of each strain was determined by measuring the number of heat-resistant CFU and comparing this number to the number of viable CFU after 24 h of sporulation. Small decreases in production of heat- and chloroform-resistant spores were observed in strains lacking PBP1 and PBP4 (Table 2), as observed previously (44). These decreases were attributed to poor initiation of sporulation as a result of decreased growth rate rather than to a specific block in the sporulation process (44). In contrast, the pbpF pbpG strain exhibited a growth rate equal to that of the wild type but a >10,000-fold decrease in spore production (Table 2, strain DPVB56). A similar sporulation block was observed in triple mutants that lacked pbpF and pbpG (Table 2, strains DPVB49 and DPVB63).
TABLE 2.
Growth and sporulation of class A PBP mutant strainsa
| Strain | PBP phenotype | Doubling timeb (min) | Cell countsc (CFU/ml)
|
||
|---|---|---|---|---|---|
| Viable | Heat-resistant spores | Chloroform-resistant spores | |||
| PS832 | Wild type | 20 | 2 × 109 | 2 × 109 | 2 × 109 |
| PS1869 | PBP2c− | 20 | 1 × 109 | 2 × 109 | 2 × 109 |
| DPVB45 | PBP2d− | 20 | 4 × 109 | 2 × 109 | 2 × 109 |
| DPVB42 | PBP4− | 20 | 3 × 109 | 2 × 109 | 1 × 109 |
| PS2062 | PBP1− | 25 | 2 × 109 | 3 × 109 | 2 × 109 |
| DPVB57 | PBP4− PBP2d− | 20 | 1 × 109 | 7 × 108 | 9 × 108 |
| DPVB56 | PBP2c− PBP2d− | 20 | 2 × 108 | 4 × 104 | 2 × 104 |
| DPVB46 | PBP2c− PBP4− | 20 | 2 × 109 | 4 × 108 | 7 × 108 |
| DPVB61 | PBP1− PBP2d− | 26 | 2 × 109 | 2 × 109 | 2 × 109 |
| PS2251 | PBP1− PBP2c− | 26 | 7 × 108 | 1 × 109 | 7 × 108 |
| DPVB68 | PBP1− PBP4− | 31 | 5 × 108 | 3 × 107 | 4 × 107 |
| DPVB49 | PBP2c− PBP4− PBP2d− | 21 | 8 × 108 | 7 × 103 | 3 × 102 |
| DPVB63 | PBP1− PBP2c− PBP2d− | 28 | 4 × 108 | 8 × 103 | 5 × 103 |
| DPVB69 | PBP1− PBP2c− PBP4− | 28 | 4 × 108 | 9 × 106 | 5 × 107 |
| DPVB62 | PBP1− PBP4− PBP2d− | 31 | 4 × 108 | 2 × 107 | 1 × 107 |
Doubling times and cell counts are averages from at least three separate experiments.
Growth was in liquid 2 × SG medium at 37°C.
Cell counts determined 24 h after initiation of sporulation.
Regulation of sporulation gene expression in the pbpF pbpG double mutant.
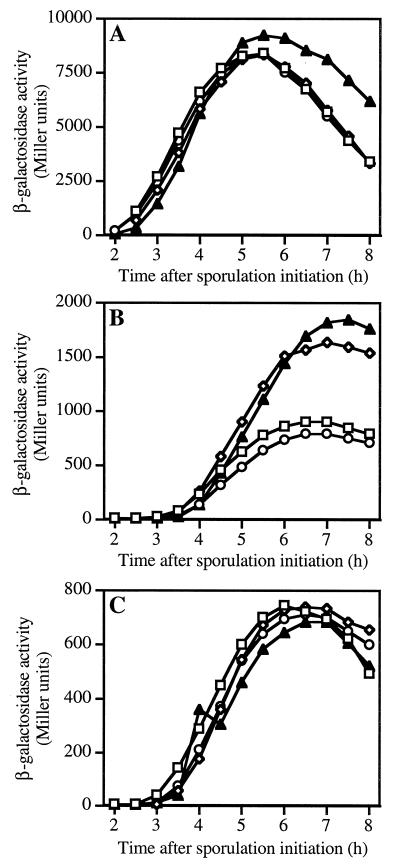
Following engulfment, activation of ςG leads to the expression of a set of sporulation genes in the forespore, some of which, in turn, are required for activation of ςK in the mother cell (reviewed in reference 51). It has been theorized that spore PG synthesis could be one component of the signal needed for the activation of ςK (58). We theorized that loss of expression of two PBPs within the forespore might disrupt spore PG synthesis, in turn blocking ςK activation and completion of sporulation. Studies were performed with wild-type, pbpF, pbpG, and pbpF pbpG strains to determine if gene expression during late sporulation was altered. ςG-dependent gene sspB (Fig. 2A) and ςK-dependent genes cotD (Fig. 2B) and gerE (Fig. 2C) were expressed at or above wild-type levels in all three mutant strains, indicating that there was no block in the signal cascade between the forespore and mother cell.
FIG. 2.
Expression of late-sporulation genes in pbpF and pbpG mutant strains. Cultures of wild-type (□), pbpF (◊), and pbpG (○) single-mutant, and pbpG pbpF double-mutant (▴) strains carrying fusions of lacZ to late sporulation genes were sampled following initiation of sporulation in 2× SG medium at 37°C. β-Galactosidase expression from sspB-lacZ under regulatory control of ςG (A) or from cotD-lacZ (B) and gerE-lacZ (C) under regulatory control of ςK was assayed using o-nitrophenyl-β-d-galactopyranoside as previously described (31). The cause of cotD overexpression in the pbpF mutants is unknown.
Spore PG synthesis in pbpF and pbpG mutants.
The amount and structure of spore PG synthesized in mutant strains were determined. Culture samples were collected every 30 min for 8 h following the initiation of sporulation. The muramic acid contents (28) of the wild-type and pbpF and pbpG single- and double-mutant cultures increased similarly throughout sporulation (data not shown). This muramic acid was present in PG strands because similar amounts of spore PG could be purified in a muramidase-sensitive form from all the sporulating cultures. PG was purified from developing forespores (28) collected throughout sporulation of each culture. Structural analysis of this forespore PG using reverse-phase high-pressure liquid chromatography (28) demonstrated that, throughout sporulation, the pbpF and pbpG strains produced spore PG with structural parameters similar to those found in the wild type (Table 3) (28). The pbpF pbpG strain produced spore PG with altered structural parameters including a twofold reduction in the percentage of muramic acid side chains that were cleaved to form muramic δ-lactam and a threefold reduction in the number of side chains cleaved to single l-alanine residues (Table 3). Increases in the numbers of both tripeptide and tetrapeptide side chains were also observed. The amount of muramic acid involved in cross-linking was slightly higher throughout the spore PG in the double mutant than in the single mutants. Although normal amounts of spore PG could be recovered from the double mutant until at least the eighth hour of sporulation, all spore PG was apparently degraded by 24 h after sporulation initiation.
TABLE 3.
Structural parameters of forespore PG produced by pbpF and pbpG mutant strainsa
| Genotype | Time in sporulation (h) | % Spore PG madeb | % Muramic acid with:
|
||||
|---|---|---|---|---|---|---|---|
| Side chain ofc:
|
Cross-linked side chain | ||||||
| Lactam | l-Ala | TriP | TP | ||||
| pbpF | 3.5 | 2 | 4.6 | 7.9 | 72.1 | 15.4 | 14.8 |
| 4 | 4.5 | 30.3 | 33.3 | 27.5 | 8.9 | 6.3 | |
| 4.5 | 13.5 | 41.4 | 44.2 | 9.5 | 4.9 | 2.7 | |
| 5 | 22.5 | 43.5 | 38.6 | 7.7 | 10.2 | 2.6 | |
| 5.5 | 33 | 44.5 | 30.9 | 5.2 | 19.3 | 2.9 | |
| 6 | 46.5 | 45.6 | 29.3 | 3.7 | 21.3 | 3.0 | |
| 6.5 | 59.5 | 45.5 | 26.5 | 3.3 | 24.7 | 3.2 | |
| 7 | 75 | 46.1 | 26.6 | 2.7 | 24.6 | 3.0 | |
| 7.5 | 89 | 46.4 | 24.8 | 2.4 | 26.4 | 3.1 | |
| 8 | 100 | 46.5 | 22.8 | 2.3 | 28.4 | 3.4 | |
| 24 | 100 | 48.5 | 21.6 | 1.5 | 28.4 | 3.4 | |
| pbpG | 3.5 | 9.5 | 13.2 | 17.1 | 56.8 | 12.9 | 11.1 |
| 4 | 18.5 | 32.4 | 34.3 | 26.2 | 7.2 | 5.5 | |
| 4.5 | 27.5 | 37.3 | 35.6 | 17.7 | 9.4 | 4.1 | |
| 5 | 37.5 | 39.8 | 29.7 | 12.0 | 18.5 | 4.0 | |
| 5.5 | 52 | 41.0 | 27.4 | 9.5 | 22.0 | 3.8 | |
| 6 | 71 | 42.7 | 26.0 | 7.0 | 24.2 | 3.9 | |
| 6.5 | 78.5 | 43.4 | 24.7 | 6.1 | 25.7 | 3.8 | |
| 7 | 86 | 44.4 | 24.6 | 5.4 | 25.6 | 3.7 | |
| 7.5 | 93.5 | 45.1 | 23.6 | 4.9 | 26.4 | 3.9 | |
| 8 | 100 | 46.1 | 23.7 | 4.2 | 26.0 | 3.6 | |
| 24 | 100 | 47.7 | 18.1 | 3.2 | 31.0 | 4.1 | |
| pbpF pbpG | 3.5 | 5 | 0.0 | 0.0 | 80.9 | 19.1 | 14.0 |
| 4 | 11 | 10.9 | 11.5 | 53.4 | 24.2 | 12.1 | |
| 4.5 | 17.5 | 18.8 | 14.9 | 29.6 | 27.7 | 9.7 | |
| 5 | 36 | 16.7 | 7.3 | 21.0 | 55.0 | 7.2 | |
| 5.5 | 57 | 14.3 | 5.4 | 11.1 | 69.3 | 6.5 | |
| 6 | 70 | 15.6 | 5.6 | 8.2 | 70.6 | 6.4 | |
| 6.5 | 81 | 16.4 | 5.5 | 7.2 | 70.9 | 6.5 | |
| 7 | 89.5 | 17.7 | 5.7 | 6.6 | 70.0 | 6.3 | |
| 7.5 | 95 | 18.7 | 6.1 | 6.1 | 69.2 | 6.1 | |
| 8 | 100 | 19.3 | 6.0 | 5.8 | 68.9 | 6.0 | |
| 24 | 0 | ||||||
Forespore PG was purified from culture samples taken every 15 min between the fourth and seventh hours of sporulation, but only data for samples taken every 30 min are shown.
These values are derived from the interpolation of culture muramic acid contents as determined by amino acid analyses (28).
Lactam, muramic δ-lactam; l-Ala, single alanine; TriP, tripeptide; TP, tetrapeptide.
Microscopic examination of mutant cells.
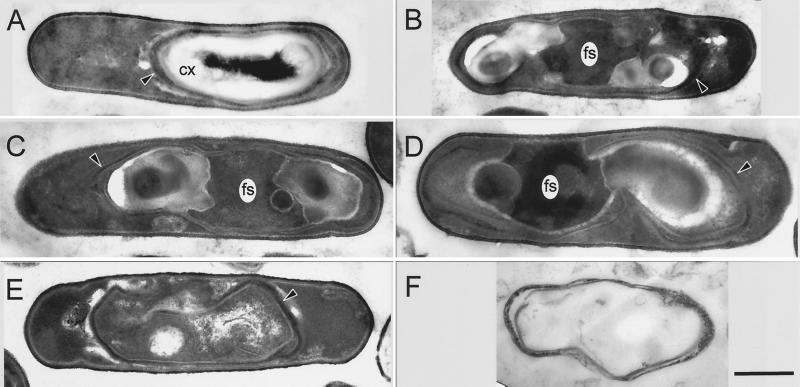
Examination of the pbpF pbpG cells under phase-contrast microscopy 6 h into sporulation revealed that >80% of the cells contained visible phase-dark forespores (data not shown). Twenty four hours following the initiation of sporulation, very few phase-bright endospores were visible. To characterize the status of the spore PG in more detail, we performed thin-section electron-microscopic analysis of mutant cells. In cultures of pbpG cells approximately 7 h after the initiation of sporulation, we observed two morphologically distinct populations. The majority of cells resembled those of a wild-type population (Fig. 3A). In particular, the cortex was clearly visible. In a subset (approximately 35%) of the cells that had clearly completed engulfment, we observed a severe and novel defect in development (Fig. 3B and C). These cells possess what appears to be a highly disorganized forespore. The central regions of these cells resemble a forespore cytoplasm in electron density and granularity. However, instead of being surrounded by a lightly staining region clearly corresponding to the cortex, one or two lightly staining masses were adjacent to the apparent forespore cytoplasm, generally at opposite ends of the forespore. The regions of the section containing these masses frequently sustained tears during electron microscopy, suggesting that the embedding resin they tended to infiltrate poorly into the sample. Spore coat material surrounded these regions. These coats possessed inner and outer layers but tended not to form a contiguous shell and to be thinner than wild-type spore coats. Those cells lacking a normal spore PG layer are not expected to achieve normal heat resistance. We believe that the proportion of defective spores in pbpG cultures is low enough (and possibly highly variable) that it was not detectable in our assays of heat-resistant spore production (Table 2).
FIG. 3.
Thin-section electron microscopy of sporulating mutant cells. Cells of pbpG (A to C) or pbpG pbpF (D to F) strains were sporulated, harvested at hour 7 (A to D) or 24 (E and F), and prepared for electron microscopy. The majority of pbpG cells had an appearance (A) similar to that of the wild type. In a minority of the pbpG cells, masses, presumed to be spore PG, do not completely surround the forespore (B and C). The majority of pbpF pbpG cells had this appearance (D). (E) A pbpF pbpG double mutant cell at the 24th hour of sporulation, in which the masses on either side of the forespore have disappeared. (F) Empty spore coat structure released by lysis of a pbpF pbpG double-mutant cell at the 24th hour of sporulation. CX, cortex; fs, forespore cytoplasm. Arrowheads, spore coat structures. Bar (F), 500 nm (all panels have the same magnification).
pbpF pbpG cells harvested at hour 7 differed from pbpG cells in that the defect was present in all the cells (Fig. 3D). At hour 24 the majority of spore structures within double-mutant sporangia no longer contained the masses (Fig. 3E) and mother cells that had lysed released what appeared to be simply shells of spore coat without any interior PG or cytoplasm (Fig. 3F). One interpretation of these data is that in the absence of pbpG a significant percentage of cells form a defective cortex, resulting in the accumulation of incorrectly assembled PG in pockets near the forespore. This defect is magnified with the addition of a mutation in pbpF, such that none of the cells form functional cortexes. As a consequence of the resulting failure in dehydration, when the mother cell lyses, the spore interior lyses as well, producing a spore that is a fragment of the coat without a core. Several lines of evidence suggest that the masses seen in the pbpF pbpG developing spores consist of disorganized spore PG. The masses are positioned between the inner forespore membrane and the spore coats, as for normal spore PG. The masses disappear by hour 24 of sporulation, and we were unable to recover any spore PG from culture samples at that time (Table 3). Two types of mutations result in stabilization of spore PG in B. subtilis: mutations in cwlJ and sleB, which encode lytic enzymes used in germination (6, 20, 29), and a mutation in cwlD (45), which is required for the production of muramic δ-lactam in the spore PG (3, 38), a recognition determinant for lytic enzymes used in germination. When cwlJ and sleB mutations (33) or a cwlD mutation (45) was introduced into the pbpF pbpG strain, the masses contained within the spore coats were still clearly visible under phase-contrast microscopy at the 24th hour of sporulation (data not shown) and the spore PG that was produced remained stable. Spore PG was isolated from the cwlJ sleB, cwlJ pbpF sleB, cwlJ pbpG sleB, and cwlJ pbpF pbpG sleB strains at both the 8th and 24th hours of sporulation. The presence of the cwlJ and sleB mutations had no effect on the spore PG structure produced by these strains (data not shown). However, the presence of these mutations allowed us to isolate spore PG from the cwlJ pbpF pbpG sleB strain at the 24th hour of sporulation, and we found it to have a structure similar to that produced by the pbpF pbpG strain at the 8th hour of sporulation (Table 3 and data not shown). Similarly, the introduction of a cwlD mutation into each pbp mutant strain resulted only in the loss of all muramic δ-lactam from the spore PG, but spore PG could still be recovered from the cwlD pbpF pbpG strain at the 24th hour of sporulation.
DISCUSSION
Previous studies performed on three class A PBPs of B. subtilis indicated that they have redundant functions in vegetative-PG polymerization but revealed no clear role in spore PG synthesis (44). Pedersen et al. (35) found that pbpG, which encodes a fourth class A PBP, is expressed only during sporulation and that a pbpG mutation had no effect on vegetative growth. We have now shown that loss of pbpG in multiple mutants lacking other class A PBPs reveals no redundant role for PBP2d in vegetative growth. This suggests that in a mutant strain lacking PBPs 1 and 4, which has a greatly reduced growth rate, pbpG is not being induced to take on a significant role in vegetative-wall synthesis.
A pbpF pbpG double-mutant strain has a severe sporulation defect in the absence of any vegetative-growth deficiency. Electron microscopy and biochemical assays of glucose dehydrogenase, dipicolinic acid (data not shown), and spore PG production revealed that this double mutant initiated and progressed through stage four of sporulation at a rate equivalent to those of the wild type and both single mutants. Consistent with a block at this stage, mutant cells never achieved full resistance properties, and the spore was degraded during the next 24 h. The fact that expression of both pbpF and pbpG is induced specifically within the forespore compartment (35, 42) suggests that these proteins might be involved in synthesis of spore PG from the surface of the inner forespore membrane. This is the site of the germ cell wall in the dormant spore, and this structure appears to be synthesized first, prior to synthesis of the cortex PG (28). However, the initial 10 to 20% of the spore PG produced by the double mutant appeared normal, having the structure expected in the germ cell wall (4, 28). The structure of the cortex PG was greatly altered in the double mutant. One major change was a twofold decrease in the amount of muramic δ-lactam, a structural marker used to differentiate cortex from germ cell wall (3, 4, 28, 37, 38). This was surprising since several lines of evidence indicate that the cortex PG is synthesized from the mother cell side. The spore PG defects revealed by electron microscopy in a minority of pbpG mutant sporangia must not reflect a major alteration of spore PG structural parameters or must have been present in too small a percentage of the cells to produce a large change in the spore PG structural parameters determined for the population.
The particular spore PG structural alterations present in the pbpF pbpG strain would not be expected to result in failure to achieve dormancy. Previous studies have shown that mutant strains that produce spore PG containing either no muramic δ-lactam (cwlD strain) (3, 38), high cross-linking (dacB strain) (37, 39), or both (cwlD dacB strain) (40) are able to achieve normal spore dehydration and dormancy. The spore PG produced by a dacB strain also has a threefold decrease in the amount of single l-alanine side chains, similar to that seen in the pbpF pbpG double mutant, but normal spore dormancy. Failure of the pbpF pbpG spores to achieve dormancy is almost certainly due to the large change in the three-dimensional PG architecture we observed in electron micrographs.
We consider several possibilities for the mechanism by which loss of pbpF and pbpG results in altered synthesis of spore cortex PG. One possibility is that cortex PG is not actually produced from the mother cell side and that PBP2c and PBP2d are required on the inner forespore membrane to synthesize this structure. While there is no direct evidence for cortex synthesis from the mother cell side, there are a variety of lines of evidence that suggest this, including the production of spore PG-specific precursors in the mother cell (53), mother cell-specific synthesis of two PBPs that have significant effects on cortex PG synthesis (8, 12, 47), and the fact that the cortex PG appears to be synthesized after the germ cell wall PG (28). A second possibility is that altered synthesis of the first layers of spore PG (alterations of a type undetectable with our current methods of analysis) could disrupt the cell-cell communication carried out by the forespore and mother cell. This communication is necessary for activation of ςK in the mother cell, and ςK activity is required for completion of spore PG synthesis (10). It is possible that spore PG synthesis could be one component of a signal transduced from the forespore to the mother cell (58). Although previous studies indicated that expression of spoIVB is the only function of forespore-specific transcription factor ςG required for activation of ςK (17), and that ςG was required for initiation of spore PG synthesis (22, 32), we felt that previous electron-microscopic examinations could have missed production of a very small amount of spore PG in a sigG mutant. However, expression of genes dependent on both ςG and ςK was normal in the pbpF pbpG strain. It is interesting that the failure of pbpF pbpG double-mutant spores to reach dormancy is similar to the phenotype produced by certain spoIVB point mutants which allow ςK activation but which are deficient in an undefined second role required for spore maturation (32). We plan to examine if similar spore PG defects are present in this type of spoIVB mutant, which might suggest that this second role of spoIVB is exerted through the spore PG synthesis machinery.
A third explanation for defective cortex synthesis in the pbpF pbpG strain is that the pbpF and pbpG products are actually required on the outer forespore membrane. These particular class A PBPs may be required for the coordination of other activities required for production of muramic δ-lactam and l-Ala side chains. Such a model would require both of these gene products to be present and functional on the outer forespore membrane in order to result in the functional redundancy seen in our genetic analysis. The pbpF gene is expressed at relatively low levels during vegetative growth, and during the process of engulfment its product, PBP2c, could be distributed to both the inner and outer forespore membranes. Previous studies of pbpG expression identified only forespore-specific transcription (35). We would have to theorize that either (i) extremely low-level mother cell expression of pbpG, below the detection limit of previous assays, was sufficient to satisfy a requirement for cortex synthesis on the surface of the outer forespore membrane or (ii) PBP2d is produced within the forespore and crosses the inner forespore membrane but, unlike other class A PBPs (16, 44), does not remain associated with this membrane and is free to move to the surface of the outer forespore membrane. Our detection of PBP2d in membrane preparations of sporulating cells argues against this idea.
Finally, the model we prefer is that alteration of the germ cell wall PG structure presents an improper “template” for synthesis of the cortex PG by proteins on the outer forespore membrane. We propose that either PBP2c or PBP2d can carry out synthesis of germ cell wall PG in a uniform shell surrounding the entire forespore. In the absence of both of these PBPs an incomplete germ cell wall is produced (potentially by class A PBPs 1 and/or 4). Cortex PG polymerization is carried out by PBPs associated with the outer forespore membrane, potentially using the germ cell wall PG as a template. We suggest that, in the absence of a proper template, the cortex is synthesized in disorganized masses, often on either side of the forespore. If this synthesis of cortex PG is specifically targeted to the forespore poles, it could possibly be due to remnants of septum PG synthesis machinery. The last known sites of PG synthesis on the membranes surrounding the forespore were at the centers of a vegetative-division septum and the asymmetric sporulation septum. An alternative explanation is that the cortex PG masses are not actually synthesized at the forespore poles but that a major elongation of the spore in one direction, due to the odd PG synthesis at any single site on the forespore surface, causes the forespore to turn within the cell so that the PG extension appears to be at a pole. Finally, PG synthesis at the apparent poles of the forespore may simply be due to the fact that this is where there is available space within the sporangium. The fact that a fraction of pbpG cells produce disorganized cortex PG may be because PBP2d expression in the forespore, directed by ςF, takes place before forespore expression of PBP2c, directed by ςG. In some pbpG cells, cortex synthesis may advance too far in an altered way before PBP2c is produced in large enough amounts to produce a normal germ cell wall. Investigation of the requirements for pbpF and pbpG expression in the mother cell and forespore compartments in order to complete spore formation is a step toward eliminating some of these alternate theories.
ACKNOWLEDGMENTS
This work was supported by grants GM56695 (D.L.P) and GM53989 (A.D.) from the National Institutes of Health.
We thank Madan Paidhungat, Peter and Barbara Setlow, and Patrick Stragier for providing strains, David Nelson and Kevin Young for advice on the use of 125I-penicillin X, Jennifer Meador-Parton and Amy Weaver for technical assistance, and Marita Seppanen Popham for editing the manuscript.
REFERENCES
- 1.Anagnostopoulos C, Spizizen J. Requirements for transformation in Bacillus subtilis. J Bacteriol. 1961;81:74–76. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archibald A R, Hancock I C, Harwood C R. Cell wall structure, synthesis, and turnover. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria. Washington, D.C.: American Society for Microbiology; 1993. pp. 381–410. [Google Scholar]
- 3.Atrih A, Zollner P, Allmaier G, Foster S J. Structural analysis of Bacillus subtilis 168 endospore peptidoglycan and its role during differentiation. J Bacteriol. 1996;178:6173–6183. doi: 10.1128/jb.178.21.6173-6183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atrih A, Zollner P, Allmaier G, Williamson M P, Foster S J. Peptidoglycan structural dynamics during germination of Bacillus subtilis 168 endospores. J Bacteriol. 1998;180:4603–4612. doi: 10.1128/jb.180.17.4603-4612.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumberg P M, Strominger J L. Five penicillin-binding components occur in Bacillus subtilis membranes. J Biol Chem. 1972;247:8107–8113. [PubMed] [Google Scholar]
- 6.Boland F M, Atrih A, Chirakkal H, Foster S J, Moir A. Complete spore-cortex hydrolysis during germination of Bacillus subtilis 168 requires SleB and YpeB. Microbiology. 2000;146:57–64. doi: 10.1099/00221287-146-1-57. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan C E, Gustafson A. Mutagenesis and mapping of the gene for a sporulation-specific penicillin-binding protein in Bacillus subtilis. J Bacteriol. 1992;174:5430–5435. doi: 10.1128/jb.174.16.5430-5435.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchanan C E, Ling M-L. Isolation and sequence analysis of dacB, which encodes a sporulation-specific penicillin-binding protein in Bacillus subtilis. J Bacteriol. 1992;174:1717–1725. doi: 10.1128/jb.174.6.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catalano F, Meador-Parton J, Popham D L, Driks A. Amino acids in the Bacillus subtilis morphogenetic protein SpoIVA with roles in spore coat and cortex formation. J Bacteriol. 2001;183:1645–1654. doi: 10.1128/JB.183.5.1645-1654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cutting S, Driks A, Schmidt R, Kunkel B, Losick R. Forespore-specific transcription of a gene in the signal transduction pathway that governs pro-ςK processing in Bacillus subtilis. Genes Dev. 1991;5:456–466. doi: 10.1101/gad.5.3.456. [DOI] [PubMed] [Google Scholar]
- 11.Cutting S, Panzer S, Losick R. Regulatory studies on the promoter for a gene governing synthesis and assembly of the spore coat in Bacillus subtilis. J Mol Biol. 1989;207:393–404. doi: 10.1016/0022-2836(89)90262-3. [DOI] [PubMed] [Google Scholar]
- 12.Daniel R A, Drake S, Buchanan C E, Scholle R, Errington J. The Bacillus subtilis spoVD gene encodes a mother-cell-specific penicillin-binding protein required for spore morphogenesis. J Mol Biol. 1994;235:209–220. doi: 10.1016/s0022-2836(05)80027-0. [DOI] [PubMed] [Google Scholar]
- 13.Denome S A, Elf P K, Henderson T A, Nelson D E, Young K D. Escherichia coli mutants lacking all possible combinations of eight penicillin binding proteins: viability, characteristics, and implications for peptidoglycan synthesis. J Bacteriol. 1999;181:3981–3993. doi: 10.1128/jb.181.13.3981-3993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster S J. The role and regulation of cell wall structural dynamics during differentiation of endospore-forming bacteria. J Appl Bacteriol Symp Suppl. 1994;76:25S–39S. doi: 10.1111/j.1365-2672.1994.tb04355.x. [DOI] [PubMed] [Google Scholar]
- 15.Ghuysen J-M. Serine β-lactamases and penicillin-binding proteins. Annu Rev Microbiol. 1991;45:37–67. doi: 10.1146/annurev.mi.45.100191.000345. [DOI] [PubMed] [Google Scholar]
- 16.Goffin C, Ghuysen J M. Multimodular penicillin-binding proteins: an enigmatic family of orthologs and paralogs. Microbiol Mol Biol Rev. 1998;62:1079–1093. doi: 10.1128/mmbr.62.4.1079-1093.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez M, Cutting S, Stragier P. Transcription of spoIVB is the only role of ςG that is essential for pro-ςK processing during spore formation in Bacillus subtilis. J Bacteriol. 1995;177:4825–4827. doi: 10.1128/jb.177.16.4825-4827.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guérout-Fleury A-M, Shazand K, Frandsen N, Stragier P. Antibiotic-resistance cassettes for Bacillus subtilis. Gene. 1995;167:335–337. doi: 10.1016/0378-1119(95)00652-4. [DOI] [PubMed] [Google Scholar]
- 19.Hoskins J, Matsushima P, Mullen D L, Tang J, Zhao G, Meier T I, Nicas T I, Jaskunas S R. Gene disruption studies of penicillin-binding proteins 1a, 1b, and 2a in Streptococcus pneumoniae. J Bacteriol. 1999;181:6552–6555. doi: 10.1128/jb.181.20.6552-6555.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishikawa S, Yamane K, Sekiguchi J. Regulation and characterization of a newly deduced cell wall hydrolase gene (cwlJ) which affects germination of Bacillus subtilis spores. J Bacteriol. 1998;180:1375–1380. doi: 10.1128/jb.180.6.1375-1380.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacoby G H, Young K D. Unequal distribution of penicillin-binding proteins among inner membrane vesicles of Escherichia coli. J Bacteriol. 1988;170:3660–3667. doi: 10.1128/jb.170.8.3660-3667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karmazyn-Campelli C, Bonamy C, Savelli B, Stragier P. Tandem genes encoding ς-factors for consecutive steps of development in Bacillus subtilis. Genes Dev. 1989;3:150–157. doi: 10.1101/gad.3.2.150. [DOI] [PubMed] [Google Scholar]
- 23.Kleppe G, Strominger J L. Studies of the high molecular weight penicillin-binding proteins of Bacillus subtilis. J Biol Chem. 1979;254:4856–4862. [PubMed] [Google Scholar]
- 24.Kunst F, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 25.Leighton T J, Doi R H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971;254:3189–3195. [PubMed] [Google Scholar]
- 26.Mason J M, Hackett R H, Setlow P. Regulation of expression of genes coding for small, acid-soluble proteins of Bacillus subtilis spores: studies using lacZ gene fusions. J Bacteriol. 1988;170:239–244. doi: 10.1128/jb.170.1.239-244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masson J M, Labia R. Synthesis of a 125I-radiolabeled penicillin for penicillin-binding proteins studies. Anal Biochem. 1983;128:164–168. doi: 10.1016/0003-2697(83)90357-3. [DOI] [PubMed] [Google Scholar]
- 28.Meador-Parton J, Popham D L. Structural analysis of Bacillus subtilis spore peptidoglycan during sporulation. J Bacteriol. 2000;182:4491–4499. doi: 10.1128/jb.182.16.4491-4499.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moriyama R, Hattori A, Miyata S, Kudoh S, Makino S. A gene (sleB) encoding a spore cortex-lytic enzyme from Bacillus subtilis and response of the enzyme to l-alanine-mediated germination. J Bacteriol. 1996;178:6059–6063. doi: 10.1128/jb.178.20.6059-6063.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray T, Popham D L, Pearson C B, Hand A R, Setlow P. Analysis of outgrowth of Bacillus subtilis spores lacking penicillin-binding protein 2a. J Bacteriol. 1998;180:6493–6502. doi: 10.1128/jb.180.24.6493-6502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholson W L, Setlow P. Sporulation, germination, and outgrowth. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, England: John Wiley & Sons Ltd.; 1990. pp. 391–450. [Google Scholar]
- 32.Oke V, Shchepetov M, Cutting S. SpoIVB has two distinct functions during spore formation in Bacillus subtilis. Mol Microbiol. 1997;23:223–230. doi: 10.1046/j.1365-2958.1997.2091573.x. [DOI] [PubMed] [Google Scholar]
- 33.Paidhungat M, Ragkousi K, Setlow P. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J Bacteriol. 2001;183:4886–4893. doi: 10.1128/JB.183.16.4886-4893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paik J, Kern I, Lurz R, Hakenbeck R. Mutational analysis of the Streptococcus pneumoniae bimodular class A penicillin-binding proteins. J Bacteriol. 1999;181:3852–3856. doi: 10.1128/jb.181.12.3852-3856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen L B, Ragkousi K, Cammett T J, Melly E, Sekowska A, Schopick E, Murray T, Setlow P. Characterization of ywhE, which encodes a putative high-molecular-weight class A penicillin-binding protein in Bacillus subtilis. Gene. 2000;246:187–196. doi: 10.1016/s0378-1119(00)00084-6. [DOI] [PubMed] [Google Scholar]
- 36.Popham D L, Gilmore M E, Setlow P. Roles of low-molecular-weight penicillin-binding proteins in Bacillus subtilis spore peptidoglycan synthesis and spore properties. J Bacteriol. 1999;181:126–132. doi: 10.1128/jb.181.1.126-132.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popham D L, Helin J, Costello C E, Setlow P. Analysis of the peptidoglycan structure of Bacillus subtilis endospores. J Bacteriol. 1996;178:6451–6458. doi: 10.1128/jb.178.22.6451-6458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popham D L, Helin J, Costello C E, Setlow P. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc Natl Acad Sci USA. 1996;93:15405–15410. doi: 10.1073/pnas.93.26.15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popham D L, Illades-Aguiar B, Setlow P. The Bacillus subtilis dacB gene, encoding penicillin-binding protein 5*, is part of a three-gene operon required for proper spore cortex synthesis and spore core dehydration. J Bacteriol. 1995;177:4721–4729. doi: 10.1128/jb.177.16.4721-4729.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Popham D L, Meador-Parton J, Costello C E, Setlow P. Spore peptidoglycan structure in a cwlD dacB double mutant of Bacillus subtilis. J Bacteriol. 1999;181:6205–6209. doi: 10.1128/jb.181.19.6205-6209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popham D L, Setlow P. Cloning, nucleotide sequence, and mutagenesis of the Bacillus subtilis ponA operon, which codes for penicillin-binding protein (PBP) 1 and a PBP-related factor. J Bacteriol. 1995;177:326–335. doi: 10.1128/jb.177.2.326-335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popham D L, Setlow P. Cloning, nucleotide sequence, and regulation of the Bacillus subtilis pbpF gene, which codes for a putative class A high-molecular-weight penicillin-binding protein. J Bacteriol. 1993;175:4870–4876. doi: 10.1128/jb.175.15.4870-4876.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Popham D L, Setlow P. Cloning, nucleotide sequence, mutagenesis, and mapping of the Bacillus subtilis pbpD gene, which codes for penicillin-binding protein 4. J Bacteriol. 1994;176:7197–7205. doi: 10.1128/jb.176.23.7197-7205.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Popham D L, Setlow P. Phenotypes of Bacillus subtilis mutants lacking multiple class A high-molecular-weight penicillin-binding proteins. J Bacteriol. 1996;178:2079–2085. doi: 10.1128/jb.178.7.2079-2085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sekiguchi J, Akeo K, Yamamoto H, Khasanov F K, Alonso J C, Kuroda A. Nucleotide sequence and regulation of a new putative cell wall hydrolase gene, cwlD, which affects germination in Bacillus subtilis. J Bacteriol. 1995;177:5582–5589. doi: 10.1128/jb.177.19.5582-5589.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Severin A, Schuster C, Hakenbeck R, Tomasz A. Altered murein composition in a dd-carboxypeptidase mutant of Streptococcus pneumoniae. J Bacteriol. 1992;174:5152–5155. doi: 10.1128/jb.174.15.5152-5155.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simpson E B, Hancock T W, Buchanan C E. Transcriptional control of dacB, which encodes a major sporulation-specific penicillin-binding protein. J Bacteriol. 1994;176:7767–7769. doi: 10.1128/jb.176.24.7767-7769.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 49.Spratt B G. Distinct penicillin-binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci USA. 1975;72:2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spratt B G. Temperature-sensitive cell division mutants of Escherichia coli with thermolabile penicillin-binding proteins. J Bacteriol. 1977;131:293–305. doi: 10.1128/jb.131.1.293-305.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki H, Nishimura Y, Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci USA. 1978;75:664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tipper D J, Linnet P E. Distribution of peptidoglycan synthetase activities between sporangia and forespores in sporulating cells of Bacillus sphaericus. J Bacteriol. 1976;126:213–221. doi: 10.1128/jb.126.1.213-221.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warth A D, Strominger J L. Structure of the peptidoglycan from spores of Bacillus subtilis. Biochemistry. 1972;11:1389–1396. doi: 10.1021/bi00758a010. [DOI] [PubMed] [Google Scholar]
- 55.Warth A D, Strominger J L. Structure of the peptidoglycan of bacterial spores: occurrence of the lactam of muramic acid. Proc Natl Acad Sci USA. 1969;64:528–535. doi: 10.1073/pnas.64.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yanouri A, Daniel R A, Errington J, Buchanan C E. Cloning and sequencing of the cell division gene pbpB, which encodes penicillin-binding protein 2B in Bacillus subtilis. J Bacteriol. 1993;175:7604–7616. doi: 10.1128/jb.175.23.7604-7616.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yousif S Y, Broome-Smith J K, Spratt B G. Lysis of Escherichia coli by β-lactam antibiotics: deletion analysis of the role of penicillin-binding proteins 1A and 1B. J Gen Microbiol. 1985;131:2839–2845. doi: 10.1099/00221287-131-10-2839. [DOI] [PubMed] [Google Scholar]
- 58.Zheng L, Losick R. Cascade regulation of spore coat gene expression in Bacillus subtilis. J Mol Biol. 1990;212:645–660. doi: 10.1016/0022-2836(90)90227-d. [DOI] [PubMed] [Google Scholar]