Abstract
Invasive candidiasis (IC), due to the yeast pathogen Candida, is still a major cause of in-hospital morbidity and mortality. The limited number of antifungal drug classes and the emergence of multi-resistant Candida species, such as Candida auris and some Candida glabrata isolates, is concerning. However, recent advances in antifungal drug development provide promising perspectives for the therapeutic approach of IC. Notably, three novel antifungal agents, currently in Phase II/III clinical trials, are expected to have an important place for the treatment of IC in the future. Rezafungin is a novel echinocandin with prolonged half-life. Ibrexafungerp and fosmanogepix are two first-in-class antifungal drugs with broad spectrum activity against Candida spp., including C. auris and echinocandin-resistant species. These novel antifungal agents also represent interesting alternative options because of their acceptable oral bioavailability (ibrexafungerp and fosmanogepix) or their large interdose interval (once weekly intravenous administration for rezafungin) for prolonged and/or outpatient treatment of complicated IC. This review discusses the potential place of these novel antifungal drugs for the treatment of IC considering their pharmacologic properties and their preclinical and clinical data.
Keywords: Candida, candidemia, rezafungin, ibrexafungerp, fosmanogepix, oteseconazole, tetrazoles, T-2307, antifungal therapy
Introduction
Candida Infections
Yeasts of the genus Candida represent the most frequent cause of fungal diseases in Europe and North America.1 Candida spp. are part of the commensal flora of the skin and gut in humans. They can cause localized infections, such as vulvovaginal or oral/esophageal candidiasis, as well as invasive infections in individuals with predisposing conditions, such as decreased immunity and/or rupture of the mechanical barriers (e.g., intravascular catheters, complicated abdominal surgery).2 Invasive candidiasis (IC) includes candidemia (i.e., bloodstream infections) and non-candidemic deep-seated candidiasis, such as intra-abdominal candidiasis (IAC) or chronic disseminated (hepatosplenic) candidiasis.1 IC can affect both immunocompromised patients (e.g., neutropenic patients) and apparently immunocompetent patients who are critically ill. It represents a major cause of healthcare-associated sepsis, in particular in the intensive care units (ICU).3 Candida albicans is the most frequent pathogenic Candida species in humans, followed by Candida glabrata and other non-albicans Candida spp. (e.g., C. parapsilosis, C. tropicalis, C. krusei).1 Candida auris has recently emerged as a novel Candida species of concern.4
The Evolving Landscape of Invasive Candidiasis
While the global epidemiological trends of IC are difficult to assess and may vary between different geographical areas, its incidence remains high and data from North America and Europe suggest an increased burden of the disease among the elderly and immunocompromised populations.1,5,6 As a consequence, a paradoxical increase of mortality rates of IC has been reported over time despite advances in diagnostic and therapeutic approaches.5,7 The epidemiology of IC has also evolved in terms of microbiology, with a decreasing proportion of Candida albicans and a progressive predominance of non-albicans Candida spp., in particular Candida glabrata, that are less susceptible to azoles.1,5 These epidemiological changes have not only been observed in Europe and in North America, but also in Asia and in the Southern hemisphere where azole-resistant C. glabrata and C. tropicalis isolates are increasingly reported.8–10
Moreover, an emerging Candida species, Candida auris, has spread in all continents since 2009.4,11 In addition to its ability to cause hospital outbreaks,12,13 C. auris has the ability to rapidly develop resistance to all antifungal drug classes with increasing reports of multi-resistant isolates.4,14,15 C. auris currently represents an important proportion of Candida bloodstream pathogens in some parts of the world, such as in South Asia and South Africa where it has been associated with an epidemiological shift.16,17
Epidemiology of Azole and Echinocandin Resistance Among Candida spp
Currently, the treatment of IC relies on echinocandin drugs (caspofungin, anidulafungin and micafungin) as empirical or first-line therapy and azoles (mainly fluconazole) as second-line or step-down therapy for susceptible isolates.2,18,19 Other triazoles (e.g., voriconazole) and amphotericin B formulations can be used in particular cases.2,18,19
Echinocandins are active against most wild-type pathogenic Candida spp.20 Although C. parapsilosis is less susceptible in vitro, echinocandins remain effective for the treatment of IC attributed to this pathogen.21 Echinocandin resistance is essentially acquired by mutations in the FKS genes, encoding for the (1,3)-beta-D-glucan synthase (the target of echinocandins).20 Its overall rate is variable according to different geographical areas, but usually does not exceed 10%. The proportion of echinocandin resistant C. glabrata may reach 5% to 15% in some US centers, but remains below 3% in Europe.22–27 Echinocandin resistance in C. albicans is much less frequent (<1%).25,27 Among C. auris, echinocandin resistance is mainly observed in clade I with a prevalence varying between 0.5% and 4%.14,15,28,29 Overall, the rate of echinocandin resistance among Candida spp. seems to increase over time.15,23,30 Regarding fluconazole resistance, besides the Candida spp. exhibiting variable intrinsic level of resistance (e.g., C. glabrata, C. krusei), acquired resistance is reported at variable rates (1% to 20%) among C. albicans and C. tropicalis, and in the majority of C. auris isolates (>90%).1,4
Current Gaps for the Treatment of Invasive Candidiasis
Limitations of the three current antifungal drug classes used for IC may be due to: 1) multiple resistance of the causative agent; 2) pharmacologic considerations, such as lack of oral formulations for outpatient treatment or poor penetration in some tissues (e.g., brain, eye); and 3) toxicity or drug-drug interactions.
Multiple resistance (i.e., ≥2 antifungal drug classes) is mainly a concern for echinocandin and azole resistant C. glabrata and C. auris. Limited pharmacologic properties can be an issue for echinocandins because of their lack of oral bioavailability and very poor penetration in eye, central nervous system (CNS) and urine. Finally, liver toxicity and drug-drug interactions (with drugs interfering with cytochrome P450) may limit the use of azoles, which are currently the only antifungals with oral bioavailability. Amphotericin B formulations also exhibit important limitations, such as nephrotoxicity and their lack of oral formulation.
Novel antifungal agents are needed to fulfill these gaps.31 Ideally, these molecules should provide broad-spectrum activity against Candida spp. including those with azole and/or echinocandin resistance, to be available as both intravenous and oral formulations, to have large tissue distribution including in sanctuary sites (CNS, deep abscesses, urine) and to have few side effects and drug-drug interactions. This review will focus on novel antifungal agents that are currently in phase II/III clinical trials for the treatment of IC.
Novel Antifungal Agents for Invasive Candidiasis
Three antifungal agents, including two first-in-class molecules (ibrexafungerp and fosmanogepix) and one molecule of a pre-existing class with improved pharmacologic properties (rezafungin), have completed or are currently in phase II/III trials for the treatment of IC (Table 1). All of them are expected to palliate some of the above mentioned gaps and to represent important therapeutic options for IC in the future. Their potential advantages and limitations are summarized in Table 2.
Table 1.
Characteristics and Stage of Development of Novel Antifungal Agents for the Treatment of IC
| Antifungal Agent (Class) | Dosage and Mode of Administration | Anti-Candida Spectrum | Current Status in Clinical Trials for ICa |
|---|---|---|---|
| Rezafungin (echinocandin) | Loading dose: 400 mg (day 1) Then: 200 mg once weekly Intravenous only |
All (↓ C. parapsilosis) Acquired resistance: 1–5% (mainly C. glabrata, C. auris) |
RCT phase II (NCT02734862): completed RCT phase III (NCT03667690): completed |
| Ibrexafungerp (triterpenoid) | Loading dose: 1000–1500 mg (day 1 ± 2, in 1 or 2 daily doses) Then: 500–750 mg qd Oral only |
All (↓ C. krusei, C. lusitaniae, C. guilliermondii) Acquired resistance: not yet reported |
Comparative open-label phase II (NCT02244606): completed Non comparative phase III C. auris (NCT03363841): ongoing RCT phase III (NCT05178862): ongoing |
| Fosmanogepix (N-phosphonooxymethylene) | Loading dose: 1000 mg bid (day 1) Then: 600 mg qd (iv) or 700–800 mg qd (po) Intravenous or oral |
All except C. krusei (↓ C. kefyr) Acquired resistance: reported in vitro (laboratory-generated) |
Non-comparative phase II (NCT03604705): completed Non-comparative phase II C. auris (NCT04148287): terminated RCT phase III (NCT05421858): not yet recruiting |
Note: aStatus as stated on www.clinicaltrials.gov (last accessed: December 14th 2022).
Abbreviations: qd, once daily; bid, twice daily; iv, intravenous; po, per os (oral); ↓, decreased susceptibility; RCT, randomized controlled trial.
Table 2.
Advantages and Limitations of Novel Antifungal Drugs for IC
| Antifungal Drug | Advantages | Limitations |
|---|---|---|
| Rezafungin | Fungicidal Prolonged half-life (once weekly administration) |
Intravenous only Lack of penetration in CNS and eye Not appropriate for short-course or initial empirical therapy (potential risk of resistance because of prolonged effect) |
| Ibrexafungerp | Fungicidal Oral mode of administration Compared to echinocandins, extended spectrum against a majority of echinocandin-resistant Candida isolates |
Oral bioavailability may be affected in some patients (e.g., proton pump inhibitors, gastro-intestinal disorders) Lack of penetration in CNS and eye |
| Fosmanogepix | Oral and intravenous mode of administration Efficient against most azole and echinocandin resistant Candida isolates Acceptable penetration in CNS and eye |
Fungistatic effect Poor or limited efficacy against some Candida spp. (C. krusei, C. kefyr) |
Abbreviation: CNS, central nervous system.
Rezafungin
Rezafungin (CD101) is a novel echinocandin drug (inhibitor of the (1,3)beta-D-glucan synthase) with enhanced stability, low clearance and prolonged half-life (130h), which allows an interdose interval of one week.32 It is fungicidal and has the same spectrum of activity as other echinocandins and, similar to them, it can be administered by intravenous route only and does not reach therapeutic concentrations in the CNS, eye and urine.32 Therefore, its main advantage consists of the convenient mode of administration (once weekly instead of once daily for other echinocandins). In vitro susceptibility testing studies of rezafungin against pathogenic Candida spp. suggested the following wild-type upper limits (WT-UL) to distinguish the wild-type and non-wild type isolates: 0.06–0.125 mg/L for C. albicans, 0.125–0.25 mg/L for C. glabrata and C. krusei, 0.25 mg/L for C. tropicalis, 0.5 mg/L for C. auris and 4 mg/L for C. parapsilosis.33,34 In murine models of IC, rezafungin demonstrated at least similar (or even better) efficacy compared to other echinocandins (e.g., micafungin) against the most relevant Candida spp. including C. auris.35–39 Notably, the area under the curve (AUC) / minimal inhibitory concentration (MIC) ratio to achieve efficacy endpoints was lower for rezafungin compared to other echinocandins.38 In a murine model of IAC, rezafungin could reach much higher concentrations in liver lesions compared to micafungin, which may be associated with improved outcomes and decreased risk of development of resistance due to suboptimal concentrations.40
The safety and efficacy of rezafungin for the treatment of IC in humans have been tested in two double-blind randomized controlled-trials against caspofungin.41,42 In a Phase II study of 207 patients (STRIVE), rezafungin was tested at two different dosing regimens (400 mg once weekly and 400 mg loading dose, then 200 mg once weekly) versus standard regimen of caspofungin.41 Overall cure rates at day 14 were comparable between the three treatment arms (60.5%, 76.1%, 67.2% for rezafungin high and low dose regimens, and caspofungin, respectively). In a Phase III study (ReSTORE), rezafungin (400 mg loading dose, then 200 mg once weekly) met non-inferiority criteria compared to caspofungin with a global cure at day 14 of 59.1% vs. 60.6%, respectively.42
Ibrexafungerp
Ibrexafungerp (SCY-078, MK-3118) is a triterpenoid derived from enfumafungin, which inhibits the (1,3)-beta-D-glucan synthase.43 Therefore, it is structurally different from the echinocandins, but exerts its fungicidal antifungal activity via the same target.43 Because its binding site on the target enzyme is not the same and only partially overlaps with that of echinocandins, ibrexafungerp can maintain antifungal activity against a majority of FKS-mutant echinocandin resistant isolates.43 In terms of pharmacologic properties, ibrexafungerp has the advantage to exhibit acceptable oral bioavailability (35–50%) to be administered by oral route.43 Its solubility and absorption is favored by low pH and co-administration of food and can be affected by clinical conditions, such as use of proton pump inhibitors, antacids, nausea, vomiting and loss of appetite.43 Similar to echinocandins, ibrexafungerp has high rate of plasma protein binding and very poor penetration in CNS and urine.43 In vitro, ibrexafungerp exhibits MICs for Candida spp. that are somewhat higher compared to other echinocandins, except for C. parapsilosis.43,44 Reported WT-UL are: 0.5 mg/L for C. albicans, 1mg/L for C. tropicalis and C. parapsilosis, 2 mg/L for C. glabrata, 4 mg/L for C. krusei.44 Relatively high MICs (≥4 mg/L) have been reported among C. lusitaniae and C. guilliermondii isolates.44 For C. auris, MIC50 and MIC90 (i.e., encompassing 50% and 90% of tested isolates, respectively) were 0.5 and 1 mg/L, respectively.45,46 Notably, ibrexafungerp demonstrated good activity against pan-resistant C. auris isolates.47 The activity of ibrexafungerp against FKS-mutant Candida spp. is variable and this variation seems to be dependent on the site/type of mutation; overall it is conserved against approximately 75% of isolates.43,44 In murine models, oral ibrexafungerp was effective in treating IC caused by different Candida spp. including C. auris and C. glabrata FKS-mutant isolates.48–50 In a murine model of IAC, ibrexafungerp achieved high concentrations in liver abscesses (~100-fold higher than in serum), which were superior to a comparative echinocandin (more than 5-fold higher compared to micafungin).51
Ibrexafungerp has been approved by the Food and Drug Administration (FDA) for the treatment of vulvovaginal candidiasis following two phase III trials.52,53 Its safety and preliminary efficacy as step-down therapy following initial echinocandin therapy for the treatment of IC was evaluated in a small (n = 27 patients) phase II study.54 Two different dosing regimens of oral ibrexafungerp (1000 mg loading dose followed by 500 mg qd, and 1250 mg loading dose followed by 750 mg qd) were compared to a standard oral fluconazole regimen with similar response rates (71%, 86%, 71%, respectively) and rates of adverse events. The population pharmacokinetic model indicated that about 85% of patients receiving the ibrexafungerp high dose regimen would achieve the pharmacodynamics target (AUC0-24 of 15.4 µM/h, based on previous murine models).48,54,55 Two phase III studies are ongoing: one randomized double-blind trial evaluating ibrexafungerp versus fluconazole as second-line oral therapy following initial echinocandin therapy (MARIO, NCT05178862) and an open-label study evaluating the efficacy of ibrexafungerp for the treatment of C. auris IC (CARES, NCT03363841).56
Fosmanogepix
Fosmanogepix (APX001, E1211), which is converted to its active moiety manogepix (APX001A, E1210) by systemic phosphatases, is a first-in-class antifungal agent that inhibits Gwt1, an enzyme localized in the endoplasmic reticulum and involved in the glycosylphosphatidylinositol (GPI) biosynthesis pathway.57 Because GPI is required for anchorage of mannoproteins in the cell wall and membrane, this results in endoplasmic reticulum stress, altered cell wall integrity, impaired morphogenesis (germ tube formation, hyphal elongation) and inhibition of adhesion and biofilm formation.57,58 Moreover, alterations in the cell wall may unmask (1,3)-beta-D-glucan and activate the host immune response.59 Fosmanogepix can be administered intravenously or orally with an oral bioavailability of >90%, which is not altered by co-administration of food.57 It has prolonged half-life (2.5 days) and excellent tissue distribution including in the brain and eye.57 Some drug-drug interactions with inhibitors or inducers of cytochrome P450 isoenzymes are possible and are under investigation.57
In vitro, manogepix has fungistatic activity with a broad spectrum against most pathogenic yeasts and molds (with the exception of Mucorales).57 MIC endpoints for yeasts are defined at 50% growth inhibition.57 The WT-UL values were 0.03 mg/L for C. albicans, 0.03–0.06 mg/L for C. parapsilosis, 0.016–0.06 mg/L for C. tropicalis, 0.03–0.12 mg/L for C. auris and 0.125–0.25 mg/L for C. glabrata, while C. krusei is considered as intrinsically resistant (MIC >2 mg/L) and C. kefyr exhibits decreased susceptibility (MIC90 0.5 mg/L).60–64 Manogepix was notably active against virtually all tested multi-resistant C. auris isolates and against echinocandin-resistant (FKS-mutant) C. glabrata and C. albicans.63–65 In vitro, manogepix also demonstrated very potent inhibition of C. albicans adherence to polystyrene and biofilm formation, which was greater compared to that of fluconazole, micafungin and amphotericin B.58 Manogepix also demonstrated prolonged post-antifungal effect in vitro and in vivo.57 While acquired resistance to manogepix was not yet reported in vivo, in vitro studies showed that resistance can be acquired following manogepix exposure by mutations in the Gwt1 gene (V162A and V163A in C. albicans and C. glabrata, respectively) or overexpression of the ATP-binding cassette (ABC) transporters CDR11 and SNQ2 resulting from a gain-of-function mutation in the transcription factor gene ZCF29.66,67
In in vivo animal models, the efficacy of fosmanogepix for IC treatment is hampered by the shorter half-life of its active moiety (manogepix) in mice compared to that in humans, which can be overcome by pre-treatment with a cytochrome P450 inhibitor.68 The 24h free-drug AUC/MIC ratio was the pharmacokinetic/pharmacodynamics (PK/PD) index correlating with efficacy in mice.69 Fosmanogepix or manogepix administered by intraperitoneal or oral routes had significant impact on kidney fungal burdens and survival in murine models of IC with wild-type C. albicans and C. glabrata compared to the untreated arms.68,70 It is noteworthy that the comparator echinocandin in these studies demonstrated better results, although the differences did not reach statistical significance.68,70 However, (fos-)manogepix was effective against echinocandin-resistant (FKS-mutant) C. albicans and C. glabrata, while the comparator echinocandin was not.68,71 (Fos-)manogepix was also effective in murine models of C. auris IC.72,73 In a murine model of IAC, repeated doses of fosmanogepix could achieve good penetration in liver abscesses after 3 days and its efficacy in reducing liver fungal burden was superior to that of micafungin.74 The penetration of fosmanogepix into the CNS was analyzed in one rabbit model of C. albicans IC and one murine model of C. auris IC.73,75 In rabbits, the CNS tissue / plasma concentration ratio was 1 and fosmanogepix treatment achieved significant reduction in CNS fungal burden (C. albicans) for concentrations ranging from 25 to 100 mg/kg bid.75 In mice, a significant decrease of CNS fungal burden (C. auris) was only achieved at high concentrations of fosmanogepix (260 mg/kg bid).73 In a rabbit model of C. albicans endophthalmitis, fosmanogepix displayed variable penetration in the different parts of the eye with liquid / plasma ratios of 0.09–0.12 in the vitreous humor and 0.02–0.04 in the choroid, which was sufficient to induce a significant decrease in fungal burden in both vitreous and choroid.75
The efficacy of fosmanogepix as first-line treatment of candidemia (all Candida spp. except C. krusei) in non-neutropenic patients was tested in a non-comparative “proof of concept” phase II trial (N = 21 patients).76 The drug was administered for 14 days with initial intravenous dosing (1000 mg bid on day 1, then 600 mg qd), with possibility for oral switch from day 4 (700 mg qd). The success rate at the end of treatment was 80% (16/20) and survival at day 30 was 85% (17/21). Fosmanogepix was well tolerated and the cases of death were not attributed to the drug. Another open label non comparative phase II study is evaluating the efficacy of fosmanogepix for the treatment of C. auris IC (APEX, NCT04148287).56 A phase III randomized controlled trial comparing fosmanogepix (initial intravenous followed by oral therapy) versus standard therapy (initial intravenous caspofungin followed by oral fluconazole therapy) for the treatment of candidemia and other IC is expected to start soon (NCT05421858).
Other Novel Antifungal Drugs Under Development
Some other antifungal drug candidates deserve mention, although they have not yet been assessed in phase II/III trials for IC. The tetrazoles (VT-1161, VT-1129 and VT-1598) are novel azole compounds that have much lower affinity for human cytochrome P450 isoenzymes (CYP2C9, CYP2C19, and CYP3A4), which results in lower potential for drug-drug interactions.77,78 Moreover, they may exhibit distinct susceptibility patterns compared to triazoles and have conserved in vitro activity against some fluconazole-resistant isolates, such as C. glabrata, C. krusei and C. auris.79–82 VT-1161 (oteseconazole) demonstrated safety and efficacy for the treatment of acute and recurrent vulvovaginal candidiasis in phase II and III trials.83–85 Its efficacy for the treatment of IC has not yet been evaluated.
Structurally modified molecules derived from fluconazole, such as aryl-1,2,4-triazol-3-ylthio analogues of fluconazole (ATTAFs), or other benzylthio analogues demonstrated some improved in vitro activity (lower MIC) compared to fluconazole, as well as some synergistic interactions, against both fluconazole-susceptible and -resistant Candida isolates.86,87
Other potential antifungal drug candidates for IC include T-2307, an arylamidine (close to pentamidine) targeting the mitochondrial membrane, which demonstrated potent in vitro activity against Candida spp. including C. auris and echinocandin-resistant C. glabrata and C. albicans, as well as in vivo efficacy in murine models of IC.88–90
The Place of Novel Antifungal Agents in the Prevention and Treatment of Invasive Candidiasis
Prophylaxis
Indications for anti-Candida prophylaxis are limited.2,18 It is recommended for some hematologic cancer patients, such as allogeneic hematopoietic stem cell transplant (HSCT) recipients during the neutropenic phase or early non-neutropenic phase and should be expanded to provide anti-mold protection in some circumstances (e.g., graft versus host disease after allogeneic HSCT or for patients with acute leukemia and prolonged chemotherapy-induced neutropenia). Fluconazole (anti-Candida only) or posaconazole (for both yeast and mold prevention) are currently the drugs of choice. One phase III randomized controlled study is currently evaluating rezafungin versus posaconazole or fluconazole prophylaxis in allogeneic HSCT recipients (ReSPECT, NCT04368559).56 The role of antifungal prophylaxis to prevent IC in non-hematologic cancer patients is more controversial and should be limited to high-risk patients (e.g., complicated abdominal surgery with anastomotic leakage).2,18 Novel antifungals seem to have little place for this indication, but might be considered in the future (e.g., ibrexafungerp) in the case of high prevalence of C. auris or azole resistance.
Empiric and Pre-Emptive Therapy
Antifungal therapy can be started empirically in patients at risk of IC with sepsis or pre-emptively in the case of a positive fungal biomarker (e.g., beta-glucan in serum). In these situations, the antifungal agent should ensure broad coverage of the most prevalent Candida spp. according to the local epidemiological context. Because of their broad spectrum of activity, echinocandins currently represent the privileged option. Because of its prolonged half-life, rezafungin does not appear to be a good candidate, as antifungal therapy should be reassessed or adapted quickly following receipt of microbiological results. Novel antifungal agents ensuring coverage of C. auris, such as ibrexafungerp and fosmanogepix, may be considered in the case of hospital outbreaks or high prevalence of this species. The poor activity of fosmanogepix against C. krusei should be kept in mind, although this species is a relatively rare cause of IC.
Targeted Therapy
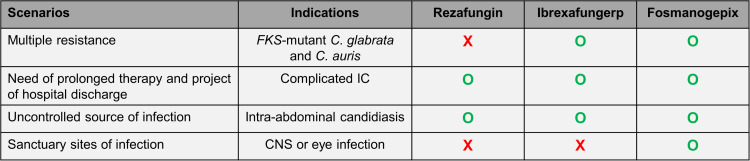
Because of their broad spectrum of activity against Candida spp., these novel antifungal agents (i.e., rezafungin, ibrexafungerp and fosmanogepix) are expected to have an important place for the treatment of IC in the future (Figure 1). However, they should be initially reserved for indications where they provide an additional value compared to current antifungal drugs. These advantages may be related to their antifungal spectrum and/or their pharmacologic properties.
Figure 1.
Potential place of novel antifungal agents for the treatment of IC.
Abbreviations: FKS, genes encoding the (1,3)-beta-D-glucan synthase; CNS, central nervous system; O (green), good candidate; X (red), not an option.
Ibrexafungerp and fosmanogepix are active against most echinocandin-resistant Candida spp. and may become the first choice for the treatment of IC caused by these species, in particular C. glabrata and C. auris that often exhibits concomitant resistance to azoles. It should be noted that cross-resistance between echinocandins and ibrexafungerp may be observed in some cases according to the type of FKS mutation.44 Whether ibrexafungerp or fosmanogepix may be more effective than echinocandins for the treatment of C. parapsilosis, which exhibits some decreased echinocandin susceptibility, should be investigated. Fosmanogepix should not be considered for the treatment of C. krusei IC because of high in vitro MICs, and some more data are needed to assess the actual efficacy of fosmanogepix against C. kefyr and of ibrexafungerp against C. krusei, C. lusitaniae and C. guilliermondii because of their decreased in vitro susceptibility to these respective drugs.
The major pharmacologic properties of these novel antifungal agents, which may provide diverse advantages over conventional echinocandins, are: 1) oral bioavailability (ibrexafungerp, fosmanogepix); 2) prolonged half-life (rezafungin, and to a lesser extend fosmanogepix); 3) better penetration in intra-abdominal abscesses according to murine models (rezafungin, ibrexafungerp, fosmanogepix); and 4) ability to cross the blood-brain barrier (fosmanogepix).
Novel antifungal drugs with acceptable oral bioavailability are particularly welcome for prompt hospital discharge of patients with uncomplicated IC due to azole-resistant Candida isolates (e.g. C. glabrata). They may be of particular interest in some areas, such as South Asia, where the rate of fluconazole resistance is particularly high due to the high prevalence of C. auris and the increasing rate of fluconazole-resistant C. tropicalis.9,10,16
IAC is relatively frequent among patients with complicated abdominal surgery and often requires prolonged antifungal therapy in case of incomplete source control.91,92 All three novel drugs appear as ideal candidates for the treatment of IAC as they seem to achieve excellent penetration in intra-abdominal abscesses and are convenient for outpatient therapy (i.e., once weekly intravenous administration for rezafungin or oral administration for ibrexafungerp and fosmanogepix). Whether these drugs could achieve improved penetration in abscesses compared to fluconazole should be further investigated. Albeit rare, Candida meningitis or chorioretinitis are difficult to treat because of the lack of penetration of echinocandins in the CNS and eye. Fosmanogepix may represent an alternative to liposomal amphotericin B and fluconazole, which are currently recommended for these infections.2,18
Conclusions
Three novel antifungal drugs are currently progressing through phase II and III trials in order to be approved for the treatment of IC. Two of them (ibrexafungerp and fosmanogepix) are first-in-class molecules and display an extended antifungal spectrum, in particular against echinocandin-resistant Candida spp. (including C. auris), and all of them have interesting pharmacologic properties that may provide advantages over current antifungal drugs, in particular for patients requiring prolonged and ambulatory treatment or those with sanctuary sites of infection (e.g., deep abscesses, CNS infection). While all of them are expected to have a place for the treatment of IC, their actual role will need to be further defined and guidelines modified accordingly.
Some caveats should be outlined regarding their future use in clinical practice. In the absence of clinical breakpoints for MIC interpretation, there may be some doubt about their actual efficacy against some rare Candida spp. exhibiting MIC values falling within a putative intermediate range. In particular, C. krusei is supposed to be resistant to fosmanogepix and also exhibits relatively high MICs to ibrexafungerp. While the possibility of oral administration for fosmanogepix and ibrexafungerp may represent a major advantage considering the very limited current options (i.e.,only azoles), there might be some concern about their oral bioavailability, in particular for ibrexafungerp under specific circumstances (e.g., concomitant administration of proton pump- inhibitors or antacids, poor appetite, gastro-intestinal disturbances).
Because IC remains a major cause of healthcare-associated infection and emergence of resistant species (e.g., C. auris) is concerning, this expansion of the antifungal armamentarium may change the epidemiology and outcome of IC in the future. Because of the ability of Candida to develop resistance to antifungal drugs, a parsimonious use of these novel agents is warranted and their indications should be restricted to situations where they provide advantages over current antifungal classes. Indeed, some in vitro data suggest the ability of Candida spp. to develop resistance to some of them (e.g., fosmanogepix) and clinical data are currently insufficient to assess their potential to induce in vivo resistance.
Finally, it is important to mention that novel therapeutic approaches of IC are not restricted to the development of novel antifungal drugs, but also include strategies to enhance the immune response.93 Interferon-gamma (IFN-γ) has been used as adjunctive therapy of standard antifungal agents in a small open-label case series with promising results.94 This approach will be evaluated in a randomized clinical trial (NCT04979052).
Funding Statement
There was no dedicated funding for this work.
Disclosure
FL has received research funding from Gilead, MSD, Pfizer and Novartis, has participated to advisory boards of Gilead, MSD and Pfizer, and has received honoraria for conferences from Gilead and Mundipharma. All contracts were made with and fees paid to his institution (CHUV). The author reports no other conflicts of interest in this work.
References
- 1.Lamoth F, Lockhart SR, Berkow EL, Calandra T. Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother. 2018;73:i4–i13. doi: 10.1093/jac/dkx444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1–e50. doi: 10.1093/cid/civ933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754 [DOI] [PubMed] [Google Scholar]
- 4.Lamoth F, Kontoyiannis DP. The Candida auris alert: facts and perspectives. J Infect Dis. 2018;217:516–520. doi: 10.1093/infdis/jix597 [DOI] [PubMed] [Google Scholar]
- 5.Koehler P, Stecher M, Cornely OA, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect. 2019;25:1200–1212. doi: 10.1016/j.cmi.2019.04.024 [DOI] [PubMed] [Google Scholar]
- 6.Tsay SV, Mu Y, Williams S, et al. Burden of Candidemia in the United States, 2017. Clin Infect Dis. 2020;71:e449–e453. doi: 10.1093/cid/ciaa193 [DOI] [PubMed] [Google Scholar]
- 7.Battistolo J, Glampedakis E, Damonti L, et al. Increasing morbidity and mortality of candidemia over one decade in a Swiss university hospital. Mycoses. 2021;64(12):1512–1520. doi: 10.1111/myc.13376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boan P, Gardam D. Epidemiology and antifungal susceptibility patterns of candidemia from a tertiary centre in Western Australia. J Chemother. 2019;31:137–140. doi: 10.1080/1120009X.2019.1595895 [DOI] [PubMed] [Google Scholar]
- 9.Verma R, Pradhan D, Hasan Z, Singh H, Jain AK, Khan LA. A systematic review on distribution and antifungal resistance pattern of Candida species in the Indian population. Med Mycol. 2021;59:1145–1165. doi: 10.1093/mmy/myab058 [DOI] [PubMed] [Google Scholar]
- 10.Xiao M, Chen SC, Kong F, et al. Distribution and antifungal susceptibility of Candida species causing candidemia in China: an update from the CHIF-NET study. J Infect Dis. 2020;221:S139–S147. doi: 10.1093/infdis/jiz573 [DOI] [PubMed] [Google Scholar]
- 11.Lockhart SR, Etienne KA, Vallabhaneni S, et al. Simultaneous emergence of multidrug-resistant candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017;64:134–140. doi: 10.1093/cid/ciw691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz-Gaitan A, Moret AM, Tasias-Pitarch M, et al. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses. 2018;61:498–505. doi: 10.1111/myc.12781 [DOI] [PubMed] [Google Scholar]
- 13.Schelenz S, Hagen F, Rhodes JL, et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control. 2016;5:35. doi: 10.1186/s13756-016-0132-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowdhary A, Prakash A, Sharma C, et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother. 2018;73:891–899. doi: 10.1093/jac/dkx480 [DOI] [PubMed] [Google Scholar]
- 15.Kilburn S, Innes G, Quinn M, et al. Antifungal resistance trends of candida auris clinical isolates in New York and New Jersey from 2016 to 2020. Antimicrob Agents Chemother. 2022;66:e0224221. doi: 10.1128/aac.02242-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudramurthy SM, Chakrabarti A, Paul RA, et al. Candida auris candidaemia in Indian ICUs: analysis of risk factors. J Antimicrob Chemother. 2017;72:1794–1801. doi: 10.1093/jac/dkx034 [DOI] [PubMed] [Google Scholar]
- 17.van Schalkwyk E, Mpembe RS, Thomas J, et al. Epidemiologic shift in candidemia driven by Candida auris, South Africa, 2016-2017(1). Emerg Infect Dis. 2019;25:1698–1707. doi: 10.3201/eid2509.190040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornely OA, Bassetti M, Calandra T, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18(Suppl 7):19–37. doi: 10.1111/1469-0691.12039 [DOI] [PubMed] [Google Scholar]
- 19.Ullmann AJ, Akova M, Herbrecht R, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect. 2012;18(Suppl 7):53–67. doi: 10.1111/1469-0691.12041 [DOI] [PubMed] [Google Scholar]
- 20.Arendrup MC, Cuenca-Estrella M, Lass-Florl C, Hope WW. Breakpoints for antifungal agents: an update from EUCAST focussing on echinocandins against Candida spp. and triazoles against Aspergillus spp. Drug Resist Updat. 2013;16:81–95. doi: 10.1016/j.drup.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Ruiz M, Aguado JM, Almirante B, et al. Initial use of echinocandins does not negatively influence outcome in Candida parapsilosis bloodstream infection: a propensity score analysis. Clin Infect Dis. 2014;58:1413–1421. doi: 10.1093/cid/ciu158 [DOI] [PubMed] [Google Scholar]
- 22.Alexander BD, Johnson MD, Pfeiffer CD, et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis. 2013;56:1724–1732. doi: 10.1093/cid/cit136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Astvad KMT, Johansen HK, Roder BL, et al. Update from a 12-year nationwide fungemia surveillance: increasing intrinsic and acquired resistance causes concern. J Clin Microbiol. 2018;56:e01564–17. doi: 10.1128/JCM.01564-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beyda ND, John J, Kilic A, Alam MJ, Lasco TM, Garey KW. FKS mutant Candida glabrata: risk factors and outcomes in patients with candidemia. Clin Infect Dis. 2014;59:819–825. doi: 10.1093/cid/ciu407 [DOI] [PubMed] [Google Scholar]
- 25.Kritikos A, Neofytos D, Khanna N, et al. Accuracy of Sensititre YeastOne echinocandins epidemiological cut-off values for identification of FKS mutant Candida albicans and Candida glabrata: a ten year national survey of the Fungal Infection Network of Switzerland (FUNGINOS). Clin Microbiol Infect. 2018;24:1214e1–1214 e4. doi: 10.1016/j.cmi.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 26.Mamali V, Siopi M, Charpantidis S, et al. Increasing incidence and shifting epidemiology of candidemia in Greece: results from the first nationwide 10-year survey. J Fungi. 2022;8(2):116. doi: 10.3390/jof8020116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shields RK, Nguyen MH, Press EG, et al. Rate of FKS mutations among consecutive candida isolates causing bloodstream infection. Antimicrob Agents Chemother. 2015;59:7465–7470. doi: 10.1128/AAC.01973-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad S, Khan Z, Al-Sweih N, Alfouzan W, Joseph L. Candida auris in various hospitals across Kuwait and their susceptibility and molecular basis of resistance to antifungal drugs. Mycoses. 2020;63:104–112. doi: 10.1111/myc.13022 [DOI] [PubMed] [Google Scholar]
- 29.Maphanga TG, Naicker SD, Kwenda S, et al. In vitro antifungal resistance of candida auris isolates from bloodstream infections, South Africa. Antimicrob Agents Chemother. 2021;65:e0051721. doi: 10.1128/AAC.00517-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coste AT, Kritikos A, Li J, et al. Emerging echinocandin-resistant Candida albicans and glabrata in Switzerland. Infection. 2020;48:761–766. doi: 10.1007/s15010-020-01475-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy MW, Kontoyiannis DP, Cornely OA, Perfect JR, Walsh TJ. Novel agents and drug targets to meet the challenges of resistant fungi. J Infect Dis. 2017;216:S474–S483. doi: 10.1093/infdis/jix130 [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Perlin DS. Review of the novel echinocandin antifungal rezafungin: animal studies and clinical data. J Fungi. 2020;6:192. doi: 10.3390/jof6040192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arendrup MC, Meletiadis J, Zaragoza O, et al. Multicentre determination of rezafungin (CD101) susceptibility of Candida species by the EUCAST method. Clin Microbiol Infect. 2018;24:1200–1204. doi: 10.1016/j.cmi.2018.02.021 [DOI] [PubMed] [Google Scholar]
- 34.Helleberg M, Jorgensen KM, Hare RK, Datcu R, Chowdhary A, Arendrup MC. Rezafungin in vitro activity against contemporary Nordic clinical candida isolates and candida auris determined by the eucast reference method. Antimicrob Agents Chemother. 2020;64:e02438–19. doi: 10.1128/AAC.02438-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hager CL, Larkin EL, Long LA, Ghannoum MA. Evaluation of the efficacy of rezafungin, a novel echinocandin, in the treatment of disseminated Candida auris infection using an immunocompromised mouse model. J Antimicrob Chemother. 2018;73:2085–2088. doi: 10.1093/jac/dky153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lepak AJ, Zhao M, Andes DR. Pharmacodynamic Evaluation of Rezafungin (CD101) against Candida auris in the Neutropenic Mouse Invasive Candidiasis Model. Antimicrob Agents Chemother. 2018;62:e01572–18. doi: 10.1128/AAC.01572-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lepak AJ, Zhao M, Andes DR. Determination of pharmacodynamic target exposures for rezafungin against Candida tropicalis and Candida dubliniensis in the neutropenic mouse disseminated candidiasis model. Antimicrob Agents Chemother. 2019;63:e01556–19. doi: 10.1128/AAC.01556-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lepak AJ, Zhao M, VanScoy B, Ambrose PG, Andes DR. Pharmacodynamics of a long-acting echinocandin, CD101, in a neutropenic invasive-candidiasis murine model using an extended-interval dosing design. Antimicrob Agents Chemother. 2018;62:e02154–17. doi: 10.1128/AAC.02154-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Y, Perez WB, Jimenez-Ortigosa C, et al. CD101: a novel long-acting echinocandin. Cell Microbiol. 2016;18:1308–1316. doi: 10.1111/cmi.12640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Y, Prideaux B, Nagasaki Y, et al. Unraveling drug penetration of echinocandin antifungals at the site of infection in an intra-abdominal abscess model. Antimicrob Agents Chemother. 2017;61:e01009–17. doi: 10.1128/AAC.01009-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson GR, Soriano A, Skoutelis A, et al. Rezafungin versus caspofungin in a Phase 2, randomized, double-blind study for the treatment of candidemia and invasive candidiasis- the STRIVE trial. Clin Infect Dis. 2021;73:e3647–e3655. doi: 10.1093/cid/ciaa1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson III GR, Soriano A, Cornely OA, et al. ReSTORE: efficacy and safety results of the Phase 3, noninferiority trial of rezafungin in the treatment of candidemia and/or invasive candidiasis (IC). ECCMID 23-26 April 2022; Poster #L0244; Lisbon, Portugal; 2022. [Google Scholar]
- 43.Jallow S, Govender NP. Ibrexafungerp: a First-in-Class Oral Triterpenoid Glucan Synthase Inhibitor. J Fungi. 2021;7:163. doi: 10.3390/jof7030163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfaller MA, Messer SA, Rhomberg PR, Borroto-Esoda K, Castanheira M. Differential activity of the oral glucan synthase inhibitor SCY-078 against wild-type and echinocandin-resistant strains of Candida species. Antimicrob Agents Chemother. 2017;61:e00161–17. doi: 10.1128/AAC.00161-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arendrup MC, Jorgensen KM, Hare RK, Chowdhary A. In vitro activity of ibrexafungerp (SCY-078) against Candida auris isolates as determined by EUCAST methodology and comparison with activity against C. albicans and C. glabrata and with the activities of six comparator agents. Antimicrob Agents Chemother. 2020;64:e02136–19. doi: 10.1128/AAC.02136-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berkow EL, Angulo D, Lockhart SR. In vitro activity of a novel glucan synthase inhibitor, SCY-078, against Clinical Isolates of Candida auris. Antimicrob Agents Chemother. 2017;61:e00435–17. doi: 10.1016/0005-2728(75)90129-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu YC, Barat SA, Borroto-Esoda K, Angulo D, Chaturvedi S, Chaturvedi V. Pan-resistant Candida auris isolates from the outbreak in New York are susceptible to ibrexafungerp (a glucan synthase inhibitor). Int J Antimicrob Agents. 2020;55:105922. doi: 10.1016/j.ijantimicag.2020.105922 [DOI] [PubMed] [Google Scholar]
- 48.Lepak AJ, Marchillo K, Andes DR. Pharmacodynamic target evaluation of a novel oral glucan synthase inhibitor, SCY-078 (MK-3118), using an in vivo murine invasive candidiasis model. Antimicrob Agents Chemother. 2015;59:1265–1272. doi: 10.1128/AAC.04445-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiederhold NP, Najvar LK, Jaramillo R, et al. Oral glucan synthase inhibitor SCY-078 is effective in an experimental murine model of invasive candidiasis caused by WT and echinocandin-resistant Candida glabrata. J Antimicrob Chemother. 2018;73:448–451. doi: 10.1093/jac/dkx422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiederhold NP, Najvar LK, Olivo M, et al. Ibrexafungerp demonstrates in vitro activity against fluconazole-resistant Candida auris and in vivo efficacy with delayed initiation of therapy in an experimental model of invasive candidiasis. Antimicrob Agents Chemother. 2021;65:e02694–20. doi: 10.1016/0005-2760(75)90159-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee A, Prideaux B, Zimmerman M, et al. Penetration of Ibrexafungerp (Formerly SCY-078) at the Site of Infection in an Intra-abdominal Candidiasis Mouse Model. Antimicrob Agents Chemother. 2020;64:e02268–19. doi: 10.1128/AAC.02268-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwebke JR, Sobel R, Gersten JK, et al. Ibrexafungerp versus placebo for vulvovaginal candidiasis treatment: a phase 3, randomized, controlled superiority trial (VANISH 303). Clin Infect Dis. 2022;74:1979–1985. doi: 10.1093/cid/ciab750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sobel R, Nyirjesy P, Ghannoum MA, et al. Efficacy and safety of oral ibrexafungerp for the treatment of acute vulvovaginal candidiasis: a global phase 3, randomised, placebo-controlled superiority study (VANISH 306). BJOG. 2022;129:412–420. doi: 10.1111/1471-0528.16972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spec A, Pullman J, Thompson GR, et al. MSG-10: a Phase 2 study of oral ibrexafungerp (SCY-078) following initial echinocandin therapy in non-neutropenic patients with invasive candidiasis. J Antimicrob Chemother. 2019;74:3056–3062. doi: 10.1093/jac/dkz277 [DOI] [PubMed] [Google Scholar]
- 55.Wring SA, Randolph R, Park S, et al. Preclinical pharmacokinetics and pharmacodynamic target of SCY-078, a first-in-class orally active antifungal glucan synthesis inhibitor, in murine models of disseminated candidiasis. Antimicrob Agents Chemother. 2017;61:e02068–16. doi: 10.1128/AAC.02068-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lamoth F, Lewis RE, Kontoyiannis DP. Investigational antifungal agents for invasive mycoses: a clinical perspective. Clin Infect Dis. 2022;75:534–544. doi: 10.1093/cid/ciab1070 [DOI] [PubMed] [Google Scholar]
- 57.Shaw KJ, Ibrahim AS. Fosmanogepix: a review of the first-in-class broad spectrum agent for the treatment of invasive fungal infections. J Fungi. 2020;6:239. doi: 10.3390/jof6040239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watanabe NA, Miyazaki M, Horii T, Sagane K, Tsukahara K, Hata K. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob Agents Chemother. 2012;56:960–971. doi: 10.1128/AAC.00731-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McLellan CA, Whitesell L, King OD, Lancaster AK, Mazitschek R, Lindquist S. Inhibiting GPI anchor biosynthesis in fungi stresses the endoplasmic reticulum and enhances immunogenicity. ACS Chem Biol. 2012;7:1520–1528. doi: 10.1021/cb300235m [DOI] [PubMed] [Google Scholar]
- 60.Arendrup MC, Chowdhary A, Astvad KMT, Jorgensen KM. APX001A In Vitro Activity against Contemporary Blood Isolates and Candida auris Determined by the EUCAST Reference Method. Antimicrob Agents Chemother. 2018;62:e01225–18. doi: 10.1128/AAC.01225-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arendrup MC, Chowdhary A, Jorgensen KM, Meletiadis J. Manogepix (APX001A) in vitro activity against candida auris: head-to-head comparison of EUCAST and CLSI MICs. Antimicrob Agents Chemother. 2020;64:e00656–20. doi: 10.1128/AAC.00656-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arendrup MC, Jorgensen KM. Manogepix (APX001A) displays potent in vitro activity against human pathogenic yeast, but with an unexpected correlation to fluconazole MICs. Antimicrob Agents Chemother. 2020;64:e00429–20. doi: 10.1128/AAC.00429-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pfaller MA, Huband MD, Flamm RK, Bien PA, Castanheira M. In vitro activity of APX001A (Manogepix) and comparator agents against 1706 fungal isolates collected during an international surveillance program in 2017. Antimicrob Agents Chemother. 2019;63:e00840–19. doi: 10.1128/AAC.00840-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu Y, Kilburn S, Kapoor M, Chaturvedi S, Shaw KJ, Chaturvedi V. In vitro activity of manogepix against multidrug-resistant and panresistant candida auris from the New York Outbreak. Antimicrob Agents Chemother. 2020;64:e01124–20. doi: 10.1128/AAC.01124-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berkow EL, Lockhart SR. Activity of novel antifungal compound APX001A against a large collection of Candida auris. J Antimicrob Chemother. 2018;73:3060–3062. doi: 10.1093/jac/dky302 [DOI] [PubMed] [Google Scholar]
- 66.Kapoor M, Moloney M, Soltow QA, Pillar CM, Shaw KJ. Evaluation of resistance development to the Gwt1 inhibitor manogepix (APX001A) in Candida species. Antimicrob Agents Chemother. 2019;64:e01387–19. doi: 10.1128/AAC.01387-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liston SD, Whitesell L, Kapoor M, Shaw KJ, Cowen LE. Enhanced efflux pump expression in Candida Mutants results in decreased manogepix susceptibility. Antimicrob Agents Chemother. 2020;64:e00261–20. doi: 10.1128/AAC.00261-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Y, Lee MH, Paderu P, et al. Significantly improved pharmacokinetics enhances in vivo efficacy of APX001 against Echinocandin- and multidrug-resistant Candida isolates in a mouse model of invasive candidiasis. Antimicrob Agents Chemother. 2018;62:e00425–18. doi: 10.1128/AAC.00425-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao M, Lepak AJ, VanScoy B, et al. In vivo pharmacokinetics and Pharmacodynamics of APX001 against Candida spp. in a neutropenic disseminated candidiasis mouse model. Antimicrob Agents Chemother. 2018;62:e02542–17. doi: 10.1128/AAC.02542-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hata K, Horii T, Miyazaki M, et al. Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis. Antimicrob Agents Chemother. 2011;55:4543–4551. doi: 10.1128/AAC.00366-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wiederhold NP, Najvar LK, Fothergill AW, et al. The investigational agent E1210 is effective in treatment of experimental invasive candidiasis caused by resistant Candida albicans. Antimicrob Agents Chemother. 2015;59:690–692. doi: 10.1128/AAC.03944-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hager CL, Larkin EL, Long L, Zohra Abidi F, Shaw KJ, Ghannoum MA. In Vitro and In Vivo Evaluation of the Antifungal Activity of APX001A/APX001 against Candida auris. Antimicrob Agents Chemother. 2018;62:e02319–17. doi: 10.1128/AAC.02319-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wiederhold NP, Najvar LK, Shaw KJ, et al. Efficacy of Delayed Therapy with Fosmanogepix (APX001) in a Murine Model of Candida auris Invasive Candidiasis. Antimicrob Agents Chemother. 2019;63:e01120–19. doi: 10.1128/AAC.01120-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee A, Wang N, Carter CL, et al. Therapeutic potential of fosmanogepix (APX001) for Intra-abdominal Candidiasis: from lesion penetration to efficacy in a mouse model. Antimicrob Agents Chemother. 2021;65:e02476–20. doi: 10.1128/AAC.02476-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petraitiene R, Petraitis V, Maung BBW, et al. Efficacy and pharmacokinetics of fosmanogepix (APX001) in the treatment of Candida Endophthalmitis and hematogenous meningoencephalitis in nonneutropenic rabbits. Antimicrob Agents Chemother. 2021;65:e01795–20. doi: 10.1128/AAC.01795-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pappas PG, Kullberg BJ, Vazquez JA, et al. Clinical safety and efficacy of novel antifungal, fosmanogepix, in the treatment of candidemia: results from a phase 2 proof of concept trial. Open Forum Infect Dis. 2020;7(Suppl 1). doi: 10.1093/ofid/ofaa439.457 [DOI] [Google Scholar]
- 77.Warrilow AG, Hull CM, Parker JE, et al. The clinical candidate VT-1161 is a highly potent inhibitor of Candida albicans CYP51 but fails to bind the human enzyme. Antimicrob Agents Chemother. 2014;58:7121–7127. doi: 10.1128/AAC.03707-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Warrilow AG, Parker JE, Price CL, et al. The investigational drug VT-1129 is a highly potent inhibitor of Cryptococcus species CYP51 but only weakly inhibits the human enzyme. Antimicrob Agents Chemother. 2016;60:4530–4538. doi: 10.1128/AAC.00349-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nishimoto AT, Whaley SG, Wiederhold NP, et al. Impact of the major candida glabrata triazole resistance determinants on the activity of the novel investigational tetrazoles VT-1598 and VT-1161. Antimicrob Agents Chemother. 2019;63:e01304–19. doi: 10.1128/AAC.01304-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nishimoto AT, Wiederhold NP, Flowers SA, et al. In vitro activities of the novel investigational tetrazoles VT-1161 and VT-1598 compared to the triazole antifungals against azole-resistant strains and clinical isolates of Candida albicans. Antimicrob Agents Chemother. 2019;63:e00341–19. doi: 10.1128/AAC.00341-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schell WA, Jones AM, Garvey EP, Hoekstra WJ, Schotzinger RJ, Alexander BD. Fungal CYP51 inhibitors VT-1161 and VT-1129 exhibit strong in vitro activity against candida glabrata and C. krusei isolates clinically resistant to azole and echinocandin antifungal compounds. Antimicrob Agents Chemother. 2017;61:e01817–16. doi: 10.1128/AAC.01817-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wiederhold NP, Lockhart SR, Najvar LK, et al. The fungal Cyp51-specific inhibitor VT-1598 demonstrates in vitro and in vivo activity against Candida auris. Antimicrob Agents Chemother. 2019;63:e02233–18. doi: 10.1128/AAC.02233-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brand SR, Degenhardt TP, Person K, et al. A phase 2, randomized, double-blind, placebo-controlled, dose-ranging study to evaluate the efficacy and safety of orally administered VT-1161 in the treatment of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2018;218:624e1–624 e9. doi: 10.1016/j.ajog.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 84.Brand SR, Sobel JD, Nyirjesy P, et al. 2 Study of VT-1161 for the treatment of acute vulvovaginal candidiasis. Clin Infect Dis. 2021;73:e1518–e1524. doi: 10.1093/cid/ciaa1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martens MG, Maximos B, Degenhardt T, et al. Phase 3 study evaluating the safety and efficacy of oteseconazole in the treatment of recurrent vulvovaginal candidiasis and acute vulvovaginal candidiasis infections. Am J Obstet Gynecol. 2022;227(6):880e1–880 e11. doi: 10.1016/j.ajog.2022.07.023 [DOI] [PubMed] [Google Scholar]
- 86.Fakhim H, Emami S, Vaezi A, et al. In Vitro Activities of Novel Azole Compounds ATTAF-1 and ATTAF-2 against Fluconazole-Susceptible and -Resistant Isolates of Candida Species. Antimicrob Agents Chemother. 2017;61:e01106–16. doi: 10.1128/AAC.01106-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Motahari K, Badali H, Hashemi SM, et al. Discovery of benzylthio analogs of fluconazole as potent antifungal agents. Future Med Chem. 2018;10:987–1002. doi: 10.4155/fmc-2017-0295 [DOI] [PubMed] [Google Scholar]
- 88.Wiederhold NP, Najvar LK, Fothergill AW, et al. The novel arylamidine T-2307 demonstrates in vitro and in vivo activity against echinocandin-resistant Candida glabrata. J Antimicrob Chemother. 2016;71:692–695. doi: 10.1093/jac/dkv398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wiederhold NP, Najvar LK, Fothergill AW, et al. The novel arylamidine T-2307 maintains in vitro and in vivo activity against echinocandin-resistant Candida albicans. Antimicrob Agents Chemother. 2015;59:1341–1343. doi: 10.1128/AAC.04228-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wiederhold NP, Najvar LK, Jaramillo R, et al. The novel arylamidine T-2307 demonstrates in vitro and in vivo activity against Candida auris. Antimicrob Agents Chemother. 2020;64:e02198–19. doi: 10.1128/AAC.02198-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bassetti M, Marchetti M, Chakrabarti A, et al. A research agenda on the management of intra-abdominal candidiasis: results from a consensus of multinational experts. Intensive Care Med. 2013;39:2092–2106. doi: 10.1007/s00134-013-3109-3 [DOI] [PubMed] [Google Scholar]
- 92.Bassetti M, Righi E, Ansaldi F, et al. A multicenter multinational study of abdominal candidiasis: epidemiology, outcomes and predictors of mortality. Intensive Care Med. 2015;41:1601–1610. doi: 10.1007/s00134-015-3866-2 [DOI] [PubMed] [Google Scholar]
- 93.Kullberg BJ, van de Veerdonk F, Netea MG. Immunotherapy: a potential adjunctive treatment for fungal infection. Curr Opin Infect Dis. 2014;27:511–516. doi: 10.1097/QCO.0000000000000105 [DOI] [PubMed] [Google Scholar]
- 94.Delsing CE, Gresnigt MS, Leentjens J, et al. Interferon-gamma as adjunctive immunotherapy for invasive fungal infections: a case series. BMC Infect Dis. 2014;14:166. doi: 10.1186/1471-2334-14-166 [DOI] [PMC free article] [PubMed] [Google Scholar]