Abstract
Purpose
The use of materials to facilitate dural closure during spina bifida (SB) repair has been a highly studied aspect of the surgical procedure. The overall objective of this review is to present key findings pertaining to the success of the materials used in clinical and pre-clinical studies. Additionally, this review aims to aid fetal surgeons as they prepare for open or fetoscopic prenatal SB repairs.
Methods
Relevant publications centered on dural substitutes used during SB repair were identified. Important information from each article was extracted including year of publication, material class and sub-class, animal model used in pre-clinical studies, whether the repair was conducted pre-or postnatally, the bioactive agent delivered, and key findings from the study.
Results
Out of 1,121 publications, 71 were selected for full review. We identified the investigation of 33 different patches where 20 and 63 publications studied synthetic and natural materials, respectively. From this library, 43.6% focused on clinical results, 36.6% focused on pre-clinical results, and 19.8% focused on tissue engineering approaches. Overall, the use of patches, irrespective of material, have shown to successfully protect the spinal cord and most have shown promising survival and neurological outcomes.
Conclusion
While most have shown significant promise as a therapeutic strategy in both clinical and pre-clinical studies, none of the patches developed so far are deemed perfect for SB repair. Therefore, there is an opportunity to develop new materials and strategies that aim to overcome these challenges and further improve the outcomes of SB patients.
Keywords: Spina bifida, Dural substitute, Myelomeningocele, Patch, Pediatric
Introduction
Spina bifida (SB) is a congenital birth defect with an incidence as high as 1 per 1000 births in which the neural tube fails to fully close during the neurulation process early in gestation [1, 2]. Myelomeningocele (MMC) and myeloschisis, both subtypes of open spina bifida, result in leakage of cerebrospinal fluid and exposure of the spinal cord to the external uterine environment, including amniotic fluid, which ultimately leads to further spinal cord damage [3]. With the hope of improving patient quality of life, surgical defect repair strategies have been a topic of great interest to the scientific and medical communities.
Surgical approaches to repair MMC defects have evolved in the neurosurgical field over the past two decades. The standard surgical MMC repair was performed after patient birth until 1997 when the first prenatal repair was performed via an endoscopic intrauterine approach and subsequently through hysterotomy [4]. The introduction of prenatal spina bifida correction has made great advancements in the surgical field. Enhanced by the advancements in ultrasound technology, it confirmed that neurological damage due to the exposed spinal cord and nerve roots at the defect was a progressive process in utero and is initiated early in gestation. This theory is often called the “two-hit hypothesis.” This hypothesis was confirmed after the Management of Myelomeningocele Study (MOMS) demonstrated a significant improvement in neurological outcomes in infants that had undergone open prenatal spina bifida repair compared to when the defect was repaired postnatally [4]. However, prenatal procedures have been associated with complications such as preterm labor and uterine dehiscence. Minimally invasive fetoscopic surgical techniques were developed to reduce these complications; however, its complexity may make this a less common procedure among pediatric neurosurgeons and fetal surgeons. Additionally, spinal cord retethering can still arise after prenatal or postnatal procedures. The neural placode should be completely released from its natural attachments, which are inherent to the congenital spinal defect, but the healing process and scarring can lead to retethering in some cases. In fact, 20–50% of children with spina bifida repaired postnatally and up to 30% of patients receiving prenatal correction experience spinal cord retethering, leading to worsening of neurological symptoms by spinal cord stretching and ultimately require further surgical intervention [5].
The use of materials to facilitate dural closure has emerged as an important aspect of all forms of spina bifida repair as they are suitable to protect the spinal cord from the external or the uterine environment, prohibit cerebrospinal fluid leakage, and based on their physical properties, may reduce the time of the surgical procedure. Importantly, recent studies show that the use of a dural substitute is a promising method to avoid spinal cord tethering. The first use of a synthetic dural substitute during postnatal spina bifida repair dates back nearly 5 decades; however, in recent years, biomedical engineers and fetal surgeons have collaborated to develop new materials and strategies that can be used during prenatal repairs to not only protect the spinal cord, but to also aid in the regeneration and repair of the neural tissue. This review aims to discuss the different types of dural substitutes that have been used for spina bifida repair using various surgical approaches and their clinical and pre-clinical outcomes. Furthermore, we will briefly discuss tissue engineering strategies that are being used to facilitate tissue regeneration after patch implantation. We also provide concluding remarks on the challenges facing currently used patches and the requirements of the “ideal” patch for spina bifida repair based on the surgical approach.
Materials and methods
Literature review strategy
A literature review was performed in February 2021, without time or language restrictions, using the PUBMED/MEDLINE database. A combination of words was strategically used by the authors to identify a library of publications that centered on the use of dural substitutes as a repair strategy for spina bifida. The key words selected by the authors were combined as follows: “Spina bifida”, “Myelomeningocele”, and “Spinal dysraphism” were all combined independently with “dura substitute”, “dural substitute”, “duraplasty”, “patch”, “graft”, “scaffold”, “scaffolds”, “matrix”, “skin substitute”, “dermal substitute”, “artificial skin”, “sealant”, “glue”, “adhesive”, “bioglue”, “fibrin”, “fibrinogen”, “skin graft”, “in utero”, and “repair”, “fetoscopy”, “intrauterine”, “prenatal”, and “repair”.
Inclusion and exclusion criteria
We primarily focused on publications that described pre-clinical and clinical studies where the patch was placed directly over the neural tissue as a spina bifida repair strategy. Publications were excluded when the patch was placed over native dura after the dura was sutured and closed, the material was not specifically identified, or the exact location of the patch placement was absent. Finally, publications focused only on the development and characterization of the material and not on its direct use as a dural substitute were also excluded from the primary library.
Study selection and data extraction
All titles and abstracts were screened for relevance and the relevant articles were selected for full report review. We extracted information from each article including year of publication, material class and sub-class, animal model used in pre-clinical studies, whether the repair was conducted pre- or postnatally, the bioactive agent delivered, and key findings from the study.
Literature review results
Using the strategy outlined, we obtained 1121 publications and from this total 263 articles were selected for full text review based on abstract relevancy with 71 publications selected for full review (Fig. 1). The materials were first categorized based on class, whether synthetic or naturally derived, and then further categorized based on sub-class, which describes the primary source of the material. Figure 2 illustrates the number of publications identified under each class and subclass of materials. We identified 20 and 63 publications that used a synthetic or natural material, respectively. These numbers do not summate to 71 as some publications investigated different types of patches within the same publication. Within these subclasses, we identified the development of 33 different patches that have been studied as a dural substitute for spina bifida repair. From this library, we determined that 31/71 (43.6%) publications focused on clinical results (Table 1), whereas 26/71 (36.6%) publications were predominantly studied in a pre-clinical setting (Table 2). Additionally, we found 14/71 (19.8%) publications that demonstrated the use of different patches to also be a bioactive agent delivery device to enhance repair (Table 3).
Fig. 1.

Flow diagram of literature selection
Fig. 2.
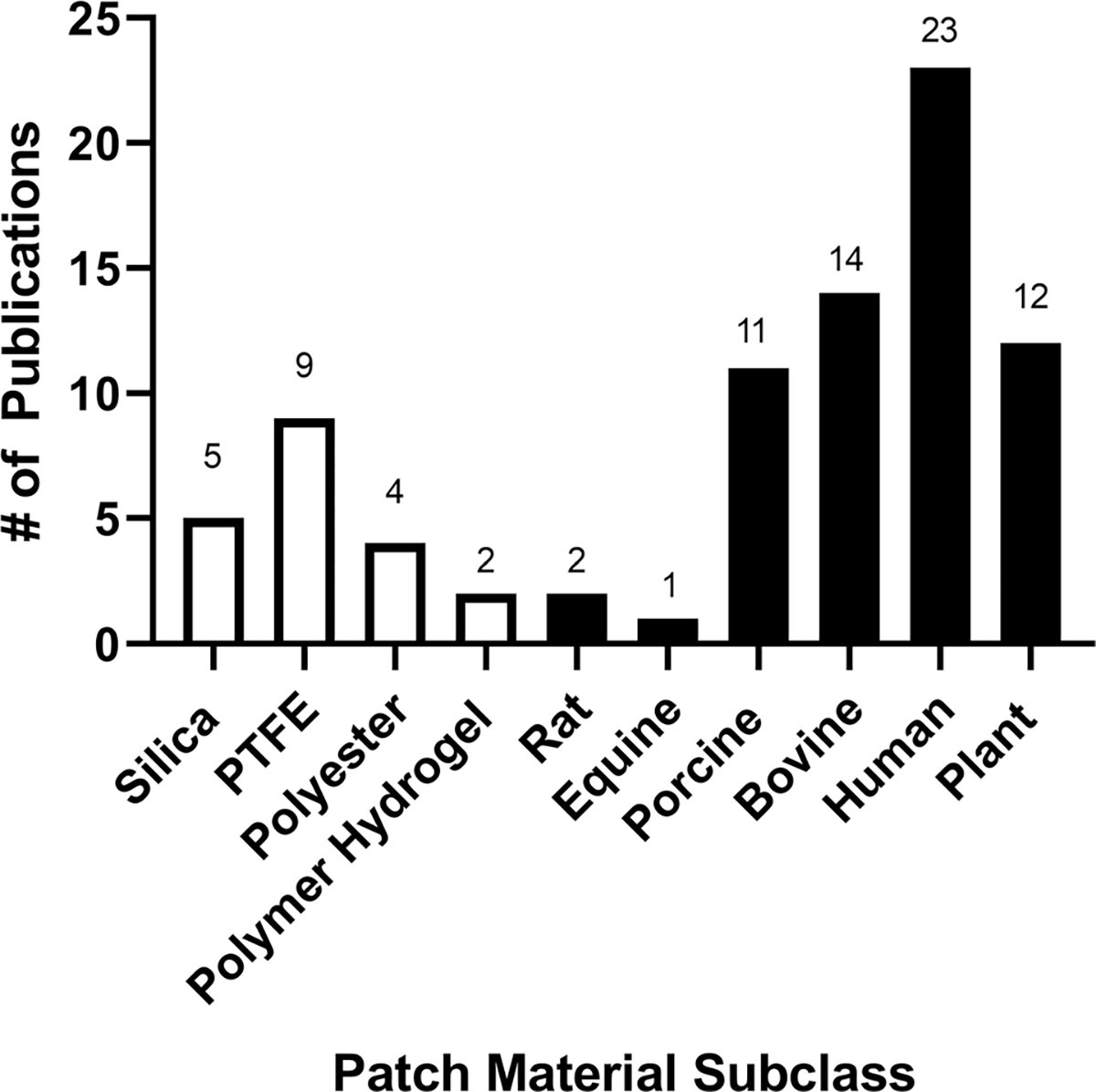
The number of publications that utilized patches developed from various subclasses of materials for spina bifida repair. White denotes synthetic materials and black denotes naturally derived materials
Table 1.
Patches utilized for spina bifida repair in a clinical setting.
| Material class | Material subclass | Patch component | Trade name | Repair | Key findings | Reference |
|---|---|---|---|---|---|---|
|
| ||||||
| Synthetic | Silica | Silicone coated polyester fabric | Dacron | Postnatal | Case report indicating satisfactory repair of a 1-day-old female with MMC using a patch of silicone coated Dacron | [6] |
| Postnatal | Dacron used to repair lipomeningocele provided an adequate barrier, but led to complications | [7] | ||||
| Medical adhesive silicone | Silastic | Postnatal | The use of Silastic to repair MMC demonstrated 5 years with no tethered cord, but there was an increased risk of CSF leakage | [8] | ||
| Postnatal | Dacron-reinforced silastic led to increased fibroblastic response compared to nonreinforced Silastic patches | [9] | ||||
| Polytretrafluoroethylene (PTFE) | Teflon | Gore-Tex, Gore | Postnatal | PTFE can prevent adhesion of the spinal cord with no significant complications | [10] | |
| Preclude | Postnatal | Case study demonstrated successful management of 29-week infant born with an MMC treated with Gore-Tex patch | [11] | |||
| Prenatal | Developed a percutaneous fetoscopic method for prenatal patch coverage that achieved tight patch attachment | [12] | ||||
| Postnatal | No difference in incidence of CSF leakage in patients that received primary dural closure, PTFE patch, or bovine pericardium patch and patches inhibited retethering compared to primary dural closure | [13] | ||||
| Prenatal | Fetoscopic surgical implantation of extracellular matrix or PTFE patches led to 13% patient complication and all operated fetuses required postnatal re-coverage | [14] | ||||
| Prenatal | 12-month follow-up of 71 patients who were repaired prenatally with teflon or collagen patches: 28% required recoverage and 45% required shunt placement | [15] | ||||
| Polyesterurethane | Polyesterurethane | Neuro-Patch | Postnatal | A Neuro-Patch was used under a Limberg Flap when primary dura closure was not possible | [16] | |
| Natural | Porcine | Small intestine submucosa-derived extracellular matrix | Durasis | Prenatal | Fetuses fetoscopically repaired with Durasis patch showed reversal of hindbrain herniation, near-normal leg function, and satisfactory bladder and bowel function | [17] |
| Prenatal | Fetoscopic surgical implantation of extracellular matrix or PTFE patches led to 13% patient complication and all operated fetuses required postnatal re-coverage | [14]* | ||||
| Prenatal | 12-month follow-up of 71 patients who were repaired prenatally with Goretex or Durasis patches: 28% required recoverage and 45% required shunt placement | [15]* | ||||
| Equine | Achilles tendon derived collagen foil | TissuDura | Postnatal | The use of TissuDura as a dural substitute did not lead to hydrocephalus, scar adhesion, or a foreign body reaction | [18] | |
| Bovine | Tendon | Duragen | Postnatal | Case study demonstrated that a combination of collagen and dermal matrix dural substitutes lead to sufficient coverage after several failed attempts of closure | [19]* | |
| Prenatal and postnatal | Prenatal surgery for myelomeningocele has better outcomes over postnatal | [4] | ||||
| Duraform | Prenatal | Duraform was used as a dural substitute when the dura could not reach over the entire defect | [20] | |||
| Dermis | Durepair | Prenatal | Three-layer closure led to lower incidence of cerebral spinal fluid leakage and increased rate of reversal of hindbrain herniation compared to single-layer closure | [21] | ||
| Postnatal | Case study demonstrated that acellular dermal matrix is a feasible option for primary coverage of MMC | [22] | ||||
| Pericardium | Dura-Guard | Postnatal | No difference in incidence of CSF leakage in patients that received primary dural closure, PTFE patch, or bovine pericardium patch and patches inhibited retethering compared to primary dural closure | [13]* | ||
| Human | Cryopreserved amniotic membrane | Postnatal | No neurological or late complications were reported and coverage was still intact 19 months after repair | [23] | ||
| Postnatal | Amniotic membrane repair did not lead to spinal cord tethering 6 months after surgery | [24] | ||||
| Dermal Matrix | Alloderm | Postnatal | Case study demonstrated that a combination of collagen and dermal matrix dural substitutes leads to sufficient coverage after several failed attempts of closure | [19]* | ||
| Prenatal | Case study demonstrated that MMC repair with Alloderm resulted in the presence of a dermoid cyst and severe tethering | [25] | ||||
| Prenatal | Fetoscopic repair, although feasible, does not yet yield optimal surgical results | [26] | ||||
| Maternal Skin Graft | Prenatal | Survivors of fetoscopic repair using maternal skin graft possessed only mild locomotor deficits | [27] | |||
| Prenatal | In utero repair through a hysterotomy may be technically superior compared to an endoscopy technique | [28] | ||||
| Autologous Amniotic Membrane | Postnatal | Case study demonstrated that three-layer autologous amnion led to a watertight closure | [29] | |||
| Cadaver Dura | Tutoplast | Postnatal | Graft of freeze-dried cadaver was successfully used as a dural substitute | [30] | ||
| Plant | Biocellulose nanofiber mesh | Bionext | Prenatal | Fetoscopic biocellulose patch MMC repair leads to reversed hindbrain herniation and favorable neurological and motor function outcomes | [31] | |
| Prenatal | Fetoscopic MMC repair with biocellulose patch led to a successful surgical closure | [32] | ||||
| Prenatal | Fetoscopic MMC repair with biocellulose led to reversal of hindbrain herniation and normal motor function | [33] | ||||
| Prenatal | Large open spina bifida defects were successfully treated with a bilaminar skin substitute over a biocellulose patch through percutaneous approach | [34] | ||||
| Sodium hyaluronate/carboxymethylcellulose | Seprafilm | Prenatal | Case study demonstrated that at 7 months of age, the child possessed no obvious neurological deficit, normal leg movement, and bladder function | [35] | ||
Indicates publications that studied multiple patches
Table 2.
Patches pre-clinically studied for spina bifida repair.
| Material class | Material sub-class | Patch components | Trade name | Animal model | Repair | Key findings | Reference | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Synthetic | Silica | Silicone coated polyester fabric | MMC surgically created | Fetal lamb | Prenatal | Repaired animals were able to walk, had sphinter continence, and almost complete defect closure compared to untreated | [36]* | |
| Medical adhesive silicone, polypropylene, and high-density polyethylene | Marlex | MMC surgically created | Fetal lamb | Prenatal | Repaired animals were able to walk, had sphinter continence, and almost complete defect closure compared to untreated | [36]* | ||
| Mytetratiuoroethylene (PIPE) | Teflon | Gore-Tex | Dural injury | Rat | Lyophilized spinal dura physically performed better than collagen mesh and PTFE patches; however, there was no difference in neurological function between the groups | [37]* | ||
| MMC surgically created | Fetal Lamb | Prenatal | The external and internal sphincter muscles developed normally in animals repaired with Gore-Tex or Alloderm | [38]* | ||||
| Gore Preclude | MMC surgically created | Fetal lamb | Prenatal | Only 38% and 14% of animals treated with PTFE and surgical adhesive in an open and fetoscopic fashion, had complete closure, respectively; however, fetal survival was greater with fetoscopic repair | [39] | |||
| Polyester | Poly(lactic-acid) nanofibers | MMC surgically created | Fetal lamb | Prenatal | The use of a nanofiber scaffold is feasible for use in large animal model of fetal MMC and did not lead to an inflammatory or fibrotic response | [40] | ||
| Poly(lactic-acid), poly(caprolactone) film | Laminectomy | Rat | Postnatal | The use of this film did not lead to inflammation, tethering cord, or any adverse effects on healthy neural function | [41] | |||
| Polymer Hydrogel | Reverse Thermal Gel (PSHU-PNIPAAm) | Congenital Grhl3 grainy head | Fetal mouse | Prenatal | The use of this film did not lead to inflammation, tethering cord, or any adverse effects on healthy neural function | [42] | ||
| Dopamine-Reverse Thermal Gel (PSHU-PNIPAAm) | Congenital Grhl3 grainy head | Fetal Mouse | Prenatal | A large variability of defect coverage was observed after gel injection | [43] | |||
| Naturai | Rat | Peritoneum | Laminectomy | Fetal rat | Prenatal | Dame rat peritoneum successfully protected the injured spinal cord | [44] | |
| Porcine | Dermis-derived gelatin, chitosan scaffold | MMC retinoic acid induced | Fetal rat | Prenatal | MMC repair using this patch led to improvements in bladder neuromuscular development | [45] | ||
| Dermal matrix | Xenoderm | MMC surgically created | Fetal lamb | Prenatal | Fetal patch MMC repair reversed hindbrain herniation after addition of a myelotomy to the sheep model | [46] | ||
| Bovine | Bovine tendon derived collagen | Duragen | MMC surgically created | Fetal lamb | Prenatal | In utero repair prevented hindbrain herniation | [47] | |
| MMC surgically created | Fetal lamb | Prenatal | Fetoscopic patch repair partially prevented bladder abnormalities associated with MMC | [48] | ||||
| MMC surgically created | Fetal lamb | Prenatal | All lambs covered with either collagen or small intestinal submucosa showed no or minor neurologic morbidity | [49] | ||||
| MMC surgically created | Fetal lamb | Prenatal | Collagen biomatrix preserved spinal cord architecture and spinal function | [50] | ||||
| Bovine dermis, nuchal ligament collagen | Matriderm | MMC surgically created | Fetal lamb | Prenatal | Lambs who underwent two port fetal repair compared to a single-access port repair did not differ significantly and none had CSF leakage, chiari malformation, or paraplegia | [51] | ||
| Human | Umbilical cord | Amnioguard | MMC surgically created | Fetal lamb | Prenatal | Cryopreserved human umbilical cord patch improved survival and neurological outcomes | [52] | |
| MMC surgically created | Fetal lamb | Prenatal | Repair with biocellulose patch and adhesive led to exposed spinal cord with CSF leakage compared to repair with human umbilical cord with suture which demonstrated full closure | [53]* | ||||
| MMC retinoic acid | Fetal rat | Prenatal | Cryopreserved human umbilical cord patch promoted cellular migration with a minimal inflammatory response compared to biocellulose film | [54]* | ||||
| MMC retinoic acid | Fetal rat | Prenatal | Repair with human acellular dermal matrix resulted in less regenerative cellular growth and more inflammation compared to human umbilical cord graft | [55]* | ||||
| Amnion | MMC surgically created | Fetal lamb | Prenatal | Amniotic membrane increased protection of the spinal cord, but decreased wound healing of the skin | [56] | |||
| Dermal Matrix | Alloderm | MMC surgically created | Fetal lamb | Prenatal | Repair using Alloderm may prevent or reverse hindbrain herniation | [57] | ||
| MMC surgically created | Fetal lamb | Prenatal | The external and internal sphincter muscles developed normally in animals repaired with Gore-Text or Alloderm | [38]* | ||||
| MMC surgically created | Fetal lamb | Prenatal | Human acellular dermal matrix led to more tissue adherence than biocellulose patch | [58]* | ||||
| MMC retinoic acid | Fetal rat | Prenatal | Repair with human acellular dermal matrix resulted in less regenerative cellular growth and more inflammation compared to human umbilical cord graft | [55]* | ||||
| Lyophilized spinal dura | Lyodura | Dural injury | Rat | Lyophilized spinal dura physically performed better than collagen mesh and PTFE patches; however, there was no difference in neurological function between the groups | [37]* | |||
| Maternal skin graft | MMC surgically created | Fetal lamb | Prenatal | In utero repair of MMC using maternal skin graft is technically feasible and resulted in spinal cord protection | [59] | |||
| Plant | Biocellulose film | Cellulose solution | MMC retinoic acid | Fetal rat | Prenatal | Cryopreserved human umbilical cord patch promoted cellular migration with a minimal inflammatory response compared to biocellulose film | [54]* | |
| Biocellulose nanofiber mesh | Bionext | MMC surgically created | Fetal lamb | Prenatal | Use of Bionext led to formation of a neoduramater which protected the nervous tissue and avoided partial injury of the medulla normally caused by the classical repair technique | [60] | ||
| Biocellulose dressing | Dermafill Nexfill Biofill | MMC surgically created | Fetal rabbit | Prenatal | The use of Biofill successfully repaired MMC in fetal rabbits | [61] | ||
| MMC surgically created | Fetal lamb | Prenatal | The use of biocellulose led to less spinal cord tethering compared to human acellular dermal matrix | [58]* | ||||
| MMC surgically created | Fetal lamb | Prenatal | Cellulose biomatrix successfully corrected 90% of repaired lambs with MMC | [62] | ||||
| MMC surgically created | Fetal lamb | Prenatal | Repair with biocellulose patch and adhesive led to exposed spinal cord with CSF leakage compared to repair with human umbilical cord with suture which demonstrated full closure | [53]* | ||||
| Alginate hydrogel (crosslinked with polyacrylamide with a bridging chitosan polymer) | MMC Surgically created | Fetal lamb | Prenatal | Hydrogel expands with the defect over time and does not lead to spinal cord tethering | [63] | |||
Indicates publications that studied multiple patches
Table 3.
Patches used during tissue engineering approaches to repair and regeneration neural function after spina bifida repair
| Material class | Material subclass | Patch component | Bioactive delivered | Animal model | Key findings | Reference |
|---|---|---|---|---|---|---|
|
| ||||||
| Synthetic | Polyester | Poly(F-lactide-co-caprolactone) + polyfpropylene glycol) + sodium acetate nanofibrous scaffold | iPSC neural crest stem cells | Surgically induced lamb MMC | Demonstrated integration of transplanted cells within the spinal cord | [64] |
| Natural | Porcine | Small intestine submucosa-derived extracellular matrix | Placenta-derived mesenchymal stromal cells | Surgically induced lamb MMC | Lambs repaired with patch and underwent postnatal bracing and physical therapy survived for 6 months after delivery and obtained rescued mobility compared to those without physical therapy | [65] |
| Surgically induced lamb MMC | Cell treatment improved motor function compared to animals that only received the ECM patch and was associated with large neuron density | [66] | ||||
| Surgically induced lamb MMC | Repair of MMC with high density cell treatment led to improved motor function and was associated with an increase in neuron density | [67] | ||||
| Retinoic acid-induced MMC in fetal rats | Spinal cord compression was reduced in animals treated with ECM and cells | [68] | ||||
| Surgically induced lamb MMC | Fetal viability, ambulation, and neuron densities were greater in animals treated with ECM and cells | [69] | ||||
| Dermis derived gelatin + chitosan scaffold | Bone marrow mesenchymal stem cells | Retinoic acid induced MMC in fetal rats | Stem cell transplant was associated with decreased defect size, stem cell survival and neuron regeneration in the defect site | [70] | ||
| Bovine | Dermis derived gelatin + bone derived collagen sponges | Basic fibroblast growth factor | Retinoic acid induced MMC in fetal rats | Release of bFGF from the sponge increased neovascularization and tissue incorporation | [71] | |
| Surgically induced lamb MMC | Release of bFGF from the sponges enhanced epithelialization, inhibited spinal cord damage, and reversed hindbrain herniation | [72] | ||||
| Artificial skin (bovine type 1 collagen gel) | Induced pluripotent stem cell derived keratinocytes | Retinoic acid induced MMC in fetal rats | 12/20 animals obtained partial or full defect coverage; however, there was not a significant difference in defect size with therapy | [73] | ||
| Rat | Rat tail collagen gel | Placenta-derived mesenchymal stromal cells | Surgically induced lamb MMC | Cells and collagen treatment improved motor outcomes and neuron quantity compared to collagen alone | [74] | |
| Human | Amnion | Placenta derived mesenchymal stromal cells | Surgically induced lamb MMC | Repair with amnion plus placental derived mesenchymal stromal cells from early-gestation placenta led to much improved motor function compared to term-gestation placenta | [75] | |
| Dermal matrix (Alloderm) | Neural stem cells | Surgically induced lamb MMC | Neural stem cells transplanted on Alloderm remained undifferentiated and produced important neurotrophic factors at the defect site | [76] | ||
| Fibrin hydrogel | Rat neural progenitor cells | Retinoic acid-induced MMC in fetal rats | Hydrogel treatment correlated with increased neuronal maturation | [77] | ||
Clinical overview
Synthetic dural substitutes
Silica
The use of a synthetic material for dural substitute was reported as early as 1971 when silicone-coated Dacron was sewn into the free dural margin after MMC excision in a 1-day old female patient [6]. Importantly, the use of the silicone-coated Dacron patch was able to be tailored to a defect of any size and led to a watertight closure of the dural canal without any spinal cord tethering [7]. Twenty years after silicone-coated Dacron was used, Silastic, medical adhesive silicone, was used to repair spina bifida defects in postnatal patients [9]. Silastic showed much potential as there were no observations of CSF leakage or other complications. Interestingly, Dacron-reinforced Silastic led to an unfavorable fibroblastic response [9]; therefore, non-reinforced Silastic was deemed as a safe dural substitute that also may prevent tethering [8]. Silica-based dural substitutes have shown promise in improving spina bifida repair procedures and outcomes; however, they are limited due to their lack of biodegradability, which is likely why they have not been a popular material used for dural substitutes in the recent decades.
Polytetrafluorethylene (PTFE)
During the turn of the twenty-first century, Gore-Tex, composed of polytetrafluorethylene (PTFE) commonly known as Teflon, emerged as a potential material to use as a dural substitute in which there were no significant complications after use during postnatal repair [10]. Clinically, the use of Gore-Tex as a dural substitute has shown promising results, in fact the first clinical report demonstrated successful management of a 29-week infant born with a MMC defect using a Gore-Tex dural substitute [11]. Furthermore, the feasibility of percutaneous fetoscopic spina bifida repair using a PTFE patch was demonstrated in 3 fetal patients in which tight patch attachment was achieved [12]. However, long-term follow-up studies indicated that approximately 28% and 45% of patients who were prenatally repaired with either Teflon or extracellular matrix-based patches required postnatal re-coverage or shunt placement, respectively [15]. Altogether, the use of PTFE patches has shown potential as a successful dural substitute; however, like silicon-based materials, they are not biodegradable which leads to secondary surgical procedures and associated emotional and economic burden.
Polyesterurethane
Based on our review of the literature, the use of a polyesterurethane-based dural substitute patches have had limited attention in the surgical community. The authors studying the success of a postnatal Limberg’s Flap as a surgical repair technique for myelomeningocele indicated that, in a few of the cases where there was not enough tissue for a primary dura closure, a Neuro-Patch was placed under the Limberg’s Flap [16]. Of the two patients that received the artificial dura substitute, one developed an infection and the patch was required to be removed. Moreover, the authors suggested that this infection was associated with the patch itself [16]. Therefore, the use of polyesterurethane-based materials for spina bifida repair has not emerged as a prominent strategy.
Natural dural substitutes
Non-human animal
Patches developed using non-human animal–derived materials have primarily been made using bovine, equine, and porcine-derived products. The use of bovine products, including collagen, dermis, and pericardium, emerged first clinically when a combination of collagen and dermal matrix dural substitutes led to sufficient coverage after several failed attempts at postnatal defect closure [19]. Most recently, a case study demonstrated acellular dermal matrix as a feasible option for postnatal MMC coverage [22]. In addition, a bovine tendon collagen patch was used in MOMS trial when it was not possible to have a primary dura suture [4] and the latest follow-up report from the MOMS study had not reported any patch-related complications after a 30-month follow-up [78]. Belfort et al. compared two fetoscopic techniques, a single-layer (primarily dura and skin in-block closure) vs three-layer closure, in which a bovine dermis collagen patch was placed over the neural tissue followed by muscle and skin closure, using a laparotomy-assisted exposed uterus fetoscopic technique [21]. Overall, the short-term results showed improved rates of CSF leakage and reversal of hindbrain herniation. Also, developed from bovine tendon collagen, Duraform® was successfully used as a dural substitute in prenatal correction when primary dural repair was not possible due to defect size [20]. Furthermore, Dura-Guard® bovine pericardium showed no difference in incidence of CSF leakage compared to the use of PTFE patch and inhibited spinal cord tethering compared to primary dural closure in postnatal repair [13]. Similarly, the use of TissuDura®, made from equine Achilles tendon collagen, inhibited hydrocephalus, scar adhesion, and foreign body response after postnatal application [18]. Finally, it was reported that a fetoscopic technique for in utero MMC repair using a porcine small intestine submucosa-derived extracellular matrix absorbable patch as a dura substitute had successfully been developed and results indicated reversal of hindbrain herniation and improved neurological outcomes [17]. However, the use of this dural substitute required that all operated fetuses receive postnatal surgery for re-coverage [14]. Overall, non-human animal-derived products as dural substitutes have led to fewer complications after clinical application.
Human
The use of human tissue for dura substitute is deemed a logical strategy. One of the first materials used as dura substitute was the human dura itself, harvested from a human cadaver [30]. It was used in the past in MMC postnatal repair of large defects [30] and was also tested for MMC intrauterine repair in a pig model [44]. However, a specific lyophilized spinal dura was discovered to cause Creutzfeldt-Jakob Disease [79], a prion disease that is a rapidly progressive and fatal neurodegenerative disorder, and therefore may be a reason why this material has not been studied or used for several years.
Human dermal tissue also presented as a good option for fast coverage of an MMC defect. Bruner et al. described a skin graft harvested from the mother during in-utero MMC hysterotomy and fetoscopic repairs and did not report any patch correlated issues [27, 28], elaborating on previous pre-clinical studies demonstrating the feasibility of this strategy [59]. Acellular dermal matrix, a patch developed as a skin burn treatment, has been used in MMC repair because of its physical characteristics, malleability, and ability to be sutured. In fact, Farmer et al. described a fetoscopic technique with acellular dermal matrix as a dural substitute for MMC repair; however, this did not yield optimal surgical outcomes [26]. On the other hand, Mazzola et al. reported 3 cases of dermoid cyst at the MMC repair site after in-utero repair with acellular dermal matrix that led to a deterioration of the neural function [25]. This ultimately raised the question about the relationship of the dermal patch to the formation of dermoid cysts.
Most recently, amniotic membrane has been used as dural substitute in prenatal MMC repair [23, 24, 29]. Amniotic membrane is harvested from donors undergoing cesarean section, rinsed in saline solution, and cryopreserved in liquid nitrogen. It is placed with amnion side down over the placode. For postnatal repair, the use of autologous amniotic membrane harvested at the moment of delivery was described by Weerd et al. in a three-layer technique closure associated with a perforator flap [29]. Another similar product being used is the human umbilical cord (HUC) patch. Papanna et al. reported two cases of large in utero myeloschisis repair using the HUC patch as skin substitute with good results although their technique included a primary dura closure and was not included in our selection list [53]. Our group is currently using this HUC as a dural substitute in both open and fetoscopic prenatal SB repair, observing good results after being used in more than 40 cases clinically.
Plant
Cellulose-based patches are the most recent alternative for MMC repair. Lapa Pedreira et al. reported a successful prenatal single-layer fetoscopy technique using a biocellulose patch and later published 10 consecutive cases without patch related complications [32, 33]. In fact, it was reported that the use of the biocellulose patch led to hindbrain herniation reversal and favorable neurological and motor function [33]. In 2018, their series of 47 consecutive cases determined that 13 cases required a bilaminar skin substitute (silicone and dermal matrix) associated with the cellulose patch [34]. In 3 of these cases, initially repaired prenatally with a percutaneous approach, postnatal repair was required, and in the other 10 cases, the silicone layer detached spontaneously, and the lesion healed by secondary intention. Ultimately, there were no adverse effects related to the biocellulose patch. Other groups reported series of cases with fetoscopic MMC repair using biocellulose as a dural substitute without patch-related issues as late as 7 months after birth [31, 35].
Pre-clinical experimental overview
Synthetic dural substitutes
Silica
There are few pre-clinical studies investigating the use of silica products as dural substitutes. Using the well-accepted surgical sheep MMC model, Fontecha et al. demonstrated that fetuses repaired via laparotomy and hysterotomy with either Silastic or Silastic decorated with Marlex in utero were able to walk, retained sphincter continence, had almost complete defect closure, and minimum Chiari malformation after birth compared to untreated animals [36]. Importantly for surgical feasibility, the average time to achieve fetal coverage was approximately 7 min. It is likely that silica lost its popularity as a dural substitute due to its inability to biodegrade.
PTFE
Pre-clinical studies indicate that the use of Gore-Tex® patches did not inhibit neurological function in healthy rats, indicating its bioavailability [37]. When used as a dural substitute in rats with a surgically induced dural injury, Gore-Tex® prevented cord tethering in direct contrast to substitutes comprised of collagen or lyophilized spinal dura which led to spinal cord adhesion [37]. Additionally, repairing MMC defects in fetal sheep using Gore-Tex® as a dural substitute led to the normal development of anal sphincter muscles, promoting normal bowel function after birth compared to untreated fetuses [38].
Clinical studies using PTFE as a dural substitute indicate that the material is routinely secured using sutures; however, other methods for securing PTFE dural substitutes should be considered with the aim of reducing time of operation. One such strategy that has been investigated is the use of surgical adhesive [39]. Using the surgical MMC lamb model, all of the animals that were repaired with sutures had complete dural closure; however, only 38% and 14% of animals that were repaired with the PTFE patch secured with surgical adhesive in either an open or fetoscopic manner, respectively, had complete dural closure [39]. These results indicate that surgical adhesive may not be the best strategy to secure patches, especially during fetoscopic repair, and that other methods of patch securement should be a priority for future research.
Polyester
Using silica and PTFE-based materials have shown promising results in the repair of MMC; however, a major limitation of these materials is that they must be removed with an additional surgery after reconstruction because they do not degrade over time in the body. To address this challenge, researchers turned to polyesters, such as poly-lactic acid (PLA) and poly-caprolactone (PCL), which degrade over time via hydrolysis, and therefore do not require removal. Nanofiber scaffolds fabricated using PLA showed feasibility of repairing MMC defects in fetal sheep [40]. Specifically, this group’s strategy incorporated one semicircular PLA nanofiber scaffold under the dural closure with a secondary rectangular PLA nanofiber scaffold above the dural closure. Importantly, histopathology revealed no significant immune cell infiltration or scar tissue formation indicative of a fibrotic response in the neural tissue; however, this repair strategy did not prevent hindbrain and cerebellar herniation [40]. Neural outcomes were significantly improved after addition of poly (propylene glycol), sodium acetate, and induced pluripotent stem cell derived neural stem cells [64]. However, scar formation was still present indicating a critical limitation of this device.
In addition to biodegradability, one significant advantage of using polyesters for the development of dural substitutes for MMC repair is that the properties of the final material developed using these polymers can be easily tailored. For example, our group has shown that by altering the ratio of PLA and PCL, the glass transition temperature can be adapted near body temperature, facilitating shape memory properties [80]. This property is expected to reduce operation time during fetoscopic MMC repair as the patch fabricated using this ratio of polymers can be easily rolled up to load into the trocar but return to its original shape upon implant. This PLA/PCL patch is also biodegradable with approximately 15% degradation after being submerged in human amniotic fluid for 16 weeks. Additionally, we demonstrated that this patch is water impermeable which ultimately protects the spinal cord from the exterior amniotic fluid and inhibits the further leak of cerebrospinal fluid. When implanted as a dural substitute in rats with a surgical dural injury, this patch did not lead to reactive astrocytes or reduced number of neurons within the neural tissue compared to untreated or uninjured controls [41]. Most interestingly, neural function was improved compared to untreated rats with dural injury. Because of these results, this PLA/PCL patch was deemed as a promising dural substitute and is currently under investigation as an MMC defect repair mechanism in larger animals.
Reverse thermal gels
The most recent advancement in synthetic dural substitute technology is the development of reverse thermal gels (RTGs). Reverse thermal gels (RTGs) are a class of biomaterials that can transition from a solution to gel state as the result of a temperature change; therefore, replacing the need for chemical reactions, such as cross-linking, and additional energy sources, such as UV administration. For example, RTGs with a polymer backbone consisting of poly(serinol hexamethylene urea) (PSHA) conjugated with poly(N-isopropylacrylamide) (PNIPAm), used to achieve reversible thermal state transition properties, are a liquid at room temperature, but quickly forms a solid at physiological temperature [42]. The use of RTGs as an MMC repair strategy is quite promising as this material can be injected onto the defect using a small gauge needle. This would decrease the invasiveness and operation time which has the potential to improve overall outcomes of the procedure. PSHA-PNIPAm RTGs are biocompatible in vitro, stable in amniotic fluid for at least up to 6 months and possess limited permeability. Researchers demonstrated the minimal invasiveness of PSHA-PNIPAm RTG application by successfully injecting the solution into Grainy Head-like (GRhl3) neural tube defect mice fetuses using a small gauge needle [42]. This technology was improved by incorporating dopamine to mimic the underwater adhesive properties of mussels [43].
Natural dural substitutes
Non-human animal
In the last two decades, much preclinical efforts have been focused on further investigating the impact of porcine, rat, and bovine derived products as dural substitutes. Porcine-derived dermal matrix was first described preclinically for MMC repair in 2003 in a sheep model for in utero repair via hysterotomy [46]. In this study, a porcine dermal matrix was sutured over the lesion and covered by the skin leading to correction of hindbrain herniation and anatomy of the vermis [46]. The next pre-clinical studies to be conducted using porcine-derived products was not until almost two decades later when Tang et al. investigated the use of scaffolds fabricated with porcine dermis derived gelatin and chitosan [45]. The authors determined that scaffold repair via an open prenatal approach improved bladder neuromuscular development in retinoic acid induced MMC rat fetuses [45]. Using a different rat model of spinal cord defect, dame rat peritoneum was also used to repair a fetal laminectomy defect and was deemed a good physical barrier to protect the injury [44].
In utero defect repair via hysterotomy with a bovine collagen matrix led to defect coverage, better neurological outcomes and prevented hindbrain herniation in the surgically created MMC sheep model [47, 49]. Furthermore, collagen matrix further preserved spinal cord architecture as well as spinal function [50]. Additionally, a single or two port fetoscopic technique was used to repair sheep with MMC using a bovine collagen patch attached with sealant which ultimately showed no Chiari malformation or paraplegia in the repaired group [51]. In addition to showing improvements in motor and neurological function, bovine collagen patches fetoscopically implanted have shown to prevent bladder abnormalities associated with this large animal model as well [48].
Human
The use of human-derived dural substitutes is extremely popular in pre-clinical study, dating back to the early 2000s. For example, repairing MMC defects in fetal sheep using human dermal matrix as a dural substitute led to the normal development of anal sphincter muscles, promoting normal bowel function after birth compared to untreated fetuses [38]. Additionally, results after open MMC repair in fetal lambs using human dermal matrix demonstrated that this patch could also reverse or prevent hindbrain herniation [57]. However, patches developed from cryopreserved human umbilical cord and cryopreserved amniotic membrane are the most recent patches created for MMC repair using human tissues. Interestingly, the use of an amniotic membrane patch increased protection of the spinal cord; however, it limited the skin wound healing, leading to a possible reason why it has not been predominantly studied in the last several years [56]. More recently, a cryopreserved human umbilical cord patch was developed through extensive processing and cryopreservation of donated full-term human placenta after cesarean delivery [52, 53]. Papanna et al. reported improvement in survival and neurological outcomes in a sheep in utero MMC repair model using this patch [52]. Further indicating its promise, Snowise et al. compared the impact of human umbilical cord and a biocellulose patch in a retinoic acid induced MMC rat model and found that human umbilical cord promotes cellular migration of native cells with minimal inflammation compared to biocellullose [54]. Furthermore, Mann et al. compared human umbilical cord with acellular dermal matrix in the same MMC rat model and demonstrated reduced acute inflammation and apoptosis together with superior organization in regenerative cellular growth in the human umbilical cord compared with the acellular dermal matrix patch [55]. Altogether, the creation of human derived umbilical cord patches has shown much promise as a dural substitute and can be deemed a great advancement in this area in the last decade.
Plant
The use of a cellulose graft has been shown to successfully repair MMC defects in both rabbits [61] and sheep [62]. In fact, it was shown to correct 90% of MMC defects in fetal lambs and it was later suggested that a cellulose patch formed a “neoduramater” [58, 60]. Interestingly, a cellulose patch was compared head to head to a human acellular dermal matrix and results indicated that there was no adhesion of the neural tissue after repair with cellulose as was found in the acellular dermal matrix [58]. Another report using the surgical MMC sheep model compared the classical neurosurgery technique of a three-layer suture (duramater, muscle and skin closure) to a simplified technique with a cellulose patch over the placode and a single-line suture of the skin over the patch. Results demonstrated a marked adherence of the medulla to the scar in the classical neurosurgery group which was not observed in the group receiving the cellulose patch [60]. Although, much data support the use of biocellulose patches as successful defect repair strategies, the use of a biocellulose film applied with adhesive led to exposed spinal cord and leakage of CSF compared to a cryopreserved human umbilical cord graft applied with suture [53]. While this also may be a result of application technique, it is possible that biocellulose patches may lead to unnecessary complications. Interestingly, a novel alginate-based hydrogel crosslinked with polyacrylamide and chitosan may alleviate these problems indicated with other plant derived patches as the hydrogel expands with the defect over time, and importantly, does not lead to spinal cord tethering [63].
Patch facilitated tissue engineering approaches for MMC repair
Tissue engineering strategies have become an increasingly popular topic for MMC repair over the last decade with the goal of improving tissue regeneration after patch application. These strategies incorporate a bioactive agent, such as cells or proteins, onto a delivery vehicle, which in this application is the patch that is also used to provide a physical barrier protecting the spinal cord from the extrauterine environment. For example, the delivery of basic fibroblast growth factor (bFGF) has been investigated to promote tissue regeneration in both small and large animal models. In fact, the release bFGF from scaffolds fabricated from bovine gelatin and collagen enhanced vascularization in a retinoic acid induced rat model of spina bifida and inhibited spinal cord damage and reversed hindbrain herniation in the surgical MMC sheep model [71, 72].
Based on our literature search, a majority of these strategies have involved the delivery of a variety of stem cells, including induced pluripotent stem cell derived neural crest stem cells, placenta derived mesenchymal stromal cells, bone marrow mesenchymal stem cells, and neural stem cells delivered on a host of different types of patches, mostly made from natural products. For example, a rat tail collagen hydrogel was used to deliver placenta derived mesenchymal stromal cells as a repair mechanism for lambs with MMC and this resulted in improved motor function and neuron quantity compared to the vehicle alone [74]. Furthermore, the positive effects of placenta-derived mesenchymal stromal cells delivered on porcine small intestine submucosa-derived extracellular matrix have been highly reported in the last 5 years. The use of this system reduced spinal cord compression in a retinoic acid induced spina bifida rat model and, more importantly, fetal viability, motor function, and neuron density were improved in fetal lambs with surgically created MMC defects [66–69]. Most recently, it was also reported that postnatal bracing and physical therapy further improved the success of this treatment leading to improved survival and mobility [65]. When using placenta-derived cells as a therapeutic strategy, the gestational age in which the placenta was isolated has been shown to be a critical variable. With human amnion used as the patch and delivery vehicle, cells derived from early gestation placenta led to much more improved motor function in MMC repaired lambs compared to those animals that received cells isolated from term gestation placenta [75]. While the use of placenta-derived mesenchymal stromal cells has seen the most success in large animal preclinical trials, bone marrow and neural stem cells have also shown potential. Using a porcine gelatin and chitosan scaffold, the delivery of bone marrow mesenchymal stem cells was associated with stem cell survival, neuron regeneration in the defect site, and decrease in defect size in a retinoic acid induced MMC rat model [70]. To enhance the neural regenerative impact of a synthetic nanofiber scaffold system fabricated from poly(caprolactone), poly(propylene glycol), and sodium acetate, induced pluripotent stem cell–derived neural stem cells were seeded onto the scaffold prior to implantation as a dural substitute in fetal sheep after MMC defect creation and results demonstrated successful transplantation within the spinal cord [64]. Additionally, human dermal matrix seeded with neural stem cells were applied to the MMC defect in fetal lambs and cells remained undifferentiated and released important neurotrophic factors into the defect site [76]. Most recently, neural stem cells were seeded onto a fibrin hydrogel and viability of both human and rat neural progenitor cells was achieved. When using an ex vivo model based on the retinoic acid induced MMC rat model, hydrogel treatment correlated with an increase in neuron maturation [77]. Lastly, a rather novel strategy that has been used to repair MMC defects in the retinoic acid rat model is the development of artificial skin using bovine type 1 collagen hydrogel and amniotic fluid cell derived induced pluripotent stem cell derived keratinocytes; however, this strategy did not perform as well as other tissue engineering strategies. In fact, only 12 out of 20 animals repaired had partial or full defect coverage and there was no significant decrease in defect size [73]. Overall, using a patch designed for spinal cord protection as a delivery vehicle for bioactive agents has shown great promise as an effective strategy to improve regeneration after MMC defect repair.
Discussion
Dural reconstruction during MMC repair is one of the most crucial steps for overall treatment success as it protects the spinal cord, prevents further CSF leakage, and avoids tethered cord. The use of a patch to aid in dural reconstruction is quite necessary in situations in both postnatal and prenatal procedures. During postnatal MMC repair, surgeons can primarily use the surrounding dura to repair the defect; however, there are times when a patch must be used because the dura itself is not sufficient to close larger defects [9]. While in prenatal repairs, the integrity of the dura is extremely weak and it is very challenging to have a water-tight close using only the primary dura; therefore, the use of dural patches is critical if surgeons aim to repair the defect as earlier as 23–26 weeks of gestation with the hopes of further improving clinical outcomes. In addition, the use of patches can be used to decrease the surgical time which is extremely important during prenatal repair as longer surgeries can increase the risks of premature membrane rupture [33]. Finally, the use of patches can also be co-administered with other prenatal approaches such as the myofascial flap closure which may lead to an even more successful creation of a water-tight barrier [21].
As we demonstrate in this review, patches from various synthetic and natural sources have been developed and used for MMC defect repair in both postnatal and prenatal situations. Patches fabricated from synthetic materials such as silica and ePTFE initially were used based on their biocompatibility and promotion of a water-tight barrier [7–9]; however, they required a secondary removal surgery because they were non-degradable, putting them at a significant disadvantage due to further financial and emotional consequences. In addition, biomedical engineers are working to develop novel patches from other synthetic materials including polyesters that are biodegradable, possess tunable properties at a low manufacturing cost, and facilitates the delivery of bioactive agents to promote tissue repair. However, these patches are still under investigation in pre-clinical studies and it could be several years before they are considered a standard method of repair. As of now, non-human collagen based patches, cellulose based patches, and patches sources from human tissues such as umbilical cord and amniotic membranes are the most common dural substitutes used in MMC repairs [14, 21, 33, 53]. Specifically, for open and postnatal approaches, we suggest that the patch chosen for repair is mainly at the discretion of the neurosurgeon. However, for fetoscopic repair, the patch chosen must be thin enough to pass through the small cannula into the amniotic cavity when rolled. Based on our experiences, we recommend patches derived from collagen matrix and human cryopreserved umbilical cord matrix for the fetoscopic approach. In addition to their physical properties, the main advantage of using such naturally derived dural substitutes is their availability; however, they do possess concerning disadvantages as well. The manufacturing cost to process these tissues can be relatively high and, importantly, the risk of disease transmission and adverse immune reaction can accompany the use of a natural foreign material. While most of the patches developed have shown significant promise as a therapeutic strategy in both clinical and pre-clinical studies, none of them are deemed the perfect patch for this application.
While the idea of using a patch for duraplasty as a dural substitute dates back to the 1970s; to this day, there is still not a “perfect” patch that has been developed for post or prenatal SB repair. The fact that no single patch has been established as the standard of care and that many neurosurgeons still prefer the use of different types of dural substitutes is unequivocal proof that no patch is perfect. Therefore, the development of a better patch for this purpose is required. There are several qualities that, based on our experience, we think characterize the ideal patch for both prenatal and postnatal MMC repair. The most basic aspects pertaining to the overall success of the patch are its biocompatibility, watertightness, and biodegradability. It must be non-toxic to the neural tissue, maintain a mechanically stable protective barrier between the spinal cord and external environment, and degrade over time avoiding the need for a secondary removal surgery. Additionally, the ideal patch would prevent scarring or spinal cord re-tethering after implantation but allows the formation of a “neo-dura” that upon patch degradation protects the spinal cord. Furthermore, from a manufacturing and market point of view, the patch should be cost-efficient and manufactured by an easily scalable process, a fact that can be challenging considering the non-few, but limited number of surgeries performed annually worldwide. Finally, its properties should facilitate the co-delivery of biological agents that leads to the regeneration of damaged neural tissue. These qualities are necessary for both postnatal and prenatal approaches; however, there are additional properties that should be considered specifically for fetoscopic in utero repair due to the complexity of the procedure. Because the fetoscopic procedure relies heavily on the use of small trocars, the patch should also be engineered in a way that allows for easy administration via these cannulas and in a simple manner that does not extend the length of the surgery. Undoubtedly, there is a significant unmet need for collaboration between the surgical and scientific communities to develop an all-encompassing patch that leads to an improvement in surgical repair procedures and overall patient quality of life.
Funding
We acknowledge the financial support by NIH/NINDS 1R01NS103992 to CY.L. and JL.P., by Structural Tissue Evaluation and Engineering Labs at University of Cincinnati to CY.L. and by the Center for Fetal and Placental Research at the Cincinnati Fetal Center at Cincinnati Children’s Hospital Medical Center to JL.P. National Institute of Neurological Disorders and Stroke, 1R01NS103992, Jose L. Peiro
Footnotes
Declarations
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. CY. L, JL. P and M. O declare that the patch for spina bifida repair is under a U.S. Patent No. WO/2018/067811.
Data availability
All data can be found in the manuscript.
References
- 1.Atta CAM, Fiest KM, Frolkis AD, Jette N, Pringsheim T, St Germaine-Smith C, Rajapakse T, Kaplan GG, Metcalfe A (2016) Global birth prevalence of spina bifida by folic acid fortification status: a systematic review and meta-analysis, Am J Public Health 106:e24–e34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, Ethen M, Frohnert B, Kirby RS (2015) Centers for Disease Control and Prevention, updated estimates of neural tube defects prevented by mandatory folic acid fortification - United States, 1995–2011, MMWR. Morb Mortal Wkly Rep 64:1–5 [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell LE, Scott Adzick N, Melchionne J, Pasquariello PS, Sutton LN, Whitehead AS (2004) Spina bifida. In: Lancet, Elsevier; 1885–1895 [DOI] [PubMed] [Google Scholar]
- 4.Adzick NS, Thom EA, Spong CY, Brock JW, Burrows PK, Johnson MP, Howell LJ, Farrell JA, Dabrowiak ME, Sutton LN, Gupta N, Tulipan NB, D’Alton ME, Farmer DL (2011) A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 364:993–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira Furtado LM, Da Costa Val Filho JA, Dantas F, Moura de Sousa C (2020) Tethered cord syndrome after myelomeningocele repair: a literature update. Cureus 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartal AD, Heilbronn YD, Plashkes YY (1971) Reconstruction of the dural canal in myelomeningocele: case report. Plast Reconstr Surg 47:87–89 [DOI] [PubMed] [Google Scholar]
- 7.Thompson DNP, Taylor WF, Hayward RD (1994) Silastic dural substitute: experience of its use in spinal and foramen magnum surgery. Br J Neurosurg 8:157–167 [DOI] [PubMed] [Google Scholar]
- 8.Venes JL (1985) Surgical considerations in the initial repair of meningomyelocele and the introduction of a technical modification. Neurosurgery 17:111–113 [DOI] [PubMed] [Google Scholar]
- 9.Boop FA, Chadduck WM (1991) Silastic duraplasty in pediatric patients. Neurosurgery 785 [DOI] [PubMed] [Google Scholar]
- 10.Ohe N, Futamura A, Kawada R, Minatsu H, Kohmura H, Hayashi K, Miwa K, Sakai N (2000) Secondary tethered cord syndrome in spinal dysraphism. Child’s Nerv Syst 16:457–461 [DOI] [PubMed] [Google Scholar]
- 11.Nakazawa H, Kikuchi Y, Honda T, Isago T, Nozaki M (2005) Successful management of a small infant born with a large meningomyelocele using a temporary artificial dermis. Scand J Plast Reconstr Surg Hand Surg 39:53–56 [DOI] [PubMed] [Google Scholar]
- 12.Kohl T, Hering R, Heep A, Schaller C, Meyer B, Greive C, Bizjak G, Buller T, van de Vondel P, Gogarten W, Bartmann P, Knöpfle G, Gembruch U (2006) Percutaneous fetoscopic patch coverage of spina bifida aperta in the human – early clinical experience and potential. Fetal Diagn Ther 21:185–193 [DOI] [PubMed] [Google Scholar]
- 13.Samuels R, McGirt MJ, Attenello FJ, Garcés Ambrossi GL, Singh N, Solakoglu C, Weingart JD, Carson BS, Jallo GI (2009) Incidence of symptomatic retethering after surgical management of pediatric tethered cord syndrome with or without duraplasty. Child’s Nerv Syst 25:1085–1089 [DOI] [PubMed] [Google Scholar]
- 14.Kohl T (2014) Percutaneous minimally invasive fetoscopic surgery for spina bifida aperta. Part I: surgical technique and perioperative outcome. Ultrasound Obstet Gynecol 44:515–524 [DOI] [PubMed] [Google Scholar]
- 15.Graf K, Kohl T, Neubauer BA, Dey F, Faas D, Wanis FA, Reinges MHT, Uhl E, Kolodziej MA (2016) Percutaneous minimally invasive fetoscopic surgery for spina bifida aperta. Part III: neurosurgical intervention in the first postnatal year. Ultrasound Obstet Gynecol 47:158–161 [DOI] [PubMed] [Google Scholar]
- 16.Shim JH, Hwang NH, Yoon ES, Dhong ES, Kim DW, Kim SD (2016) Closure of myelomeningocele defects using a limberg flap or direct repair. Arch Plast Surg 43:26–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohl T, Tchatcheva K, Merz W, Wartenberg HC, Heep A, Müller A, Franz A, Stressig R, Willinek W, Gembruch U (2009) Percutaneous fetoscopic patch closure of human spina bifida aperta: advances in fetal surgical techniques may obviate the need for early postnatal neurosurgical intervention. Surg Endosc Other Interv Tech 23:890–895 [DOI] [PubMed] [Google Scholar]
- 18.Pettorini BL, Tamburrini G, Massimi L, Paternoster G, Caldarelli M, Di Rocco C (2010) The use of a reconstituted collagen foil dura mater substitute in paediatric neurosurgical procedures - experience in 47 patients. Br J Neurosurg 24:51–54 [DOI] [PubMed] [Google Scholar]
- 19.Grigoryants V, Jane JA Jr, Lin KY (2007) Salvage of a complicated myelomeningocele using collagen (Duragen) and Dermal (Alloderm) matrix substitutes. Pediatr Neurosurg 43:512–515 [DOI] [PubMed] [Google Scholar]
- 20.Bennett KA, Carroll MA, Shannon CN, Braun SA, Dabrowiak ME, Crum AK, Paschall RL, Kavanaugh-Mchugh AL, Wellons JC, Tulipan NB (2014) Reducing perinatal complications and preterm delivery for patients undergoing in utero closure of fetal myelomeningocele: further modifications to the multidisciplinary surgical technique: Clinical article. J Neurosurg Pediatr 14:108–114 [DOI] [PubMed] [Google Scholar]
- 21.Belfort MA, Whitehead WE, Shamshirsaz AA, Espinoza J, Nassr AA, Lee TC, Olutoye OO, Keswani SG, Cortes MS (2019) Comparison of two fetoscopic open neural tube defect (ONTD) repair techniques: single-layer vs three-layer closure, Ultrasound Obstet. Gynecol uog.21915 [DOI] [PubMed] [Google Scholar]
- 22.Susarla SM, Hauptman J, Ettinger R, Sittler B, Ellenbogen RG (2019) Acellular dermal matrix as a definitive reconstructive option for management of a large myelomeningocele defect in the setting of severe lumbar kyphosis. World Neurosurg 129:363–366 [DOI] [PubMed] [Google Scholar]
- 23.Pignatti M, Feletti A, Sapino G, Marotti F, Pavesi G, De Santis G (2020) Myelomeningocele repair combining a double cryopreserved amniotic membrane homograft and the keystone flap in a 3-year-old child: a case report. Pediatr Neurosurg 55:106–112 [DOI] [PubMed] [Google Scholar]
- 24.Marton E, Giordan E, Gioffrè G, Canova G, Paolin A, Mazzucco MG, Longatti P (2018) Homologous cryopreserved amniotic membrane in the repair of myelomeningocele: preliminary experience. Acta Neurochir (Wien) 160:1625–1631 [DOI] [PubMed] [Google Scholar]
- 25.Mazzola CA, Albright AL, Sutton LN, Tuite GF, Hamilton RL, Pollack IF (2002) Dermoid inclusion cysts and early spinal cord tethering after fetal surgery for myelomeningocele. N Engl J Med 347:256–259 [DOI] [PubMed] [Google Scholar]
- 26.Farmer DL, Von Koch CS, Peacock WJ, Danielpour M, Gupta N, Lee H, Harrison MR, Sawin RS, Mullins RJ (2003) In utero repair of myelomeningocele: experimental pathophysiology, initial clinical experience, and outcomes. In: Arch Surg, Am Med Assoc 872–878 [DOI] [PubMed] [Google Scholar]
- 27.Bruner JP, Tulipan NE, Richards WO (1997) Endoscopic coverage of fetal open myelomeningocele in utero. Am J Obstet Gynecol 176:256–257 [DOI] [PubMed] [Google Scholar]
- 28.Bruner JP, Tulipan NB, Richards WO, Walsh WF, Boehm FH, Vrabcak EK (2000) In utero repair of myelomeningocele: a comparison of endoscopy and hysterotomy. Fetal Diagn Ther 15:83–88 [DOI] [PubMed] [Google Scholar]
- 29.De Weerd L, Sjåvik K, Pedersen LK, Weum S (2020) Hennig, Triple use of autologous amnion graft in the treatment of meningomyelocele and split cord malformation. Plast Reconstr Surg - Glob Open 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffman HJ, Taecholarn C, Hendrick EB, Humphreys RP (1985) Management of lipomyelomeningoceles. Experience at the hospital for sick children, Toronto. J Neurosurg 62:1–8 [DOI] [PubMed] [Google Scholar]
- 31.Carrabba G, Macchini F, Fabietti I, Schisano L, Meccariello G, Campanella R, Bertani G, Locatelli M, Boito S, Porro GA, Gabetta L, Picciolini O, Cinnante C, Triulzi F, Ciralli F, Mosca F, Lapa DA, Leva E, Rampini P, Persico N (2019) Minimally invasive fetal surgery for myelomeningocele: preliminary report from a single center. Neurosurg Focus 47:12. [DOI] [PubMed] [Google Scholar]
- 32.Pedreira DAL, Zanon N, de Sá RAM, Acacio GL, Ogeda E, Belem TMLOU, Chmait RH, Kontopoulos E, Quintero RA (2014) Fetoscopic single-layer repair of open spina bifida using a cellulose patch: preliminary clinical experience. J Matern Neonatal Med 27:1613–1619 [DOI] [PubMed] [Google Scholar]
- 33.Pedreira DAL, Zanon N, Nishikuni K, Moreira De Sá RA, Acacio GL, Chmait RH, Kontopoulos EV, Quintero RA (2016) Endoscopic surgery for the antenatal treatment of myelomeningocele: the CECAM trial. Am J Obstet Gynecol 214(111):e1–111.e11 [DOI] [PubMed] [Google Scholar]
- 34.Lapa Pedreira DA, Acacio GL, Gonçalves RT, Sá RAM, Brandt RA, Chmait RH, Kontopoulos EV, Quintero RA (2018) Percutaneous fetoscopic closure of large open spina bifida using a bilaminar skin substitute, Ultrasound Obstet. Gynecol 52:458–466 [DOI] [PubMed] [Google Scholar]
- 35.Kahn L, Mbabuike N, Valle-Giler EP, Garces J, Clifton Moore R, Hilaire HS, Bui CJ (2014) Fetal surgery: the Ochsner experience with in utero spina bifida repair. Ochsner J 14:112–118 [PMC free article] [PubMed] [Google Scholar]
- 36.Fontecha CG, Peiro JL, Aguirre M, Soldado F, Añor S, Fresno L, Martinez-Ibañez V (2009) Inert patch with bioadhesive for gentle foetal surgery of myelomeningocele in a sheep model. Eur J Obstet Gynecol Reprod Biol 146:174–179 [DOI] [PubMed] [Google Scholar]
- 37.Park Y-K, Tator CH (1998) Prevention of arachnoiditis and postoperative tethering of the spinal cord with Gore-Tex surgical membrane: an experimental study with rats. Neurosurgery 42:813–823 [DOI] [PubMed] [Google Scholar]
- 38.Yoshizawa J, Sbragia L, Paek BW, Sydorak RM, Yamazaki Y, Harrison MR, Farmer DL (2004) Fetal surgery for repair of myelomeningocele allows normal development of anal sphincter muscles in sheep. Pediatr Surg Int 20:14–18 [DOI] [PubMed] [Google Scholar]
- 39.Guilbaud L, Roux N, Friszer S, Garabedian C, Dhombres F, Bessières B, Fallet-Bianco C, Di Rocco F, Zerah M, Jouannic JM (2017) Fetoscopic patch coverage of experimental myelomenigocele using a two-port access in fetal sheep. Child’s Nerv Syst 33:1177–1184 [DOI] [PubMed] [Google Scholar]
- 40.Saadai P, Nout YS, Encinas J, Wang A, Downing TL, Beattie MS, Bresnahan JC, Li S, Farmer DL (2011) Prenatal repair of myelomeningocele with aligned nanofibrous scaffolds - a pilot study in sheep. In: Saunders WB (ed) J Pediatr Surg pp 2279–2283 [DOI] [PubMed] [Google Scholar]
- 41.Oria M, Tatu RR, Lin CY, Peiro JL (2019) In vivo evaluation of novel PLA/PCL polymeric patch in rats for potential spina bifida coverage. J Surg Res 242:62–69 [DOI] [PubMed] [Google Scholar]
- 42.Bardill J, Williams SM, Shabeka U, Niswander L, Park D, Marwan AI (2019) An injectable reverse thermal gel for minimally invasive coverage of mouse myelomeningocele. J Surg Res 235:227–236 [DOI] [PubMed] [Google Scholar]
- 43.Bardill JR, Park D, Marwan AI (2020) Improved coverage of mouse myelomeningocele with a mussel inspired reverse thermal gel. J Surg Res 251:262–274 [DOI] [PubMed] [Google Scholar]
- 44.Heffez DS, Aryanpur J, Rotellini NAC, Hutchins GM, Freeman JM (1993) Intrauterine repair of experimental surgically created dysraphism. Neurosurgery 32:1005–1010 [DOI] [PubMed] [Google Scholar]
- 45.Tang L, Zhong H, Chen H, Shen J, Bi Y, Xiao X (2017) In utero repair of fetal rat myelomeningocele affects neuromuscular development in the bladder. Exp Ther Med 14:3681–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouchard S, Davey MG, Rintoul NE, Walsh DS, Rorke LB, Adzick NS, Farmer D, O’Neill J (2003) Correction of hindbrain herniation and anatomy of the vermis after in utero repair of myelomeningocele in sheep. In: Saunders WB (ed) J Pediatr Surg pp 451–458 [DOI] [PubMed] [Google Scholar]
- 47.Von Koch CS, Compagnone N, Hirose S, Yoder S, Harrison MR, Farmer DL (2005) Myelomeningocele: characterization of a surgically induced sheep model and its central nervous system similarities and differences to the human disease. Am J Obstet Gynecol 193:1456–1462 [DOI] [PubMed] [Google Scholar]
- 48.Burgos L, Encinas JL, García-Cabezas MA, Peiró JL, López-Santamaría M, Jaureguízar E (2014) Cambios en la vejiga después de varias modalidades de cobertura en el modelo de mielomeningocele inducido quirúrgicamente en corderos. Actas Urol Esp 38:55–61 [DOI] [PubMed] [Google Scholar]
- 49.Eggink AJ, Roelofs LAJ, Feitz WFJ, Wijnen RMH, Mullaart RA, Grotenhuis JA, van Kuppevelt TH, Lammens MMY, Crevels AJ, Hanssen A, van den Berg PP (2005) In utero repair of an experimental neural tube defect in a chronic sheep model using biomatrices. Fetal Diagn Ther 20:335–340 [DOI] [PubMed] [Google Scholar]
- 50.Eggink AJ, Roelofs LAJ, Feitz WFJ, Wijnen RMH, Lammens MMY, Mullaart RA, van Moerkerk HTB, van Kuppevelt TH, Crevels AJ, Verrijp K, Lotgering FK, van den Berg PP (2008) Delayed intrauterine repair of an experimental spina bifida with a collagen biomatrix. Pediatr Neurosurg 44:29–35 [DOI] [PubMed] [Google Scholar]
- 51.Peiro JL, Fontecha CG, Ruano R, Esteves M, Fonseca C, Marotta M, Haeri S, Belfort MA (2013) Single-Access Fetal Endoscopy (SAFE) for myelomeningocele in sheep model I: Amniotic carbon dioxide gas approach. Surg Endosc 27:3835–3840 [DOI] [PubMed] [Google Scholar]
- 52.Papanna R, Mann L, Snowise S, Morales Y, Prabhu S, Tseng S, Grill R, Fletcher S, Moise K (2016) Neurological outcomes after human umbilical cord patch for in utero spina bifida repair in a sheep model. Am J Perinatol Reports 06:e309–e317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papanna R, Moise KJ, Mann LK, Fletcher S, Schniederjan R, Bhattacharjee MB, Stewart RJ, Kaur S, Prabhu SP, Tseng SCG (2016) Cryopreserved human umbilical cord patch for in-utero spina bifida repair. Ultrasound Obstet Gynecol 47:168–176 [DOI] [PubMed] [Google Scholar]
- 54.Snowise S, Mann L, Morales Y, Moise KJ, Johnson A, Fletcher S, Grill RJ, Tseng SCG, Papanna R (2017) Cryopreserved human umbilical cord versus biocellulose film for prenatal spina bifida repair in a physiologic rat model. Prenat Diagn 37:473–481 [DOI] [PubMed] [Google Scholar]
- 55.Mann LK, Won JH, Trenton NJ, Garnett J, Snowise S, Fletcher SA, Tseng SCG, Diehl MR, Papanna R (2020) Cryopreserved human umbilical cord versus acellular dermal matrix patches for in utero fetal spina bifida repair in a pregnant rat model. J Neurosurg Spine 32:321–331 [DOI] [PubMed] [Google Scholar]
- 56.Brown EG, Saadai P, Pivetti CD, Beattie MS, Bresnahan JC, Wang A, Farmer DL (2014) in utero repair of myelomeningocele with autologous amniotic membrane in the fetal lamb model [DOI] [PubMed]
- 57.Paek BW, Farmer DL, Wilkinson CC, Albanese CT, Peacock W, Harrison MR, Jennings RW (2000) Hindbrain herniation develops in surgically created myelomeningocele but is absent after repair in fetal lambs. Am J Obstet Gynecol 183:1119–1123 [DOI] [PubMed] [Google Scholar]
- 58.Sanchez E Oliveira RDC, Valente PR, Abou-Jamra RC, Araújo A, Saldiva PH, Pedreira DAL (2007) Biosynthetic cellulose induces the formation of a neoduramater following prenatal correction of meningomyelocele in fetal sheep. Acta Cir Bras 22:174–181 [DOI] [PubMed] [Google Scholar]
- 59.Copeland ML, Bruner JP, Richards WO, Sundell HW, Tulipan NB (1993) A model for in utero endoscopic treatment of myelomeningocele. Neurosurgery 33:542–545 [DOI] [PubMed] [Google Scholar]
- 60.Herrera SRF, de A Leme RJ, Valente PR, Caldini EG, Saldiva PHN, Pedreira DAL (2012) Comparison between two surgical techniques for prenatal correction of meningomyelocele in sheep. Einstein (Sao Paulo) 10:455–461 [DOI] [PubMed] [Google Scholar]
- 61.Pedreira DAL, Valente PR, Abou-Jamra RC, Pelarigo CL, Silva LM, Goldenberg S (2003) Successful fetal surgery for the repair of a ‘myelomeningocele-like’ defect created in the fetal rabbit. Fetal Diagn Ther 18:201–206 [DOI] [PubMed] [Google Scholar]
- 62.Abou-Jamra RC, Valente PR, Araújo A, Oliveira RDCS, Saldiva PH, Pedreira DAL (2009) Simplified correction of a meningomyelocele-like defect in the ovine fetus. Acta Cir Bras 24:239–244 [DOI] [PubMed] [Google Scholar]
- 63.Lazow SP, Labuz DF, Freedman BR, Rock A, Zurakowski D, Mooney DJ, Fauza DO (2021) A novel two-component, expandable bioadhesive for exposed defect coverage: applicability to prenatal procedures. J Pediatr Surg 56:165–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saadai P, Wang A, Nout YS, Downing TL, Lofberg K, Beattie MS, Bresnahan JC, Li S, Farmer DL (2013) Human induced pluripotent stem cell-derived neural crest stem cells integrate into the injured spinal cord in the fetal lamb model of myelomeningocele [DOI] [PubMed]
- 65.Yamashiro K, Galganski LA, Peyton J, Haynes K, Vicuna V, Kumar P, Keller B, Becker J, Pivetti C, Stokes S, Theodorou C, Jackson J, Wang A, Farmer D (2020) Surviving lambs with myelomeningocele repaired in utero with placental mesenchymal stromal cells for 6 months: a pilot study. Fetal Diagn Ther 47:912–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galganski LA, Kumar P, Vanover MA, Pivetti CD, Anderson JE, Lankford L, Paxton ZJ, Chung K, Lee C, Hegazi MS, Yamashiro KJ, Wang A, Farmer DL (2019) In utero treatment of myelomeningocele with placental mesenchymal stromal cells — selection of an optimal cell line in preparation for clinical trials. J Pediatr Surg [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanover M, Pivetti C, Lankford L, Kumar P, Galganski L, Kabagambe S, Keller B, Becker J, Chen YJ, Chung K, Lee C, Paxton Z, Deal B, Goodman L, Anderson J, Jensen G, Wang A, Farmer D (2019) High density placental mesenchymal stromal cells provide neuronal preservation and improve motor function following in utero treatment of ovine myelomeningocele. J Pediatr Surg 54:75–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen YJ, Chung K, Pivetti C, Lankford L, Kabagambe SK, Vanover M, Becker J, Lee C, Tsang J, Wang A, Farmer DL (2017) Fetal surgical repair with placenta-derived mesenchymal stromal cell engineered patch in a rodent model of myelomeningocele [DOI] [PubMed]
- 69.Kabagambe S, Keller B, Becker J, Goodman L, Pivetti C, Lankford L, Chung K, Lee C, Chen YJ, Kumar P, Vanover M, Wang A, Farmer D (2018) Placental mesenchymal stromal cells seeded on clinical grade extracellular matrix improve ambulation in ovine myelomeningocele. J Pediatr Surg 53:178–182 [DOI] [PubMed] [Google Scholar]
- 70.Li X, Yuan Z, Wei X, Li H, Zhao G, Miao J, Wu D, Liu B, Cao S, An D, Ma W, Zhang H, Wang W, Wang Q, Gu H (2016) Application potential of bone marrow mesenchymal stem cell (BMSCs) based tissue-engineering for spinal cord defect repair in rat fetuses with spina bifida aperta. J Mater Sci Mater Med 27:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watanabe M, Jo JI, Radu A, Kaneko M, Tabata Y, Flake AW (2010) A tissue engineering approach for prenatal closure of myelomeningocele with gelatin sponges incorporating basic fibroblast growth factor. Tissue Eng - Part A 16:1645–1655 [DOI] [PubMed] [Google Scholar]
- 72.Watanabe M, Li H, Kim AG, Weilerstein A, Radu A, Davey M, Loukogeorgakis S, Sánchez MD, Sumita K, Morimoto N, Yamamoto M, Tabata Y, Flake AW (2016) Complete tissue coverage achieved by scaffold-based tissue engineering in the fetal sheep model of Myelomeningocele. Biomaterials 76:133–143 [DOI] [PubMed] [Google Scholar]
- 73.Kajiwara K, Tanemoto T, Wada S, Karibe J, Ihara N, Ikemoto Y, Kawasaki T, Oishi Y, Samura O, Okamura K, Takada S, Akutsu H, Sago H, Okamoto A, Umezawa A (2017) Fetal therapy model of myelomeningocele with three-dimensional skin using amniotic fluid cell-derived induced pluripotent stem cells. Stem Cell Reports 8:1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang A, Brown EG, Lankford L, Keller BA, Pivetti CD, Sitkin NA, Beattie MS, Bresnahan JC, Farmer DL (2015) placental mesenchymal stromal cells rescue ambulation in ovine myelomeningocele. Stem Cells Transl Med 4:659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brown EG, Keller BA, Lankford L, Pivetti CD, Hirose S, Farmer DL, Wang A (2016) Age Does Matter: A pilot comparison of placenta-derived stromal cells for in utero repair of myelomeningocele using a lamb model. Fetal Diagn Ther 39:179–185 [DOI] [PubMed] [Google Scholar]
- 76.Fauza DO, Jennings RW, Teng YD, Snyder EY (2008) Neural stem cell delivery to the spinal cord in an ovine model of fetal surgery for spina bifida. Surgery 144:367–373 [DOI] [PubMed] [Google Scholar]
- 77.Biancotti JC, Walker KA, Jiang G, Di Bernardo J, Shea LD, Kunisaki SM (2020) Hydrogel and neural progenitor cell delivery supports organotypic fetal spinal cord development in an ex vivo model of prenatal spina bifida repair. J Tissue Eng 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Farmer DL, Thom EA, Brock JW, Burrows PK, Johnson MP, Howell LJ, Farrell JA, Gupta N, Adzick NS (2018) The management of myelomeningocele study: full cohort 30-month pediatric outcomes. Am J Obstet Gynecol 218(256):e1–256.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ae R, Hamaguchi T, Nakamura Y, Yamada M, Tsukamoto T, Mizusawa H, Belay ED, Schonberger LB (2018) Update: dura mater graft–associated Creutzfeldt-Jakob disease — Japan, 1975–2017, MMWR. Morb Mortal Wkly Rep 67:274–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tatu R, Oria M, Pulliam S, Signey L, Rao MB, Peiro JL, Lin CY (2019) Using poly(l-lactic acid) and poly(ɛ-caprolactone) blends to fabricate self-expanding, watertight and biodegradable surgical patches for potential fetoscopic myelomeningocele repair. J Biomed Mater Res - Part B Appl Biomater 107:295–305 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data can be found in the manuscript.


