Abstract
Background
In the context of the coronavirus disease 2019 (COVID-19) pandemic, a rapid and reliable point-of-care test is an essential tool for controlling the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In particular, an immunochromatography test (ICT) that uses saliva specimens for rapid antigen detection not only reduces the risk of secondary infections but also reduces the burden on medical personnel.
Methods
The newly developed salivary antigen test kit “Inspecter Kowa® SARS-CoV-2” is an ICT to which saliva specimens can be directly applied. We evaluated its usefulness in comparison with reverse transcription quantitative PCR (RT-qPCR) and the Espline® SARS-CoV-2 Kit for the detection of SARS-CoV-2 using nasopharyngeal swab specimens. In this study, 140 patients with suspected symptomatic COVID-19 who visited our hospital were enrolled, and nasopharyngeal swab and saliva specimens were collected after they consented to participate in the study.
Results
Inspector Kowa SARS-CoV-2 was positive in 45 of 61 (73.8%) saliva that were positive by RT-qPCR and the Espline® SARS-CoV-2 Kit was also positive in 56 of 60 (93.3%) Np swabs that were positive by RT-qPCR. Good antigen detection was achieved by ICT with saliva and nasopharyngeal swab specimens when viral load was ≥105 copies/mL, whereas detection sensitivity was low when viral load was <105 copies/mL, especially in saliva specimens.
Conclusion
This ICT for the detection of SARS-CoV-2 salivary antigen is an attractive tool that does not require specialized equipment and allows patients to perform the entire process from sample collection to self-diagnose and to reduce the burden on medical care during a pandemic.
Keywords: SARS-CoV-2, COVID-19, Antigen detection, Immunochromatography, Saliva
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, Hubei province, central China, and immediately became a worldwide public health threat. The COVID-19 is still estimated to be causing 30,000 or more deaths weekly as of April 2022 [1]. In fact, the emergence of mutant strains of SARS-CoV-2 has resulted in further morbidity and mortality worldwide; therefore, disease control strategies still depend upon the availability of rapid and reliable tests for SARS-CoV-2 and the early isolation of patients [2,3].
Reverse transcription quantitative PCR (RT-qPCR) has been suggested as the most reliable nucleic acid amplification test for the detection of SARS-CoV-2, and nasopharyngeal (Np) swabs are also considered to be the most suitable specimen for diagnoses [4]. However, the acquisition of Np swab specimens is an invasive process that poses a potential risk for SARS-CoV-2 transmission among healthcare workers. In contrast, saliva can be self-collected safely by anyone, including children, and can be used in place of Np swabs to resolve these issues because saliva collection is non-invasive and does not require specialized technical skills [5,6]. There are various reports on the detection sensitivity, specificity, and limitations of saliva-based tests [[7], [8], [9]]. Unlike Np swab specimens, saliva can be affected by changes in the oral environment due to the patient's condition, timing of eating and drinking, and the steps used to process samples.
Immunochromatography test (ICT) kits for COVID-19 antigens have become popular because they take only 10–30 min to produce a result. The need for point-of-care tests, such as ICTs for antigen detection, is essential for strengthening the setup of testing for future pandemics [10]. Especially, ICT devices that use saliva specimens for rapid antigen detection not only reduce the risk of secondary infections but also reduce the burden on medical personnel, the time for diagnosis, and the requirement for expensive specialized laboratory equipment.
In this study, we carried out independent validation of point-of-care tests for the detection of SARS-CoV-2 antigen to evaluate the usefulness of a newly developed antigen test kit, which is dedicated exclusively to saliva specimens (SARS CoV-2 Ag Diagnostic Test Kit; Inspecter Kowa® SARS-CoV-2, Kowa Company, Ltd., Japan) compared with RT-qPCR and an already established and widely used ICT kit with Np swab specimens.
2. Materials and methods
2.1. Study design
This single-center prospective study was conducted to evaluate the diagnostic value of an ICT kit using saliva for the diagnosis of COVID-19. This study was conducted at Saitama Medical University Hospital, a secondary emergency medical institution, from February 14 to March 5, 2022. During the study period, two sets of Np swab specimens and additional saliva specimens were simultaneously collected from patients referred to our hospital because of close contact with COVID-19 patients or the presence of some symptoms of upper respiratory tract inflammation including fever. Non-Japanese residents who did not fully understand Japanese, asymptomatic patients, children under the age of 15 years, and patients who did not consent to participate were excluded from the study. Ethics approval was obtained from the Institutional Review Board of Saitama Medical University Hospital (Ref No: 2021–105).
2.2. Sample collection and preparation
After cessation of eating or drinking for 30 min, ≥1.5 mL salivary fluid was collected by the patients themselves in sterile containers through the drooling technique. One of the two Np swabs was suspended in 1,000 μL phosphate-buffered saline (PBS) for RNA extraction followed by RT-qPCR. The remaining swab was used directly for rapid antigen testing with an Espline® SARS-CoV-2 Kit (Fujirebio, Inc., Tokyo, Japan). Half of the saliva sample was set aside for ICT using Inspecter Kowa® SARS-CoV-2, and the other half was diluted 1:1 with PBS and vortexed for 15 s for homogenization, centrifuged at 15,000 rpm for 20 min, and the supernatant was used for subsequent RNA extraction followed by RT-qPCR. Thereafter, RNA extraction was performed with a QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany) using 140 μL of both the PBS suspension of Np swabs and saliva supernatants respectively, and RNA was finally eluted in 60 μL of the provided AVE buffer. To avoid a significant impact on the results due to the storage condition of the specimens after collection, both ICT analysis were performed immediately after sample collection according to the manufacturer's instructions and the results were determined. On the other hand, PBS suspensions of Np swabs and saliva supernatants for RNA extraction were temporarily stored at −80 °C until use.
2.3. RT-qPCR
RT-qPCR for the specific amplification of the N2 gene of SARS-CoV-2 was performed using TaqMan-based real-time RT-PCR with the following sets of primers and probe (2.4 μM forward primer, 5′-AAA TTT TGG GGA CCA GGA AC-3′; 3.2 μM reverse primer, 5′-TGG CAG CTG TGT AGG TCA AC-3′; 0.4 μM probe, 5′-FAM-ATG TCG CGC ATT GGC ATG GA-BHQ-3′) [11]. RT-qPCR amplification was performed using a QuantiTect Probe RT-PCR Kit (Qiagen). In brief, 5 μL extracted RNA was added to the amplification mixture, and distilled water was added to a final volume of 25 μL. Amplification was performed under the following conditions: reverse transcription at 50 °C for 30 min; initial denaturation at 95 °C for 15 min; and 40 cycles of denaturation at 94 °C for 15 s and annealing/extension at 60 °C for 60 s. Positive RNA controls were prepared in 10-fold serial dilutions ranging from 5.0 × 105 to 5.0 × 10°.1 copies/reaction using in vitro synthesized SARS-CoV-2 RNA. A calibration assay was carried out in parallel to create a calibration curve by RT-qPCR.
2.4. Antigen tests
Inspecter Kowa® SARS-CoV-2, an ICT based on a colloidal gold-enhanced double antibody sandwich immunoassay for the qualitative determination of the nucleocapsid protein of SARS-CoV-2, was performed using saliva immediately after sample collection according to the manufacturer's instructions, and the results were determined by visual examination. Briefly, the head of the attached swab was inserted into a saliva specimen. The swab was then moved up and down the side of a tube containing the suspension buffer at least 10 times and the tube was squeezed five times by hand to completely dissolve the specimen in the swab in the buffer. The suspension (100 μL, 3 drops) was added to the well of the test cassette. After 15 min, the results were determined.
Espline® SARS-CoV-2, an ICT based on a sandwich enzyme immunoassay targeting the SARS-CoV-2 nucleocapsid antigen, was performed immediately after sample collection according to the manufacturer's instructions [10]. Briefly, Np swabs were soaked directly in the kit's pretreatment solution, mixed approximately 10 times, and 20 μL (2 drops) was added dropwise to the test cassette after incubation for 5 min. After 30 min, the results were determined as positive when both the reference and judgment lines could be confirmed visually, and negative when only the reference line was observed.
2.5. Clinical data
Patients’ clinical data were collected retrospectively from the electronic medical records, and the date of symptom onset was defined as the first day of symptoms caused by COVID-19. We also investigated the number of days from symptom onset to when the patient visited our hospital as well as the severity of disease at the time of consultation.
3. Results
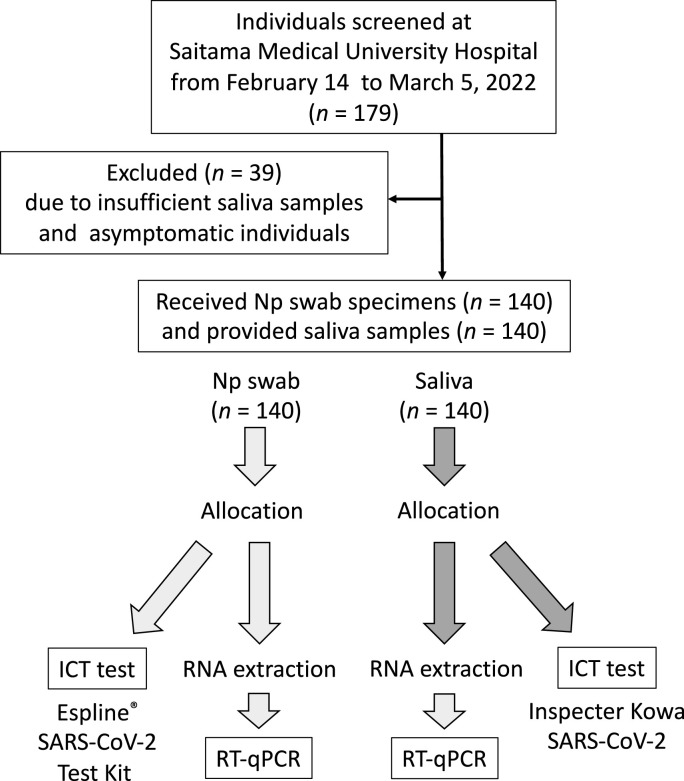
A total of 179 referred first-visit patients who had provided Np swab specimens, additional saliva samples, and informed consent were enrolled in the study. We retrospectively excluded 39 patients due to an insufficiently low volume of saliva and asymptomatic individuals; therefore, we finally included 140 patients in the study (Fig. 1 ). The median age of the enrolled patients was 36.0 years (interquartile range [IQR]: 25.0–49.3) and 73 (52.1%) were women. The median time from onset to hospital visit and sampling was 2.0 days (IQR: 2.0–3.0).
Fig. 1.
Flow chart summarizing the study design. Initially, 179 patients were enrolled, but 39 were excluded due to insufficient sample volume, leaving 140 patients for the analysis.
3.1. RT-qPCR
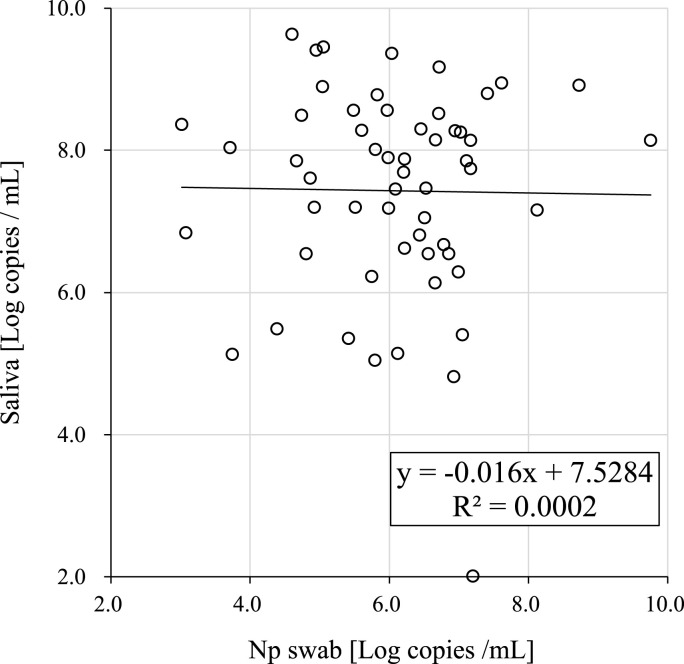
All 140 Np swab specimens and saliva specimens were subjected to RT-qPCR, and the number of SARS-CoV-2 RNA copies in the reactions was calculated from Ct values using the formula based on values obtained with reference standard RNA. SARS-CoV-2 was detected by RT-qPCR in 61 of the 140 saliva samples (43.6%) and 60 of the 140 Np swab specimens (42.9%). Fig. 2 shows the correlation of viral load between the Np swab and saliva specimens collected at the same time. The correlation coefficient was R2 = 0.0002 and there was no significant correlation between the two sets of samples.
Fig. 2.
Correlation of viral load analyzed using RT-qPCR between saliva and Np swab specimens.
3.2. Inspecter Kowa® SARS-CoV-2 using saliva
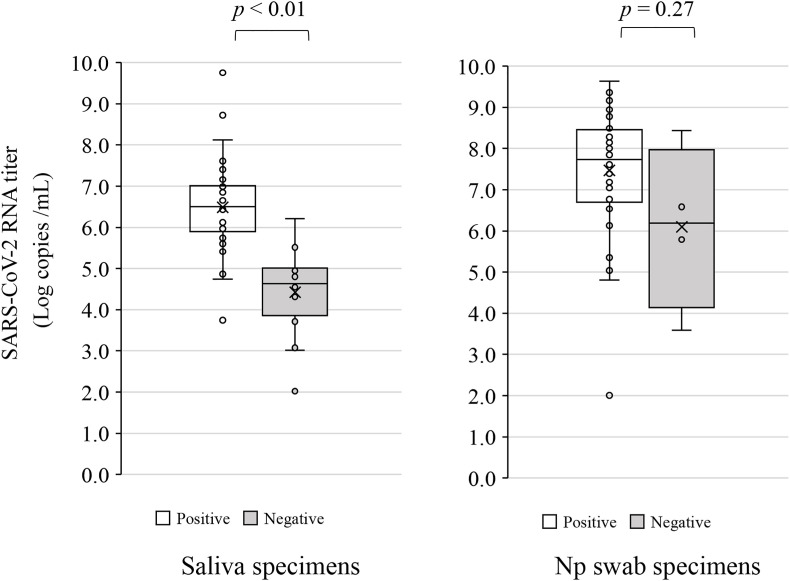
In the analysis with Inspecter Kowa® SARS-CoV-2 using saliva, all RT-qPCR-negative samples (n = 79) gave negative results (100% specificity), whereas antigens were detected in 45 of the 61 RT-qPCR-positive samples (73.8% sensitivity) (Table 1 a). The median number of SARS-CoV-2 RNA copies in the positive Inspecter Kowa® SARS-CoV-2 samples was 6.50 log copies/mL (IQR: 0.97–6.99) (Fig. 3 a). Fig. 3a also shows the viral load in the Inspecter Kowa® SARS-CoV-2-positive and -negative saliva specimens. Median viral load was significantly different between the antigen-positive specimens (6.50 log copies/mL) and antigen-negative samples (64 log copies/mL; p < 0.01).
Table 1a.
Concordance between Inspecter Kowa® SARS-CoV-2 and RT-qPCR.
| RT-qPCR |
Overall agreement (%) (95% CI) | Kappa value (95% CI) | |||
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Inspecter Kowa® SARS-CoV-2 | Positive | 45 | 0 | 88.6 (83.3–93.8) | 0.764 (66.1–86.7) |
| Negative |
16 |
79 |
|||
| Total | 61 | 79 | |||
Fig. 3.
Box-and-whisker plot of SARS-CoV-2 levels in saliva and Np swab specimens. The results for SARS-CoV-2 antigen-positive (Ag+)/RT-PCR-positive (PCR+) and SARS-CoV-2 antigen-negative (Ag−)/RT-PCR-positive (PCR+) samples were plotted.
3.3. Espline® SARS-CoV-2 test Kit using Np swab specimens
In the Espline® SARS-CoV-2, all RT-qPCR-negative samples (n = 80) also gave negative test results (100% specificity), whereas antigens were detected in 56 of the 60 RT-qPCR-positive samples (93.3% sensitivity) (Table 1 b). The median number of SARS-CoV-2 RNA copies in the positive Espline® SARS-CoV-2 Test Kit samples was 7.74 log copies/mL (IQR: 6.74-8.39) (Fig. 3b). Fig. 3b also shows the viral load in the Espline® SARS-CoV-2-positive and -negative Np swab specimens. There was no significant difference in median viral load between the antigen-positive specimens (7.74 log copies/mL) and antigen-negative samples (6.19 log copies/mL; p = 0.27).
Table 1b.
Concordance between Espline® SARS-CoV-2 and RT-qPCR.
| RT-qPCR |
Overall agreement (%) (95% CI) | Kappa value (95% CI) | |||
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Espline® SARS-CoV-2 | Positive | 56 | 0 | 97.1 (94.4–99.9) | 0.941 (88.4–99.8) |
| Negative |
4 |
80 |
|||
| Total | 60 | 80 | |||
3.4. Comparative verification of antigen tests
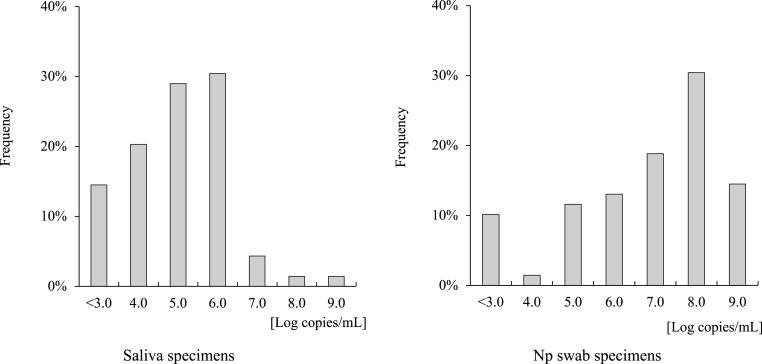
Overall agreement between the Inspecter Kowa® SARS-CoV-2 and Espline® SARS-CoV-2 Kits was 87.9% (Table 1 c). Table 2 shows the changes in the positive concordance rate of each rapid antigen detection kit and RT-qPCR according to viral load in each sample. Saliva specimens in particular were shown to produce false negative results when viral load was decreased to <1.0 × 105 copies/mL using Inspecter Kowa® SARS-CoV-2, and Np swab specimens were also shown to produce false negative results when viral load was decreased to <1.0 × 104 copies/mL using the Espline® SARS-CoV-2 Test Kit. Comparing the histograms of viral load, saliva samples had peaks between 1.0 × 105 and 106 copies/mL, while Np swab had peaks at 1.0 × 108 copies/mL (Fig. 4 ).
Table 1c.
Concordance between Inspecter Kowa® SARS-CoV-2 and Espline® SARS-CoV-2.
| Espline® SARS-CoV-2 |
Overall agreement (%) (95% CI) | Kappa value (95% CI) | |||
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Inspecter Kowa® SARS-CoV-2 | Positive | 42 | 3 | 87.9 (82.4–93.3) | 0.738 (62.2–85.5) |
| Negative |
14 |
81 |
|||
| Total | 56 | 84 | |||
Table 2.
Percentage of positive matches with RT-qPCR test results according to viral load in each sample.
| Viral load (copies/mL) | Saliva |
Np swab |
||
|---|---|---|---|---|
| n | Positive saliva antigen test (positive rate, %) | n | Positive Np swab antigen test (positive rate, %) | |
| ≤103 | 3 | 0 (0.0) | 1 | 0 (0.0) |
| 103–104 | 2 | 1 (50.0) | 1 | 1 (100) |
| 104–105 | 12 | 2 (16.7) | 7 | 6 (85.7) |
| 105–106 | 18 | 16 (88.9) | 8 | 7 (87.5) |
| 106–107 | 21 | 21 (100) | 13 | 12 (92.3) |
| 107–108 | 3 | 3 (100) | 20 | 20 (100) |
| 108–109 |
2 |
2 (100) |
10 |
10 (100) |
| Total | 61 | 45 | 60 | 56 |
Fig. 4.
Histograms of SARS-CoV-2 viral load in each specimen.
4. Discussion
In this study, we compared the performance of the Inspecter Kowa® SARS-CoV-2 Kit using saliva specimens with conventional RT-qPCR and the Espline® SARS-CoV-2 Antigen Test Kit using Np swab specimens. The clinical advantage of Inspecter Kowa® SARS-CoV-2 is that it can be performed using saliva samples collected by patients without the need for expert skills and equipment. Therefore, by introducing an ICT-based test kit, it is possible to establish an immediate COVID-19 rapid screening system at low cost, not only in hospitals but also in all facilities including schools, workplaces, and dormitories, where regular screening is especially essential for safe daily operations. Patients with asymptomatic SARS-CoV-2 infection still have a significant impact on the spread of the virus and are important targets for controlling the pandemic. In addition, some patients have substantial viral shedding before the onset of symptoms [12]. Because it is important to recognize SARS-CoV-2 infection early and stop its transmission using active screening, the test kit verified in this study may be ideal for infection control owing to its ease of use, as it could allow patients to perform the entire process from saliva collection to self-diagnose and to reduce the burden on medical care during a pandemic.
The overall sensitivity and specificity of Inspecter Kowa® SARS-CoV-2 with saliva specimens was 88.6% and 100%, respectively. In this study, the results of both antigen tests using saliva and Np swab specimens were directly influenced by the amount of virus in the samples: when viral load was ≤1.0 × 103 copies, the sensitivity of both antigen tests was significantly reduced. Unexpectedly, there was no correlation between the amount of virus in saliva and Np swab specimens in this study, whereas it has been reported that in patients with low viral load, such as asymptomatic patients, the viral load is lower in saliva specimens than in Np swab specimens [13]. Compared with the viral load of the saliva specimens,1.0 × 104 to 1.0 × 105 more virus was detected in the Np swab specimens. This discrepancy between saliva and Np swab specimens might depend on the sensitivity of the reagents or the presence of reaction inhibitors in the specimen as well as the possibility that viral RNA fragments or antigen accumulation in the absence of infectivity might be detected in Np swab specimens [14].
Several antigen-based rapid diagnostic test kits also are reported to have difficulty in detecting SARS-CoV-2 in samples with a low viral load, regardless of whether a saliva or Np swab specimen is used [15,16]. Additionally, it has been reported that a high viral load results in a shorter time to generate an antigen-positive test result; therefore, antigen-based test kits should be used to detect and estimate viral load in clinical specimens [17]. In addition, rapid antigen tests, although less sensitive than RT-qPCR, have been suggested to accurately reflect both the presence of infectious virus and the potential risk of SARS-CoV-2 infection [18]. Further studies using a larger number of specimens are warranted to determine the detailed detection sensitivity and quantitative evaluation when using Inspector Kowa SARS-CoV-2 with saliva specimens as well as its association with infectivity.
Notably, Inspecter Kowa® SARS-CoV-2 did not generate any false positive results, despite the presence of various symbiotic pathogens in the oral cavity and the different physical backgrounds of the patients [19]. Saliva specimens have been suggested to be useful for the diagnosis of COVID-19 in children as well as in adults, whereas the collection of Np swab specimens is an invasive and specialized procedure that is especially difficult to perform on children [20]. In the long-running COVID-19 pandemic, ICTs with saliva are attractive point-of-care test that can be performed immediately using specimens that can be easily collected by anyone. In this study, saliva was self-collected in a tube and used for analysis. According to the kit's instructions, saliva can be produced by pressing the tip of the tongue against the lower jaw, and then collected by absorption with a cotton swab. A limitation of this study is that we did not obtain data for the saliva swab pool testing (lollipop method) using cotton swabs, so we cannot assess the impact of different collection methods on detection sensitivity. Evaluation of efficacy in children also remains a future issue.
It is obvious that not all tests are suitable for diagnosis in all clinical settings. Finally, the role of ICT-based salivary diagnosis in the detection of SARS-CoV-2 antigen is to easily identify viral shedding and reduce the medical burden as an on-site direct test that can be performed by anyone.
Authorship statement
TM was responsible for the organization and coordination of the study. MK, YO, MT, RT, NM, NK, KO, RK, and TKa performed data analysis. ST, NT, TKo, and HN prepared the study design. RSh, RA, MS, TKu, NN, SK, HM, YU, HY, RSe, KK, SM, and TS obtained informed consent and collected the clinical specimens. All the authors contributed to the writing of the manuscript.
Declaration of competing interest
None.
Acknowledgments
We thank everyone involved in the COVID-19 Care Task Force at Saitama Medical University Hospital in Japan.
References
- 1.WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/
- 2.Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182(4):812–817 e819. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Challen R., Brooks-Pollock E., Read J.M., Dyson L., Tsaneva-Atanasova K., Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372:n579. doi: 10.1136/bmj.n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jayakody H., Kiddle G., Perera S., Tisi L., Leese L.S. Molecular diagnostics in the era of COVID-19. Anal Methods. 2021;13(34):3744–3763. doi: 10.1039/d1ay00947h. [DOI] [PubMed] [Google Scholar]
- 5.Allicock O.M., Petrone M.E., Yolda-Carr D., Breban M., Walsh H., Watkins A.E., et al. Evaluation of saliva self-collection devices for SARS-CoV-2 diagnostics. BMC Infect Dis. 2022;22(1):284. doi: 10.1186/s12879-022-07285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyllie A.L., Fournier J., Casanovas-Massana A., et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med. 2020;383:1283–1286. doi: 10.1056/NEJMc2016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakanashi D., Asai N., Nakamura A., Miyazaki N., Kawamoto Y., et al. Comparative evaluation of nasopharyngeal swab and saliva specimens for the molecular detection of sars-cov-2 rna in Japanese patients with covid-19. J Infect Chemother. 2021;27:126–129. doi: 10.1016/j.jiac.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kodana M., Kitagawa Y., Takahashi R., Matsuoka M., Fushimi N., et al. Concerns about the clinical usefulness of saliva specimens for the diagnosis of COVID-19. J Infect. 2021;83:134–136. doi: 10.1016/j.jinf.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagura-Ikeda M., Imai K., Tabata S., Miyoshi K., Murahara N., Mizuno T., et al. Clinical evaluation of self-collected saliva by quantitative reverse transcription-PCR (RT-qPCR), direct RT-qPCR, reverse transcription-loop-mediated isothermal amplification, and a rapid antigen test to diagnose COVID-19. J Clin Microbiol. 2020;58(9) doi: 10.1128/JCM.01438-20. e01438-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoki K., Nagasawa T., Ishii Y., Yagi S., Kashiwagi K., Miyazaki T., et al. Evaluation of clinical utility of novel coronavirus antigen detection reagent, Espline SARS-CoV-2. J Infect Chemother. 2021;27(2):319–322. doi: 10.1016/j.jiac.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirato K., Nao N., Katano H., Takayama I., Saito S., Kato F., et al. Development of genetic diagnostic methods for detection for Novel Coronavirus 2019 (nCoV-2019) in Japan. Jpn J Infect Dis. 2020;73:304–307. doi: 10.7883/yoken.JJID.2020.061. [DOI] [PubMed] [Google Scholar]
- 12.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alkhateeb K.J., Cahill M.N., Ross A.S., Arnold F.W., Snyder J.W. The reliability of saliva for the detection of sars-cov-2 in symptomatic and asymptomatic patients: insights on the diagnostic performance and utility for covid-19 screening. Diagn Microbiol Infect Dis. 2021;101 doi: 10.1016/j.diagmicrobio.2021.115450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohn Y., Jeong S.J., Chung W.S., Hyun J.H., Baek Y.J., Cho Y., et al. Assessing viral shedding and infectivity of asymptomatic or mildly symptomatic patients with COVID-19 in a later phase. J Clin Med. 2020;9(9):2924. doi: 10.3390/jcm9092924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patriquin G., LeBlanc J., Williams C., Hatchette F., Ross J., Barrett L., et al. Comparison between nasal and nasopharyngeal swabs for SARS-CoV-2 rapid antigen detection in an asymptomatic population, and direct confirmation by RT-PCR from the residual buffer. Microbiol Spectr. 2022;10(1) doi: 10.1128/spectrum.02455-21. e02455-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Marinis Y., Pesola A.K., Söderlund Strand A., Norman A., Pernow G., et al. Detection of SARS-CoV-2 by rapid antigen tests on saliva in hospitalized patients with COVID-19. Infect Ecol Epidemiol. 2021;11(1) doi: 10.1080/20008686.2021.1993535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akashi Y., Kiyasu Y., Takeuchi Y., Kato D., Kuwahara M., Muramatsu S., et al. Evaluation and clinical implications of the time to a positive results of antigen testing for sars-cov-2. J Infect Chemother. 2022;28:248–251. doi: 10.1016/j.jiac.2021.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pekosz A., Parvu V., Li M., Andrews J.C., Manabe Y.C., Kodsi S., et al. Antigen-based testing but not real-time polymerase chain reaction correlates with severe acute respiratory syndrome coronavirus 2 viral culture. Clin Infect Dis. 2021;73:e2861–e2866. doi: 10.1093/cid/ciaa1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi R., Murai R., Moriai M., Nirasawa S., Yonezawa H., Kondoh T., et al. Evaluation of false positives in the SARS-CoV-2 quantitative antigen test. J Infect Chemother. 2021;27(10):1477–1481. doi: 10.1016/j.jiac.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al Suwaidi H., Senok A., Varghese R., Deesi Z., Khansaheb H., Pokasirakath S., et al. Saliva for molecular detection of SARS-Cov-2 in school-age children. Clin Microbiol Infect. 2021;27:1330–1335. doi: 10.1016/j.cmi.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]