Abstract
Although coronavirus disease 2019 (COVID-19) remains an ongoing threat, concerns regarding other respiratory infections remain. Throughout the COVID-19 pandemic various epidemiologic trends have been observed in other respiratory viruses including a reduction in influenza and respiratory syncytial virus infections following onset of the COVID-19 pandemic. Observations suggest that infections with other respiratory viruses were reduced with social distancing, mask wearing, eye protection, and hand hygiene practices. Coinfections with COVID-19 exist not only with other respiratory viruses but also with bacterial pneumonias and other nosocomial and opportunistic infections. Coinfections have been associated with increased severity of illness and other adverse outcomes.
Keywords: Coinfections, COVID-19, Influenza, Respiratory syncytial virus, Social distancing, Bacterial pneumonia
Key points
-
•
The COVID-19 pandemic impacted the trends of other common respiratory viral illnesses between 2020 and 2022.
-
•
Implementation of social distancing, mask wearing, and other behavioral interventions for COVID impacted levels of respiratory virus spread.
-
•
Coinfection with other respiratory viruses can occur with COVID-19 infections, with influenza and RSV being among the most common.
-
•
Coinfection with bacterial pneumonia can occur with COVID-19 infections, including community-acquired pneumonia and hospital-acquired pneumonia.
-
•
Ventilator-associated pneumonia is a common nosocomial infection associated with COVID-19 infections and increased mortality.
Introduction
Since the emergence of the coronavirus disease 2019 (COVID-19) pandemic in Wuhan, China, in December 2019, there has been a significant focus placed on its transmission, pathogenesis, treatment, and prevention.1 Although COVID-19 continues to have a global impact, concerns about other respiratory infections, including those caused by viruses and bacteria, remain.
The COVID-19 pandemic has shed unique light on the epidemiologic trends of common community-acquired respiratory viruses and the impacts of nonpharmacologic practices including social distancing and mask wearing. In addition, coinfections of COVID-19 with other respiratory viruses and bacterial organisms, along with nosocomial and opportunistic fungal infections, have been observed with variable outcomes.
This article highlights epidemiologic trends of common respiratory viruses during the COVID-19 pandemic and coinfections with common respiratory viruses and other infectious agents.
Epidemiologic trends of common respiratory viruses during the severe acute respiratory syndrome coronavirus 2 pandemic
Although there has been a primary focus on the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) respiratory virus since the COVID-19 pandemic emerged in 2019, concern for other common community-acquired respiratory viruses remains. Community-acquired respiratory viruses include influenza, respiratory syncytial virus (RSV), paramyxoviruses, rhinovirus, and adenovirus, among others; all may have varied clinical presentations/severities depending on host factors. In the absence of laboratory testing, it may be difficult to distinguish between various respiratory viruses. Often respiratory viruses cause upper respiratory tract infections all with similar symptoms of fever, chills, myalgias, cough, and shortness of breath. However, several respiratory viruses including influenza may lead to lower respiratory tract infections with pneumonia, hypoxemic respiratory failure, and superimposed infections. Given the wide range of symptom severity, including those with asymptomatic infections, respiratory viral illnesses may be underestimated because many patients do not undergo diagnostic testing for non-COVID infections. Thus, the authors provide an overview of the epidemiologic trends in respiratory viruses during the SARS-CoV-2 pandemic and discuss recent updates and the impact of the pandemic on influenza, RSV, and other respiratory viruses.
Influenza Virus
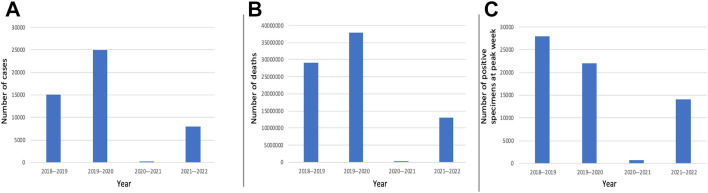
Influenza is seasonal in North America occurring frequently in the winter but can occur year-round in tropical countries. The emergence of the H3N2 strain occurred in 1968 near Hong Kong, and since that time the origin of several antigenically diverse strains of seasonal influenza a (H3N2) has been attributed to the densely populous East, South, and Southeast Asia regions. Seasonal influenza epidemics are influenced by antigenic drift or small mutations in hemagglutinin and neuraminidase proteins, which produce closely related viruses. Antigenic shift occurs less frequently and is an abrupt change in the influenza virus that can cause pandemics due to lack of immunity in the general population to this genetic shift. Annual epidemics can result in up to 650,000 deaths worldwide according to the World Health Organization (WHO), and the last influenza pandemic was in 2009 to 2010, caused by the H1N1 virus with 284,000 deaths in more than 214 countries.2 , 3 Surveillance done by WHO FluNet and Centers for Disease Control and Prevention ([CDC] FluView) include the most robust data on influenza; however, because influenza is not a reportable disease in the United States, these are compiled estimates (Fig. 1 ).
Fig. 1.
Comparison of influenza seasons in the United States with compilation of data from WHO FluNet and CDC FluView between 2018 and 20224, 5, 6, 7, 8 including (A) total number of cases, (B) total number of deaths, and (C) total number of positive specimens at peak week.
Indeed, studies early in the pandemic demonstrated that changes in population behavior were associated with both reduced transmission of SARS-CoV-2 and decreased influenza transmission.4 , 5 In the United States, between September 2020 and May 2021, there was a marked reported reduction in reported influenza cases: the CDC reported that only 1899 (0.2%) of 1,081,671 clinical samples tested positive for influenza virus; influenza B comprised most of the reported cases at 62.5%.6 In contrast to the 2020 to 2021 season, the CDC reported more than 250,000 positive influenza specimens of 1,491,430 total specimens in the 2019 to 2020 season7 with a similar global trend.8 , 9 Prediction models after the light 2020 to 2021 season anticipated a heavier compensatory season in 2021 to 2022 due to decreased immunity.10
Respiratory Syncytial Virus
RSV contributed substantially to the respiratory viral disease burden before the pandemic. RSV is common in the pediatric population where it causes significant mortality in children younger than 2 years due to bronchiolitis and pneumonia, although there are increasing data demonstrating significant burden in elderly, chronically ill, or immunocompromised adults.11 , 12 Influenza is typically associated with more deaths than RSV in all age groups except for children younger than 1 year.13 The CDC collects information on RSV in the United States using the National Respiratory and Enteric Virus Surveillance System (NREVSS).14 According to the CDC, RSV contributes to 58,000 hospitalizations among children younger than 5 years and 14,000 deaths among adults older than 65 years annually.15 RSV follows a similar seasonal trend to influenza in the United States,16 starting in early December and peaking in February in the United States.14 Longer infection seasons have been associated with more northern latitude.17 Infection can be seen outside a seasonal trend in tropical countries and also when infection mitigation measures disrupt seasonal patterns as noted earlier. RSV activity can correlate with rainfall and humidity in tropical regions, as in Australia during 2020 when widespread spring RSV outbreaks extended into summer.18 In addition, in the United States and France, RSV was reported later and extended into the spring and summer months.14 , 19
Similar to influenza, epidemiologic trends demonstrate a reduction in RSV cases across various countries from March 202020, 21, 22, 23 that was also attributed to social distancing and nonpharmacologic interventions. Despite lifting social distancing restrictions in April 2020, no RSV cases were detected in western Australia until august 2020, inferring that increased hygienic measures, such as hand washing, may have sustained prevention of viral transmission.24 Another hypothesis for reduction in RSV was viral interference from SARS-CoV-2 because interferon-stimulated immunity by one virus reduces infectivity of additional viruses. In 2021 as pandemic measures were relaxed, the reemergence of RSV was noted in various regions even out of the usual seasonal trends in both northern and southern hemispheres where outbreaks began later than seasonally expected.14 , 19 Another hypothesis for the reemergence of RSV was that fewer viral infections in 2020 led to lower concentrations of antibodies in pregnant women, subsequently impacting acquired immunity of infants.25 Indeed, lack of immunity coupled with the resumption of normal societal activities is likely responsible for the surge of RSV cases in children that is overwhelming pediatric facilities in the fall of 2022.26
Epidemiologic trends of other respiratory viruses
Throughout the SARS-CoV-2 pandemic there remained concerns for other respiratory viruses including paramyxoviruses, respiratory adenovirus, seasonal coronavirus, and rhinovirus, among others. The NREVSS database showed that the epidemiology of non-RSV paramyxoviruses (including human metapneumovirus and parainfluenza virus) and respiratory adenovirus varied during the pandemic.14
The common seasonal coronavirus (the strains 229E, NL63, OC43, and HKU1) had a persistent role during the SARS-CoV-2 pandemic and was often found in cocirculation with SARS-CoV-2.14 The data on the diagnosis of seasonal coronavirus may have been limited because it is often not part of the diagnostic panels. Seasonal coronavirus follows a trend of peaking during winter months, which may suggest that factors including low temperature and low sunlight favor survival, and remains more common in the pediatric and adolescent populations.27 , 28
Rhinovirus is another commonly observed virus that is also noted to have persistence during the COVID-19 pandemic in various regions including the United Kingdom and Singapore.29 , 30 Rhinovirus reductions were only seen after lockdown, and levels rebounded earlier than other respiratory viruses, which suggests that social distancing practices were more effective at suppressing other respiratory viruses.30 The hypothesis surrounding the early reemergence of rhinovirus infections included its viral structure as a small, hydrophilic, nonenveloped virus with propensity for contact and droplet transmission.29
These various viruses have demonstrated some regional differences during the COVID-19 pandemic, but overall many regions exhibited similar trends of decreased annual seasonal respiratory viruses in 2020 to 2021 with reemergence in the 2021 to 2022 season.31 , 32
Impact of social distancing and vaccination on the spread of common respiratory viral infections during the coronavirus disease 2019 pandemic
Several interventions have influenced epidemiologic trends of respiratory infections since the pandemic began, including social distancing and implementation of nonpharmacologic measures such as wearing masks, eye protection, and strict hand hygiene practices.
Even before the COVID-19 pandemic, interventions to limit contact and droplet-related spread were studied. A systematic review of social distancing measures including crowd avoidance, workplace/school closures, lockdowns, isolation of infected persons, quarantining of exposed persons, and contract tracing had some benefit against influenza spread.33, 34, 35, 36 Transmission of viruses was noted to be lower when physical distance was greater than 1 m, and protection was increased as distance increased.37 Hand hygiene has long been a key infection prevention method and has been associated with reduction in respiratory viral illnesses.38 Chan and colleagues39 observed an 88.9% decrease in the prototypical community-acquired pneumonia (CAP) syndrome with pneumococcal pneumonia from pandemic years 2020 to 2021 when compared with the 5 years before the pandemic, hypothesizing that public health policies (eg, universal masking and social distancing) may have been the primary drivers of such a decrease. A reduction in bronchiectasis exacerbations was noted during the first 12 months of the COVID-19 pandemic upon implementation of social distancing measures.40
In addition, the implementation of mask wearing resulted in a large reduction in risk of COVID-19 infection. The use of respirator masks (like N95 masks) was associated with stronger protection when compared with the use of disposable surgical masks.37 An N95 respirator mask has efficient airborne filtration with a tight-fitting design allowing for a seal around the nose and mouth. In contrast, surgical masks are loose fitting and less efficiently filter airborne particles.
Apart from masks, eye protection provided an added layer for infection prevention. In a study of frontline emergency room workers, mandatory eye protection in conjunction with universal masking was effective at reducing COVID-19; additional reviews have suggested that use of eye protection may help prevent eye inoculation, which could lead to respiratory infections.41 , 42
Severe acute respiratory syndrome coronavirus 2 and viral coinfections
Severe Acute Respiratory Syndrome Coronavirus 2 and Influenza
It can be expected that SARS-CoV-2 will become endemic and cocirculate with influenza, and therefore their similarities and differences should be reviewed. Before SARS-CoV-2, influenza was one of the largest public health challenges, resulting in approximately half a million deaths annually.43 Several studies have compared both viruses given their similarity of symptoms and propensity to each cause severe illness (table 1 ).
Table 1.
Comparison of characteristics of severe acute respiratory syndrome coronavirus 2, influenza, and respiratory syncytial virus
| SARS-CoV-2 | Influenza | RSV | |
|---|---|---|---|
| Viral structure | Single-stranded, positive-sense RNA | Negative-sense, single-stranded RNA with surface glycoproteins integral in determining influenza type | Filamentous enveloped, negative-sense, single-stranded RNA |
| Zoonotic infection | Bats | Avian and swine | Animal models of RSV infections in rodents and nonhumans primates |
| Virulence | Median r0 2.79 | Median r0 1.28 | Median r0 1.2–.2.1 |
| Population at risk |
|
|
|
| Time until postexposure presentation | 3–7 d | 2–5 d | 4–6 d |
| Clinical symptoms |
|
|
|
| Duration of symptoms | Depends on severity of illness | Typically resolves by day 8 | Typically resolves by 3–7 d |
| Complications | Post-COVID syndrome and severe cases can lead to ARDS | Severe cases can lead to ARDS | Severe cases can lead to bronchiolitis and pneumonia (especially in pediatric cases) |
Clinical presentation may be similar for both influenza and SARS-CoV-2; however, symptoms that tend to be more unique to SARS-CoV-2 include loss of taste and smell as a symptom. Additional variants of SARS-CoV-2, including the omicron variant, have been associated with less severe symptoms when compared with alpha and delta variants, and many individuals are asymptomatic and may be unaware of their infection. A large study demonstrated that patients with SARS-CoV-2 more frequently had conditions including obesity, diabetes, hypertension, and dyslipidemia when compared with patients with influenza, indicating the importance of metabolic syndrome as a risk factor for SARS-CoV-2 infection. Those with influenza more frequently had heart failure, chronic respiratory disease, and cirrhosis. This comparative study also demonstrated that patients hospitalized with SARS-CoV-2 infection more frequently developed acute respiratory failure, pulmonary embolism, septic shock, or hemorrhagic stroke than patients with influenza, although they were noted to have a lower incidence of myocardial infarction or atrial fibrillation. In-hospital mortality was higher in patients with SARS-CoV-2 than in patients with influenza,62 and patients with SARS-CoV-2 more frequently have abnormal chest radiology and longer duration of stay during hospitalizations.63
Apart from the clinical presentations, the treatment and prevention for SARS-CoV-2 and influenza also differ. SARS-CoV-2 therapeutics include novel antiviral therapies such as remdesivir and vaccinations, which are currently the mainstay of outpatient therapy and prevention of SARS-CoV-2. For influenza, interferons and neuraminidase inhibitors play roles in the prophylaxis and treatment. Interferon types 1 and type 3 function to inhibit viral replication,64 and neuraminidase inhibitors assist in preventing virions from being released from the surface of infected cells and are effective for both prophylaxis and treatment.65 Antiviral medications for influenza are approved for use within the United States and have a mechanism of action based via neuraminidase inhibition and can lessen symptoms and shorten duration of illness. Annual influenza vaccination is recommended for everyone aged six months and older (Table 2 ), whereas CDC recommendations for SARS-CoV-2 vaccines include initial series and boosters. The efficacy for influenza vaccines varies by season and aids to reduce severity of illness.66 , 67 There has been evidence that has also suggested that flu vaccination has the potential to reduce mortality of SARS-CoV-2 with lower risk of death at 60 days in patients who had received flu vaccinations.68
Table 2.
Influenza and severe acute respiratory syndrome coronavirus 2 vaccine comparison
| Influenza Vaccine | SARS-CoV-2 Vaccine | |||
|---|---|---|---|---|
| Type | Inactivated, quadrivalent vaccine (live attenuated or recombinant vaccines also available) | mRNA vaccine | DNA viral vector | |
| Mechanism | Surface glycoproteins hemagglutinin | RNA leads to generation of spike protein found on coronavirus and creation of antibodies against it | Modified virus is a vector carrying for COVID spike protein to trigger immune response | |
| Brands | Not applicable | Pfizer-BioNTech | Moderna | Johnson & Johnson’s Janssen (J&J) |
| Frequency of dosing | Annual | 2 injections 21 d apart + booster | 2 injections 28 d apart + booster | 1 dose + booster |
| % Effectiveness | 40–60 | 90 | 95 | 66 |
| Population recommended for | 6 mo and older | 6 mo and older | 6 mo and older | > 18 years old |
In cases of severe disease with refractory hypoxemia in both SARS-CoV-2 and influenza, extracorporeal membrane oxygenation can be considered as a salvage therapy.72 When comparing symptoms after acute illness of SARS-CoV-2 and influenza infections, postacute sequalae of COVID ([PASC] or long COVID) has been associated with symptoms of prolonged dyspnea, fatigue/malaise, chest/throat pain, headache, abdominal symptoms, myalgias, cognitive symptoms, and anxiety/depression.73
Systematic reviews report variable rates of coinfection, one stating rates of influenza infection were 0.8% in patients with confirmed SARS-CoV-2 with fever, cough, and shortness of breath being most common clinical manifestations.74 Analyses also demonstrate higher coinfection rates in pediatrics compared with adults.75 In one small pediatric study, nearly half of SARS-CoV-2 infected children had coinfection with other common respiratory pathogens.76 Although there is no clear consensus on the implications of coinfection, one study suggests there is a potential for added harm with viral coinfection, because posttranslational changes in angiotensin-converting enzyme-2 by influenza A may increase vulnerability to lung injury and acute respiratory distress syndrome (ARDS) during coinfections.77 Although potential targets for therapy are under investigation,78 no single medication is known to treat both SARS-CoV-2 and influenza simultaneously, although supportive care can be given for both. Similar to influenza infections, as many as one-fifth of patients with SARS-CoV-2 are found to have coinfection or superinfection with other pathogens, which increases mortality, and these coinfections and superinfections are discussed further in this article. Influenza is also associated with bacterial superinfection, specifically Staphylococcus aureus and Streptococcus pneumoniae.79
In the future, coinfection of SARS-CoV-2 and influenza virus will likely continue to occur because influenza epidemics may happen concurrently with the ongoing SARS-CoV-2 pandemic. WHO now recommends countries to prepare for the cocirculation of influenza and SARS-CoV-2 viruses with surveillance, monitoring, and vaccination programs for both SARS-CoV-2 and influenza.
Severe Acute Respiratory Syndrome Coronavirus 2 and Respiratory Syncytial Virus
Similar to influenza, we can expect that SARS-CoV-2 and RSV will start to cocirculate globally. Both RSV and SARS-CoV-2 can present with typical viral symptoms including fever, chills, myalgias, rhinorrhea, cough, and sore throat. Apart from cold-type symptoms (see Table 2) RSV can also lead to serious conditions such as bronchiolitis and predominantly affects pediatric and elderly patient populations.
Treatment of RSV is largely supportive and includes antipyretics and hydration. However, severe cases of bronchiolitis or pneumonia may require hospitalization and respiratory support. For prophylaxis against RSV, the medication palivizumab can be given to select pediatric patient populations who are at high risk of serious complications including lower respiratory tract infections from RSV and has been shown to reduce hospitalizations.80 Ribavarin is approved for severe RSV disease, but its effectiveness is unclear and not well studied, including in patients who are immunocompromised. At this time, there are limited effective antiviral medications targeted to RSV infections; however, there are ongoing trials to evaluate new antivirals and vaccines for RSV, including during pregnancy.81
RSV is among the most common coinfections in patients with SARS-CoV-2 pneumonia.82 Viral coinfection of RSV and SARS-CoV-2 may be associated with prolonged hospitalization, need for higher level of care, complicated lower respiratory infections,83 and elevated procalcitonin levels84; however, its contribution to increased mortality remains unclear.85, 86, 87, 88
Severe Acute Respiratory Syndrome Coronavirus 2 and Epstein-Barr Virus
SARS-CoV-2 has also demonstrated interaction with other viruses, although data for these viruses is based on smaller studies and case reports. Immune dysregulation from SARS-CoV-2 may potentiate other viral infections, particularly those with an asymptomatic course with potential to reactivate.
Epstein-Barr virus (EBV) can cause a wide range of diseases, from infectious mononucleosis in younger adults to various cancers or lymphoproliferative disorders, or it may be asymptomatic. Coinfection with both viruses demonstrated increased inflammation and consequently increased steroid use.89 Indeed, both SARS-CoV-2 and EBV can infect epithelial cells of the respiratory tract or cause liver function abnormalities, thus elevated liver function tests in coinfection can be expected, especially in setting of EBV reactivation.90 One study demonstrated up to 25% reactivation rate of EBV in patients with SARS-CoV-2, particularly in older and female persons, and suggested an associated increased mortality.91 This enhanced inflammation from EBV viral reactivation is thought to play a significant role in the development of PASC symptoms,92 possibly due to alternations in mitochondrial function and senescence.93
Severe Acute Respiratory Syndrome Coronavirus 2 and Cytomegalovirus
There are case reports of SARS-CoV-2 and cytomegalovirus (CMV), which have been associated with various clinical presentations including the development of CMV pneumonitis and presentations of gastrointestinal symptoms.94 CMV seropositivity is associated with increased risk of hospitalization with SARS-CoV-2 infection15 and increased severe bacterial infections.95 Furthermore, there has been suggestion to consider secondary infection with CMV in the differential of transaminitis for those with SARS-CoV-2 and EBV infections, because reactivation of CMV has also been described,96 although EBV is most consistently shown among opportunistic viruses.97
Additional considerations for viral reactivation include the alterations of the immune system associated with SARS-CoV-2 vaccination, which have been associated with sequelae of viral reactivation in CMV and varicella zoster virus.98, 99, 100, 101, 102
Severe Acute Respiratory Syndrome Coronavirus 2 and Other Respiratory Viruses
Although viral coinfections remains relatively rare, case reports have described coinfections of SARS-CoV-2 with human metapneumovirus,103 human parainfluenza virus,104 and adenovirus,105 among others. Case reports of SARS-CoV-2 and coinfection with adenovirus have suggested a more severe hospital course than when with isolated infections.106 It should be noted that studies reviewed here are not specific to patients who undergo transplant, although transplant recipients are already at increased risk of reactivation of certain viruses due to immunosuppression.
Severe acute respiratory syndrome coronavirus 2, bacterial coinfections, and superinfections
Bacterial coinfection and superinfection during severe viral illness has been a prominent issue documented as early as the 1918 influenza pandemic. Autopsies showed that virtually all influenza deaths were complicated, and perhaps caused by bacterial pneumonia coinfection; Streptococcus pneumoniae was a particularly significant pathogen with high mortality.107
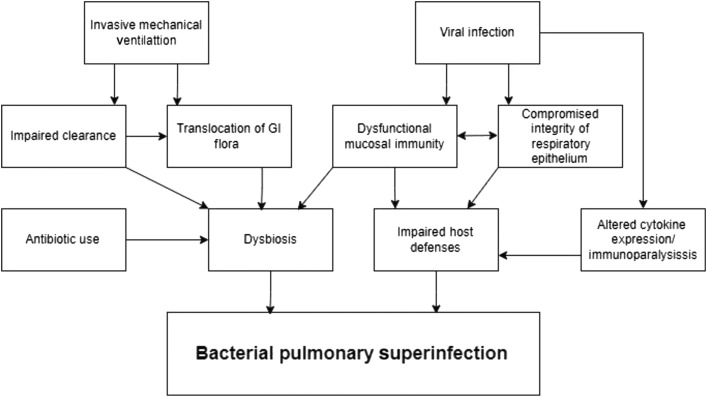
The reasons for bacterial involvement in viral disease are unclear and can occur through several mechanisms (Fig. 2 ). Dysbiosis of the pulmonary microbiome during invasive mechanical ventilation has been demonstrated and hypothesized to be due to a combination of antibiotic use, translocation of gastrointestinal flora, dysfunction of pulmonary mucosal immunity, and impaired microbial clearance.108 Viruses also impair host defenses resulting in increased susceptibility to bacterial infection, namely, by compromising the integrity of the respiratory epithelium, which allows for better adherence and invasion of bacteria.109 In addition, there is preliminary evidence in extrapulmonary infection models that viruses alter host cytokine expression potentially “distracting” the immune system away from a bacterial pathogen leading to worsening of bacterial infection.110 Because it pertains to SARS-CoV-2 specifically, some evidence also points toward an early immunoparalysis driven by virally infected monocytes and macrophages.111
Fig. 2.
The complex interplay of factors predisposing to bacterial pulmonary superinfection. GI, gastrointestinal.
The diagnosis of contemporaneous and secondary bacterial infections during SARS-CoV-2 infections has implications for patient outcomes, antibiotic stewardship, and health care costs.
Severe acute respiratory syndrome coronavirus 2 and community-acquired pneumonia
Definition
CAP is defined in clinical terms as the presence of symptoms attributable to pneumonia (eg, dyspnea, cough) plus radiographic confirmation.112
Epidemiology
A multicenter study done by the CDC in 2015 showed that among approximately 2300 patients presenting with CAP, a bacterial cause was only identified 17% of the time.113 Patients presenting with SARS-CoV-2 have bacterial coinfection even less frequently. Lehmann and colleagues114 found bacterial coinfection to occur in 7 of 321 SARS-CoV-2 presentations, with 4 cases of S pneumoniae, 2 cases of S aureus, and 1 case of Proteus mirabilis. This finding was further confirmed when an additional study showed that community-acquired bacterial coinfection occurred in only 59 of 1705 SARS-CoV-2 admissions.
The contrast between higher rates of coinfection during the influenza pandemic and the lower rate of bacterial CAP as a coinfection in SARS-CoV-2 is potentially explained by differing public health landscapes. Amin-Choudhury and colleagues115 confirmed that public health policies, such as social distancing practices, can lead to of decreased rates of pneumococcal CAP in the setting of coinfection; however, they noted that when coinfection happened it was associated with a 7-fold increase in 28-day mortality.39
Diagnosis
Discerning a patient presenting from the community with solitary COVID-19 pneumonia versus one with a bacterial coinfection remains difficult. Unfortunately, procalcitonin level drawn at admission is neither sensitive nor specific for this purpose.116 Radiographically, there are some findings that are more “typical” for COVID-19 than for bacterial pneumonia. In COVID-19, computed tomography (CT) of the chest shows bilateral, multilobar ground-glass opacities (GGOS) in a peripheral and subpleural distribution and focal areas of consolidation may be found within areas of GGOS.117 In the second week of illness the GGOS may continue to expand and evolve to contain areas of irregular linear opacities signaling the transformation into an organizing pneumonia.117
In contrast, bacterial pneumonia may more frequently have centrilobular nodules and bronchial wall thickening with mucoid impactions, as well as the characteristic lobar consolidation.116
Treatment
Treatment of suspected bacterial CAP coinfection should be according to published Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) CAP guidelines.112 Targeted diagnostics should include sputum culture and blood culture for severe disease or if treatment is targeting Pseudomonas aeruginosa or methicillin-resistant S aureus. Although guidelines only recommend pneumococcal urinary antigen testing for severe cases, it should likely be done whenever coinfection with SARS-CoV-2 is being considered given the higher odds of severe disease developing. Current IDSA guidelines for management of COVID-19 do not recommend for or against antibiotics for CAP, but do describe mostly negative effects of indiscriminate antibiotic use at the time of admission such as resistant superinfection.118
Coronavirus disease 2019 and ventilator-associated pneumonia
Definition
Hospital-acquired pneumonia (HAP) is specified as a pneumonia not incubating at the time of hospital admission and occurring 48 hours or more after admission. Ventilator-associated pneumonia (VAP) is defined as a pneumonia developing greater than 48 hours after endotracheal intubation.119 Diagnosis of HAP or VAP is based on finding a new lung infiltrate plus clinical evidence that the infiltrate is of an infectious origin (new fever, purulent sputum, leukocytosis, and decline in oxygenation).
Epidemiology
There are considerably more data regarding VAP infections in the setting of COVID-19 when compared with CAP. Mechanically ventilated patients with COVID-19 are at higher risk for VAP than mechanically ventilated patients without COVID-19, with a virtually universal agreement among large studies using strict definitions and microbiological confirmation.120 Furthermore, autopsy studies of patients who died of severe COVID-19 show that at least 32% had a bacterial superinfection.121
VAP was found to be the cause of more than 50% of hospital-acquired infections with P aeruginosa and Klebsiella pneumoniae being the most common isolated organisms.122 In a retrospective study observing 192 patients intubated for COVID-19 with early bronchoalveolar lavage (BAL) occurring before intubation in most cases, 11.6% had a superinfection meeting diagnostic criteria for HAP before intubation, with at least one episode of VAP occurring in 44.4% of patients. The average time to diagnosis was 10.8 days postintubation.123 In a study of more than 70,000 patients in the medical intensive care unit (ICU) divided into groups of prepandemic, pandemic without COVID-19 infection, and pandemic with COVID-19 infection, incidence of VAP was found to be higher in COVID-19-infected patients and had a higher attributable mortality. Attributable mortality for COVID-19 with VAP was 9.17%, whereas attributable mortality for prepandemic and pandemic COVID-19-negative patients was 3.15% and 2.91%, respectively.124
The explanation for such a high incidence of VAP in COVID-19 is likely multifactorial. Patients with COVID-19 who are intubated commonly have one or more of the traditional risk factors for VAP such as high lengths of stay, prolonged duration of mechanical ventilation, the presence of ARDS, sedation, neuromuscular blocking agents, and not least the high utilization of prone positioning.125 Stress on both human and material resources during peak surges has also been argued to have a detrimental impact on the rate of secondary infection.126
Diagnosis
HAP/VAP in COVID-19 should be diagnosed in accordance with IDSA/ATS guidelines.119 , 120 Although a spot test of procalcitonin on admission to the hospital was not found to be helpful for the diagnosis of bacterial CAP coinfection,116 a procalcitonin trend for patients in the ICU may be more useful. Richards and colleagues127 found that a 50% increase in procalcitonin levels from its previous value was independently associated with the presence of a secondary bacterial infection (VAP, ventilator-associated tracheobronchitis, or bacteremia) compared with increases in either white blood cell count or C-reactive peptide.
Treatment
Treatment of suspected bacterial HAP/VAP coinfections should be according to published IDSA/ATS guidelines.120
Coronavirus disease 2019 and other nosocomial infections
Bloodstream Infections
In a study comparing nosocomial bloodstream infections (BSIs) in non-COVID-19 and COVID-19 patients,128 Candida species accounted for nearly 50% of infections with next commonest being Enterococcus (Table 3 ). A comparison between the COVID-19 and non-COVID-19 groups showed that patients with COVID-19 were more than 2 times as likely to have candidemia than non-COVID-19 patients. Enterococcus rates were similar; however, the investigators note that the typical risk factors for candidemia and enterococcal BSIs such as gastrointestinal surgeries, malignancies, and chemotherapy were present in the non-COVID-19 patients but tended to be absent in the patients with COVID-19, suggesting that these were distinct complications of COVID-19. The investigators also noted that these BSI organisms occurred later in hospitalization, proposing that perhaps antimicrobials, steroids, and the interleukin (IL)-6 inhibitor tocilizumab may have promoted translocations of these organisms over time (see Table 3).
Table 3.
Most common etiologic organisms in coronavirus disease 2019-associated bloodstream infections
| Early | Late |
|---|---|
| S aureus | Enterococcus |
| Gram-negatives | Candida |
In a study of 212 patients with severe COVID-19 in Brazil, Silva and colleagues129 found bacteremia to carry an odds ratio for mortality of 21. Gago and colleagues128 went further to specify that although there was not an observed statistically significant difference in mortality among patients with BSI due to Candida or Enterococcus, there was an increase in mortality clustered around cases of BSI that occurred earlier in hospitalization with the less common S aureus and gram-negative organisms.
Bhatt and colleagues130 published a multicenter case-control study that highlighted some of the risk factors for secondary BSIs in a sample composed of all hospitalized patients: higher need of supplemental oxygen on admission, higher admission serum creatinine levels, and presentation with encephalopathy. Other potential risk factors include the use of antimicrobials, systemic corticosteroids, and IL-6 blockade. The study did, however, have a high proportion of coagulase-negative staphylococci, raising the concern of inappropriately screened contaminants.130
Coronavirus disease 2019 and invasive fungal infections
Aspergillosis and mucormycosis are diseases caused by fungal organisms that infect humans as molds. Although they are both recognized entities occurring in patients with COVID-19, considerable debate exists regarding the rate of occurrence, as it concerns COVID-19-associated pulmonary aspergillosis (CAPA).
Coronavirus Disease 2019-Associated Pulmonary Aspergillosis
One autopsy review series reports a CAPA rate of occurrence of approximately 2% in both mechanically ventilated and nonmechanically ventilated COVID-19 decedents.131 Published incidences of CAPA ranges from 0% to 30% in studies without standardized definitions, and 2% to 11% in studies with standardized definitions.132
A definition of “probable CAPA” has been devised to include more than one of the following: new cavitary lung lesions on chest CT without alternative explanation, positive serum galactomannan Enzyme Immunoassay (EIA) index greater than or equal to 0.5, positive BAL galactomannan EIA index greater than or equal to 1.0, or positive aspergillus cultures from BAL specimen.133, 134, 135 In their retrospective analysis of mechanically ventilated patients with COVID-19 in 5 Johns Hopkins Medicine health system hospitals, Permpalung and colleagues136 added a diagnosis of “possible” CAPA for patients who had more than 1 of the following: positive BAL galactomannan index greater than or equal to 0.5 (lower threshold), positive serum (1, 3)-β-d-glucan (BDG) level greater than or equal to 80 pg/mL without alternate explanation, and/or non-BAL sample culture with growth of Aspergillus. The expanded definition was intended for centers that do not use bronchoscopy for BAL liberally and that use BDG as a fungal marker in addition to galactomannan. Table 4 shows a comparison of diagnostics in probable versus possible CAPA.
Table 4.
Diagnostics in “probable” compared with “possible” coronavirus disease 2019-associated pulmonary aspergillosis
| Probable CAPA | Possible CAPA | |
|---|---|---|
| Imaging | New cavitary lung lesions on chest CT without alternative explanation | Not required |
| Serum markers | Serum galactomannan EIA index ≥ 0.5 | Serum (1, 3)-β-d-glucan ≥ 80 pg/mL without alternate explanation |
| BAL markers | BAL galactomannan EIA index ≥ 1.0 | BAL galactomannan EIA index ≥ 0.5 |
| Lung specimen cultures | Positive Aspergillus cultures from BAL | Non-BAL sample culture with growth of Aspergillus |
In the study by Permpalung and colleagues,136 rates of CAPA ranged from 5% to 10% and patients with CAPA had higher severity of illness, need for more ventilatory and hemodynamic support, and longer duration of hospitalization, although mortality was no different from non-CAPA patients. It is worth noting, however, that only 48.7% of patients with CAPA received antifungal therapy, likely representing the challenge of diagnosis.
Treatment of CAPA may be extrapolated from IDSA guidelines for invasive aspergillosis: triazoles (voriconazole, posaconazole, isavuconazole, itraconazole) are recommended as first-line agents, amphotericin B and derivatives can be used for salvage therapy and when voriconazole cannot be used, whereas echinocandins (eg, micafungin, anidulafungin) are not recommended to be used as initial monotherapy but may be effective as salvage therapy either alone or in combination with others.137
Coronavirus Disease 2019-Associated Mucormycosis
Mucormycosis is caused by several molds, but species Rhizopus, Lichtheimia, and Mucor account for 75% of infections.138 Cases of COVID-19-associated mucormycosis are more commonly sinonasal and rhino-orbito-cerebral than pulmonary or disseminated.139 Patients in the developing world have accounted for most cases reported thus far.
Mucormycosis is more diagnostically challenging than aspergillosis, in part because there are no approved biomarkers to aid in diagnosis. The etiologic agents of mucormycosis neither produce galactomannan nor do they have BDG in their cell walls. Diagnosis must be undertaken first with clinical suspicion of site of infection (eg, rhino-orbital) followed by surgical sampling and histopathology of the involved site.140
Treatment of mucormycosis also differs slightly from aspergillosis, and is likewise more limiting. In contrast to aspergillosis, amphotericin B and derivatives are considered first line for mucormycosis with triazoles being suitable for salvage therapy. Also unlike aspergillosis, echinocandins demonstrate breakthrough infection and may not be appropriate monotherapy for salvage or otherwise. Importantly, voriconazole and itraconazole do not have activity against mucormycosis organisms, therefore a clinician hoping to select coverage for both mucormycosis and aspergillosis without needing to concern themselves with the potential of toxicity from amphotericin B would have only isavuconazole and posaconazole as options.140 At that point of consideration, consultation with an infectious disease specialist would be recommended.
Summary
The COVID-19 pandemic had a significant impact on the epidemiology of other respiratory infections. The implementation of social distancing and wearing masks and eye protection was associated with a decline in rates of influenza and RSV during 2020 to 2021; now there is a reemergence of these and other community-acquired respiratory viruses. Although coinfections of COVID-19 with other respiratory viruses remain relatively rare, there have been some evidence that coinfection is associated with increased severity of illness. Treatment of coinfections includes a specific antiviral agents if available for the virus and supportive care.
Regarding bacterial pneumonia and COVID-19, CAP and nonintubated HAP are infrequent coinfections; an exception is perhaps merited for CAP caused by S pneumoniae. Once patients are endotracheally intubated, however, they become highly susceptible to VAP development in a manner out of proportion to similar patients with non-COVID-19 indications for intubation. About 60% to 70% of COVID-19-related VAPs involve gram-negative organisms126; treatment should be according to local antibiograms and resistance patterns in combination with published guidelines.120 A procalcitonin trend may be a useful adjunct in diagnosis.
Last, nosocomial infections including BSI and opportunistic invasive fungal infections can occur in patients with COVID-19 especially in patients with higher severity of illness.
Clinics care points
-
•
Changes in population behaviour early in the COVID-19 pandemic including; social distancing and masking; were associated with reduced transmission of SARS-CoV-2, influenza, respiratory syncytial virus, along with other seasonal coronavirus and rhinovirus infections.
-
•
Symptoms of many respiratory viral illnesses are similar to SARS-CoV-2 infection. Symptoms that are more unique to SARS-CoV-2 infection than other respiratory viral illness include loss of taste and smell.
-
•
Vaccination and treatment options are available for prevention and management of SARS-CoV-2 and influenza infections.
-
•
Bacterial coinfection and superinfection during SARS-CoV-2 infections have implications for patient outcomes, antibiotic stewardship and health care costs. Bacterial infections include community-acquired pneumonias, hospital-acquired pneumonia, ventilator-associated pneumonias and blood stream infections.
-
•
Fungal infections including COVID-19-associated pulmonary aspergillosis (CAPA) and mucormycosis were identified amongst patients infected with SARS-CoV-2.
Disclosure
Authors involved in this chapter have nothing to disclose.
References
- 1.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China of novel Coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyrrell CS A.J., Gkrania-klotsas E. Influenza: epidemiology and hospital management. Medicine (Abingdon) 2021;49:797–804. doi: 10.1016/j.mpmed.2021.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shieh W., Blau D., Denison A., et al., 2009 Pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States, Am J Pathol, 177, 2010, 166–175. [DOI] [PMC free article] [PubMed]
- 4.Cowling B.J., Ali S., Ng T., et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020;5(5):e279–e288. doi: 10.1016/S2468-2667(20)30090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tyrrell C.S., Allen J.L.Y., Gkrania-Klotsas E. Influenza: epidemiology and hospital management. Medicine (Abingdon) 2021;49(12):797–804. doi: 10.1016/j.mpmed.2021.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prevention C. 2020-2021 Flu season summary. 2022. https://www.cdc.gov/flu/season/faq-flu-season-2020-2021.htm Available from:
- 7.Prevention, C.f.D.C.a. Archived Estimated influenza illnesses, medical visits, hospitalizations, and deaths in the United States — 2019–2020 influenza season. 2022. https://www.cdc.gov/flu/about/burden/2019-2020/archive-09292021.html June 10, 2022]; Available from:
- 8.Adlhoch C., Mook P., Lamb F., et al., Very little influenza in the WHO European Region during the 2020/21 season, weeks 40 2020 to 8 2021, Euro Surveill, 26 (11), 2021. [DOI] [PMC free article] [PubMed]
- 9.Olsen S.J., Azziz-Baumgartner E., Budd A., et al., Decreased influenza activity during the COVID-19 pandemic-United States, Australia, Chile, and South Africa, 2020, Am J Transplant, 20 (12), 2020, 3681–3685. [DOI] [PMC free article] [PubMed]
- 10.Krauland M.G., Galloway D., Raviotta J., et al., Impact of low rates of influenza on next-season influenza infections, Am J Prev Med, 62 (4), 2022, 503–510. [DOI] [PMC free article] [PubMed]
- 11.Falsey A.R., Hennessey P., Formica M., et al., Respiratory syncytial virus infection in elderly and high-risk adults, N Engl J Med, 352 (17), 2005, 1749–1759. [DOI] [PubMed]
- 12.Tin Tin Htar M., Yerramalla M. Moisi J., et al., The burden of respiratory syncytial virus in adults: a systematic review and meta-analysis. Epidemiol Infect. 2020;148:e48. doi: 10.1017/S0950268820000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson W.W., Shay D., Weintraub E., et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 14.Available at: https://www.cdc.gov/surveillance/nrevss/index.html. Updated January 25, 2023.
- 15.Alanio C., Verma A., Mathew D., et al., Cytomegalovirus latent infection is associated with an increased risk of COVID-19-related hospitalization, J Infect Dis, 226 (3), 2022, 463–473. [DOI] [PMC free article] [PubMed]
- 16.Paes B.A., Mitchell I., Banerji A., et al. A decade of respiratory syncytial virus epidemiology and prophylaxis: translating evidence into everyday clinical practice. Can Respir J. 2011;18(2) doi: 10.1155/2011/493056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broberg E.K., Waris M., Johansen K., et al., Seasonality and geographical spread of respiratory syncytial virus epidemics in 15 European countries, 2010 to 2016, Euro Surveill, 23 (5), 2018, 00217–00284. [DOI] [PMC free article] [PubMed]
- 18.Eden JE e.a. Off-season RSV epidemics after easing of COVID-19 restrictions. Nat Commun. 2022;13(2884):2884. doi: 10.1038/s41467-022-30485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delestrain C e.a. Impact of COVID-19 social distancing on viral infection in France: a delayed outbreak of RSV. Pediatr Pulmonol. 2021;56(12):3669–3673. doi: 10.1002/ppul.25644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calderaro A., Canto F., Buttrini M., et al. Human respiratory viruses, including SARS-CoV-2, circulating in the winter season 2019-2020 in Parma, Northern Italy. Int J Infect Dis. 2021;102:79–84. doi: 10.1016/j.ijid.2020.09.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherman A.C., Babiker A., Seiben A., et al., The effect of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mitigation strategies on seasonal respiratory viruses: a tale of 2 large metropolitan centers in the United States, Clin Infect Dis, 72 (5), 2021, e154–e157. [DOI] [PMC free article] [PubMed]
- 22.Kuitunen I., Artama M., Makela L., et al., Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020, Pediatr Infect Dis J, 39 (12), 2020, e423–e427. [DOI] [PubMed]
- 23.Britton P.N., Hu N., Saravanos G., et al., COVID-19 public health measures and respiratory syncytial virus, Lancet Child Adolesc Health, 4 (11), 2020, e42–e43. [DOI] [PMC free article] [PubMed]
- 24.Yeoh D., Foley D., Minney-Smith C., et al. Impact of coronavirus disease 2019 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis. 2021;72(12):2199–2202. doi: 10.1093/cid/ciaa1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogilvie M., Vathenen A., Radford M., et al. Maternal antibody and respiratory syncytial virus infection in infancy. J Med Virol. 1981;7(4):263–271. doi: 10.1002/jmv.1890070403. [DOI] [PubMed] [Google Scholar]
- 26.Romo V. Children's hospitals grapple with a nationwide surge in RSV infections. 2022. https://www.npr.org/2022/10/24/1130764314/childrens-hospitals-rsv-sur Available from:
- 27.Nichols G.L. Coronavirus seasonality, respiratory infections and weather. BMC Infect Dis. 2021;21(1011):1101. doi: 10.1186/s12879-021-06785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ljubin-Sternak S e.a. Seasonal coronaviruses and other neglected respiratory viruses: a global perspective and a local snapshot. Front Public Health. 2021;9:691163. doi: 10.3389/fpubh.2021.691163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan W., Thoon K., Loo L., et al. Trends in respiratory virus infection during the COVID-19 pandemic in Singapore, 2020. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teo KW e.a. Rhinovirus persistence during the COVID-19 pandemic on pediatric acute wheezing presentations. J Med virology. 2022;94(11):5547–5552. doi: 10.1002/jmv.27986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groves H.E., Piche-Renaud P., Peci A., et al. The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada: a population-based study, Lancet Reg Health Am. 2021;1:100015. doi: 10.1016/j.lana.2021.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poole S., Brendish N.J., Clark T.W. SARS-CoV-2 has displaced other seasonal respiratory viruses: results from a prospective cohort study. J Infect. 2020;81(6):966–972. doi: 10.1016/j.jinf.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fong M.W., Gao H., Wong J., et al., Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings-social distancing measures, Emerg Infect Dis, 26 (5), 2020, 976–984. [DOI] [PMC free article] [PubMed]
- 34.Cowling B.J., Ng D., Ip D., et al. Community psychological and behavioral responses through the first wave of the 2009 influenza A(H1N1) pandemic in Hong Kong. J Infect Dis. 2010;202(6):867–876. doi: 10.1086/655811. [DOI] [PubMed] [Google Scholar]
- 35.Ali S., Cowling B., Lao E., et al., Mitigation of influenza B epidemic with school closures, Hong Kong, 2018, Emerg Infect Dis, 24 (11), 2018, 2071–2073. [DOI] [PMC free article] [PubMed]
- 36.Honein M., Christie A.M, Rose D., et al., Summary of guidance for public health strategies to address high levels of community transmission of SARS-CoV-2 and related deaths, december 2020, MMWR Morb Mortal Wkly Rep, 69 (49), 2020, 1860–1867. [DOI] [PMC free article] [PubMed]
- 37.Chu D., Akl E., Duda S., et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jefferson T., Del Mar C., Dooley L., et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2020;11:CD006207. doi: 10.1002/14651858.CD006207.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan KF M.T., Ip M.S., Ho P.L. Invasive pneumococcal disease, pneumococcal pneumonia and all-cause pneumonia in Hong Kong during the COVID-19 pandemic compared with the preceding 5 years: a retrospective observational study. BMJ Open. 2021;11(10):e055575. doi: 10.1136/bmjopen-2021-055575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crichton ML S.A., Chalmers J.D. The impact of the COVID-19 pandemic on exacerbations and symptoms in bronchiectasis: a prospective study. Am J Respir Crit Care Med. 2021;204:857–859. doi: 10.1164/rccm.202105-1137LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hawkins E., Fertal B., Muir S., et al., Adding eye protection to universal masking reduces COVID-19 among frontline emergency clinicians to the level of community spread, Am J Emerg Med, 46, 2020, 792–793. [DOI] [PMC free article] [PubMed]
- 42.Mermel L., Eye protection for preventing transmission of respiratory viral infections to healthcare workers, Infect Control Hosp Epidemiol, 39, 2018, 1387. [DOI] [PubMed]
- 43.Javanian M., Barary M., Ghebrehewat S., et al., A brief review of influenza virus infection, J Med Virol, 93 (8), 2021, 4638–4646. [DOI] [PubMed]
- 44.Khorramdelazad H., Kozemi M., Najafi A., et al. Immunopathological similarities between COVID-19 and influenza: investigating the consequences of Co-infection. Microb Pathog. 2021;152:104554. doi: 10.1016/j.micpath.2020.104554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y., Gayle A., Wilder-Smith A., et al., The reproductive number of COVID-19 is higher compared to SARS coronavirus, J Trav Med, 27 (2), 2020, taaa021. [DOI] [PMC free article] [PubMed]
- 46.Rahman A., Sathi N.J. Risk factors of the severity of COVID-19: a meta-analysis. Int J Clin Pract. 2021;75(7):e13916. doi: 10.1111/ijcp.13916. [DOI] [PubMed] [Google Scholar]
- 47.Hobbs A., Turner N., Omer I.,et al., Risk factors for mortality and progression to severe COVID-19 disease in the Southeast region in the United States: a report from the SEUS Study Group, Infect Control Hosp Epidemiol, 42 (12), 2021, 1464–1472. [DOI] [PMC free article] [PubMed]
- 48.Celewicz A., Celewicz M., Michalczyk M., et al., Pregnancy as a risk factor of severe COVID-19, J Clin Med, 10 (22), 2021, 5458. [DOI] [PMC free article] [PubMed]
- 49.Guan W., Ni Z., Hu Y., et al., Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med, 382 (18), 2020, 1708–1720. [DOI] [PMC free article] [PubMed]
- 50.Gao Z., Xu Y., Sun C., et al., A systematic review of asymptomatic infections with COVID-19, J Microbiol Immunol Infect, 54 (1), 2021, 12–16. [DOI] [PMC free article] [PubMed]
- 51.Esakandari H., Nabi-Afjadi M., Fakkari-Afjadi J., et al. A comprehensive review of COVID-19 characteristics. Biol Proced Online. 2020;22:19. doi: 10.1186/s12575-020-00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mehta P., McAuley D., Brown M., et al., COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet, 395 (10229), 2020, 1033–1034. [DOI] [PMC free article] [PubMed]
- 53.Mehandru S., Merad M. Pathological sequelae of long-haul COVID. Nat Immunol. 2022;23(2):194–202. doi: 10.1038/s41590-021-01104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biggerstaff M., Cauchemaz S., Reed C., et al. Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: a systematic review of the literature. BMC Infect Dis. 2014;14:480. doi: 10.1186/1471-2334-14-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Kerkhove M.D., Vandemaele K., Shinde V., et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. Plos Med. 2011;8(7) doi: 10.1371/journal.pmed.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mertz D., Kim T., Johnstone J., et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347:f5061. doi: 10.1136/bmj.f5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clark N.M., Lynch J.P., 3rd Influenza: epidemiology, clinical features, therapy, and prevention. Semin Respir Crit Care Med. 2011;32(4):373–392. doi: 10.1055/s-0031-1283278. [DOI] [PubMed] [Google Scholar]
- 58.Julkunen I., Melen K., Nyqvist M., et al., Inflammatory responses in influenza A virus infection, Vaccine, 19 (Suppl 1), 2000, S32–S37. [DOI] [PubMed]
- 59.Altamirano-Lagos M.J., Diaz F., Manseilla M.A., et al. Current animal models for understanding the pathology caused by respiratory syncytial virus. Front Microbiol. 2019;10:873. doi: 10.3389/fmicb.2019.00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Otomaru H., Kamigaki T., Tamaki R., et al. Transmission of respiratory syncytial virus among children under 5 Years in households of rural communities, the Philippines. Open Forum Infect Dis. 2016;6:ofz045. doi: 10.1093/ofid/ofz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weber A., Milligan P. Modeling epidemic caused by respiratory syncytial virus. Math Biosci. 2001;172:95–113. doi: 10.1016/s0025-5564(01)00066-9. [DOI] [PubMed] [Google Scholar]
- 62.Piroth L., Cottenet J., Marriet A-S., et al., Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study, Lancet Respir Med, 9 (3), 2021, 251–259. [DOI] [PMC free article] [PubMed]
- 63.Pormohammad A., Ghorbani S., Khatami A., et al., Comparison of influenza type A and B with COVID-19: a global systematic review and meta-analysis on clinical, laboratory and radiographic findings, Rev Med Virol, 31 (3), 2021, e2179. [DOI] [PMC free article] [PubMed]
- 64.Crotta S., Davidson S., Mahlakoiv T., et al. Type I and type III interferons drive redundant amplification loops to induce a transcriptional signature in influenza-infected airway epithelia. Plos Pathog. 2013;9(11) doi: 10.1371/journal.ppat.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okoli G.N., Otete H., Beck C., et al. Use of neuraminidase inhibitors for rapid containment of influenza: a systematic review and meta-analysis of individual and household transmission studies. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0113633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grijalva C.G., Feldstein L., Talbot H., et al., Influenza vaccine effectiveness for prevention of severe influenza-associated illness among adults in the United States, 2019-2020: a test-negative study, Clin Infect Dis, 73 (8), 2021, 1459–1468. [DOI] [PMC free article] [PubMed]
- 67.Tenforde M.W., Talbot H., Trabue C., et al., Influenza vaccine effectiveness against hospitalization in the United States, 2019-2020, J Infect Dis, 224 (5), 2021, 813–820. [DOI] [PMC free article] [PubMed]
- 68.Candelli M., Pignataro G., Torelli E., et al., Effect of influenza vaccine on COVID-19 mortality: a retrospective study, Intern Emerg Med, 16 (7), 2021, 1849–1855. [DOI] [PMC free article] [PubMed]
- 69.Fiore A.E., Bridges C.B., Cox N.J. Seasonal influenza vaccines. Curr Top Microbiol Immunol. 2009;333:43–82. doi: 10.1007/978-3-540-92165-3_3. [DOI] [PubMed] [Google Scholar]
- 70.Mascellino M.T., Timoteo F., Angelis M., et al. Overview of the main anti-SARS-CoV-2 vaccines: mechanism of action, efficacy and safety. Infect Drug Resist. 2021;14:3459–3476. doi: 10.2147/IDR.S315727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.MD J. Mechanism of action of the janssen COVID-19 vaccine. 2022. https://www.janssenmd.com/janssen-covid19-vaccine/pharmacology/mechanism-of-action/mechanism-of-action-of-the-janssen-covid19-vaccine June 29, 2022]; Available from:
- 72.Chong W.H., Saha B.K., Medarov B.I. A systematic review and meta-analysis comparing the clinical characteristics and outcomes of COVID-19 and influenza patients on ECMO. Respir Investig. 2021;59(6):748–756. doi: 10.1016/j.resinv.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taquet M., Dercon Q., Luciano S., et al., Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19, Plos Med, 18 (9), 2021, e1003773. [DOI] [PMC free article] [PubMed]
- 74.Dadashi M., Khaleghnejad S., Elkhichi P., et al. COVID-19 and influenza Co-infection: a systematic review and meta-analysis. Front Med (Lausanne) 2021;8:681469. doi: 10.3389/fmed.2021.681469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dao T.L., Hoang V., Colson P., et al., Co-infection of SARS-CoV-2 and influenza viruses: a systematic review and meta-analysis, J Clin Virol Plus, 1 (3), 2021, 100036. [DOI] [PMC free article] [PubMed]
- 76.Wu Q., Xing Y., Shi L., et al., Coinfection and other clinical characteristics of COVID-19 in children, Pediatrics, 146 (1), 2020, e20200961. [DOI] [PubMed]
- 77.Schweitzer K.S., Crue T., Nall J., et al. Influenza virus infection increases ACE2 expression and shedding in human small airway epithelial cells. Eur Respir J. 2021;58(1):2003988. doi: 10.1183/13993003.03988-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lai Y., Han T., Lao Z., et al. Phillyrin for COVID-19 and influenza Co-infection: a potential therapeutic strategy targeting host based on bioinformatics analysis. Front Pharmacol. 2021;12:754241. doi: 10.3389/fphar.2021.754241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rynda-Apple A., Robinson K.M., Alcorn J.F. Influenza and bacterial superinfection: illuminating the immunologic mechanisms of disease. Infect Immun. 2015;83(10):3764–3770. doi: 10.1128/IAI.00298-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brady M., Byington C., Davies D., et al. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:e620–e638. doi: 10.1542/peds.2014-1666. [DOI] [PubMed] [Google Scholar]
- 81.Brand S., Munywoki P., Walumbe D., et al. Reducing respiratory syncytial virus hospitalization in a lower-income country by vaccinating mothers-to-be and their households. eLife. 2020;9:e47003. doi: 10.7554/eLife.47003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang M., Li Y., Chen X., et al., Co-Infection with common respiratory pathogens and SARS-CoV-2 in patients with COVID-19 pneumonia and laboratory biochemistry findings: a retrospective cross-sectional study of 78 patients from a single center in China, Med Sci Monit, 27, 2021, e929783-1-e929783-8. [DOI] [PMC free article] [PubMed]
- 83.Zandi M., Soltani S., Fani M., et al. Severe acute respiratory syndrome coronavirus 2 and respiratory syncytial virus coinfection in children. Osong Public Health Res Perspect. 2021;12:286–292. doi: 10.24171/j.phrp.2021.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang M.L., Li Y., Chen X., et al. Co-infection with common respiratory pathogens and SARS-CoV-2 in patients with COVID-19 pneumonia and laboratory biochemistry findings: a retrospective cross-sectional study of 78 patients from a single center in China. Med Sci Monit. 2021;27 doi: 10.12659/MSM.929783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alvares P.A. SARS-CoV-2 and respiratory syncytial virus coinfection in hospitalized pediatric patients. Pediatr Infect Dis J. 2021;40(4):e164–e166. doi: 10.1097/INF.0000000000003057. [DOI] [PubMed] [Google Scholar]
- 86.Chekuri S., Szymczak W., Goldstein D., et al., SARS-CoV-2 coinfection with additional respiratory virus does not predict severe disease: a retrospective cohort study, J Antimicrob Chemother, 76 (Supplement_3), 2021, iii12-iii19. [DOI] [PMC free article] [PubMed]
- 87.Ma L., Wang W., Le Grange J., et al. Coinfection of SARS-CoV-2 and other respiratory pathogens. Infect Drug Resist. 2020;13:3045–3053. doi: 10.2147/IDR.S267238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goldberg E.M., Hasegawa K., Lawrence A., et al., Viral coinfection is associated with improved outcomes in emergency department patients with SARS-CoV-2, West J Emerg Med, 22 (6), 2021, 1262–1269. [DOI] [PMC free article] [PubMed]
- 89.Chen T., Song J., Liu H., et al., Positive Epstein-Barr virus detection in coronavirus disease 2019 (COVID-19) patients, Sci Rep, 11 (1), 2021, 10902. [DOI] [PMC free article] [PubMed]
- 90.Nadeem A., Suresh K., Awais H., et al. Epstein-barr virus coinfection in COVID-19. J Investig Med High Impact Case Rep. 2021;9 doi: 10.1177/23247096211040626. 23247096211040626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meng M., Zhang S., Dong X., et al., COVID-19 associated EBV reactivation and effects of ganciclovir treatment, Immun Inflamm Dis, 10 (4), 2022, e597. [DOI] [PMC free article] [PubMed]
- 92.Gold J.E., Okyay R., Licht W., et al., Investigation of long COVID prevalence and its relationship to epstein-barr virus reactivation, Pathogens, 10 (6), 2021, 763. [DOI] [PMC free article] [PubMed]
- 93.Nunn A., Guy G., Botchway S., et al., SARS-CoV-2 and EBV; the cost of a second mitochondrial “whammy”?, Immun Ageing, 18 (1), 2021, 40. [DOI] [PMC free article] [PubMed]
- 94.Amaral P., Ferraira B., Roll S., et al. COVID-19 and cytomegalovirus Co-infection: a challenging case of a critically ill patient with gastrointestinal symptoms. Eur J Case Rep Intern Med. 2020;7(10):001911. doi: 10.12890/2020_001911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gatto I., Biagioni E., Coloretti I., et al., Cytomegalovirus blood reactivation in COVID-19 critically ill patients: risk factors and impact on mortality, Intensive Care Med, 48 (6), 2022, 706–713. [DOI] [PMC free article] [PubMed]
- 96.Naendrup J., Borrega J., Eichenauer D., et al. Reactivation of EBV and CMV in severe COVID-19 epiphenomena or trigger of hyperinflammation in need of treatment? A large case series of critically ill patients. J Intensive Care Med. 2021;37(9):1152–1158. doi: 10.1177/08850666211053990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paolucci S., Cassaniti I., Novazzi F., et al., EBV DNA increase in COVID-19 patients with impaired lymphocyte subpopulation count, Int J Infect Dis, 104, 2021, 315–319. [DOI] [PMC free article] [PubMed]
- 98.Pluss M., Mese K., Kowallick J., et al. Case report: cytomegalovirus reactivation and pericarditis following ChAdOx1 nCoV-19 vaccination against SARS-CoV-2. Front Immunol. 2021;12:784145. doi: 10.3389/fimmu.2021.784145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Katsikas Triantafyllidis K., Giannos P., Mian I., et al., Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports, Vaccines (Basel), 9 (9), 2021, 1013. [DOI] [PMC free article] [PubMed]
- 100.Munasinghe B., Fernando U., Mathurageethan M., et al. Reactivation of varicella-zoster virus following mRNA COVID-19 vaccination in a patient with moderately differentiated adenocarcinoma of rectum: a case report. SAGE Open Med Case Rep. 2022;10 doi: 10.1177/2050313X221077737. 2050313X221077737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abu-Rumeileh S., Mayer B., Still V., et al., Varicella zoster virus-induced neurological disease after COVID-19 vaccination: a retrospective monocentric study, J Neurol, 269 (4), 2022, 1751–1757. [DOI] [PMC free article] [PubMed]
- 102.Buranasakda M., Kotruchin P., Phanthachai K., et al. Varicella zoster meningitis following COVID-19 vaccination: a report of two cases. Int J Infect Dis. 2022;119:214–216. doi: 10.1016/j.ijid.2022.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Touzard-Romo F.,T.C., Lonks J.R. Co-infection with SARS-CoV-2 and human metapneumovirus. R Med J. 2020;103(6):23–24. [PubMed] [Google Scholar]
- 104.He H., Liao C., Wang R., et al. Co-infection with SARS-CoV-2 and parainfluenza virus in a hemodialysis patient: a Case report. Clin Nephrol. 2020;94:207–211. doi: 10.5414/CN110164. [DOI] [PubMed] [Google Scholar]
- 105.Swets M., Russell C., Harrison E., et al. SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet. 2022;399(10334):1463–1464. doi: 10.1016/S0140-6736(22)00383-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Motta JC G.C. Adenovirus and novel coronavirus (SARS-Cov2) coinfection: a case report. ID Cases. 2020;22:e00936. doi: 10.1016/j.idcr.2020.e00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morens DM T.J., Fauci A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198(7):962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Roquilly A., Torres A., Villadangos J.A., et al., Pathophysiological role of respiratory dysbiosis in hospital-acquired pneumonia, Lancet Respir Med, 7 (8), 2019, 710–720. [DOI] [PubMed]
- 109.Howard L. Is there an association between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and Streptococcus pneumoniae? Clin Infect Dis. 2021;72(5):e76–e78. doi: 10.1093/cid/ciaa1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sweere J.,Van Bellenghem H.J., Ishak H., et al.,Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection, Science, 363, (6434), 2019,eaat9691. [DOI] [PMC free article] [PubMed]
- 111.Boumaza A., Gay L., Mezouar S., et al., Monocytes and macrophages, targets of severe acute respiratory syndrome coronavirus 2: the clue for coronavirus disease 2019 immunoparalysis, J Infect Dis, 224 (3), 2019, 395–406. [DOI] [PMC free article] [PubMed]
- 112.Metlay J., Waterer G., Long A., et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jain S., Self W., Wunderink R., et al. CDC EPIC Study Team Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lehmann C., Pho M., Lehmann C., Pitrak D., et al. Community-acquired coinfection in coronavirus disease 2019: a retrospective observational experience. Clin Infect Dis. 2021;72(8):1450–1452. doi: 10.1093/cid/ciaa902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Amin-Chowdhury Z., Aiano F., Mensah A., et al., Impact of the coronavirus disease 2019 (COVID-19) pandemic on invasive pneumococcal disease and risk of pneumococcal coinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): prospective national cohort study, England, Clin Infect Dis, 72 (5), 2021, e65–e75. [DOI] [PMC free article] [PubMed]
- 116.Relph K., Russel C., Fairfield C., et al. International severe acute respiratory and emerging infections consortium coronavirus clinical characterization consortium (ISARIC4C) investigators. Procalcitonin is not a reliable biomarker of bacterial coinfection in people with coronavirus disease 2019 undergoing microbiological investigation at the time of hospital admission. Open Forum Infect Dis. 2022;9(5):ofac179. doi: 10.1093/ofid/ofac179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hani C., Trieu N., Saab I., et al., COVID-19 pneumonia: a review of typical CT findings and differential diagnosis, Diagn Interv Imaging, 101 (5), 2020, 263–268. [DOI] [PMC free article] [PubMed]
- 118.Bhimraj A. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Infect Dis Soc America. 2022:ciaa478. doi: 10.1093/cid/ciaa478. Version 9.0.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ryder J, Kalil A. The puzzles of ventilator-associated pneumonia and COVID-19: absolute knowns and relative unknowns. Crit Care Med. 2022;50(5):894–896. doi: 10.1097/CCM.0000000000005475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kalil A., Metersky M., Klompas M., et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the american thoracic society. Clin Infect Dis. 2017;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clancy C., Schwartz I., Kula B., et al., Bacterial superinfections among persons with coronavirus disease 2019: a comprehensive review of data from postmortem studies, Open Forum Infect Dis, 8 (Suppl 1), 2021, 296. [DOI] [PMC free article] [PubMed]
- 122.Castañeda-Méndez P., Cabrera-Ruiz M., Barragán-Reyes A., et al. Epidemiologic and microbiologic characteristics of hospital-acquired infections in patients with COVID-19 at intensive care unit, Mexico City. Open Forum Infect Dis. 2021;8:296. [Google Scholar]
- 123.Pickens C.O., Gao C., Cuttica M.J., et al., Bacterial superinfection pneumonia in patients mechanically ventilated for COVID-19 pneumonia, Am J Respir Crit Care Med, 204 (8), 2021, 921–932. [DOI] [PMC free article] [PubMed]
- 124.Vacheron C., Lepape A., Savey A., et al. Attributable mortality of ventilator-associated pneumonia among COVID-19 patients. Am J Respir Crit Care Med. 2022;206(2):161–169. doi: 10.1164/rccm.202202-0357OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Povoa P M.-L.I., Nseir S. Secondary pneumonias in critically ill patients with COVID-19: risk factors and outcomes. Curr Opin Crit Care. 2021;27:468–473. doi: 10.1097/MCC.0000000000000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kalil AC Cawcutt K. Is ventilator-associated pneumonia more frequent in patients with coronavirus disease 2019? Crit Care Med. 2022;50(3):522–524. doi: 10.1097/CCM.0000000000005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Richards O., Pallman P., King C., et al. Procalcitonin increase is associated with the development of critical care-acquired infections in COVID-19 ARDS. Antibiotics (Basel) 2021;10(11):1425. doi: 10.3390/antibiotics10111425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gago J., Filardo T., Conderino S., et al. Pathogen species is associated with mortality in nosocomial bloodstream infection in patients with COVID-19. Crit Care Med. 2022;9:ofac083. doi: 10.1093/ofid/ofac083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Silva D.L., Lima C.,Magalhães V.,et al., Fungal and bacterial coinfections increase mortality of severely ill COVID-19 patients, J Hosp Infect, 113, 2021, 145–154. [DOI] [PMC free article] [PubMed]
- 130.Bhatt P.J., Shiau S., Brunetti L., et al. Risk factors and outcomes of hospitalized patients with severe coronavirus disease 2019 (COVID-19) and secondary bloodstream infections: a multicenter case-control study. Clin Infect Dis. 2021;72:e995–e1003. doi: 10.1093/cid/ciaa1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kula B.E., Clancy C., Hong Nguyen M., et al. Invasive mould disease in fatal COVID-19: a systematic review of autopsies. Lancet Microbe. 2021;2:e405–e414. doi: 10.1016/S2666-5247(21)00091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Clancy C.J., Hong Nguyen M. Coronavirus disease 2019-associated pulmonary aspergillosis: reframing the debate. Open Forum Infect Dis. 2022;9:ofac081. doi: 10.1093/ofid/ofac081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bartoletti M., Pascale R., Cricca M., et al. PREDICO Study Group (2021), Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID-19: a prospective study. Clin Infect Dis. 2021;73:e3606–e3614. doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koehler P., Cornerly O., Böttiger B.W., et al. COVID-19 associated pulmonary aspergillosis. Mycoses. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.White P., Dhillon R., Cordey A., et al. A national strategy to diagnose coronavirus disease 2019-associated invasive fungal disease in the intensive care unit. Clin Infect Dis. 2021;73:e1634–e1644. doi: 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Permpalung N., Po-Yu Chiang T., Massie A., et al., Coronavirus disease 2019-associated pulmonary aspergillosis in mechanically ventilated patients, Clin Infect Dis, 74, 2022, 83–91. [DOI] [PMC free article] [PubMed]
- 137.Patterson T., Thompson G., Denning D.W., et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;63:e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Patel A., Agarwal R., Rudramurthy S.M., et al. MucoCovi Network3, Multicenter epidemiologic study of coronavirus disease-associated mucormycosis. Emerg Infect Dis. 2021;27:2349–2359. doi: 10.3201/eid2709.210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Narayanan S., Chua J., Baddley J.W. Coronavirus disease 2019-associated mucormycosis: risk factors and mechanisms of disease. Clin Infect Dis. 2022;74:1279–1283. doi: 10.1093/cid/ciab726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Farmakiotis D. Mucormycoses. Infect Dis Clin North Am. 2016;30:143–163. doi: 10.1016/j.idc.2015.10.011. [DOI] [PubMed] [Google Scholar]