Abstract
An important design aspect of electronic cigarettes (“e-cigarettes”) is the nature of the acid/base chemistry in the e-liquid phase. E-liquids having formulations similar to those of early products are mixes of propylene glycol/glycerol (PG/GL) plus free-base (fb) nicotine and (usually) flavor chemicals that are either rather weak or non-acid/base actors in PG/GL. The fraction of nicotine in the fb form is denoted (αfb)e-liquid, with a possible range of 0 < (αfb)e-liquid < 1. For e-liquids of an early design, (αfb)e-liquid ≈ 1. Because e-cigarette aerosols high in fb nicotine are harsh upon inhalation, many commercial e-liquids now also contain variable levels of an acid additive (e.g., benzoic acid, levulinic acid, etc.) to protonate the nicotine and form dissolved “nicotine salts”: (αfb)e-liquid values significantly less than 1 are now common. A framework is developed for predicting αfb values in a given medium based on the following: (1) acid/nicotine ratios and (2) overall acid + nicotine protonation constant (Koa) values. This framework is required for understanding (1) e-liquid design in regard to how acid additives affect (αfb)e-liquid values, and (2) why (αfb)e-liquid values cannot, in general, be measured by any method that involves significant dilution with water.
Graphical Abstract

INTRODUCTION
Electronic cigarette (“e-cigarette”) use has increased greatly during the past decade.1 Because nicotine is an alkaloid, its behavior in an e-cigarette system and its sensory propeties in the resulting e-cigarette aerosol are affected by the acid/base characteristics of the “e-liquid” used. There is a need to understand nicotine protonation chemistry in e-liquids and their associated aerosol liquids;2 measurement efforts have begun.3–8
Two distinctly different but complementary approaches for probing the acid/base chemistry of e-liquids and their aerosols have emerged: (1) native solution methods (NSMs) and (2) diluted solution methods (DSMs). The explicit goal of NSMs is to understand the acid/base chemistry of an unaltered e-liquid or the associated aerosol liquid. NSMs are best capable of examining the connection between product design and the immediate aspects of nicotine delivery to the user.3,4 In contrast, DSMs cannot (by definition) directly access the state of the acid/base chemistry of nicotine (or other constiutents) in a native e-liquid or aerosol liquid phase. However, if the dilution water is free of dissolved CO23 then DSMs will allow the study of the composition of a given e-liquid mix. For example, a 1:10 dilution of an e-liquid is mostly (~90%) water, so measurement with a glass pH electrode will provide a good approximation of the pH that would be present in a solution of the same resultant mix of acids and nicotine in ~100% water, wherein the needed equilibrium constants are known. In particular, for acids and protonated nicotine, tabulated pKa (= −log Ka) values in water can then be used to help work out the identities and levels of the acids and nicotine present. To summarize, while the results from a CO2-excluded DSM for a given e-liquid must not be overinterpreted, those results will provide a valuable characterization for the e-liquid.
Nicotine is a dibasic alkaloid. Whether in propylene glycol (PG), glycerol (GL), PG/GL, diluted PG/GL, or 100% water, nicotine can exist in three forms (Figure 1), including (1) free-base (fb, also known as unprotonated or bound), (2) monoprotonated (mp), and (3) diprotonated (dp). Gaseous nicotine is solely in the fb form. In a liquid phase, the fraction of the nicotine that is in the fb form is denoted αfb:2–4,9
| (1) |
Figure 1.

Nicotine in its three forms: diprotonated (dp), monoprotonated (mp), and unprotonated/free-base (fb).
The dp species can be important when (1) the level of protonating acid (eq/L) is greater than the level of nicotine (eq/L), and (2) diprotonation is sufficiently favorable in the medium of interest.
The nicotine protonation degree affects nicotine aerosol inhalation harshness3,4,10 and can also affect the uptake kinetics of aerosolized nicotine.10 In the absence of a protonating acid, (αfb)e-liquid = 1, as in early style PG/GL + nicotine e-liquids.11 At high-nicotine levels such as ~60 mg/mL, (αfb)e-liquid ≈ 1 gives an aerosol that is harsh upon inhalation;3,4,10 adding an acid will lower (αfb)e-liquid and moderate harshness. Currently, important carboxylic acids used in e-liquids include benzoic acid and levulinic acid.4,12 Flavor chemical phenols like vanillin and ethyl vanillin are also acidic; however, these are much weaker in both water and PG/GL than carboxylic acids.4 Vanillin and ethyl vanillin have been found at levels as high as 1 to 3% in multiple e-liquid products.13,14 No other common flavor chemical ingredients are acids nor is vitamin E acetate, as used in vaping cannabis products.15,16 However, the degradation of ingredients during vaping may produce levels of acids that are relevant for protonation of nicotine, as illustrated by the degradation of sucralose to HCl and other products.11
Duell et al. (2019)4 discuss how the incorporation of acids in e-cigarette liquids is a “déjà vu” reprise of the shift, in the mid-1800s for smoked tobacco, from air-cured tobacco to flue-cured tobacco. While the exceedingly complex nature of the liquid matter comprising tobacco smoke particulate material (PM) precludes any easy mathematical framework for predicting αfb values for such PM, e-liquids are much simpler making such a framework possible. Here, we combine acid/base chemical principles with recent data on nicotine protonation constants in PG/GL4 to develop and apply that framework.
RESULTS
General Considerations.
The medium- and temperature-dependent nicotine acidity equilibria are
| (2) |
| (3) |
Each bracketed term here is a molar concentration and not a chemical activity: all K values here are medium-specific equilibrium constants, as with cK values used in solution chemistry.17 Manipulation of eq 1 gives
| (4) |
wherein
| (5) |
and
| (6) |
If can be neglected
| (7) |
Ka values in 100% water are known for acids relevant here (e.g., see Table 1). The measurement of [H+] values in aqueous solutions is straightforward, and in ~100% water, [H+] = 10−pH. In non-aqueous solvents, Ka values are not generally known, and the measurement of [H+] is difficult. Fortunately, as discussed by Duell et al.4 and elaborated below, only the overall nicotine protonation constants (and not individual Ka values) are needed to predict αfb values.
Table 1.
pKa Values for Acids in 100% Water (Low Ionic Strength)
Because αfb in a solution mixture depends on the exact nature of the solution, the determination of a given (αfb)e-liquid value must proceed by a NSM, as by 1H nuclear magnetic resonance (1H NMR) spectroscopy.3,4 In contrast, a DSM (e.g., with 1:10 dilution)5,7,8 will unequivocally alter the solution αfb. Indeed, the fact that solution thermodynamics is affected by the solvent is embodied in the prerequisite that dilution with water be carried out if there is to be an unequivocal interpretation of a pH measurement using a water-calibrated electrode. Defending DSMs, Gholap et al.8 state, “…it can be argued that the purpose of the dilution approach is to provide a pH relevant scale for classifying e-liquids based on their free base nicotine yields.” This is true only when the focus is the level of free-base nicotine in respiratory tract fluids after the inhaled e-cigarette aerosol droplets have been deposited and diluted. It is not true if the focus is either the immediate user perceptions of the inhaled nicotine aerosol or the early stages in the processes of nicotine deposition from the inhaled aerosol.10
DSMs with water-calibrated pH measurements have proceeded based on the “Henderson–Hasselbalch” equation (HHE) for the determination of the [Nic]/[NicH+] ratio for use in eq 7. The derivation of the HHE equation is simple. As used here, taking the log of both sides of eq 5 for water gives the HHE for nicotine in 100% water:
| (8) |
| (9) |
As noted above, for a DSM with 1:10 dilution,5,7 it is reasonable to assume that neither the water-based calibration of the pH electrode response nor the value of is substantially affected by the remaining (~10%) PG/GL. That is, the [Nic]/[NicH+] ratio in a 1:10 dilution can be estimated based on the response of a water-calibrated pH electrode and the water-based value of :
| (10) |
However, as suggested above, it is fatally problematic to assume further that the right hand side (RHS) of eq 10 gives [Nic]/[NicH+] in the e-liquid. The validity of that circumstance would require that both the pH calibration and the value of are the same for water as for ~100% PG/GL or at least that . As a general matter, this will certainly not be the case: as shown below, pKa values are greatly affected by the nature of the solvent, and changing the pKa can greatly alter nicotine protonation. Some special stoichiometric circumstances do allow (αfb)water (1:10) ≈ (αfb)e-liquid: see Table 2.
Table 2.
Special Circumstances Allowing (αfb)water (1:10) ≈ (αfb)e-liquida
| case 1 | no protonating acid is present in the e-liquid, so (αfb)e-liquid = 1, and the nicotine concentration after dilution is high enough to yield a relatively high aqueous pH, giving (αfb)water (1:10) ≈ 1 |
| case 2 | a protonating acid is present in the e-liquid and is sufficiently strong to react stoichiometrically with free-base nicotine in both the e-liquid and in water |
| 2a | (eq/L acid/eq/L nicotine) = y < 1: (αfb)e-liquid ≈ (αfb)water (1:10) ≈ 1 − y |
| 2b | (eq/L acid/eq/L nicotine) ≥ 1: (αfb)e-liquid ≈ (αfb)water (1:10) ≈ 0 |
Assumes the use of CO2-free water.
Protonation by a Single Monoprotic Acid HA: General Equations.
For HA
| (11) |
The first overall nicotine protonation reaction is4
| (12) |
The second overall protonation reaction is
| (13) |
Because Debye–Hückel effects can favor ion formation, both and can increase with increasing total ion concentration; this has likely been observed for .4 Comparisons of the protonation chemistry of nicotine in any given solution type by different acids (or in different solution types by the same acid) should be done using K values for the same temperature. The three temperatures relevant here are ~20 °C (lab-bench), 25 °C (many tabulated K values), and 37 °C (the human body).
On the basis of eq 12 and the pKa values in Table 1, values for Koa,1 in water are given in Table 3 for benzoic acid and vanillin. While and values in PG/GL mixtures are not known, Duell et al.4 measured (αfb)e-liquid values at 40 °C (≈ 37 °C), and these were used (with the assumption that could be neglected) to estimate the Koa,1 values in PG/GL for benzoic acid and vanillin at 40 °C (≈ 37 °C). They are much smaller than the corresponding values in water. For benzoic acid, for a 43/57 mol % mixture of PG/GL, Koa,1 = 101.41. In JUUL product e-liquids (approximated as ~31/69 wt % PG/GL or ~35/65 mol % PG/GL), on average, Koa,1 = 101.81. It is clear that nicotine protonation by benzoic acid is much more favored in water than in PG/GL, which is why dilution with water creates enormous problems when seeking to determine (αfb)e-liquid values: (αfb)water (1:10) values obtained by DSM methods will be too low (except under the circumstances in Table 2).
Table 3.
Log Koa,1 Values for Nicotine Protonation by Benzoic Acid and Vanillin in ~100% Water and in Propylene Glycol/Glycerol Solutions
In a non-aqueous Nic + HA system, there are six concentration unknowns: [H+], [HA], [A−], [Nic], [NicH+], and . Ion formation by autoprotolysis of PG and GL is assumed to be negligible. When HA is a weak acid (benzoic acid, levulinic acid, acetic acid, etc.), [H+] can generally be neglected in the electroneutrality equation (ENE) for the solution, especially in PG/GL, leaving five unknowns. The five equations needed for solving for the speciation values are then (1) the ENE, (2) CHA = total added HA concentration (mol/L), (3) CNic = total added Nic concentration (mol/L), (4) the expression for Koa,1, and (5) the expression for Koa,2.
With [H+] neglected, the general ENE is
| (14) |
By definition
| (15) |
Similarly
| (16) |
Eq 14 can then be represented as
| (17) |
| (18) |
| (19) |
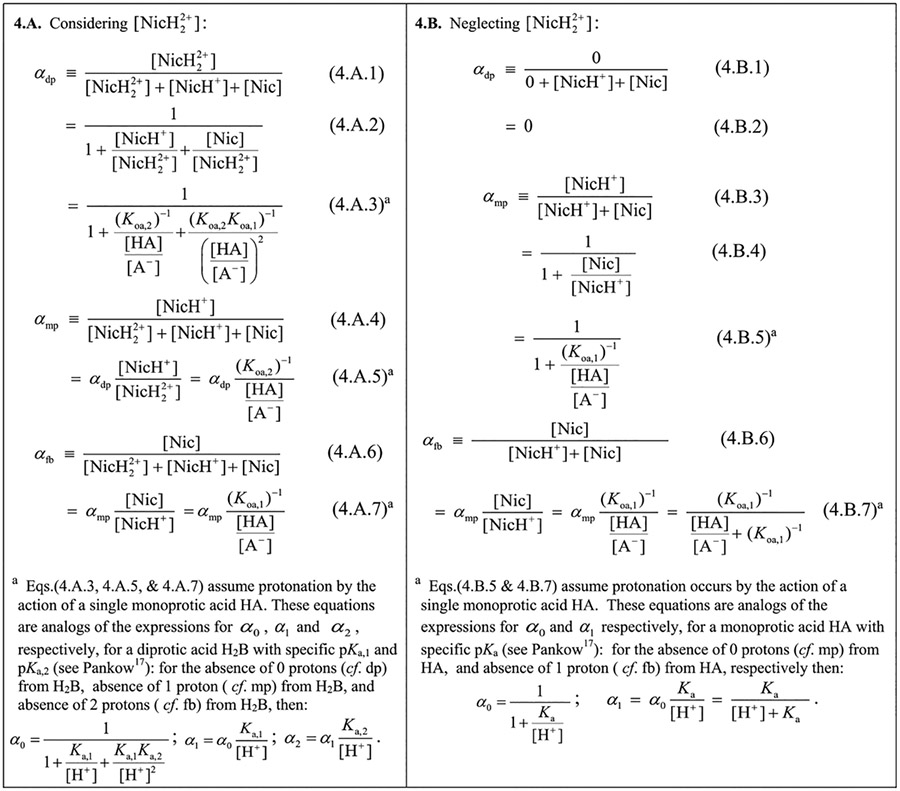
Since αdp and αmp (and αfb) are available as functions of [HA]/[A−] (Table 4), then with input values of CHA, CNic, Koa,1, and Koa,2, a numerical solution of eq 19 for the root value of [HA]/[A−] can be obtained; the root value is then used to evaluate αdp, αmp, and αfb. The numerical solution can be achieved using a spreadsheet optimization solver that varies log([HA]/[A−]) to find LHS – RHS = 0 for eq 19. This is analogous to varying pH = −log [H+] in the ENE of an acid/base problem.17 Varying log([HA]/[A−]) rather than [HA]/[A−] itself facilitates the numerical process because it speeds the search for the root and automatically excludes negative values of [HA]/[A−].
Table 4.
Definitions and Expressions for αdp, αmp, and αfb for Nicotine
|
Neglecting .
When is neglected, eq 14 reduces to
| (20) |
This approximation permits the quadratic solution for αfb given in Table 5, as obtained by Duell et al.4 In terms of a mathematical solution based on the ratio [HA]/[A−], the solution can equivalently be obtained by writing eq 20 as
| (21) |
so that
| (22) |
The solution proceeds as given in Table 6.
Table 5.
Equation for αfb in a Solution Containing Nicotine and a Single Monoprotic Acid HA when Neglecting a

|
From Duell et al.;4 CHA/CNic = molar concentration ratio of HA to nicotine.
Table 6.
Development of an [HA]/[A−]-Based Equation for αfb in a Solution Containing Nicotine and a Single Monoprotic Acid HA when Neglecting a

|
CHA/CNic = molar concentration ratio.
Protonation by Two Monoprotic Acids, HA and HA′.
When two monoprotic acids are present, the general ENE (neglecting [H+]) is
| (23) |
| (24) |
For HA′
| (25) |
As one pair, HA and HA′ might be benzoic acid and levulinic acid, respectively. The proton exchange reaction between the A and A′ systems is
| (26) |
where f is the strength factor for HA′ over HA. Then
| (27) |
Equation 24 becomes
| (28) |
| (29) |
If the two monoprotic acids have similar strengths (f ≈ 1) then the two acids can be lumped as one with a concentration of CHA + CHA′. In any case, the Table 4 equations for αdp, αmp, and αfb remain the same. Knowing Koa,1 and Koa,2 for HA along with f fully reveals how well HA′ can protonate nicotine: the value of f and the values of Koa,1 and Koa,2 for HA can be used to obtain the values of Koa,1 and Koa,2 for HA′.
αfb as a Function of Log Koa,1 and CHA/CNic.
Figure 1 provides isopleths of αfb in a log Koa,1 versus CHA/CNic format for the case of a single monoprotic acid. The calculations were made by assuming Koa,2 = Koa,1/105 because in water is ~105 and is likely of a similar magnitude in PG/GL mixtures (, so the reaction Nic + H+ = NicH+ is more inherently favored than ). is negligible for all areas where αfb ≥ 0.01; for the isopleths shown, αdp is largest along the αfb = 0.01 isopleth, yet αdp is only 0.00098. Along αfb = 0.1, αdp= 0.00008. At the upper right corner (log Koa,1 = 4 and CHA/CNic = 3), αdp = 0.14, αmp = 0.86, and αfb = 0.00005.
Differences Between (αfb)e-liquid and (αfb)water (1:10).
For 10 JUUL products, Pankow et al.18 and Duell et al.4 found the dominant acid to be benzoic acid with a benzoic acid/nicotine ratio of ~1 (i.e., CHA/CNic ≈ 1). For JUUL products, logKoa,1 ≈ 1.8 (40 °C),4 giving (αfb)e-liquid ≈ 0.1 and the point BAJUUL in Figure 2. However, after a 1:10 dilution with water, with CHA/CNic remaining at ~1, Koa,1 now moves to ~103.45, giving (αfb)water (1:10) ≈ 0.02 (and point BAw): the analytical error from presuming (αfb)water (1:10) = (αfb)e-liquid with JUUL products is large.
Figure 2.

Isopleths of αfb in a log Koa,1 vs CHA/CNic format assuming Koa,2 = Koa,1/105. Additional points: 10 JUUL e-liquid data points (40 °C) from Duell et al.;4 BAJUUL = average (40 °C) for benzoic acid + nicotine in JUUL e-liquid at CHA/CNic = 1; BAw = benzoic acid + nicotine in water-diluted e-liquid at CHA/CNic = 1, assuming dilution at 37 °C with CO2-free water; Vanilline = vanillin + nicotine in ~1:1 propylene glycol (PG) and glycerol (GL) at CHA/CNic = 1 (40 °C); and Vanillinw = vanillin + nicotine in water-diluted e-liquid at CHA/CNic = 1, assuming dilution at 37 °C with CO2-free water.
For vanillin, Koa,1 in 50/50 mol % (43/57 wt % PG/GL) PG/GL has been measured to be ~10−2.07 (40 °C).4 In a 50/50 PG/GL e-liquid with CHA/CNic = 1.0 for the case of vanillin, (αfb)e-liquid ≈ 0.92 at the point Vanilline (Figure 2). After a 1:10 dilution with water (with CHA/CNic again remaining at ~1), Koa,1 moves to ~100.38 so that (αfb)water (1:10) ≈ 0.39 at the point Vanillinw: the error from presuming (αfb)water (1:10) = (αfb)e-liquid is again large.
CONCLUSIONS
The comprehensive framework for predicting (αfb)e-liquid values developed here will be useful in understanding (1) how the manipulation of e-liquid acid/base chemistry can alter consumer perceptions, (2) the chemistry underlying any associated regulatory actions, and (3) the necessity for using native solution methods (NSMs) when determining (αfb)e-liquid values.
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health, Grant R01ES025257. Research reported was supported by the NIEHS and FDA Center for Tobacco Products (CTP). The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the FDA.
Footnotes
The authors declare no competing financial interest.
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.chemrestox.0c00008
Contributor Information
James F. Pankow, Department of Chemistry Portland and Department of Civil and Environmental Engineering Portland, State University Portland, Oregon 97207-0751, United States;
Anna K. Duell, Department of Chemistry Portland, State University Portland, Oregon 97207-0751, United States;
David H. Peyton, Department of Chemistry Portland, State University Portland, Oregon 97207-0751, United States;
REFERENCES
- (1).Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, and King BA (2018) Notes from the Field: Use of Electronic Cigarettes and Any Tobacco Product Among Middle and High School Students - United States, 2011–2018. MMWR Morb Mortal Wkly Rep 67 (45), 1276–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Pankow JF (2017) Calculating Compound Dependent Gas-Droplet Distributions in Aerosols of Propylene Glycol and Glycerol from Electronic Cigarettes. J. Aerosol Sci 107, 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Duell AK, Pankow JF, and Peyton DH (2018) Free-Base Nicotine Determination in Electronic Cigarette Liquids by (1)H NMR Spectroscopy. Chem. Res. Toxicol 31 (6), 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Duell AK, Pankow JF, and Peyton DH (2019) Nicotine in tobacco product aerosols: “It’s déjà vu all over again”. Tob Control, 055275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).El-Hellani A, El-Hage R, Baalbaki R, Salman R, Talih S, Shihadeh A, and Saliba NA (2015) Free-Base and Protonated Nicotine in Electronic Cigarette Liquids and Aerosols. Chem. Res. Toxicol 28 (8), 1532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lisko JG, Tran H, Stanfill SB, Blount BC, and Watson CH (2015) Chemical Composition and Evaluation of Nicotine, Tobacco Alkaloids, pH, and Selected Flavors in E-Cigarette Cartridges and Refill Solutions. Nicotine Tob. Res 17 (10), 1270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Stepanov I, and Fujioka N (2015) Bringing attention to e-cigarette pH as an important element for research and regulation. Tob Control 24 (4), 413–4. [DOI] [PubMed] [Google Scholar]
- (8).Gholap VV, Heyder RS, Kosmider L, and Halquist MS (2020) An Analytical Perspective on Determination of Free Base Nicotine in E-Liquids. J. Anal. Methods Chem 2020, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Pankow JF, Mader BT, Isabelle LM, Luo WT, Pavlick A, and Liang CK (1997) Conversion of nicotine in tobacco smoke to its volatile and available free-base form through the action of gaseous ammonia. Environ. Sci. Technol 31 (8), 2428–2433. [Google Scholar]
- (10).Pankow JF (2001) A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chem. Res. Toxicol 14 (11), 1465–81. [DOI] [PubMed] [Google Scholar]
- (11).Duell AK, McWhirter KJ, Korzun T, Strongin RM, and Peyton DH (2019) Sucralose-Enhanced Degradation of Electronic Cigarette Liquids during Vaping. Chem. Res. Toxicol 32 (6), 1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Harvanko AM, Havel CM, Jacob P, and Benowitz NL (2019) Characterization of Nicotine Salts in 23 Electronic Cigarette Refill Liquids, Nicotine Tob Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Omaiye EE, McWhirter KJ, Luo W, Tierney PA, Pankow JF, and Talbot P (2019) High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci. Rep 9 (1), 2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Tierney PA, Karpinski CD, Brown JE, Luo W, and Pankow JF (2016) Flavour chemicals in electronic cigarette fluids. Tob Control 25 (e1), e10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Heinzerling A, Armatas C, Karmarkar E, Attfield K, Guo W, Wang Y, Vrdoljak G, Moezzi B, Xu D, Wagner J, Fowles J, Dean C, Cummings KJ, and Wilken JA (2020) Severe Lung Injury Associated With Use of e-Cigarette, or Vaping, Products–California, 2019, JAMA Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Pray IW, Atti SK, Tomasallo C, and Meiman JG (2020) E-cigarette, or Vaping, Product Use-Associated Lung Injury Among Clusters of Patients Reporting Shared Product Use - Wisconsin, 2019. MMWR Morb Mortal Wkly Rep 69 (9), 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Pankow JF (2019) Aquatic Chemistry Concepts, 2nd ed., p 554, CRC Press, Boca Raton, FL. [Google Scholar]
- (18).Pankow JF, Kim K, McWhirter KJ, Luo W, Escobedo JO, Strongin RM, Duell AK, and Peyton DH (2017) Benzene formation in electronic cigarettes. PLoS One 12 (3), No. e0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Barlow RB, and Hamilton JT (1962) Effects of ph on the activity of nicotine and nicotine monomethiodide on the rat diaphragm preparation. Br. J. Pharmacol. Chemother 18 (3), 543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Clayton PM, Vas CA, Bui TT, Drake AF, and McAdam K (2013) Spectroscopic studies on nicotine and nornicotine in the UV region. Chirality 25 (5), 288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Christensen JJ, and Hansen LD (1976) Handbook of Proton Ionization Heats and Related Thermodynamic Quantities, J. Wiley and Sons, New York. [Google Scholar]
- (22).Kortüm G, Vogel W, and Andrussow K (1961) Dissociation Constants of Organic Acids in Aqueous Solution, Butterworths, London, UK. [Google Scholar]


