Abstract
Fritillariae thunbergii bulbus (FTB) is a popular Chinese herbal medicine with various applications in respiratory diseases. The quality evaluation of FTB has been insufficient to date, as the active ingredients and mechanisms of action of FTB remain unclear. This study proposes a novel strategy for exploring the quality markers (Q-markers) of FTB based on UPLC-QTOF-MS analysis, network pharmacology, molecular docking, and molecular dynamics (MD) simulation. A total of 26 compounds in FTB were identified by UPLC-QTOF-MS. Ten of these compounds were screened as Q-markers based on network pharmacology for their anti-pneumonia effects, including imperialine, peimisine, peiminine, ebeiedinone, zhebeirine, puqiedine, 9-hydroxy-10,12-octadecadienoic acid, (9Z,12Z,15Z)-13-hydroxy-9,12,15-octadecatrienoic acid, 9,12,15-octadecatrienoic acid, and (2E,4Z,7Z,10Z,13Z,16Z,19Z)-2,4,7,10,13,16,19-docosaheptaenoic acid methyl ester (DAME). These Q-markers were predicted to act on multiple targets and pathways associated with pneumonia. Molecular docking results revealed that most of the Q-markers showed high affinity with at least one of the main targets of pneumonia, and the top ten complexes were confirmed with MD simulation. Network pharmacology indicated that FTB may act on the TNF signaling pathway, HIF-1 signaling pathway, JAK-STAT signaling pathway, etc. The results demonstrated that imperialine (P8), peimisine (P9), peiminine (P11), ebeiedine (P15), zhebeirine (P16), and puqiedine (P18) may be potential Q-markers of FTB, and AKT1, IL-6, VEGFA, TP53, EGFR, STAT3, PPARG, MMP9, and CASP3 may be promising therapeutic targets for pneumonia treatment that are worthy of further research.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s11030-023-10620-y.
Keywords: Fritillariae thunbergii bulbus, Quality markers, Pneumonia, UPLC-QTOF-MS, Network pharmacology, Molecular docking
Introduction
Fritillariae Thunbergii bulbus (FTB), also known as “Zhe beimu” in Chinese, is derived from the Fritillaria genus of the Liliaceae family and is mainly cultivated in Zhejiang province in China [1]. FTB is a traditional Chinese medicine (TCM) that has been to relieve cough and expectorate sputum for more than 2000 years and is listed in the Pharmacopoeia of the People’s Republic of China [2, 3]. Current studies have confirmed that the pharmacological effects of FTB include anti-cancer effects [4, 5], tracheobronchial relaxation [6, 7], and expectorant [8], antitussive [8, 9], and anti-inflammatory effects [10–12]. Therefore, FTB has widely used for several clinical conditions, such as pneumonia, cough, lung abscess, lung atrophy, dyspnea, anal fistula, pharyngitis, scrofula, hemorrhoids, acute mastitis, and other inflammation and infectious diseases [2].
With an increase in market demand for FTB, quality problems, such as counterfeit FTB, have emerged and have dramatically affected human health. Quality control plays a key role in the application of FTB, and great progress has been made to improve its quality standardization. However, few studies have focused on bridging the chemical profiles with the effects of FTB. In the Chinese Pharmacopoeia (2020 edition), peimine and peiminine are listed as identification and quality control indicators for FTB. It is well acknowledged that herbal medicines are inherently complex mixtures, containing various chemical components that usually exhibit synergistic effects on multiple therapeutic targets. Therefore, it is of great significance to establish a comprehensive quality control method for FTB.
Recently, a concept of quality marker (Q-marker) has been proposed to identify quality indicators for TCMs that reflect their safety and therapeutic effects [13, 14]. This Q-marker requirement has led to the development of a novel quality evaluation system of the ingredients and bioactivities in TCMs. This study aimed to uncover the Q-markers that underly the anti-pneumonic effects of FTB by utilizing diverse chemical analysis, data processing, and network pharmacology. TOF-MS with high-resolution properties is an attractive analytical technique for elucidating or confirming the structures of complex matrices [15]. Network pharmacology is a comprehensive approach that generates information that can be used to identify drugs with pleiotropic mechanisms [16, 17]. It is popularly used to screen potential bioactive compounds, discover targets, and explore the mechanisms of TCMs in biomolecular networks [18]. Molecular docking [19] and dynamics simulation [20] are important technical means used to explore potential active candidate compounds in TCM. Combining network pharmacology, molecular docking, and dynamics simulation provides novel ideas for TCM research based on a complex system. Network pharmacology has been successful in elucidating multi-target drug mechanisms and in predicting potential biomarkers for drug discovery and clinical assessments [21, 22].
In this study, a universal strategy to explore the Q-markers of herbal medicines was developed by combing UPLC-QTOF-MS with network pharmacology, MD, and molecular dynamic simulation, and then successfully apply this strategy to qualify FTB.
Materials and methods
Chemical components analysis of FTB
Chemicals and reagents
The bulbus of Fritillaria thunbergii Miq. was purchased from Yinzhou Pharmaceutical Co., Ltd. (Ningbo, Zhejiang, China). A voucher specimen of raw material (no. FTB-20200506) was deposited at the Museum of TCM at Zhejiang Pharmaceutical University. Reference standard components, peimine (99.4%) and peiminine (99.2%), were obtained from Yuanye Bio-Technology Co., Ltd. (Shanghai, China). HPLC-grade acetonitrile and methanol were purchased from Merck (Darmstadt, Germany). LC-MS formic acid (FA) was purchased from Thermo Fisher Scientific (Fair Lawn, NJ, USA). Distilled water (DW) was obtained from Watsons Corporation (Guangzhou, China). All other reagents were analytical grade and were purchased from commercial sources.
Standard and sample solutions preparation
The standards were weighed accurately and then dissolved in methanol to prepare separate stock solutions. The mixed reference substance solution was made by diluting the stock solution tenfold, and the final concentrations of peimine 0.1022 mg/mL and peiminine 0.1008 mg/mL were achieved. All standard solutions were stored at 4 °C.
The sample solutions were processed according to the previously reported sample preparation protocol [3]. First, the raw FTB materials were crushed and sieved (65 mesh) to obtain sample powder for further preparation. One gram of crude powder was weighed and soaked in 2.0 mL 25% ammonia solution for 1 h. 20 mL chloroform–methanol 4:1(v/v) was used as the extraction solvent, and the total weight was determined prior to extraction. Then, the FTB sample powder was extracted under reflux boiling for 2 h at 80 °C. After cooling, the sample weight was obtained again, and the appropriate amount of solvent was added to keep the weight unchanged compared to the weight prior to reflux boiling. Next, the extraction solution of FTB was filtered and concentrated by drying via rotary evaporation at 50 °C. The extracted residue was dissolved in methanol at a final volume of 10 mL to obtain the sample solution, which was filtered through a 0.22 µm membrane (Millipore, USA) before UPLC analysis.
UPLC-Q-TOF-MS determination
An ACQUITY UPLC system (Waters, Milford, MA, USA) equipped with an Agilent ZORBAX Eclipse Plus C18 column (150 mm × 3 mm, 1.8 μm) was used for chromatographic separation. The mobile phases consisted of 0.1% (v/v) formic acid water (solvent A) and acetonitrile (solvent B), which were delivered at a flow rate of 0.4 mL/min. The optimized linear elution gradient was performed as follows: 0–5 min, 2–25% B; 5–10 min, 25–45% B; 10–15 min, 45–80% B; 15–25 min, 80–98% B; and 25–30 min, 98% B; 30–33 min, 98–2% B; and 33–35 min, 2% B. The separation temperature was at 30 °C, and the sample injection volume was 2 µL.
The mass spectrum analysis was performed using a Q-TOF-MS system (Waters, Milford, MA, USA) equipped with an electrospray ionization (ESI) source. The detection was implemented under ESI− and ESI+ modes. A full-scan range of 50–1200 Da was set with a 0.2 s scan time. The main working parameters were set as follows: spray voltage, 3000 V; source temperature, 100 °C; desolvation temperature, 400 °C; desolvation gas flow, 800 L/h; cone gas flow, 50 L/h; and collision energies were 10, 20, and 30 eV for MS/MS.
UPLC-Q-TOF-MS analysis
The raw data from UPLC-Q-TOF-MS analysis were processed using Waters Masslynx 4.1 software. The molecular weight, elemental composition, and the molecular formula of the FTB compounds were determined by comparing the mass spectrometry information of reference substances, matching the self-built chemical composition database, and searching different chemical databases such as PubChem (https://pubchem.ncbi.nlm.nih.gov), ChemSpider (https://www.chemspider.com), and NIST Chemistry WebBook (https://webbook.nist.gov/chemistry). The screening conditions included a mass error < 3 ppm for all precursor ions.
Network pharmacology prediction
Screening of components-related targets of FTB and pneumonia-related targets
According to the above UPLC-Q-TOF-MS analysis results, network pharmacology research was used to screen for Q-markers of FTB. The mol file of the above compounds was uploaded into the SwissTargetPrediction (http://www.swisstargetprediction.ch/) to find matching targets with a probability value ≥ 0.1 [23, 24]. Matched targets were verified using the UniProt database with "Homo sapiens" set as the species. Targets were merged, and replicate targets were deleted. Using “pneumonia" and/or "pneumonitis" as search terms, related targets were acquired in the TTD (http://db.idrblab.net/ttd/), Drugbank (https://go.drugbank.com/), and DisGeNet (https://www.disgenet.org/) databases. The standard gene names of pneumonia-related targets were also acquired from the UniprotKB database. The targets of the three databases were merged, and duplicate targets were deleted so that each item was unique. Finally, overlapping parts of potential component targets and disease targets were mapped using Venny 2.1 software.
Construction of the target protein interaction network
The above common targets were introduced into the STRING database to clarify their interactivity. Analysis was carried out with “Homo sapiens” as the organism with a required interaction score of 0.700 (higher confidence) [25]. Results were exported in tab-separated value (TSV) format, and the unconnected targets were filtered out. Subsequently, visualization of the PPI data was constructed in Cytoscape 3.9.1 [26]. The topology parameters of the network were analyzed using the Network Analyzer function in Cytoscape 3.9.1. “Degree” was used as a key parameter to reflect the importance of nodes and was used to screen key targets [27].
GO and KEGG pathway enrichment analyses
DAVID Bioinformatics Resources (2021 Update, https://david.ncifcrf.gov/) was adopted to investigate key GO terms (BP, MF, and CC) and KEGG pathways to gain insight into the main physiological functions and actions of potential targets. P value < 0.01 was set as the significance level for GO and KEGG enrichment [28]. Histograms and bubble charts were plotted using a free online platform (https://www.bioinformatics. com.cn) to visualize the results of the top 10 GO terms and top 20 KEGG pathways.
Prediction of Q-markers
Pathways with significant changes (P < 0.01) were chosen to construct the drug-target-pathway network using Cytoscape 3.9.1 software. The topological properties of the network were analyzed using the Network Analyzer plugin in Cytoscape 3.9.1 to explore the key compounds, targets, and pathways of FTB in the treatment of pneumonia. The topological properties of the network, including betweenness centrality (BC), closeness centrality (CC), and degree centrality (DC), were also analyzed by the Network Analyzer plugin in the Cytoscape 3.9.1. The key compounds of FTB in the treatment of pneumonia were chosen according to the scores that were higher than the median in three topological parameters.
The validation of Q-markers by molecular docking
The top nine targets predicted by network pharmacology were selected for molecular docking were AKT1, IL-6, VEGFA, TP53, EGFR, STAT3, PPARG, MMP9, and CASP3. The three-dimensional (3D) protein structures of AKT1(PDB ID: 3O96), IL-6 (PDB ID: 1ALU), VEGFA (PDB ID: 3QTK), TP53 (PDB ID: 5O1H), EGFR (PDB ID: 5Y25), STAT3 (PDB ID: 6NJS), PPARG (PDB ID: 2ZNO), MMP9 (PDB ID: 1GKC), and CASP3 (PDB ID: 7RNB) were downloaded from the RCSB PDB database (https://www.rcsb.org//home) and used as receptors. Through ChemBio 3D Ultra 12.0 software, the energy of the ten Q-markers was minimized and converted into PDB format for use as molecular ligands. AutoDock Tools 1.5.6 software was used to perform hydrogenation and dehydration of the molecules and calculate the charges of the receptor proteins [29]. Autodock Vina 1.1.2 was used to dock with the ten Q-markers and the top nine targets [30]. The coordinate and box size for molecular docking of AKT1 were finalized according to the X-ray co-crystalized ligand. The coordinate and box size for molecular docking of IL-6 were finalized according to the active residues and key amino acid residues (Leu4, Met1, Asn 35, Gly 2) [31]. The grid maps were centered at the active site pocket of the two proteins by running the Auto Grid file. The grid box sizes of the nine proteins were 40 × 44 × 40 Å, with a grid-point spacing of 0.375 Å. The docking center grid parameters of the nine targets are presented in Table S2. The conformation with the highest affinity was visualized in Pymol 2.3.
Molecular dynamic simulation study
The Molecular dynamic (MD) simulation study was performed using the AMBER18 software package to examine the stability and dynamic fluctuations of the ligand–protein complexes and further validate the docking results. The compound that had the strongest affinity with proteins in the molecular docking results was chosen for downstream experiments. The protein–ligand complexes were pre-treated before starting the MD simulation. The system was minimized for 10,000 steps in a constant number of atoms. The temperature was set to 300 K, and then a production run in the NPT ensemble was performed for 100 ns. The protein–ligand complexes prepared in tleap were analyzed using a CPPTRAJ module [32]. The binding free energy of the ligand–protein interaction was calculated using the MMPBSA.py module [33].
Results
Analysis and Identification of chemical components from FTB by UPLC-Q-TOF-MS
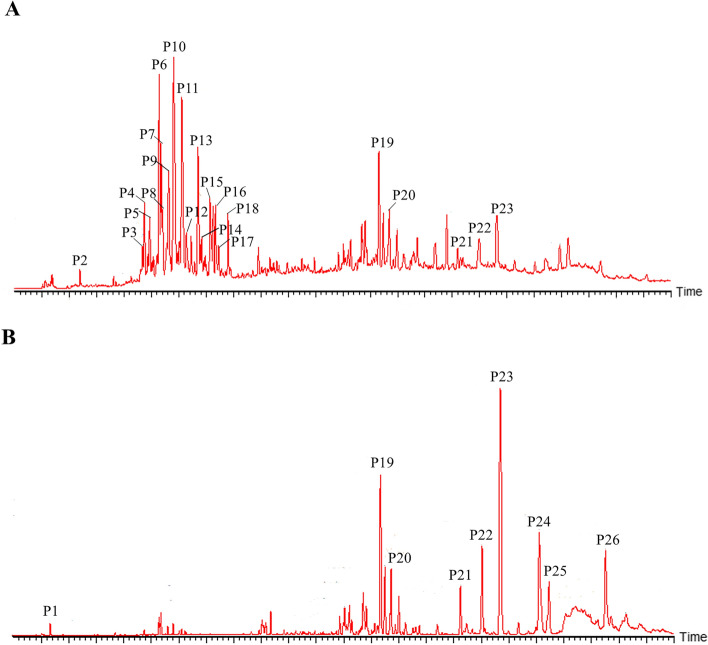
In this study, 26 compounds from FTB were identified using UPLC-MS, of which 2 were also identified in the reference substances. Compounds were mainly divided into four categories: carbohydrates (P1), nucleosides (P2), alkaloids (P3–P18), and fatty acids (P19–P26). The total ion chromatograms in the positive- and negative-ion modes are shown in Fig. 1. The retention time, chemical formula, and other major related information for the 26 identified compounds are presented in Table 1. Their structures are shown in Fig. S1. Isosteroidal alkaloids were the main chemical components in FTB. According to the linkage patterns between rings E and F, the isosteroidal alkaloids could be further classified into cevanine, jervine, and veratramine types [34]. The identified alkaloids from FTB were mainly cevanine and jervine types.
Fig. 1.
The total ion chromatograms (TIC) of FTB. A TIC in positive-ion mode. B TIC in negative-ion mode
Table 1.
Identification of major chemical compounds from FTB by UPLC-QTOF-MS
| Peak no | RT (min) | Compounds | Molecular formula | Ion mode | Measured value (m/z) | Caculated value (m/z) | Error (ppm) | Major fragments |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.67 | Sucrose | C12H22O11 | Negative | 341.1084 | 341.1094 | 0.29 | 179.0567, 142.9669 |
| 2 | 2.98 | Adenosine | C10H13N5O4 | Positive | 268.1051 | 268.1046 | − 0.19 | 136.0625, 119.0357, 94.0405, 92.0248 |
| 3 | 5.83 | Edpetiline | C33H53NO8 | Positive | 592.3854 | 592.3849 | − 0.08 | 574.3740, 430.3329, 157.1019,114.0920 |
| 4 | 5.93 | Peimisine-3-β-d-glucoside | C33H52NO8 | Positive | 590.3730 | 590.3693 | − 0.63 | 572.3584, 428.3155, 114.0917,67.0554 |
| 5 | 6.17 | Yibeissine | C27H41NO4 | Positive | 444.3154 | 444.3114 | − 0.90 | 426.3007, 157.1018, 114.0924, 81.0705 |
| 6 | 6.60 | Zhebeininoside | C33H55NO8 | Positive | 594.4093 | 594.4006 | − 1.46 | 576.3962, 414.3376, 396.3264, 157.1015 |
| 7 | 6.69 | Puqietinonoside | C34H57NO7 | Positive | 592.3939 | 592.3849 | − 1.52 | 574.3818, 412.3226, 394.3116 |
| 8 | 6.75 | Imperialine | C27H43NO3 | Positive | 430.3369 | 430.3321 | − 1.12 | 412.3214, 157.1017, 114.0924, 109.1021 |
| 9 | 7.03 | Peimisine | C27H41NO3 | Positive | 428.3261 | 428.3165 | − 2.24 | 410.3067, 114.0925, 81.0703,67.0552 |
| 10 | 7.25 | Peiminea | C27H45NO3 | Positive | 432.3593 | 432.3478 | − 2.66 | 414.3459, 398.3069, 396.3243, 138.1286 |
| 11 | 7.63 | Peimininea | C27H43NO3 | Positive | 430.3432 | 430.3321 | − 2.58 | 412.3300, 396.2911, 256.2069, 176.1447 |
| 12 | 7.84 | Yibeinoside B | C33H53NO7 | Positive | 576.3915 | 576.3900 | − 0.26 | 414.3376, 396.3264, 258.2223, 112.1129 |
| 13 | 8.37 | Isoverticine | C27H45NO3 | Positive | 432.3579 | 432.3478 | − 2.34 | 414.3441, 398.3060, 396.3239, 148.1132 |
| 14 | 8.47 | Hupeheninoside | C33H55NO7 | Positive | 578.4065 | 578.4057 | − 0.14 | 416.3524, 414.3376, 398.3417 |
| 15 | 8.92 | Ebeiedinone | C27H43NO2 | Positive | 414.3431 | 414.3372 | − 1.42 | 396.3266, 145.1019, 105.0708, 98.0973 |
| 16 | 9.17 | Zhebeirine | C27H43NO2 | Positive | 414.3416 | 414.3372 | − 1.06 | 396.3273, 290.2127, 136.0767 |
| 17 | 9.32 | Ebeiedine | C27H45NO2 | Positive | 416.3539 | 416.3529 | − 0.24 | 147.1179, 145.1024, 131.0866, 105.0707 |
| 18 | 9.74 | Puqiedine | C27H45NO2 | Positive | 416.3571 | 416.3529 | − 1.01 | 398.3422, 145.1019, 105.0707, 91.0549 |
| 19 | 16.66 | 9-Hydroxy-10,12-octadecadienoic acid | C18H32O3 | Negative | 295.2299 | 295.2273 | − 0.88 | 277.2171, 195.1391, 116.9295 |
| 20 | 17.15 | (9Z,12Z,15Z)-13-Hydroxy-9,12,15-octadecatrienoic acid | C18H30O3 | Negative | 293.2130 | 293.2117 | − 0.44 | 279.2314, 146.9660, 113.0987 |
| 21 | 20.30 | 9,12,15-Octadecatrienoic acid | C18H30O2 | Negative | 277.2181 | 277.2168 | − 0.47 | 255.2328, 225.9259, 116.9293 |
| 22 | 21.28 | (2E,4Z,7Z,10Z,13Z,16Z,19Z)-2,4,7,10,13,16,19-Docosaheptaenoic acid methyl ester (DAME) | C23H32O2 | Negative | 339.2338 | 339.2324 | − 0.41 | 279.2325, 164.1170, 163.1135, 147.0823 |
| 23 | 22.11 | Linoleic acid | C18H32O2 | Negative | 279.2361 | 279.2324 | − 1.33 | 255.2335, 116.9294, 99.9265 |
| 24 | 23.88 | Palmitic acid | C16H32O2 | Negative | 255.2334 | 255.2324 | − 0.39 | 183.0128, 116.9296, 96.9612 |
| 25 | 24.32 | Oleic acid | C18H34O2 | Negative | 281.2495 | 281.2481 | − 0.50 | 265.1478, 183.0127, 116.9297 |
| 26 | 26.89 | Stearic acid | C18H36O2 | Negative | 283.2661 | 283.2637 | − 0.85 | 279.2342, 255.2345, 183.0133, 96.9615 |
aCompared with standard compounds
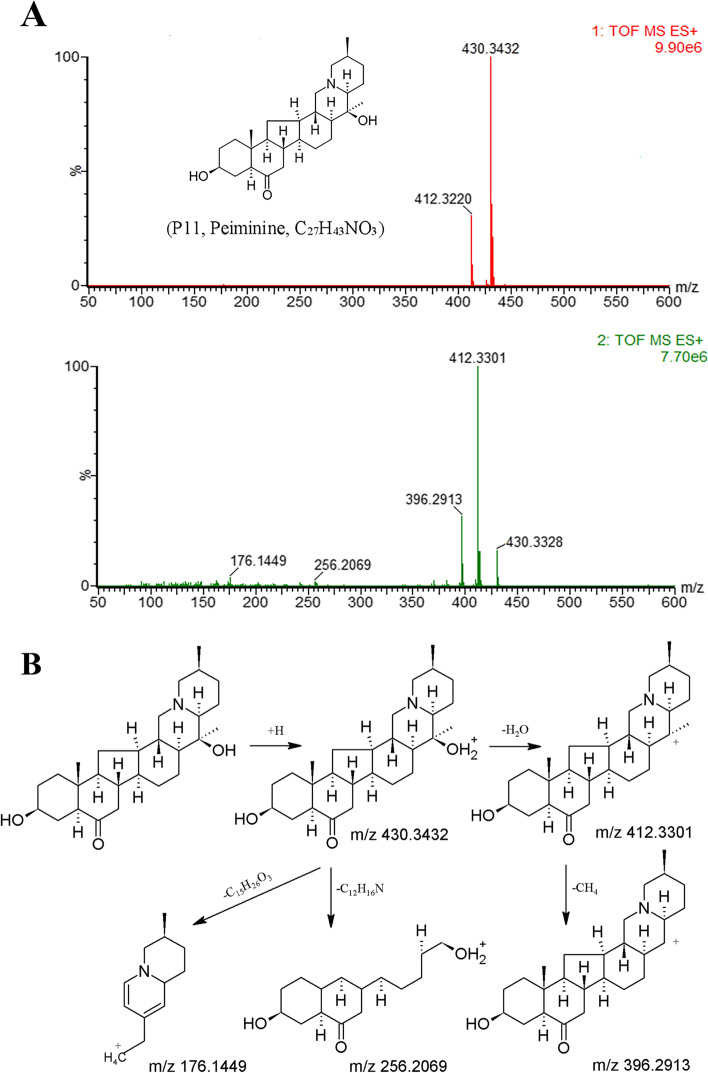
Cevanine alkaloids, the largest representative group from the genus Fritillaria, have similar structural characteristics to the hexacyclobenzofluorene quinolizine nucleus [35, 36]. In the positive-ion mode, groups containing C20-OH substituents easily lose a molecule of H2O (m/z 18.0106 Da). In the No-C20-OH substituent group, strong [M + H]+ ions are observed [15]. Moreover, if the compound has a glycosyl group in its structure, a fragment ion that loses glycosyl (C6H10O5, m/z 162.0528) will be generated. This fragmentation mechanism may be explained by the presence of the compound peiminine (C27H43NO3, P11). The MS spectra and fragmentation pathway for peiminine is shown in Fig. 2A and B, respectively.
Fig. 2.
The MS spectra in positive mode (A) and the proposed fragmentation pathway of peiminine (B)
Peiminine exhibited a parent ion [M + H]+ at m/z 430.3432, which produced a strong [M + H − H2O]+ ion (m/z 412.3300) and then further demethylated to form [M + H − H2O − CH4]+ ions (m/z 396.2911). Accompanied by the fragmentation and hydrogen rearrangement of the rings in the framework structure, characteristic ions, [M + H − C12H16N]+ ions (m/z 256.2069) and [M + H − C15H26O3]+ ions (m/z 176.1447), are generated. Based on the above rules, a total of 12 cevanine alkaloids of FTB were identified (P3, 6, 8, 10–18). Among them, compounds P10 and P11 were further identified based on the standards.
Jervine alkaloids are distinct from cevanine types; the furan ring (E) is fused to a piperidine ring system, forming an ether bridge between C17 and C23 [15]. Peimisine (C27H41NO3, P9) is the representative jervine type in FTB (Fig. 3). The protonated molecule [M + H]+ (m/z 428.3261) occurred with high abundance via primary mass spectra. Due to the ring cleavage in the basic skeleton on the MS2 spectrum, a strong [M + H − H2O]+ ion (m/z 410.3067) and characteristic fragment ions at m/z 114.0925, 81.0703, and 67.0552 were formed, which were consistent with previous literature reports [37]. P4 and P5 were inferred according to the above rules.
Fig. 3.
The MS spectra in positive mode (A) and the proposed fragmentation pathway f peimisine (B)
Fatty acids were another major ingredient in FTB that exhibited a higher response value in the negative-ion mode. Compound database searches in PubChem and NIST, combined with mass spectrographic analysis and relevant literature [38–40], lead to the identification of eight fatty acids compounds (P19–26). All eight of the identified fatty acids were long-chain fatty acids, of which P19–23 and P25 were unsaturated fatty acids and P24 and P26 were saturated fatty acids.
Results of network pharmacology
Screening of potential targets of FTB for pneumonia treatment
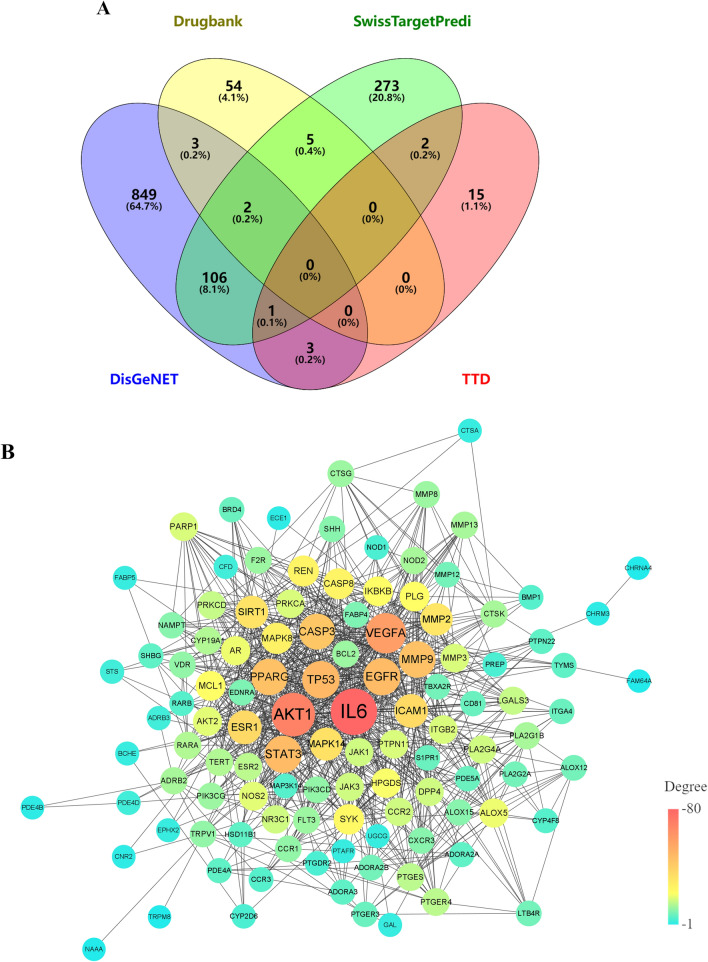
Including the results with a probability ≥ 0.1 and excluding duplicates, a total of 390 potential targets related to the identified 26 compounds were obtained from the SwissTarget Prediction database. Nine hundred and sixty-four pneumonia-related targets were obtained from DisGeNET, 64 from the Drugbank database, and 21 from the TTD database. After removing duplicates, a total of 1040 disease targets remained. The component potential and disease targets were mapped using Venny 2.1 software, and 116 interacting proteins were identified (Fig. 4A).
Fig. 4.
The potential targets of FTB for treating pneumonia. A The Venn analysis between component targets and disease targets. B The PPI network analysis of interacting targets
Construction and analysis of the PPI network
The analysis results of interactions from the STRING database were introduced into Cytoscape 3.9.1 to draw the PPI network. After removing three targets (FAM64A, CYP4F8, and HPGDS) that had no interactions, a total of 113 target nodes exhibited interactive associations, and 891 interaction edges were observed in the PPI network (Fig. 4B). Sixty-seven disease targets were highly correlated (degree ≥ 10), and AKT1 and IL-6 were the crucial hub targets in the network, followed by VEGFA, TP53, EGFR, STAT3, PPARG, MMP9, etc. These data provide effective instruction for identifying Q-markers of FTB. The top 20 targets with a higher degree of interaction are shown Table S1.
GO enrichment and KEGG pathway analysis of FTB for pneumonia treatment
GO analysis of 116 gene targets was performed using the David database. A total of 177 biological processes (BP), 48 cell compositions (CC), and 45 molecular functions (MF) were identified (P < 0.01). According to the ascending order of − log10(P value), the top ten BP, CC, and MF were selected and are shown in Fig. 5A. These targets are mainly distributed in the plasma membrane, cytoplasm and its perinuclear region, cytosol, extracellular space, and within other cellular components. BPs were mainly related to the inflammatory response, positive regulation of IL-6 production, extracellular matrix disassembly, positive regulation of transcription from the RNA polymerase II promoter, and positive regulation of cytosolic calcium ion concentration. The MF was mainly involved in enzyme binding, endopeptidase activity, serine-type endopeptidase activity, RNA polymerase II transcription factor activity, ligand-activated sequence-specific DNA binding, and protein phosphatase binding.
Fig. 5.
GO Enrichment and KEGG Pathway analyses. A The results of GO enrichment analysis. B The results of KEGG pathway enrichment analysis
The KEGG pathway enrichment analysis using the David database showed that 62 signaling pathways were selected (P < 0.01). The top 20 signaling pathways with the most significant P value are shown in Fig. 5B and mainly include pathways involved in cancer, TNF signaling, endocrine resistance, chemical carcinogenesis-receptor activation, and relaxin signaling.
Prediction of potential Q-markers in FTB
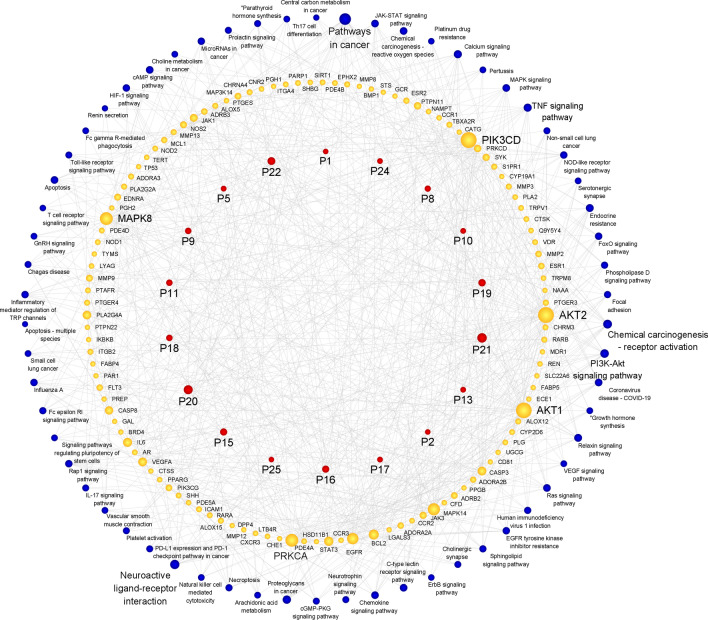
The compound-target-pathway network for FTB in the treatment of pneumonia was drawn using Cytoscape 3.9.1 software, based on target prediction and pathway enrichment analyses. The network involved 196 nodes (18 compounds, 116 targets, and 62 pathways) and 704 edges (Fig. 6). According to the results of the topological analysis from this network, ten key active compounds (P21, P20, P22, P19, P16, P15, P9, P18, P11, P8) with higher degrees than the median were identified as potential Q-markers of FTB for pneumonia treatment.
Fig. 6.
The compound-target-pathway network of FTB for the treatment of pneumonia
Molecular docking results
Molecular docking is a novel technology in the computer-aided molecular design field, which is being increasing used to identify unknown active compounds, and modeling interactions between inhibitor and target molecules [41, 42]. To provide further evidence to support the identification of the potential Q-markers, the binding of ten Q-markers to the top nine targets was verified using molecular docking analysis. According to the docking results, all ten Q-markers exhibited strong affinity for AKT1 and EGFR. Compounds P11 and P15 had high affinity with for all nine targets. Compounds P8, P9, P16, and P18 showed high affinity for eight of the nine targets. Compounds P19–P22 also showed high affinity for eight of the nine targets but had lower binding energy values than the other compounds. The binding energies are shown in Table 2.
Table 2.
The binding energy of 10 Q-markers with the top 9 targets (Kcal/mol)
| Compounds | AKT1 | IL-6 | VEGFA | TP53 | EGFR | STAT3 | PPARG | MMP9 | CASP3 |
|---|---|---|---|---|---|---|---|---|---|
| X-ray ligand | − 14.6 | – | − 3.1 | − 6.9 | − 8.6 | − 9.0 | − 9.9 | − 6.9 | − 6.1 |
| P8 | − 10.2 | − 6.9 | − 4.5 | − 6.6 | − 8.5 | − 6.8 | − 7.3 | − 6.9 | − 6.5 |
| P9 | − 8.5 | − 7.5 | − 4.7 | − 7.3 | − 8.5 | − 7.3 | − 7.5 | − 6.9 | − 6.3 |
| P11 | − 11.1 | − 6.9 | − 5.4 | − 6.0 | − 8.9 | − 7.1 | − 8.4 | − 6.9 | − 6.2 |
| P15 | − 9.0 | − 7.4 | − 5.2 | − 5.2 | − 8.3 | − 6.8 | − 7.3 | − 7.0 | − 6.2 |
| P16 | − 10.1 | − 7.1 | − 5.2 | − 4.7 | − 8.7 | − 6.6 | − 7.9 | − 6.9 | − 6.0 |
| P18 | − 10.2 | − 7.1 | − 5.4 | − 4.8 | − 8.5 | − 5.3 | − 8.3 | − 6.9 | − 6.0 |
| P19 | − 6.7 | − 4.8 | − 3.6 | − 5.7 | − 5.1 | − 5.2 | − 5.6 | − 6.9 | − 6.0 |
| P20 | − 7.2 | − 5.1 | − 3.3 | − 6.0 | − 5.4 | − 5.4 | − 5.7 | − 6.9 | − 6.3 |
| P21 | − 6.8 | − 5.2 | − 3.6 | − 6.0 | − 5.5 | − 4.5 | − 6.1 | − 7.0 | − 6.9 |
| P22 | − 7.8 | − 5.5 | − 3.9 | − 6.7 | − 5.8 | − 5.3 | − 6.7 | − 6.9 | − 6.5 |
– without X-ray ligand
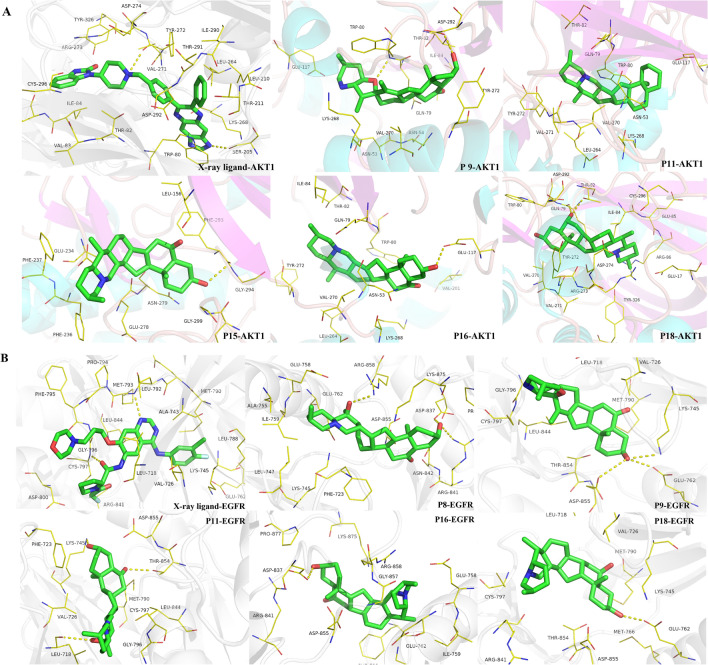
The AutoDock score is a final estimator of interactions between compounds and targets. The docking scores of six alkaloid compounds (P8, P9, P11, P15, P16, and P18) with six targets (AKT1, IL-6, VEGFA, EGFR, STAT3, and PPARG) were higher than those of four fatty acid compounds (P19–P22), and the docking scores with the other three targets were equal to that of the four fatty acid compounds. Hence, ten Q-markers represent possible multi-target compounds that may be important for preventing and treating pneumonia. Furthermore, alkaloids may be more effective than fatty acids in preventing pneumonia. In summary, P9, P11, P15, P16, P18 with AKT1, and P8, P9, P11, P16, P18 with EGFR were the promising complexes. The docking patterns of these Q-markers with the response targets are shown in Fig. 7A and B. AutoDock results also supported the results obtained from the network pharmacology experiment.
Fig. 7.
The docking complex of ten Q-markers with AKT1 and EGFR proteins
Molecular dynamics simulation results
According to the molecular docking results in Table 2, P9, P11, P15, P16, and P18 with AKT1, and P8, P9, P11, P16, and P18 with EGFR were the top ten complexes. These complexes were subjected to MD simulation, and the root-mean-square deviations (RMSDs) for each simulation were calculated (Fig. 8A and B). The root-mean-square fluctuation (RMSF) and Radius of Gyration (Rog) are shown in Fig. S2. The binding free energy (ΔGbind) reflects the affinity of a ligand to its target. Here, the ΔGbind of the complexes were calculated using MM-GBSA in Ambertools 18. The ΔGbind of complexes X-ray ligand, P9, P11, P15, P16, and P18 with AKT1 were − 55, − 36, − 57, − 31, − 37, and − 52 kcal/mol, respectively, and complexes X-ray ligand, P8, P9, P11, P16, and P18 with EGFR were − 49, − 25, − 30, − 28, − 28, and − 26 kcal/mol, respectively. These data imply that the binding interactions are spontaneous. Moreover, the contributions favoring ligand binding are van der Waals and electrostatic interactions.
Fig. 8.
Molecular dynamics simulation. A RMSD of X-ray ligand, P9, P11, P15, P16, P18 with AKT1; B RMSD of X-ray ligand, P8, P9, P11, P16, P18 with EGFR; C Amino acid interactions of P11-AKT1; D Amino acid interactions of P11-EGFR
The active sites of the protein–ligand interactions of P11-AKT1 and P11-EGRF were analyzed, as they showed the lowest ΔGbind. The stability of the protein–ligand complex is predominantly determined by its hydrogen bonds, which are the major stabilizers of the docked complexes. As shown in Fig. 8C, P11 formed hydrophilic interactions with Lys268 and Glu117 of AKT1 and hydrophobic interactions with Tyr272, Val271, Val270, and Leu264 of AKT1. Two H-bonds were formed between the hydroxyl (OH) and carbonyl (C=O) of P11 with Leu718 and Thr854 residues of EGFR, respectively. Additionally, the hydrophobic amino acids Gly796, Met790, ASP855, of EGFR formed van der Waals interaction with P11, and were also conducive to ligand binding (Fig. 8D). Temperature and pressure had no effect on the structural conformations. In summary, these results prove the validity of the docking results.
Discussion
Pneumonia is an acute inflammatory disease, which is characterized by acute onset and severe symptoms and leads to a great deal of infection-related deaths [43, 44]. FTB is mainly used for airway inflammatory diseases, such as bronchitis and pneumonia [45]. However, the active compounds, Q-markers, and molecular mechanisms of FTB and its effects in the treatment of pneumonia remain unknown. In this study, phytochemical investigation identified 26 compounds in FTB using UPLC-QTOF-MS. These compounds were mainly isosteroidal alkaloids and fatty acids. These results are consistent with the literature [2]. Network pharmacology, molecular docking, and MD simulation were conducted to acquire system-level information on the activity of FTB against pneumonia.
Through network pharmacology, a compounds-targets-pathway network of FTB with 18 compounds, 116 targets, and 62 pathways was constructed. The PPI network and critical network analyses identified 67 core targets, the top nine being IL-6, AKT1, VEGFA, TP53, EGFR, STAT3, PPARG, MMP9, and CASP3. These targets were prominently enriched in many immune pathways. Bioinformatics analysis of the 18 bioactive ingredients in FTB and the 116 corresponding pneumonia-related targets revealed that these ingredients were involved in the regulation of inflammation-related pathways, such as the TNF signaling pathway, HIF-1 signaling pathway, JAK-STAT signaling pathway, FoxO signaling pathway, and PI3K-Akt signaling pathway. Changes in IL-6, VEGFA, TNF, HIF, STAT, and the various identified signaling pathways have all been reported in pneumonia. IL-6 may serve as a risk factor for pneumonia and contributes to the development of pneumonia [46]. One animal study indicated that genetic deletion of AKT1 enhances neutrophil apoptosis, attenuates neutrophil influx into the lungs in a mouse model of pneumonia, and diminishes IL-6 expression [47]. Increased secretion of VEGFA accelerates the progression of lung tissue injury, including exacerbating vascular permeability and edema [48, 49]. As a vital activator of inflammation, TNF can induce a wide range of inflammatory and immune signaling pathways via the NF-κB pathway and the MAPK signaling cascade [50]. It has been demonstrated that inhibition of the TNF-α pathway has a selective protective effect on lung injury due to radiation [51]. The absence of HIF-1α has no beneficial effect on lung injury in mice during stereotactic radiotherapy [52]. Yu et al. suggested that the protective effect of delayed treatment of WP1066 suggests that the STAT3 signal may be a therapeutic target for radiation-induced lung injury [53].
Network pharmacology provides a multidimensional pathway for predicting and understanding the interactions and mechanisms of action for multiple components in FTB. Through component analysis, octadecatrienoic acid derivatives (P19–21), DAME, zhebeirine, ebeiedinone, peimisine, puqiedine, peiminine, and imperialine were identified as essential for FBT’s action against pneumonia. Among these compounds, peimine and peiminine (A2) are usually used for the identification and quality control of FBT, as listed in the Pharmacopeia of People's Republic of China [3]; however, the rationality and completeness of using these compounds for this purpose may require further research. The Q-marker concept is a novel theory of quality control applied to TCM and refers to the material inherent in Chinese herbal medicines and products related to Chinese medicines or produced during processing and preparation [54]. Q-markers are more closely related to the functional properties of Chinese medicine. The results provided herein may provide more reasonable Q-markers for controlling FTB quality.
Growing evidence has proven that AKT and IL-6 are important targets for the treatment of pneumonia [17, 18, 55]. It has been reported that lobar pneumonia and bronchial pneumonia patients have elevated IL-6 levels relative to those without pneumonia [56]. EGFR plays a role in virus entry and replication, including in SARS-CoV-2 infections, due to its effect on the growth and proliferation of fibroblasts, keratinocytes, and vascular endothelial cells. One study demonstrated that EGFR was highly expressed in 78% of patients with COVID-19, and those patients were found to have pulmonary fibrosis when examined radiologically and pathologically postmortem [57]. One molecular docking study revealed that AKT1 was a promising drug target to reduce lung injury, lung fibrogenesis, and viral infection [58]. In the present study, molecular docking was used to verify the interactions between the top nine crucial pneumonia targets (AKT1, IL-6, VEGFA, TP53, EGFR, STAT3, PPARG, MMP9, and CASP3) and the Q-markers in FTB. The results of this study showed that all the other eight targets except VEGFA had good binding affinity (≤ − 5 kcal/mol) with all ten Q-markers. Also, all Q-markers have almost identical docking scores with MMP9 and CASP3. It might be mainly due to the hydrophobic interaction between all the Q-markers with the same Nfh1449, Met422, and Pro421 amino acid residues of MMP9, and with the same Ser209, Asn208, Asp211, Phe252, Phe250 amino acid residues of CASP3. In summary, the docking results indicated that the Q-markers play a beneficial role in the treatment of pneumonia. Furthermore, the six alkaloid compounds (P8, P9, P11, P15, P16, and P18) may be more effective than the four fatty acids (P19–P22) in preventing pneumonia, as the six alkaloid compounds had lower docking scores. Finally, MD simulation was employed to assess the stability and flexibility of P9, P11, P15, P16, and P18 with AKT1, and P8, P9, P11, P16, and P18 with EGFR. The lowest binding free energy was found between AKT1-peiminine (− 57 kcal/mol). Accordingly, AKT1-peiminine had the greatest stability and flexibility. Interestingly, this study's findings were similar to several previous reports. Imperialine (P8) has been shown to have activity against non-small cell lung cancer and inflammation [59]. Based on molecular docking, drug-likeness, and toxicity prediction, peimisine (P9) was found to be a potential inhibitor of SARS-CoV-2 [60]. Peiminine (P11) significantly attenuates LPS-induced AKT and PI3K phosphorylation, and inhibits the expression of TNF-α, IL-1β, and IL-6 in acute lung injury mice [61]. Peiminine (P11) has also been verified to be a therapeutic agent for chronic obstructive pulmonary disease (COPD) that acts on the EGFR and TP53 targets and that inhibits CASP3 [62, 63]. Peimine (P10), peiminine (P11), and ebeiedine (P15) derived from FTB have been reported to exert antitussive activity by relaxing the tracheobronchial and tracheal rings of rats [7]. Peimine (P10) and ebeiedine (P15) reduce the expression and production of MUC5AC mucin in human pulmonary mucoepidermoid NCI-H292 cells [10]. Zhebeirine (P16) and peimine (P10) exhibited distinct drug actions due to their strong hydrogen bonding with the hot spots of SARS-CoV-2 RBD. They also showed significant efficacy in inhibiting the infection of SARSCoV-2 [64]. In addition, in vivo and in vitro experimental studies have demonstrated the biological activities of peimine (P10) and peiminine (P11). These activities include anti-cancer activity [65], tracheobronchial relaxation [6], and anti-inflammatory activity [12]. Verification experiments that may be absent in the present study will be conducted in subsequent studies to elucidate the effect of Q-markers and molecular mechanisms in treating pneumonia.
Conclusion
In this paper, a strategy to discover the potential Q-markers of FTB was developed using a combination of UPLC-QTOF-MS, network pharmacology, and molecular docking. As a result, a total of 26 compounds were identified, including 16 alkaloids, eight fatty acids, one carbohydrate, and one nucleoside. Of these, ten ingredients were selected as potential Q-markers by network pharmacology. The molecular docking results revealed that six of the Q-markers (P8, P9, P11, P15, P16, P18) exhibited strong affinity to the crucial targets AKT1, IL-6, VEGFA, TP53, EGFR, STAT3, PPARG, MMP9, and CASP3 that are important for their potential medical application in pneumonia. In addition, it is worthwhile to note that although the unsaturated fatty acids of FTB exhibited higher degrees in the component-target-pathway network than alkaloids, they have weaker affinities with the nine targets in molecular docking. The above results provide technical support for identifying Q-markers in FTB. Imperialine (P8), peimisine (P9), peiminine (P11), ebeiedine (P15), zhebeirine (P16), and puqiedine (P18) may be potential Q-markers of FTB, and AKT1, IL-6, VEGFA, TP53, EGFR, STAT3, PPARG, MMP9, and CASP3 are promising therapeutic targets for pneumonia that are worthy of further research.
Supplementary Information
Below is the link to the electronic supplementary material.
Authors contributions
Z-AZ contributed to perform the experimental work, analyzed the data and wrote the manuscript. W-LL and X-DL prepared the samples. BC coordinated the experiment and collect the data. JW coordinated the experiment and edited the article. All authors have read and agreed to the published version of the manuscript.
Funding
The work was financially supported by Zhejiang Provincial Public Welfare Program (LGN22H280009, LTGN23H280002, LTGN23H280003), Zhejiang Provincial Drug Administration Science and technology plan project (2022008), School-enterprise Cooperation Project from Domestic Visiting Engineers of Colleges and Universities (FG2020002, FG2020003, FG2022003).
Data availability
The data that support the findings of this study are available from the first author upon reasonable request.
Declarations
Conflict of interest
The authors have declared that no competing interest exists.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bin Cheng, Email: chengb@zjpu.edu.cn.
Juan Wang, Email: wangjuan@zjpu.edu.cn.
References
- 1.Liu YH, Bei K, Zheng WR, Yu GG, Sun CX. Assessment of health risks associated with pesticide and heavy metal contents in Fritillaria thunbergii Miq. (Zhe Beimu) Environ Sci Pollut Res Int. 2022 doi: 10.1007/s11356-022-23995-6. [DOI] [PubMed] [Google Scholar]
- 2.Li H, Hung A, Li MD, Yang AWH. Fritillariae thunbergii bulbus: traditional uses, phytochemistry, pharmacodynamics, pharmacokinetics and toxicity. Int J Mol Sci. 2019;20(7):1667. doi: 10.3390/ijms20071667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.State Pharmacopoeia Commission of the PRC . Pharmacopoeia of the people’s republic of China. Beijing: China Medical Science and Technology Press; 2020. [Google Scholar]
- 4.Li ZH, An C, Hu KW, Zhou KH, Duan HH, Tang MK. Multidrug resistance reversal activity of total alkaloid from Fritillaria thunbergii on cisplatin-resistant human lung adenocarcinoma A549/DDP cells. Chin J Pharmacol Toxicol. 2013;27(3):315–320. doi: 10.3867/j.issn.1000-3002.2013.03.002. [DOI] [Google Scholar]
- 5.Yang Q, Nie SQ, Weng XG, Li LF, Huang LQ. Experiment studies on anti-tumor effect in vivo and in vitro of Aconitum carmichaelii Debx. and Fritillaria thunbergii Miq. used singly or matched. Chin J Exp Tradit Med Formulae. 2005;11(4):25–28. doi: 10.13422/j.cnki.syfjx.2005.04.013. [DOI] [Google Scholar]
- 6.Chan SW (2000) Pharmacological and chemical investigations into Bulbus Fritillariae. Ph.D. Thesis, Chinese University of Hong Kong, Hong Kong, China
- 7.Wu X, Chan SW, Ma J, Li P, Shaw PC, Lin G. Investigation of association of chemical profiles with the tracheobronchial relaxant activity of Chinese medicinal herb Beimu derived from various Fritillaria species. J Ethnopharmacol. 2018;210:39–46. doi: 10.1016/j.jep.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Yan XY, Tong ZY, Luo ZJ, Tang L, Wu SS, Yang J, Peng C. Pharmacodynamic comparison of Fritillaria unibracteata, Fritillaria unibracteata var. wabensis and Fritillaria thunbergii cultivated. Chin J Exp Tradit Med Formulae. 2012;18(10):244–248. doi: 10.13422/j.cnki.syfjx.2012.10.072. [DOI] [Google Scholar]
- 9.Yan ZJ, Luo YQ, Li ZY, Tang L, Wu SS, Yan XY, Peng C. Comparative studies on antiussive effect between Fritillaria unibracteata Hisao et K. C. Hisa and Fritillaria thunbergii Miq with chemical stimulation induced cough method. Lishizhen Med Mater Med Res. 2012;23(10):2522–2525. doi: 10.3969/j.issn.1008-0805.2012.10.060. [DOI] [Google Scholar]
- 10.Kim EJ, Yoon YP, Woo KW, Kim JH, Min SY, Lee HJ, Lee SK, Hong JH, Lee KR, Lee CJ. Verticine, ebeiedine and suchengbeisine isolated from the bulbs of Fritillaria thunbergii Miq. inhibited the gene expression and production of MUC5AC mucin from human airway epithelial cells. Phytomedicine. 2016;23(2):95–104. doi: 10.1016/j.phymed.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Zhou MG, Ma XY, Ding GY, Wang ZY, Liu D, Tong YL, Zhou H, Gao J, Hou YY, Jiang M, Bai G. Comparison and evaluation of antimuscarinic and anti-inflammatory effects of five Bulbus fritillariae species based on UPLC-Q/TOF integrated dual-luciferase reporter assay, PCA and ANN analysis. J Chromatogr B. 2017;1041:60–69. doi: 10.1016/j.jchromb.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Xu JW, Zhao W, Pan LY, Zhang AL, Chen QM, Xu K, Lu HY, Chen Y. Peimine, a main active ingredient of Fritillaria, exhibits anti-inflammatory and pain suppression properties at the cellular level. Fitoterapia. 2016;111:1–6. doi: 10.1016/j.fitote.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Liu CX. Construction of traceability system of Chinese materia medica product quality based on quality marker. Chin Tradit Herb Drugs. 2017;48(18):3669–3676. doi: 10.7501/j.issn.0253-2670.2017.18.001. [DOI] [Google Scholar]
- 14.Liu CX. Five-year review on development of quality markers of traditional Chinese medicine. Chin Tradit Herb Drugs. 2021;52(9):2511–2518. doi: 10.7501/j.issn.0253-2670.2021.09.001. [DOI] [Google Scholar]
- 15.Wang L, Yao ZP, Li P, Chen SB, So PK, Shi ZQ, Hu B, Liu LF, Xin GZ. Global detection and semi-quantification of Fritillaria alkaloids in Fritillariae ussuriensis Bulbus by a non-targeted multiple reaction monitoring approach. J Sep Sci. 2016;39(2):287–295. doi: 10.1002/jssc.201500880. [DOI] [PubMed] [Google Scholar]
- 16.Mou X, Zhou DY, Zhou D, Liu K, Chen LJ, Liu WH. A Bioinformatics and network pharmacology approach to the mechanisms of action of Shenxiao decoction for the treatment of diabetic nephropathy. Phytomedicine. 2020;69:153192. doi: 10.1016/j.phymed.2020.153192. [DOI] [PubMed] [Google Scholar]
- 17.Li P, Xia XC, Zhou JD, Wu JC. Exploring the pharmacological mechanism of Radix Salvia miltiorrhizae in the treatment of radiation pneumonia by using network pharmacology. Front Oncol. 2021;11:684315. doi: 10.3389/fonc.202.684315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng JQ, Chen XX, Hou M, Yang K, Yang B, Wang P, Du Y, Yu QY, Ren JG, Liu JX. The TCM preparation feilike mixture for the treatment of pneumonia: network analysis, pharmacological assessment and silico simulation. Front Pharmacol. 2022;13:794405. doi: 10.3389/fphar.2022.794405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan J, Fu A, Zhang L. Progress in molecular docking. Quant Biol. 2019;7(2):83–89. doi: 10.1007/s40484-019-0172-y. [DOI] [Google Scholar]
- 20.Nunes-Alves A, Kokh DB, Wade RC. Recent progress in molecular simulation methods for drug binding kinetics. Curr Opin Struct Biol. 2020;64:126–133. doi: 10.1016/j.sbi.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4(11):682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 22.Wang NN, Zhu FF, Shen MX, Qiu LP, Tang M, Xia HC, Chen L, Yuan Y, Ma SS, Chen KP. Network pharmacology-based analysis on bioactive anti-diabetic compounds in Potentilla discolor bunge. J Ethnopharmacol. 2019;241:111905. doi: 10.1016/j.jep.2019.111905. [DOI] [PubMed] [Google Scholar]
- 23.Antoine D, Olivier M, Vincent Z. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucl Acids Res. 2019;47:W357–W364. doi: 10.1093/nar/gkz382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lv QY, Lin JR, Wu XY, Pu HH, Guan YW, Xiao PG, He CN, Jiang BP. Novel active compounds and the anti-diabetic mechanism of mulberry leaves. Front Pharmacol. 2022;13:1–26. doi: 10.3389/fphar.2022.986931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bozhilova LV, Whitmore AV, Wray J, Reinert G, Deane CM. Measuring rank robustness in scored protein interaction networks. BMC Bioinform. 2019;20(1):446. doi: 10.1186/s12859-019-3036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su HH, Wu GS, Zhan LL, Xu F, Qian HQ, Li YL, Zhu XM. Exploration of the mechanism of Lianhua Qingwen in treating influenza virus pneumonia and new coronavirus pneumonia with the concept of "different diseases with the same treatment" based on network pharmacology. Evid Based Complement Altern Med. 2022;2022:5536266. doi: 10.1155/2022/5536266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan CH, Li Y, Dong XR, Xu WB, Ma YL. Network pharmacology and reverse molecular docking-based prediction of the molecular targets and pathways for avicularin against cancer. Comb Chem High Throughput Screen. 2019;22(1):4–12. doi: 10.2174/1386207322666190206163409. [DOI] [PubMed] [Google Scholar]
- 28.Wu FZ, Liu J, Cao ZT, Wang TQ, Ye L, Zhu MM, Wang ZG. Molecular mechanism of the Saposhnikovia divaricata–Angelica dahurica herb pair in migraine therapy based on network pharmacology and molecular docking. Evid Based Complement Alternat Med. 2022;2022:1994575. doi: 10.1155/2022/1994575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris GM, Hueym R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30(16):2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dan WC, Wu C, Xue CY, Liu JL, Guo XY, Lian YJ. Rules of Chinese herbal intervention of radiation pneumonia based on network pharmacology and data mining. Evid Based Complement Altern Med. 2022;2022:7313864. doi: 10.1155/2022/7313864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roe DR, Cheatham TE. PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J Chem Theory Comput. 2013;9(7):3084–3095. doi: 10.1021/ct400341p. [DOI] [PubMed] [Google Scholar]
- 33.Vishakha S, Poonam D, Vikram D, Shailly T, Pravindra K. In-silico functional and structural annotation of hypothetical protein from Klebsiella pneumonia: a potential drug target. J Mol Graph Model. 2022;116:108262. doi: 10.1016/j.jmgm.2022.108262. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Hou H, Ren Q, Hu HY, Yang TC, Li XW. Natural drug sources for respiratory diseases from Fritillaria: chemical and biological analyses. Chin Med. 2021;16(1):40. doi: 10.1186/s13020-021-00450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atta-ur-Rahman, Akhtar MN, Choudhary MI, Tsuda Y, Sener B, Khalid A, Parvez M. New steroidal alkaloids from Fritillaria imperialis and their cholinesterase inhibiting activities. Chem Pharm Bull. 2002;50(8):1013–1016. doi: 10.1248/cpb.50.1013. [DOI] [PubMed] [Google Scholar]
- 36.Li QH, Wu ZH. Isolation and identification of alkaloids from Fritillaria anhuiensis S. C. Chen et S. F. YIN. Acta Pharm Sin. 1986;21(10):767–771. [PubMed] [Google Scholar]
- 37.Jiang Y, Li HJ, Li P, Cai ZH, Ye WC. Steroidal alkaloids from the Bulbs of Fritillaria puqiensis. J Nat Prod. 2005;68(2):264–267. doi: 10.1021/np0497649. [DOI] [PubMed] [Google Scholar]
- 38.Liang JL, Cao XJ, Li JW, Ren HX, Wu SH. Analysis of volatile components of flowers of Fritillaria thunbergii by GC-TOF- MS. Chin J Chin Mate Med. 2011;19(36):2689–2692. doi: 10.4268/cjcmm20111918. [DOI] [PubMed] [Google Scholar]
- 39.Du WF, Zhang H, Yue XK, Zhu T, Ge WH. The analysis on volatile components of Zhejiang Fritillary slices with different primary processing methods. Lishizhen Med Mater Med Res. 2018;29(1):73–76. doi: 10.3969/j.issn.1008-0805.2018.01.024. [DOI] [Google Scholar]
- 40.Shang WJ, Wu J. Analysis of fatty acids components in Fritillaria cirrhosa D. Don by GC-MS. Chin Mod Med. 2016;23(33):135–139. [Google Scholar]
- 41.Veselinovic JB, Veselinovic AM, Ilic-Tomicb T, Davisc R, O’Connorc K, Pavic A, Nikodinovic-Runic J. Potent anti-melanogenic activity and favorable toxicityprofile of selected 4-phenyl hydroxycoumarins in the zebrafish model and the computational molecular modeling studies. Bioorg Med Chem. 2017;25(24):6286–6296. doi: 10.1016/j.bmc.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Ge W, Peng X, Yuan LX, He SB, Fu XY. Investigating the active compounds and mechanism of HuaShi XuanFei formula for prevention and treatment of COVID-19 based on network pharmacology and molecular docking analysis. Mol Divers. 2022;26(2):1175–1190. doi: 10.1007/s11030-021-10244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinton LJ, Walkey AJ, Mizgerd JP. Integrative physiology of pneumonia. Physiol Rev. 2018;98(3):1417–1464. doi: 10.1152/physrev.00032.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hespanhol V, Bárbara C. Pneumonia mortality, comorbidities matter? Pulmonology. 2020;26(3):123–129. doi: 10.1016/j.pulmoe.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Wang D, Zhu J, Wang S, Wang X, Ou Y, Wei D, Li X. Antitussive, expectorant and anti-inflammatory alkaloids from Bulbus Fritillariae Cirrhosae. Fitoterapia. 2011;82(8):1290–1294. doi: 10.1016/j.fitote.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Fu ZZ, Peng Y, Cao LY, Chen YS, Li K, Fu BH. Correlations between serum IL-6 levels and radiation pneumonitis in lung cancer patients: a meta analysis. J Clin Lab Anal. 2016;30(2):145–154. doi: 10.1002/jcla.21828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao H, Ma Y, Zhang LF. Low-molecular-mass hyaluronan induces pulmonary inflammation by up-regulation of Mcl-1 to inhibit neutrophil apoptosis via PI3K/Akt1 pathway. Immunology. 2018;155(3):387–395. doi: 10.1111/imm.12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barratt S, Medford AR, Millar AB. Vascular endothelial growth factor in acute lung injury and acute respiratory distress syndrome. Respiration. 2014;87(4):329–342. doi: 10.1159/000356034. [DOI] [PubMed] [Google Scholar]
- 49.Varet J, Douglas SK, Gilmartin L, Medford AR, Bates DO, Harper SJ, Millar AB. VEGF in the lung: a role for novel isoforms. Am J Physiol Lung Cel Mol Physiol. 2010;298(6):L768–L774. doi: 10.1152/ajplung.00353.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang M, Qian J, Xing X, Kong FM, Zhao L, Chen M, Lawrence TS. Inhibition of the tumor necrosis factor-alpha pathway is radioprotective for the lung. Clin Cancer Res. 2008;14(6):1868–1876. doi: 10.1158/1078-0432.CCR-07-1894. [DOI] [PubMed] [Google Scholar]
- 52.Lavigne J, Suissa A, Verger N, Dos Santos M, Benadjaoud M, Mille-Hamard L, et al. Lung stereotactic arc therapy in mice: development of radiation pneumopathy and influence of HIF-1alpha endothelial deletion. Int J Radiat Oncol Biol Phys. 2019;104(2):279–290. doi: 10.1016/j.ijrobp.2019.01.081. [DOI] [PubMed] [Google Scholar]
- 53.Yu JH, Yuan XP, Liu Y, Zhang KS, Wang J, Zhang HW, Liu FJ. Delayed administration of WP1066, an STAT3 inhibitor, ameliorates radiation-induced lung injury in mice. Lung. 2016;194(1):67–74. doi: 10.1007/s00408-015-9821-8. [DOI] [PubMed] [Google Scholar]
- 54.Liu CX, Chen SL, Xiao XH, Zhang TJ, Hou WB, Liao ML. A new concept on quality marker of Chinese materia medica: quality control for Chinese medicinal products. Chin Tradit Herb Drugs. 2016;47(9):1443–1457. doi: 10.7501/j.issn.0253-2670.2016.09.001. [DOI] [Google Scholar]
- 55.Jin L, Zhang Y, Yang J, Zhou H, Jia G, He Y, Wan H. Investigation of pharmacological mechanisms of Yinhua Pinggan granule on the treatment of pneumonia through network pharmacology and in vitro. Biomed Res Int. 2022;2022:1602447. doi: 10.1155/2022/1602447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tian F, Chen LP, Yuan G, Zhang AM, Jiang Y, Li S. Differences of TNF-α, IL-6 and Gal-3 in lobar pneumonia and bronchial pneumonia caused by mycoplasma pneumoniae. Technol Health Care. 2020;28(6):711–719. doi: 10.3233/THC-192011. [DOI] [PubMed] [Google Scholar]
- 57.Dülger SU, Mutlu N, Ceylan İ, Özhan E. The relationship between lung fibrosis, the epidermal growth factor receptor, and disease outcomes in COVID-19 pneumonia: a postmortem evaluation. Clin Exp Med. 2022;20:1–8. doi: 10.1007/s10238-022-00872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xia QD, Xun Y, Lu JL, Lu YC, Yang YY, Zhou P, Hu J, Li C, Wang SG. Network pharmacology and molecular docking analyses on Lianhua Qingwen capsule indicate Akt1 is a potential target to treat and prevent COVID-19. Cell Prolif. 2020;53(12):e12949. doi: 10.1111/cpr.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin Q, Qua MK, Zhou BJ, Patra HK, SunZH LQ, Yang WY, Wu YC, Zhang Y, Li L, Deng L, Wang LL, Gong T, He Q, Zhang L, Sun X, Zhang ZR. Exosome-like nanoplatform modified with targeting ligand improves anti-cancer and anti-inflammation effects of imperialine. J Control Release. 2019;311–312:104–116. doi: 10.1016/j.jconrel.2019.08.037. [DOI] [PubMed] [Google Scholar]
- 60.Priyanka S, Tushar J, Shalini M, Tanuja J, Hemlata P, Subhash C, Sushma T. Identification of natural inhibitors against Mpro of SARS-CoV-2 by molecular docking, molecular dynamics simulation, and MM/PBSA methods. J Biomol Struct Dyn. 2022;40(6):2757–2768. doi: 10.1080/07391102.2020.1842806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du BX, Cao L, Wang K, Miu JJ, Yao L, Xu ZH, Song J. Peiminine attenuates acute lung injury induced by LPS through inhibiting lipid rafts formation. Inflammation. 2020;43(3):1110–1119. doi: 10.1007/s10753-020-01198-w. [DOI] [PubMed] [Google Scholar]
- 62.Li J, Ma J, Tian Y, Zhao P, Liu X, Dong H, Zheng W, Feng S, Zhang L, Wu M, Zhu L, Liu S. Effective-component compatibility of Bufei Yishen formula II inhibits mucus hypersecretion of chronic obstructive pulmonary disease rats by regulating EGFR/PI3K/mTOR signaling. J Ethnopharmacol. 2020;257:112796. doi: 10.1016/j.jep.2020.112796. [DOI] [PubMed] [Google Scholar]
- 63.Ma X, Liu A, Liu W, Wang Z, Chang N, Li S, Li J, Hou Y, Bai G. Analyze and identify peiminine target EGFR improve lung function and alleviate pulmonary fibrosis to prevent exacerbation of chronic obstructive pulmonary disease by phosphoproteomics analysis. Front Pharmacol. 2019;10:737. doi: 10.3389/fphar.2019.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stalin A, Lin D, Kannan BS, Feng Y, Wang YJ, Zhao W, Ignacimuthu S, Wei DQ, Chen Y. An in-silico approach to identify the potential hot spots in SARS-CoV-2 spike RBD to block the interaction with ACE2 receptor. J Biomol Struct Dyn. 2022;40(16):7408–7423. doi: 10.1080/07391102.2021.1897682. [DOI] [PubMed] [Google Scholar]
- 65.Tong X (2016) Reversing multi-drug resistance on tumor cells and pharmacokinetics study on ingredients in a traditional Chinese medicine Fritillaria thunbergii Miq. Master’s Thesis, Yunnan University of Traditional Chinese Medicine, Kunming, China
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the first author upon reasonable request.