Abstract
Objectives:
Sleep disturbance negatively impacts quality of life and recovery. Our objective was to evaluate the relationship between individual patient and surgical factors with greater sleep disturbance following breast surgery.
Methods:
In this prospective longitudinal study, patients completed validated measures regarding sleep disturbance, pain, opioid use, and psychological symptoms preoperatively and then 2 weeks, 6 and 12 months postoperatively.Univariable and multivariable generalized estimating equations (GEE) evaluated demographic, surgical, pain, and psychological predictors of sleep disturbance during the first year after breast surgery.
Results:
Female patients (n=259) reported varying degrees of sleep disturbance, which were longitudinally associated with pain and psychosocial factors (e.g., anxiety, depression, and affect). Independent preoperative predictors of worse sleep disturbance included younger age (B=−.09, p=.006), opioid use (B=3.09, p=.02), higher pain (B=.19, p=<.001) and anxiety (B=.45, p=<.001) at baseline. Additionally, higher basline positive affect (B=−.14, p=<.012) and the surgical category total mastectomy without reconstruction (B=−2.81, p=<.006) were independently associated with lower sleep disturbance. Those with worse baseline sleep required more opioid analgesics during surgical recovery, and continued use of opioids at 2 weeks post-surgery was associated with disturbed sleep.
Discussion:
Certain patient characteristics including younger age and baseline anxiety, positive affect, pain, and opioid use were associated with greater sleep disturbance in the first year after breast surgery. Sleep disturbance was also associated with greater perioperative and postoperative opioid requirement. Preoperative interventions (e.g. anxiety management, cultivating positive affect, and multimodal pain management) in high risk individuals may enhance sleep and recovery postoperatively, and allow more moderate and less prolonged opioid use.
Keywords: sleep, acute pain, chronic pain, opioid use
Introduction:
Sleep disturbance and insomnia result in disability,1 increased health care costs,2 longer hospital stays,3 worse disease outcomes, lower quality of life,4 and even greater mortality.5, 6 Sleep disturbance and chronic pain often co-occur, with 70–80% of chronic pain patients reporting impaired sleep,7, 8 and approximately 50% of patients with insomnia suffering from chronic pain.9 A growing body of research suggests a bidirectional relationship between sleep and pain, in which a vicious cycle may form, with impaired sleep worsening pain and increased pain subsequently impairing sleep. In lab settings, sleep deprivation results in increased pain perception and impairments in pain modulation.10 Sleep and postsurgical pain also appear to be tightly linked: disturbed sleep the night before surgery and ongoing sleep disturbance postoperatively are both associated with increased postoperative pain.11–13
Around 33% of patients develop persistent pain following mastectomy,14, 15 paralleling forty percent of patients who develop chronic pain symptoms after surgery or trauma.3 With persistent pain occurring in a substantial minority following mastectomy and baseline sleep disturbance being a consistent independent predictor of worse pain outcomes after surgery,15 clarifying the relationship between postoperative sleep disturbance and pain may illuminate important insights and targets for intervention to improve quality of life in patients with breast surgery. Postsurgical pain is often managed using opioids, although more recently, efforts to reduce post-surgical opioid use have increased. Sleep disturbance itself is associated with higher opioid requirements,16 and opioid use is sometimes characterized as an adjuctive aid to allow sleep in the days-weeks after surgery, despite some evidence that opioid use may further disrupt sleep.17, 18 Therefore, efforts to reduce post-surgical pain and opioid use may benefit from studying postoperative sleep disturbance and other patient-specific factors associated with it.
While sleep, pain, and opioid use appear to be tightly linked, their relationship after breast surgery has not been closely examined. Identifying predictors and patient factors that may place individuals at higher risk for sleep disturbance following surgery may allow for insights into how to break the cycle of poor sleep, worsened pain, and inappropriate, persistent, or escalating opioid use. The role of individual patient demographics and psychosocial variables in predicting persistent sleep disturbance has yet to be well characterized. Prospective longitudinal postsurgical studies may be utilized as a relatively structured platform, with a standardized, timed traumatic injury event, to allow for a unique avenue to better understand those at risk for worse sleep and pain outcomes post-surgically.
The primary aim of this prospective, longitudinal study was to identify variations in the course and degree of sleep disturbance following mastectomy, and to identify the longitudinal relationship of pain and other modifying factors to sleep disturbance following breast surgery. The secondary aim was to identify baseline demographic, surgical, pain, and psychological risk factors that are consistently associated with worse sleep disturbance to inform targeting of peri- and post-operative interventions to improve sleep, and in turn decrease pain and opioid use.
2. Materials and Methods:
2.1. Study design and subjects
This prospective, observational cohort study evaluating biopsychosocial modulators of persistent post-mastectomy pain (PPMP) and other longitudinal postoperative outcomes was approved by the Partners Healthcare Institutional Review Board. Patients aged 18–80 years old, who were scheduled to undergo breast cancer surgery at Brigham and Women’s Hospital, were recruited during their visit at the Weiner Center for Preoperative Evaluation between September 2014 and March 2017. Patients with an inability to complete questionnaires because of cognitive impairment or English improficiency were excluded as identified by the treatment team. Previous methods and findings from this cohort reporting on acute postsurgical pain and opioid use (2-weeks post-surgery),19, 20 6-month preliminary post-surgical outcomes,21 and chronic pain 1 year after surgery have been published.22
Data collection
After providing informed consent, participants received a secure email link to complete validated questionnaires via the REDCap electronic data entry system, assessing psychosocial characteristics, demographics, and pain in surgical sites and other body areas before their scheduled surgery (baseline). Patients received automated reminders to complete these same measures at 2 weeks, 6 and 12 months after surgery using the same secure electronic data capture system.
2.2. Surgical Procedures
Patients underwent a range of surgical procedures, reflective of the clinical diversity treated at this tertiary referral academic hospital that is proximate to a cancer center. Procedure details, including type, laterality, duration of surgery, complications, reconstruction type, axillary node dissection and subsequent surgeries were extracted from the patient’s medical records. Surgical type was categorized as (1) breast conservation surgery including partial mastectomy and excisional biopsy, (2) mastectomy with reconstruction (immediate or delayed). Axillary surgery was evaluated independently and categorized as: (0) no axillary surgery, (1) sentinel lymph node biopsy, or (2) axillary lymph node dissection (ALND). Medical record review also documented any subsequent surgeries occurring within the first year after index surgery. Patients with ALND occurring after index surgery were considered in the ALND group. Similarly, patients undergoing subsequent total mastectomy after initial lumpectomy were considered to be part of the total mastectomy group. Patients electronically reported use of other breast cancer treatment(s) including radiation, chemotherapy, or endocrine therapy at one year after surgery.
2.3. Anesthetic care and perioperative pain management
Most patients received general anesthesia (96.1%) or sedation (3.9%). Approximately 40% of patients had a primarily propofol-based anesthetic (>800mg). Surgeons injected local anesthetic (0.25% bupivacaine) at the incision site at the end of the surgery in 70% of cases. Regional anesthesia (RA, ultrasound guided thoracic paravertebral block, proximal intercostal block, and/or pectoralis nerve block) was offered preoperatively to most patients undergoing total mastectomy, with 25.5% of patients receiving regional anesthesia. Additional intraoperative and postoperative analgesics were administered according to current practice and anesthesia and surgical provider preference. These included opioids and non-opioid analgesics such as celecoxib (17.8%), gabapentin (13.9%), ketamine (8.1%) and acetaminophen. Patients self-reported whether they were using opioids during follow-up assessments.
2.4. Sleep quality assessment
Subjects completed the Patient-Reported Outcomes Information System (PROMIS) Sleep Disturbance short-form, which was developed to assess self-reported perceptions of sleep quality, sleep depth, and any perceived difficulties related to getting and staying asleep over a 7-day period.23 The resulting PROMIS Sleep Disturbance short form contains 8 items, with a 6-point Likert scale response items where 0=“not at all” and 5=“very much.” Higher scores represent higher sleep disturbance ranging from 8–40. The raw sum score is rescaled on the PROMIS score conversion table to determine the standardized T-score,24 calibrated such that the mean is 50 and a standard deviation is 10 points. The development of this instrument was validated (1993) on a sample of adults recruited from clinical and community settings. Subsequent validations of a 8-item short-form version,25 validated this scale in a sample with greater prevalence of chronic illnesses, and thus the PROMIS scoring manual advises that a score of 50 may be reflective of a higher presence of sleep disturbances than is likely in an average healthy population. For example, this sample concurrently completed the Pittsburgh Sleep Quality Index.26 More than half (55%) of the derivation cohort were over the PSQI cut-off for poor sleep quality (>5).
2.3. Pain Assessment
Breast Cancer Pain Questionnaire (BCPQ):
Persistent pain was measured using the extended version of a surgery-specific questionnaire, the Breast Cancer Pain Questionnaire (BCPQ) (Appendix A), first developed by Gartner et al.27 and used in subsequent studies.28–37 The BCPQ queries about pain severity (1–10) and frequency (constantly=5, daily=4, occasionally=3, weekly=2, monthly=1, and never=0) in 4 surgically-related body areas (breast, axilla, chest wall, arm). Similar to our previous reports,14, 38, 39 a Pain Severity Index (PSI) score was calculated using: PSI = Σ[Pain score at each site (0–10)] x [frequency (1–5)], with a possible score range of 0–200. The BCPQ also includes questions about the impact of pain on physical activities (Physical Impact of Pain, scores range between 0–38). All questions from the BCPQ are asked directly in reference to the surgical area, including opioid and other analgesic use (use for surgical-related pain).
2.4. Psychological measures
Psychological measures previously associated with persistent pain in a retrospective cohort14 and those with strong psychometric properties and brevity were selected.19 The Pain Catastrophizing Scale (PCS),40, 41 was used to measure pain-associated exaggerated negative, maladaptive thoughts about pain. This 13-item scale uses a 6-point Likert scale where higher scores equate to higher pain catastrophizing, ranging between 0–56. Depressive symptoms and sleep disturbance were assessed using NIH PROMIS short-forms, that also use a 6-point Likert scale for 8-items with higher scores indicating higher symptomatology measured (range 8–40); anxiety PROMIS-SF uses a similar Likert scale and includes 7 items (range 7–35).42 The Brief Symptom Index 18-Somatization Scale43 measured somatization using a 5-point Likert scale for 6 items (range 6–30). The Positive Affect Negative Affect Scale (PANAS)44 is a 20-item scale, uses a 5-point Likert scale, and assesses both symptoms of positive and negative affect; higher scores represent higher affect, ranging between 0–50 for each subscale.
2.5. Sociodemographic variables
Patients self-reported characteristics before surgery including age, ethnicity, highest educational training, and gender.
2.6. Statistical analysis
Descriptive statistics for patient demographics, psychosocial, psychophysical, and pain outcomes are reported as either frequencies and percentages for categorical variables or means and standard deviations for continuous variables. Study sample size (n=200) was determined according previously determined effect sizes for association with the primary outcome of the parent study (persistent post-surgical pain severity). Repeated measure correlations were conducted between sleep disturbance and other psychological and pain measures that were assessed longitudinally, to determine the overall within-individual significant associations between sleep and other variables.45
To identify which baseline demographic, surgical, treatment, opioid, pain, and psychosocial variables were associated with longitudinal sleep disturbance, we conducted simple univariable linear regressions using general estimating equations (GEE) analysis. The GEE method uses an autoregressive correlation structure, which takes into account the correlation between repeated measurements on the same patient and can accommodate missing data across timepoints if this missingness is random (e.g., if patient skipped a survey at a timepoint). To then inform the development of our multivariable model, which allows for the concurrent analysis baseline independent variables with our longitudinal outcome of sleep disturbance, we included variables that were associated with sleep disturbance at the level of significance set at p≤0.1 on univariable analyses. The multivariable model allows for the evaluation of each unique predictor variable on the dependent variable (longitudinal sleep disturbance), while controlling for the impact of other variables included in the multivariable model. Effect sizes are reported as GEE model beta coefficients (B) and confidence intervals (CI). Differences in sleep disturbance between those taking or not taking opioids were evaluated using Mann-Whitney U test, and correlations between perioperative opioid use and sleep disturbance were evaluated using Spearman Correlation analysis. All statistical analyses were performed using SPSS v27, except repeated measure correlations which were conducted using R.
3. Results
Description of the Cohort
Details of the enrollment and retention for this prospective, observational cohort have been previously described.15 Briefly, 259 patients approached at the preoperative clinic consented and completed baseline questionnaires prior to surgery with 218, 216, and 201 patients completing questionnaires at 2-week, 6, and 12 month questionnaires after surgery, respectively. Subjects had a mean age of 55.5 years old, were predominantly Caucasian (86.4%), and 76% reported education of a college degree or higher (Table 1). Indications for breast surgery among these patients included invasive cancer (77%), ductal carcinoma in situ (15%), prophylactic mastectomy (4.5%) and benign lesions (6.1%). Patients underwent a range of surgical procedures. Surgeries were performed by a group of 11 breast surgeons and 12 plastic surgeons. The most common surgery was breast conservation surgery/lumpectomy (54% of patients). The remaining 46% of patients underwent total mastectomy, and 36% also had immediate or delayed reconstruction (28% reconstruction involving tissue expander placement/implant, 8% autologous reconstruction (DIEP or TRAM flap). The most common axillary procedure was sentinel lymph node biopsy (63% of cases), followed by 20% of patients having no axillary procedure, and 16% having axillary lymph node dissection. The majority (96%) of patients received general anesthesia, and 4% received local anesthesia with sedation. Of patients receiving total mastectomy, 44% received a peripheral nerve block (proximal intercostal or paravertebral). Data regarding impact of nerve blocks in mastectomy only cohort are reported in a separate publication.20, 46 Thirteen patients (5%) were taking opioids before surgery, 63 (24%) at 14 days post-surgery, and 8 (3.1%) at 6 and 12 months.
Table 1.
Demographics of patients undergoing breast surgery
| Variable | N (%), mean ± SD |
|---|---|
| Demographics | |
| Age | 55.5±12.4 |
| Body Mass Index (BMI) | 27.4±6.2 |
| Education | |
| Some HS | 4 (1.5%) |
| HS grad/GED | 20 (7.7%) |
| Tech School | 2 (0.8%) |
| Some College | 35 (13.5%) |
| College Graduate | 104 (40.2%) |
| Master’s Degree | 68 (26.3%) |
| Doctoral Degree | 24 (9.3%) |
| Race/ethnicity* | |
| Caucasian | 223 (86.4%) |
| African American | 7 (2.7%) |
| Hispanic/Latina | 5 (1.9) |
| Asian | 11 (4.3%) |
| Mixed race | 8 (3.1%) |
| Other | 4 (1.6%) |
| Surgical Variables | |
| Surgery/reconstruction type, n (%) | |
| Breast conserving surgery (Lumpectomy) | 136 (52.5%) |
| Mastectomy | 34 (13.1%) |
| Mastectomy with reconstruction - tissue expander | 68 (26.3%) |
| Mastectomy with reconstruction - autologous | 21 (8.1%) |
| Node surgery type, n (%) | |
| No axillary surgery | 52 (20.1%) |
| Sentinel lymph node procedure | 165 (63.7%) |
| Axillary lymph node dissection | 42 (16.2%) |
| Medical Treatment within first year after surgery | |
| Radiation therapy, n(%) | 146 (57.3%) |
| Chemotherapy, n (%) | 92 (35.9%) |
| Hormone therapy, n (%) | 126 (49.6%) |
| Baseline pain (Breast Cancer Pain questionnaire, BCPQ) | |
| Pain Severity Index (PSI) (severity x frequency in 4 surgical areas), mean±SD | 4.1±9.3 |
| Surgical Area Pain Severity, mean±SD | 1.2±1.8 |
| Physical Impact, mean±SD | 1.0±2.6 |
| BPI Average Pain, mean±SD | 1.1±1.6 |
| BPI Impairment mean±SD | 9.3±17.5 |
| Severity of chronic pain in other area(s), mean±SD | 1.7±2.5 |
| Opioid use at baseline, n (%) | 13 (5.1%) |
| Psychosocial Variables at baseline | |
| Pain Catastrophizing Scale (PCS total), mean±SD | 6.4±6.9 |
| Anxiety (PROMIS SF), mean t score ±SD | 46.2±6.5 |
| Depression (PROMIS SF), mean t score±SD | 38.9±7.4 |
| Sleep Disturbance (PROMIS SF), mean t score ±SD | 50.62±8.9 |
| Negative Affect (PANAS; range: 10–50), mean±SD | 17.5±5.9 |
| Positive Affect (PANAS; range: 10–50), mean±SD | 34.1±7.4 |
| Somatization (BSI; range: 6–30), mean±SD | 7.0±2.2 |
Sleep Disturbance at baseline and in the first postoperative year
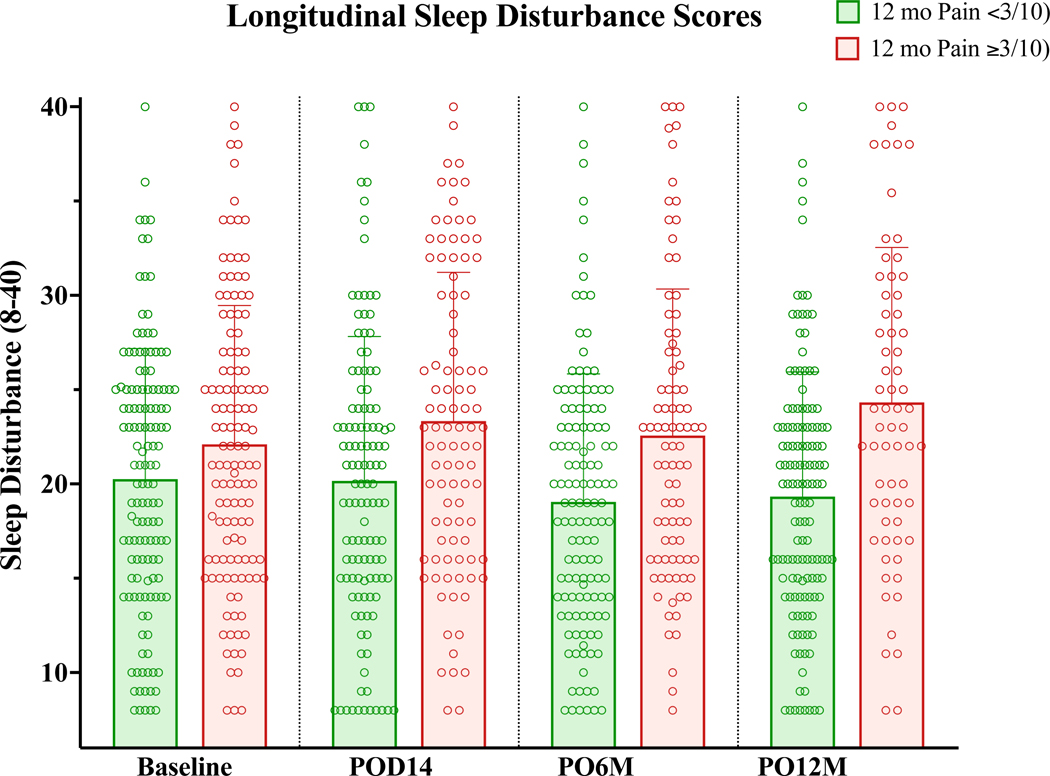
In the current analysis, we were interested in understanding the patterns and predictors of worse sleep during the postsurgical course. We observed a wide variability in sleep disturbance scores among patients at baseline and throughout the first postsurgical year. Sleep disturbance throughout the first year following survey was slightly higher among those who ultimately went on to report persistent pain at 12 months (Figure 1). Approximately 13% (33/259) of patients reported elevated sleep disturbance at baseline (t-scores ≥60, sleep disturbance 1 standard deviation above the mean of the PROMIS derivation cohort). At 2 weeks, 18% (40/218) had reported elevated sleep disturbance (t-scores ≥60), and subsequently this number decreased to baseline levels at 6 months (26/216, 12%) and 12 months (26/201, 13%). Overall sleep disturbance did not change significantly over time (univariable longitudinal GEE analysis, time: B=−0.035, p=.315, 95% CI(−0.103, 0.033)]. See Table 2.
Figure 1:
Variation in Sleep Disturbance in patients who did or did not develop persistent pain (≥ 3/10 severity) at 1 year, showing mean sleep disturbance with standard deviation (bars) and individual patient scores (circles).
Table 2.
Variables measured across time points at baseline, 2-week post-op, 6 months, and 12 months following breast surgery
| Time points, mean ± sd or t-score (se) |
||||
|---|---|---|---|---|
| Clinical characteristics | Baseline | 2 weeks post-op | 6 months | 12 months |
| Sleep disturbance (PROMIS SF, t-score, range) | 51.2 (2.5) | 52.2 (2.5) | 51.2 (2.5) | 51.2 (2.5) |
| Pain Variables | ||||
| Pain Severity Index (PSI) | 4.4 (9.9) | 26.0 (28.4) | 11.7 (20.4) | 9.9 (19.5) |
| Surgical Area Pain Severity | 1.2 (1.9) | 3.4 (2.4) | 2.0 (2.2) | 1.8 (2.3) |
| Physical Impact | 1.4 (3.7) | 8.0 (8.3) | 2.7 (4.6) | 2.6 (4.6) |
| BPI Average Pain | 1.3 (1.8) | 2.7 (2.2) | 1.8 (2.0) | 1.7 (2.1) |
| BPI Impairment | 9.3 (17.5) | 26.0 (24.1) | 11.2 (17.3) | 10.6 (18.0) |
| Psychosocial Variables | ||||
| Pain Catastrophizing Scale (PCS total) | 6.4 (6.9) | 4.8 (6.2) | 4.0 (5.9) | 3.65 (5.3) |
| Anxiety (PROMIS SF, t-score) |
56.3 (2.2) | 53.8 (2.2) | 51.3 (2.3) | 51.3 (2.3) |
| Depression (PROMIS SF, t-score) | 51.2 (2.0) | 51.2 (2.0) | 49.8 (2.2) | 49.8 (2.2) |
| Negative Affect (PANAS; range: 10–50) | 17.5 (5.9) | 16.1 (5.5) | 15.1 (5.2) | 15.4 (5.4) |
| Positive Affect (PANAS; range: 10–50) | 34.1 (7.4) | 32.3 (8.3) | 34.6 (7.9) | 34.4 (8.1) |
| Somatization (BSI; range: 6–30), | 7.6 (2.3) | 9.0 (3.4) | 8.2 (2.7) | 8.2 (2.8) |
| Self-reported opioid use, n (%) | 12 (5%) | 63 (24%) | 8 (3%) | 8 (3%) |
Abbreviations: sd=standard deviation; se=standard error; PSI=severity x frequency in 4 surgical areas.
Longitudinal associations of sleep disturbance with pain outcomes and psychological measures over time
Psychosocial factors measured at equivalent longitudinal timepoints, including pain catastrophizing, anxiety, depression, positive and negative affect and somatization, were significantly correlated with sleep disturbance scores from baseline to 1 year post-surgery (Table 2&3). While symptoms of anxiety were slightly elevated, pain catastrophizing scores were low in this sample. Similarly, persistent pain severity and impact were significantly correlated with sleep disturbance (Table 2&3), such that the degree of sleep disturbance during the first postoperative year was significantly lower among those reporting lower pain scores.
Table 3.
Paired correlations of longitudinal sleep disturbance with pain and psychological assessment across baseline, 2 weeks, 6 and 12 months postoperatively.
| PROMIS Sleep Disturbance | r (95% CI) | df | p |
|---|---|---|---|
| Pain related measures | |||
| Worst Pain Severity (0–10) | 0.143 (0.065, 0.218) | 633 | <.001 |
| Pain Severity Index (0–200) | 0.152 (0.075, 0.228) | 623 | <.001 |
| Physical Impact of Pain | 0.255 (0.175, 0.332) | 540 | <.001 |
| Psychosocial Measures | |||
| Pain Catastrophizing | 0.148 (0.069, 0.226) | 600 | <.001 |
| Anxiety | 0.265 (0.190, 0.337) | 619 | <.001 |
| Depression | 0.289 (0.216, 0.359) | 634 | <.001 |
| PANAS Negative | 0.260 (0.183, 0.335) | 578 | <.001 |
| PANAS Positive | −0.300 (−0.371, −0.225) | 597 | <.001 |
| Somatization | 0.247 (0.171, 0.320) | 611 | <.001 |
Predictors of sleep disturbance in the first postoperative year: univariable linear regressions
Younger age, lower education level, axillary lymph node dissection, higher baseline pain and opioid use, lower pressure pain tolerance, negative affect, positive affect, pain catastrophizing, depression, anxiety, and somatization were significantly associated with greater sleep disturbance (Table 4).
Table 4:
Univariable GEE analysis of baseline variables and PROMIS sleep disturbance across 4 time points (baseline, 2 weeks, 6 months, and 12 months)
| Baseline Variable | B (CI) | SE | Test Statistic | p |
|---|---|---|---|---|
| Univariable GEE | ||||
| Sociodemographics | ||||
| Age | −0.15 (−0.21, −0.09) | 0.03 | 22.26 | <.001 |
| Education | −0.78 (−1.45, −.011) | 0.34 | 5.21 | .022 |
| Non-Caucasian | −0.61 (−3.31, 2.10) | 1.38 | 0.19 | .661 |
| Surgical Variables | ||||
| Surgery/reconstruction type | ||||
| Breast conserving surgery (Lumpectomy) | Ref | Ref | Ref | Ref |
| Mastectomy | −0.95 (−3.40, 1.49) | 1.25 | 0.59 | .444 |
| Mastectomy with reconstruction | 1.70 (−0.06, 3.45) | 0.90 | 3.59 | .058 |
| Axillary procedure | 3.65 (1.42, 5.88) | 1.14 | 10.30 | .001 |
| Treatment variables | ||||
| Radiation therapy | −0.62 (−2.22, 0.99) | 0.82 | 0.57 | .451 |
| Chemotherapy* | 1.70 (0.03, 3.33) | 0.84 | 3.98 | .046 |
| Breast Cancer Pain questionnaire (BCPQ) | ||||
| Pain Severity Index (PSI) | 0.22 (0.10, 0.35) | 0.06 | 11.87 | .001 |
| Opioid use | 4.97 ( 2.19, 7.74) | 1.42 | 12.31 | <.001 |
| Psychological Variables | ||||
| Pain Catastrophizing | 0.25 (0.13, 0.37) | 0.06 | 15.66 | <.001 |
| Anxiety (PROMIS-SF) | 0.49 (0.34, 0.65) | 0.08 | 40.20 | <.001 |
| Depression (PROMIS-SF) | 0.45 (0.26, 0.65) | 0.10 | 21.46 | <.001 |
| Negative Affect (PANAS) | 0.35 (0.19, 0.50) | 0.08 | 18.23 | <.001 |
| Positive Affect (PANAS) | −0.26 (−0.38, −0.15) | 0.06 | 20.41 | <.001 |
| Somatization (BSI) | 0.89 (00.50, 1.27) | 0.20 | 20.65 | <.001 |
Predictors of sleep disturbance in the first postoperative year: Multivariable linear regression
Because many of the identified predictors are covariates, we performed a multivariable linear regression analysis using GEE to identify which of these predictors were independently associated with greater sleep disturbance in the first year after surgery. This analysis identified younger age, baseline opioid use, higher baseline pain severity, and higher baseline anxiety as being independently associated with greater sleep disturbance over time. On the other hand, higher basline positive affect and being in the surgical category of total mastectomy without reconstruction were both independently associated with lower sleep disturbance (Table 5).
Table 5.
Multivariable GEE analysis of baseline variables and PROMIS sleep disturbance across 4 time points (baseline, 2 weeks, 6 months, and 12 months)
| Baseline Variable | B (CI) | SE | Test Statistic | p |
|---|---|---|---|---|
| Multivariable GEE | ||||
|
| ||||
| Sociodemographics | ||||
| Age** | −0.09 (−0.15, −0.02) | 0.03 | 6.99 | .008 |
| Education | −0.56 (−1.10, 0.02) | 0.27 | 4.20 | .04 |
| Surgical Variables | ||||
| Surgery/reconstruction type | ||||
| Breast conserving surgery (Lumpectomy) | Ref | Ref | Ref | Ref |
| Mastectomy** | −2.74 (−4.77, −0.71) | 1.03 | 7.02 | .008 |
| Mastectomy with reconstruction | −0.32 (−1.88, 1.23) | 0.80 | 0.16 | .689 |
| Axillary surgery | 1.78 (−0.23, 3.79) | 1.02 | 3.03 | .082 |
| Treatment variables | ||||
| Chemotherapy | 0.08 (−1.63, 1.79) | 0.87 | 0.01 | .925 |
| Baseline pain (Breast Cancer Pain questionnaire, BCPQ) | ||||
| Pain Severity Index (PSI)*** | 0.19 (0.08, 0.30) | 0.05 | 12.46 | <.001 |
| Opioid use* | 3.14 (0.57, 5.72) | 1.32 | 5.71 | .017 |
| Psychological Variables | ||||
| Pain Catastrophizing Scale (PCS) | 0.01 (−0.08, 0.10) | 0.05 | 0.06 | .810 |
| Anxiety (PROMIS-SF)*** | 0.45 (0.25, 0.65) | 0.10 | 19.26 | <.001 |
| Depression (PROMIS-SF) | −0.02 (−0.24, 0.19) | 0.11 | 0.05 | .816 |
| Negative Affect (PANAS) | −0.05 (−0.23, 0.13) | 0.09 | 0.25 | .615 |
| Positive Affect (PANAS)** | −0.14 (−0.25, −0.03) | 0.06 | 6.57 | .010 |
| Somatization (BSI) | 0.16 (−0.19, 0.51) | 0.18 | 0.80 | .372 |
Relationship of Opioid use and Sleep Disturbance
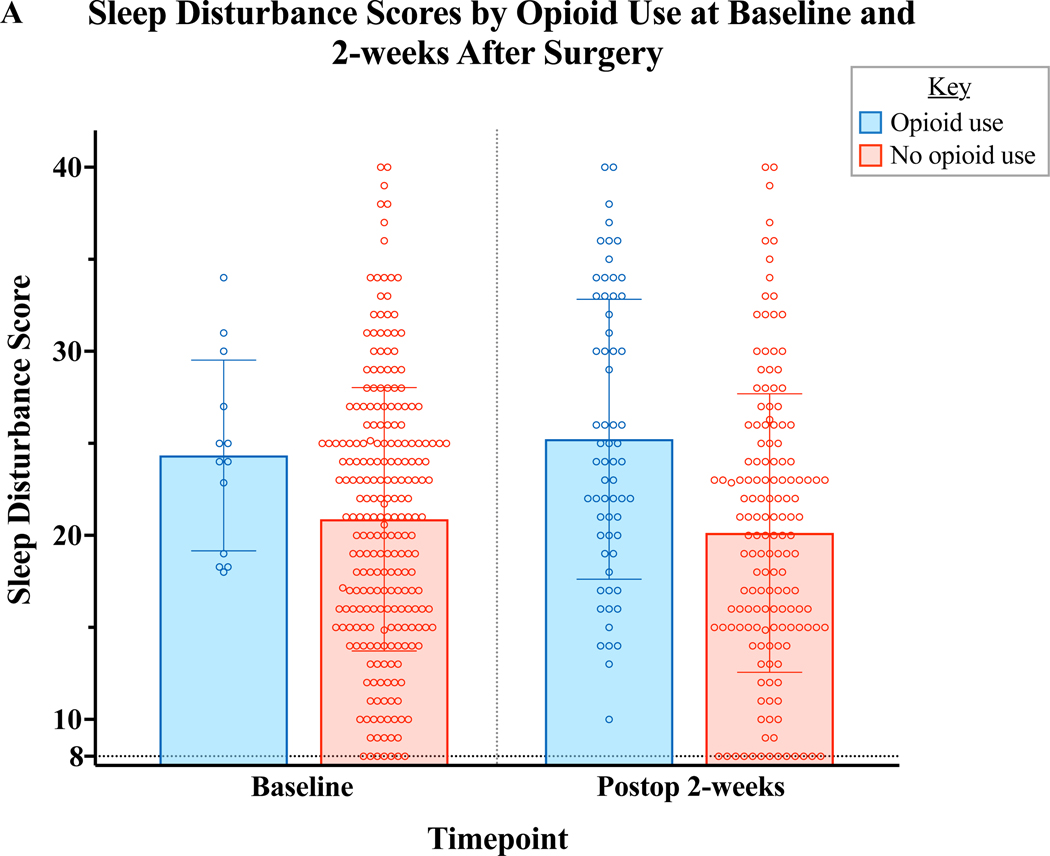
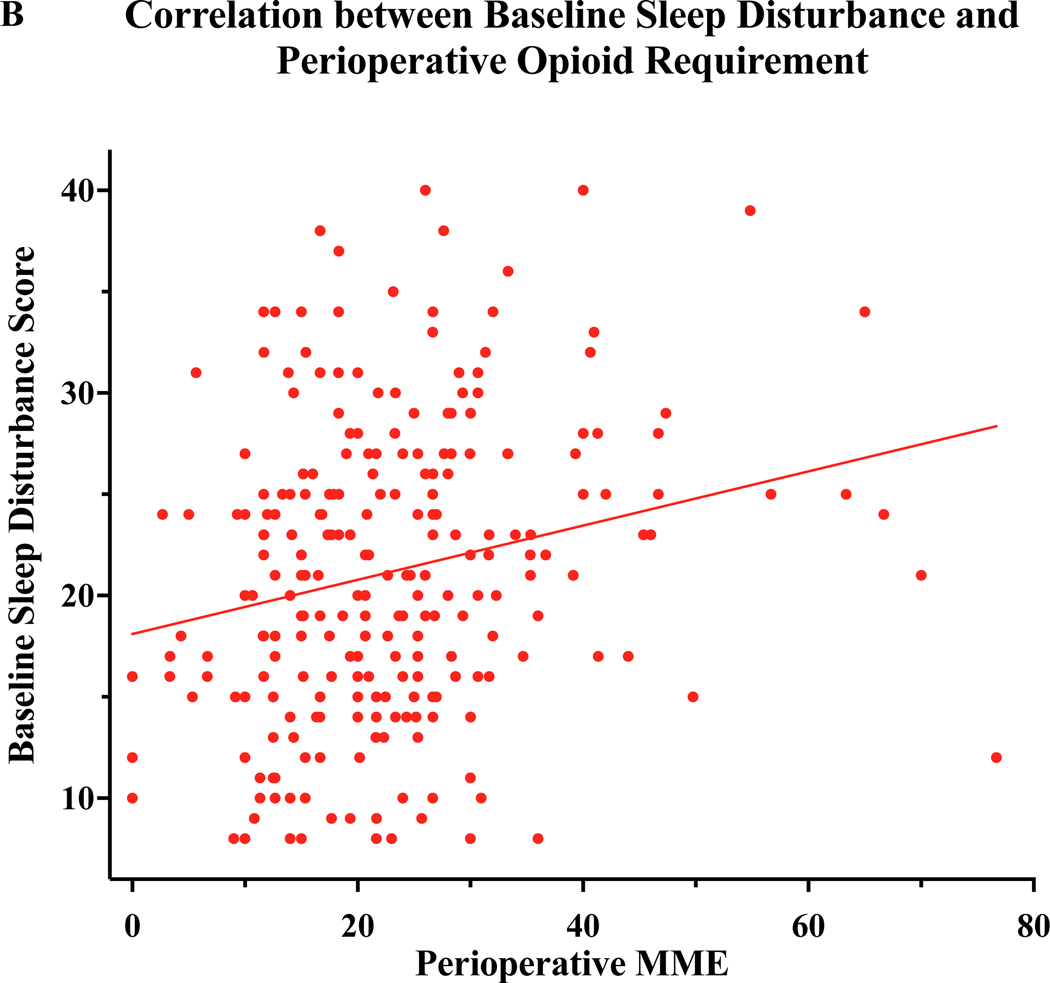
The multivariable regression analysis revealed baseline opioid use as a significant predictor of worse sleep disturbance over the course of the 1st year following breast surgery. In order to more closely investigate the relationship of sleep and opioid utilization, we compared sleep disturbance scores among those who were or were not taking opioids both at baseline and at 2 weeks after surgery. Although few patients reported baseline opioid use, there was a trend toward baseline opioid use and baseline sleep disturbance (Figure 2A). At 2 weeks after surgery, the time at which the largest number of participants reported taking opioids, there was a significant association of disturbed sleep and opioid use (Figure 2A). We also observed that those with worse baseline sleep required more opioid analgesics during the perioperative period (Figure 2B). Very few patients continued to take opioids at 6 and 12 months (n=8/215, 3.1% patients at 6 months and 12 months), therefore associations were tested at 2 week post-op.
Figure 2:
Relationship of Sleep disturbance and Opioid Use. A) Preoperative sleep disturbance scores were marginally higher among patient taking opioids at baseline (Mann-Whitney U=1080, p=0.06). At 2 weeks after surgery, disturbed sleep was strongly associated with continued use of opioids to manage surgical pain (Mann-Whitney U=3100, p<0.001) B) Preoperative sleep disturbance score correlated to a larger perioperative opioid requirement (Spearman Rho=0.23, p<.001). MME:Milligram Morphine Equivalents.
4. Discussion:
Disturbed sleep and surgical area pain are potential persistent complications following breast surgery. In this prospective, observational cohort study of patients undergoing breast surgery, more disturbed sleep parallelled greater pain severity and impact, anxiety, depression, pain catastrophizing, somatization, and opioid use during the 12 months following surgery. In our final multivariable model, baseline factors significantly predicted worsened sleep throughout one year following surgery. Specifically, younger age, baseline opioid use, and higher baseline pain and anxiety were independently predictive of greater sleep disturbance, while the surgical category of those who received total mastectomy without reconstruction (compared to breast conserving surgery) and higher baseline positive affect were independently predictive of lower sleep disturbance in the first year after surgery. Efforts to improve sleep following breast surgery would benefit from integrating pain, anxiety, and resilience/positive psychology interventions – especially for younger patients or those undergoing mastectomy with reconstruction or breast conserving surgery.
Higher anxiety, depression, catastrophizing, and sleep disturbance have all been connected to higher pain after surgery in breast cancer patients,14, 47 19 and may also be associated with a greater likelihood of developing chronic pain post-surgery.39, 47 In a previous analysis of this cohort, sleep disturbance presented as a more consistent independent predictor across different persistent pain outcomes (severity, physical and psychological impact) 12 months after surgery, compared to pain catastrophizing, anxiety, or depression.15 Building on this previous research, we sought to identify the relevant patient-centered factors evaluated at baseline, before surgery, that were predictive of worse sleep disturbance in the 12 months following surgery. Our results suggest that certain individual characteristics predispose patients to worse sleep following surgery; specifically, younger age, surgical type, and baseline anxiety, pain, and opioid use were important predictors of sleep disturbance following breast surgery. Many of these same individual characteristics overlap with predictors of worse pain, underscoring the strong relationship between sleep, pain, and psychological health during recovery. Despite the tendency for sleep to generally worsen with age, being younger was related to more disturbed sleep in the year after surgery. This is likely related to the fact that younger patients experiences greater postsurgical pain in this cohort.19, 47, 48 Although most surgical factors were not significant predictors of sleep disturbance (including axillary lymph node dissection, which is consistently associated with greater pain in this cohort15), patients receiving total mastectomy without reconstruction had less disturbed sleep in the first postoperative year. This was a somewhat surprising finding, given that this group also reported equivalent or somewhat worse pain than those having surgery with reconstruction or those having breast conserving surgery.15 It is conceivable that the removal of all breast tissue, and the absence of subsequent concerns about reconstruction-related focus on breast appearance may facilitate closure in the path of breast cancer management, although this hypothesis requires further investigation. Further vigilance may be needed to continue patient surveillance and management of their reconstruction-related concerns. Identifying patients before surgery and providing pre-surgical interventions for individuals at particular risk could be especially important in modifying post-surgical sleep and pain outcomes.
To our knowledge, few studies have evaluated the co-occuring, longitudinal relationship between pain and sleep following mastectomy that may underscore particular intervention targets to prevent persistent post-mastectomy pain and improve subsequent quality of life. Our findings highlight that changes in sleep and pain occur in tandem, likely fluctuating with psychological symptoms following mastectomy. Other studies have supported our findings that sleep is related to worse pain outcomes and psychological symptomatology,49–52 and biological changes occurring with sleep deprivation result in increased pain sensitivity and impaired conditioned pain modulation.10 Although more studies are needed to specify the directionality of these relationships, our findings suggest that over time there lies a unique overlap between sleep, pain, psychological symptoms, and opioid use following mastectomy.
Opioids are often utilized to treat acute postoperative pain and promote restorative sleep. Both pain and psychological factors may influence postoperative opioid use,53 and sleep disturbance specifically has been associated with greater likelihood of using opioids.16, 50 It is possible that disturbed sleep causes more pain, increased pain leads to more opioid use, and more opioid use leads to disturbed sleep, thus fueling a viscious cycle of worse sleep and pain post-surgically. Patients with more severe pain after surgery are in fact more likely to require opioids, but some evidence suggests opioids further disrupt sleep.16, 17 Although it may be the case that individuals with worse pain are more likely to use more opioids and have worse sleep, we also observed an independent association of opioid use with sleep disturbance, even with postoperative pain severity included in the multivariable model, indicating a significant relationship between opioid use and sleep. From a biological standpoint, opioids have been shown to inhibit several aspects of sleep, including both rapid eye movement (REM) sleep as well as deeper restorative non-REM sleep,18, 54–56 possibly through a mu-opioid receptor dependent suppression of GABA-ergic sleep promoting neurons.57
Preliminary evidence suggests that behavioral interventions such as CBT may improve both sleep and pain outcomes in patients with chronic pain.58 In the perioperative context, behavioral interventions in breast surgery patient may also improve the ability to self-taper from opioids.59 Interventions are often provided post-surgically in response to the incidence of these symptoms; however, evidence suggests that pre-surgical interventions may be a better approach to prevent incidence or worsening of symptoms for patient phenotypes with particular risk. For example, patients with pre-surgical profiles including individuals with baseline opioid use, who are young or Caucasian, and report higher pain and anxiety could benefit from targeted pre-surgical, pre-habilitation interventions to potentially reduce negative sleep and pain outcomes following breast surgery. Future research should explicitly evaluate the role of pre-habilitation programs directed at proactively improving sleep, and assessing the resultant impact on pain and opioid use.
Limitations:
Despite its strengths in prospective, longitudinal, and systematic methodology in measuring a wide variety of biopsychosocial factors simultaneously on sleep disturbance, this study has limitations. First, as an observational study, any associations seen between variables should not be interpreted as causal. While sleep and pain have been shown to influence each other in other studies, no conclusions regarding the existence or directionality of effect can be inferred from this study. Second, observations within the context of surgical pain and injury may not generalize to other types of chronic pain. Surgical interventions often reduce sleep and worsen pain, adding salience and relevance to the interaction between the two, which may not be present in other contexts – for example, the relationship between sleep and pain was not found in chronic musculoskeletal pain.60, 61 Third, the patient sample, while reflecting the patients at our institution, may not reflect that of other institutions; a more demographically diverse sample would allow greater generalization of findings.
Conclusion:
Individuals who are taking opioids, are younger, report lower positive affect and higher baseline anxiety and pain experience greater sleep disturbance throughout the first year following breast surgery; those who underwent mastectomy without reconstruction in turn endorsed lower sleep disturbance. Patients with these pre-surgical phenotypes could benefit from pre-surgical sleep or anxiety interventions to target modifiable factors prior to surgery that may improve sleep disturbance and other relevant pain outcomes. This study provides unique insights in understanding the relationships between post surgical sleep, pain, opioid use, and relevant psychological factors and developing chronic pain and sleep disturbance following breast surgery.
Supplementary Material
References
- 1.Cremeans-Smith JK, Millington K, Sledjeski E, Greene K, Delahanty DL. Sleep disruptions mediate the relationship between early postoperative pain and later functioning following total knee replacement surgery. Journal of behavioral medicine. 2006;29(2):215–222. [DOI] [PubMed] [Google Scholar]
- 2.Sarsour K, Kalsekar A, Swindle R, Foley K, Walsh JK. The association between insomnia severity and healthcare and productivity costs in a health plan sample. Sleep. 2011;34(4):443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J-p Lu S-f, Guo L-n Ren C-g, Zhang Z-w . Poor preoperative sleep quality is a risk factor for severe postoperative pain after breast cancer surgery: a prospective cohort study. Medicine. 2019;98(44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poole L, Kidd T, Leigh E, Ronaldson A, Jahangiri M, Steptoe A. Preoperative sleep complaints are associated with poor physical recovery in the months following cardiac surgery. Annals of Behavioral Medicine. 2014;47(3):347–357. [DOI] [PubMed] [Google Scholar]
- 5.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosomatic medicine. 2003;65(1):63–73. [DOI] [PubMed] [Google Scholar]
- 7.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. The Journal of Pain. 2013;14(12):1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep medicine reviews. 2004;8(2):119–132. [DOI] [PubMed] [Google Scholar]
- 9.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30(2):213–218. [DOI] [PubMed] [Google Scholar]
- 10.Staffe AT, Bech MW, Clemmensen SLK, Nielsen HT, Larsen DB, Petersen KK. Total sleep deprivation increases pain sensitivity, impairs conditioned pain modulation and facilitates temporal summation of pain in healthy participants. PloS one. 2019;14(12):e0225849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright CE, Bovbjerg DH, Montgomery GH, et al. Disrupted sleep the night before breast surgery is associated with increased postoperative pain. Journal of pain and symptom management. 2009;37(3):352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lautenbacher S, Kundermann B, Krieg J-C. Sleep deprivation and pain perception. Sleep medicine reviews. 2006;10(5):357–369. [DOI] [PubMed] [Google Scholar]
- 13.Skarpsno ES, Mork PJ, Nilsen TIL, Steingrímsdóttir ÓA, Zwart JA, Nilsen KB. The interplay between sleeplessness and high-sensitivity C-reactive protein on risk of chronic musculoskeletal pain: longitudinal data from the Tromsø Study. Sleep. 2019;42(9):zsz127. [DOI] [PubMed] [Google Scholar]
- 14.Belfer I, Schreiber KL, Shaffer JR, et al. Persistent postmastectomy pain in breast cancer survivors: analysis of clinical, demographic, and psychosocial factors. J Pain. Oct 2013;14(10):1185–95. doi: 10.1016/j.jpain.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 15.Schreiber KL, Zinboonyahgoon N, Flowers KM, et al. Prediction of Persistent Pain Severity and Impact 12 Months After Breast Surgery Using Comprehensive Preoperative Assessment of Biopsychosocial Pain Modulators. Annals of Surgical Oncology. 2021/January/15 2021;doi: 10.1245/s10434-020-09479-2 [DOI] [PMC free article] [PubMed]
- 16.Webster LR, Choi Y, Desai H, Webster L, Grant BJ. Sleep-disordered breathing and chronic opioid therapy. Pain Medicine. 2008;9(4):425–432. [DOI] [PubMed] [Google Scholar]
- 17.Kjølhede P, Langström P, Nilsson P, Wodlin NB, Nilsson L. The impact of quality of sleep on recovery from fast-track abdominal hysterectomy. Journal of Clinical Sleep Medicine. 2012;8(4):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimsdale JE, Norman D, DeJardin D, Wallace MS. The effect of opioids on sleep architecture. Journal of clinical sleep medicine. 2007;3(01):33–36. [PubMed] [Google Scholar]
- 19.Schreiber KL, Zinboonyahgoon N, Xu X, et al. Preoperative Psychosocial and Psychophysical Phenotypes as Predictors of Acute Pain Outcomes After Breast Surgery. J Pain. May 2019;20(5):540–556. doi: 10.1016/j.jpain.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zinboonyahgoon N, Vlassakov K, Lirk P, et al. Benefit of regional anaesthesia on postoperative pain following mastectomy: the influence of catastrophising. Br J Anaesth. Aug 2019;123(2):e293–e302. doi: 10.1016/j.bja.2019.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spivey TL, Gutowski ED, Zinboonyahgoon N, et al. Chronic pain after breast surgery: a prospective, observational study. Annals of Surgical Oncology. 2018;25(10):2917–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber K, Zinboonyahgoon N, Flowers KM, et al. Prediction of Persistent Pain Severity and Impact 12 months after Breast Surgery using comprehensive Preoperative Assessment of Biopsychosocial Pain Modulators. Annals of Surgical Oncology. in press;[accepted 27-Oct-2020] [DOI] [PMC free article] [PubMed]
- 23.Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33(6):781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Group PC. User Manual: Patient-reported outcomes measurement information system (PROMIS), Version 1.1. Unpublished Manual for the Patient Reported Outcomes Measurement Information System (PROMIS). 2008;
- 25.Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS™ sleep disturbance and sleep-related impairment item banks. Behavioral sleep medicine. 2012;10(1):6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds III CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 27.Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. Nov 11 2009;302(18):1985–92. doi: 10.1001/jama.2009.1568 [DOI] [PubMed] [Google Scholar]
- 28.Ammitzboll G, Andersen KG, Bidstrup PE, et al. Effect of progressive resistance training on persistent pain after axillary dissection in breast cancer: a randomized controlled trial. Breast Cancer Res Treat. Jan 2020;179(1):173–183. doi: 10.1007/s10549-019-05461-z [DOI] [PubMed] [Google Scholar]
- 29.Andersen KG, Gartner R, Kroman N, Flyger H, Kehlet H. Persistent pain after targeted intraoperative radiotherapy (TARGIT) or external breast radiotherapy for breast cancer: a randomized trial. Breast. Feb 2012;21(1):46–9. doi: 10.1016/j.breast.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 30.Andersen KG, Jensen MB, Kehlet H, Gartner R, Eckhoff L, Kroman N. Persistent pain, sensory disturbances and functional impairment after adjuvant chemotherapy for breast cancer: cyclophosphamide, epirubicin and fluorouracil compared with docetaxel + epirubicin and cyclophosphamide. Acta Oncol. Nov 2012;51(8):1036–44. doi: 10.3109/0284186X.2012.692884 [DOI] [PubMed] [Google Scholar]
- 31.Andersen KG, Christensen KB, Kehlet H, Bidstup PE. The Effect of Pain on Physical Functioning After Breast Cancer Treatment: Development and Validation of an Assessment Tool. Clin J Pain. Sep 2015;31(9):794–802. doi: 10.1097/AJP.0000000000000156 [DOI] [PubMed] [Google Scholar]
- 32.Andersen KG, Jensen MB, Tvedskov TF, Kehlet H, Gartner R, Kroman N. Persistent pain, sensory disturbances and functional impairment after immediate or delayed axillary lymph node dissection. Eur J Surg Oncol. Jan 2013;39(1):31–5. doi: 10.1016/j.ejso.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 33.Gartner R, Jensen MB, Kronborg L, Ewertz M, Kehlet H, Kroman N. Self-reported arm-lymphedema and functional impairment after breast cancer treatment--a nationwide study of prevalence and associated factors. Breast. Dec 2010;19(6):506–15. doi: 10.1016/j.breast.2010.05.015 [DOI] [PubMed] [Google Scholar]
- 34.Hamood R, Hamood H, Merhasin I, Keinan-Boker L. Chronic pain and other symptoms among breast cancer survivors: prevalence, predictors, and effects on quality of life. Breast Cancer Res Treat. Jan 2018;167(1):157–169. doi: 10.1007/s10549-017-4485-0 [DOI] [PubMed] [Google Scholar]
- 35.Klit A, Mejdahl MK, Gartner R, Elberg JJ, Kroman N, Andersen KG. Breast reconstruction with an expander prosthesis following mastectomy does not cause additional persistent pain: a nationwide cross-sectional study. J Plast Reconstr Aesthet Surg. Dec 2013;66(12):1652–8. doi: 10.1016/j.bjps.2013.07.015 [DOI] [PubMed] [Google Scholar]
- 36.Mejdahl MK, Andersen KG, Gartner R, Kroman N, Kehlet H. Persistent pain and sensory disturbances after treatment for breast cancer: six year nationwide follow-up study. BMJ. Apr 11 2013;346:f1865. doi: 10.1136/bmj.f1865 [DOI] [PubMed] [Google Scholar]
- 37.Mertz BG, Duriaud HM, Kroman N, Andersen KG. Pain, sensory disturbances and psychological distress are common sequelae after treatment of ductal carcinoma in situ: a cross-sectional study. Acta Oncol. May 2017;56(5):724–729. doi: 10.1080/0284186X.2017.1295167 [DOI] [PubMed] [Google Scholar]
- 38.Schreiber KL, Belfer I, Miaskowski C, Schumacher M, Stacey BR, Van De Ven T. AAAPT Diagnostic Criteria for Acute Pain Following Breast Surgery. J Pain. Sep 5 2019;doi: 10.1016/j.jpain.2019.08.008 [DOI] [PMC free article] [PubMed]
- 39.Schreiber KL, Martel MO, Shnol H, et al. Persistent pain in postmastectomy patients: comparison of psychophysical, medical, surgical, and psychosocial characteristics between patients with and without pain. Pain. May 2013;154(5):660–8. doi: 10.1016/j.pain.2012.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychological assessment. 1995;7(4):524. [Google Scholar]
- 41.Darnall BD, Sturgeon JA, Cook KF, et al. Development and validation of a daily pain catastrophizing scale. The Journal of Pain. 2017;18(9):1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. Nov 2010;63(11):1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dura E, Andreu Y, Galdon MJ, et al. Psychological assessment of patients with temporomandibular disorders: confirmatory analysis of the dimensional structure of the Brief Symptoms Inventory 18. J Psychosom Res. Apr 2006;60(4):365–70. doi: 10.1016/j.jpsychores.2005.10.013 [DOI] [PubMed] [Google Scholar]
- 44.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. Jun 1988;54(6):1063–70. [DOI] [PubMed] [Google Scholar]
- 45.Bakdash JZ, Marusich LR, Marusich MLR. Package ‘rmcorr’. 2016;
- 46.Zinboonyahgoon N, Patton ME, Chen Y-YK, Edwards RR, Schreiber KL. Persistent Post-Mastectomy Pain: The Impact of Regional Anesthesia Among Patients with High vs Low Baseline Catastrophizing. Pain Medicine. 2021; [DOI] [PMC free article] [PubMed]
- 47.Miaskowski C, Paul SM, Cooper B, et al. Identification of patient subgroups and risk factors for persistent arm/shoulder pain following breast cancer surgery. Eur J Oncol Nurs. Jun 2014;18(3):242–53. doi: 10.1016/j.ejon.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. Jul 2011;12(7):725–46. doi: 10.1016/j.jpain.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 49.Bulls HW, Hoogland AI, Small BJ, et al. Lagged Relationships Among Chemotherapy-Induced Peripheral Neuropathy, Sleep Quality, and Physical Activity During and After Chemotherapy. Annals of Behavioral Medicine. 2020; [DOI] [PMC free article] [PubMed]
- 50.Azizoddin DR, Schreiber K, Beck M, et al. Chronic Pain Severity, Impact, and Opioid Use Among Patients with Cancer: An analysis of biopsychosocial factors using the CHOIR learning healthcare system. Cancer. 2021; [DOI] [PMC free article] [PubMed]
- 51.Stepanski EJ, Walker MS, Schwartzberg LS, Blakely LJ, Ong JC, Houts AC. The relation of trouble sleeping, depressed mood, pain, and fatigue in patients with cancer. Journal of Clinical Sleep Medicine. 2009;5(2):132–136. [PMC free article] [PubMed] [Google Scholar]
- 52.Palesh OG, Collie K, Batiuchok D, et al. A longitudinal study of depression, pain, and stress as predictors of sleep disturbance among women with metastatic breast cancer. Biological psychology. 2007;75(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Özalp G, Sarioglu R, Tuncel G, Aslan K, Kadiogullari N. Preoperative emotional states in patients with breast cancer and postoperative pain. Acta Anaesthesiologica Scandinavica. 2003;47(1):26–29. [DOI] [PubMed] [Google Scholar]
- 54.Nelson AM, Battersby AS, Baghdoyan HA, Lydic R. Opioid-induced decreases in rat brain adenosine levels are reversed by inhibiting adenosine deaminase. The Journal of the American Society of Anesthesiologists. 2009;111(6):1327–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore JT, Kelz MB. Opiates, sleep, and pain: the adenosinergic link. The Journal of the American Society of Anesthesiologists. 2009;111(6):1175–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis S, Oswald I, Evans J, Akindele M, Tompsett S. Heroin and human sleep. Electroencephalography and clinical neurophysiology. 1970;28(4):374–381. [PubMed] [Google Scholar]
- 57.Wang Q, Yue X-F, Qu W-M, et al. Morphine inhibits sleep-promoting neurons in the ventrolateral preoptic area via mu receptors and induces wakefulness in rats. Neuropsychopharmacology. 2013;38(5):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vitiello MV, McCurry SM, Shortreed SM, et al. Cognitive‐behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: The lifestyles randomized controlled trial. Journal of the American Geriatrics Society. 2013;61(6):947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Darnall BD, Ziadni MS, Krishnamurthy P, et al. “My surgical success”: effect of a digital behavioral pain medicine intervention on time to opioid cessation after breast cancer surgery—a pilot randomized controlled clinical trial. Pain Medicine. 2019;20(11):2228–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stroemel-Scheder C, Karmann AJ, Ziegler E, et al. Sleep, experimental pain and clinical pain in patients with chronic musculoskeletal pain and healthy controls. Journal of pain research. 2019;12:3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karmann AJ, Lauer C, Ziegler E, Killian L, Horn-Hofmann C, Lautenbacher S. Associations of nocturnal sleep with experimental pain and pain catastrophizing in healthy volunteers. Biological psychology. 2018;135:1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.