Abstract
Tuberous sclerosis complex (TSC) is a multiple system neurocutaneous syndrome with a genetic disorder caused by different mutations in TSC1 or TSC2. Usually, TSC causes tumors in the heart, brain, kidneys, eyes, and lungs. However, tumors can also develop in any other organs. The prenatal diagnosis of TCS is based on the identification of fetal cardiac tumors by ultrasound and brain subependymal nodules, usually identified by fetal magnetic resonance imaging (MRI). We present two case reports of the prenatal diagnosis of TCS using both ultrasound and MRI, which were confirmed by clinical and radiological methods in the postnatal period accordingly.
Keywords: prenatal diagnosis, tuberous sclerosis complex, ultrasound, magnetic resonance imaging
Introduction
Prenatal diagnosis of tuberous sclerosis complex (TSC) can show rhabdomyomas, subependymal nodules, cortical tubers, and renal angiomyolipomas, which are the major features of TSC. 1 2 3 Ultrasound, being a noninvasive and repeatable examination, can be used to detect multiple nodules or a hyperechogenic myocardium, usually from the 20th week of pregnancy. 3 4 Currently, fetal magnetic resonance imaging (MRI) can identify brain lesions and sometimes even renal lesions, thus enabling parental counselling and postnatal management with target therapy. 5 Subependymal nodules and cortical tubers are T1-hyperintense and T2-hypointense, the former being more easily identified on fetal imaging. 5 6 However, subcortical lesions are more commonly detected in postnatal MRI. 7
In this article, we present two cases of prenatal diagnosis of TSC using both ultrasound and MRI.
Case Report
Case 1
This case involved a primiparous (G1-P0) 35-year-old pregnant woman with no family history of TSC. Ultrasound at 24 weeks of gestational age showed an echogenic mass in the left ventricle of the fetal heart, consistent with a rhabdomyoma ( Fig. 1 ). Fetal MRI at 26 weeks of gestational age showed a typical brain subependymal nodule ( Fig. 2 ). She underwent a cesarean section due to breech presentation at 39 weeks' gestation and delivered a male newborn weighing 3,250 g. Apgar scores at the 1st and 5th minutes were 9 and 10, respectively. Postnatal clinical and imaging diagnoses confirmed the same findings seen in prenatal examinations. Genetic tests have not been performed to date.
Fig. 1.
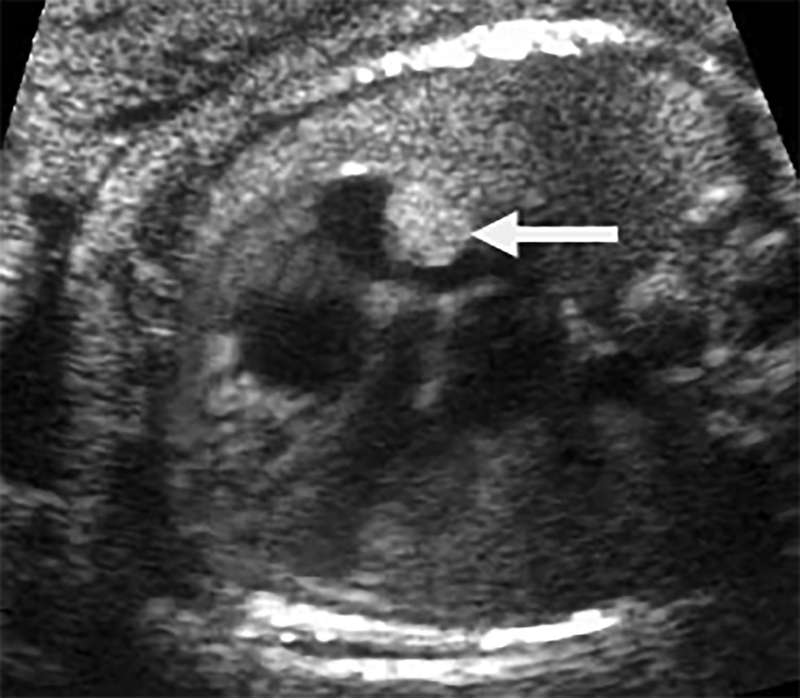
Case 1. Ultrasound image showing a cardiac rhabdomyoma (arrow) in the right atrium (26 weeks).
Fig. 2.
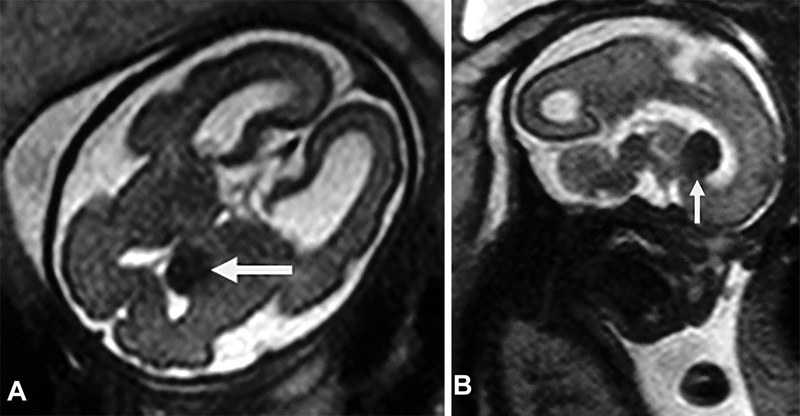
Case 1. ( A ) Axial T2-weighted magnetic resonance image showing a brain subependymal nodule (arrow). ( B ) Sagittal T2-weighted image showing a typical brain subependymal nodule (arrow).
Case 2
This case involved a G1-P0 32-year-old pregnant woman, with no family history of TSC. Ultrasound at 32 weeks of gestational age showed an echogenic mass in the fetal heart, consistent with a rhabdomyoma, and hyperechoic lesions in the fetal brain ( Fig. 3 ). Fetal MRI at 35 weeks of gestational age showed typical brain periventricular and subependymal nodules as well as a cardiac rhabdomyoma ( Fig. 4 ). She underwent a cesarean section at 38 weeks' gestation due to low placental implantation and delivered a male weighing 3,100 g. Apgar scores at the 1st and 5th minutes were 9 and 10, respectively. Postnatal clinical and prenatal imaging diagnoses confirmed the same findings. Genetic tests have not been performed to date.
Fig. 3.
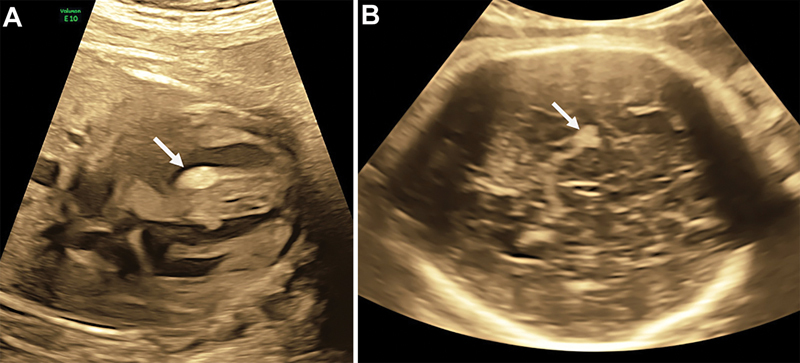
Case 2. ( A ) Ultrasound image showing a cardiac rhabdomyoma (arrow) in the interventricular septum (35 weeks). ( B ) Ultrasound image showing a brain cortical nodule (arrow).
Fig. 4.
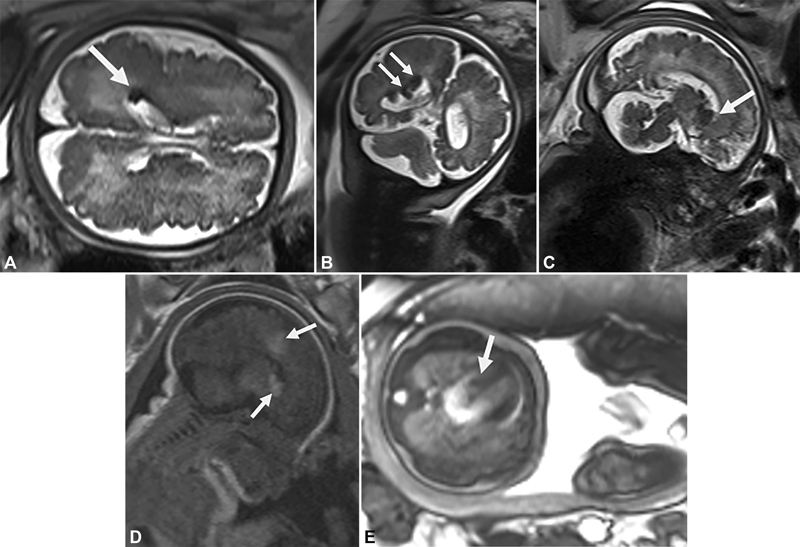
Case 2 ( A ) Axial T2-weighted magnetic resonance image (half Fourier single-shot turbo spin-echo [HASTE]) at 35 weeks of gestational age showing a brain periventricular low-signal-intensity nodule (arrow). ( B ) Coronal T2 image (HASTE) at 35 weeks of gestational age showing brain periventricular low-signal-intensity nodules (arrows). ( C ) Sagittal T2-weighted image (HASTE) at 35 weeks of gestational age showing a brain subependymal low-signal-intensity nodule (arrow). ( D ) Sagittal T1-weighted image at 35 weeks of gestational age showing a brain subependymal and cortical high-signal-intensity nodule (arrows). ( E ): Axial T2-weighted image showing a cardiac rhabdomyoma in the interventricular septum (35 weeks) (arrow).
Discussion
After the diagnosis of TSC, a multidisciplinary approach, depending on the most affected systems or organs, is required. Testing for subclinical features with clinical examination, MRI, echocardiogram, electrocardiogram, chest computed tomography (CT), and blood workup needs to be carried out accordingly. 8
Most rhabdomyomas are benign, but they can lead to the obstruction of the cardiac flow depending on the size or location. Sometime this may be the only feature present in the prenatal period, with others developing later in life. They also have a high rate of spontaneous regression up to the age of 5 years. 5
Prenatal diagnosis allows the children to be tested even before the onset of epilepsy symptoms, leading to easier surveillance or targeted treatment. 5 Early findings can make parents aware of the risk of development of autism spectrum disorders and more specifically, of the early signs of the disorder. 9
Some brain lesions that suggest neurodevelopmental diseases can be detected on a third trimester ultrasound, despite this not being a routine exam for low-risk pregnancies in most countries. 10 Signs of renal impairment during the prenatal period are rare, and the reported cases showed cystic formations (unilateral or even diffuse polycystic involvement), accompanied by other findings in the fetal brain or heart. 5
In our case reports, we observed the brain periventricular and subependymal nodules on fetal MRI. Sonigo et al 11 assessed eight fetuses with multiple cardiac tumors, five of which showed hyperintense subependymal and cortical nodules on Tl-weighted images. They concluded that MRI was a valuable tool for diagnosing TSC in fetuses with multiple cardiac tumors.
Age does not compromise a good imaging assessment, but it facilitates the diagnosis of TSC by MRI. Most subependymal nodules detected in the fetus and neonate are noncalcified. If TSC is clinically suspected in the first 3 months of life, imaging should not be delayed. TSC nodules in fetus and neonates are hyperintense on T1-weighted images and hypointense on T2-weighted images. The low degree of myelination helps to identify white matter abnormalities, which become less visible as myelination progresses. Since nodules calcify in childhood, they are more easily detected on CT and MRI susceptibility weighted images. 12
Chen et al 13 demonstrated that ultrafast MRI can detect small subependymal nodules in fetuses with cardiac tumors. The accuracy of ultrasound in identifying brain periventricular/cortical nodules was low. However, we identified a typical brain hyperechogenic cortical nodule on ultrasound in case 2.
Conclusion
In summary, we presented two case reports of prenatal diagnosis of TSC using both ultrasound and MRI. Ultrasound is the first-line screening method for TSC by the identification of cardiac tumors, and MRI is important for identifying typical brain subependymal nodules accordingly.
Footnotes
Conflict of Interest None declared.
References
- 1.Wang C C, Wang C Y, Lai Y J, Chang T Y, Su H Y. Prenatal diagnosis of tuberous sclerosis complex using fetal ultrasonography and magnetic resonance imaging and genetic testing. Taiwan J Obstet Gynecol. 2018;57(01):163–165. doi: 10.1016/j.tjog.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 2.Sharma N, Sharma S, Thiek J L, Ahanthem S S, Kalita A, Lynser D. Maternal and fetal tuberous sclerosis: do we know enough as an obstetrician? J Reprod Infertil. 2017;18(02):257–260. [PMC free article] [PubMed] [Google Scholar]
- 3.Onay ÖS, Sağlık AÇ, Köşger P. Maternal and fetal tuberous sclerosis complex: a case report questioning clinical approach. Turk J Pediatr. 2020;62(02):332–337. doi: 10.24953/turkjped.2020.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Cotaina G L, Lázaro G E, Jiménez M I, Savirón C R, Lerma P D. [Diagnosis of cardiac rhabdomyoma in the first trimester of pregnancy] Ginecol Obstet Mex. 2016;84(03):180–185. [PubMed] [Google Scholar]
- 5.Dragoumi P, O'Callaghan F, Zafeiriou D I. Diagnosis of tuberous sclerosis complex in the fetus. Eur J Paediatr Neurol. 2018;22(06):1027–1034. doi: 10.1016/j.ejpn.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Goel R, Aggarwal N, Lemmon M E, Bosemani T. Fetal and maternal manifestations of tuberous sclerosis complex: value of fetal MRI. Neuroradiol J. 2016;29(01):57–60. doi: 10.1177/1971400915621323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y, Dong S Z, Zhong Y M, Sun A M. Prenatal and postnatal diagnosis of rhabdomyomas and tuberous sclerosis complex by ultrafast and standard MRI. Indian J Pediatr. 2018;85(09):729–737. doi: 10.1007/s12098-017-2592-x. [DOI] [PubMed] [Google Scholar]
- 8.Sarafidis P A, Bikos A, Loutradis C. Diagnosis of tuberous sclerosis complex in a patient referred for uncontrolled hypertension and renal dysfunction: a case highlighting the importance of proper diagnostic work-up of hypertensive patients. J Hypertens. 2017;35(10):2109–2114. doi: 10.1097/HJH.0000000000001423. [DOI] [PubMed] [Google Scholar]
- 9.Ornoy A, Weinstein-Fudim L, Ergaz Z. Genetic syndromes, maternal diseases and antenatal factors associated with autism spectrum disorders (ASD) Front Neurosci. 2016;10:316. doi: 10.3389/fnins.2016.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mongrain V, van Doesburg N H, Rypens F. A case report of severe tuberous sclerosis complex detected in utero and linked to a novel duplication in the TSC2 gene. BMC Neurol. 2020;20(01):324. doi: 10.1186/s12883-020-01905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonigo P, Elmaleh A, Fermont L, Delezoíde A L, Mirlesse V, Brunelle F. Prenatal MRI diagnosis of fetal cerebral tuberous sclerosis. Pediatr Radiol. 1996;26(01):1–4. doi: 10.1007/BF01403693. [DOI] [PubMed] [Google Scholar]
- 12.Baron Y, Barkovich A J. MR imaging of tuberous sclerosis in neonates and young infants. AJNR Am J Neuroradiol. 1999;20(05):907–916. [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C P, Liu Y P, Huang J K. Contribution of ultrafast magnetic resonance imaging in prenatal diagnosis of sonographically undetected cerebral tuberous sclerosis associated with cardiac rhabdomyomas. Prenat Diagn. 2005;25(06):523–524. doi: 10.1002/pd.1182. [DOI] [PubMed] [Google Scholar]


