Abstract
The sustained epidemic of Omicron subvariants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a worldwide concern, and older adults are at high risk. We conducted a prospective cohort study to assess the immunogenicity of COVID-19 mRNA vaccines (BNT162b2 or mRNA-1273) in nursing home residents and staff between May 2021 and December 2022. A total of 335 SARS-CoV-2 naïve individuals, including 141 residents (median age: 88 years) and 194 staff (median age: 44 years) participated. Receptor-binding domain (RBD) and nucleocapsid (N) protein IgG and neutralizing titer (NT) against the Wuhan strain, Alpha and Delta variants, and Omicron BA.1 and BA.5 subvariants were measured in serum samples drawn from participants after the second and third doses of mRNA vaccine using SARS-CoV-2 pseudotyped virus. Breakthrough infection (BTI) was confirmed by a notification of COVID-19 or a positive anti-N IgG result in serum after mRNA vaccination. Fifty-one participants experienced SARS-CoV-2 BTI during the study period. The RBD IgG and NTs against Omicron BA.1 and BA.5 were markedly increased in SARS CoV-2 naïve participants 2 months after the third dose of mRNA vaccine, compared to those 5 months after the second dose, and declined 5 months after the third dose. The decline in RBD IgG and NT against Omicron BA.1 and BA.5 in SARS-CoV-2 naïve participants after the second and the third dose was particularly marked in those aged ≥ 80 years. BTIs during the BA.5 epidemic period, which occurred between 2 and 5 months after the third dose, induced a robust NT against BA.5 even five months after the booster dose vaccination. Further studies are required to assess the sustainability of NTs elicited by Omicron-containing bivalent mRNA booster vaccine in older adults.
Keywords: COVID-19, Omicron subvariants, mRNA vaccine, Older adults, Neutralizing antibody, Pseudotyped virus
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by severe respiratory syndrome coronavirus 2 (SARS-CoV-2), which was first detected in a patient with pneumonia in December 2019 in Wuhan, China, and became pandemic in early 2020 [1]. Older adults who live in nursing homes are at high risk of COVID-19 [2], and it has been reported that 14 % and 26.6 % of COVID-19 deaths in Japan and the United States, respectively, have occurred among nursing home residents [3], [4], [5]. A previous study on the clusters of COVID-19 in long-term care facilities (LTCFs) during early 2020 found that both a larger number and size of clusters in LTCFs were independently linked to higher mortality [5], highlighting the importance of preventing COVID-19 outbreaks in LTCFs.
COVID-19 vaccine development was rapid and vaccines have been introduced worldwide since the end of 2020. In randomized, controlled trials conducted in 2020, the COVID-19 messenger RNA (mRNA) vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna), were more than 90 % effective at preventing COVID-19 approximately 2 months after vaccination [6], [7]. In Japan, the 1st and 2nd dose of mRNA vaccines (BNT162b2 vaccine) have been administered to healthcare workers since February 2021, followed by adults aged ≥ 65 years, adults with underlying medical conditions, adults aged 60–64 years, and nursing home staff. The booster dose by BNT162b2 vaccine or mRNA-1273 vaccine has been administered to the same vaccine targets more than 6 months after the 2nd dose.
Several SARS-CoV-2 variants have been reported worldwide since December 2020, including B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), B.1.617.2 (Delta), and B.1.1.529 (Omicron) [8]. These variants of concern which have altered virus characteristics, such as transmissibility and antigenicity, may have evolved in response to herd immunity [9]. The initial mRNA vaccines targeted the Wuhan strain, and certain SARS-CoV-2 variants have shown evidence of immune evasion [10], [11]. A recent study reported high mortality and morbidity among vaccinated residents in a nursing home in Kyoto Prefecture, Japan during an outbreak of the Omicron variant [12].
We conducted a cohort study of residents and staff of nursing homes in Toyama Prefecture, Japan to evaluate the antibody responses against the SARS-CoV-2 Omicron subvariants after receiving an mRNA vaccine in people at high risk of COVID-19.
2. Materials and methods
2.1. Study participants
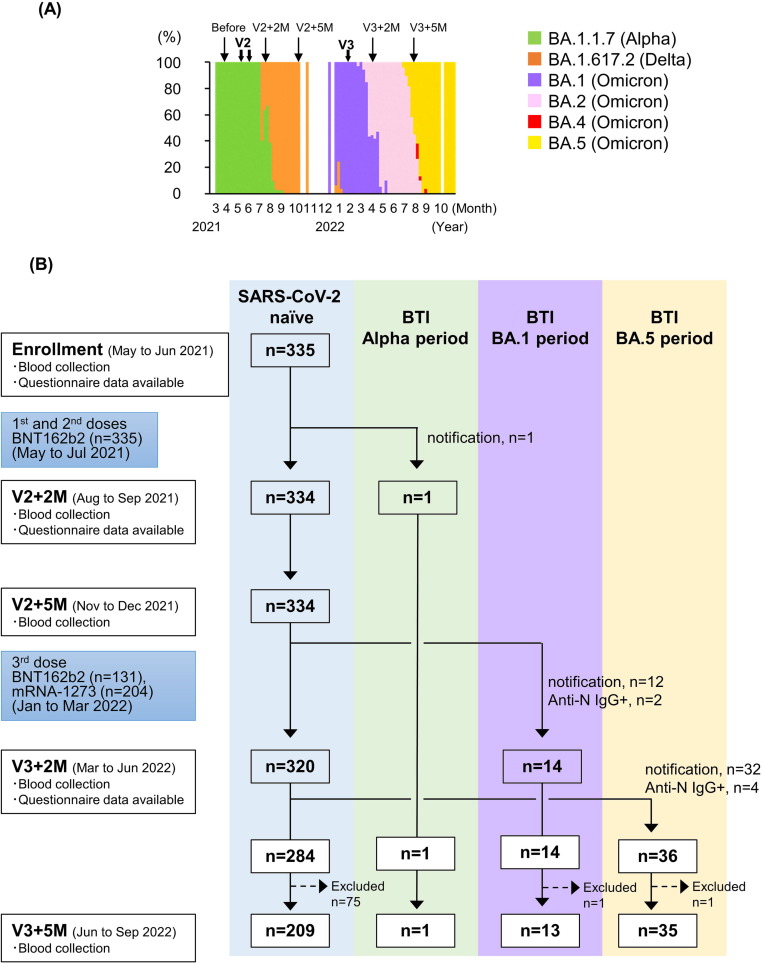
This study was conducted between May 2021 and December 2022, and a total of 335 SARS-CoV-2 naïve individuals were enrolled from six nursing homes in Toyama Prefecture, Japan, on May 2021. The study participants included 141 residents (median age: 88 years; range: 57–103 years) and 194 facility staff (median age: 44 years, range: 18–79 years). We defined a case of breakthrough infection (BTI) as a notification of COVID-19 confirmed by a positive result of reverse transcription polymerase chain reaction (RT-PCR) or immunochromatography test for SARS CoV-2, or a positive result of anti-N IgG in serum, a biomarker of natural infection [13], following COVID-19 mRNA vaccination. Consequently, the study participants consisted of 284 SARS CoV-2 naïve (161 staff and 123 residents) and 51 SARS-CoV-2 BTI participants which included one BTI participant during epidemic period of Alpha variant, 14 BTI participants during epidemic period of BA.1 subvariant, and 36 BTIs during epidemic period of BA.5 subvariant 2 months after the third dose (Table, Fig. 1 B).
Fig. 1.
Genomic epidemiology and study design. (A) Genomic epidemiology of SARS-CoV-2 variants or Omicron subvariants in Toyama Prefecture. The proportions of each variant or Omicron subvariant were determined by analyzing 0 to 178 SARS-CoV-2 positive samples in each month. (B) Flow chart of the nursing home cohort study. After the enrollment of 335 SARS CoV-2 naïve participants, participants with BTI were detected during epidemic periods of Alpha variant (n = 1), BA.1 subvariant (n = 14) and BA.5 subvariant (n = 36). Six participants had positive results for anti-N IgG 2 months (n = 2) and 5 months (n = 4) after the third dose. Seventy-seven study participants were excluded from the study because 49 received a fourth dose of mRNA vaccine before blood collection 5 months after the third dose. Fourteen staff members and seven residents declined blood collection 5 months after the third dose of mRNA vaccine, and seven residents died of old age. Abbreviations: V2, 1st and 2nd dose of vaccine; V3, 3rd dose of vaccine, V2 + 2 M, 2 months after the 2nd dose of vaccine; V2 + 5 M, 5 months after the 2nd dose of vaccine; V3 + 2 M, 2 months after the 3rd dose of vaccine; V3 + 5 M, 5 months after the 3rd dose of vaccine, BTI; breakthrough infection.
All participants received two doses of the BNT162b2 vaccine (30 μg per dose) intramuscularly as the initial series vaccination between April and June 2021. For the third dose, 131 participants received the BNT162b2 vaccine, and 204 participants received the mRNA-1273 vaccine (100 μg per dose) between January and March 2022 (Fig. 1A and B). Blood sampling was carried out from May to June 2021 prior to COVID-19 vaccination, and two and five months following the primary vaccination series from August to September 2021 and November to December 2021, respectively. Blood sampling was carried out two and five months following the booster (third dose) vaccination from March to June 2022 and June to September 2022, respectively.
2.2. Whole-genome sequencing of SARS-CoV-2
Clinical samples of nasopharyngeal swabs or saliva, which tested positive by PCR or antigen test at hospitals in Toyama Prefecture, were collected at Toyama Institute of Health under the national surveillance of COVID-19. A total of 1,356 samples were subjected to the genomic analyses between March 2021 and October 2022. Viral RNA was isolated using a QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. Whole-genome amplification of escape mutants was performed using a modified version of the ARTIC Network protocol for SARS-CoV-2 genome sequencing [14]. A next-generation sequencing (NGS) library was constructed using the QIAseq FX DNA Library kit (Qiagen) and sequenced using the iSeq platform (Illumina, San Diego, CA, USA). NGS reads were mapped to the SARS-CoV-2 Wuhan-Hu-1 reference genome sequence (GenBank accession no. MN908947.3) using the BWA-MEM algorithm (version 0.7.13-r1126) [15], and variant allele frequency analysis was conducted using VarScan version 2.4.3 [16], [17]. Subsequently, the variant of Alpha (B.1.1.7) or Delta (B.1.617.2), or the subvariant of Omicron BA.1 (B.1.1.529.1) or Omicron BA.5 (B.1.1.529.5) were determined using COG-UK (https://pangolin.cog-uk.io/) for each SARS-CoV-2 positive sample. We were, however, unable to determine the genotype of participants with BTI notified from the nursing homes in this study.
2.3. Neutralizing activity against SARS-CoV-2 pseudotyped virus
Pseudotyped vesicular stomatitis viruses (VSVs) bearing SARS-CoV-2 S proteins were generated as previously described [18]. The expression plasmids for the truncated spike (S) protein of SARS-CoV-2 variants, pCAGG-pm3-SARS2-SHu-d19_Eng (Alpha variant), pCAGG-pm3-SARS2-SHu-d19_B.1.617.2 (Delta variant), pCAGG-pm3-SARS2-SHu-d19_B.1.1.529.1 (Omicron BA.1 subvariant), and pCAGG-pm3-SARS2-SHu-d19_B.1.1.529.5 (Omicron BA.5 subvariant), were provided by Drs. C. Ono and Y. Matsuura of the Research Institute for Microbial Diseases, Osaka University, Japan. The SARS-CoV-2 pseudotyped VSVs (SARS-CoV-2pv) were stored at − 80 °C until use.
Serum samples were collected from participants 2 and 5 months after two doses of the initial vaccination series, and 2 and 5 months after the third vaccination. Neutralizing activity was measured using a chemiluminescence reduction neutralizing test of SARS-CoV2pv bearing S proteins of the ancestral strain (Wuhan), Alpha (B.1.1.7), Delta (B.1.617.2), Omicron BA.1 (B.1.1.529.1), and Omicron BA.5 (B.1.1.529.5). Briefly, VeroE6/TMPRSS2 cells on 96-well plates were mixed with 1 μL of participant serum in Dulbecco’s Modified Eagle’s Medium containing 8 % fetal bovine serum and 10 μL of SARS-CoV-2pv, and incubated at 37 ℃, 5 %CO2 overnight. The negative and positive controls were assayed in the same manner as the test samples. The level of infection inhibition was calculated using luciferase activity as an indicator of neutralizing activity, and each specimen was measured in duplicate. Neutralizing activity was expressed as the neutralizing titer (NT) by dividing the value of luciferase activity in the negative control serum by the value of luciferase activity in the test serum using SARS-CoV-2pv. The chemiluminescent assay was performed using a Pica gene BrillianStar-LT (TOYO B-Net, Tokyo, Japan), and measured using a GloMax navigator system (Promega, WI, USA). We confirmed significant correlations between the NTs against SARS-CoV2pv and those against SARS-CoV-2 live virus using rabbit and human immune serum (Supplementary Fig. 1). SARS-CoV-2 live virus and rabbit immune serum were kindly provided by Dr. S. Fukushi, National Institute of Infectious Diseases, Japan [19].
2.4. SARS-CoV-2 IgG ELISA
Receptor-binding domain (RBD) protein (1 μg/mL, 50 μL/well) (ACRO Biosystems, Newark, DE, USA) of the SARS-CoV-2 Wuhan strain or nucleocapsid (N) protein (1 μg/mL, 50 μL/well) (ACRO Biosystems, Newark, DE, USA) was coated onto enzyme-linked immunosorbent assay plates overnight at 4 °C. The plates were washed with phosphate-buffered saline containing 0.1 % Tween 20 (PBST) and incubated with 20 % Blocking-One plus PBST. After blocking, serum diluted 400- or 1600-fold for RBD protein, and 100-fold for N protein was added, and the cells were washed. Next, anti-human IgG horseradish peroxidase (Promega, Madison, WI, USA) was added, washed, and ABTS solution (Merck, Darmstadt, Germany) was added. After stopping the reaction with 4 N sulfuric acid, the enzyme activity was detected at 405/490 nm using a plate reader. An absorbance of 1.0 or higher was interpreted as positive for anti-N protein IgG. The dynamic ranges were 1 to 1.0 × 106 for neutralizing antibody titers, and 0 to 8.0 × 104 U/mL for anti-RBD IgG and anti-N protein IgG, respectively.
2.5. Statistical analysis
RBD IgG values and NT activity by virus strain, age, and sex were analyzed using IBM SPSS Statistics 27.0 (IBM, Armonk, NY). The NT value was log-transformed. Statistical significance was defined as p < 0.05. The Mann–Whitney U test and Wilcoxon signed-rank test were used to compare non-parametric variables between groups. Correlations between the test findings were expressed using Pearson’s correlation coefficient. The Jonckheere–Terpstra test was used to determine the trend in titer by age group.
2.6. Ethical approval
This study was approved by the Ethical Review Committee of the Toyama Institute of Health (approval number #R3-11) and was conducted according to the principles expressed in the Declaration of Helsinki. Informed consent was obtained from the participants or their surrogates.
3. Results
3.1. Genomic epidemiology of SARS-CoV-2
In Toyama Prefecture, the COVID-19 epidemic caused by the Alpha and the Delta variants emerged between March and August and between July and October 2021, respectively. The COVID-19 epidemic caused by the Omicron variant (BA.1 subvariant) began in December 2021 in Japan. Omicron BA.1 was replaced by BA.2 between April and July 2022, which was then replaced by Omicron BA.5 between August and December 2022 (Fig. 1A).
3.2. RBD IgG and NT response in SARS-CoV-2 naïve participants
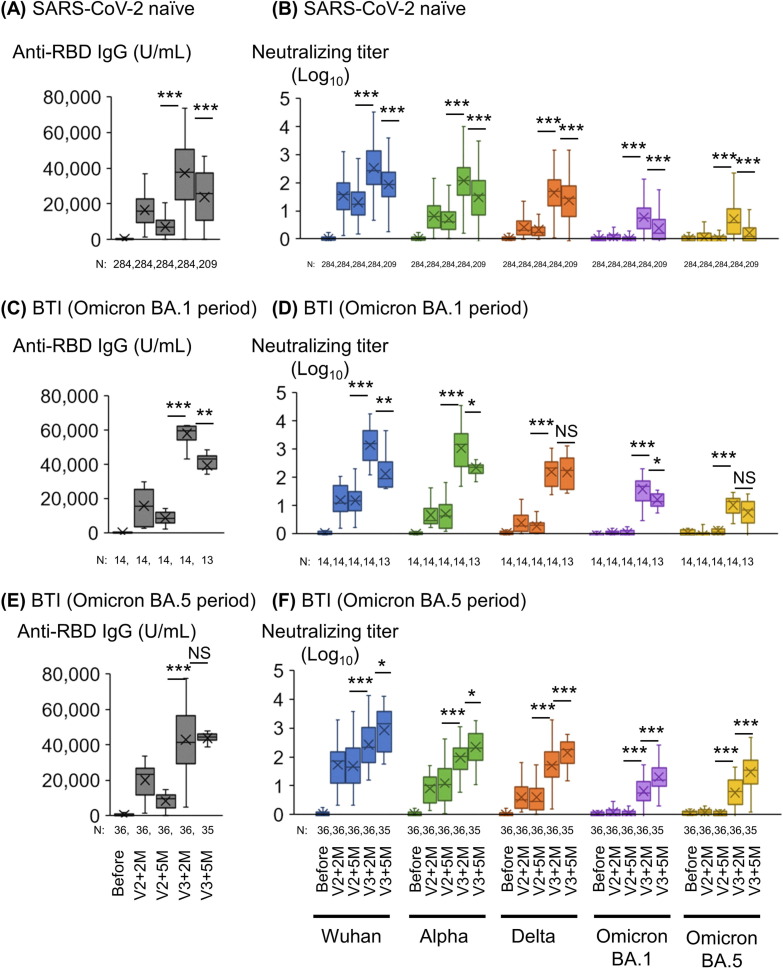
The geometric mean RBD IgG titer in serum samples from 284 SARS-CoV-2 naïve participants increased from the baseline value to 1.6 × 104 U/mL 2 months after the second dose and decreased to 7.1 × 103 U/mL 5 months after the second dose (Fig. 2 A). The geometric mean RBD IgG titer of 284 SARS-CoV-2 naïve participants increased to 3.7 × 104 U/mL 2 months after the third dose, and the geometric mean RBD IgG titer of 211 participants who were SARS-CoV-2 naïve declined to 2.3 × 104 U/mL 5 months after the third dose. In 284 SARS-CoV-2 naïve participants, the geometric mean NTs against Wuhan, and Alpha, Delta, and Omicron BA.1 and BA.5 against SARS-CoV-2pv increased from the baseline value to 33.8, 6.4, 2.7, 1.2 and 1.1, respectively, 2 months after the second dose, and declined to 19.9, 5.2, 2.1, 1.0 and 1.0, respectively, 5 months after the second dose of mRNA vaccine (Fig. 2B). In contrast, the geometric mean NTs against Wuhan, and Alpha, Delta, and Omicron BA.1, and BA.5 in 284 SARS-CoV-2 naïve participants increased noticeably to 336.0, 119.4, 43.1, 6.0, and 5.2, respectively, 2 months after the third vaccination, and declined to 88.0, 30.1, 23.2, 2.4, and 1.7, respectively, 5 months after the third dose of mRNA vaccine.
Fig. 2.
Anti-RBD IgG titers and neutralizing titers at 2 and 5 months after two doses and the third dose of mRNA vaccine in participants with or without BTI. (A) SARS-CoV-2 RBD IgG antibody titers, (B) Neutralizing titers against SARS-CoV-2pv of SARS-CoV-2 naïve participants. (C) SARS-CoV-2 RBD IgG antibody titers, (D) Neutralizing titers of participants with BTI during BA.1 epidemic period (January to March 2022). (E) SARS-CoV-2 RBD IgG antibody titers, (F) Neutralizing titers of participants with BTI during BA.5 epidemic period (June to September 2022). 14 participants were infected from January to March 2022 (during the Omicron BA.1 epidemic period); and 36 participants were infected from June to September 2022 (during the Omicron BA.5 epidemic period). ***, p < 0.001; **, p < 0.01; *, p < 0.05; NS, not significant. Abbreviations: RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; V2, 2nd dose of vaccine; V2 + 2 M, 2 months after the 2nd dose of vaccine; V2 + 5 M, 5 months after the 2nd dose of vaccine; V3, 3rd dose of vaccine; V3 + 2 M, 2 months after the 3rd dose of vaccine; V3 + 5 M, 5 months after the 3rd dose of vaccine; BTI, breakthrough infection.
One participant, a facility staff member aged 41 years, had BTI during the Alpha epidemic period (August 2021), and the RBD IgG and NTs in this participant increased 2 months after the third dose of mRNA vaccine (Supplementary Fig. 2). During the Omicron BA.1 epidemic period (Jan to Mar 2022), 14 participants had BTI before the third dose of COVID-19 mRNA vaccine. The geometric mean RBD IgG titer in these 14 participants with BTI markedly increased (5.8 × 104 U/mL) two months after the third dose, from the baseline 5 months after the second dose (3.9 × 104 U/mL, p < 0.001) (Fig. 2C). The geometric mean NTs against Wuhan, Alpha, Delta, and Omicron BA.1 and BA.5 also significantly increased (1373.4, 1077.8, 155.7, 38.1, and 10.5, respectively) in these 14 participants with BTI from the baseline 5 months after the second dose (p < 0.001, Fig. 2D). The geometric mean NTs in these participants with BTI significantly declined 5 months after the third dose, compared to those 2 months after the third dose (p < 0.01 for Wuhan, p < 0.05 for Alpha and Omicron BA.1).
During the Omicron BA.5 epidemic period (Jun to Sep 2022), the geometric mean RBD IgG titer (4.4 × 104 U/mL) in 35 participants with BTI five months after the third dose did not differ from those 2 months after the third dose (4.3 × 104 U/mL, p > 0.05, Fig. 2E). The geometric mean NTs against Wuhan, Alpha, Delta, and Omicron BA.1 and BA.5 significantly increased in these 35 participants two months after the third dose (266.3, 95.2, 50.6, 6.4, and 5.7) from the baseline 5 months after the second dose (p < 0.001, Fig. 2F). Because 35 participants had BTI between 2 and 5 months after the third dose, the geometric mean NTs against Wuhan, Alpha, Delta, and Omicron BA.1 and BA.5 significantly increased (860.0, 220.7, 141.0, 20.1, and 30.0) in these 35 participants 5 months after the third dose, compared with those 2 months after the third dose (p < 0.001 for Delta, Omicron BA.1 and BA.5, p < 0.05 for Wuhan and Alpha).
3.3. RBD IgG and NT response in the participants SARS-CoV-2 naïve participants by age group
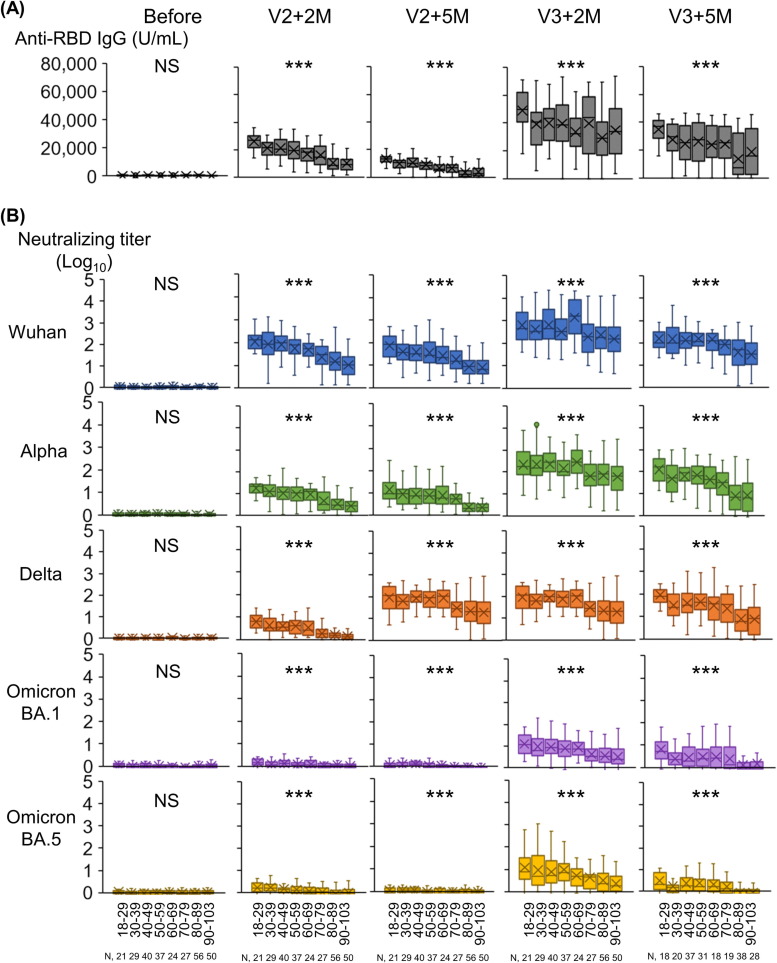
The geometric mean RBD IgG titers and NTs by age group of the SARS-CoV-2 naïve participants are shown in Fig. 3 . Two and 5 months after the second and third doses, the decline in the geometric mean of RBD IgG titers was significantly correlated with age (p < 0.001, Fig. 3A). Two and 5 months after the second dose, the decline in the geometric mean NTs against Wuhan, Alpha, Delta, Omicron BA.1 and BA.5 were similarly correlated with age (p < 0.001, Fig. 3B). Two and 5 months after the second dose, the NTs against Omicron BA.1 and BA.5 were at negligible levels in all age groups. In contrast, the NTs against Omicron BA.1 and BA.5 were increased 2 months after the third dose but waned 5 months after the third dose, especially in those aged 80 years and older. Two and 5 months after the third dose, the NTs against Omicron BA.1 and BA.5 were significantly correlated with age (p < 0.001, Fig. 3B).
Fig. 3.
Anti-RBD IgG titers and neutralizing titers 2 and 5 months after two and three doses of mRNA vaccine in SARS-CoV2 naïve participants by age group. (A) SARS-CoV-2 RBD IgG titer. (B) Neutralizing titers against SARS-CoV-2pv. N = 284. ***, p < 0.001; *, p < 0.05; NS, not significant, using the Jonckheere-Terpstra test. Abbreviations: RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SARS-CoV-2pv, severe acute respiratory syndrome coronavirus 2 pseudotyped virus.
We also examined whether the immune responses after the second and third dose of COVID-19 mRNA vaccine differed between the staff and the residents in the SARS CoV-2 naïve participants in a common age group of 57–79 years (Table 1 ). Although NTs against Wuhan, Alpha, Delta, and Omicron BA.5 were significantly higher in the staff than in the residents after the second dose of mRNA vaccine, no difference was found in enhanced levels of RBD IgG and NTs against Wuhan and all variants including BA.5 between the two groups 2 months after the third dose (median age: 64 years for the staff and 75 years for the residents) (Supplementary Fig. 3).
Table 1.
Sex and age distribution and SARS-CoV-2 infection status of study participants.
| SARS-CoV-2 naïve |
SARS-CoV-2 breakthrough infection |
Total | |||||
|---|---|---|---|---|---|---|---|
| Staff | Residents | Subtotal | Staff | Residents | Subtotal | ||
| Participants (n) | 161 | 123 | 284 | 33 | 18 | 51 | 335 |
| Female (%) | 114 (71) | 92 (75) | 206 (73) | 28 (85) | 16 (89) | 44 (86) | 250 (75) |
| Age, years, median (IQR) | 47(36–58) | 88 (83–93) | 66 (45–86) | 38 (26–43) | 90 (85–94) | 43 (35–86) | 62 (43–86) |
| Age group | |||||||
| 18–29 years | 21 | 21 | 9 | 9 | 30 | ||
| 30–39 years | 29 | 29 | 9 | 9 | 38 | ||
| 40–49 years | 40 | 40 | 11 | 11 | 51 | ||
| 50–59 years | 36 | 1 | 37 | 2 | 2 | 39 | |
| 60–69 years | 22 | 2 | 24 | 2 | 2 | 26 | |
| 70–79 years | 13 | 14 | 27 | 2 | 2 | 29 | |
| 80–89 years | 56 | 56 | 7 | 7 | 63 | ||
| 90–103 years | 50 | 50 | 9 | 9 | 59 | ||
IQR: interquartile range.
4. Discussion
In this study, a booster dose of mRNA vaccine resulted in a marked increase in RBD IgG titers and NTs in nursing home residents and staff. The NTs after the second and third doses were highest against the Wuhan strain, followed by the Alpha, Delta and Omicron BA.1, and BA.5 variants, indicating immune evasion by SARS-CoV-2 variants from the herd immunity raised by three doses of mRNA-based COVID-19 vaccine [9], [10], [11]. Our results showing that a third dose of mRNA vaccine elicited an enhanced immune response in RBD IgG and NTs against variant strains, including the Omicron BA.1 variant, are consistent with the results of previous studies in healthcare workers [20], [21], [22] and nursing home residents [23], [24]. In this study, we also found that a third dose of mRNA vaccine induced an enhanced NT against Omicron BA.5 which was comparable to those against BA.1. A recent study reported that two doses of mRNA vaccine induced an expanded antibody breadth for approximately 5 months after vaccination. The authors suggested that the neutralizing breadth of the resting memory B cell subset may induce Omicron-neutralizing antibodies after a third dose of mRNA vaccine [25].
We also found that BTIs that occurred in the 35 participants during the BA.5 epidemic period augmented NTs against Wuhan, Alpha, and Delta, and Omicron BA.1 and BA.5, induced by the third dose of COVID-19 mRNA vaccine. Previous reports on the boosting antibody response in healthcare workers after one or two doses of mRNA vaccine are consistent with our findings [26], [27]. A recent study reported that the effectiveness of previous infection and three doses of BNT162b2 vaccine was 77.3 %, whereas the effectiveness of previous infection and two doses of BNT162b2 vaccine was 55.1 % [28]. The authors concluded that hybrid immunity resulting from previous infection and recent booster vaccination provides the strongest protection. In our study, it is necessary to clarify how long the NT against BA.5 lasts in sera of the 35 participants with BTI due to the hybrid immunity.
In this study, the NTs of the SARS-CoV-2 naïve participants were 36.7-fold lower for BA.1 and 51.8-fold lower for BA.5, than to the Wuhan strain, and the NTs of participants with BTI during the Omicron BA.5 period were 42.8-fold lower for BA.1 and 28.7-fold lower for BA.5 than to the Wuhan strain. A previous study found that the NTs of SARS-CoV-2 naïve individuals (median age of 35 years) after three doses of mRNA vaccine were 6.4-fold lower for BA.1 and 21-fold lower for BA.5, compared with wild-type SARS-CoV-2 [29]. Differences in NTs for BA.1 and BA.5 between our study (more than 30-fold lower for BA.1 and BA.5) and a previous study (no more than 21-fold lower for BA.1 and BA.5) may be explained, in part, by the difference in the age distribution of the participants.
We found that the NTs against Omicron BA.1 and BA.5 increased noticeably 2 months after the third dose of mRNA vaccine, but rapidly declined in SARS-CoV-2 naïve participants 5 months after the third dose in this study. These findings contrast with those of a recently published study [30], which found a somewhat less steep rate of decay in NTs in individuals with prior SARS-CoV-2 infection, including individuals infected with the Omicron variant, 9 months after the third dose of mRNA vaccine [31]. The authors reported that the 30-day rates of decline in the NT were 17.1 % against prototypic virus with the D614G mutation and 12.1 % against BA.4/5, and noted that individuals with prior SARS-CoV-2 infection had high NTs against Omicron subvariants, especially BA.4/5.
A previous study found an age-dependent negative correlation between spike-specific IgG levels among adults in Germany after a second dose of BNT162b2 vaccine [32]. Another study reported an age-dependent decrease in RBD IgG and NTs against variants among healthcare workers in a teaching hospital, whose age ranged from 20 to 70 years [21]. Collectively, these findings are in agreement with our findings of age-dependent decrease in RBD IgG and NTs after the second and third doses of the mRNA vaccine in nursing homes. In our study, RBD IgG and NTs differed significantly between the staff and the residents after the second dose of COVID-19 mRNA vaccine, but no difference was found in the increase levels of antibody responses between the two groups after the third dose. These findings suggest that the booster dose of COVID-19 mRNA vaccine affords high levels of NTs against Omicron subvariants even in the frail residents in the nursing home, which is consistent with a recent report [33].
In this study, we also found that the NTs were at very low levels 5 months after the third dose of mRNA vaccine especially in SARS-CoV-2 naïve participants aged ≥ 80 years in this study. Because the prevalence of Omicron BA.5 remained high from August until the end of 2022 in Japan, the BA.1 or BA.5 bivalent mRNA vaccine is currently (in February 2023) being administered to persons aged ≥ 12 years who have completed the first and second dose of mRNA vaccine since October 2022. A recently published paper and a preprint paper reported that NTs against BA.5 from the BA.1 or the BA.5 bivalent mRNA vaccine booster was superior or comparable to those by monovalent mRNA vaccine boosters [34], [35]. The sustainability of the NTs against BA.5 elicited by BA.1 or the BA.5 bivalent mRNA vaccine boosters in adults aged ≥ 80 years remains unclear. Further studies are needed to evaluate the sustainability of NTs in older adults following administration of an Omicron-containing bivalent mRNA booster vaccine.
Our study has some limitations. First, we analyzed the data of antibody responses in nursing home staff living in the community and residents of nursing homes as one cohort. Second, we did not collect information of the medical history of each participant, including the presence of chronic medical conditions. These factors might have affected antibody responses. Third, although certain chronic medical conditions, such as autoimmune diseases and the use of immunosuppressants, may affect the antibody response [36], we were unable to evaluate the influence of such chronic medical conditions on the antibody response in this study.
5. Conclusions
In SARS CoV-2 naïve participants, the RBD IgG and NTs against Omicron BA.1 and BA.5 were noticeably increased 2 months after the booster dose of COVID-19 mRNA vaccine. The RBD IgG and NT against Omicron BA.1 and BA.5 in SARS-CoV-2 naïve participants declined 2 and 5 months after the third dose in an age-dependent manner, and was particularly marked in those aged ≥ 80 years. BTIs during the epidemic period of BA.5 (between 2 and 5 months after the booster dose) induced robust NTs against Omicron BA.5 even 5 months after the booster dose vaccination.
Funding
This study was supported in part by a grant-in-aid from the Japan Society of Public Health (2021) (H.T.), Daido Life Insurance Company (2021–14) (H.T.), and Kurozumi Medical Foundation (2021) (M.I.).
CRediT authorship contribution statement
Masae Itamochi: Funding acquisition, Writing – review & editing, Investigation, Writing – original draft, Conceptualization, Methodology. Shunsuke Yazawa: Writing – original draft, Conceptualization, Methodology, Investigation. Noriko Inasaki: Investigation. Yumiko Saga: Investigation. Emiko Yamazaki: Investigation. Takahisa Shimada: Investigation. Kosuke Tamura: Investigation. Emi Maenishi: . Junko Isobe: . Masahiko Nakamura: . Misuzu Takaoka: . Hitoshi Sasajima: Supervision, Writing – review & editing. Chikako Kawashiri: Supervision. Hideki Tani: Funding acquisition, Supervision, Writing – review & editing, Writing – original draft, Conceptualization, Methodology. Kazunori Oishi: Writing – original draft, Conceptualization, Methodology, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank the residents and staff of the nursing homes and nurses from the Toyama City Medical Association. We are grateful to Sumiyo Hasegawa, Izumi Kawaguchi, and Yoko Kanamori for their excellent technical and secretarial assistance. We thank Yukari Murotani (Alpen Rehabilitation Hospital, Toyama), Yoichi Sugimori and Mayumi Ishikuro (Nursing Home Asitanenomori, Toyama), Yosuke Hirata (Nursing Home Miyabi, Toyama), Miwa Ise (Nursing Home Harukaze, Toyama), Maki Sakai (Nursing Home Kagayaki, Toyama), Eijiro Minami, and Kazuyoshi Haba (Nursing Home Kurehaen, Toyama) for their kind cooperation in this study. We thank Makoto Kuroda and Tsuyoshi Sekizuka at the Pathogen Genomics Center, National Institute of Infectious Diseases, Tokyo, for their technical support in sequence analysis. We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.02.068.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary Fig. 1. Correlation between NT titer against live SARS-CoV-2 and NTs against SARS-CoV-2pv using standard rabbit and human serum samples. (A) Diluted series of rabbit immune serum samples. (B) Dilution series of human immune serum samples. * NT titer = Value of SARS-CoV-2pv luciferase activity in the negative serum /the value in test serum. Abbreviations: NT, neutralizing titer; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SARS-CoV-2pv, severe acute respiratory syndrome coronavirus 2 pseudotyped vesicular stomatitis virus. Supplementary Fig. 2. Anti-RBD IgG titers and NTs 2 and 5 months after two doses and the third dose of COVID-19 mRNA vaccine in one participant with BTI during the epidemic period of the Alpha variant (August 2021). (A) SARS-CoV-2 RBD IgG titers, (B) Neutralizing titers against SARS-CoV-2pv. Abbreviations: RBD, receptor-binding domain; NT, neutralizing titer; BTI, breakthrough infection; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SARS-CoV-2pv, severe acute respiratory syndrome coronavirus 2 pseudotyped virus; V2+2M, 2 months after the 2nd dose of vaccine; V2+5M, 5 months after the 2nd dose of vaccine; V3+2M, 2 months after the 3rd dose of vaccine; V3+5M, 5 months after the 3rd dose of vaccine. Supplementary Fig. 3. Anti-RBD IgG titers and NTs 2 and 5 months after two doses and the third dose of COVID-19 mRNA vaccine in participants of the staff and resident participants aged 57 to 79 years. ***, p<0.001; **, p<0.01; *, p<0.05 (comparing the staff vs residents, using the Mann Whitney U test). Abbreviations: RBD, receptor-binding domain; NT, neutralizing titer; V2+2M, 2 months after the 2nd dose of vaccine; V2+5M, 5 months after the 2nd dose of vaccine; V3+2M, 2 months after the 3rd dose of vaccine; V3+5M, 5 months after the 3rd dose of vaccine.
Data availability
Data will be made available on request.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A Novel Coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White EM, Kosar CM, Feifer RA, Blackman C, Gravenstein S, Ouslander J, et al. Variation in SARS-CoV-2 prevalence in U.S. skilled nursing facilities. Am J Geriatric Society 2020; 68: 2167–73. 10.1111/jgs.16752. [DOI] [PMC free article] [PubMed]
- 3.The kyodo news service [Internet]. COVID-19 deaths in the nursing facilities account for 14% of overall CIVID-19 deaths [cited 2023 Feb 20]. 47NEWS. Available from: https://47news/4808143.html.
- 4.Comas-Herrera A, Marczak J, Byrd W, Lorenz-Dant K, Patel D, Pharoah D (eds.) and LTC covid contributors. LT Covid International living report on COVID-19 and Long-Term Care. Available at: https://ltccovid.org/international-living-report-covid-ltc/.
- 5.Iritani O., Okuno T., Hama D., Kane A., Kodera K., Morinaga K., et al. Clusters of COVID-19 in long-term care hospitals and facilities in Japan from 16 January to 9 May 2020. Geriatr Gerontol Int. 2020;20(7):715–719. doi: 10.1111/ggi.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Tracking SARS-CoV-2 variants. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ [Accessed on November 4, 2022].
- 9.Lucas C., Vogels C.B., Yilddirim I., Rothman J., Lu P., Monteiro V., et al. Impact of circulating SARS-CoV-2 variants on mRNA vaccine-induced immunity. Nature. 2021 Dec;600(7889):523–529. doi: 10.1038/s41586-021-04085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iketani S., Liu L., Guo Y., Liu L., Chan J.F.W., Huang Y., et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature. 2022;604:553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumura Y., Yamamoto M., Shinohara K., Tsuchido Y., Yukawa S., Noguchi T., et al. High mortality and morbidity among vaccinated residents infected with the SARS-CoV-2 Omicron variant during an outbreak in a nursing home in Kyoto City. Japan Am J Infect Control. 2022;S0196–6553(22):00675–00677. doi: 10.1016/j.ajic.2022.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee N., Jeong S., Lee S.K., Cho E.J., Hyun J., Park M.J., et al. Quantitative Analysis of anti-N and anti-S antibody titers of SARS-CoV-2 infection after the third dose of COVID-19 vaccination. Vaccines. 2022;10:1143. doi: 10.3390/vaccines10071143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itokawa K., Sekizuka T., Hashino M., Tanaka R., Kuroda M. Disentangling primer interactions improves SARS-CoV-2 genome sequencing by multiplex tiling PCR. PLoS One. 2020;15(9):e0239403. doi: 10.1371/journal.pone.0239403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H., Durbin R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 2012; 22(3): 568–76. 10.1101/ gr.129684.111. [DOI] [PMC free article] [PubMed]
- 17.Melero R., Sorzano C.O.S., Foster B., Vilas J.L., Martinez M., Marabini R., et al. Continuous flexibility analysis of SARS-CoV-2 spike prefusion structures. IUCrJ. 2020;7:1059–1069. doi: 10.1107/S2052252520012725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tani H., Kimura M., Tan L., Yoshida Y., Ozawa T., Kishi H., et al. Evaluation of SARS-CoV-2 neutralizing antibodies using a vesicular stomatitis virus possessing SARS-CoV-2 spike protein. Virol J. 2021;18:16. doi: 10.1186/s12985-021-01490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada S., Fukushi S., Kinoshita H., Ohnishi M., Suzuki T., Fujimoto T., et al. Assessment of SARS-CoV-2 infectivity of upper respiratory specimens from COVID-19 patients by virus isolation using VeroE6/TMPRSS2 cells. BMJ Open Respir Res. 2021;8:e000830. doi: 10.1136/bmjresp-2020-000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haveri A., Solastie A., Ekstrom N., Osterlund P., Nohynek H., Nieminen T., et al. Neutralizing antibodies to SARS-CoV-2 Omicron variant after third mRNA vaccination and health care workers and elderly subjects. Eur J Immunol. 2022;52:816–824. doi: 10.1002/eji.202149785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawasuji H., Morinaga Y., Tani H., Saga Y., Kaneda M., Murai Y., et al. Age-dependent reduction in neutralization against Alpha and Beta variants of BNT162b2 SARS-CoV-2 vaccine-induced immunity. Microbiol Spectr. 2021;9(3):e0056121. doi: 10.1128/Spectrum.00561-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furukawa K., Handayani T., Kurahashi Y., Sutandhio S., Nishimura M., Aril J., et al. Assessment of Neutralizing Antibody Response Against SARS-CoV-2 Variants After 2 to 3 Doses of the BNT162b2 mRNA COVID-19 Vaccine. JAMA Netw Open. 2022;5(5):e2210780. doi: 10.1001/jamanetworkopen.2022.10780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chong Y., Goto T., Tani N., Yonekawa A., Ikematsu H., Shimono N., et al. Pronounced antibody elevation after SARS-CoV-2 BNT162b2 mRNA booster vaccination in nursing home residents. Influenza Other Respir Viruses. 2022;16:1066–1071. doi: 10.1111/irv.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canaday D.H., Oyebanji O.A., White E., Keresztesy D., Payne M., Wilk D., et al. COVID-19 vaccine booster dose needed to achieve Omicron-specific neutralisation in nursing home residents. EBioMedicine. 2022;80 doi: 10.1016/j.ebiom.2022.104066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotaki R, Adachi Y, Moriyama S, Onodera T, Fukushi S, Nagakura T, et al. SARS-CoV-2 Omicron-neutralization memory cells are elicited by two doses of BNT162b2 mRNA vaccine. Science Immunol 2022 Apr 22; 7(70): eabn8590. 10.1126/sciimmunol.abn8590. [DOI] [PMC free article] [PubMed]
- 26.Vicenti I., Gatti F., Scaggiante R., Boccuto A., Zago D., Basso M., et al. Single-dose BNT162b2 mRNA COVID-19 vaccine significantly boosts neutralizing antibody response in health care workers recovering from asymptomatic or mild natural SARS-CoV-2 infection. Int J Infect Dis. 2021;108:176–178. doi: 10.1016/j.ijid.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ontanon J., Blas J., de Cabo C., Santos C., Ruiz-Escribano E., Garcia A., et al. Influence of past infection with SARS-CoV-2 on the response to the BNT162 mRNA vaccine in healthy health care workers: Kinetics and durability of the humoral immune response. EBioMedicine. 2021;73 doi: 10.1016/j.ebiom.2021.103656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altarawneh H.N., Chemaitelly H., Ayoub H.H., Tang P., Hasan M.R., Yassine H.M., et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Eng J Med. 2022;387(1):21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hachmann N.P., Miller J., Collier A.Y., Yu J., Rowe M., Bondzie E.A., et al. Neutralization escape by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4, and BA.5. N Eng J Med. 2022;387(1):86–88. doi: 10.1056/NEJMc2206576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyke K.E., Atmar R.L., Islas C.D., Posavad C.M., Szydlo D., Paul Chourdhury R., et al. Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant. Cell Rep Med. 2022;3(7) doi: 10.1016/j.xcrm.2022.100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qu P., Faraone J.N., Evans J.P., Zheng Y.-M., Yu L., Ma Q., et al. Durability of booster mRNA vaccine against SARS-CoV-2 BA.2.12.1, BA.4, and BA.5 subvariants. N Eng J Med. 2022;387(14):1329–1331. doi: 10.1056/NEJMc2210546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Müller L., Andrée M., Moskorz W., Drexler I., Walotka L., Grothmann R., et al. Age-dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin Infect Dis. 2021;73:2065–2072. doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro J.R., Sitaras I., Park H.-S., Aytenfisu T.Y., Caputo C., Li M., et al. Association of frailty, age, and biological sex with severe acute respiratory syndrome coronavirus 2 messenger RNA vaccine-induced immunity in older adults. Clin Infect Dis. 2022;75(Suppl 1):S61–S71. doi: 10.1093/cid/ciac397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chalkias S., Harper C., Vrbicky K., Walsh S.R., Essink B., Brosz A., et al. A bivalent Omicron-containing booster vaccine against Covid-19. N Eng J Med. 2022;387(14):1279–1291. doi: 10.1056/NEJMoa2208343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collier AY, Miller J, Hachmann NP, McMahan K, Liu J, Bondzie EA, et al. Immunogenicity of the BA.5 bivalent mRNA vaccine boosters. bioRxiv. 2022 Oct 25:2022.10.24.513619. 10.1101/2022.10.24.513619. Preprint.
- 36.Furer V., Eviatar T., Zisman D., Peleg H., Paran D., Levartovsky D., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80(10):1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Correlation between NT titer against live SARS-CoV-2 and NTs against SARS-CoV-2pv using standard rabbit and human serum samples. (A) Diluted series of rabbit immune serum samples. (B) Dilution series of human immune serum samples. * NT titer = Value of SARS-CoV-2pv luciferase activity in the negative serum /the value in test serum. Abbreviations: NT, neutralizing titer; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SARS-CoV-2pv, severe acute respiratory syndrome coronavirus 2 pseudotyped vesicular stomatitis virus. Supplementary Fig. 2. Anti-RBD IgG titers and NTs 2 and 5 months after two doses and the third dose of COVID-19 mRNA vaccine in one participant with BTI during the epidemic period of the Alpha variant (August 2021). (A) SARS-CoV-2 RBD IgG titers, (B) Neutralizing titers against SARS-CoV-2pv. Abbreviations: RBD, receptor-binding domain; NT, neutralizing titer; BTI, breakthrough infection; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SARS-CoV-2pv, severe acute respiratory syndrome coronavirus 2 pseudotyped virus; V2+2M, 2 months after the 2nd dose of vaccine; V2+5M, 5 months after the 2nd dose of vaccine; V3+2M, 2 months after the 3rd dose of vaccine; V3+5M, 5 months after the 3rd dose of vaccine. Supplementary Fig. 3. Anti-RBD IgG titers and NTs 2 and 5 months after two doses and the third dose of COVID-19 mRNA vaccine in participants of the staff and resident participants aged 57 to 79 years. ***, p<0.001; **, p<0.01; *, p<0.05 (comparing the staff vs residents, using the Mann Whitney U test). Abbreviations: RBD, receptor-binding domain; NT, neutralizing titer; V2+2M, 2 months after the 2nd dose of vaccine; V2+5M, 5 months after the 2nd dose of vaccine; V3+2M, 2 months after the 3rd dose of vaccine; V3+5M, 5 months after the 3rd dose of vaccine.
Data Availability Statement
Data will be made available on request.