Abstract
Aim
The present study aimed to estimate the anaphylaxis rates following mRNA COVID-19 vaccination in children and adolescents in Europe.
Methods
We retrieved data on 371 anaphylaxis cases following mRNA COVID-19 vaccination in children ≤ 17 years old notified to EudraVigilance as of October 8, 2022. Overall, 27,120,512 doses of BNT162b2 vaccine and 1,400,300 doses of mRNA-1273 vaccine have been delivered to children during the study period.
Results
The overall mean anaphylaxis rate was 12.81 [95% confidence interval (CI): 11.49–14.12] per 106 mRNA vaccine doses [12.14 (95% CI: 6.37–17.91) per 106 doses for mRNA-1273 and 12.84 (95% CI: 11.49–14.19) per 106 doses for BNT162b2]. Children 12–17 years old accounted for 317 anaphylaxis cases, followed by 48 cases in children 3–11 years old, and 6 cases in children 0–2 years old. Children 10–17 years old had a mean anaphylaxis rate of 13.52 (95% CI: 12.03–15.00) cases per 106 mRNA vaccine doses and children 5–9 years old had a mean anaphylaxis rate of 9.51 (95% CI: 6.82–12.20) cases per 106 mRNA vaccine doses. There were two fatalities, both in the 12–17 years age group. The fatal anaphylaxis rate was 0.07 cases per 106 mRNA vaccine doses.
Conclusions
Anaphylaxis is a rare adverse event after receiving an mRNA COVID-19 vaccine in children. Continuous surveillance of serious adverse events is needed to guide vaccination policies as we move towards SARS-CoV-2 endemicity. Larger real-world studies on COVID-19 vaccination in children, using clinical case confirmation, are imperative.
Keywords: mRNA COVID-19 vaccine, Anaphylaxis, Adverse event, Children, Adolescents, Infants, Pediatric, EudraVigilance
1. Introduction
Early studies from the beginning of the COVID-19 pandemic demonstrated that children mostly develop mild manifestations while the true prevalence of SARS-CoV-2 infection in the pediatric age group was rather underestimated at that time [1]. Soon it became evident that children may develop severe COVID-19 complications and outcomes and participate in the transmission cycle of SARS-CoV-2 [1], [2], [3], [4]. Vaccines based on the novel mRNA platform were the first to receive authorization for use in successively decreasing age groups of the pediatric population. Results from large COVID-19 vaccine trials in children showed good tolerability, immunogenicity, and efficacy against serious morbidity outcomes and an acceptable safety profile [5], [6], [7], [8], [9], [10]. Currently, several COVID-19 vaccines have been approved for pediatric use globally, aiming to reduce the associated morbidity and mortality and for children to resume full educational and social activities [11]. Nonetheless, although, more than 13 billion COVID-19 vaccine doses have been delivered globally [12], in many countries COVID-19 vaccine uptake among children has been largely slow, mainly due to parental concerns about safety but also due to uncertainty about the role of vaccination in the reduction of community transmission [13], [14]. Our previous studies using preliminary data from the US Vaccine Adverse Event Reporting System (VAERS) and the European EudraVigilance estimated a mean of 10.67 anaphylaxis cases per 106 doses of COVID-19 vaccines administered to adults, with significant variation by vaccine platform and brand [15], [16]. Two main vaccine excipients (polyethylene glycol 2000 and polysorbate 80) have been implicated for triggering allergic reactions post vaccination, but the true cause(s) remain elusive [17]. A recent study of safety outcomes in US vaccine recipients 12–64 years of age using three databases of a total of 60 million persons per year, found that anaphylaxis met the statistical threshold for a signal following mRNA vaccination in all three databases compared to historical rates prior to COVID-19 vaccination [18].
The aim of the present study was to estimate the anaphylaxis rate after receipt of mRNA COVID-19 vaccines among children and adolescents in Europe, using data reported to EudraVigilance [19].
2. Methods
EudraVigilance is the European Medicine Agency (EMA) pharmacovigilance system for monitoring and analyzing suspected adverse reactions to medicines, including post-vaccination adverse events. Adverse events are reported by healthcare professionals, patients, and other stakeholders; due to its passive reporting nature and possibility of misreporting biases, the reported data are subject to internal evaluation, and detection and merge of duplicated cases [19], [20]. Information about each case of suspected adverse event is provided in the Individual Case Safety Report (ICSR). The duration-outcome-seriousness criteria of reaction, the suspect/interacting drug list, and the concomitant/not administered drug list are also reported in each ICSR.
2.1. Estimation of anaphylaxis rates
Anaphylaxis cases (anaphylactic reaction and anaphylactic shock cases that were listed in immune system disorders) following mRNA COVID-19 vaccination in children and adolescents ≤ 17 years of age reported as of October 8, 2022, were retrieved from EudraVigilance [19]. The following mRNA vaccines are licensed for pediatric use in Europe: BNT162b2 vaccine (Comirnaty; Pfizer-BioNTech) and mRNA-1273 vaccine (Spikevax; Moderna). The mean anaphylaxis rate following mRNA COVID-19 vaccination was estimated by summing all reported post-COVID-19 vaccination anaphylaxis cases, and then dividing by the corresponding total number of administered mRNA COVID-19 vaccine doses. The number of mRNA COVID-19 vaccine doses administered to children in the European Economic Area (EEA) was retrieved from the European Centre for Disease Prevention and Control (ECDC) website [21]. Mean anaphylaxis rates were also estimated per age group and per vaccine brand. Given that different age groups are used for data presentation in EudraVigilance (0–1 month, 2 months-2 years, 3–11 years, 12–17 years) and ECDC (0–4 years, 5–9 years, 10–14 years, 15–17 years), data were grouped as follows for the present analysis: 0–4 years, 5–9 years, and 10–17 years. EudraVigilance and ECDC data are publicly available and ethical approval was not required.
2.2. Statistical analysis
The anaphylactic reaction and anaphylactic shock rates for the two mRNA vaccines were compared with the Pearson 2-sided chi-square test. P-values lower than 0.05 were considered statistically significant. The 95% confidence interval (CI) was calculated using the SPSS statistical program (version 27; IBM, NY, USA).
3. Results
3.1. Vaccines administered to children and adolescents in Europe
As of October 6, 2022, a total of 27,120,512 doses of the BNT162b2 vaccine and of 1,400,300 doses of the mRNA-1273 vaccine have been administered to children ≤ 17 years of age in Europe [21]. Table 1 shows the mRNA vaccine doses delivered to children per age group and vaccine brand.
Table 1.
mRNA COVID-19 vaccine doses administered to children and adolescents in Europe by vaccine brand and age group (ECDC, as of October 6, 2022).
| Vaccine Age group | mRNA-1273a | BNT162b2b | Total by age |
|---|---|---|---|
| 0–4 years | 56 | 16,688 | 16,744 |
| 5–9 years | 271 | 5,048,705 | 5,048,976 |
| 10–14 years | 750,984 | 11,974,524 | 12,725,508 |
| 15–17 years | 648,989 | 10,080,595 | 10,729,604 |
| Total by vaccine | 1,400,300 | 27,120,512 | 28,512,812 |
Spikevax (Moderna).
Comirnaty (Pfizer-BioNTech).
3.2. Anaphylactic reaction and anaphylactic shock cases
A total of 371 anaphylaxis cases following mRNA COVID-19 vaccination in children and adolescents ≤ 17 years of age were reported to EudraVigilance as of October 8, 2022, of which 293 were anaphylactic reactions and 78 were anaphylactic shock cases. Table 2 shows the numbers of anaphylactic reactions and anaphylactic shock cases by COVID-19 vaccine, age group, and sex. There were 353 anaphylaxis cases following a dose of BNT162b2 vaccine and 18 anaphylaxis cases following a dose of mRNA-1273 vaccine. Children 12–17 years of age accounted for 317 notified anaphylaxis cases, followed by 48 anaphylaxis cases in the 3–11 years age group, and 6 anaphylaxis cases in the 0–2 years age group. Females accounted for 222 of 364 (60.9%) anaphylaxis cases for which the sex was recorded.
Table 2.
Anaphylactic reactions (A) and anaphylactic shock (Β) cases following mRNA COVID-19 vaccination in children and adolescents by vaccine brand, age group, and sex (EudraVigilance, as of October 8, 2022).
| (A) Anaphylactic reactions |
mRNA-1273a |
BNT162b2b |
||||||
|---|---|---|---|---|---|---|---|---|
| Age group | Females | Males | Unknown | Total by age | Females | Males | Unknown | Total by age |
| 0–2 years | 1 | 0 | 0 | 1 | 2 | 1 | 1 | 4 |
| 3–11 years | 0 | 0 | 0 | 0 | 21 | 20 | 1 | 42 |
| 12–17 years | 7 | 6 | 0 | 13 | 146 | 84 | 3 | 233 |
|
Total by sex |
8 | 6 | 0 | 14 | 169 | 105 | 5 | 279 |
| (Β) Anaphylactic shock |
mRNA-1273a |
BNT162b2b |
||||||
|
Age group |
Females | Males | Unknown | Total by age | Females | Males | Unknown | Total by age |
| 0–2 years | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| 3–11 years | 0 | 0 | 0 | 0 | 4 | 2 | 0 | 6 |
| 12–17 years | 2 | 2 | 0 | 4 | 38 | 27 | 2 | 67 |
| Total by sex | 2 | 2 | 0 | 4 | 43 | 29 | 2 | 74 |
Spikevax (Moderna).
Comirnaty (Pfizer-BioNTech).
3.3. Anaphylaxis rates following mRNA COVID-19 vaccination in children
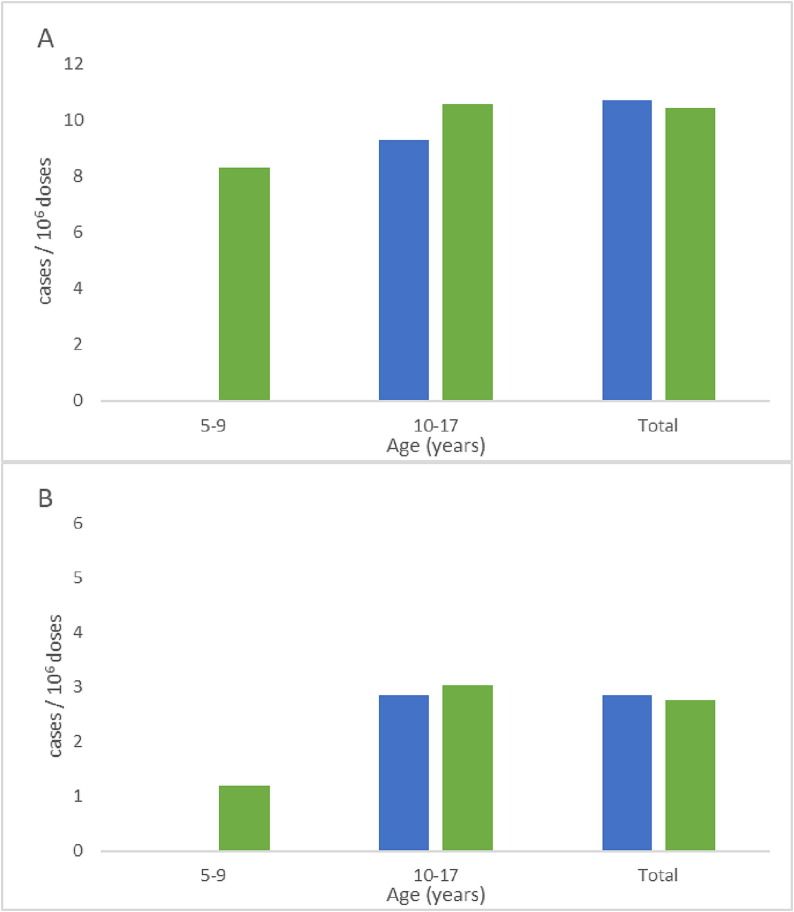
Table 3 shows the estimated anaphylaxis rates in children and adolescents ≤ 17 years of age by vaccine brand and age group. Given the small number of COVID-19 vaccine doses administered to young children, the 0–4 year old group was excluded from the estimation of anaphylaxis rates. The overall mean anaphylaxis rate was 12.81 (95% CI: 11.49–14.12) per 106 delivered mRNA COVID-19 vaccine doses, and in particular, 12.14 (95% CI: 6.37–17.91) per 106 mRNA-1273 vaccine doses and 12.84 (95% CI: 11.49–14.19) per 106 BNT162b2 vaccine doses. Children 10–17 years of age had the highest overall anaphylaxis rate, followed by children 5–9 years of age [13.52 (95% CI: 12.03–15.00) and 9.51 (95% CI: 6.82–12.20) anaphylaxis cases per 106 mRNA COVID-19 vaccine doses, respectively] (Fig. 1 ). There were no statistically significant differences in anaphylaxis rates by vaccine brand, either in the whole pediatric population or by age group. Lastly, there were two fatalities (1 male and 1 female, both 12–17 years of age) following vaccination with BNT162b2. The following findings were reported: shortness of breath, fatigue, dizziness, nausea, cyanosis, anaphylactic reaction, and cardiovascular collapse (first case); and fever (second fatal case); no other information was available. The fatal anaphylaxis rate was 0.07 cases per 106 delivered mRNA COVID-19 vaccine doses.
Table 3.
Anaphylactic reaction and anaphylactic shock cases per 106 mRNA COVID-19 vaccine doses in children 5–17 years of age by vaccine and age group, EudraVigilance (as of October 8, 2022).
| Vaccine | mRNA-1273a | BNT162b2b | Total |
|---|---|---|---|
| Anaphylactic reaction cases / 106 doses (95% CI) | |||
| 5–9 years | 0.00 (0.00–0.00) | 8.32 (5.80–10.83) | 8.32 (5.80–10.83) |
| 10–17 years | 9.29 (4.24–14.33) | 10.56 (9.21–11.92) | 10.49 (9.18–11.80) |
| Total | 9.28 (4.24–14.33) | 10.15 (8.95–11.35) | 10.10 (8.94–11.27) |
| Anaphylactic shock cases / 106doses (95% CI) | |||
| 5–9 years | 0.00 (0.00–0.00) | 1.19 (0.24–2.14) | 1.19 (0.24–2.14) |
| 10–17 years | 2.86 (0.06–5.66) | 3.04 (2.31–3.77) | 3.03 (2.32–3.73) |
| Total | 2.86 (0.06–5.66) | 2.69 (2.08–3.31) | 2.70 (2.10–3.30) |
CI: confidence interval.
Spikevax (Moderna).
Comirnaty (Pfizer-BioNTech).
Fig. 1.
(A) anaphylactic reaction and (B) anaphylactic shock cases per 106 vaccine doses in children 5–17 years old by age group and vaccine brand [mRNA-1273 ( ), BNT162b2 (
), BNT162b2 ( )].
)].
4. Discussion
In align with the US Food and Drug Administration, starting in May 2021 the EMA granted an extension of indication for using the BNT162b2 vaccine in adolescents, which was followed by indication of also using the mRNA-1273 vaccine in adolescents and subsequently a gradual expansion to younger age groups. This is in contrast to other novel vaccines approved between January 2010 and June 2020 under regular licensure procedures, which required approximately eight years of clinical development and were based on evidence from a median of seven clinical trials [22].
During the past seventeen months more than 28.5 million mRNA COVID-19 vaccine doses have been delivered to children across Europe. Currently all children ≥ 6 months of age are potentially eligible for COVID-19 vaccination. However, the available real-life evidence on mRNA COVID-19 vaccines comes mainly from adult or adolescent recipients [15], [16], [17], [18], [22], [23], [24], while safety data for young children ≤ 3 years of age and infants are scarce [25]. Therefore, post-licensure notification of very rare life-threatening adverse events following vaccination is critical, particularly during public health threats such as the COVID-19 pandemic, given the restricted number of participants in phase 3 trials. We estimated a mean anaphylaxis rate of 12.81 cases per 106 delivered mRNA vaccine doses in children and adolescents 5–17 years of age, based on data notified to EudraVigilance. Our findings indicate that anaphylaxis is a rather rare adverse event following mRNA COVID-19 vaccination in childhood, although above the range of 1 anaphylaxis case per 106 to 105 doses of other routine pediatric vaccines, according to the International Consensus Document on Allergic Reactions to Vaccines [26]. In particular, reported anaphylaxis rates following routine pediatric vaccines are as follows: 0.51–3.6 per 106 million doses of Diphtheria, Tetanus, acellular Pertussis vaccine, 0.6 – 5.14 per 106 million doses of measles-mumps-rubella vaccine, 1.2–10.3 per 106 million doses of varicella vaccine, and 0–1.67 per 106 million doses of hepatitis B vaccine [26], [27]. The increased overall anaphylaxis rate following mRNA COVID-19 vaccination in children and adolescents may be partially attributed to the increased awareness of healthcare professionals and parents regarding safety of COVID-19 vaccines. We have to notice however, that different methodologies were used in the abovementioned studies on anaphylaxis rates following routine pediatric vaccinations [26], [27]. Overall, anaphylaxis-associated fatalities following COVID-19 vaccination were extremely rare in our pediatric study group, as also noted among adult vaccine recipients [15], [16].
During the past months indications for mRNA COVID-19 vaccination were expanded to younger pediatric ages targeting mostly children with underlying conditions rather than the general pediatric population. It is possible that several of these young children who developed anaphylaxis were referred for COVID-19 vaccination because of underlying conditions. Unfortunately, relevant data to support our hypothesis were available only in 5 of 371 children with anaphylaxis notified to EudraVigilance, all of whom had asthma or allergies and were on anti-allergic medications. Vaccine recipients with a past history of allergies and/or anaphylaxis have higher risk of anaphylaxis following COVID-19 mRNA vaccination [28]. Nonetheless, a history of allergy is not a contraindication for COVID-19 vaccination per se, and an allergic assessment should be requested [29]. In our study, the small number of mRNA COVID-19 vaccine doses delivered to children up to 2 years old precluded the estimation of anaphylaxis rates in this age group and therefore any conclusions.
A clear strength of the present study is the use of real-life data retrieved from EudraVigilance, which is one of the largest pharmacovigilance platforms for reporting vaccine-associated adverse events. An additional strength is the use of a large number of vaccine doses administered from a geographically diverse population for the denominator, including data on vaccine manufacturer. Overall, our study highlights the intrinsic limitations of passive vaccine-safety reporting systems. EudraVigilance relies on passive reporting, which could potentially underestimate or overestimate true anaphylaxis rates [15]. Given that healthcare professionals and vaccine policy makers were highly sensitized about any adverse event following COVID-19 vaccination in children, such a possibility is much disputed. In addition, passive reporting systems are subject to misreporting biases, which could preclude a clear cause and effect association. This include unavailability of clinical information, including information on timing from vaccination to onset of anaphylaxis symptoms, whether reported cases met Brighton case definitions for anaphylaxis, and whether patients received other vaccines, medications or foods that could have contributed to anaphylaxis. Another limitation is that EudraVigilance and ECDC COVID-vaccine data are presented in different age groups.
In conclusion, we studied anaphylaxis rates following mRNA COVID-19 vaccination in children and adolescents reported to EudraVigilance. The overall mean anaphylaxis rate was 12.81 per 106 delivered mRNA COVID-19 vaccine doses to children and adolescents 5–17 years old. Our findings highlight the need for continuous surveillance of serious adverse events to guide vaccination policies and raise confidence on vaccinations. Large real-world studies on COVID-19 vaccination in the pediatric population using clinical case confirmation are imperative.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The opinions presented in this article are those of the authors and do not necessarily represent those of their institutions.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Data will be made available on request.
References
- 1.Nikolopoulou G.B., Maltezou H.C. COVID-19 in children: where do we stand? Arch Med Res. 2022;53:1–8. doi: 10.1016/j.arcmed.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., et al. Multisystem Inflammatory Syndrome in US children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi D.S., Whitaker M., Marks K.J., Anglin O., Milucky J., Patel K., et al. Hospitalizations of children aged 5–11 years with laboratory-confirmed COVID-19 – COVID-NET, 14 States, March 2020-February 2022. Morb Mortal Wkly Rep. 2022;71:574–581. doi: 10.15585/mmwr.mm7116e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anastassopoulou C., Spanakis N., Tsakris A. SARS-CoV-2 transmission, the ambiguous role of children and considerations for the reopening of schools in the fall. Future Microbiol. 2020;15:1201–1206. doi: 10.2217/fmb-2020-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadeghi S., Kalantari Y., Shokri S., Fallahpour M., Nafissi N., Goodarzi A., et al. Immunologic response, efficacy, and safety of vaccines against COVID-19 infection in healthy and immunosuppressed children and adolescents aged 2–21 years old: a systematic review and meta-analysis. J Clin Virol. 2022;153 doi: 10.1016/j.jcv.2022.105196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hause AM, Shay DK, Klein NP, Abara WE, Baggs J, Cortese MM, et al. Safety of COVID-19 vaccination in United States children ages 5 to 11 Years. Pediatr 2022;150:e2022057313. [DOI] [PMC free article] [PubMed]
- 7.Hause A.M., Baggs J., Marquez P., Myers T.R., Gee J., Su J.R., et al. COVID-19 vaccine safety in children aged 5–11 years - United States, November 3-December 19, 2021. Morb Mortal Wkly Rep. 2021;70:1755–1760. doi: 10.15585/mmwr.mm705152a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zambrano L.D., Newhams M.M., Olson S.M., Halasa N.B., Price A.M., Boom J.A., et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12–18 years - United States, July-December 2021. Morb Mortal Wkly Rep. 2022;71:52–58. doi: 10.15585/mmwr.mm7102e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olson S.M., Newhams M.M., Halasa N.B., Price A.M., Boom J.A., Sahni L.C., et al. Effectiveness of Pfizer-BioNTech mRNA vaccination against COVID-19 hospitalization among persons aged 12–18 years - United States, June-September 2021. Morb Mortal Wkly Rep. 2021;70:1483–1488. doi: 10.15585/mmwr.mm7042e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hause A.M., Marquez P., Zhang B., Myers T.R., Gee J., Su J.R., et al. COVID-19 mRNA vaccine safety among children aged 6 months-5 years - United States, June 18, 2022-August 21, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1115–1120. doi: 10.15585/mmwr.mm7135a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Interim statement for COVID-19 vaccination in children. Available at: https://www.who.int/news/item/11-08-2022-interim-statement-on-covid-19-vaccination-for-children (last accessed: February 14, 2023).
- 12.Our World data. Coronavirus (COVID-19) vaccinations. Available at: https://ourworldindata.org/covid-vaccinations (last accessed: February 14, 2023).
- 13.Byrne A., Thompson L.A., Filipp S.L., Ryan K. COVID-19 vaccine perceptions and hesitancy amongst parents of school-aged children during the pediatric vaccine rollout. Vaccine. 2022;40:6680–6687. doi: 10.1016/j.vaccine.2022.09.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul S., Mishra C.M. Do we need to vaccinate every child against COVID-19: what evidence suggests-a systematic review of opinions. Front Public Health. 2022;10:1002992. doi: 10.3389/fpubh.2022.1002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maltezou H.C., Anastassopoulou C., Hatziantoniou S., Poland G.A., Tsakris A. Anaphylaxis rates associated with COVID-19 vaccines are comparable to those of other vaccines. Vaccine. 2022;40:183–186. doi: 10.1016/j.vaccine.2021.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatziantoniou S., Anastassopoulou C., Lampropoulou V., Maltezou H.C., Andreakos E., Poland G.A., et al. Comparative assessment of allergic reactions to COVID-19 vaccines in Europe and the United States. Allergy. 2022;77:1630–1633. doi: 10.1111/all.15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatziantoniou S., Maltezou H.C., Tsakris A., Poland G.A., Anastassopoulou C. Anaphylactic reactions to mRNA COVID-19 vaccines: a call for further study. Vaccine. 2021;39:2605–2607. doi: 10.1016/j.vaccine.2021.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lloyd P.C., Hu M., Wong H.L., Shoaibi A., Zhou C.K., Lo A.C., et al. Near real-time surveillance of safety outcomes in US COVID-19 vaccine recipients aged 12 to 64 years. Vaccine. 2022;40:6481–6488. doi: 10.1016/j.vaccine.2022.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Medicines Agency. EudraVigilance system overview. Available at: https://www.ema.europa.eu/en/human-regulatory/research-development/pharmacovigilance/eudravigilance/eudravigilance-system-overview (last accessed: February 14, 2023).
- 20.Kreimeyer K., Menschik D., Winiecki S., Paul W., Barash F., Woo E.J., et al. Probabilistic record linkage of structured and unstructured data to identify duplicate cases in spontaneous adverse event reporting systems. Drug Saf. 2017;40:571–582. doi: 10.1007/s40264-017-0523-4. [DOI] [PubMed] [Google Scholar]
- 21.European Centre for Disease Prevention and Control. COVID-19 vaccine tracker. Available at: https://vaccinetracker.ecdc.europa.eu (last accessed: February 14, 2023).
- 22.Puthumana J., Egilman A.C., Zhang A.D., Schwartz J.L., Ross J.S. Speed, evidence, and safety characteristics of vaccine approvals by the US Food and Drug Administration. JAMA Intern Med. 2021;181:559–560. doi: 10.1001/jamainternmed.2020.7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alhumaid S, Al MutairA, Al AlawiZ, A Rabaan A, Tirupathi P, Alomari ΜA, et al. Anaphylactic and nonanaphylactic reactions to SARS-CoV-2 vaccines: a systematic review and meta-analysis. Allergy Asthma Clin Immunol 2021;17:109. [DOI] [PMC free article] [PubMed]
- 24.Katoto P.D.M.C., Brand A.S., Byamungu L.N., Tamuzi J.L., Mahwire T.C., Kitenage M.K., et al. Safety of COVID-19 Pfizer-BioNtech (BNT162b2) mRNA vaccination in adolescents aged 12–17 years: a systematic review and meta-analysis. Hum Vaccin Immunother. 2022;11:2144039. doi: 10.1080/21645515.2022.2144039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du Y., Chen L., Shi Y. Safety, immunogenicity, and efficacy of COVID-19 vaccines in adolescents, children, and infants: a systematic review and meta-analysis. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dreskin S.C., Halsey N.A., Kelso J.M., Wood R.A., Hummell D.S., Edwards K.M., et al. International Consensus (ICON): allergic reactions to vaccines. World Allergy Organ J. 2016;9:32. doi: 10.1186/s40413-016-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampath V., Rabinowitz G., Shah M., Jain S., Diamant Z., Jesenak M., et al. Vaccines and allergic reactions: the past, the current COVID-19 pandemic, and future perspectives. Allergy. 2021;76:1640–1660. doi: 10.1111/all.14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai A.P., Desai A.P., Loomis G.J. Relationship between pre-existing allergies and anaphylactic reactions post mRNA COVID-19 vaccine administration. Vaccine. 2021;39:4407–4409. doi: 10.1016/j.vaccine.2021.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novembre E., Tosca M., Caffarelli C., Calvani M., Cardinale F., Castagnoli R., et al. Management of BNT162b2 mRNA COVID–19 vaccine in children aged 5–11 years with allergies, asthma, and immunodeficiency: consensus of the Italian Society of Pediatric Allergy and Immunology (SIAIP) Ital J Pediatr. 2022;48:76. doi: 10.1186/s13052-022-01272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.