Abstract
The emergence of SARS-CoV-2 variants calls for continuous monitoring of vaccine effectiveness (VE). We estimated the absolute effectiveness of complete 2-dose primary vaccination and booster vaccination with COVID-19 mRNA vaccines, and the duration of protection against Delta and Omicron BA.1 symptomatic infection and severe outcomes. French residents aged ≥50 years, who presented with SARS-CoV-2-like symptoms and tested for SARS-CoV-2 between June 6, 2021 and February 10, 2022 were included. A test-negative study was conducted to estimate VE against symptomatic infection, using conditional logistic regression models. Cox proportional hazard regressions were performed to assess additional protection against severe COVID-19 outcomes (any hospitalization, and intensive care units [ICU] admission or in-hospital death). In total, 273 732 cases and 735 919 controls were included. VE against symptomatic infection after 2-doses vaccination was 86% (95% CI: 75–92%) for Delta and 70% (58–79%) for Omicron, 7–30 days post vaccination. Protection waned over time, reaching 60% (57–63%) against Delta and 20% (16.–24%) for Omicron BA.1 > 120 days after vaccination. The booster dose fully restored protection against symtpomatic Delta infection (95% [81–99%]) but only partially against symptomatic Omicron BA.1 infection (63% [59–67%]). VE against Delta-related severe outcomes was above 95% with 2 doses, and persisted for at least four months. Protection against any Omicron BA.1-hospitalization was 92% (65%-99%) at 8–30 days, and 82% (67%-91%) > 120 days from the second dose. Against BA.1 ICU admission or in-patient death, VE stood at 98% (0–100%) at 8–30 days, and was 90% (40–99%) > 120 days from the second dose. Protection confered by mRNA vaccines against severe disease caused by either Delta or Omicron BA.1 appeared high and sustained over time. Protection against symptomatic diseases after 2 doses decreased rapidly, especially against Omicron BA.1. A booster dose restored high protection against Delta but only a partial one against Omicron BA.1.
Keywords: COVID-19, SARS-CoV-2, Vaccine, Booster, Effectiveness
1. Introduction
From the end of 2020, one year following the emergence of the SARS-CoV-2 virus, causative agent of the COVID-19 disease, several vaccines targeted against the original SARS-CoV-2 strain were made available. These vaccines served to implement worldwide national mass vaccination campaigns, as a key strategy to mitigate the COVID-19 pandemic. Real-world setting studies performed, few months into vaccination campaigns, showed high effectiveness of these vaccines [1], [2], [3], [4], [5], [6], [7], [8]. However, as vaccination campaigns progressed, additional vaccine effectiveness (VE) studies revealed waning of the vaccine-induced protection over time [8], [9], [10], [11], [12], [13], more so against symptomatic infection than against severe COVID-19 disease [14].
Due to the continued widespread and genetic evolution of the SARS-CoV-2 virus new viral variants emerged, notably the variant Delta in mid 2021 and the variant Omicron in late 2021. The Delta variant was characterized by its higher transmissibility and severity compared to the previously circulating strains [15], [16] and Omicron showed very high transmissibility but lower severity [17]. Real-word VE studies published during the periods of circulation of these two variants showed differences in vaccine-induced protection against Delta and Omicron variants-related infections, with less protection against Omicron than Delta [18], [19], [20], [21], [22].
In France, the COVID-19 vaccination campaign started in December 27, 2020 with the rollout of the Pfizer/BioNTech BNT162b2 and Moderna mRNA-1273 vaccines, which had received emergency use authorisations by the European Medicines Agency. The vaccines were distributed at first to those most likely to develop severe COVID-19 disease such as the elderly population (people aged ≥75 years old), residents of nursing homes and long-term care facilities, people with chronic conditions, and healthcare workers. The campaign gradually opened up to people aged 50 years and older, first with comorbidities, in February 19, 2021, and was extended to all adults by mid-May 2021 and to children ≥12 years old in mid-June 2021. The Oxford/AstraZeneca ChAdOx1-S vaccine became available in February 2021 but was restricted to ≥55 years old individuals from March 19, 2021, similarly to the Johnson & Johnson Ad26.COV2.S vaccine which became available from April 24, 2021. The country faced two large epidemic waves caused by the Delta variant between June and December 2021, followed by a third wave due to the Omicron variant which peaked in January 2022. To date, only a few studies [12], [23], [24] have reported on the protection afforded by vaccines in the French population, especially among the most at risk of severe COVID-19 disease. This observational study provides additional insights on vaccine protection provided by the COVID-19 mRNA vaccines Pfizer-BioNTech BNT162b2, and Moderna mRNA-1273 through the Delta- and Omicron BA.1-driven epidemic waves among immunocompetents adults 50 years and older, in France.
2. Materials and methods
2.1. Study design
This retrospective observational study was conducted between June 6, 2021 and February 10, 2022. During this period, France experienced three pandemic waves caused by the variants Delta (B.1.617.2) and Omicron (B.1.1.529), mainly its sub-lineage BA.1. The analysis relied on two complementary studies. First, a case-control study, based on the test-negative-design (TND), was implemented to estimate the risk (expressed as odds ratio) of developing a symptomatic SARS-CoV-2 infection, according to the vaccination status of participants. Individuals with self-declared COVID-19-like symptoms at sampling, and a SARS-CoV-2 diagnostic test result available during the study period, were considered. Symptomatic individuals with a positive SARS-CoV-2 reverse-transcriptase polymerase chain reaction (RT-PCR) were enrolled as cases, and those with a negative SARS-CoV-2 RT-PCR test were considered as controls. Second, a cohort study was implemented to estimate the risk (expressed as hazard ratio) of developing severe COVID-19 outcomes according to their vaccination status, among the symptomatic SARS-CoV-2 positive cases identified in the TND study.
2.2. Data sources
Three health information systems available in France [12], [15] served as sources of data. These information systems were set up in the context of the pandemic, and included:
-
(1)
SI-DEP which records results of all SARS-CoV-2 tests (RT-PCR, serologic, and antigen tests, excluding self-administered tests) performed in France. For each test carried out, regardless of the reason of testing (contact with someone who has tested positive for SARS-CoV-2, routine testing at work, etc…) and the testing site (hospital, pharmacy, diagnostic laboratory, long term care facility, etc…), the presence or absence (yes or no) of COVID-19-like symptoms is recorded. The database is intended to be exhaustive with regards to SARS-CoV-2 testing.
-
(2)
VAC-SI which provides information on the entire French population regarding vaccination status, the type of vaccines administered, vaccine brand name, number of doses administered, dates of the vaccine doses administered, and vaccine priority status (presence of comorbidities, healthcare professionals or social workers, retirement homes residents); and
-
(3)
SI-VIC which holds information on all hospital admissions for COVID-19, the type of hospital admissions (conventional wards, intensive care units [ICU]), and inpatient deaths linked to COVID-19.
The three databases were linked using a unique personal identifying number (a pseudonym i.e. a character string constructed through concatenation and encryption of individuals’s surname, first name, sex and date of birth) found in each database.
2.3. Outcomes of interest
We evaluated the absolute VE of complete 2-dose primary vaccination and booster vaccination against Delta and Omicron BA.1-related: (1) symptomatic infection through the TND study, and (2) severe outcomes following a symptomatic infection through the nested cohort studiy. Two levels of severity of COVID-19 disease were examined: i) any hospitalization, and ii) ICU admissions or in-hospital deaths.
2.4. Definition of vaccination status
By February 9, 2022, 71.1% of doses administered in the ≥50 years old population represented the Pfizer-BioNTech BNT162b2 vaccine; 16.8% were Moderna mRNA-1273; 10.7% accounted for Astrazeneca ChAdOx1-S vaccine; and 1.4% represented Johnson & Johnson Ad26.COV2.S vaccine. The study focused on mRNA vaccines, in the context of national vaccination guidelines which recommended mRNA vaccines for the booster dose.
Vaccination status was defined, before onset of symptoms, regarding the two mRNA COVID-19 vaccines namely Pfizer/BioNTech BNT162b2 and Moderna mRNA-1273. Based on French recommendations, individuals were classified as: (1) unvaccinated, (2) complete primary vaccination (if they had received two doses of vaccine), and (3) complete primary vaccination plus one booster dose (if they had received complete primary vaccination and an additional dose of vaccine). Vaccinated individuals were further classified according to the time since the last vacine dose administered, to assess waning over time.
2.5. Study period and study population
Individuals aged 50 years and above, with self-reported COVID-19-like symptoms and SARS-CoV-2 RT-PCR tests recorded within the period of June 6, 2021 and February 10, 2022 were considered. Cases were defined as individuals with at least one SARS-CoV-2-positive RT-PCR test, provided that the RT-PCR test was performed within seven days following the onset of symptoms, or <10 days after a positive antigenic test performed within seven days from the onset of symptoms. Where an individual had more than one positive RT-PCR test performed within the study period, the first test meeting the inclusion criteria was retained for each case. Negative tests recorded for cases during the study period were excluded. Eligible controls were those with a negative SARS-CoV-2 PCR test performed within the seven days following the onset of symptoms, and neither preceded nor followed by a positive RT-PCR test during the study period. The first negative test meeting the inclusion criteria was considered for each control. These criteria ensured that the same person could not be included as a case and a control.
Indidivuals were retrieved in both SI-DEP and the VAC-SI registers, based on the following inclusion criteria: (i) presence of self-reported COVID-19-like symptoms, (ii) absence of very-high-risk, immunocompromising comorbidities, as previously described [15], (iii) adherence to the case and control definition set above, (iv) complete primary vaccination schedule with two doses of mRNA vaccines, as defined by vaccination guidelines, and (v) homologous vaccination schedules i.e. the administration of the same vaccine, Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273, as 2-dose primary vaccination or 2-dose primary vaccination and booster. The selection process of the studied population is described in Fig. S1.
For the TND study, symptomatic RT-PCR positive individuals (cases) were randomly matched to symptomatic RT-PCR negative individuals (controls) with a ratio of up to five controls per case, depending on the number of controls available. Matching criteria included: the week of SARS-CoV-2 testing, the age (five-year age brackets), and the area of residence (Public inter-municipal cooperation establishment/EPCI level). The cohort study focused on symptomatic positive SARS-CoV-2 cases, identified in the TND study, who were retrieved in SI-VIC with a COVID-19-related hospitalization outcome of interest.
2.6. Characterization of variants
Variants were characterized using mutation screening; which is performed with a multiplex RT-qPCR targeting a set of predefined mutations associated with Delta and Omicron BA.1 variants. At the time of the study, the presence of the mutation L452R was indicative of the Delta variant; and the deletion 69–70 or mutations N501Y, K417N, S371L-S373P or Q493R were indivative of the Omicron BA.1 variant.
In absence of mutation screening results or where these results were inconclusive, individuals were assigned to a SARS-CoV-2 variant based on the dominant variant at the time of SARS-CoV-2 testing. The dominant variant was defined as the variant found at least at a prevalence of 90% in weekly representative whole genome sequencing (WGS) surveillance data available at both national and regional level [25]. Based on WGS results, the period of dominance of Delta, at a national level, started on 07/06/2021 and ended on 13/12/2021. Dominance of Omicron BA.1 started on 17/01/2022. The period between 13/12/2021 and 17/01/2022 was assigned to none of the two variants, as they co-circulated during that time. Depending on the region and based on regional WGS data, these periods of dominance of a variant shifted by one or two weeks.
2.7. Statistical analysis
Data (jointure of SI-DEP, VAC-SI and SI-VIC) for analysis was extracted on March 16, 2022. VE against symptomatic COVID-19 disease was estimated with a conditional logistic regression model, with the status of the individuals (cases or control) as the dependent variable and vaccination status as an independent variable. The models included the variables age (as a continuous variable modelled using multiple fractional polynomials), sex, type of residence, presence of at least one low or medium-risk comorbidity (yes or no) [15], and healthcare professional status (yes or no) as covariates. Low and medium-risk comorbidities included obesity, diabetes, chronic renal failure, chronic obstructive pulmonary disease, respiratory failure, hypertension, and heart failure. VE was estimated as (1 - odds of symptomatic positive SARS-CoV-2 test) × 100%.
Cox proportional hazard models were fitted to estimate the risk of occurrence of the two severe COVID-19 outcomes (any hospitalization, and ICU admissions or in-hospital deaths) studied among symptomatic SARS-CoV-2 positive cases. The follow-up period started on the RT-PCR test date and stopped 21 days following the index test; this represented the date of March 3, 2022 for those included on the last day of the study period (February 10, 2022). When the outcome occurred on the date of testing, the follow-up period was considered equal to 0.5 days. The model was adjusted for the same variables used in the conditional logistic regression model described above. VE against severe COVID-19 disease was deduced by combining the reduction in the odds of symptomatic disease with the reduction in the risk of severe outcome among cases, conferred by vaccination: [1 – (odds ratiosymptomatic disease × hazard ratiohospitalization)] × 100%. The Delta method was applied to obtain two-sided 95% confidence intervals of the VE estimates.
VE was estimated according to the time elapsed since the last vaccine dose administered: 15–28 days post first dose; 0–7, 8–30, 31–60, 61–90, 91–120, and > 120 days post second dose; 0–7, 8–60, and 61–120 days post first booster dose. To account for the time needed to mount a protective immune response, estimates in the first two weeks after the first dose of vaccine were not considered. Additional subgroup analyses by age groups (50–79 years and ≥80 years), and by variant (Delta and Omicron BA.1) were performed. All analyses were conducted using R Version 4.04.
2.8. Ethical considerations
This study was conducted under the legal mandate of Santé publique France, as the public health agency in France. The analyses made use of pseudononymised data collected by the French Ministry of Health through COVID-19 surveillance tools put in place to manage and assess the toll of the COVID-19 epidemic on the French population and monitor vaccination activities. The pseudonymisation process ensures that personal data cannot be linked to a specific individual without the use of additional data. This process is done in accordance to French law and is compliant to the provisions of the GDPR. In this context, ethical approval was not required to perform this study.
3. Results
3.1. Description of the study population
A total of 1 009 651 individuals 50 years and older was identified for the TND study. Among these individuals, 273 732 were cases (48.4% (132 467) Delta, and 51.6% (141 265) Omicron BA.1) and 735 919 were controls. Within the symptomatic SARS-CoV-2 cases, 7.5% were 80 years and older, 53.5% were females, 25.6% had at least one comorbidity, and 20.9% were not vaccinated at onset of symptoms. Among the symptomatic SARS-CoV-2 controls, 8.6% were 80 years and older, 59% were females, 34.1% had at least one comorbidity, and 39.9% were not vaccinated at symptoms onset. Further characteristic of the studied population are summarized in Table 1 . The survival analysis considered 273 695 cases (37 cases were excluded due to inconsistent dates), among whom 34 442 (12.6%) had been hospitalized.
Table 1.
Characteristics of cases and controls at symptoms onset.
| Variables | Overall N = 1 009 6511 | Cases N = 273 7321 | Controls N = 735 9191 | p-value2 |
|---|---|---|---|---|
| Age group | <0.001 | |||
| 50–79 | 926 121 (91.7%) | 253 126 (92.5%) | 672 995 (91.4%) | |
| ≥80 | 83 530 (8.3%) | 20 606 (7.5%) | 62 924 (8.6%) | |
| Sex | <0.001 | |||
| Female | 580 392 (57.5%) | 146 540 (53.5%) | 433 852 (59.0%) | |
| Male | 429 256 (42.5%) | 127 192 (46.5%) | 302 064 (41.0%) | |
| Unknown | 3 (0.0%) | 0 (0.0%) | 3 (0.0%) | |
| Region | <0.001 | |||
| Auvergne-Rhône-Alpes | 135 442(13.4%) | 38 502 (14.1%) | 96 940 (13.2%) | |
| Bourgogne-Franche-Comté | 39 987 (4.0%) | 10 826 (4.0%) | 29 161 (4.0%) | |
| Bretagne | 57 754 (5.7%) | 13 720 (5.0%) | 44 034 (6.0%) | |
| Centre-Val de Loire | 29 740 (2.9%) | 6 764 (2.5%) | 22 976 (3.1%) | |
| Corse | 4 252 (0.4%) | 1 504.0 (0.5%) | 2 748 (0.4%) | |
| Grand Est | 98 139 (9.7%) | 30 139.0 (11.0%) | 68 000 (9.2%) | |
| Guadeloupe | 1 095 (0.1%) | 413 (0.2%) | 682 (0.1%) | |
| Guyane | 1 755 (0.2%) | 388 (0.1%) | 1 367 (0.2%) | |
| Hauts-de-France | 61 409 (6.1%) | 15 607 (5.7%) | 45 802 (6.2%) | |
| Île-de-France | 132 426 (13.1%) | 35 070 (12.8%) | 97 356 (13.2%) | |
| La Réunion | 8 932 (0.9%) | 1 952 (0.7%) | 6 980 (0.9%) | |
| Martinique | 1 180 (0.1%) | 363 (0.1%) | 817 (0.1%) | |
| Mayotte | 74 (0.0%) | 1 (0.0%) | 73 (0.0%) | |
| Normandie | 48 325 (4.8%) | 9 980 (3.6%) | 38 345 (5.2%) | |
| Nouvelle-Aquitaine | 81 096 (8.0%) | 23 055 (8.4%) | 58 041 (7.9%) | |
| Occitanie | 112 782 (11.2%) | 33 803 (12.3%) | 78 979 (10.7%) | |
| Pays de la Loire | 54 295 (5.4%) | 13 177 (4.8%) | 41 118 (5.6%) | |
| Provence-Alpes-Côte d'Azur | 89 291 (8.8%) | 25 736 (9.4%) | 63 555 (8.6%) | |
| Unknown | 51 677 (5.1%) | 12 732 (4.7%) | 38 945 (5.3%) | |
| Healthcare worker status | <0.001 | |||
| No | 923 548 (91.5%) | 251 142 (91.7%) | 672 406 (91.4%) | |
| Yes | 51 194 (5.1%) | 11 910 (4.4%) | 39 284 (5.3%) | |
| Unknown | 34 909 (3.5%) | 10 680 (3.9%) | 24 229 (3.3%) | |
| Type of residence | <0.001 | |||
| Individual housing | 942 750 (93.4%) | 258 247 (94.3%) | 684 503 (93.0%) | |
| Hospitalized | 31 188 (3.1%) | 5 181 (1.9%) | 26 007 (3.5%) | |
| Prison | 210 (0.0%) | 48 (0.0%) | 162 (0.0%) | |
| Nursing homes | 11 231 (1.1%) | 3 032 (1.1%) | 8 199 (1.1%) | |
| Unknown | 24 272 (2.4%) | 7 224 (2.6%) | 17 048 (2.3%) | |
| Presence of comorbidities* | <0.001 | |||
| No | 688 915 (68.2%) | 203 616 (74.4%) | 485 299 (65.9%) | |
| Yes | 320 736 (31.8%) | 70 116 (25.6%) | 250 620 (34.1%) | |
| SARS-CoV-2 variants | <0.001 | |||
| SARS-CoV-2–negative | 735 919 (72.9%) | 0 (0.0%) | 735 919 (100.0%) | |
| Delta | 132 467 (13.1%) | 132 467 (48.4%) | 0 (0.0%) | |
| Omicron BA.1 | 141 265 (14.0%) | 141 265 (51.6%) | 0 (0.0%) | |
| Vaccination status & intervals between doses | <0.001 | |||
| Not vaccinated | 350 697 (34.7%) | 57 271 (20.9%) | 293 426 (39.9%) | |
| First dose 0–14 days | 20 571 (2.0%) | 3 507 (1.3%) | 17 064 (2.3%) | |
| First dose 15–28 days | 15 493 (1.5%) | 1 894 (0.7%) | 13 599 (1.8%) | |
| First dose > 28 days | 39 703 (3.9%) | 10 056 (3.7%) | 29 647 (4.0%) | |
| Second dose 0–7 days | 6 928 (0.7%) | 1 437 (0.5%) | 5 491 (0.7%) | |
| Second dose 8–30 days | 20 476 (2%) | 3 161 (1.2%) | 17 315 (2.4%) | |
| Second dose 31–60 days | 29 413 (2.9%) | 6 789 (2.5%) | 22 624 (3.1%) | |
| Second dose 61–90 days | 29 810 (3.0%) | 8 282 (3.0%) | 21 528 (2.9%) | |
| Second dose 91–120 days | 38 345 (3.8%) | 12 903 (4.7%) | 25 442 (3.5%) | |
| Second dose > 120 days | 210 924 (20.9%) | 89 668 (32.8%) | 121 256 (16.5%) | |
| Booster dose 0–7 days | 27 168 (2.7%) | 10 629 (3.9%) | 16 539 (2.2%) | |
| Booster dose 8–60 days | 168 213 (16.7%) | 48 384 (17.7%) | 119 829 (16.3%) | |
| Booster dose 61–120 days | 45 976 (4.6%) | 16 818 (6.1%) | 29 158.0 (4.0%) | |
| Booster dose > 120 days | 5 934 (0.6%) | 2 933 (1.1%) | 3 001 (0.4%) | |
*: low and medium-risk morbidities include obesity, diabetes, chronic renal failure, chronic obstructive pulmonary disease, respiratory failure, hypertension, and heart failure.
n (%).
Pearson's Chi-squared test.
3.2. Vaccine effectiveness against symptomatic SARS-CoV-2 infection
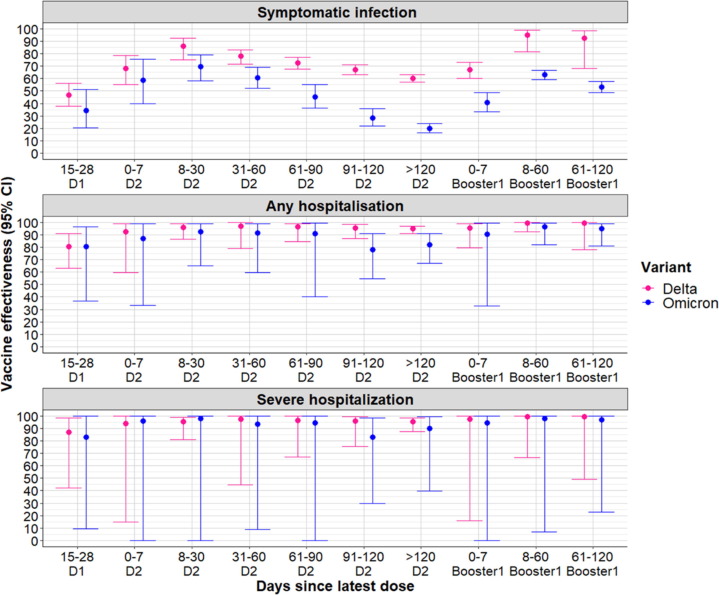
Vaccine effectiveness against symptomatic SARS-CoV-2 infection of both mRNA vaccines (BNT162b2 and mRNA-1273) reached high levels following completion of the primary vaccination schedule. Adjusted VE against Delta reached 86% (95% confidence interval (CI): 75−92%) within a month (8–30 days) after the second dose of any mRNA vaccine. However protection waned over time dropping to 78% (95% CI: 72−83%) at 31–60 days and subsequently reaching 60% (57−63%) > 120 days after the second dose. Effectiveness against Omicron BA.1 symptomatic infection was markedly lower and waned faster in comparison to Delta. Protection against this variant stood at 70% (95% CI: 58−79%) 8–30 days after the second dose. The decline in VE accelerated from the third month of receving the second dose of mRNA vaccine and reached 20% (95% CI: 16−24%) > 120 days post second dose.
The booster dose was very effective at restoring immunity against Delta; VE reached 95% (95% CI: 81−99%) at 8–60 days post vaccination. However only partial restitution of protection against Omicron BA.1 was noted: 63% (95% CI: 59−67%) at 8–60 days post the booster dose. Morever while little waning of protection against SARS-CoV-2 infection with Delta occurred following administration of the booster dose vaccine-induced protection against SARS-CoV-2 infection with Omicron BA.1 decreased significantly to 53% (49−58%) at 61–120 days following administration of the booster dose (Fig. 1 and Table S1).
Fig. 1.
Vaccine effectiveness (all mRNA vaccines combined) against symptomatic COVID-19 disease, any hospitalization and severe hospitalization (ICU admission or in-hospital death) according to the number of doses received and the time since the last dose among ≥50 years old individuals. D1 = first dose of vaccine; D2 = second dose of vaccine.
3.3. Vaccine effectiveness against hospitalizations for COVID-19
The survival analysis (Cox poportional hazard model) showed a reduction in the relative risks for any hospitalization and ICU admission or in-hospital death following symptomatic SARS-CoV-2 infection; this reduction was particularly marked ≥30 days after the second dose. At 31–60 days post second dose the risk of any hospitalisation, and ICU admission or in-hospital death due to Delta was significantly reduced to 0.14 (95% CI: 0.10–0.18) and 0.12 (95% CI: 0.08–0.19) respectively. Risk estimates against Omicron BA.1 were 0.21 (95% CI: 0.14–0.32) for any hospitalization and 0.16 (95% CI: 0.08–0.35) for ICU admission or in-hospital death (Table S1). The adjusted VE against hospitalization which combined the odds of developing a symptomatic SARS-CoV-2 infection and the risk of hospitalization reached 97% (95% CI: 79−100%) against any hospitalization and 97% (95% CI: 45−100%) against ICU admission or in-hospital death related to Delta within 31–60 days of receving the second dose. For Omicron BA.1, VE against any hospitalization was 92% (95% CI: 59−99%) and was 94% (95% CI: 9−100%) against ICU admission or in-hospital death related to COVID-19 within the same vaccination time interval.
While vaccine-induced protection against both Delta-related severe outcomes remained constant over time, estimates decreased over time against Omicron BA.1. Between 91 and 120 days following the second dose VE levels against Omicron BA.1 were 78% (95% CI: 55−91%) against any hospitalization and 83% (95% CI: 30−98%) against ICU admission or in-hospital death. The booster dose increased VE again to levels above 95% for both outcomes related to Omicron BA.1 (Fig. 1 Table S1).
3.4. Vaccine effectiveness by age groups
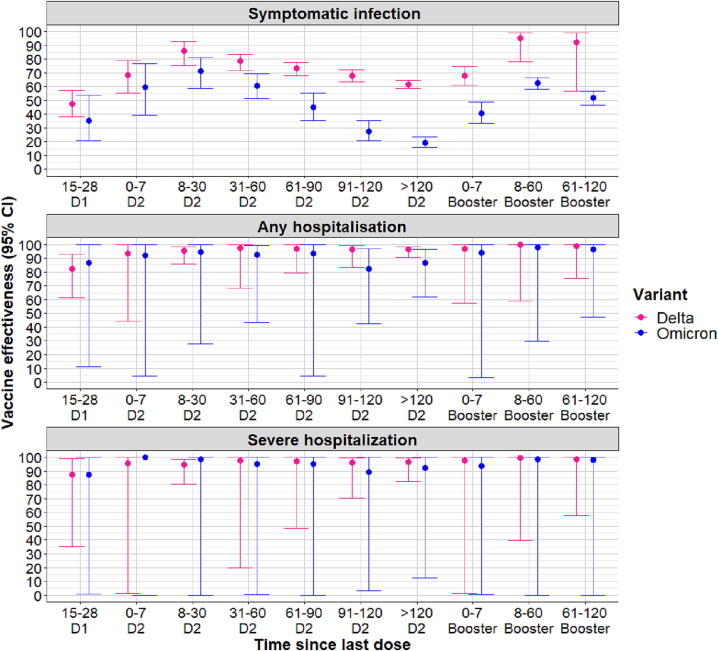
Against Delta symptomatic infection, similar levels of protection were noted in both age groups but with significant waning among ≥80 years old. Levels of protection within this age group decreased from 86% (95% CI: 11%-100%) 8–30 days to 39% (95% CI: 29−50%) at > 120 days post second dose whereas it decreased from 86% (95% CI: 75−93%) at 8–30 days to 62% (59%-65%) at > 120 days post second dose among 50–79 years old. The booster dose increased VE to 95% (95% CI: 78−99%) among 50–79 years old individuals and to 91% (95% CI: 48−99%) among ≥80 years old individuals 8–60 days after vaccination (Tables S2 and S3).
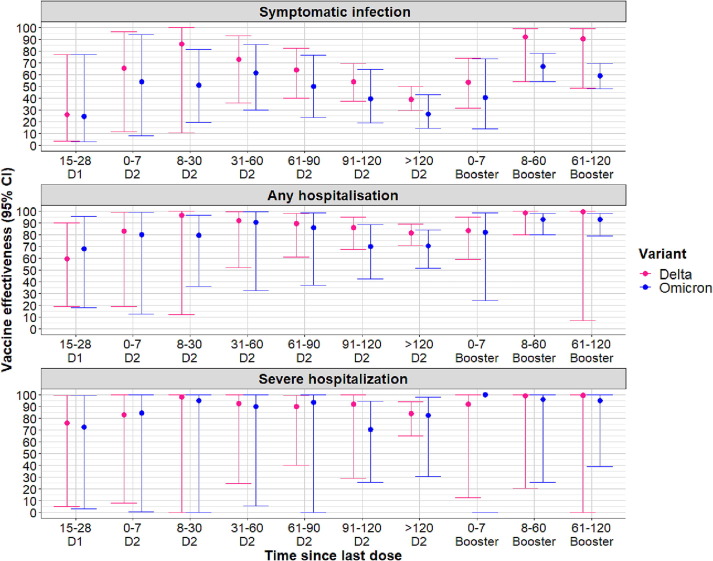
The adjusted VE against Omicron BA.1-related symptomatic infection in 50–79 years old was estimated at 71% (95% CI: 59−81%) 8–30 days following the second dose. This estimate decreased to 19% (95% CI: 16−24%) > 120 days since vaccination (Fig. 2 ). Among ≥80 years olds vaccine protection against the same outcome was estimated at 51% (95% CI: 20−82%) at the same vaccination time interval and decreased to 26% (95% CI: 15−43%) past 120 days from receiving the second dose (Fig. 3 ). The booster dose reinstaured protection levels against Omicron BA.1 to 63% (95% CI: 58−66%) in 50–79 years old and 67% (95% CI: 54−78%) in ≥80 years old at 8–60 days following vaccination.
Fig. 2.
Vaccine effectiveness (all mRNA vaccines combined) against symptomatic COVID-19 disease, any hospitalization and severe hospitalization (ICU admissions or in-hospital deaths) according to the time since last vaccine dose and variant of infection among 50–79 years old individuals. D1 = first dose of vaccine; D2 = second dose of vaccine.
Fig. 3.
Vaccine effectiveness (all mRNA vaccines combined) against symptomatic COVID-19 disease, any hospitalization and severe hospitalization (ICU admissions or in-hospital deaths) according to the time since last vaccine dose and variant of infection among individuals ≥80 years old. D1 = first dose of vaccine; D2 = second dose of vaccine.
With regards to protection from severe outcomes, slower and limited waning of protection was observed for the 50–79 years old compared to the ≥80 years old. Among 50–79 years old, effectiveness against Delta-related hospitalization outcomes remained at levels above 90% over time; against Omicron BA.1-related any hospitalization, it decreased to 87% (95% CI: 62−97%) > 120 days post second dose. Among ≥80 years old vaccine-induced protection against severe outcomes was also high across all vaccination time intervals. In this age group, VE against Delta-related hospitalization outcomes decreased to levels below 90%, from 61 days post vaccination. Against Omicron-related hospitalization outcomes, the levels of protection were also high but lower compared to Delta. Still, limited waning of protection was observed in comparison to the protection against symptomatic disease (Tables S2 and S3).
4. Discussion
Findings from this study confirmed high effectiveness of primary vaccination with two doses of mRNA COVID-19 vaccines BNT162b2 Pfizer/BioNTech and mRNA-1273 Moderna against symptomatic SARS-CoV-2 infection in the population aged 50 years and above. As documented in the literature [19], [22], [26], VE during the Delta-dominant period was higher compared to the Omicron BA.1-dominant period: two doses of either BNT162b2 Pfizer/BioNTech or mRNA-1273 Moderna vaccines were 86% effective against Delta symptomatic infection but were only 70% effective against Omicron BA.1 symptomatic infection 8–30 days post second dose.
Several studies have described a drop over time of two-dose vaccine effectiveness against SARS-CoV-2 symptomatic infection; this decrease in protection was substantially higher against Omicron BA.1 compared to Delta [3], [8], [12], [19], [26]. Similar patterns of waning were observed in our study, with lower estimates of VE against Omicron BA.1 than Delta at each studied interval after the second dose. Certainly, waning of vaccine-induced immunity was particularly marked against Omicron BA.1 for which effectiveness against related symptomatic infection declined from 70% at 8–30 days to 20% >120 days following completion of the 2-dose primary vaccination series. The decrease in protection against Delta has been mainly attributed to a decay of vaccine-induced neutralizing antibodies over time rather than an intrinsic capacity of the Delta variant to escape immunity [27], [28]. This is different with Omicron BA.1 which possesses a greater capacity of immune escape [29], [30], [31]; which when coupled to a decay of neutralizing antibodies further reduces VE against this variant. Still, numerous studies conducted during the Omicron BA.1 period have reported an increase in VE against infection caused by this variant following a booster dose [19], [26], [32], [33], [34], [35]. Indeed in our study, VE increased to 63% against Omicron BA.1 in the two months (7–60 days) subsequent to a mRNA vaccine booster dose. These results further illustrate the significant impact of mRNA vaccine booster dose in restoring a high level of protection, particularly during the Omicron BA.1-dominant period.
Our study also confirmed high protection against both Delta and Omicron BA.1 variants-related hospitalizations provided by mRNA vaccines. Our estimated VE against Delta-related hospitalizations are comparable to results from a recent French observational study [23]. Bouillon and colleagues estimated an adjusted VE of 89% (95% CI: 87−90%) provided by a two-dose BNT162b2 vaccination schedule following an infection during the Delta dominant period in France. The adjusted VE with the mRNA-1273 vaccine was 95% (95% CI: 90−98%) [23]. Moreover, as observed in this study, Castillo and colleagues noted that waning of protection following infection with Delta was less pronounced against COVID-19 related hospitalizations than against symptomatic infection [12]. Our study did not differentiate between the two vaccines brands, as we focused on investigating the effectiveness of the vaccination campaign using mRNA vaccines. Nevertheless our results were comparable to those from studies in other countries [19], [20], [34]. In the USA, Lauring and colleagues estimated VE at 85% (95% CI: 83−87%) with 2 doses of any mRNA vaccine and 94% (95% CI: 92−95%) following the booster dose against Delta [20]. In the Canadian study by Gram et al, protection induced by any mRNA vaccine against COVID-19 hospitalization with Delta variant after 2 doses, among individuals aged 60 years or above, was estimated at 87.5% (95% CI: 85.6−89.2%) >120 days since vaccination. Following the booster dose, protection increased to 97.2% (95% CI: 94.6−98.5%) 14–30 days and 91.7% (95% CI: 78.7−96.7%) 61–90 days since vaccination [34]. Point estimates of the adjusted VE against Omicron BA.1 described in this study should be interpreted with cautious due to the wide 95% confidence intervals.
Throughout this fast-evolving COVID-19 pandemic the test-negative design (TND) has been commonly used to evaluate real-world vaccine effectiveness of COVID-19 vaccines. This study design offers the ability (1) to select individuals (cases and controls) with symptoms suggestive of COVID-19 who present at a health-care facility for diagnostic testing thereby reducing selection bias and other confounding biases associated with health-care seeking behaviours. The TND also enables to monitor waning of VE over time and to assess the performance of these vaccines against new viral strains; two very pertinent aspects of the COVID-19 pandemic that we could evaluate in this study. As with any case-control study, minimizing false-positive and false-negative results was crucial; thus, cautions was taken in the selection of cases (confirmed PCR positive test within seven days following the onset of symptoms or <10 days after a positive antigenic test performed within seven days after the onset of symptoms) and controls (exclusion of negative antigenic tests without follow-up negative PCR confirmed test and negative PCR tests performed more than seven days from the onsets of symptoms). The exhaustiveness of the databases used and being able to link data at an individual level across these databases provided us with the ability to investigate vaccine effectiveness at a population level. Other advantages of using these databases were the ability (1) to differentiate between individuals hospitalized for COVID-19 and those hospitalized for other medical conditions and infected with COVID-19 (the former were selected for our analysis), and (2) to identify and exclude immunocompromised individuals from the study.
Nonetheless, the study also bears some limitations inherent to its observational nature, which may have limited our consideration of the difference in the risks of infection between vaccinated and unvaccinated individuals [36]. Indeed cases and controls were matched according to the week of testing to ensure that both groups of individuals were exposed to a similar virus circulation; and age which is an important factor in the risk of infection. Other factors such as diagnostic testing frequency between the two groups may still have confounded our estimates. For instance health-conscious vaccinated individuals may test more frequently (at the onset of slight symptoms related to COVID-19) and therefore be more likely to document an infection. Conversely being vaccinated may lead to changes in behaviours such as less diagnostic testing in case of symptoms or less adherence to protective barriers. We were unable to control for previous laboratory-confirmed infections due to technical difficulties in creating the unique identifier in the SI-DEP database in 2020. This could have led to an underestimation of VE if individuals with knowledge of a previous infection have been less prone to get vaccinated. However, this situation is likely to be marginal, due to (1) the exclusion in our study of individuals who had received only one vaccine dose as a complete primary vaccination, in presence of a history of documented previous infection and (2) the requisite, in France, of a 2-doses primary vaccination for all social activities, in absence of a documented previous infection. Furthermore, differences in socio-demographics may have potentially influenced access to vaccine and the decision to be vaccinated among individuals – socially disadvantaged individuals are less likely to be vaccinated and may be more exposed for example in a professional context or due to living conditions [23]. To minimize this bias, cases and controls were also matched according to a fine geographical level (>1250 adminitrative units) which served as a proxy of socio-economic status and to ensure comparable exposition to vaccination and different levels of virus circulation. Additionally, all models performed were adjusted for other potential confounders such as sex (male sex has been associated with higher morbidity and mortality related to COVID-19 disease), and type of residence (individual housing versus housing in long-term care facilities which is likely related to both individual vaccination status and level of exposition to the virus). The presence of comorbidities was also considered, in the study, as a potential confounder; risky behaviors, the risk of severe COVID-19 disease, as well as the decision to get vaccinated may differed between individuals with and without these comorbidities. Another limitation, common to almost all COVID-19 VE studies assessing duration of protection, is linked to a higher depletion of susceptible individuals, through undocumented asymptomatic infections, among the unvaccinated as compared to the vaccinated individuals. This may lead to underestimate the residual vaccine-induced protection when moving away from vaccination [37]. However, this bias is likely to be limited in our study due the low proportion of asymptomatic infections in the studied age groups.
5. Conclusion
This study confirmed the high effectiveness of original mRNA vaccines used in France against both symptomatic SARS-CoV-2 infection and severe complications related to COVID-19 disease among immunocompetent adults aged ≥50 years old. In contrast to the high levels of protection induced by complete 2-dose primary vaccination against Delta, protection against Omicron BA.1 remained however modest and short-term. A booster dose restored protection against Omicron BA.1 symptomatic infection for at least two months and induced very high protection against severe outcomes caused by both variants.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authours would like to thank Alessandro PINI for his valuable contribution to the early phases of implementation of this study at Santé Publique France.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.02.062.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
The data that has been used is confidential.
References
- 1.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charmet T., Schaeffer L., Grant R., Galmiche S., Cheny O., Von Platen C., et al. Impact of original, B.1.1.7, and B.1.351/P.1 SARS-CoV-2 lineages on vaccine effectiveness of two doses of COVID-19 mRNA vaccines: Results from a nationwide case-control study in France. Lancet Reg Health Eur. 2021;8 doi: 10.1016/j.lanepe.2021.100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27:1614-21. doi:10.1038/s41591-021-01446-y. [DOI] [PubMed]
- 4.Haas E.J., Angulo F.J., McLaughlin J.M., Anis E., Singer S.R., Khan F., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moline HL, Whitaker M, Deng L, Rhodes JC, Milucky J, Pham H, et al. Effectiveness of COVID-19 Vaccines in Preventing Hospitalization Among Adults Aged >/=65 Years - COVID-NET, 13 States, February-April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1088–93. doi:10.15585/mmwr.mm7032e3. [DOI] [PMC free article] [PubMed]
- 6.Self WH, Tenforde MW, Rhoads JP, Gaglani M, Ginde AA, Douin DJ, et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions - United States, March-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1337–43. doi:10.15585/mmwr.mm7038e1. [DOI] [PMC free article] [PubMed]
- 7.Tang P., Hasan M.R., Chemaitelly H., Yassine H.M., Benslimane F.M., Al Khatib H.A., et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021;27:2136–2143. doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 8.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews N., Tessier E., Stowe J., Gower C., Kirsebom F., Simmons R., et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med. 2022;386:340–350. doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chemaitelly H., Tang P., Hasan M.R., AlMukdad S., Yassine H.M., Benslimane F.M., et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castillo M.S., Khaoua H., Courtejoie N. Vaccine effectiveness and duration of protection against symptomatic infections and severe Covid-19 outcomes in adults aged 50 years and over, France, January to mid-December 2021. Global. Epidemiology. 2022: doi: 10.1016/j.gloepi.2022.100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suah J.L., Husin M., Tok P.S.K., Tng B.H., Thevananthan T., Low E.V., et al. Waning COVID-19 vaccine effectiveness for BNT162b2 and CoronaVac in Malaysia: an observational study. Int J Infect Dis. 2022;119:69–76. doi: 10.1016/j.ijid.2022.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feikin D.R., Higdon M.M., Abu-Raddad L.J., Andrews N., Araos R., Goldberg Y., et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/s0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auvigne V., Vaux S., Strat Y.L., Schaeffer J., Fournier L., Tamandjou C., et al. Severe hospital events following symptomatic infection with Sars-CoV-2 Omicron and Delta variants in France, December 2021-January 2022: a retrospective, population-based, matched cohort study. EClinicalMedicine. 2022;48 doi: 10.1016/j.eclinm.2022.101455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wrenn J.O., Pakala S.B., Vestal G., Shilts M.H., Brown H.M., Bowen S.M., et al. COVID-19 severity from Omicron and Delta SARS-CoV-2 variants. Influenza Other Respir Viruses. 2022;16:832–836. doi: 10.1111/irv.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Araf Y., Akter F., Tang Y.D., Fatemi R., Parvez M.S.A., Zheng C., et al. Omicron variant of SARS-CoV-2: genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol. 2022;94:1825–1832. doi: 10.1002/jmv.27588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Accorsi E.K., Britton A., Fleming-Dutra K.E., Smith Z.R., Shang N., Derado G., et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022;327:639–651. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchan S.A., Chung H., Brown K.A., Austin P.C., Fell D.B., Gubbay J.B., et al. Estimated effectiveness of COVID-19 vaccines against omicron or delta symptomatic infection and severe outcomes. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.32760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauring A.S., Tenforde M.W., Chappell J.D., Gaglani M., Ginde A.A., McNeal T., et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376 doi: 10.1136/bmj-2021-069761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) Variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng H.F., Ackerson B.K., Luo Y., Sy L.S., Talarico C.A., Tian Y., et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med. 2022;28:1063–1071. doi: 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouillon K., Baricault B., Botton J., Jabagi M.-J., Bertrand M., Semenzato L., et al. Effectiveness of BNT162b2, mRNA-1273, and ChAdOx1-S vaccines against severe covid-19 outcomes in a nationwide mass vaccination setting: cohort study. BMJ Medicine. 2022:1. doi: 10.1136/bmjmed-2021-000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semenzato L., Botton J., Baricault B., Deloumeaux J., Joachim C., Sylvestre E., et al. Vaccine effectiveness against severe COVID-19 outcomes within the French overseas territories: a cohort study of 2-doses vaccinated individuals matched to unvaccinated ones followed up until September 2021 and based on the National Health Data System. PLoS One. 2022;17 doi: 10.1371/journal.pone.0274309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.France SP. Coronavirus: circulation des variants du SARS-CoV-2; 2021. <https://www.santepubliquefrance.fr/dossiers/coronavirus-covid-19/coronavirus-circulation-des-variants-du-sars-cov-2> [accessed 25 November 2022].
- 26.Hansen CH, Schelde AB, Moustsen-Helm IR, Emborg H-D, Krause TG, Mølbak K, et al. Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: a Danish cohort study. medRxiv. 2021:2021.12.20.21267966. doi:10.1101/2021.12.20.21267966.
- 27.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newman J., Thakur N., Peacock T.P., Bialy D., Elrefaey A.M.E., Bogaardt C., et al. Neutralizing antibody activity against 21 SARS-CoV-2 variants in older adults vaccinated with BNT162b2. Nat Microbiol. 2022 doi: 10.1038/s41564-022-01163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rössler A., Riepler L., Bante D., von Laer D., Kimpel J. SARS-CoV-2 Omicron variant neutralization in serum from vaccinated and convalescent persons. N Engl J Med. 2022;386:698–700. doi: 10.1056/NEJMc2119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu J., Peng P., Cao X., Wu K., Chen J., Wang K., et al. Increased immune escape of the new SARS-CoV-2 variant of concern Omicron. Cell Mol Immunol. 2022;19:293–295. doi: 10.1038/s41423-021-00836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sokal A., Broketa M., Barba-Spaeth G., Meola A., Fernández I., Fourati S., et al. Analysis of mRNA vaccination-elicited RBD-specific memory B cells reveals strong but incomplete immune escape of the SARS-CoV-2 Omicron variant. Immunity. 2022 doi: 10.1016/j.immuni.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abu-Raddad L.J., Chemaitelly H., Ayoub H.H., AlMukdad S., Yassine H.M., Al-Khatib H.A., et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron Infection in Qatar. N Engl J Med. 2022;386:1804–1816. doi: 10.1056/NEJMoa2200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrews N., Stowe J., Kirsebom F., Toffa S., Sachdeva R., Gower C., et al. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;28:831–837. doi: 10.1038/s41591-022-01699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gram M.A., Emborg H.-D., Schelde A.B., Friis N.U., Nielsen K.F., Moustsen-Helms I.R., et al. Vaccine effectiveness against SARS-CoV-2 infection or COVID-19 hospitalization with the Alpha, Delta, or Omicron SARS-CoV-2 variant: a nationwide Danish cohort study. PLoS Med. 2022;19 doi: 10.1371/journal.pmed.1003992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monge S., Rojas-Benedicto A., Olmedo C., Mazagatos C., Jose Sierra M., Limia A., et al. Effectiveness of mRNA vaccine boosters against infection with the SARS-CoV-2 omicron (B.1.1.529) variant in Spain: a nationwide cohort study. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewnard J.A., Patel M.M., Jewell N.P., Verani J.R., Kobayashi M., Tenforde M.W., et al. Theoretical framework for retrospective studies of the effectiveness of SARS-CoV-2 vaccines. Epidemiology. 2021;32:508–517. doi: 10.1097/ede.0000000000001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahn R., Schrag S.J., Verani J.R., Lipsitch M. Identifying and alleviating bias due to differential depletion of susceptible people in postmarketing evaluations of COVID-19 vaccines. Am J Epidemiol. 2022;191:800–811. doi: 10.1093/aje/kwac015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.