Abstract
The severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) induced coronavirus disease 2019 (COVID-19) has recently caused a pandemic. Patients with COVID-19 presented with a wide spectrum of symptoms for the disease, from entirely asymptomatic disease to full-blown pneumonia and multiorgan failures. More evidence emerged, showing the production of interferons (IFNs) in the severe cases were significantly lower than their milder counterparts, suggesting linkage of COVID-19 to impaired innate immunity. This review presents a brief overview of how coronaviruses evade innate immunity, according to the current studies about SARS-CoV and middle-east respiratory syndrome-coronavirus (MERS-CoV). The coronaviruses manage to block, escape, or dampen the innate immune response by antagonizing double-stranded RNA (dsRNA) sensor, mitochondrial antiviral-signaling protein (MAVS) and stimulator of IFN genes (STING) pathways, epigenetic modification, posttranslational modifications, and host mRNA translation. We provide novel insights into a comprehensive therapy to combat SARS-CoV-2 infection.
Keywords: COVID-19, Coronavirus, Innate immunity, Immune evasion, Epigenetics
1. Introduction
As the outbreak of coronavirus disease 2019 (COVID-19) becomes pandemic worldwide, more than 190 countries are currently affected, confirming over 537.8 million cases, and mortality exceeds 6.3 million people [1]. Patients presented with a wide spectrum of symptoms for the disease, from entirely asymptomatic disease to full-blown pneumonia and multiorgan failures [2]. Half of the COVID-19-infected individuals were asymptomatic, but highly contagious [3]. Their viral loads were comparable to that in patients with symptoms, therefore possess a hidden threat to the health-care workers and general population [4], [5]. Immune cells (including monocyte, lymphocyte and natural killer cells [NKs]) involved in innate immune response were crucial in systemic inflammatory induced by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection, and the key immune cell subsets were revealed to decrease significantly in COVID-19 cases. A clinical study reported a landscape of single-cell mRNA sequencing data of clonotypic T cells, clonotypic B cells and peripheral blood mononuclear cells (PBMCs) from 16 patients with COVID-19. A rapid decline of naïve CD4+ T lymphocytes, as well as a decrease of CD8+ T lymphocytes and NKs in severe cases [6]. Another single-cell profiling of immune cells from patients during the early recovery stage also found that CD4+ T cells and CD8+ T cells diminished along with a high level of inflammatory factors in patients with COVID-19 [7]. More evidence emerged, showing the production of interferons (IFNs) in the severe cases were significantly lower than their milder counterparts, suggesting linkage of COVID-19 to impaired innate immunity [8]. By investigating type I IFNs (IFN-α and IFN-β) and type III IFNs (IFN-λ) in 32 moderate-to-severe hospitalized patients diagnosed with COVID-19, evidence revealed that the production of type I and III IFNs both delayed and decreased in patients who finally became critically ill [9]. In contrast, type I IFNs and IFN-λ were intensively induced earlier and at a higher level in patients with milder symptoms, which were independently of disease severity. This phenomenon underlined the importance of a thorough investigation into the possible mechanisms of SARS-CoV-2 escaping the human innate immune system and continuous endeavor into the development of therapeutic strategy [10]. This review presents a brief overview of how coronaviruses evade innate immunity, according to the information from SARS-CoV and middle-east respiratory syndrome-coronavirus (MERS-CoV) as they share significant sequence and structural similarities with SARS-CoV-2 [11].
2. Coronavirus
Coronavirus is a large RNA virus marked with a 26–32 kb positive-sense single-stranded RNA genome. Four human coronavirus genera (α, β, γ and δ) have been distinguished, including two α coronavirus (CoV-NL63 and 229E) and four β coronavirus (SARS-CoV, MERS-CoV, CoV-OC43 and CoV-HKU1) [12]. The newly discovered novel coronavirus, SARS-CoV-2, is the third most pathogenic coronavirus to human population, right after the SARS-CoV and the MERS-CoV. SARS-CoV-2 has a typical CoV genome that contains at least ten ORFs with 75–80 % sequence similarity to SARS-CoV [11].
3. Mechanisms of innate immune evasion
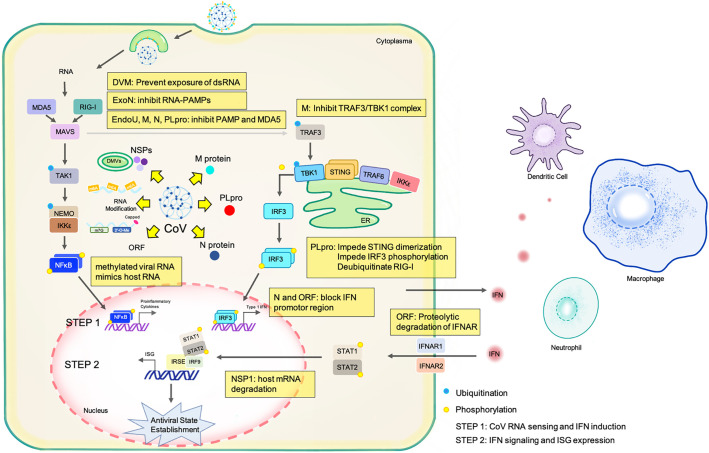
The innate immunity activates a serial of antiviral and proinflammatory signaling. The subsequent induction of IFNs in turn recruit macrophages and dendritic cells to the infection site, impair the efficacy of virus replication, and possibly lead to the elimination of infected cells. Several key factors such as IFN regulatory factor 3 (IRF3), nuclear factor of kappa light polypeptide gene enhancer in B cells (NF-κB), and stimulator of IFN genes (STING) can detect both DNA and RNA viruses and fine-tune the immune response to specifically inhibit or remove the virus without damaging the host. Virus may evolve elaborate mechanisms to either evade or inactivate the host innate immune response as summarized in Fig. 1 and Table 1 .
Fig. 1.
Schematic diagram of mechanisms by which coronavirus evade or inactivate the host innate immune response. Sequestering of viral RNA in double membrane vesicles (DMVs) prevents dsRNA from being recognized by retinoic acid-inducible gene-I (RIG-I)-like-receptors (RLRs). The the 3′ → 5′ exonuclease (ExoN) in RNA-PAMPs degradation acts as an interferon (IFN) antagonist. Endonuclease, Poly(U) Specific (EndoU) targets dsRNA sensor to cleave polyU sequences. Both protein M and N inhibit the PAMP and MDA5 to recognize the virus. Papain-like protease (PLpro) impedes STING dimerization, IRF3 phosphorylation, and deubiquitinate RIG-I. Protein N and ORF block IFN promotor region. ORF promotes proteolytic degradation of interferon alpha and beta receptor subunit (IFNAR). Non-structural protein 1 (NSP1) promotes host mRNA degradation.
Table 1.
Summary of mechanisms by which coronavirus evade or inactivate the host innate immune response.
| Mechanism | Function | |
|---|---|---|
| Targeting RNA Sensor | (1) DMVs | Isolate viral RNA from the host sensors |
| (2) EndoU | Limit PAMP formation (NSP15); Impede MDA5 activation | |
| (3) Exon | Degrade RNA-PAMPs (NSP14); Antagonize PKR activation; Inhibit stress granule formation | |
| (4) ORF4a | Impede MDA5 activation | |
| Targeting STING and MAVS pathways | (1) PLpro | Impede STING dimerization; PLpro-™ inhibit IRF3 phosphorylation; Deubiquitinate RIG-I |
| (2) M protein | Inhibit TRAF3/TBK1 complex formation | |
| (3) N protein | Block the promotor region of IFN-γ | |
| (4) ORF4a\4b\5 | Block the promotor region of IFN-β; prevent IRF3 translocation | |
| (5) ORF4b | bind to TBK1 and IKKε | |
| (6) ORF9b | proteasomal degradation of MAVS, TRAF3, TRAF6 | |
| RNA Modification | (1) m6A | Decrease CD80, CD83, CD86, MHC class II, TNFα, IL-12 |
| (2) m7G and 2′-O-Me | Catalyze the methylation steps of 5′-methylated-blocked cap structure | |
| (3) RNA editing | Related to the intensity of host innate immune response | |
| (4) microRNA | Hijack the host miRNA machinery to regulate the interferon-mediated immune response | |
| Post-translational Modification | (1) Macrodomain | Reverse the activity of ADP-ribosyltransferase |
| Inhibiting host mRNA translation | (1) NSP1 | Bind to cellular factors; facilitate host mRNA degradation |
DMVs: double membrane vesicles; EndoU: Endonuclease, Poly(U) Specific; NSP: Non-structural protein; PAMP: pathogen-associated molecular pattern; PKR: protein kinase R; ORF: open reading frames; MDA5: melanoma differentiation-associated gene 5; IFN: interferon; STING: stimulator of IFN genes; MAVS: mitochondrial antiviral-signaling protein; PLpro: papain-like protease; IRF3: IFN regulatory factor 3; RIG-I: retinoic acid-inducible gene I; TRAF: TNF receptor associated factor; TBK1: TANK binding kinase 1; IKKε: NF-κB kinase epsilon; m6A: N6-methyladenosine; MHC: major histocompatibility complex; TNF: tumor necrosis factor; IL: interleukin; m7G: 7-methylguanosine; 2′-O-Me: 2′-O-methylation; ADP: adenosine diphosphate.
3.1. Target dsRNA sensor
CoVs that utilize double-stranded RNA (dsRNA) intermediate can be perceived as “non-self RNA” by the dsRNA sensors, such as retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) [13]. Upon activation, dsRNA sensors promote the expression of IFN-stimulated genes (ISGs) and the subsequent execution of antiviral activity [14]. In response, CoVs evolved unique strategies to alleviate the early activation of dsRNA sensors. In addition to the RNA, CoVs contain structure proteins (spike [S], envelope [E], membrane [M], and nucleocapsid [N]), non-structural proteins (NSPs) and a set of accessory proteins. Some of these proteins act as antagonists to interrupt the recognition process. Two main mechanisms are involved: the sequestering of viral RNA in double membrane vesicles (DMVs), which prevents dsRNA from being recognized by RIG-I-like-receptors (RLRs), or some special factors expressed by the CoVs antagonize the innate response directly [15].
A hallmark of CoVs replication process is the formation of the endoplasmic reticulum (ER)-derived DMVs, which are the sites of viral RNA synthesis [16]. The NSPs, processed from replicase polyprotein, involve in assembling the host ER to form intricate membranes and DMVs [16]. In the early stages of viral infection, SARS-CoV and MERS-CoV dsRNA associates with replication complexes sequestered in DMVs, which are considered to isolate the viral RNA from antiviral defense mechanisms [17], [18]. The “replication network” resulting from DMV merger uncovered the process on how CoVs extensively rearrange the host ER infrastructure to coordinate and synchronize the replication cycle, and potentially hide viral RNA from being detected [18]. Previous evidence revealed that SARS-CoV nsp3, 4, 6, and MERS-CoV nsp3 and 4, which contain transmembrane domains, are required for DMV generation [19]. Also, coronavirus nsp15, which is an endoribonuclease designated endonuclease poly(U) specific (EndoU), derails the activation of dsRNA sensors in macrophages, possibly by burying the viral dsRNA in DMVs [20].
Besides these “hiding” strategies inside the DMVs, the “attacking” mechanism targeting the dsRNA sensor by encoding anti-immune response antagonists, are also essential in innate immune evasion. Efficient removal of RNA-pathogen-associated molecular patterns (PAMPs) inhibits the innate immune response and thus facilitating efficient replication in the infected cells [16]. Therefore, another mechanism for EndoU targeting dsRNA sensor is to cleave polyU sequences from 5′-polyU-containing negative-sense RNAs, thus inhibiting the generation of PAMPs and limiting MDA5 to activate the innate immune system towards viral infection [21].
Also, NSP4a of MERS-CoV may prohibit IFN production by activating MDA5 via direct interaction with dsRNA [22]. As IFN induction is potently impaired in CoV infection, the function of the 3′ → 5′ exonuclease (ExoN) in RNA-PAMPs degradation might be feasible as well as complement known IFN-antagonistic mechanisms during viral infection [16]. Protein 4a exerts its antagonistic role to inhibit protein kinase R (PKR) activation and stress granule formation by binding to dsRNA in viral [23].
3.2. Antagonism to STING and MAVS pathways
RNA and DNA viruses are recognized by distinct signaling pathways. The viral dsRNA engages the MAVS adaptor to promote IFN expression, whereas cGMP-AMP synthase (cGAS) binds viral DNA and activates an analogous pathway via the STING protein. STING resides in the ER, and upon activation, generates dimers that assemble with mitochondrial antiviral-signaling protein (MAVS), TANK binding kinase 1 (TBK1), and inhibitor of NF-κB kinase epsilon (IKKε). These lead to IRF3 and NF-κB activation, and subsequently, production of type 1 IFN and tumor necrosis factor-α (TNF-α) [24]. Besides, cGAS-STING pathway also restricts RNA virus infection, suggesting the existence of crosstalk between DNA and RNA sensing pathways [14]. The masking of STING and MAVS signaling is an important mechanism adopted by coronavirus to circumvent the antiviral effect of innate immunity.
In contrast to the NSPs of coronaviruses that are largely homogeneous, the 5′ sequences of nsp1 and nsp3 are the most heterogeneous region, which have encoded the most important genes accounting for innate immune antagonizing properties [25]. Papain-like protease (PLpro) acts as a multifaceted protein that can function as protease, deubiquitinase and de-ISGylatinase (for ISG15-removing), and the inhibition of PLpro does interrupt NF-kB-dependent reporter but does not influence the antagonism of IRF-3 dependent reporters [26], [27]. Evidence demonstrated that the SARS-CoV PLpro may impede STING dimerization and the subsequent activation of IFN signaling through protein-protein interactions [28]. The expression of the membrane-anchored PLpro transmembrane (PLpro-TM) of SARS-CoV could inhibit TBK1/IKKε-mediated phosphorylation of IRF-3 and the activation of type I IFN [28]. Also, an adaptive mutation is found in M protein to enhance the ability of viral replication or infection [29]. MERS-CoV M protein physically interacts with TNF receptor associated factor 3 (TRAF3), therefore impedes the activation of IRF-3 by disrupting the association between TRAF3 and TBK1 [30]. The N-terminal of MERS-CoV M protein was sufficient to inhibit the expression of IFNs [31]. As a molecular chaperon, SARS-CoV N protein can block the signaling cascade of NF-kB and the induction of reporter gene expression at the promoter region of IFN-β, hence down-regulates its mRNA transcription [32].
CoVs also express a set of accessory proteins antagonizing the IFN signaling [33]. The MERS-CoV accessory protein 4a (nonstructural [NS]4a), a dsRNA-binding protein, shows strong antagonistic activity to interferon response by the inhibition of IRF-3 activity at IFN-β promoter region to turn down the transcription and production of IFN-β [30]. Besides, accessory protein 4b (NS4b) and accessory protein 5 (NS5) have shown similar effects [30]. Importantly, the ORFs of SARS-CoV-2 may serve as accessory proteins that modulate the interaction between viral replication and host immune response [34].
3.3. Epigenetic modifications
RNA epigenetic modifications are engaged in pathological processes by regulating RNA metabolism in SARS-CoV-2 [35]. Modified nucleosides are located in the internal regions of viral RNAs which present more frequent than those in host cells [36]. Since the innate immune system has been found to be less sensitive to RNAs with nucleoside modification, the modified RNA may promote viral survival and innate immune evasion [37]. In this section, we summarized the effects of major methylation modifications on viral RNAs, including N6-methyladenosine (m6A), 7-methylguanosine (m7G) and 2′-O-methylation (2′-O-Me), and how they facilitate the SARS-CoV-2 to escape innate immunity recognition. Among the modified bases, the m6A is most frequent in the internal base modifications and methylated viral RNA can't be recognized by host RIG-I by utilizing host m6A methyltransferase to modify the RNA of CoVs and make it undistinguishable from the RNA in host cells [38], [39].
Methylated RNA immunoprecipitation sequencing and Nanopore direct RNA sequencing revealed that m6A RNA modification was particularly enriched at the 3′ -end of the SARS-COV-2 viral genome [40]. Notably, the m6A modification sites were detected to be widely distributed as well as dynamically regulated in both positive-sense genome RNA and negative-sense RNA intermediates of SARS-CoV-2 [36], [40]. Interestingly, the m6A modification of SARS-CoV-2 RNA is regulated by host's methyltransferases called ‘writer’ including methyltransferase-like 3 (METTL3), methyltransferase-like 14 (METTL14), WT1 associated protein (WATAP) and vir like m6A methyltransferase associated (VIRMA), demethylases called ‘erasers' including FTO alpha-ketoglutarate dependent dioxygenase (FTO), alkB homolog 5 (ALKBH5) and m6A-binding proteins called ‘reader’ [41], [42], [43]. Recent studies uncovered that knockout of the ‘writer’ METTL3 significantly reduces m6A levels of SARS-COV-2 genome, increases RIG-I binding, and subsequently promotes inflammatory genes expression [40], [43], [44]. Particularly, the expressions of METTL3 were examined to be reduced and inflammatory genes were activated in COVID-19 cases [40]. The m6A modification mechanisms in SARS-COV-2 will contribute to a further understanding of COVID-19 and offer new strategies for antiviral therapy. Specifically, small molecules that modulate the catalytic activity of enzymes can regulate SARS-COV-2 infection [36].
Similar to other coronaviruses, there is a standard 5′-end cap structure and a 3′-polyadenylate tail in the genome of SARS-CoV-2 [45]. The 5′ end of SARS-CoV-2 RNA mimics native host cellular mRNAs and inhibits being degraded by 5′ exoribonucleases, ensures effective process of its translation, and evade host innate immune [46]. According to the capping pathway of CoVs [38], [47], [48], the cap structure is sequentially formed by four steps, and the last two steps of RNA capping were methylation catalyzed by two methyltransferases (MTases) from the virus itself, which is distinct from host-derived m6A methyltransferases mentioned above. The MTases of SARS-CoV-2, including guanine-N7-methyltransferase (N7-MTase) and 2′-O-methyltransferase (2′-O-MTase), sequentially catalyze the methylation steps of 5′-methylated-blocked cap structure. The coronaviral N7-MTase was located at the C terminus of nsp14 to form a N7-methyl guanine RNA cap, and the N-terminal domain bears the ExoN activity [48]. 2′-O-MTase is a heterodimeric complex made up of nsp16 (for catalyzing the methylation process) and nsp10 (for activating the catalytic subunit) confers upon it 2′-O-MTase activity [48]. The cap impedes the detection of viral RNA recognized by Mda5/RIG-I sensors as well as viral translation by interferon-induced protein with tetratricopetide repeats 1 (IFIT1). One study demonstrated that the N7-methylated defective virus protected mice against the deadly attack of SARS-COV-2, suggesting that the N7-methylase might be a potential target for drug design [49]. Similarly, the absence of 2′-O-MTase activity results in a significant attenuation of SARS-CoV infection, which is characterized by decreased viral replication and limited breathing difficulties in animal models [44]. Therefore, pharmacological exploitation of 2′-O-MTase activity might open new treatment and prevention avenues to restore viral RNA recognition and activate intrinsic cell immunity against SARS-CoV-2. Similarly, loss of 2’-O-MT enzyme activity leads to a marked reduction in SARS-COV-2 infection. Hence, targeting 2’-O-MT enzyme activity may open up new therapeutic approaches for COVID-19 treatment [50].
In addition to the typical methylation epigenetic marks, RNA modification of SARS-CoV-2 includes uncanonical nucleotides with implications for antiviral natural immunity and related signaling pathways [51]. Studies revealed the global evidence that the genome of SARS-CoV-2 undergoes the RNA editing (deamination of adenosine to inosine, A-to-I) catalyzed by the enzyme——adenosine deaminase acting on RNA (ADAR) in host cells (both p110 and p150 isoforms) [52]. Another study found that A-to-I editing in SARS-CoV-2 was related to the intensity of host innate immune response by analyzing RNA virus-specific editing identification data from samples of patients infected with COVID-19 [53]. Also, another experimental evidence demonstrates that apolipoprotein B MRNA editing enzyme catalytic subunit (APOBEC) 3A and C1 modulate C-to-U mutation at specific sites of SARS-CoV-2 RNA, and may resulting in evading the immune monitor [54]. In addition to RNA editing, studies reported that SARS-CoV-2 can hijack the host miRNA by virus-derived microRNA to regulate interferon-related genes in host cells [55], [56]. For example, during SARS-COV2 infection, ISG expression in host cells was down-regulated by CoV-microRNA-O7a.2 [56].
3.4. Posttranslational modifications
Post-translational modifications (PTMs) of proteins modulate a series of cellular activities and processes. Viruses are well studied for their capacity to manipulate PTMs (phosphorylation, ubiquitination, ADP-ribosylation, for example) to their advantages. ADP-ribosylation is one of the most common PTMs that may induce antiviral effect and impact innate immune system [57]. All CoVs contain a macrodomain, a ubiquitous structural domain that removes ADP-ribose from proteins and reverse the function of ADP-ribosyltransferases. It was found to be an important virulence factor for SARS-CoV to suppress the innate immune response during infection [58]. The phosphorylation and ubiquitination regulation have been explained above.
3.5. Inhibiting host mRNA translation
Host shut-off, which desists host protein from expressing by virus, is an efficient way to restrain innate immune stimulation against the virus invasion [59], [60]. SARS-CoV and MERS-CoV achieve host shut-off at the transcriptional as well as the translational level. Nsp1 was reported to induce host shut-off by blocking host factors in charge of translation and result in inhibiting the translation of host mRNAs [23]. The nsp1 encoded by MERS-CoV is able to distinguish between the host mRNAs generated in the nucleus and the viral mRNA exist in the cytoplasm, and only suppresses the host mRNA. By this way, specificity towards interrupting host mRNA translation is achieved. [61]. Nevertheless, the nsp1 of SARS-CoV execute as an inhibitor to both host and viral mRNA translations [23].
Notably, in addition to SARS-CoVs, other respiratory RNA viruses share some mechanisms for escaping innate immunity. Viruses with either positive-stranded genome (e.g. rhinovirus) or negative-stranded genome (e.g. respiratory syncytial virus [RSV]) possesses a vesicle structure similar to the DMV-like capsule to make themselves unreachable for the innate immune sensors [62], [63], [64]. Similar to CoVs, rhinoviruses have a virally encoded 5′-end cap structure to protect it from recognition and degraded by 5′ exoribonucleases [65]. Influenza viruses also utilizes a combination of approaches to achieve host shut-off at the level of transcription and translation by protein NS1 which is analogous to the nsp1 in CoVs [66], [67].
4. Prospect
Though effective treatment options for COVID-19 remain scarce, The drugs adopted includes antivirals, immunomodulators and host-directed therapies were promising effective treatment options for COVID-19 [68]. While nucleoside analog is currently being explored (clinical improvement was found in 68% cases treated with compassionate-use of remdesivir, an adenosine analogue that inhibits viral RNA polymerases), using antiviral agents alone seems not to be potent enough to inhibit the cytokine storm [69]. Moreover, analyzing the viral structure information, such as a recent study determined cryo-electron microscopy structures of the SARS-CoV-2 S ectodomain trimer, may provide a direction for the development of protease inhibitor of CoVs [70].
Host-directed therapies mainly aim to improving of the host status and immune response or handling host-related factors during the process of viral replication [71]. Since certain host factors participate entirely the process of the CoV replication cycle [72], the host immunity is supposed to be taken into account. There may be a correlation between the disease prognosis and the hosts’ innate immune reactivity, but needs to be confirmed by further studies. A comprehensive understanding of CoV-host interaction may help us to ascertain crucial viral and host factors that modulate the pathogenesis of CoV, and to identify an appropriate target for effective therapy against CoV infection.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by The Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (No. TP2018046 to J.S.), Shanghai Municipal Education Commission-Two Hundred Talent (No. 20191817 to J.S.), General Program of National Natural Science Foundation of China (No. 81972667 to J.S.), Special Medical Innovation Research of Shanghai Science and Technology Commission (No. 22Y11910100 to Z.W.), Interdisciplinary Joint Project of Tongji University (No. 2022-4-ZD-09 to Z.W), the Natural Science Foundation of Tianjin (16JCQNJC10400 to Z.Y.).
Data availability
No data was used for the research described in the article.
References
- 1.Johns Hopkins University Coronavirus research center, Global tracking map: COVID-19 cases and data visualization. https://coronavirus.jhu.edu/map.html
- 2.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., Fan Y., Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 2020;20(4):425–434. doi: 10.1016/s1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma Q., Liu J., Liu Q., Kang L., Liu R., Jing W., Wu Y., Liu M. Global percentage of asymptomatic SARS-CoV-2 infections among the tested population and individuals with confirmed COVID-19 diagnosis: a systematic review and meta-analysis. JAMA Netw. Open. 2021;4(12) doi: 10.1001/jamanetworkopen.2021.37257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.L., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuin M., Gentili V., Cervellati C., Rizzo R., Zuliani G. Viral load difference between symptomatic and asymptomatic COVID-19 patients: systematic review and meta-analysis. Infect. Dis. Rep. 2021;13(3):645–653. doi: 10.3390/idr13030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang L., Shi Y., Gong B., Jiang L., Zhang Z., Liu X., Yang J., He Y., Jiang Z., Zhong L., Tang J., You C., Jiang Q., Long B., Zeng T., Luo M., Zeng F., Zeng F., Wang S., Yang X., Yang Z. Dynamic blood single-cell immune responses in patients with COVID-19. Signal Transduct. Target. Ther. 2021;6(1):110. doi: 10.1038/s41392-021-00526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen W., Su W., Tang H., Le W., Zhang X., Zheng Y., Liu X., Xie L., Li J., Ye J., Dong L., Cui X., Miao Y., Wang D., Dong J., Xiao C., Chen W., Wang H. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020;6:31. doi: 10.1038/s41421-020-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guang Chen D.W., Guo Wei, Cao Yong, Da Huang Hongwu Wang, Wang Tao, Zhang Xiaoyun, Chen Huilong, Yu Haijing, Zhang Xiaoping, Zhang Minxia, Wu Shiji, Song Jianxin, Chen Tao, Han Meifang, Li Shusheng, Luo Xiaoping, Zhao Jianping, Ning Qin. Clinical and immunologic features in severe and moderate forms of Coronavirus Disease 2019. 2020. [DOI] [PMC free article] [PubMed]
- 9.Galani I.E., Rovina N., Lampropoulou V., Triantafyllia V., Manioudaki M., Pavlos E., Koukaki E., Fragkou P.C., Panou V., Rapti V., Koltsida O., Mentis A., Koulouris N., Tsiodras S., Koutsoukou A., Andreakos E. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat. Immunol. 2021;22(1):32–40. doi: 10.1038/s41590-020-00840-x. [DOI] [PubMed] [Google Scholar]
- 10.Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/s0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7(6):439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volk A., Hackbart M., Deng X., Cruz-Pulido Y., O'Brien A., Baker S.C. Coronavirus endoribonuclease and deubiquitinating interferon antagonists differentially modulate the host response during replication in macrophages. J. Virol. 2020 doi: 10.1128/jvi.00178-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maringer K., Fernandez-Sesma A. Message in a bottle: lessons learned from antagonism of STING signalling during RNA virus infection. Cytokine Growth Factor Rev. 2014;25(6):669–679. doi: 10.1016/j.cytogfr.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X., Yang X., Zheng Y., Yang Y., Xing Y., Chen Z. SARS coronavirus papain-like protease inhibits the type I interferon signaling pathway through interaction with the STING-TRAF3-TBK1 complex. Protein Cell. 2014;5(5):369–381. doi: 10.1007/s13238-014-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kindler E., Thiel V. To sense or not to sense viral RNA--essentials of coronavirus innate immune evasion. Curr. Opin. Microbiol. 2014;20:69–75. doi: 10.1016/j.mib.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagemeijer M.C., Verheije M.H., Ulasli M., Shaltiël I.A., de Vries L.A., Reggiori F., Rottier P.J., de Haan C.A. Dynamics of coronavirus replication-transcription complexes. J. Virol. 2010;84(4):2134–2149. doi: 10.1128/jvi.01716-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knoops K., Kikkert M., Worm S.H., Zevenhoven-Dobbe J.C., van der Meer Y., Koster A.J., Mommaas A.M., Snijder E.J. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 2008;6(9) doi: 10.1371/journal.pbio.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oudshoorn D., Rijs K., Limpens R., Groen K., Koster A.J., Snijder E.J., Kikkert M., Barcena M. Expression and cleavage of middle east respiratory syndrome coronavirus nsp3-4 polyprotein induce the formation of double-membrane vesicles that mimic those associated with coronaviral RNA replication. mBio. 2017;8(6) doi: 10.1128/mBio.01658-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng X., Hackbart M., Mettelman R.C., O'Brien A., Mielech A.M., Yi G., Kao C.C., Baker S.C. Coronavirus nonstructural protein 15 mediates evasion of dsRNA sensors and limits apoptosis in macrophages. Proc. Natl. Acad. Sci. U. S. A. 2017;114(21):E4251–e4260. doi: 10.1073/pnas.1618310114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hackbart M., Deng X., Baker S.C. Coronavirus endoribonuclease targets viral polyuridine sequences to evade activating host sensors. Proc. Natl. Acad. Sci. U. S. A. 2020;117(14):8094–8103. doi: 10.1073/pnas.1921485117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niemeyer D., Zillinger T., Muth D., Zielecki F., Horvath G., Suliman T., Barchet W., Weber F., Drosten C., Muller M.A. Middle East respiratory syndrome coronavirus accessory protein 4a is a type I interferon antagonist. J. Virol. 2013;87(22):12489–12495. doi: 10.1128/jvi.01845-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kikkert M. Innate immune evasion by human respiratory RNA viruses. J. Innate Immun. 2020;12(1):4–20. doi: 10.1159/000503030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motwani M., Pesiridis S., Fitzgerald K.A. DNA sensing by the cGAS-STING pathway in health and disease. Nat. Rev. Genet. 2019;20(11):657–674. doi: 10.1038/s41576-019-0151-1. [DOI] [PubMed] [Google Scholar]
- 25.Magiorkinis G., Magiorkinis E., Paraskevis D., Vandamme A.M., Van Ranst M., Moulton V., Hatzakis A. Phylogenetic analysis of the full-length SARS-CoV sequences: evidence for phylogenetic discordance in three genomic regions. J. Med. Virol. 2004;74(3):369–372. doi: 10.1002/jmv.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng X., Mettelman R.C., O'Brien A., Thompson J.A., O'Brien T.E., Baker S.C. Analysis of coronavirus temperature-sensitive mutants reveals an interplay between the macrodomain and papain-like protease impacting replication and pathogenesis. J. Virol. 2019;93(12) doi: 10.1128/jvi.02140-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rui Y., Su J., Shen S., Hu Y., Huang D., Zheng W., Lou M., Shi Y., Wang M., Chen S., Zhao N., Dong Q., Cai Y., Xu R., Zheng S., Yu X.F. Unique and complementary suppression of cGAS-STING and RNA sensing- triggered innate immune responses by SARS-CoV-2 proteins. Signal Transduct. Target. Ther. 2021;6(1):123. doi: 10.1038/s41392-021-00515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X., Wang K., Xing Y., Tu J., Yang X., Zhao Q., Li K., Chen Z. Coronavirus membrane-associated papain-like proteases induce autophagy through interacting with Beclin1 to negatively regulate antiviral innate immunity. Protein Cell. 2014;5(12):912–927. doi: 10.1007/s13238-014-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang H., Zeqiraj E., Dong B., Jha B.K., Duffy N.M., Orlicky S., Thevakumaran N., Talukdar M., Pillon M.C., Ceccarelli D.F., Wan L.C., Juang Y.C., Mao D.Y., Gaughan C., Brinton M.A., Perelygin A.A., Kourinov I., Guarne A., Silverman R.H., Sicheri F. Dimeric structure of pseudokinase RNase L bound to 2-5A reveals a basis for interferon-induced antiviral activity. Mol. Cell. 2014;53(2):221–234. doi: 10.1016/j.molcel.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thornbrough J.M., Jha B.K., Yount B., Goldstein S.A., Li Y., Elliott R., Sims A.C., Baric R.S., Silverman R.H., Weiss S.R. Middle east respiratory syndrome coronavirus NS4b protein inhibits host rnase L activation. mBio. 2016;7(2) doi: 10.1128/mBio.00258-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lui P.Y., Wong L.Y., Fung C.L., Siu K.L., Yeung M.L., Yuen K.S., Chan C.P., Woo P.C., Yuen K.Y., Jin D.Y. Middle East respiratory syndrome coronavirus M protein suppresses type I interferon expression through the inhibition of TBK1-dependent phosphorylation of IRF3. Emerg. Microbes Infect. 2016;5 doi: 10.1038/emi.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopecky-Bromberg S.A., Martínez-Sobrido L., Frieman M., Baric R.A., Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J. Virol. 2007;81(2):548–557. doi: 10.1128/jvi.01782-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun L., Xing Y., Chen X., Zheng Y., Yang Y., Nichols D.B., Clementz M.A., Banach B.S., Li K., Baker S.C., Chen Z. Coronavirus papain-like proteases negatively regulate antiviral innate immune response through disruption of STING-mediated signaling. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020 doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim D., Lee J.Y., Yang J.S., Kim J.W., Kim V.N., Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181(4):914–921.e10. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J., Xu Y.P., Li K., Ye Q., Zhou H.Y., Sun H., Li X., Yu L., Deng Y.Q., Li R.T., Cheng M.L., He B., Zhou J., Li X.F., Wu A., Yi C., Qin C.F. The m(6)A methylome of SARS-CoV-2 in host cells. Cell Res. 2021;31(4):404–414. doi: 10.1038/s41422-020-00465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kariko K., Buckstein M., Ni H., Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Yan L., Yang Y., Li M., Zhang Y., Zheng L., Ge J., Huang Y.C., Liu Z., Wang T., Gao S., Zhang R., Huang Y.Y., Guddat L.W., Gao Y., Rao Z., Lou Z. Coupling of N7-methyltransferase and 3'-5' exoribonuclease with SARS-CoV-2 polymerase reveals mechanisms for capping and proofreading. Cell. 2021;184(13):3474–3485.e11. doi: 10.1016/j.cell.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilamowski M., Sherrell D.A., Minasov G., Kim Y., Shuvalova L., Lavens A., Chard R., Maltseva N., Jedrzejczak R., Rosas-Lemus M., Saint N., Foster I.T., Michalska K., Satchell K.J.F., Joachimiak A. 2'-O methylation of RNA cap in SARS-CoV-2 captured by serial crystallography. Proc. Natl. Acad. Sci. U. S. A. 2021;118(21) doi: 10.1073/pnas.2100170118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li N., Hui H., Bray B., Gonzalez G.M., Zeller M., Anderson K.G., Knight R., Smith D., Wang Y., Carlin A.F., Rana T.M. METTL3 regulates viral m6A RNA modification and host cell innate immune responses during SARS-CoV-2 infection. Cell Rep. 2021;35(6) doi: 10.1016/j.celrep.2021.109091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paramasivam A., Priyadharsini J.V. Epigenetic modifications of RNA and their implications in antiviral immunity. Epigenomics. 2020;12(19):1673–1675. doi: 10.2217/epi-2020-0307. [DOI] [PubMed] [Google Scholar]
- 42.Kim G.W., Imam H., Khan M., Siddiqui A. N (6)-methyladenosine modification of hepatitis B and C viral RNAs attenuates host innate immunity via RIG-I signaling. J. Biol. Chem. 2020;295(37):13123–13133. doi: 10.1074/jbc.RA120.014260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campos J.H.C., Maricato J.T., Braconi C.T., Antoneli F., Janini L.M.R., Briones M.R.S. Direct RNA sequencing reveals SARS-CoV-2 m6A sites and possible differential DRACH motif methylation among variants. Viruses. 2021;13(11) doi: 10.3390/v13112108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu W., Zhang Q., Zhang R., Lu Y., Wang X., Tian H., Yang Y., Gu Z., Gao Y., Yang X., Cui G., Sun B., Peng Y., Deng H., Peng H., Yang A., Yang Y.G., Yang P. N(6)-methyladenosine RNA modification suppresses antiviral innate sensing pathways via reshaping double-stranded RNA. Nat. Commun. 2021;12(1):1582. doi: 10.1038/s41467-021-21904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benoni R., Krafcikova P., Baranowski M.R., Kowalska J., Boura E., Cahová H. Substrate specificity of SARS-CoV-2 Nsp10-Nsp16 methyltransferase. Viruses. 2021;13(9) doi: 10.3390/v13091722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Encinar J.A., Menendez J.A. Potential drugs targeting early innate immune evasion of SARS-coronavirus 2 via 2'-O-methylation of viral RNA. Viruses. 2020;12(5) doi: 10.3390/v12050525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viswanathan T., Arya S., Chan S.H., Qi S., Dai N., Misra A., Park J.G., Oladunni F., Kovalskyy D., Hromas R.A., Martinez-Sobrido L., Gupta Y.K. Structural basis of RNA cap modification by SARS-CoV-2. Nat. Commun. 2020;11(1):3718. doi: 10.1038/s41467-020-17496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nencka R., Silhan J., Klima M., Otava T., Kocek H., Krafcikova P., Boura E. Coronaviral RNA-methyltransferases: function, structure and inhibition. Nucleic Acids Res. 2022;50(2):635–650. doi: 10.1093/nar/gkab1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan R., Kindler E., Cao L., Zhou Y., Zhang Z., Liu Q., Ebert N., Züst R., Sun Y., Gorbalenya A.E., Perlman S., Thiel V., Chen Y., Guo D., et al. mBio. 2022;13(1) doi: 10.1128/mbio.03662-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Menachery V.D., Yount B.L., Jr., Josset L., Gralinski L.E., Scobey T., Agnihothram S., Katze M.G., Baric R.S. Attenuation and restoration of severe acute respiratory syndrome coronavirus mutant lacking 2'-o-methyltransferase activity. J. Virol. 2014;88(8):4251–4264. doi: 10.1128/jvi.03571-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tong J., Zhang W., Chen Y., Yuan Q., Qin N.N., Qu G. The emerging role of RNA modifications in the regulation of antiviral innate immunity. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.845625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng X., Luo Y., Li H., Guo X., Chen H., Ji X., Liang H. RNA editing increases the nucleotide diversity of SARS-CoV-2 in human host cells. PLoS Genet. 2022;18(3) doi: 10.1371/journal.pgen.1010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song Y., He X., Yang W., Wu Y., Cui J., Tang T., Zhang R. Virus-specific editing identification approach reveals the landscape of A-to-I editing and its impacts on SARS-CoV-2 characteristics and evolution. Nucleic Acids Res. 2022;50(5):2509–2521. doi: 10.1093/nar/gkac120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim K., Calabrese P., Wang S., Qin C., Rao Y., Feng P., Chen X.S. APOBEC-mediated editing of SARS-CoV-2 genomic RNA impacts viral replication and fitness. bioRxiv. 2021 doi: 10.1101/2021.12.18.473309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grehl C., Schultheiß C., Hoffmann K., Binder M., Altmann T., Grosse I., Kuhlmann M. Detection of SARS-CoV-2 derived small RNAs and changes in circulating small RNAs associated with COVID-19. Viruses. 2021;13(8) doi: 10.3390/v13081593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh M., Chazal M., Quarato P., Bourdon L., Malabat C., Vallet T., Vignuzzi M., van der Werf S., Behillil S., Donati F., Sauvonnet N., Nigro G., Bourgine M., Jouvenet N., Cecere G. A virus-derived microRNA targets immune response genes during SARS-CoV-2 infection. EMBO Rep. 2022;23(2) doi: 10.15252/embr.202154341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daugherty M.D., Young J.M., Kerns J.A., Malik H.S. Rapid evolution of PARP genes suggests a broad role for ADP-ribosylation in host-virus conflicts. PLoS Genet. 2014;10(5) doi: 10.1371/journal.pgen.1004403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grunewald M.E., Fehr A.R., Athmer J., Perlman S. The coronavirus nucleocapsid protein is ADP-ribosylated. Virology. 2018;517:62–68. doi: 10.1016/j.virol.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dunagan M.M., Hardy K., Takimoto T. Impact of influenza A virus shutoff proteins on host immune responses. Vaccines (Basel) 2021;9(6) doi: 10.3390/vaccines9060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schubert K., Karousis E.D., Jomaa A., Scaiola A., Echeverria B., Gurzeler L.A., Leibundgut M., Thiel V., Mühlemann O., Ban N. SARS-CoV-2 Nsp1 binds the ribosomal mRNA channel to inhibit translation. Nat. Struct. Mol. Biol. 2020;27(10):959–966. doi: 10.1038/s41594-020-0511-8. [DOI] [PubMed] [Google Scholar]
- 61.Lokugamage K.G., Narayanan K., Nakagawa K., Terasaki K., Ramirez S.I., Tseng C.T., Makino S. Middle east respiratory syndrome coronavirus nsp1 inhibits host gene expression by selectively targeting mRNAs transcribed in the nucleus while sparing mRNAs of cytoplasmic origin. J. Virol. 2015;89(21):10970–10981. doi: 10.1128/jvi.01352-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.den Boon J.A., Ahlquist P. Organelle-like membrane compartmentalization of positive-strand RNA virus replication factories. Annu. Rev. Microbiol. 2010;64:241–256. doi: 10.1146/annurev.micro.112408.134012. [DOI] [PubMed] [Google Scholar]
- 63.Romero-Brey I., Bartenschlager R. Membranous replication factories induced by plus-strand RNA viruses. Viruses. 2014;6(7):2826–2857. doi: 10.3390/v6072826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lifland A.W., Jung J., Alonas E., Zurla C., Crowe J.E., Jr., Santangelo P.J. Human respiratory syncytial virus nucleoprotein and inclusion bodies antagonize the innate immune response mediated by MDA5 and MAVS. J. Virol. 2012;86(15):8245–8258. doi: 10.1128/jvi.00215-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flanegan J.B., Petterson R.F., Ambros V., Hewlett N.J., Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5′-terminus of virion and replicative intermediate RNAs of poliovirus. Proc. Natl. Acad. Sci. U. S. A. 1977;74(3):961–965. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levene R.E., Gaglia M.M. Host shutoff in influenza A virus: many means to an end. Viruses. 2018;10(9) doi: 10.3390/v10090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nogales A., Martinez-Sobrido L., Topham D.J., DeDiego M.L. Modulation of innate immune responses by the influenza A NS1 and PA-X proteins. Viruses. 2018;10(12) doi: 10.3390/v10120708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sarma P., Prajapat M., Avti P., Kaur H., Kumar S., Medhi B. Therapeutic options for the treatment of 2019-novel coronavirus: an evidence-based approach. Indian J. Pharm. 2020;52(1):1–5. doi: 10.4103/ijp.IJP_119_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D'Arminio Monforte A., Ismail S., Kato H., Lapadula G., L'Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fung T.S., Liu D.X. Human coronavirus: host-pathogen interaction. Annu. Rev. Microbiol. 2019;73:529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.