Abstract
Objective
Autoimmune autonomic ganglionopathy (AAG) is a rare disorder characterized by autonomic failure associated with the presence of anti-ganglionic acetylcholine receptor (gAChR) antibodies; however, several studies have reported that individuals with anti-gAChR antibodies present with central nervous system (CNS) symptoms such as impaired consciousness and seizures. In the present study, we investigated whether the presence of serum anti-gAChR antibodies correlated with autonomic symptoms in patients with functional neurological symptom disorder/conversion disorder (FNSD/CD).
Methods
Clinical data were collected for 59 patients presenting with neurologically unexplained motor and sensory symptoms at the Department of Neurology and Geriatrics between January 2013 and October 2017 and who were ultimately diagnosed with FNSD/CD according to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. Correlations between serum anti-gAChR antibodies and clinical symptoms and laboratory data were analyzed. Data analysis was conducted in 2021.
Results
Of the 59 patients with FNSD/CD, 52 (88.1%) exhibited autonomic disturbances and 16 (27.1%) were positive for serum anti-gAChR antibodies. Cardiovascular autonomic dysfunction, including orthostatic hypotension, was significantly more prevalent (75.0 vs. 34.9%, P = 0.008), whereas involuntary movements were significantly less prevalent (31.3 vs. 69.8%, P = 0.007), among anti-gAChR antibody-positive compared with -negative patients. Anti-gAChR antibody serostatus did not correlate significantly with the frequency of other autonomic, sensory, or motor symptoms analyzed.
Conclusions
An autoimmune mechanism mediated by anti-gAChR antibodies may be involved in disease etiology in a subgroup of FNSD/CD patients.
Keywords: autonomic symptoms, functional neurological symptom disorder, conversion disorder, ganglionic acetylcholine receptor, antibody, LIPS
1. Introduction
Functional neurological symptom disorder/conversion disorder (FNSD/CD) is a neurological disorder of voluntary movement or sensory function with no medial or neurological cause; and is described in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5); as a long-term impairment that significantly impairs quality of life and requires assistive and supportive services (1). Because the etiopathogenesis of FNSD/CD is unknown and abnormalities in clinical tests are rare, FNSD/CD is generally considered to be a psychological disorder (2, 3). Diagnosis of FNSD/CD in patients with non-specific motor-sensory symptoms can be difficult, and some are ultimately determined to have tumor-associated neurological syndrome or autoimmune encephalitis (3–6). In addition, elevated blood inflammatory markers have been reported in some FNSD/CD patients (7, 8), leading to the hypothesis that FNSD/CD symptoms may correlate with immune dysfunction. To avoid misdiagnosis, physicians are recommended to not assign a diagnosis of psychogenic reactions to neurological symptoms that are difficult to explain, such as dystonia or atypical seizures. Indeed, DSM-5 and International Classification of Diseases, 11th Revision (ICD-11) no longer supports the term psychogenic factors as a cause of symptoms of neurological disorders such as involuntary movement.
Although patients with FNSD/CD often exhibit autonomic symptoms such as orthostatic hypotension and gastrointestinal dysfunction and dysuria, the exact frequency of autonomic complications in patients with FNSD/CD is unknown (9, 10). The autonomic nervous system is broadly divided into sympathetic and parasympathetic components, with the main neurotransmitters being norepinephrine and acetylcholine (ACh). The two ACh receptor (AChR) subtypes, nicotinic and muscarinic, are widely expressed throughout the body. In both the sympathetic and parasympathetic ganglia, preganglionic to postganglionic fiber neurotransmission is predominantly mediated by ACh and the major receptor involved is the nicotinic AChR (nAChR) (11). nAChRs exist as homodimeric or heteromeric complexes of nine subunits (α2–7 and β2–4) in the mammalian peripheral and central nervous systems (12). The distribution of gAChR in organs other than the nervous system is not clear. In humans, autonomic ganglia nAChRs generally exist as pentameric heterocomplexes, with α3 and β4 subunits playing important roles (13, 14). Autoantibodies against nAChRs have been associated with several diseases, including myasthenia gravis, in which the antibodies target the α1 subunit of nAChRs at the neuromuscular junction (15). Serum antibodies against the autonomic ganglion nAChR (anti-gAChR) were first detected in patients with autonomic dysfunction in 1998 and resulted in the concept of autoimmune autonomic ganglionopathy (AAG), a term that refers to autonomic neuropathy mediated by an autoimmune mechanism (16).
In the present study, we retrospectively reviewed clinicopathological data, including autonomic, motor, and sensory symptoms and anti-gAChR antibody serostatus, in patients who were admitted to our Neurology Department with neurologically unexplainable motor and sensory symptoms and were ultimately diagnosed with FNSD/CD.
2. Materials and methods
2.1. Patients
Patients with the main complaint of motor and sensory disorders who were admitted to the Department of Neurology and Geriatrics at Kagoshima University Hospital between January 2013 and October 2017 were included in this study. Of those patients, 68 met the DSM-5 diagnostic criteria for FNSD/CD (1). Medical records were retrospectively reviewed for clinical symptoms and blood and cerebrospinal fluid (CSF) test results. After exclusion of patients with concomitant autoimmune neurological disease including Sjögren's syndrome, myasthenia gravis, systemic lupus erythematosus, chronic inflammatory demyelinating polyneuropathy, a total of 59 patients were included in the final analysis set.
2.2. Neurological examinations
All patients who presented with motor and sensory symptoms were evaluated neurologically and no neurologic lesion sites were found. Nine of the patients with sensory symptoms had nerve conduction studies performed because of difficulties in determining the site of the lesion by the neurologist. Nerve conduction studies showed that none of the patients had decreased nerve conduction velocity or sensory nerve action potentials.
Routine neurological examinations included autonomic symptoms, which were categorized into four types: gastrointestinal dysfunction (constipation, diarrhea, early satiety, nausea, vomiting, anorexia, abdominal pain, and feeling abdominally bloated); cardiovascular autonomic dysfunction (orthostatic hypotension, postural orthostatic tachycardia syndrome [POTS], tachycardia, and palpitations); bladder dysfunction; and anhidrosis. The diagnostic criteria of the European Society of Cardiology in 2018 were used for the diagnosis of orthostatic hypotension and POTS. Bladder dysfunction was assessed by the presence or absence of incontinence, frequent urination that interfered with daily activities, and overactive bladder as assessed by OABSS (17, 18). Motor symptoms were categorized into three types: weakness, involuntary movements, and ataxia. Sensory symptoms were categorized as either sensory disturbance (abnormal or decreased sensation) or pain (headache, pain in extremities and trunk).
2.3. Detection of anti-gAChR antibodies using a luciferase immunoprecipitation system
Serum antibodies against gAChR α3 and β4 subunits were quantified using a LIPS assay (13, 19), in which specific antibodies are bound to a Gaussia luciferase-tagged antigen and the bound antibody is quantified by measuring luciferase activity with a luminometer. The results are expressed as relative luminescence units (RLU). Serum was considered positive for anti-gAChR α3 or β4 antibodies if the RLU of the test sample exceeded a cut-off value of three standard deviations (SDs) greater than the mean RLU of the control sample (sera obtained from healthy individuals). We evaluated the antibody levels as an antibody index (A.I.). A.I.= [measurement value of the sample serum (RLU)]/[the cut-off value (RLU)].
2.4. Statistical analysis
Mean values were compared between anti-gAChR antibody-positive and -negative patient groups. Age and laboratory findings were compared using t-tests, and gender, clinical symptoms, and other autoantibodies were compared using Fisher's exact test. IBM SPSS, version 27, was used for statistical analyses. P < 0.05 was considered significant.
3. Results
Of the 68 patients who presented with motor or sensory deficits and met the diagnostic criteria for FNSD/CD, 9 had concomitant neuroimmune disease and were excluded. The final analysis set consisted of 59 patients (Table 1), of whom 51 (86.4%) were female. The mean (±SD) age at admission was 26.4 ± 15.4 years, the age at symptom onset was 22.0 ± 14.9 years, and the duration of illness was 4.4 ± 5.9 years. We examined the prevalence of motor, sensory, and autonomic symptoms, and found that 45 of the 59 patients (76.3%) had all three symptom types, one (1.7%) had only motor symptoms, and none had only sensory symptoms (Figure 1). Of the 59 patients, 52 (88.1%) had autonomic symptoms that included gastrointestinal symptoms (40 patients, 67.8%), cardiovascular autonomic symptoms (27 patients, 45.8%), bladder dysfunction (23 patients, 39.0%), and anhidrosis (16 patients, 27.1%) (Table 1).
Table 1.
Characteristics of patients with FNSD/CD (N = 59).
| Male | 8 (13.6%) | |
|---|---|---|
| Age at admission (y.o.) | 26.4 ± 15.4 | |
| Age at onset (y.o.) | 22.0 ± 14.9 | |
| Disease duration (year) | 4.4 ± 5.9 | |
| Autonomic dysfunction | 52 (88.1%) | |
| Gastrointestinal dysfunction | 40 (67.8%) | |
| Cardiovascular autonomic dysfunction | 27 (45.8%) | |
| Bladder dysfunction | 23 (39.0%) | |
| Anhidrosis | 16 (27.1%) | |
| Sensory symptom | 56 (95.0%) | |
| Pain | 52 (88.1%) | |
| Sensory disturbance | 43 (72.9%) | |
| Motor symptom | 54 (91.5%) | |
| Weakness | 47 (79.7%) | |
| Involuntary movement | 35 (59.3%) | |
| Ataxia | 9 (15.3%) | |
Figure 1.
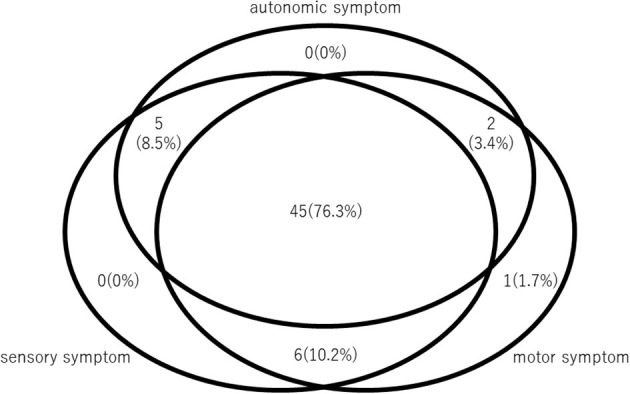
Prevalence of symptoms in 59 patients with FNSD/CD. Venn diagram shows the number and percentage of patients with autonomic, sensory, and motor symptoms.
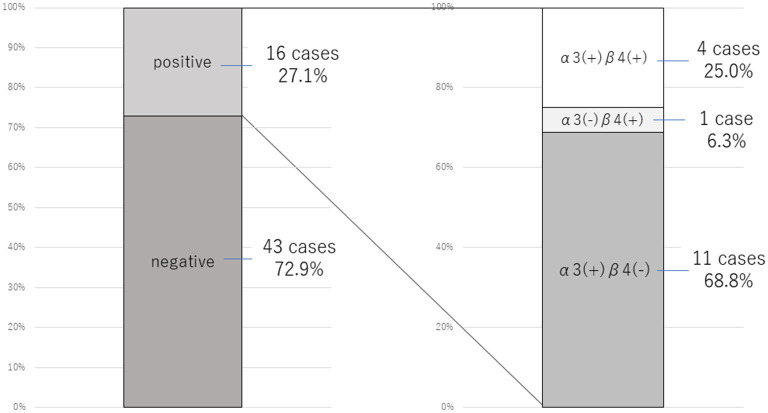
Because of the prevalence of autonomic symptoms among our patient cohort, we investigated the possible relationship between FNSD/CD and anti-gAChR antibodies. Of the total cohort of 59 patients, 16 (27.1%) were positive for anti-gAChR antibodies, and of these, 11 were positive for antibodies against the α3 subunit only, one patient was positive for antibodies against the β4 subunit only, and four patients were positive for antibodies against both the α3 and β4 subunits (Figure 2).
Figure 2.
Prevalence of anti-gAChR antibodies in 59 patients with FNSD/CD. The proportion of patients positive for anti-gAChR antibodies (left) and for antibodies specifically against the α3 and β4 subunits (right) are shown.
We next compared clinical symptoms between patients who were positive (n = 16) and negative (n = 43) for anti-gAChR antibodies (Table 2). There were no significant differences between the antibody-positive and -negative groups with respect to sex ratio (3 males [18.8%] and 5 males [11.6%], respectively), mean age at admission (30.7 ± 17.8 years and 24.8 ± 14.5 years, respectively), or mean age at disease onset (23.3 ± 16.8 years and 21.5 ± 14.4 years, respectively). There was no significant difference in the prevalence of autonomic dysfunction between the anti-gAChR antibody-positive group (15 of 16 patients, 93.8%) and the antibody-negative group (37 of 43 patients, 86.0%); however cardiovascular autonomic symptoms were significantly more common in the antibody-positive group (75.0 vs. 34.9%, P = 0.008). Among the individual cardiovascular autonomic symptoms, POTS and orthostatic hypotension were both more commonly seen among the antibody-positive group, but only the frequency of POTS reached the level of statistical significance (50.0 vs. 9.3%, P = 0.002). The prevalence of POTS is 0.5–1.0% of the population, mostly female (female/male: 5:1), so the prevalence in our patients is high regardless of the presence of antibodies. There were no significant association between anti-gAChR antibody serostatus and other autonomic dysfunctions. Although most motor and sensory symptoms were less common in the anti-gAChR antibody-positive group than the antibody-negative group, only the frequency of patients with involuntary movements was significantly different between the two groups (31.3 vs. 69.8%, P = 0.007).
Table 2.
Characteristics of FNSD/CD patients according to anti-gAChR antibody serostatus.
| Anti-gAChR antibody | |||||
|---|---|---|---|---|---|
| Positive (N = 16) | Negative (N = 43) | P value | |||
| Male | 3 (18.8%) | 5 (11.6%) | 0.670 | ||
| Age at admission (y.o.) | 30.7 ± 17.8 | 24.8 ± 14.5 | 0.192 | ||
| Age at onset (y.o.) | 23.3 ± 16.8 | 21.5 ± 14.4 | 0.699 | ||
| Disease duration (year) | 7.5 ± 8.8 | 3.2 ± 4.0 | 0.079 | ||
| Anti-gAChR α3 antibodies (A.I.) | 1.71 ± 0.59 | 0.49 ± 0.23 | |||
| Anti-gAChR β4 antibodies (A.I.) | 0.93 ± 0.46 | 0.38 ± 0.18 | |||
| Autonomic dysfunction | 15 (93.8%) | 37 (86.0%) | 0.661 | ||
| Gastrointestinal dysfunction | 12 (75.0%) | 28 (65.1%) | 0.545 | ||
| Cardiovascular autonomic dysfunction | 12 (75.0%) | 15 (34.9%) | 0.008 | ||
| Orthostatic hypotension | 8 (50%) | 9 (20.9%) | 0.05 | ||
| POTS | 8 (50%) | 4 (9.3%) | 0.002 | ||
| Palpitation | 2 (12.5%) | 3 (7.0%) | 0.606 | ||
| Bladder dysfunction | 7 (43.8%) | 16 (37.2%) | 0.647 | ||
| Anhidrosis | 7 (43.8%) | 9 (20.9%) | 0.104 | ||
| Sensory symptom | 14 (87.5%) | 42 (97.7%) | 0.176 | ||
| Pain | 13 (81.3%) | 39 (90.7%) | 0.375 | ||
| Sensory disturbance | 12 (75.0%) | 31 (72.1%) | 1.000 | ||
| Motor symptom | 14 (87.5%) | 40 (93.0%) | 0.606 | ||
| Weakness | 12 (75.0%) | 35 (81.4%) | 0.718 | ||
| Involuntary movement | 5 (31.3%) | 30 (69.8%) | 0.007 | ||
| Ataxia | 2 (12.5%) | 7 (16.3%) | 1.000 | ||
POTS, postural orthostatic tachycardia syndrome; A.I., antibody index.
The results of common laboratory tests revealed no significant differences in peripheral blood inflammatory markers, such as white blood cell count and C-reactive protein level, or in CSF inflammatory markers, such as total cell count and total protein level, between the anti-gAChR antibody-positive and -negative patient groups. No patient had low CSF pressure (< 60 mmH2O) on lumbar puncture examination. The IgG index, which measures the ratio of IgG and albumin levels in the CSF vs. serum and is an indicator of IgG production in the CNS, was also within normal limits in both patient groups (Table 3). Analysis of serum autoantibodies revealed that the presence of anti-nuclear antibodies was significantly more common in the anti-gAChR antibody-positive than -negative group (46.7 vs. 12.5%, p = 0.006).
Table 3.
Laboratory data for FNSD/CD patients according to anti-gAChR antibody serostatus.
| Anti gAChR antibody | |||
|---|---|---|---|
| Positive | Negative | P value | |
| Serum WBC count (/μl) | 5,800.0 ± 1,065.6 | 6,248.1 ± 1,645.9 | 0.317 |
| Serum CRP (mg/dl) | 0.101 ± 0.178 | 0.037 ± 0.447 | 0.147 |
| CSF cell count (/μl) | 1.25 ± 0.45 | 1.22 ± 0.47 | 0.826 |
| CSF protein (mg/dl) | 22.60 ± 6.45 | 24.40 ± 11.97 | 0.573 |
| IgG index | 0.490 ± 0.041 | 0.483 ± 0.046 | 0.597 |
| Anti nuclear antibody | 7/15 (46.7%) | 5/40 (12.5%) | 0.006 |
| Anti Tg antibody | 0/15 (0%) | 3/40 (7.5%) | NA |
| anti TPO antibody | 0/15 (0%) | 3/41 (7.3%) | NA |
| Anti GAD antibody | 0/7 (0%) | 0/25 (0%) | NA |
| Anti SS-A antibody | 1/12 (8.3%) | 0/39 (0%) | NA |
| Anti SS-B antibody | 0/13 (0%) | 0/38 (0%) | NA |
Tg, thyroglobulin; TPO, thyroid peroxidase; GAD, glutamic acid decarboxylase.
4. Discussion
Among our cohort of FNSD/CD patients, more than two-thirds (88.1%) presented with autonomic symptoms and, notably, more than a quarter (27.1%) were positive for serum anti-gAChR antibodies. Autonomic symptoms have been reported to be a precursor to FNSD/CD and have been positively associated with autism (10, 20). However, to our knowledge, there have been no formal investigations of autonomic dysfunction in patients with FNSD/CD, possibly because the DSM-5 diagnostic criteria for FNSD/CD require the presence of motor and sensory symptoms but not of autonomic symptoms. Although motor and sensory symptoms in FNSD/CD patients can be ruled in or out as consistent with neurological dysfunction by neurologists, it is more difficult to rule out a neurological basis for autonomic symptoms. In the present study, we found high rates of autonomic symptoms (gastrointestinal and cardiovascular dysfunction) in addition to motor and sensory symptoms, were the main symptoms of FNSD/CD, suggesting that evaluation of autonomic symptoms is important for both the diagnosis of FNSD/CD and for understanding its pathophysiology.
Anti-gAChR antibodies were initially identified in sera from patients with autonomic symptoms, leading to the discovery of autoimmune autonomic neuropathy, however, they are rarely detected in other disorders or in healthy individuals. McKeon et al. reported that serum anti-gAChR antibodies were present in 155 of 15,000 patients (1.03%) with suspected paraneoplastic autoimmune neurological syndrome but in only 1 of 173 healthy subjects (0.58%) (21). More recently, 0% of 2,628 patients with non-AAG neurological diseases were positive for antibodies to α3-nAChR (22). These reports suggest that anti-gAChR antibodies are usually undetectable or present at very low levels in the serum (16, 19, 23). Although these reports used radioimmunoprecipitation assay (RIA) as the measurement method, it has been proven that RIA and LIPS show the similar performance to detect anti-gAChR antibody (19). The high prevalence of anti-gAChR antibodies among our FNSD/CD patient cohort (27.1%) prompted us to hypothesize that anti-gAChR antibodies may be directly associated with autonomic symptoms in these patients. A previous study suggests that patients' symptoms may differ depending on the antibody subtype (24). Unfortunately, the small number of our cases did not allow us to examine this point in the present study. Interestingly, we detected no significant difference in the frequency of autonomic symptoms between patients who were positive or negative for serum anti-gAChR antibodies, suggesting that a direct relationship between the antibodies and autonomic symptoms is unlikely.
Anti-nuclear antibodies were significantly elevated in patients positive for anti-gAChR antibodies in the present study. Previous studies have identified antibodies against nAChR α4 and α7 subunits, VGKC channels, amphiphysin, and SS-B in anti-gAChR antibody-positive sera, suggesting the presence of a variety of humoral immune responses in these individuals (25–27). Interestingly, some patients with CNS symptoms, such as impaired consciousness and seizures, are positive for anti-gAChR antibodies, and several reports suggest that α7 and α4β2 nAChRs are involved in memory and pain (12, 16, 21, 24, 28–31). Notably, recent functional MRI studies have revealed that disturbances in the central autonomic network (CAN) can lead to psychiatric symptoms (32–34). These observations suggest that antibodies targeting nAChR subunits may affect not only the peripheral autonomic nervous system but also CNS function. The detection of anti-gAChR antibodies in patients with FNSD/CD with or without autonomic symptoms in the present study suggests that anti-gAChR antibodies do not simply affect peripheral autonomic function. However, whether anti-gAChR antibodies are a pathogenic factor or simply correlate with disease status remains unclear.
5. Conclusions
More than 80% of our FNSD/CD patient cohort had autonomic symptoms, and more than 25% of the cohort were positive for serum anti-gAChR antibodies, whether or not autonomic symptoms were present. To clarify the relationship between this psychiatric disorder and anti-gAChR antibodies, further investigation of clinical symptoms and the establishment of a measurement system for antibodies against the anti-nAChR subunit are needed.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Review Board on Bioethics and Genetic Research Reference of Kagoshima University (Approval No. 492). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
RN, EM, and HT conceived and designed the study. SNo, MD, YN, MA, YHir, YHig, YS, HA, and KH provided the laboratory data for patients at Kagoshima University Hospital. SNa provided the laboratory data for control subjects. DK, MT, RK, MY, and TH analyzed the data, take responsibility for the integrity of the data, and the accuracy of the data analysis. All authors critically revised the manuscript and approved of the final version.
Acknowledgments
The authors thank the staff of the Department of Neurology and Geriatrics, Kagoshima University Hospital, including Katsunori Takahata, Yoshimitsu Maki, Michiyoshi Yoshimura, Yuichi Tashiro, Akihiro Hashiguchi, and Kumiko Michizono. We thank Anne M. O'Rourke, PhD, from Edanz (www.edanz.com/ac) for editing a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Guha M. Diagnostic and Statistical Manual of Mental Disorders: DSM-5 (5th edition). Ref. Rev. 28:36–7. 10.1108/RR-10-2013-025625478714 [DOI] [Google Scholar]
- 2.Boulet C, Lopez-Castroman J, Mouchabac S, Olié E, Courtet P, Thouvenot E, et al. Stress response in dissociation and conversion disorders: A systematic review. Neurosci Biobehav Rev. (2022) 132:957–67. 10.1016/j.neubiorev.2021.10.049 [DOI] [PubMed] [Google Scholar]
- 3.Ludwig L, Pasman JA, Nicholson T, Aybek S, David AS, Tuck S, et al. Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. Lancet Psychiatry. (2018) 5:307–20. 10.1016/S2215-0366(18)30051-8 [DOI] [PubMed] [Google Scholar]
- 4.Parthasarathi UD, Harrower T, Tempest M, Hodges JR, Walsh C, McKenna PJ, et al. Psychiatric presentation of voltage-gated potassium channel antibody-associated encephalopathy: Case report. Br J Psychiatry. (2006) 189:182–3. 10.1192/bjp.bp.105.012864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iizuka T, Sakai F, Ide T, Monzen T, Yoshii S, Iigaya M, et al. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology. (2008) 70:504–11. 10.1212/01.wnl.0000278388.90370.c3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsutsui K, Kanbayashi T, Tanaka K, Boku S, Ito W, Tokunaga J, et al. Anti-NMDA-receptor antibody detected in encephalitis, schizophrenia, and narcolepsy with psychotic features. BMC Psychiatry. (2012) 12:37. 10.1186/1471-244X-12-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Feltz-Cornelis C, Brabyn S, Ratcliff J, Varley D, Allgar V, Gilbody S, et al. Assessment of cytokines, microRNA and patient related outcome measures in conversion disorder/functional neurological disorder (CD/FND): The CANDO clinical feasibility study. Brain Behav Immun Health. (2021) 13:100228. 10.1016/j.bbih.2021.100228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozlowska K, Chung J, Cruickshank B, McLean L, Scher S, Dale RC, et al. Blood CRP levels are elevated in children and adolescents with functional neurological symptom disorder. Eur Child Adolesc Psychiatry. (2019) 28:491–504. 10.1007/s00787-018-1212-2 [DOI] [PubMed] [Google Scholar]
- 9.Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. (2010) 133:1537–51. 10.1093/brain/awq068 [DOI] [PubMed] [Google Scholar]
- 10.Leger R. Dysautonomia-predominant functional neurological symptom disorder. Am J Psychiat Residents' J. (2020) 16:12–4. 10.1176/appi.ajp-rj.2020.1602078723190 [DOI] [Google Scholar]
- 11.Goldstein DS, Cheshire WP. The autonomic medical history. Clin Auton Res. (2017) 27:223–33. 10.1007/s10286-017-0425-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dineley KT, Pandya AA, Yakel JL. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol Sci. (2015) 36:96–108. 10.1016/j.tips.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakane S, Mukaino A, Higuchi O, Watari M, Maeda Y, Yamakawa M, et al. Autoimmune autonomic ganglionopathy: an update on diagnosis and treatment. Expert Rev Neurother. (2018) 18:953–65. 10.1080/14737175.2018.1540304 [DOI] [PubMed] [Google Scholar]
- 14.Skok MV, Voitenko LP, Voitenko SV, Lykhmus EY, Kalashnik EN, Litvin TI, et al. Alpha subunit composition of nicotinic acetylcholine receptors in the rat autonomic ganglia neurons as determined with subunit-specific anti-alpha(181-192) peptide antibodies. Neuroscience. (1999) 93:1427–36. 10.1016/S0306-4522(99)00160-8 [DOI] [PubMed] [Google Scholar]
- 15.Noridomi K, Watanabe G, Hansen MN, Han GW, Chen L. Structural insights into the molecular mechanisms of myasthenia gravis and their therapeutic implications. Elife. (2017) 6:e23043. 10.7554/eLife.23043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vernino S, Adamski J, Kryzer TJ, Fealey RD, Lennon VA. Neuronal nicotinic ACh receptor antibody in subacute autonomic neuropathy and cancer-related syndromes. Neurology. (1998) 50:1806–13. 10.1212/WNL.50.6.1806 [DOI] [PubMed] [Google Scholar]
- 17.Chapple CR, Artibani W, Cardozo LD, Castro-Diaz D, Craggs M, Haab F, et al. The role of urinary urgency and its measurement in the overactive bladder symptom syndrome: current concepts and future prospects. BJU Int. (2005) 95:335–40. 10.1111/j.1464-410X.2005.05294.x [DOI] [PubMed] [Google Scholar]
- 18.Homma Y, Kakizaki H, Yamaguchi O, Yamanishi T, Nishizawa O, Yokoyama O, et al. Assessment of overactive bladder symptoms: comparison of 3-day bladder diary and the overactive bladder symptoms score. Urology. (2011) 77:60–4. 10.1016/j.urology.2010.06.044 [DOI] [PubMed] [Google Scholar]
- 19.Nakane S, Higuchi O, Koga M, Kanda T, Murata K, Suzuki T, et al. Clinical features of autoimmune autonomic ganglionopathy and the detection of subunit-specific autoantibodies to the ganglionic acetylcholine receptor in Japanese patients. PLoS ONE. (2015) 10:e0118312. 10.1371/journal.pone.0118312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owens AP, Mathias CJ, Iodice V. Autonomic dysfunction in autism spectrum disorder. Front Integr Neurosci. (2021) 15:787037. 10.3389/fnint.2021.787037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKeon A, Lennon VA, Lachance DH, Fealey RD, Pittock SJ. Ganglionic acetylcholine receptor autoantibody: oncological, neurological, and serological accompaniments. Arch Neurol. (2009) 66:735–41. 10.1001/archneurol.2009.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karagiorgou K, Dandoulaki M, Mantegazza R, Andreetta F, Furlan R, Lindstrom J, et al. Novel Cell-Based Assay for Alpha-3 Nicotinic Receptor Antibodies Detects Antibodies Exclusively in Autoimmune Autonomic Ganglionopathy. Neurol Neuroimmunol Neuroinflamm. (2022) 9:e1162. 10.1212/NXI.0000000000001162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. (2000) 343:847–55. 10.1056/NEJM200009213431204 [DOI] [PubMed] [Google Scholar]
- 24.Nakane S, Mukaino A, Higuchi O, Yasuhiro M, Takamatsu K, Yamakawa M, et al. A comprehensive analysis of the clinical characteristics and laboratory features in 179 patients with autoimmune autonomic ganglionopathy. J Autoimmun. (2020) 108:102403. 10.1016/j.jaut.2020.102403 [DOI] [PubMed] [Google Scholar]
- 25.Baker SK, Morillo C, Vernino S. Autoimmune autonomic ganglionopathy with late-onset encephalopathy. Auton Neurosci. (2009) 146:29–32. 10.1016/j.autneu.2008.10.016 [DOI] [PubMed] [Google Scholar]
- 26.Horta ES, Lennon VA, Lachance DH, Jenkins SM, Smith CY, McKeon A, et al. Neural autoantibody clusters aid diagnosis of cancer. Clin Cancer Res. (2014) 20:3862–9. 10.1158/1078-0432.CCR-14-0652 [DOI] [PubMed] [Google Scholar]
- 27.Kondo T, Inoue H, Usui T, Mimori T, Tomimoto H, Vernino S, et al. Autoimmune autonomic ganglionopathy with Sjögren's syndrome: significance of ganglionic acetylcholine receptor antibody and therapeutic approach. Auton Neurosci. (2009) 146:33–5. 10.1016/j.autneu.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 28.Watson R, Jepson JE, Bermudez I, Alexander S, Hart Y, McKnight K, et al. Alpha7-acetylcholine receptor antibodies in two patients with Rasmussen encephalitis. Neurology. (2005) 65:1802–4. 10.1212/01.wnl.0000191566.86977.04 [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Jammoul A, Mente K, Li J, Shields RW, Vernino S, et al. Clinical experience of seropositive ganglionic acetylcholine receptor antibody in a tertiary neurology referral center. Muscle Nerve. (2015) 52:386–91. 10.1002/mus.24559 [DOI] [PubMed] [Google Scholar]
- 30.Nakane S, Mukaino A, Maeda Y, Higuchi O, Matsuo H, Ando Y. Extra-autonomic manifestations in autoimmune autonomic ganglionopathy: A Japanese survey. J Neurol Neurosurg Psychiatry. (2017) 88:367–8. 10.1136/jnnp-2016-314707 [DOI] [PubMed] [Google Scholar]
- 31.Hurst R, Rollema H, Bertrand D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol Ther. (2013) 137:22–54. 10.1016/j.pharmthera.2012.08.012 [DOI] [PubMed] [Google Scholar]
- 32.Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. (1993) 68:988–1001. 10.1016/S0025-6196(12)62272-1 [DOI] [PubMed] [Google Scholar]
- 33.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. (2012) 36:747–56. 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- 34.Umeda S, Harrison NA, Gray MA, Mathias CJ, Critchley HD. Structural brain abnormalities in postural tachycardia syndrome: A VBM-DARTEL study. Front Neurosci. (2015) 9:34. 10.3389/fnins.2015.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.