Abstract
A leading cause of white matter (WM) injury in older individuals is cerebral small vessel disease (SVD). Cerebral SVD is the most prevalent vascular contributor to cognitive impairment and dementia. Therapeutic progress for cerebral SVD and other WM disorders depends on the development and validation of neuroimaging markers suitable as outcome measures in future interventional trials. Diffusion-tensor imaging (DTI) is one of the best-suited MRI techniques for assessing the extent of WM damage in the brain. But the optimal method to analyze individual DTI data remains hindered by labor-intensive and time-consuming processes. Peak width of skeletonized mean diffusivity (PSMD), a recently developed fast, fully automated DTI marker, was designed to quantify the WM damage secondary to cerebral SVD and reflect related cognitive impairment. Despite its promising results, knowledge about PSMD is still limited in the radiologic community. This focused review provides an overview of the technical details of PSMD while synthesizing the available data on its clinical and neuroimaging associations. From a critical expert viewpoint, the authors discuss the limitations of PSMD and its current validation status as a neuroimaging marker for vascular cognitive impairment. Finally, they point out the gaps to be addressed to further advance the field.
© RSNA, 2023
Summary
Peak width of skeletonized mean diffusivity is an automated quantitative diffusion-tensor imaging marker for assessing white matter injury and predicting cognitive outcomes of neurologic disorders.
Essentials
■ Peak width of skeletonized mean diffusivity (PSMD) is an automated quantitative diffusion-tensor imaging marker for cerebral white matter (WM) injury.
■ PSMD shows consistent cognitive associations across studies and is most strongly associated with the domain of processing speed.
■ PSMD appears robust against different scanners and MRI protocols, and promising data support high test-retest repeatability.
■ Further technical and longitudinal studies are necessary to validate PSMD as a surrogate marker of cerebral WM injury for future clinical trials in vascular cognitive impairment.
Introduction
Cerebral white matter (WM) regions harbor organized structural and functional networks essential for neurobehavioral operations. These WM networks are susceptible to a wide range of disorders (1). A leading cause of WM injury in older individuals is cerebral small vessel disease (SVD) (2). Cerebral SVD triggers widespread ischemic and hemorrhagic lesions that contribute to vascular cognitive impairment and dementia (3).
Advances in neuroimaging have enabled in vivo assessment of WM structural integrity. Lesions visible with routinely applied MRI sequences (ie, T1-weighted, T2-weighted, fluid-attenuated inversion recovery, and susceptibility-weighted imaging), such as WM hyperintensities (WMHs), lacunes, and hemorrhages, play a pivotal role in defining cerebral SVD and other WM disorders and have been incorporated into clinical practice (2,4). However, these lesions explain only a small portion of the variance in cognitive impairment and clinical disability related to highly prevalent WM disorders (5). Also, abnormalities in normal-appearing WM known to impact clinical outcomes (6) go undetected at conventional MRI. Thus, there is a need for advanced techniques capable of better capturing the whole spectrum of WM injury (7).
Diffusion-tensor imaging (DTI) is one of the best-suited MRI techniques for assessing the extent of WM damage (8). Specifically, DTI provides quantitative parameters on the microstructural integrity and directionality of WM tracts. But the optimal method to analyze individual DTI data remains undefined, hindered by labor-intensive and time-consuming options. Thus, future therapeutic development for several WM disorders, including cerebral SVD, depends on the validation of neuroimaging markers fit for large clinical trials (9).
In this context, a recently developed DTI marker offers several advantages that align with current scientific needs and priorities. Peak width of skeletonized mean diffusivity (PSMD) is a fully automated and rapidly computed histogram marker that reflects the heterogeneity in voxel-based mean diffusivity (MD) values across the main WM tracts. It was originally designed to quantify the severity of cerebral SVD and related cognitive impairment (10). A substantial number of studies applying PSMD in different neurologic conditions are finally available and offer robust data and promising results. Nonetheless, the relevance of PSMD and its utility as a neuroimaging marker for cerebral SVD and neurodegenerative disorders remain unclear, and knowledge about PSMD is limited in the radiologic community.
Thus, we reviewed the literature to gather and synthesize the available data on the neurocognitive, clinical, and neuroimaging associations of PSMD in vascular and neurodegenerative disorders. We also explored the current state of validating PSMD as a neuroimaging marker for vascular cognitive impairment and dementia, its limitations, and promising future steps from a critical expert viewpoint.
Literature Search and Data Extraction
The literature search was performed by a librarian on February 1, 2021. Details of the search strategy, selection criteria, and data extraction are provided in Appendix S1 and Table S1. We selected only studies applying PSMD in the context of vascular and neurodegenerative disorders.
To compare PSMD values across different samples, we used the Box-Cox method (11) to calculate the mean and SD from studies originally reporting only the median and IQR (10,12–15). Specifically for the samples investigated by Beaudet et al (16), because PSMD values were available only per stratum of age, we identified the most prevalent age range per sample and displayed PSMD values with respect to that specific stratum. Whenever there was known or suspected overlap between the samples (10,13,14,16,17) (Appendix S2), only data from the largest reported sample was included in the pooled presentation.
We evaluated the risk of bias using the Newcastle-Ottawa scale for case-control studies and its adapted version for cross-sectional and longitudinal studies (18). We further included key technical parameters in the scales, assessing the clarity and availability of information on the multidirectional diffusion-weighted imaging (DWI) acquisition protocols and preprocessing steps (18). Based on previously defined thresholds, studies were rated as very good, good, satisfactory, and unsatisfactory (18,19) (Appendix S3).
Technical Overview
In Appendix S4, we provide a detailed guide through the main technical steps of PSMD (Fig 1), including prerequisites, preprocessing, skeletonization, custom mask application (Fig S1), histogram analysis, known sources of bias, and how to contour them.
Figure 1:
Schematic overview of the peak width of skeletonized mean diffusivity (PSMD) pipeline. (A) The preprocessing steps included in the pipeline perform motion and eddy currents correction, brain extraction, and tensor fitting. The computation of PSMD further relies on three main steps: skeletonization, application of a custom mask, and histogram analysis. (B) The skeletonization procedure is performed through tract-based spatial statistics by registering the fractional anisotropy (FA) map to common space (functional MRI of the brain [FMRIB] 1-mm fractional anisotropy template) and projecting it onto the skeleton (derived from the same template, thresholded at a fractional anisotropy value of 0.2). The same transformation matrices are used for mean diffusivity (MD) data to obtain a skeletonized MD map. (C) This map is further masked using the template thresholded at a fractional anisotropy value of 0.3 and a custom-made mask. (D) Finally, the width of the histogram derived from the MD values of all voxels included in the skeleton (ie, the difference between percentiles 95 and 5) represents the PSMD. Created with BioRender (https://biorender.com). 4D = four-dimensional, MD-DWI = multidirectional diffusion-weighted imaging, NIFTI = Neuroimaging Informatics Technology Initiative.
Study Characteristics
Overall, 15 studies met eligibility criteria and were included in this review (Fig 2). All were analytical observational studies. The main characteristics of the studies are summarized in Table 1. Regarding the quality assessment, all included studies were classified between satisfactory and very good (Table S2).
Figure 2:
PRISMA flow diagram of the included articles. MS = multiple sclerosis.
Table 1:
Overview of the 15 Included Studies
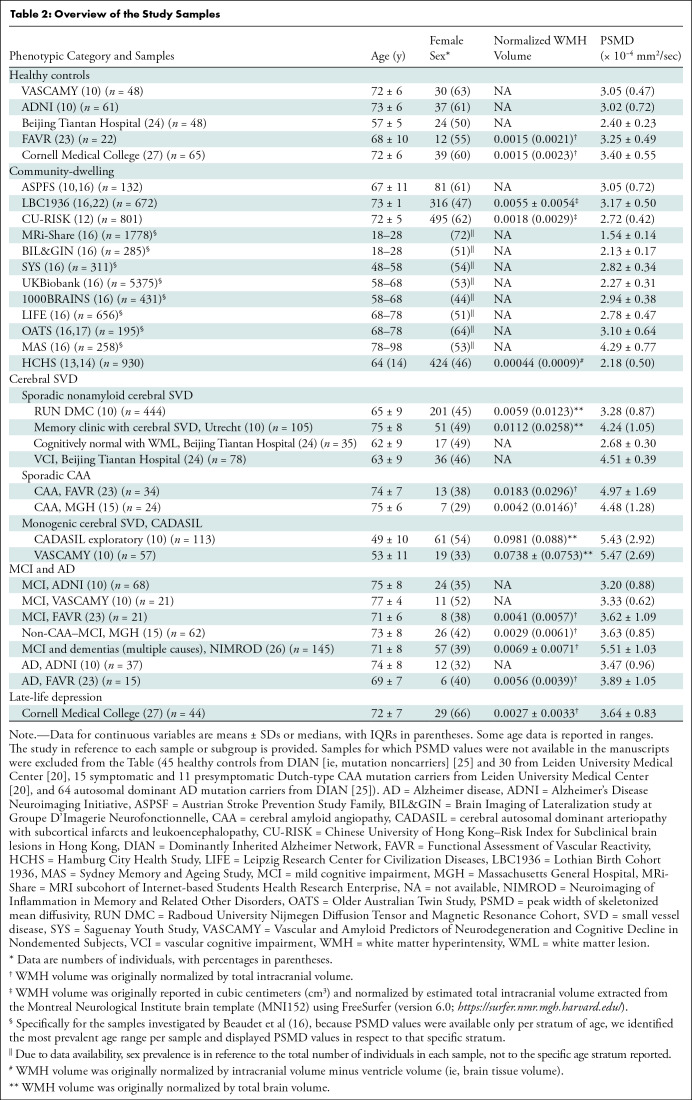
After selecting only the largest samples from overlapping studies (10,13,14,16,17), the PSMD pipeline was applied to a total of 23 489 individuals and more than 25 samples and 39 subsamples. The prevalence of women ranged from 29% (seven of 24 in a sample of patients with cerebral amyloid angiopathy [CAA]) (15) to 73% (eight of 11 among presymptomatic patients with Dutch-type hereditary CAA) (20) across samples and was overall estimated to be 54% (12 550 of 23 448, excluding samples in which the prevalence of women was not reported) (21). All studies came from North America, Europe, Australia, and China. Table 2 summarizes the main demographic and imaging variables extracted per sample and/or subgroup.
Table 2:
Overview of the Study Samples
PSMD in Different Neurologic Conditions
The neurologic conditions investigated with the PSMD marker were organized into four phenotypic categories: community-dwelling (n = 21 736) (10,12–14,16,22), cerebral SVD (n = 916; arteriolosclerosis [n = 662], CAA [n = 58], and monogenic cerebral SVD [n = 196]) (10,15,20,23,24), Alzheimer disease (n = 116) and mild cognitive impairment (n = 317) (10,15,23,25,26), and late-life depression (n = 44) (27).
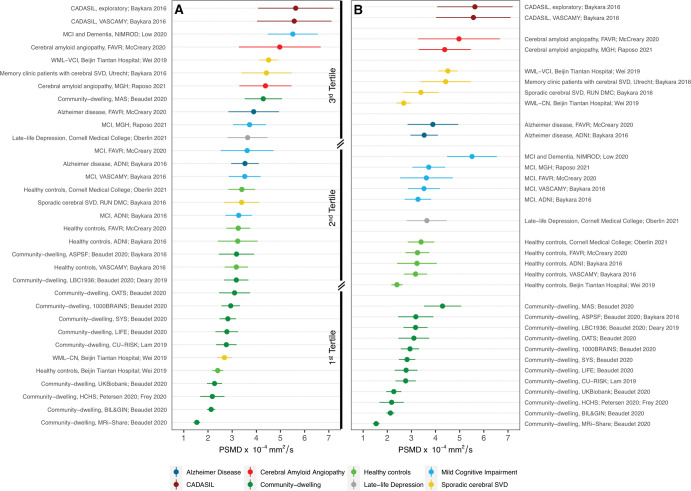
The mean PSMD values from all subgroups ranged from 1.54 × 10−4 mm2/sec ± 0.14 (SD) to 5.63 × 10−4 mm2/sec ± 1.56 (Fig 3A). For the most part, samples with similar diagnoses presented comparable PSMD values but with substantial overlap across various samples and pathologic conditions (Fig 3B). Lower PSMD values were observed among community-dwelling individuals and healthy controls (mean range, 1.54 × 10−4 mm2/sec ± 0.14 to 4.29 × 10−4 mm2/sec ± 0.77). Higher PSMD values were observed among individuals with more widespread WM injury, such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL; mean range, 5.57 × 10−4 mm2/sec ± 1.54 to 5.63 × 10−4 mm2/sec ± 1.56) and CAA (mean range, 4.38 × 10−4 mm2/sec ± 1.08 to 4.97 × 10−4 mm2/sec ± 1.69).
Figure 3:
(A) Cleveland dot plot shows the peak width of skeletonized mean diffusivity (PSMD) values obtained from each sample in descending order. Dots represent the mean PSMD values and bars represent plus or minus the SD. The underlying neurologic conditions are depicted in different colors and listed below the figure. The samples are further divided into tertiles, which demonstrates that community-dwelling and healthy control groups are represented mainly in the low tertile, whereas the middle tertile comprises primarily samples with mild cognitive impairment and less advanced sporadic cerebral small vessel disease (SVD). Pathologic conditions known to present more severe forms of white matter (WM) injury (eg, CADASIL, cerebral amyloid angiopathy, and advanced sporadic cerebral SVD) appear mainly in the highest tertile of PSMD values. (B) Cleveland dot plot shows samples grouped according to the main underlying condition. Despite extensive heterogeneity in technical and clinical variables, there is reasonable homogeneity in the values obtained from equivalent samples. The overlap of values derived from multiple neurologic diseases suggests that PSMD is not specific to any pathologic condition, but likely reflects WM injury irrespective of cause. Results were plotted using the R ggplot2 package (The R Foundation) (38). ADNI = Alzheimer’s Disease Neuroimaging Initiative, ASPSF = Austrian Stroke Prevention Study Family, BIL&GIN = Brain Imaging of Lateralization study at Groupe D’Imagerie Neurofonctionnelle, CADASIL = cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, CN = cognitively normal, CU-RISK = Chinese University of Hong Kong–Risk Index for Subclinical Brain Lesions in Hong Kong, FAVR = Functional Assessment of Vascular Reactivity Study, HCHS = Hamburg City Health Study, LBC1936 = Lothian Birth Cohort 1936, LIFE = Leipzig Research Center for Civilization Diseases, MAS = Sydney Memory and Ageing Study, MCI = mild cognitive impairment, MGH = Massachusetts General Hospital, MRi-Share = MRI subcohort of Internet-based Students Health Research Enterprise, NIMROD = Neuroimaging of Inflammation in Memory and Related Other Disorders, OATS = Older Australian Twin Study, RUN DMC = Radboud University Nijmegen Diffusion Tensor and Magnetic Resonance Cohort, SYS = Saguenay Youth Study, VASCAMY = Vascular and Amyloid Predictors of Neurodegeneration and Cognitive Decline in Nondemented Subjects, VCI = vascular cognitive impairment, WML = white matter lesion.
As expected, PSMD was not an exclusive marker of any disease but appeared sensitive to WM injury from multiple causes. Nonetheless, when applied specifically in older adult populations, PSMD may reflect microvascular disease to the detriment of primary neurodegenerative pathologic abnormality. Microvascular disease predominantly affects WM and PSMD is particularly sensitive to its core lesions (ie, WMH). In recent analyses across multiple samples, including genetically defined diseases, other DTI measures (ie, MD, fractional anisotropy, and free water) were predominantly influenced by cerebral SVD–related, rather than Alzheimer disease–related, abnormalities (28). PSMD appears to behave similarly (10,26). Evidence does suggest that PSMD can detect WM changes in neurodegenerative disorders (10,23,25,26). Yet, in patients with Alzheimer disease, only those with increased WMH burden presented with higher PSMD values (10). Thus, PSMD has been considered a marker of cerebral SVD–induced WM injury in older adult populations and has been employed as a surrogate for cerebral SVD in several studies (13,14,17,27). In one of these studies, PSMD values were unexpectedly lower in a sample of nondemented community-dwelling individuals classified as having presumed “vascular” cognitive impairment according to their plasma lipid profile, which was similar to that observed in patients with vascular dementia (17). Importantly, this “vascular” group displayed similar WMH volume and standard mean DTI metrics (ie, fractional anisotropy, MD, axial and radial diffusivity) compared with the “control” group. This result implies that other pathologic conditions than cerebral SVD, mainly neurodegenerative diseases, were likely in place, which could help explain this discrepant result (17).
Instrumental Properties
There was substantial variability in multidirectional DWI acquisition protocols and preprocessing steps across studies (Table S3). Primary DTI parameters (ie, fractional anisotropy and MD) display good interscanner reproducibility in both healthy controls (29) and patients with cerebral SVD (30). Thus, they do not require extensive harmonization and are considered well suited for multicenter trials (16,31,32). PSMD could be similarly robust based on its comparable values in similar diagnoses, contrasting multidirectional DWI acquisition protocols, and good interscanner reproducibility assessments (10,21,23).
Three studies (27 patients total) specifically investigated the precision of replicate PSMD measurements using different MRI scanners and protocols (interscanner reproducibility) (10,21,23). Among seven patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) consecutively scanned with 3-T and 1.5-T MRI scanners, PSMD offered a higher intraclass correlation coefficient (0.95) than other histogram MD measures (10). McCreary et al (23) examined four patients with two different imaging protocols (11-gradient and 25-gradient directions) and observed no evidence of a significant difference in the derived PSMD values (P = .25). Maillard et al (21) specifically investigated the interscanner reproducibility of PSMD among patients with cerebral SVD. The authors observed an excellent intraclass correlation coefficient among 16 individuals examined with four different MRI scanners (3 T, Philips; 3 T, Siemens Healthineers [×2]; 3 T, GE HealthCare) using harmonized multidirectional DWI protocols (21). The same single study investigated the variability of PSMD values measured with the same individual, scanner, and protocol within a short period of time (test-retest repeatability) in 41 participants and found a high intraclass correlation coefficient between test and retest PSMD values (21). To our knowledge, no studies to date have investigated the impact that different preprocessing steps may have on PSMD values.
PSMD and Age
Previous studies have reported nonlinear associations between age and DTI metrics, with MD being the most age-sensitive measure (33–35). Three cross-sectional studies reported a positive association between PSMD and age. Compared with controls, a greater increase in PSMD values over age was observed in Dutch-type hereditary CAA (20) and carriers of the autosomal dominant mutation in Alzheimer disease (25). Beaudet et al (16) observed a significant effect of age on PSMD among community-dwelling individuals (P < .01 for all age categories except for 28–38 years). In this multicenter study, PSMD displayed a specific lifespan profile, distinct from other DTI metrics, characterized by constant and accelerating increases with age (16). This particular behavior supports a promising role for PMSD as a marker of brain aging (12,16) and may explain some discrepancies in PSMD values observed among nonpathologic samples (Fig 3B). For instance, while the mean age across all healthy control samples ranged from 57 years ± 5 to 73 years ± 6, the MRI subcohort of the Internet-based Students Health Research Enterprise (MRi-Share) study comprised much younger individuals (mean age, 22 years ± 2) (16), which could explain the unexpectedly lower values in this sample. However, in the community-based sample with the highest PSMD values (ie, Sydney Memory and Ageing Study), participants were older (mean age, 80 years ± 5) than most healthy control groups (16).
Neuroimaging Associations
Multiple studies have tried to identify which brain lesions preponderantly drive changes in PSMD values. Groups with a larger burden of WMH had reportedly higher PSMD values (10,24). Studies specifically addressing the relationship of PSMD with conventional MRI markers of cerebral SVD (Table S4) (15,22,23,26,27) found significant associations with WMH volume (P < .001) (15,22,23,26,27), lobar lacunes (P < .001) (26), and a composite burden score of cerebral SVD (P = .004) (23). Perivascular spaces did not show evidence of a significant correlation with PSMD in adjusted models (P > .05) (15,26). Association with cerebral microbleeds was reported in one memory clinic study (26). Conversely, PSMD did not show evidence of an association with cerebral microbleeds or cortical superficial siderosis in patients with CAA (15,23). Markers of global neurodegeneration, such as atrophy, gray matter and WM volumes, and cortical thickness, did not show evidence of a correlation with PSMD (15,23,26).
Overall, PSMD was more strongly related to ischemic than hemorrhagic markers of cerebral SVD, especially with WMH. Dominance analyses have shown that WMH volume contributes substantially more to PSMD than gray matter and WM volumes (26). This is in line with the hypothesis that PSMD, like other diffusion metrics (28), is more sensitive to cerebral SVD than neurodegenerative abnormalities. This strong association with WMH may help explain the higher PSMD values observed in the Neuroimaging of Inflammation in Memory and Related Other Disorders (NIMROD) study (26) in comparison with other mild cognitive impairment and Alzheimer disease samples (Fig 3B). Although there were no striking differences in age or technical parameters, most patients from the NIMROD study were cognitively impaired and the reported mean WMH volume was higher than in other samples. Fractional anisotropy and MD measures (22,27), along with global and topologic network parameters computed using graph theoretical analysis (13,14), were also associated with PSMD.
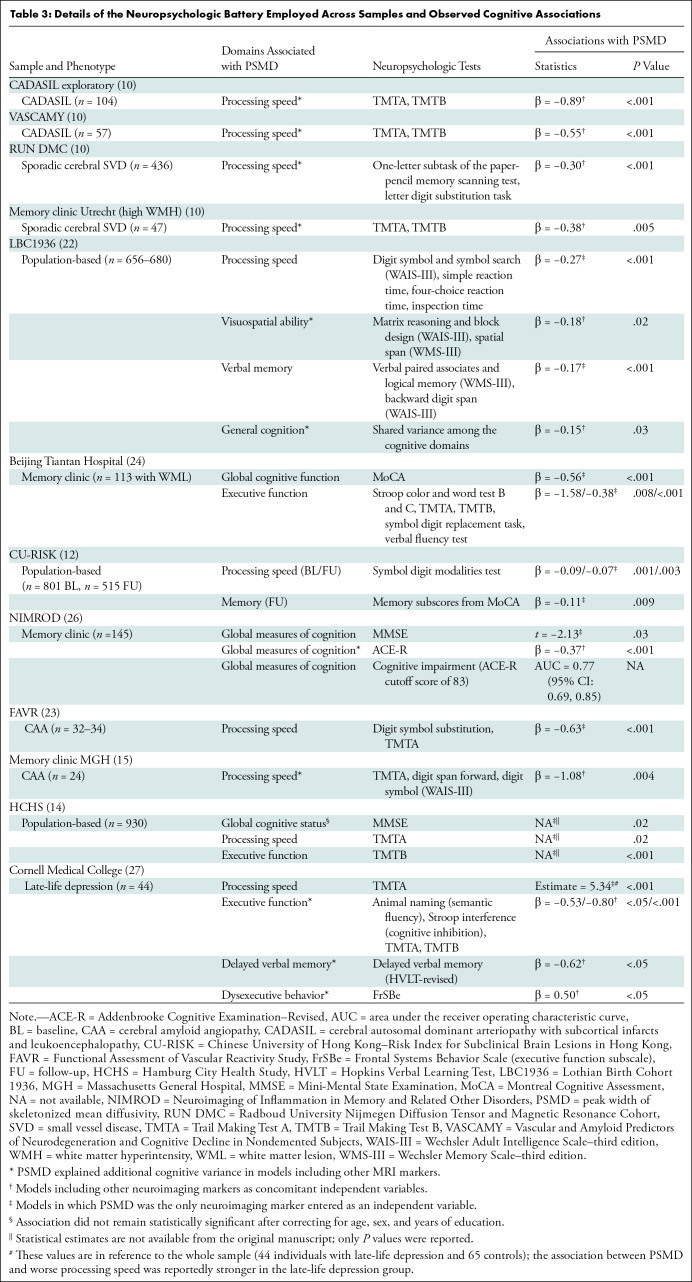
Neurocognitive Associations
The neuropsychologic tests and statistical procedures used to examine the cognitive associations of PSMD are highly variable across studies (Table 3). PSMD has shown a consistent association with cognitive end points, especially in samples with a severe burden of WM lesions, in which it explained 8.8%–46% of the variance in cognitive performance (10,15,23). These associations were frequently absent in samples with a low burden of cerebral SVD (10,15,24), probably because WM injury is less likely the primary mechanism impacting cognition in that context. Nonetheless, strong associations were found in population-based samples with a low burden of cerebral SVD, probably driven by abnormalities in the normal-appearing WM detectable due to the increased statistical power of large samples (12,14,22).
Table 3:
Details of the Neuropsychologic Battery Employed Across Samples and Observed Cognitive Associations
Processing speed was the domain most consistently correlated with PSMD (10,14,15,22,23,27). Baykara et al (10) suggested a particular link between PSMD and processing speed, further supported by two independent studies in sporadic CAA and a control sample of nondepressed individuals in whom PSMD was exclusively associated with processing speed (15,23,27). By specifically assessing the microstructural integrity in the main WM tracts, PSMD could be reflecting how preserved these pathways are and, hence, how fast the information is likely to navigate through them. However, in larger samples examined with more comprehensive neuropsychologic batteries, PSMD correlated with multiple outcomes, such as global cognition and general cognitive ability (14,22,24,26), executive function (14,24,27), visuospatial ability (22), verbal and semantic fluency (27), cognitive inhibition (27), memory (12,22,27), and dysexecutive behavior (27). This could be an advantageous feature because age-related cognitive impairment often manifests with heterogeneous cognitive profiles (2).
PSMD outperformed conventional MRI measures by explaining additional variance in clinical end points in several studies (10,15,22,26,27). In six samples, the strength of the cognitive associations of PSMD was compared against that of other MRI markers using multivariable models. PSMD explained additional variance in at least one domain, including global cognition and general cognitive ability (22,26), processing speed (10,14,15), executive function (14), visuospatial ability (22), semantic fluency (27), cognitive inhibition (27), and memory (27). PSMD also excelled in helping to predict dysexecutive behavior (27) and to discern patients with and without cognitive impairment (26) (Table 3). Although this could be simply secondary to the general superiority of DTI, PSMD also outperformed primary DTI parameters, such as the mean fractional anisotropy and other established MD metrics, in predicting cognitive variance (10,22,26,27). Importantly, unlike the mean MD and fractional anisotropy, PSMD represents a dispersion rather than central tendency statistic (16), reflecting the heterogeneity of MD values across the WM skeleton. It has been hypothesized that PSMD could capture multiple sources of heterogeneity in diffusion parameters and be more susceptible to regional MD variability than primary DTI markers, such as the average MD, which could help explain the superior results of PSMD (16). Although the histopathologic correlates of PSMD remain unexplored, studies in CAA and multiple sclerosis show that MD correlates with tissue rarefaction, myelin density, axonal count, and WM microinfarcts (36,37). PSMD could be associated with similar histopathologic changes, reflecting disruption of connectivity and slowing of synaptic transmission, thus relating to cognitive impairment. Accordingly, higher PSMD was associated with widespread impaired structural connectivity in a population-based sample (13). PSMD also correlated with impairment in the brain’s capacity to integrate information in the form of a reduced diffusion-derived global efficiency measure, which has been reported to mediate the association between cerebral SVD and cognitive decline (14).
Longitudinal Changes in PSMD
Only two studies have investigated longitudinal changes in PSMD. In a sample of 58 patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) followed over 18 months, PSMD displayed significant longitudinal change (P < .001) and required smaller sample size estimates than other variables (10). In a sample of 64 patients followed over approximately 1.1 years, changes in PSMD were also detectable (mean, 0.42 × 10−4 mm2/sec ± 0.53; P < .001) and larger than in skeletonized mean MD (mean, 0.11 × 10−4 mm2/sec ± 0.21; P = .001). However, these changes in PSMD did not show an association with changes in psychomotor speed scores (P = .22) or WMH volume (r = 0.01, P = .95) (23).
Limitations of PSMD
The weaknesses of PSMD include the loss of anatomic information in favor of a single global metric and the lack of specificity concerning the underlying pathologic abnormality. PSMD is also susceptible to motion artifacts and large ischemic or hemorrhagic brain lesions, but several approaches can be taken to mitigate these biases, as mentioned in Appendix S4.
PSMD Validation Status as a Neuroimaging Marker for Vascular Cognitive Impairment and Dementia
To assess the current status of the validation of PSMD as a neuroimaging marker for vascular cognitive impairment and dementia, the available evidence addressing each step of the framework was organized as proposed by the Harmonizing Brain Imaging Methods for Vascular Contributions to Neurodegeneration (HARNESS) initiative in 2019 (9). Substantial evidence for proof of concept, principle, effectiveness, and reproducibility is available in the literature. However, data on monitoring and surrogate steps are largely absent (Table S5). Specifically, there is insufficient longitudinal data. Although two studies investigated how much PSMD changes over time (10,23), only one study assessed whether these changes were related to disease progression or the aggravation of clinical end points, with negative results (23).
Harmonization Techniques
The intense heterogeneity observed across studies indicates the need for standardizing methods to homogenize neuroimaging and clinical data for future multicenter trials and meta-analyses. For instance, technical heterogeneity probably played a relevant role in explaining interstudy PSMD variability, particularly among nonpathologic samples with similar age ranges such as the MRI subcohort of the Internet-based Students Health Research Enterprise (MRi-Share) study, Brain Imaging of Lateralization study at Groupe D’Imagerie Neurofonctionnelle (BIL&GIL), 1000BRAINS study, and Hamburg City Health Study (HCHS) (Fig 3). Accordingly, PSMD was recently included as a candidate marker in initiatives to disseminate and standardize neuroimaging techniques for the study of vascular cognitive impairment and dementia (ie, https://markvcid.partners.org, https://harness-neuroimaging.org), leading to the development of several harmonizing tools currently available for the scientific community (Appendix S5).
Conclusion and Future Directions
Our review presents a detailed overview of a promising quantitative marker, PSMD, and covers its relevant technical aspects and research applications. The main limitation of our study was the striking heterogeneity in MRI protocols, preprocessing methods, cognitive tests, and statistical analyses across studies, which prevented specific comparisons. Other limitations included the nonavailability of some neuroimaging, cognitive, and technical data (eg, PSMD values for the Beaudet et al [16] samples were only available per age stratum, and detailed statistics and the strength of cognitive associations were not available for the Frey et al study [14]). By organizing the available evidence around the steps required for validation as a neuroimaging marker, we could provide an up-to-date synthesis of the role of PSMD as a neuroimaging marker in vascular cognitive impairment and dementia.
In conclusion, evidence suggests that peak width of skeletonized mean diffusivity (PSMD) could play the critical role of a reliable outcome measure for clinical trials in cerebral small vessel disease and favors its potential future application in clinical practice. Clinical translation would further benefit from future development of standardized multidirectional diffusion-weighted imaging protocols for 1.5-T scanners and optimized postacquisition harmonizing techniques. Future research should prioritize the complete validation of PSMD as a surrogate marker, dependent on further longitudinal and technical investigations. Determining if changes in PSMD over time reflect changes in the burden of disease or clinical outcomes is a research priority. Investigating how different preprocessing techniques may impact PSMD values would help better define the extent of technical harmonization needed for multicenter and longitudinal studies. In addition, the histopathologic correlations of PSMD are unexplored, which underscores the need for a more purposeful biologic understanding of the marker in context of different underlying pathologic conditions. Given the promising results already observed in several vascular and neurodegenerative disorders, PSMD could potentially be useful in other yet-to-be-investigated neurologic diseases, such as traumatic brain injury and leukodystrophies.
M.C.Z.Z. and P.Y. contributed equally to this work.
Supported by the National Institutes of Health (R01AG047975, R01AG026484, P50AG005134, and K23AG02872605). L.S. supported by the Swiss National Science Foundation postdoctoral scholarship (P2GEP3_191584).
Disclosures of conflicts of interest: M.C.Z.Z. Payment and travel support from Massachusetts General Hospital. P.Y. No relevant relationships. L.S. No relevant relationships. D.S. Fellowship from the American Heart Association. S.J.v.V. No relevant relationships. M.R.E. No relevant relationships. A.C. No relevant relationships. S.M.G. Grants from National Institutes of Health; royalties from Up-To-Date; consulting fees from Roche; advisory board, IQVIA–Washington University, Bayer, and Biogen. M.D. Institutional payments from Roche Pharma, Hovid Berhad, Sanofi Genyzme, and Bayer Vital; executive committee, The International Society of Vascular Behavioural and Cognitive Disorders; scientific advisory board, Biogen. A.V. Advisory board, Alnylam Pharmaceuticals and Biogen.
Abbreviations:
- CAA
- cerebral amyloid angiopathy
- DTI
- diffusion-tensor imaging
- DWI
- diffusion-weighted imaging
- MD
- mean diffusivity
- PSMD
- peak width of skeletonized MD
- SVD
- small vessel disease
- WM
- white matter
- WMH
- WM hyperintensity
References
- 1. Filley CM , Fields RD . White matter and cognition: making the connection . J Neurophysiol 2016. ; 116 ( 5 ): 2093 – 2104 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zanon Zotin MC , Sveikata L , Viswanathan A , Yilmaz P . Cerebral small vessel disease and vascular cognitive impairment: from diagnosis to management . Curr Opin Neurol 2021. ; 34 ( 2 ): 246 – 257 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wardlaw JM , Smith C , Dichgans M . Small vessel disease: mechanisms and clinical implications . Lancet Neurol 2019. ; 18 ( 7 ): 684 – 696 . [DOI] [PubMed] [Google Scholar]
- 4. Greenberg SM , Charidimou A . Diagnosis of Cerebral Amyloid Angiopathy: Evolution of the Boston Criteria . Stroke 2018. ; 49 ( 2 ): 491 – 497 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reijmer YD , van Veluw SJ , Greenberg SM . Ischemic brain injury in cerebral amyloid angiopathy . J Cereb Blood Flow Metab 2016. ; 36 ( 1 ): 40 – 54 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Etherton MR , Wu O , Cougo P , et al . Integrity of normal-appearing white matter and functional outcomes after acute ischemic stroke . Neurology 2017. ; 88 ( 18 ): 1701 – 1708 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maniega SM , Valdés Hernández MC , Clayden JD , et al . White matter hyperintensities and normal-appearing white matter integrity in the aging brain . Neurobiol Aging 2015. ; 36 ( 2 ): 909 – 918 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raja R , Rosenberg G , Caprihan A . Review of diffusion MRI studies in chronic white matter diseases . Neurosci Lett 2019. ; 694 : 198 – 207 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith EE , Biessels GJ , De Guio F , et al . Harmonizing brain magnetic resonance imaging methods for vascular contributions to neurodegeneration . Alzheimers Dement (Amst) 2019. ; 11 ( 1 ): 191 – 204 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baykara E , Gesierich B , Adam R , et al. ; Alzheimer’s Disease Neuroimaging Initiative. A Novel Imaging Marker for Small Vessel Disease Based on Skeletonization of White Matter Tracts and Diffusion Histograms . Ann Neurol 2016. ; 80 ( 4 ): 581 – 592 . [DOI] [PubMed] [Google Scholar]
- 11. McGrath S , Zhao X , Steele R , Thombs BD , Benedetti A ; DEPRESsion Screening Data (DEPRESSD) Collaboration . Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis . Stat Methods Med Res 2020. ; 29 ( 9 ): 2520 – 2537 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lam BYK , Leung KT , Yiu B , et al . Peak width of skeletonized mean diffusivity and its association with age-related cognitive alterations and vascular risk factors . Alzheimers Dement (Amst) 2019. ; 11 ( 1 ): 721 – 729 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petersen M , Frey BM , Schlemm E , et al . Network Localisation of White Matter Damage in Cerebral Small Vessel Disease . Sci Rep 2020. ; 10 ( 1 ): 9210 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frey BM , Petersen M , Schlemm E , et al . White matter integrity and structural brain network topology in cerebral small vessel disease: The Hamburg city health study . Hum Brain Mapp 2021. ; 42 ( 5 ): 1406 – 1415 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raposo N , Zanon Zotin MC , Schoemaker D , et al . Peak Width of Skeletonized Mean Diffusivity as Neuroimaging Biomarker in Cerebral Amyloid Angiopathy . AJNR Am J Neuroradiol 2021. ; 42 ( 5 ): 875 – 881 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beaudet G , Tsuchida A , Petit L , et al . Age-Related Changes of Peak Width Skeletonized Mean Diffusivity (PSMD) Across the Adult Lifespan: A Multi-Cohort Study . Front Psychiatry 2020. ; 11 : 342 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Y , Chan DKY , Thalamuthu A , et al . Plasma lipidomic biomarker analysis reveals distinct lipid changes in vascular dementia . Comput Struct Biotechnol J 2020. ; 18 : 1613 – 1624 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herzog R , Álvarez-Pasquin MJ , Díaz C , Del Barrio JL , Estrada JM , Gil Á . Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review . BMC Public Health 2013. ; 13 ( 1 ): 154 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yuan T , Fitzpatrick T , Ko NY , et al . Circumcision to prevent HIV and other sexually transmitted infections in men who have sex with men: a systematic review and meta-analysis of global data . Lancet Glob Health 2019. ; 7 ( 4 ): e436 – e447 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schouten TM , de Vos F , van Rooden S , et al . Multiple Approaches to Diffusion Magnetic Resonance Imaging in Hereditary Cerebral Amyloid Angiopathy Mutation Carriers . J Am Heart Assoc 2019. ; 8 ( 3 ): e011288 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maillard P , Lu H , Arfanakis K , et al. MarkVCID Consortium . Instrumental validation of free water, peak-width of skeletonized mean diffusivity, and white matter hyperintensities: MarkVCID neuroimaging kits . Alzheimers Dement (Amst) 2022. ; 14 ( 1 ): e12261 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deary IJ , Ritchie SJ , Muñoz Maniega S , et al . Brain Peak Width of Skeletonized Mean Diffusivity (PSMD) and Cognitive Function in Later Life . Front Psychiatry 2019. ; 10 : 524 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCreary CR , Beaudin AE , Subotic A , et al . Cross-sectional and longitudinal differences in peak skeletonized white matter mean diffusivity in cerebral amyloid angiopathy . Neuroimage Clin 2020. ; 27 : 102280 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei N , Deng Y , Yao L , et al . A Neuroimaging Marker Based on Diffusion Tensor Imaging and Cognitive Impairment Due to Cerebral White Matter Lesions . Front Neurol 2019. ; 10 : 81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Araque Caballero MÁ , Suárez-Calvet M , Duering M , et al . White matter diffusion alterations precede symptom onset in autosomal dominant Alzheimer’s disease . Brain 2018. ; 141 ( 10 ): 3065 – 3080 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Low A , Mak E , Stefaniak JD , et al . Peak Width of Skeletonized Mean Diffusivity as a Marker of Diffuse Cerebrovascular Damage . Front Neurosci 2020. ; 14 : 238 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oberlin LE , Respino M , Victoria L , et al . Late-life depression accentuates cognitive weaknesses in older adults with small vessel disease . Neuropsychopharmacology 2022. ; 47 ( 2 ): 580 – 587 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Finsterwalder S , Vlegels N , Gesierich B , et al. Utrecht VCI study group . Small vessel disease more than Alzheimer’s disease determines diffusion MRI alterations in memory clinic patients . Alzheimers Dement 2020. ; 16 ( 11 ): 1504 – 1514 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou X , Sakaie KE , Debbins JP , Narayanan S , Fox RJ , Lowe MJ . Scan-rescan repeatability and cross-scanner comparability of DTI metrics in healthy subjects in the SPRINT-MS multicenter trial . Magn Reson Imaging 2018. ; 53 : 105 – 111 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Konieczny MJ , Dewenter A , Ter Telgte A , et al . Multi-shell Diffusion MRI Models for White Matter Characterization in Cerebral Small Vessel Disease . Neurology 2020. ; 96 ( 5 ): e698 – e708 . [DOI] [PubMed] [Google Scholar]
- 31. Grech-Sollars M , Hales PW , Miyazaki K , et al . Multi-centre reproducibility of diffusion MRI parameters for clinical sequences in the brain . NMR Biomed 2015. ; 28 ( 4 ): 468 – 485 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prohl AK , Scherrer B , Tomas-Fernandez X , et al . Reproducibility of Structural and Diffusion Tensor Imaging in the TACERN Multi-Center Study . Front Integr Neurosci 2019. ; 13 : 24 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lebel C , Gee M , Camicioli R , Wieler M , Martin W , Beaulieu C . Diffusion tensor imaging of white matter tract evolution over the lifespan . Neuroimage 2012. ; 60 ( 1 ): 340 – 352 . [DOI] [PubMed] [Google Scholar]
- 34. Hasan KM , Sankar A , Halphen C , et al . Development and organization of the human brain tissue compartments across the lifespan using diffusion tensor imaging . Neuroreport 2007. ; 18 ( 16 ): 1735 – 1739 . [DOI] [PubMed] [Google Scholar]
- 35. Cox SR , Ritchie SJ , Tucker-Drob EM , et al . Ageing and brain white matter structure in 3,513 UK Biobank participants . Nat Commun 2016. ; 7 ( 1 ): 13629 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Veluw SJ , Reijmer YD , van der Kouwe AJ , et al . Histopathology of diffusion imaging abnormalities in cerebral amyloid angiopathy . Neurology 2019. ; 92 ( 9 ): e933 – e943 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moll NM , Rietsch AM , Thomas S , et al . Multiple sclerosis normal-appearing white matter: pathology-imaging correlations . Ann Neurol 2011. ; 70 ( 5 ): 764 – 773 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wickham H . Positioning . In: ggplot2: Elegant Graphics for Data Analysis . 2nd ed . Cham, Switzerland: : Springer-Verlag; , 2016. ; 147 – 168 . [Google Scholar]




![Schematic overview of the peak width of skeletonized mean diffusivity (PSMD) pipeline. (A) The preprocessing steps included in the pipeline perform motion and eddy currents correction, brain extraction, and tensor fitting. The computation of PSMD further relies on three main steps: skeletonization, application of a custom mask, and histogram analysis. (B) The skeletonization procedure is performed through tract-based spatial statistics by registering the fractional anisotropy (FA) map to common space (functional MRI of the brain [FMRIB] 1-mm fractional anisotropy template) and projecting it onto the skeleton (derived from the same template, thresholded at a fractional anisotropy value of 0.2). The same transformation matrices are used for mean diffusivity (MD) data to obtain a skeletonized MD map. (C) This map is further masked using the template thresholded at a fractional anisotropy value of 0.3 and a custom-made mask. (D) Finally, the width of the histogram derived from the MD values of all voxels included in the skeleton (ie, the difference between percentiles 95 and 5) represents the PSMD. Created with BioRender (https://biorender.com). 4D = four-dimensional, MD-DWI = multidirectional diffusion-weighted imaging, NIFTI = Neuroimaging Informatics Technology Initiative.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c1b4/9968775/d9f134fddd39/radiol.212780.fig1.jpg)