Abstract
Objective
To determine if treatment with a 5-HT3 antagonist (ondansetron) reduces need for opioid therapy in infants at risk for neonatal opioid withdrawal syndrome (NOWS).
Study Design
A multicenter, randomized, placebo controlled, double blind clinical trial of ninety (90) infants. The intervention arms were intravenous ondansetron or placebo during labor followed by a daily dose of ondansetron or placebo in infants for five days.
Results
Twenty-two (49%) ondansetron-treated and 26 (63%) placebo-treated infants required pharmacologic treatment (p>0.05). The Finnegan score was lower in the ondansetron-treated group (4.6 vs. 5.6, p=0.02). A non-significant trend was noted for the duration of hospitalization. There was no difference in need for phenobarbital or clonidine therapy, or total dose of morphine in the first 15 days of NOWS treatment.
Conclusions
Ondansetron treatment reduced the severity of NOWS symptoms; and there was an indication that it could reduce the length of stay.
INTRODUCTION
In utero exposure to opioids predisposes infants to a postnatal opioid withdrawal syndrome, which is referred to as the neonatal opioid withdrawal syndrome (NOWS). Pharmacotherapy is the standard of care when withdrawal symptoms cannot be controlled by supportive non-pharmacologic approaches. While meta-analysis(1) and expert opinion(2) have identified opioids as the primary pharmacologic therapy for reducing NOWS symptoms, there are concerns about the long-term consequences of their use as the primary therapy for NOWS.(3) We previously examined 18 inbred mouse strains that were physically dependent on opioids. The inbred strains exhibit a highly heritable level of variability in the extent of experimentally induced (i.e., naloxone-precipitated) opiate withdrawal (NPOW) symptoms, which can range from nonexistent to maximal. Computational genetic analysis of the measured severity of NPOW identified allelic variation within the gene (Htr3a) encoding the 5-HT3 receptor as most highly correlated with the response pattern. Consistent with this genetic finding, administration of a 5-HT3 antagonist (ondansetron) significantly decreased NPOW in mice.(4) Subsequent studies in healthy human volunteers demonstrated that NPOW symptoms were greatly reduced by prior administration of ondansetron,(4) or the structurally unrelated 5-HT3 antagonist palonosetron.(5) These findings led to a dose finding study that characterized ondansetron pharmacokinetics in pregnant women and placental transfer to neonates.(6) The dose identified in that study was used in this clinical trial, which was designed to produce ondansetron blood levels that were shown to be sufficient for its anti-emetic effect. Our underlying hypothesis is that a short course of ondansetron treatment administered in the perinatal period could reduce the NOWS severity in at risk infants.
METHODS
This study (NCT01965704) was a double-blind, placebo-controlled, multisite clinical trial that tested the hypothesis that a brief period of ondansetron treatment could reduce the severity of withdrawal symptoms in infants who had in utero opioid exposure. The primary endpoint was the fraction of infants requiring pharmacologic therapy with morphine. The secondary endpoints were NOWS severity as defined by duration of hospitalization, severity of symptoms as measured by Finnegan scores, the total dose of morphine required in the first 15 days of treatment, and the need for adjunctive phenobarbital or clonidine therapy for NOWS treatment.
After providing informed consent, pregnant women and their infants were randomized in a 1:1 fashion to the ondansetron or placebo treatment arms using a RedCap allocation. Research staff, subjects and treating providers were blinded to group status. Unblinded pharmacists at each site managed drug preparation. Both mother and infant received the same treatment allocation. In the active treatment arm, mothers received an 8 mg dose of ondansetron intravenously (IV) within 4 hours prior to delivery. This dose could be repeated one time if the delivery did not occur within the 4 hr time frame. Infants had an electrocardiogram (ECG) before receiving the first dose of the study drug. If the QTc was not elevated and no contraindications for participation were identified, infants born to mothers who received active treatment received a daily oral dose of ondansetron 0.1 mg/kg daily for five days, a duration at which symptoms of NOWS requiring opioid therapy are manifest Mothers in the placebo arm received a saline infusion and their infants received placebo oral medication. All other treatments and procedures were identical. The first infant dose of ondansetron or placebo was initiated within 4-8 hours of delivery. An ECG was performed on the infants within 2-5 hours after each dose of study drug. Figure 1
Figure 1.
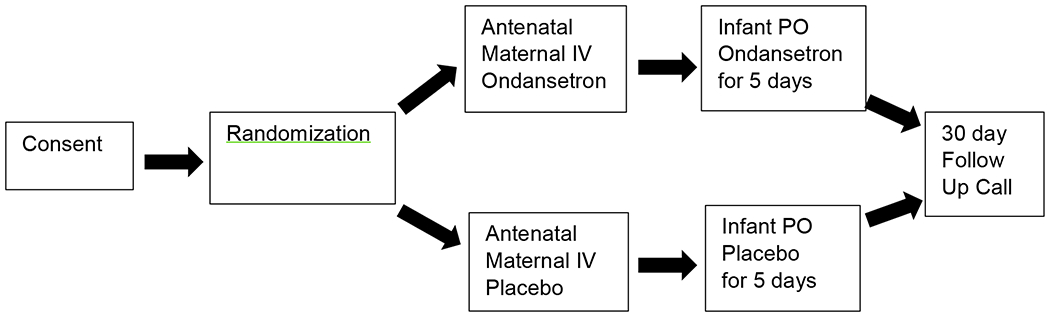
Study design
Maternal ondansetron (Zofran, Sandoz or generic equivalent) or saline placebo was administered as an IV infusion. Infant ondansetron was a commercially available oral formulation (Apotex generic or equivalent). Oral placebo for infants was simple syrup USP (Humco, Sucrose 85% W/V; Purified Water; Citric Acid; 0.1% Methylparaben or equivalent). If infants could not take oral medication, they received the ondansetron 0.04 mg/kg or placebo intravenously. Maternal inclusion criteria were daily opioid use for at least three weeks prior to delivery and known or estimated gestational age of ≥ 37 and < 42 weeks. Exclusion criteria were ingestion of ondansetron within 24 hours prior to delivery, known prior QTc prolongation, or other medical concern of the investigator. Infants were ineligible if they had a prolonged QTc; a medical condition, or administration of a concomitant drug that would impact the ondansetron metabolism. All infants were provided non-pharmacologic care delivered according to the local hospital protocols. NOWS symptoms were monitored using the local site’s modified Finnegan Scoring instrument. The threshold for pharmacologic treatment with morphine was according to local protocols with standardized rater assessments. Phenobarbital or clonidine was added as an adjunct medication according to local protocol when symptoms were not adequately controlled at a maximum morphine dose. Morphine treatment approaches remained uniform during the time period the study was conducted. If infants were discharged before day 15 of life, mothers received daily follow-up telephone calls to assess for NOWS symptoms and adverse events using a standardized study questionnaire. A final follow-up phone call was made 30 days after the baby’s last dose of study medication. The study was approved by the IRB at each of the enrolling sites and informed consent was obtained for each mother and infant enrolled in the study. The study was performed in accordance with the Declaration of Helsinki. A certificate of confidentiality protected the privacy of subject research data.
Statistics
All infants who received at least one dose of study medication and met the inclusion criteria were included in the efficacy analysis. All infants and mothers who were allocated and received at least one dose of study medication in any form were included in the safety analysis. Power analysis effect size was based upon NPOW response data that was obtained from the healthy opioid-naïve adult volunteers under controlled conditions(4). In that study, there was a difference of 2.38 between the means of the control and ondansetron-treated withdrawal responses, with standard deviations of 2.23 and 0.89, respectively. Based upon these numbers, 13 patients per treatment arm would have 90% power to ensure that a treatment difference (ondansetron vs. control) could be detected at the p=0.05 level. However, because of the very large of differences between opioid-naive adults analyzed under experimentally controlled conditions and infants that are exposed in utero to opioids, the actual relative effect size of ondansetron treatment was assumed to be 25-33% of that estimated from the pilot data in normal subjects. This revised effect size estimate provided 45 infants in each arm to provide the same statistical power. For categorical outcomes (the fraction of infants requiring pharmacologic therapy with morphine and the need for adjunctive phenobarbital or clonidine therapy), Fisher’s Exact test was used to perform the analysis of independence. Because the need for morphine was not impacted by the sex of the neonates or by the race/ethnicity of the mother, these stratification factors were not corrected for in the analysis of primary outcome (Table S1).
The null hypothesis for the Fisher’s Exact test was that the need for morphine treatment and study drug allocation were independent. The continuous outcomes of secondary endpoints were examined using the two-sided Wilcoxon Rank-Sum test (equivalent to the Mann-Whitney test), where the null hypothesis is stated as the outcomes in the ondansetron and placebo arms are equal. QTc values in the safety population were analyzed by two sample t-test to determine if the mean QTc of two groups were equal. All tests were two-sided, and the p-values obtained from statistical tests were compared with the significance level (α=0.05) to determine whether null hypothesis could be rejected or retained. Statistical analyses were performed using R software (version 3.6.1).
RESULTS
The study took place between September 2014 and August 2020. Ninety-eight mothers provided consent to reach our goal of 90 infants receiving at least a single dose of ondansetron/placebo. Twelve infants were removed from the efficacy analysis. In the ondansetron group, three mother-neonate pairs were excluded because 1 mother delivered at home and 2 mothers withdrew consent prior to receipt of the study drug. In the placebo group, 9 mother-neonate pairs were excluded because: 1 mother revealed that she was not taking any opioids, 1 mother and her baby did not receive study drug due to a prolonged QTc, 1 mother withdrew consent for her baby prior to study drug administration, 1 mother refused her study drug but allowed her infant to be in the study, however, this infant was excluded after delivery due to respiratory concerns; 1 neonate was withdrawn due hypoxic encephalopathy, and 4 mother-neonate pairs did not meet inclusion criteria (3 received off-trial ondansetron that was ordered by the clinical teams within 24 hours of delivery, and one mother had opioid use but none in the 8 days before delivery). The study flow is diagrammed in Figure 2 and subject demographic data are provided in Table 1. As described in the supplemental note, the ondansetron dosing regimen produced plasma ondansetron concentrations that are consistent with those required for anti-emetic efficacy (Fig. S1).
Figure 2.
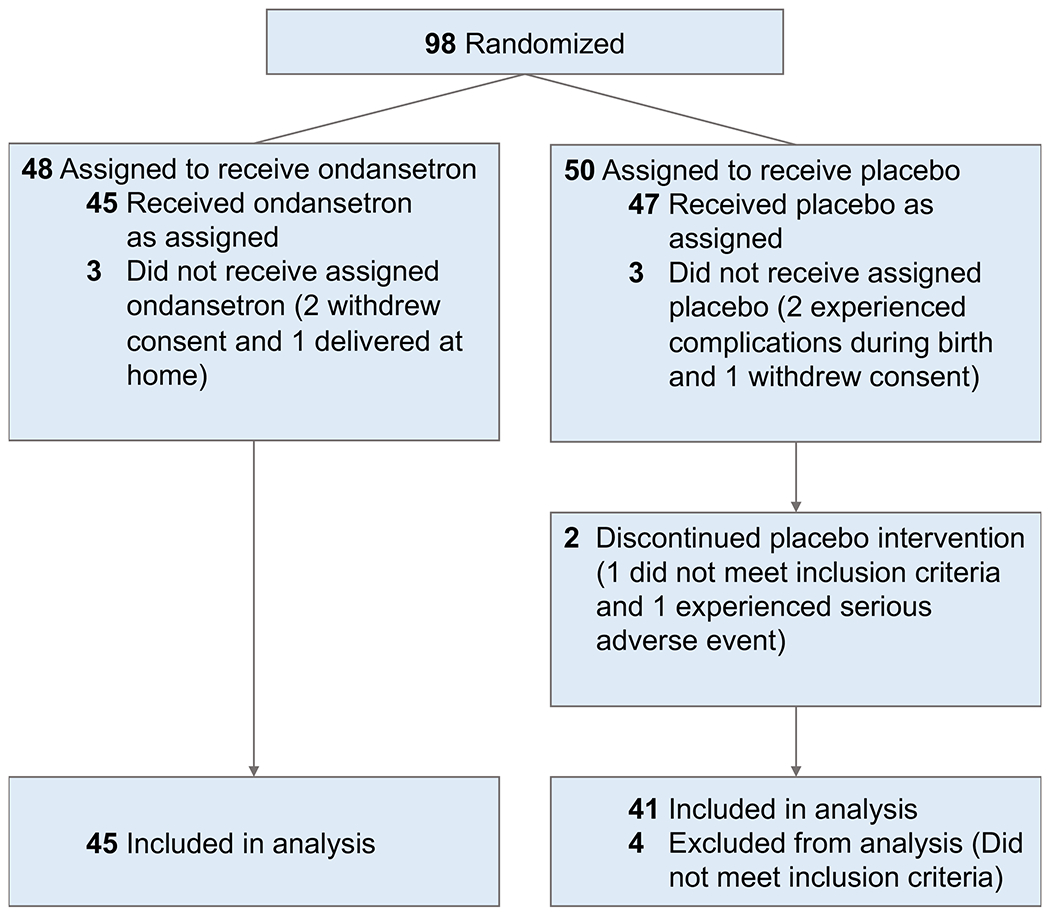
Patient flow CONSORT diagram
Table 1.
Characteristics of the infants and mothers (Per-Protocol Population).
| Characteristic | Ondansetron (N=45) | Placebo (N=41) |
|---|---|---|
| Infants | ||
| Male sex - # (%) | 23 (51%) | 22 (54%) |
| Median birth weight (range) - grams | 3053 (1940 to 4017) | 3060 (2017 to 4120) |
| Median gestational age (range) - weeks/days | 39/1 (36/0 to 41/2) | 39/0 (37/0 to 40/6) |
| Median Apgar scores at 1 minute (range) | 8 (2 to 9) | 8 (5 to 9) |
| Median Apgar score at 5 minutes (range) | 9 (6 to 9) | 9 (5 to 10) |
| Receipt of any breastmilk in hospital (%) | 18 (40%) | 17 (41%) |
| Mothers | ||
| Ethnicity and race - # (%) | ||
| White non-Hispanic | 35 (78%) | 34 (83%) |
| Black non-Hispanic | 6 (13%) | 4 (10%) |
| White Hispanic | 3 (7%) | 3 (7%) |
| Black + other non-Hispanic | 1 (2%) | 0 (0%) |
| Maternal substance exposure | ||
| Methadone (%) | 38 (84%) | 34 (83%) |
| Heroin (%) | 23 (51%) | 20 (49%) |
| Methamphetamine (%) | 7 (16%) | 5 (12%) |
| Tobacco use (>5 cigarettes day) (%) | 24 (53%) | 18 (44%) |
| Marijuana (%) | 10 (22%) | 7 (17%) |
| Cocaine (%) | 8 (18%) | 9 (22%) |
| Benzodiazepine (%) | 10 (22%) | 7 (17%) |
| PCP (%) | 1 (2%) | 0 (0%) |
| Mother did not receive study drug during labor (%) | 5 (11%) | 7 (17%) |
Efficacy
For the primary endpoint, 22 (49%) ondansetron-treated and 26 (63%) placebo-treated infants required pharmacologic treatment for NOWS. While the number of infants requiring opioid pharmacotherapy for NOWS was reduced in the ondansetron-treated group, this difference was not statistically significant. However, the mean modified Finnegan score (defined as the mean of all modified Finnegan scores recorded for that infant) was significantly reduced in the ondansetron-treated versus the placebo group (p=0.02) (Figure 3, Table 2). Given the high cost of treating infants with NOWS, we investigated whether ondansetron treatment would reduce the duration of hospital stay among the at-risk neonates. A graphic analysis of the duration of hospital stay revealed that the number of ondansetron-treated neonates whose duration of hospitalization was ≤5 days or 6-10 days in duration was increased (vs. placebo) and was decreased among those with hospital stays ≥16 days (vs. placebo) (Fig. 4). A clear reversal in the ratios of the number of placebo and ondansetron-treated subjects became readily apparent among those whose length of stay was ≤10 days or ≥16 days. There was a trend toward a reduction in the overall mean length of hospital stay for the ondansetron-treated group (15.5 days) versus the placebo group (17.4 days), but this difference did not achieve statistical significance (p=0.56) (Table 2). However, it was possible that the large number of subjects whose hospital stay was ≥16 days in the ondansetron (33%) and placebo-treated (56%) groups could have obscured the change in the mean number of hospital days, which was seen in the graph in the subjects with a shorter duration of hospitalization. Therefore, when the maximum hospital stay was capped at 15 days, the calculated p-value (p=0.07) for the difference in mean hospital stay between the two groups was very near to the threshold for statistical significance. There was not a statistically significant difference between the placebo and treatment arms in the amount of morphine administered to infants in the first 15 days of treatment, or in the need for adjunctive phenobarbital or clonidine therapy (Figure 3, Table 2). Among the infants who did not require pharmacologic therapy, the maximum Finnegan scores in the ondansetron-treated group were below those in the placebo-treated group, but these reductions did not reach statistical significance. Three infants that were assigned to the placebo arm, but whose mother had received ondansetron for clinically indicated reasons within 24 hours of birth were excluded from the analysis. In a sensitivity analysis, when these infants were included in an as-treated comparison, 59% of placebo treated infants required pharmacologic treatment, compared to the 49% in the ondansetron group (P>0.05).
Figure 3.
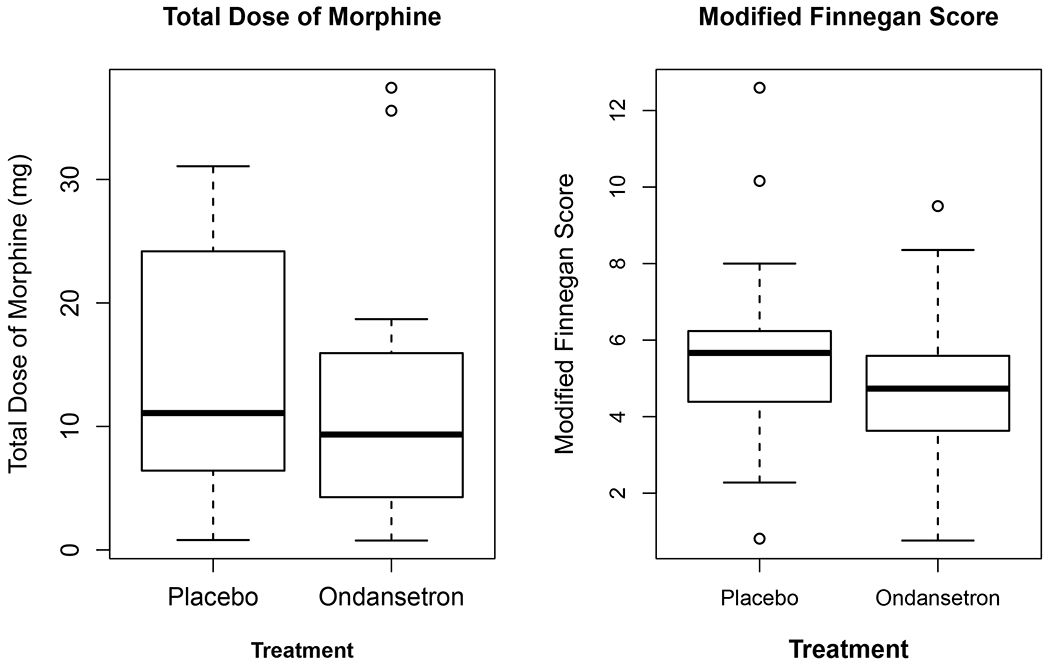
These graphs compare the secondary outcomes of total dose of morphine administered and the average modified Finnegan score in the placebo and ondansetron-treated groups.
Table 2.
Primary and Secondary Outcomes
| Outcome | Ondansetron (N=45) | Placebo (N=41) | Difference (95% CI) a | P Value |
|---|---|---|---|---|
| Primary outcome | ||||
| Need for morphine to treat NOWS – #. (%) | 22 (49%) | 26 (63%) | −4 | 0.2c |
| Secondary outcomes | ||||
| Mean hospital stay (range) | 15.5 (2 to 64) | 17.4 (3 to 63) | −1.9 (−8.0 to 4.3) | 0.56d |
| Mean hospital stay (range) with a 15-day maximume | 9.3 (2 to 15) | 11.2 (3 to 15) | −1.9 (−4.0 to 0.2) | 0.07 |
| Median dose of morphine in 15 days (range) – mg | 9.3 (0.8 to 37.4) | 11.1 (0.8 to 31.1) | −1.8 (−8.8 to 2.7) | 0.31d |
| Mean modified Finnegan scoreb (standard deviation) | 4.6 (1.6) | 5.6 (2.1) | −1.0 (−1.8 to −0.2) | 0.02f |
| Use of adjunctive medicationg to treat NOWS – # (%) | 5 (11%) | 5 (12%) | 0 | 1c |
The 95% confidence interval (CI) between the ondansetron group and the placebo group was calculated with the use of Hodges-Lehmann estimator.
The modified Finnegan score of a neonate is represented by the mean of all modified Finnegan scores recorded for that neonate. The mean modified Finnegan score by ondansetron/placebo allocation reported in the table is the mean of all neonates’ scores in the corresponding allocation.
Fishers exact test.
Wilcoxon Rank-Sum test
By capping the maximum stay at 15 days, the statistical significance of the treatment effect is improved.
Two-sample t-test.
phenobarbital or clonidine
Figure 4.
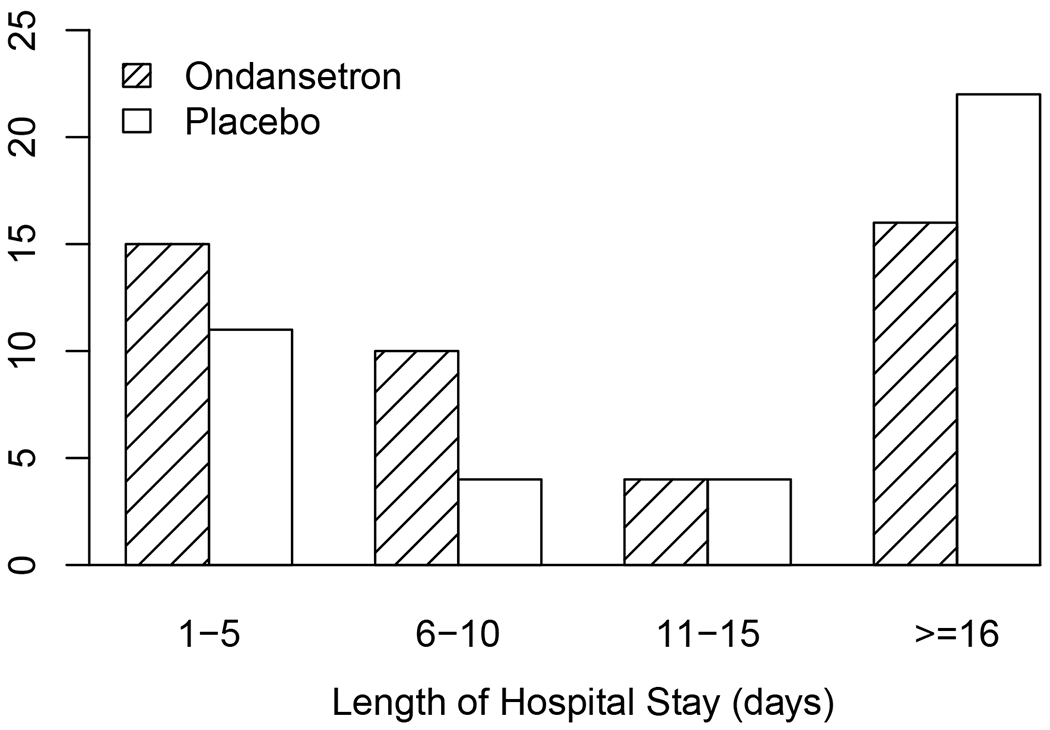
This bar graph shows the number subjects in the placebo and ondansetron-treated groups with a duration of hospitalization for each indicated time. It is noteworthy that the number of ondansetron-treated subjects with a duration of hospitalization that was less than ≤5 days or 6-10 days was increased but was decreased among those whose hospital stays was ≥16 days (vs. placebo).
Safety
Any infant exposed to study drug, either indirectly via administration to the mother or directly to the infant, was included in the safety cohort. Six infants had a serious adverse event (SAE); 2 were in the ondansetron group and 4 were in the placebo group. There was 1 maternal serious event in each treatment arm (Table 3). No SAEs were judged to be due to ondansetron treatment. One neonate had a SAE (hypoxic encephalopathy) and four additional AEs (seizure, sepsis, laryngomalacia, and left brachial plexus injury). This neonate was excluded from the per-protocol analysis due to complications during the birth. One infant allocated to ondansetron had recurrent seizures graded as possibly related to the study drug; and another had late onset NOWS symptoms (the mother reported irritability and the infant’s weight was below the expected trajectory). Two infants in the placebo group were readmitted for NOWS symptoms, but both were managed without the need for pharmacologic therapy. Fourteen (29%) infants in the ondansetron arm and 12 (27%) in the placebo arm were withdrawn from the study due to a prolonged QTc interval. The initial protocol-specified that the maximum QTc for infants was 440 milliseconds (ms) based upon FDA input. A prespecified safety and pharmacokinetic interim analysis took place in May 2016 after 27 infants had been enrolled. The average QTc was 418 ms in the ondansetron group and 437 ms in the placebo group, with 26% of infants having a QTc >440 ms. After discussion with the Data Safety Monitoring Board and subsequent approval by the FDA, the maximum allowed QTc value was increased to 480 ms. Of particular importance, no clinically significant arrhythmias were noted and there was no significant difference in the QTc of infants in the ondansetron and placebo groups. (Table 4, Figure S2) The baseline QTc in infants whose mothers received at least one dose of ondansetron in labor was 424 ms and compared to 419 ms in those who did not for a difference of 5 ms (95% CI −9.4 to 19.4, P =0.49).
Table 3.
Adverse Events.
| Category (number of patients in safety analysis) | Ondansetron (n=48) | Placebo (n=44) |
|---|---|---|
| no. of events | ||
| Serious Adverse Events (SAEs) | ||
| Maternal | ||
| Suicidal ideation | 1 | |
| C-section superficial wound infection | 1 | |
| Neonatal | ||
| Readmission for NOWS observation | 2 | |
| Hyperbilirubinemia | 1 | |
| Late onset NOWS | 1 | |
| Intermittent seizures | 1 | |
| Hypoxic encephalopathy | 1 b | |
| Adverse Events (AEs) | ||
| Maternal | ||
| Subcutaneous hematoma drainage from C-section incision | 1 | |
| Vaginal bleeding | 1 | |
| Neonatal | ||
| Prolonged QTc | 14 a | 12 |
| Short QTc | 3 | 2 |
| Cyanotic episode | 1 | |
| Seizure | 1 b | |
| Sepsis | 1 b | |
| Laryngomalacia | 1 b | |
| Left brachial plexus injury | 1 b | |
| Secundum atrial septal defect | 1 a | |
| Total AE and SAE | 23 | 24 |
One neonate had two AEs (prolonged QTc and secundum atrial septal defect).
One neonate had one SAE (hypoxic encephalopathy) and four AEs (seizure, sepsis, laryngomalacia, and left brachial plexus injury). The neonate was excluded for per-protocol analysis due to complications during the birth.
Table 4.
Infant QTc values in the safety population.
| Outcome | Ondansetron (N=48) | Placebo (N=44) | Difference (95% CI) | P Value |
|---|---|---|---|---|
| Mean number of ECG (range) | 3.3 (0 to 5) | 3.6 (0 to 5) | −0.3 (−1.1 to 0.5) | 0.46 b |
| Baseline QTc (in ms) | 423.6 | 418.9 | 4.7 (−9.7 to 19.1) | 0.52 b |
| Mean QTc after receipt of first infant dose a (in ms) | 435.3 | 429.1 | 6.2 (−9.9 to 22.3) | 0.45 b |
| Mean QTc change from baseline (initial QTc – mean of all post dose values) | −15.7 | −17.7 | 2.0 (−15.8 to 19.8) | 0.83 b |
This refers to mean of all QTc values measured for post doses
Two-sample t-test.
DISCUSSION
NOWS treatment, which is focused on reducing symptom severity in at-risk infants, initially involves the use of non-pharmacologic approaches. However, opioids are administered to many of these infants because their symptoms are not controlled by the non-pharmacologic measures. While NOWS symptoms can be controlled by opioid administration, there are concerns about the potential detrimental effects that opioid treatment, which is associated with a prolonged and costly period of hospitalization, can have on infant development and maternal bonding. (7–11) It is therefore essential to develop non-opioid therapies that can reduce or prevent NOWS symptoms. Based on the striking results obtained in rodents(4) and in opioid naïve adults,(5) we investigated whether ondansetron could provide a non-opiate based treatment for reducing NOWS symptoms and incidence. Of importance, the internal validity of this trial was good with a low risk of bias from randomization, robust local protocols were used for standardization of scoring, and there were few deviations from intended therapy or missing outcomes. The external validity of this trial was strengthened by a multicenter design at high functioning sites serving a variety of patient populations. Since ondansetron treatment produced a statistically significant reduction in the severity of NOWS symptoms, this study demonstrates that ondansetron treatment reduces NOWS symptom scores. While the impact of a symptom reduction of a 20% magnitude (4.6 vs. 5.6) on an individual patient may be uncertain, the importance of this decrease is magnified when viewed across the NOWS patient population. Since symptom scores determine whether opioid therapy is initiated, a 20% decrease in symptom scores would in many patients alter a protocol-driven decision about whether to initiate opioid treatment. It was also noteworthy that there was a trend toward a reduction in the duration of hospitalization in the ondansetron treated group, which was observed in the decreased number of ondansetron-treated subjects requiring a longer hospital stay and an increase in the number of ondansetron-treated subjects with a short hospital stay.
Ondansetron treatment caused a statistically significant decrease in the Finnegan scores but not in the total dose of morphine administered. This divergence is not surprising since the Finnegan score is an elastic measurement (i.e., it is determined at each time point without other constraints), while the morphine dose administered is constrained by the treatment protocol, and it is altered in a stepwise fashion based upon the prior morphine dose. Thus, a change in the morphine dose will be less responsive to a change in NOWS expression than will a change in the Finnegan score and so a change in the morphine dose is likely less likely to differ between treatment groups than will the Finnegan scores. It was also noteworthy that ondansetron’s efficacy in this clinical trial was far less than what we observed previously in mice or human subjects.(4),(5) There are several potential reasons for this. First, unlike that during experimentally induced opioid withdrawal, NOWS expression in any infant can be influenced by factors that are known and measurable and by others that are unknown. While there could be differences in the adult versus neonatal opioid withdrawal physiology, the similarities in buprenorphine ED50 for amelioration of opioid withdrawal symptomatology in adults and infants(12) argues against this possibility. Another factor that could reduce the efficacy of ondansetron in this trial could be that a suboptimal level of ondansetron was achieved with the ondansetron dosing regimen used here. While the ondansetron exposures in this trial were adequate for achieving anti-emetic efficacy, its anti-emetic effect is mediated by drug action at sites (vagal afferent nerves in the gastrointestinal tract) outside of the CNS.(13,14) Our intra-ventricular injection study in mice demonstrates that ondansetron’s effect on opioid withdrawal occurs within the CNS.(4) Moreover, a peripherally acting opioid antagonist (methylnaltrexone), which is used to treat opioid-induced constipation, does not precipitate withdrawal.(15,16) In addition, ondansetron has a slow rate of CNS entry.(17) Although ondansetron has moderate levels of protein binding and ionization at plasma pH and good lipid solubility, its rate of blood brain barrier penetration is below that predicted by its intrinsic properties.(17) Ondansetron CNS kinetics have not been established in either neonates or adults. Thus, it is likely that if a sufficient level of ondansetron binding to its target within the CNS could be achieved, it could have an even larger effect on reducing opioid withdrawal symptomatology and incidence which is consistent with our observation that intrathecal ondansetron administration in an animal model demonstrated such marked efficacy in reducing opioid withdrawal behavior.(4)
This clinical trial did not identify any safety issues. These results suggest that it may be possible to administer ondansetron to the mother for a longer period prior to delivery or possibly to the neonate at higher doses after delivery. This would increase the concentration of ondansetron that is present at its site of action in the CNS for a period of time, which may generate a more marked reduction in NOWS symptoms. If so, this would impact a significant and costly public health problem that arises from the prevalence of opioid use disorder, which generates a severe clinical condition that could have a downstream effect on the infant’s subsequent development. Of course, the effect of a more prolonged period of ondansetron treatment of the expectant mother prior to giving birth must be tested in a subsequent clinical trial.
Supplementary Material
Acknowledgment
The authors acknowledge the work of the following site principal investigators who assisted in the conduct of the study. Lori A. Devlin, MD (Univ. of Louisville), Ramasubbareddy Dhanireddy, MD (University of Tennessee Health Science Center), Ronald S. Cohen, MD (Lucile Packard Children’s Hospital), Camille M. Fung, MD (University of Utah), Juan E. Vargas, MD (University of California, San Francisco General Hospital)
Funding
This trial was supported by National Institute of Drugs of Abuse (NIDA) R01 HD070795-06A1. Gary Petlz and Manhong Wu were also supported by a NIH/NIDA award 5U01DA04439902. The other authors received no additional funding. The NIH had no role in the design and conduct of the study.
Footnotes
Clinical Trial Registration
Conflict of interest disclosure
The authors have no conflicts of interest relevant to this article to disclose.
Participating sites (number of subjects enrolled)
Thomas Jefferson University (42)
Johns Hopkins Bayview Medical Center (31)
Santa Clara Valley Medical Center (13)
University of Louisville (4)
University of Tennessee Health Science Center (3)
Lucile Packard Children’s Hospital (2)
University of Utah (2)
University of California, San Francisco General Hospital (1)
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- (1).Disher T, Gullickson C, Singh B, Cameron C, Boulos L, Beaubien L, et al. Pharmacological Treatments for Neonatal Abstinence Syndrome: A Systematic Review and Network Meta-analysis. JAMA Pediatr 2019. Mar 1;173(3):234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Patrick SW, Barfield WD, Poindexter BB, COMMITTEE ON FETUS AND NEWBORN, COMMITTEE ON SUBSTANCE USE AND PREVENTION. Neonatal Opioid Withdrawal Syndrome. Pediatrics 2020. Nov;146(5): 10.1542/peds.2020-029074. [DOI] [PubMed] [Google Scholar]
- (3).Peltz G, Sudhof TC. The Neurobiology of Opioid Addiction and the Potential for Prevention Strategies. JAMA 2018. May 22;319(20):2071–2072. [DOI] [PubMed] [Google Scholar]
- (4).Chu LF, Liang DY, Li X, Sahbaie P, D’arcy N, Liao G, et al. From mouse to man: the 5-HT3 receptor modulates physical dependence on opioid narcotics. Pharmacogenet Genomics 2009. Mar;19(3):193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Erlendson MJ, D’Arcy N, Encisco EM, Yu JJ, Rincon-Cruz L, Peltz G, et al. Palonosetron and hydroxyzine pre-treatment reduces the objective signs of experimentally-induced acute opioid withdrawal in humans: a double-blinded, randomized, placebo-controlled crossover study. Am J Drug Alcohol Abuse 2017. Jan;43(1):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Elkomy MH, Sultan P, Carvalho B, Peltz G, Wu M, Clavijo C, et al. Ondansetron pharmacokinetics in pregnant women and neonates: towards a new treatment for neonatal abstinence syndrome. Clin Pharmacol Ther 2015. Feb;97(2):167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Jones HE, O’Grady KE, Kaltenbach K. Reconsidering retrospective review of neurodevelopmental outcomes in infants treated for neonatal abstinence syndrome. J Perinatol 2018. Sep;38(9):1280–1281. [DOI] [PubMed] [Google Scholar]
- (8).Merhar SL, McAllister JM, Wedig-Stevie KE, Klein AC, Meinzen-Derr J, Poindexter BB. Retrospective review of neurodevelopmental outcomes in infants treated for neonatal abstinence syndrome. J Perinatol 2018. May;38(5):587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Oei JL, Melhuish E, Uebel H, Azzam N, Breen C, Burns L, et al. Neonatal Abstinence Syndrome and High School Performance. Pediatrics 2017. Feb;139(2): 10.1542/peds.2016-2651. Epub 2017 Jan 16. [DOI] [PubMed] [Google Scholar]
- (10).Ornoy A The impact of intrauterine exposure versus postnatal environment in neurodevelopmental toxicity: long-term neurobehavioral studies in children at risk for developmental disorders. Toxicol Lett 2003. Apr 11;140–141:171–181. [DOI] [PubMed] [Google Scholar]
- (11).Hunt RW, Tzioumi D, Collins E, Jeffery HE. Adverse neurodevelopmental outcome of infants exposed to opiate in-utero. Early Hum Dev 2008. Jan;84(1):29–35. [DOI] [PubMed] [Google Scholar]
- (12).Moore JN, Gastonguay MR, Ng CM, Adeniyi-Jones SC, Moody DE, Fang WB, et al. The Pharmacokinetics and Pharmacodynamics of Buprenorphine in Neonatal Abstinence Syndrome. Clin Pharmacol Ther 2018. Jun;103(6):1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Tyers MB, Freeman AJ. Mechanism of the anti-emetic activity of 5-HT3 receptor antagonists. Oncology 1992;49(4):263–268. [DOI] [PubMed] [Google Scholar]
- (14).Tyers MB. Site(s) and mechanisms of the anti-emetic action of 5-HT3 receptor antagonists: a discussion of Professor Naylor’s paper. Br J Cancer Suppl 1992. Dec;19:S12–3. [PMC free article] [PubMed] [Google Scholar]
- (15).Portenoy RK, Thomas J, Moehl Boatwright ML, Tran D, Galasso FL, Stambler N, et al. Subcutaneous methylnaltrexone for the treatment of opioid-induced constipation in patients with advanced illness: a double-blind, randomized, parallel group, dose-ranging study. J Pain Symptom Manage 2008. May;35(5):458–468. [DOI] [PubMed] [Google Scholar]
- (16).Thomas J, Karver S, Cooney GA, Chamberlain BH, Watt CK, Slatkin NE, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med 2008. May 29;358(22):2332–2343. [DOI] [PubMed] [Google Scholar]
- (17).Simpson KH, Murphy P, Colthup PV, Whelan P. Concentration of ondansetron in cerebrospinal fluid following oral dosing in volunteers. Psychopharmacology (Berl) 1992;109(4):497–498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


