Abstract
Iatrogenic tracheobronchial injury (ITI) is an infrequent but potentially life-threatening disease, with significant morbidity and mortality rates. Its incidence is presumably underestimated since several cases are underrecognized and underreported. Causes of ITI include endotracheal intubation (EI) or percutaneous tracheostomy (PT). Most frequent clinical manifestations are subcutaneous emphysema, pneumomediastinum and unilateral or bilateral pneumothorax, even if occasionally ITI can occur without significant symptoms. Diagnosis mainly relies on clinical suspicion and CT scan, although flexible bronchoscopy remains the gold standard, allowing to identify location and size of the injury. EI and PT related ITIs more commonly consist of longitudinal tear involving the pars membranacea. Based on the depth of tracheal wall injury, Cardillo and colleagues proposed a morphologic classification of ITIs, attempting to standardize their management. Nevertheless, in literature there are no unambiguous guidelines on the best therapeutic modality: management and its timing remain controversial. Historically, surgical repair was considered the gold standard, mainly in high-grade lesions (IIIa-IIIb), carrying high morbi-mortality rates, but currently the development of promising endoscopic techniques through rigid bronchoscopy and stenting could allow for bridge treatment, delaying surgical approach after improving general conditions of the patient, or even for definitive repair, ensuring lower morbi-mortality rates especially in high-risk surgical candidates. Our perspective review will cover all the above issues, aiming at providing an updated and clear diagnostic-therapeutic pathway protocol, which could be applied in case of unexpected ITI.
Keywords: iatrogenic tracheal injury, tracheal surgery, thoracic surgery, endoscopy, tracheobronchial laceration
Introduction
Iatrogenic tracheobronchial injury (ITI) can be defined as any lesion occurring in the airway due to invasive medical or surgical procedure. Main causes are orotracheal intubation and tracheostomy, defining the post-intubation ITIs, and this review will focus on them (1, 2). Other causes include thoracic and neck surgeries but discussing them is beyond the aims of the present review. Globally, post-intubation ITI is considered rare (2), thanks to advancements in medical devices and development of innovative less-invasive procedures; nonetheless its consequences could be awful (3). The recent SARS-CoV-2 pandemic has well raised the issue since the high rate of emergency intubation and close radiological imaging monitoring have brought out several ITI cases (3–5). However, experiences in this field are limited just by the rarity of this condition, and literature is still lacking updated definitive indications on its identification and management. Therefore, the following perspective review aims at discussing the main aspect of post-intubation ITIs, eventually proposing an updated diagnostic-therapeutic algorithm, appliable in case of unexpected ITI.
Epidemiology
The real incidence of ITIs is unknown, but it is estimated to be 0.005% for all endotracheal (ET) intubation, up to 0.5% for double-lumen tube procedures, and 1% for tracheostomy (6, 7). These rates are likely underestimated, due to several cases are underrecognized or even underreported. Surely, emergency procedures increase the risk for accidental injury; in such settings, the incidence is reported to be up to 15% (2, 8).
Risk factors
Predisposing factors can be divided into patient-related and procedure-related. The formers are largely unmodifiable and consist of advanced age, female gender, obesity, chronic use of inhaled or systemic steroids, local inflammation and all those conditions leading to tissue malacia (3, 7, 9–11); furthermore, anatomic variations or alterations, such as tracheal diverticula or neoplasms, neck or mediastinal masses dislocating the trachea, marked cervical lordosis or scoliosis, fall into this category (7). The latters include multiple attempts or limited experience in intubation, misuse of a stylet or rigid-guide, as well as incorrect choice of tube or cannula size, double-lumen tube, mishandling of cuff pressure or leverage of the tube (2). Emergency procedures can enhance each of the described risk factors, explaining the higher prevalence of ITIs in emergency settings (12).
Pathophysiology
Usually, post-intubation ITIs are caused by friction of the endotracheal tube against the pars membranacea of the tracheal wall, at the midline along the posterior membrane or at the cartilaginous-membrane junction, whereas cartilaginous rings and ligaments offer relative protection from injury to the anterior wall (13). Typically, the injury consists of longitudinal tear at the tracheal middle third, which may spread to the lower third or even to the main bronchi: the length is highly variable (14). The depth of the laceration is similarly variable, but it is critical to be assessed. Indeed, based on depth of the lesion, in 2010 Cardillo and colleagues (8) proposed a morphological classification for patients-risk-stratification, aiming at standardizing treatments. According to that classification, post-intubation ITIs were categorized as follows: I, partial-thickness lesion (limited to mucose or submucose) without mediastinal or subcutaneous emphysema; II, full-thickness lesion with mediastinal or subcutaneous emphysema, but without esophageal or mediastinal soft-tissue involvement; IIIA, full-thickness lesion with esophageal or mediastinal soft-tissue herniation, but without esophageal injury or mediastinitis; IIIB, full-thickness lesion with esophageal injury or mediastinitis. Recently this classification has been revised, adding level IV lesions, characterized by extensive loss of substance or fracture of tracheal rings (8, 15, 16).
Clinical features
Usual clinical presentation consists of facial and upper-trunk subcutaneous emphysema together with cough, occurring within a variable interval of time from ET intubation, generally up to 3 days (14, 17). Dyspnoea can variably occur, from breathing discomfort up to real acute respiratory failure, depending on severity of the lesion and association of unilateral or bilateral pneumothorax (2). ITI may also have asymptomatic course, especially in case of partial-thickness lacerations (14, 17, 18). In mechanical ventilated patients, ITIs can have either subtle development (17), with delayed occurrence in case the cuff overcomes or covers the lesion as well as with ventilatory leaks needing for over-cuffing the tube, or catastrophic presentation (2, 14), with rapid-onset massive pneumomediastinum, tension pneumothorax and difficult ventilation, mainly depending on extent of the tear. Based on mediastinal involvement degree and extent of pneumomediastinum, pneumopericardium, angina or even hypovolemic or cardiogenic shock may occur (4). Haemoptysis or pneumoperitoneum are seldom reported in literature (2, 18, 19).
Diagnosis
Nowadays, several ITIs are likely misdiagnosed, leading to delayed workup and late treatment, with detrimental effects on patients' outcome (2). To overcome this issue, it is recommended keeping high suspicion in case of suggestive symptoms in patients under mechanical ventilation or with medical history of recent intubation. To define and characterize the suspected lesion, radiologic imaging and endoscopic visualization are two complementary pillars of the diagnostic workup (20). Imaging can be obtained either through chest x-ray or CT-scan. The former allows to promptly rule out pneumothorax, large pneumomediastinum, pneumoperitoneum or subcutaneous emphysema, and it can be very useful in emergency settings to shrink differential diagnosis (21). The latter allows to detect the same findings as x-ray does, with greater sensitivity and accuracy (20–22).
Furthermore, contrast-enhanced CT scan may directly reveal tracheal laceration, approximately defining its site and extent, assessing alterations or deformities of the tracheal wall and cartilaginous rings, as well as identifying collateral damages to mediastinal organs or mediastinitis (12, 23). Typically, a tracheal tear may be highlighted as follows: discontinuity in the tracheal wall, localized pneumomediastinum, overdistension or herniation of the cuff, or tube displacement (24); in case of laceration expanding towards a main bronchus, the fallen lung sign could be noted (25). Another crucial role of the CT scan is to provide a non-invasive evaluation of the tube location and cuff inflation (22). Eventually, double-contrast-enhanced CT scan may even reveal oesophageal injury with mediastinal contrast spreading (23, 26, 27). Despite the valuable information that can be gained through CT, endoscopy remains the gold standard in properly characterizing the tracheal tear and it is mandatory to perform it as soon as possible. Flexible bronchoscopy allows to dynamically evaluate location, length, and depth of the lesion, ruling out involvement of mediastinal soft-tissue or oesophagus and correctly classifying the injury. In mechanical ventilated patients, basic bronchoscopic assessment may fail identifying the lesion, which could be hidden by the cuff or the tube itself; therefore, in such setting, it is recommended to perform a thorough evaluation with cuff deflation and tube manipulation. It is worth underlining that a correct ITI management cannot be planned without performing both CT-scan and bronchoscopy (21, 26).
Management
ITIs are burdened by significant morbidity and mortality rates (28), which impose an early and efficacious treatment. Nowadays, therapeutic options for management of post-intubation ITIs are the following: conservative, endoscopic, and surgical treatments (27, 28). Currently, definitive indications on best treatment option are still demanded. However, it is broadly recommended to personalize treatment case-by-case, depending on characteristics of the laceration, patient's clinical features, general conditions, and comorbidities, as well as experience of the centre. Main experiences on the management of this disease are reported in Table 1. Traditionally, surgical repair has long been considered the gold standard, praised to be the only procedure preventing mediastinitis or further tracheal scarring stenosis (30, 38). Nevertheless, due to technical difficulties and non-negligible complications rate affecting surgery, there has recently been a shift towards conservative or less-invasive management of ITIs, which has been allowed by development of innovative materials and spread of minimally invasive procedures (27, 30, 39, 40).. Eventually, multidisciplinary assessment is recommended to choose the best treatment option for each patient, invariably depending on his clinical and respiratory conditions.
Table 1.
Significant studies focusing on management of post-intubation iatrogenic tracheal injuries.
| Reference | No of patients | Cause | Site | Lenght | Cardillo grade | Management | Type of treatment | Success rate |
|---|---|---|---|---|---|---|---|---|
| Conti et al. (13) | 30 | Elective intubation 16 | Posterior membrane 30 | 4.5 ± 1.5 | NA | Conservative 28 | Observation 15 | 86% |
| Intubation 13 | ||||||||
| Emergency intubation 14 | — | Endoscopic 0 | — | — | ||||
| NA | Surgical 2 | Posterolateral thoracotomy 2 | 0% | |||||
| Cardillo et al. (8) | 30 | Elective intubation 22 | NA | 3.2 ± 1.1 | I 3 | Conservative 29 | Glue application 29 | 100% |
| II 24 | ||||||||
| IIIA 2 | ||||||||
| Emergency intubation 8 | — | Endoscopic 0 | — | — | ||||
| IIIB 1 | Surgical 1 | Posterolateral thoracotomy 1 | 100% | |||||
| Schneider et al. (1) | 29 | Elective intubation 6 | NA | 4 | NA | Conservative 11 | Observation 3 | 100% |
| Emergency intubation 10 | Intubation 8 | |||||||
| Tracheostomy 10 | — | Endoscopic 0 | — | — | ||||
| Other 3 | NA | Surgical 18 | Transtracheal 7 | 100% | ||||
| Posterolateral thoracotomy 11 | ||||||||
| Gomez-Caro Andrés et al. (29) | 18 | Elective intubation 14 | Posterior membrane 17 | 2.83 ± 1.02 | NA | Conservative 17 | NA | 82% |
| Emergency intubation 1 | Carina 1 | — | Endoscopic 0 | — | — | |||
| Tracheostomy 3 | NA | Surgical 1 | Cervicotomy 1 | 0% | ||||
| Sippel et al. (30) | 13 | Elective intubation 4 | Posterior membrane 9 | 4.4 ± 2.9 | NA | Conservative 2 | Intubation 2 | 100% |
| Emergency intubation 8 | — | Endoscopic 0 | — | — | ||||
| Tracheostomy 1 | Membranous-cartilaginous 4 | NA | Surgical 11 | Lateral thoracotomy 11 | 73% | |||
| Cardillo et al. (16) | 62a | Elective intubation 51 | NA | 2.54 | I 8 | Conservative 55 | Glue application 55 | 100% |
| Emergency intubation 11 | II 36 | |||||||
| IIIA 11 | ||||||||
| — | Endoscopic 0 | — | — | |||||
| IIIB 6 | Surgical 7 | Posterolateral thoracotomy 5 | 100% | |||||
| IV 1 | VATS 1 | |||||||
| Cervicotomy 1 | ||||||||
| Herrmann et al. (31) | 64 | Elective intubation 19 | NA | 4 | I 2 | Conservative 21 | Observation 2 | 100% |
| Emergency intubation 17 | II 14 | Glue application 19 | ||||||
| Tracheostomy 26 | IIIA 5 | |||||||
| Other 2 | — | Endoscopic 0 | — | — | ||||
| IIIA 23 | Surgical 43 | Transcervical 29 | 77% | |||||
| IIIB 20 | Lateral thoracotomy 14 | |||||||
| Fiorelli et al. (32) | 6 | Elective intubation 6 | NA | 3.5 | II 3 | Conservative 6 | Glue application 6 | 83% |
| IIIA 3 | ||||||||
| — | Endoscopic 0 | — | — | |||||
| — | Surgical 0 | — | — | |||||
| Tazi-Mezalek et al. (33)] | 35 | Elective intubation 19 | Posterior membrane 20 | 3.74 ± 1.76 | NA | Conservative 24 | Observation 7 | 96% |
| Tracheostomy 16 | Membranous-cartilaginous 14 | Intubation 17 | ||||||
| Anterior wall 1 | NA (IIIB 1) | Endoscopic 8 | Silicon Y stenting 7 | 62% | ||||
| Oesophageal stenting 1 | ||||||||
| IIIB 3 | Surgical 3 | NA | 0% | |||||
| Hussein et al. (34) | 4 | Emergency intubation 3 | Posterior membrane 4 | 5.62 | — | Conservative 0 | — | — |
| Tracheostomy 1 | IIIA 4 | Endoscopic 4 | Nitinol stenting 4 | 100% | ||||
| — | Surgical 0 | — | — | |||||
| Carretta et al. (35) | 36 | Elective intubation 23 | NA | 3.5 ± 1 | NA | Conservative 16 | Observationb | 94% |
| Emergency intubation 7 | Tracheostomyb | |||||||
| Tracheostomy 6 | — | Endoscopic 0 | — | — | ||||
| NA | Surgical 20 | Lateral thoracotomyb | 90% | |||||
| Cervicotomyb Transtrachealb | ||||||||
| da Silva Costa et al. (36) | 2 | Emergency intubation 2 | NA | 5.5 | NA | Conservative 0 | — | — |
| NA | Endoscopic 0 | — | — | |||||
| NA | Surgical 2 | Combined VATS/transtracheal | 100% | |||||
| Welter et al. (37) | 17 | NA | NA | NA | NA | Conservative 0 | — | — |
| NA | Endoscopic 0 | — | — | |||||
| NA | Surgical 18 | Endotracheal 18 | 94% | |||||
| San Gerardo Hospitalc | 14 | Elective intubation 6 | Posterior membrane 8 | 3.14 ± 1.09 | II 4 | Conservative 4 | Intubation 4 | 100% |
| Emergency intubation 4 | IIIA 9 | Endoscopic 10 | Nitinol stenting 10 | 100% | ||||
| Tracheostomy 4 | Membranous-cartilaginous 6 | IIIB 1 | Surgical 0 | — | — |
NA, not available data.
30 patients from previous publication were included.
Type of treatment is specified, whereas number of patients treated by a specific approach cannot be derived.
Authors' personal experience.
Conservative treatment
Conservative approach is widely suggested in asymptomatic patients with small partial-thickness laceration (level I), hemodynamical and respiratory stability, without mediastinal involvement (8, 17). However, indications to conservative management are now spreading to larger (up to 9 cm) or even deeper (up to level IIIA) tears (30, 41). Conservative options consist of observation, intubation, tracheostomy, fibrin glue application. Whatever the chosen conservative technique, strictly follow-up of patients is of paramount importance to early detect any clinical worsening.
-
•
Observation is based on rest, antitussive drugs, and broad-spectrum antibiotics. This management may be adopted in case of small (< 2 cm) level I tears in asymptomatic or pauci-symptomatic patients (40).
-
•
Intubation allows to overcome the injured segment, by placing the cuff distally in healthy tissue, ensuring ventilation support (22, 27). This management may be adopted in case of level I-IIIa tears in patients needing for ventilation. Anyway, ventilator setting should provide protective ventilation, by minimizing airway pressures. If the length of the lesion does not allow to place the cuff distally, after considering a double-lumen tube, and the respiratory failure is not otherwise manageable, ECMO support is worth to be accounted (42).
-
•
Tracheostomy (40, 43) is considered a fallback option, due to significant side effects, but may be indicated in long level I–II tears, since it decreases endotracheal pressure ensuring progressive healing of the injury.
-
•
Glue application is an innovative procedure proposed by Cardillo and colleagues (8); it consists of instillation of fibrin sealant to directly cover the tear through flexible bronchoscopy. It is generally appliable in level I–IIIA tears. Recently, the same authors (16) have presented an updated series of 55 patients treated by glue application, showing 100% success rate, when the procedure is performed in experienced centres on fit patients.
Endoscopic treatment
Several cases of patients with endoscopically managed ITIs have been reported in literature with encouraging results (40, 41). In the recent past, this option was reserved to poor surgical candidates (12), deemed unfit for surgery, due to comorbidities. The reported satisfactory results have prompted some physicians to spread the indications for this technique (2, 27, 40). Nowadays, endoscopic treatment may be suggested for treating level IIIA or even selected IIIB lesions instead of surgical approach, in patients with worsening clinical conditions, such as expanding pneumomediastinum/subcutaneous emphysema, high risk for mediastinitis, if without signs of actual mediastinitis, or prolonged mechanical ventilation without short-term perspective of weaning (40, 41, 43–45). The technique consists of rigid bronchoscopy and temporary placement of covered metallic or silicone stent over the laceration, allowing for granulation tissue to close the defect. It is suggested to keep the stent in position from 4 to 8 weeks, then it can be removed (46). This procedure is inherited by lung transplantation field, where it is applied in case of post-transplant tracheobronchial dehiscence (44). Complications reported in literature include stent migration, tracheal stenosis, mucus plugging and local infections (44, 47, 48). If benefits overcome these risks, stenting could be a valid surrogate of surgery, allowing for bridge treatment and delaying surgical approach after improving general conditions of the patient, or even for definitive repair, ensuring lower morbi-mortality rates especially in high-risk surgical candidates (34, 49, 50). The placement of nitinol-coated self-expandable metallic stents (n-SEMS) seems to be particularly interesting (33, 41, 43, 45) since it could apparently fit better in the airway than silicone ones, decreasing the risk of migration, while preserving the tracheal segment from air leakage.
Authors' personal experience
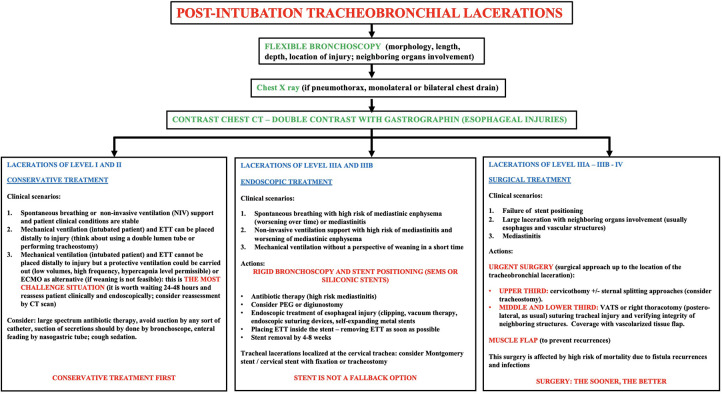
In the last three years, 14 patients with post-intubation ITIs were referred to the Department of Thoracic Surgery of our tertiary centre (San Gerardo Hospital, Monza, Italy). The injury was due to endotracheal tube mispositioning in 10 patients and emergency tracheotomy in 4 patients. It was along the tracheal posterior membrane in 8 cases (57%) and at the tracheal membrane-cartilaginous junction in the remaining patients. 4 lacerations were classified as level II, 9 as IIIA, 1 as IIIB. Upon multidisciplinary discussion, we have successfully treated all the patients, through conservative or endoscopic treatment, depending on patients' clinical and respiratory conditions, according to the in-hospital protocol reported in Figure 1: 4 patients (level II) were conservatively treated, 10 patients (9 IIIA and 1 IIIB) were endoscopically managed. The conservative treatment consisted of endotracheal tube proper positioning and observation. On the other hand, the endoscopic treatment consisted of n-SEMS placement through rigid bronchoscopy, within 72 h from detection of ITIs; 30-day morbidity and mortality rates were null, and the stent was removed 4–6 weeks later without complications. All the injuries were completely healed at 1-month, without any relapse at 6-month follow-up. We were prompted to endoscopically handle patients with level III injuries, instead of adopting a conservative strategy, because of their respiratory conditions: all the patients were still ventilatory-dependent, due to primary lung failure, without a perspective of weaning in short time. In such setting, we strongly believe that a conservative treatment could be hardly feasible.
Figure 1.
Diagnostic-therapeutic algorithm proposal for management of post-intubation tracheobronchial injuries.
Surgical treatment
Surgery is recommended for highly symptomatic patients with large level IIIA, especially in case of ineffective mechanical ventilation, or level IIIB lacerations, mainly when involving vascular structures or esophagus, as well as for level IV tears, or any lesion occurring with mediastinitis (22, 35, 38). Most authors agree that fit patients with rapidly worsening clinical conditions, despite previous conservative or endoscopic treatment, should undergo surgery, preferably within 48–72 h from the original event, to mitigate morbidity and mortality rates (2, 35, 50). Different surgical approaches are described in literature (2, 12): open, video-assisted thoracoscopy surgery (VATS), and endotracheal. The decision to perform one rather than others approach relies on the site and extent of injury, the emergency or elective setting, the experience of the center. Open techniques consist of posterolateral right thoracotomy (28), which was traditionally the approach of choice for emergency procedures and for middle or lower thirds tracheal injuries, and cervicotomy, as introduced by Angelillo-Mackinlay (51) in case of upper third lesions, possibly associated with sternal split if middle third is involved. VATS techniques include right thoracoscopy, as well as video-assisted transcervical-transtracheal approach, which was proposed by da Silva Costa and colleagues (36) introducing an endoscopic needle holder and a 0-degree camera though the tracheal incision. Either in open or VATS approach, continuous running or interrupted sutures are used, based on surgeons' choice. To prevent recurrences or fistulas, mainly in case of mediastinal inflammation or infection, pedicled muscle flaps are placed over the suture line (50). Another promising technique is the endotracheal repair, firstly described in 2011 by Welter and colleagues (37). It is performed using an endoscopic needle holder through rigid tracheoscopy, leading to a totally intraluminal repair, with lower surgical trauma and postoperative pain (37).
Conclusions
Post-intubation ITIs are rare complications of intubation or tracheostomy, nevertheless they are clinically significant due to their high morbidity and mortality rates. Keeping high clinical suspicion is of utmost importance, and patients with suggestive symptoms should early undergo thorough diagnostic workup, through radiologic and endoscopic assessment to detect and characterize the suspected injury. The management of post-intubation ITIs is still a matter of debate and definitive guidelines are still lacking. Procedural and instrumental innovation, as well as medical development, have likely revolutionized traditional management of post-intubation ITIs, broadening the use of conservative treatment and introducing the opportunity of endoscopic approach, with interesting success and reasonable complication rates. In such setting, endoscopic stenting may be a viable alternative to surgery and no more a fallback option, limiting surgical management to advanced stages or in case of failure of other treatments. On the other hand, surgery has become less and less invasive, leading to lower morbidity and mortality rates than in the past. Patients' general clinical and respiratory conditions must be considered in the management pathway. Anyway, multidisciplinary evaluation and personalized treatment of each patient at experienced centres are strongly recommended.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author contributions
RO, FR, and EP contributed to conception and design of the study. RO and EP wrote the first draft of the manuscript. RO, FR, EP, MC wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Schneider T, Volz K, Dienemann H, Hoffmann H. Incidence and treatment modalities of tracheobronchial injuries in Germany. Interact Cardiovasc Thorac Surg. (2009) 8(5):571–6. 10.1510/icvts.2008.196790 [DOI] [PubMed] [Google Scholar]
- 2.Miñambres E, Burón J, Ballesteros MA, Llorca J, Muñoz P, González-Castro A. Tracheal rupture after endotracheal intubation: a literature systematic review. Eur J Cardiothorac Surg. (2009) 35(6):1056–62. 10.1016/j.ejcts.2009.01.053 [DOI] [PubMed] [Google Scholar]
- 3.Fiacchini G, Tricò D, Ribechini A, Forfori F, Brogi E, Lucchi M, et al. Evaluation of the incidence and potential mechanisms of tracheal complications in patients with COVID-19. JAMA Otolaryngol Head Neck Surg. (2021) 147(1):70. 10.1001/jamaoto.2020.4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prokakis C, Koletsis EN, Dedeilias P, Fligou F, Filos K, Dougenis D. Airway trauma: a review on epidemiology, mechanisms of injury, diagnosis and treatment. J Cardiothorac Surg. (2014) 9:117. 10.1186/1749-8090-9-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Massard G, Rougé C, Dabbagh A, Kessler R, Hentz JG, Roeslin N, et al. Tracheobronchial lacerations after intubation and tracheostomy. Ann Thorac Surg. (1996) 61(5):1483–7. 10.1016/0003-4975(96)00083-5 [DOI] [PubMed] [Google Scholar]
- 6.Trottier SJ, Hazard PB, Sakabu SA, Levine JH, Troop BR, Thompson JA, et al. Posterior tracheal wall perforation during percutaneous dilational tracheostomy: an investigation into its mechanism and prevention. Chest. (1999) 115(5):1383–9. 10.1378/chest.115.5.1383 [DOI] [PubMed] [Google Scholar]
- 7.Misak VB, Beraković AP, Vukusić I, Kogler J, Pazanin L, Ozegović SO. Postintubation tracheal injuries–case series and literature review. Acta Clin Croat. (2012) 51(3):467–71. PMID: [PubMed] [Google Scholar]
- 8.Cardillo G, Carbone L, Carleo F, Batzella S, Jacono Rd, Lucantoni G, et al. Tracheal lacerations after endotracheal intubation: a proposed morphological classification to guide non-surgical treatment. Eur J Cardiothorac Surg. (2010) 37(3):581–7. 10.1016/j.ejcts.2009.07.034 [DOI] [PubMed] [Google Scholar]
- 9.Jougon J, Ballester M, Choukroun E, Dubrez J, Reboul G, Velly JF. Conservative treatment for postintubation tracheobronchial rupture. Ann Thorac Surg. (2000) 69(1):216–20. 10.1016/S0003-4975(99)01129-7 [DOI] [PubMed] [Google Scholar]
- 10.Singh P, Wojnar M, Malhotra A. Iatrogenic tracheal laceration in the setting of chronic steroids. J Clin Anesth. (2017) 37:38–42. 10.1016/j.jclinane.2016.10.043 [DOI] [PubMed] [Google Scholar]
- 11.Farzanegan R, Alijanipour P, Akbarshahi H, Abbasidezfouli A, Pejhan S, Daneshvar A, et al. Major airways trauma, management and long term results. Ann Thorac Cardiovasc Surg. (2011) 17(6):544–51. 10.5761/atcs.oa.11.01679 [DOI] [PubMed] [Google Scholar]
- 12.Grewal HS, Dangayach NS, Ahmad U, Ghosh S, Gildea T, Mehta AC. Treatment of tracheobronchial injuries: a contemporary review. Chest. (2019) 155(3):595–604. 10.1016/j.chest.2018.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conti M, Fournier C, Hysi I, Ramon PP, Wurtz A. Conservative management of postintubation tracheal membrane ruptures. Intensive Care Med. (2010) 36(9):1622–3. 10.1007/s00134-010-1906-5 [DOI] [PubMed] [Google Scholar]
- 14.Boutros J, Marquette CH, Ichai C, Leroy S, Benzaquen J. Multidisciplinary management of tracheobronchial injury. Eur Respir Rev. (2022) 31(163):210126. 10.1183/16000617.0126-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marty-Ané CH, Picard E, Jonquet O, Mary H. Membranous tracheal rupture after endotracheal intubation. Ann Thorac Surg. (1995) 60(5):1367–71. 10.1016/0003-4975(95)00643-Y [DOI] [PubMed] [Google Scholar]
- 16.Cardillo G, Ricciardi S, Forcione AR, Carbone L, Carleo F, di Martino M, et al. Post-intubation tracheal lacerations: risk-stratification and treatment protocol according to morphological classification. Front Surg. (2022) 9:1049126. 10.3389/fsurg.2022.1049126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mussi A, Ambrogi MC, Ribechini A, Lucchi M, Menoni F, Angeletti CA. Acute major airway injuries: clinical features and management. Eur J Cardiothorac Surg. (2001) 20(1):46–51. discussion 51-2. 10.1016/S1010-7940(01)00702-3 [DOI] [PubMed] [Google Scholar]
- 18.Koletsis E, Prokakis C, Baltayiannis N, Apostolakis E, Chatzimichalis A, Dougenis D. Surgical decision making in tracheobronchial injuries on the basis of clinical evidences and the injury's anatomical setting: a retrospective analysis. Injury. (2012) 43(9):1437–41. 10.1016/j.injury.2010.08.038 [DOI] [PubMed] [Google Scholar]
- 19.Barnhart GR, Brooks JW, Kellum JM. Pneumoperitoneum resulting from tracheal rupture following blunt chest trauma. The journal of trauma: injury. Infect Crit Care. (1986) 26(5):486–8. 10.1097/00005373-198605000-00015 [DOI] [PubMed] [Google Scholar]
- 20.Chen JD, Shanmuganathan K, Mirvis SE, Killeen KL, Dutton RP. Using CT to diagnose tracheal rupture. AJR Am J Roentgenol. (2001) 176(5):1273–80. 10.2214/ajr.176.5.1761273 [DOI] [PubMed] [Google Scholar]
- 21.Palas J, Matos AP, Mascarenhas V, Herédia V, Ramalho M. Multidetector computer tomography: evaluation of blunt chest trauma in adults. Radiol Res Pract. (2014) 2014:864369. 10.1155/2014/864369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deja M, Menk M, Heidenhain C, Spies CD, Heymann A, Weidemann H, et al. Strategies for diagnosis and treatment of iatrogenic tracheal ruptures. Minerva Anestesiol. (2011) 77(12):1155–66. [PubMed] [Google Scholar]
- 23.Exarhos DN, Malagari K, Tsatalou EG, Benakis SV, Peppas C, Kotanidou A, et al. Acute mediastinitis: spectrum of computed tomography findings. Eur Radiol. (2005) 15(8):1569–74. 10.1007/s00330-004-2538-3 [DOI] [PubMed] [Google Scholar]
- 24.Scaglione M, Romano S, Pinto A, Sparano A, Scialpi M, Rotondo A. Acute tracheobronchial injuries: impact of imaging on diagnosis and management implications. Eur J Radiol. (2006) 59(3):336–43. 10.1016/j.ejrad.2006.04.026 [DOI] [PubMed] [Google Scholar]
- 25.Tack D, Defrance P, Delcour C, Gevenois PA. The CT fallen-lung sign. Eur Radiol. (2000) 10(5):719–21. 10.1007/s003300050992 [DOI] [PubMed] [Google Scholar]
- 26.Gray ND, Miller K, Brath L, Shepherd W. Tracheal ring herniation. J Bronchology Interv Pulmonol. (2010) 17(1):54–5. 10.1097/LBR.0b013e3181c7fe9c [DOI] [PubMed] [Google Scholar]
- 27.Ross HM, Grant FJ, Wilson RS, Burt ME. Nonoperative management of tracheal laceration during endotracheal intubation. Ann Thorac Surg. (1997) 63(1):240–2. 10.1016/S0003-4975(96)01077-6 [DOI] [PubMed] [Google Scholar]
- 28.Grillo HC. Surgical treatment of postintubation tracheal injuries. J Thorac Cardiovasc Surg. (1979) 78(6):860–75. 10.1016/S0022-5223(19)38030-4 [DOI] [PubMed] [Google Scholar]
- 29.Gómez-Caro Andrés A, Moradiellos Díez FJ, Ausín Herrero P, Díaz-Hellín Gude V, Larrú Cabrero E, de Miguel Porch E, et al. Successful conservative management in iatrogenic tracheobronchial injury. Ann Thorac Surg. (2005) 79(6):1872–8. 10.1016/j.athoracsur.2004.10.006 [DOI] [PubMed] [Google Scholar]
- 30.Sippel M, Putensen C, Hirner A, Wolff M. Tracheal rupture after endotracheal intubation: experience with management in 13 cases. Thorac Cardiovasc Surg. (2006) 54(1):51–6. 10.1055/s-2005-865917 [DOI] [PubMed] [Google Scholar]
- 31.Herrmann D, Volmerig J, Al-Turki A, Braun M, Herrmann A, Ewig S, et al. Does less surgical trauma result in better outcome in management of iatrogenic tracheobronchial laceration? J Thorac Dis. (2019) 11(11):4772–81. 10.21037/jtd.2019.10.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiorelli A, Cascone R, di Natale D, Pierdiluca M, Mastromarino R, Natale G, et al. Endoscopic treatment with fibrin glue of post-intubation tracheal laceration. J Vis Surg. (2017) 3:102. 10.21037/jovs.2017.06.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tazi-Mezalek R, Musani AI, Laroumagne S, Astoul PJ, D’Journo XB, Thomas PA, et al. Airway stenting in the management of iatrogenic tracheal injuries: 10-year experience. Respirology. (2016) 21(8):1452–8. 10.1111/resp.12853 [DOI] [PubMed] [Google Scholar]
- 34.Hussein E, Pathak V, Shepherd RW, Shojaee S. Bronchoscopic management of iatrogenic tracheal laceration using polyurethane-covered nitinol tracheal stents. J Trauma Acute Care Surg. (2016) 81(5):979–83. 10.1097/TA.0000000000001233 [DOI] [PubMed] [Google Scholar]
- 35.Carretta A, Melloni G, Bandiera A, Negri G, Voci C, Zannini P. Conservative and surgical treatment of acute posttraumatic tracheobronchial injuries. World J Surg. (2011) 35(11):2568–74. 10.1007/s00268-011-1227-z [DOI] [PubMed] [Google Scholar]
- 36.da Silva Costa A, Juliano Perfeito JA, Succi JE, Villaça Leão LE, Rymkiewicz E, da Matta CAS, et al. A video-assisted endotracheal suture technique for correction of distal tracheal laceration after intubation. Ann Thorac Surg. (2012) 93(6):2073–5. 10.1016/j.athoracsur.2011.11.018 [DOI] [PubMed] [Google Scholar]
- 37.Welter S, Krbek T, Halder R, Stamatis G. A new technique for complete intraluminal repair of iatrogenic posterior tracheal lacerations. Interact Cardiovasc Thorac Surg. (2011) 12(1):6–9. 10.1510/icvts.2010.248641 [DOI] [PubMed] [Google Scholar]
- 38.Mussi A, Ambrogi MC, Menconi G, Ribechini A, Angeletti CA. Surgical approaches to membranous tracheal wall lacerations. J Thorac Cardiovasc Surg. (2000) 120(1):115–8. 10.1067/mtc.2000.107122 [DOI] [PubMed] [Google Scholar]
- 39.Borasio P, Ardissone F, Chiampo G. Post-intubation tracheal rupture. A report on ten cases. Eur J Cardiothorac Surg. (1997) 12(1):98–100. 10.1016/S1010-7940(97)00111-5 [DOI] [PubMed] [Google Scholar]
- 40.Marquette CH, Bocquillon N, Roumilhac D, Nevière R, Mathieu D, Ramon P. Conservative treatment of tracheal rupture. J Thorac Cardiovasc Surg. (1999) 117(2):399–401. 10.1016/S0022-5223(99)70443-5 [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto S, Endo S, Endo T, Mitsuda S. Successful silicon stent for life-threatening tracheal wall laceration. Ann Thorac Cardiovasc Surg. (2013) 19(1):49–51. 10.5761/atcs.cr.11.01768 [DOI] [PubMed] [Google Scholar]
- 42.Son BS, Cho WH, Kim CW, Cho HM, Kim SH, Lee SK, et al. Conservative extracorporeal membrane oxygenation treatment in a tracheal injury: a case report. J Cardiothorac Surg. (2015) 10:48. 10.1186/s13019-015-0252-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conti M, Pougeoise M, Wurtz A, Porte H, Fourrier F, Ramon P, et al. Management of postintubation tracheobronchial ruptures. Chest. (2006) 130(2):412–8. 10.1378/chest.130.2.412 [DOI] [PubMed] [Google Scholar]
- 44.Fruchter O, Raviv Y, Fox BD, Kramer MR. Removal of metallic tracheobronchial stents in lung transplantation with flexible bronchoscopy. J Cardiothorac Surg. (2010) 5:72. 10.1186/1749-8090-5-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mughal MM, Gildea TR, Murthy S, Pettersson G, DeCamp M, Mehta AC. Short-term deployment of self-expanding metallic stents facilitates healing of bronchial dehiscence. Am J Respir Crit Care Med. (2005) 172(6):768–71. 10.1164/rccm.200410-1388OC [DOI] [PubMed] [Google Scholar]
- 46.Murthy SC, Gildea TR, Mehta AC. Removal of self-expandable metallic stents: is it possible? Semin Respir Crit Care Med. (2004) 25(4):381–5. 10.1055/s-2004-832711 [DOI] [PubMed] [Google Scholar]
- 47.Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc. (2009) 6(1):79–93. 10.1513/pats.200808-094GO [DOI] [PubMed] [Google Scholar]
- 48.Bottero S, Meucci D, Trozzi M, Carotti A. Dehiscence of bronchial anastomosis after lung transplantation: a successful unconventional treatment. Ann Thorac Surg. (2018) 106(2):e81–3. 10.1016/j.athoracsur.2018.02.058 [DOI] [PubMed] [Google Scholar]
- 49.Marchese R, Mercadante S, Paglino G, Agozzino C. Villari P, di giacomo G. Tracheal stent to repair tracheal laceration after a double-lumen intubation. Ann Thorac Surg. (2012) 94(3):1001–3. 10.1016/j.athoracsur.2011.12.080 [DOI] [PubMed] [Google Scholar]
- 50.Gabor S, Renner H, Pinter H, Sankin O, Maier A, Tomaselli F, et al. Indications for surgery in tracheobronchial ruptures. Eur J Cardiothorac Surg. (2001) 20(2):399–404. 10.1016/S1010-7940(01)00798-9 [DOI] [PubMed] [Google Scholar]
- 51.Angelillo-Mackinlay T. Transcervical repair of distal membranous tracheal laceration. Ann Thorac Surg. (1995) 59(2):531–2. 10.1016/0003-4975(94)00882-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.