Abstract
Percutaneous coronary intervention and acute coronary syndrome are both closely tied to the frequently occurring complication of coronary microembolization (CME). Resveratrol (RES) has been shown to have a substantial cardioprotective influence in a variety of cardiac diseases, though its function and potential mechanistic involvement in CME are still unclear. The forty Sprague–Dawley rats were divided into four groups randomly: CME, CME + RES (25 mg/kg), CME + RES (50 mg/kg), and sham (10 rats per group). The CME model was developed. Echocardiography, levels of myocardial injury markers in the serum, and histopathology of the myocardium were used to assess the function of the cardiac muscle. For the detection of the signaling of TLR4/MyD88/NF-κB along with the expression of pyroptosis-related molecules, ELISA, qRT-PCR, immunofluorescence, and Western blotting were used, among other techniques. The findings revealed that myocardial injury and pyroptosis occurred in the myocardium following CME, with a decreased function of cardiac, increased levels of serum myocardial injury markers, increased area of micro-infarct, as well as a rise in the expression levels of pyroptosis-related molecules. In addition to this, pretreatment with resveratrol reduced the severity of myocardial injury after CME by improving cardiac dysfunction, decreasing serum myocardial injury markers, decreasing microinfarct area, and decreasing cardiomyocyte pyroptosis, primarily by blocking the signaling of TLR4/MyD88/NF-κB and also reducing the NLRP3 inflammasome activation. Resveratrol may be able to alleviate CME-induced myocardial pyroptosis and cardiac dysfunction by impeding the activation of NLRP3 inflammasome and the signaling pathway of TLR4/MyD88/NF-κB.
Keywords: Coronary microembolization, NLRP3, Pyroptosis, Resveratrol, TLR4/MyD88/NF-κB
INTRODUCTION
When an atherosclerotic plaque spontaneously ruptures or percutaneous coronary intervention (PCI) is performed, coronary microembolization (CME) and myocardial microinfarction may occur [1,2]. CME frequently results in cardiac dysfunction, including regional myocardial inflammation, myocardial microinfarction, cardiomyocyte necrosis and apoptosis, and a decrease in coronary flow reserve. Consequently, CME is generally considered a significant cause leading the way to myocardial injury and cardiac dysfunction. CME is closely tied to an increased risk of coronary artery diseases [3-5]. As a result, the treatment of CME is still a difficult problem to solve, and the molecular mechanisms regulating the pathophysiology of CME have yet to be discovered [6,7].
Pyroptosis is a type of inflammatory-mediated cellular death, triggered by caspase-1 activation downstream of the cell death pathway. It eventually leads to the rupture of cells and the liberation of pro-inflammatory cytokines [8,9]. Cell death due to pyroptosis is strictly controlled and associated with a high risk of advancing towards many cardiovascular ailments, including coronary artery disease [10-12]. As a result, subduing pyroptosis of cardiomyocytes could be a promising therapeutic objective for CME. Nevertheless, the underlying mechanical controls mediating cardiomyocyte pyroptosis within the CME context are still not completely comprehended.
Toll-like receptors (TLRs) belong to the category of pattern recognition receptors and are critical components of the immune system's inherent response to pathogens [13]. The inflammatory response within the myocardium is regulated by the foremost member of the TLR family to be discovered, namely toll-like receptor 4 (TLR4). Furthermore, the inflammatory signaling pathway that it mediates, is important in the development of myocardial infarction, myocarditis, and ischemia-reperfusion injury [14-16]. The signaling of TLR4/myeloid differentiation factor 88 (MyD88)/nuclear factor-kappa B (NF-κB) has been demonstrated in several studies to regulate the generation of pro-inflammatory factors and induce the inflammatory reaction within the tissues of the myocardial, precisely the primary source of myocardial tissue injury [17,18]. An earlier work demonstrated that local inflammation of the myocardial induced by CME is the critical link in the chain that leads to injury to the myocardium and progressive cardiac dysfunction [19]. Furthermore, it was discovered that following CME, TLR4 expression is increased, which stimulates the activation of the adaptive immune responses by activating the signaling pathway of the NF-κB, which is controlled through the dependent linker protein MYD88 [20]. Because of the triggering of the TLR4 receptor and NF-κB, some endogenous ligands caused the release of inflammatory factors such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), among others. Furthermore, the release and elevated production of inflammatory factors activate NF-κB, which subsequently activates nod-like receptor protein 3 (NLRP3) inflammatory bodies. Consequently, the early inflammatory signals were continuously amplified, leading to a cascade-like inflammation reaction and consequently a myocardial injury resulting from this phenomenon. Specific inhibitors of the TLR4 signaling pathway were found to significantly reduce the injury of CME-induced myocardial while also improving the function of the cardiac [21]. As a result, we assume that hindering the signaling pathway of TLR4/MyD88/NF-κB might improve cardiac activity following CME while also alleviating myocardial inflammation in the heart.
Resveratrol (RES), a molecule of polyphenolic origin, detected in many plants, has many medicinal properties [22]. According to recent cardiovascular disease studies, resveratrol has many cardiovascular protective effects, for instance, antioxidative stress [23], suppression of platelet aggregation [24], modulation of blood lipids [25], anti-inflammatory [26], and anti-myocardial ischemia-reperfusion injury [27]. Nonetheless, there is little known about resveratrol's cardioprotective properties and putative molecular processes in the treatment of CME-induced myocardial damage. Furthermore, resveratrol has been shown to reduce inflammation caused by cardiac ischemia-reperfusion injury via the signaling pathway of TLR4/NF-κB in a prior study. The preventive benefits of resveratrol are linked to an increase in nitrogen monoxide generation, a reduction in neutrophil infiltration, and a reduction in TNF-α production [28]. Resveratrol also protected against ischemia-induced mice cardiac injury and hypoxia-induced neonatal rat cardiomyocytes (NRCMs) injury in vitro through modulating Sirt1/p53-mediated cell senescence and decreasing NLRP3-mediated inflammasome activation, according to another study [29]. Consequently, this work aimed to investigate if resveratrol administration reduced CME-induced myocardial damage and pyroptosis by hindering the signaling cascade of TLR4/MyD88/NF-κB.
METHODS
Animal preparation
The Clinical and Animal Research Ethics Committee of Guangxi Medical University granted permission to use the animals (application no: 202006015), and all procedures followed the National Institutes of Health Guidelines for utilization of Laboratory Animals. As a result, the Experimental Animal Center of Guangxi Medical University purchased 40 male Sprague–Dawley (SD) rats, all 8-week-old, healthy and having a weight ranging between 250–300 g. All of the rats were later placed in an enclosed area with access to rat chow and tap water. Additionally, the temperature was kept at 23°C, and the level of humidity was maintained at 50%–60%, with a 12-h dark cycle and 12-h light.
Animal groups and CME model establishment
The 40 SD rats were randomly placed within one of four categories, each with ten individuals: sham, CME, CME + RES (25 mg/kg), and CME + RES (50 mg/kg). Before generating CME, rats in the CME + RES (25 mg/kg) and CME + RES (50 mg/kg) groups were given resveratrol (Sigma Chemical Co) at a dosage of 25 mg/kg or 50 mg/kg per day by gavage for 7 days. Previous studies were used to determine the resveratrol dose and duration [30]. Following a protocol published earlier by Mao et al. [31] the rats were given 30–40 mg/kg of pentobarbital sodium intraperitoneally for anesthetizing them. To enhance aerobic respiration, the rats were subjected to incubating and ventilating with a tiny animal ventilator. A thoracotomy was then carried out at the left margin of the sternum in the fourth and third intercostal spaces. The ascending aorta was then entirely separated and exposed before being clamped for about 10 sec implementing a vascular clamp. An injection comprising three thousand 42 μm diameter polyethylene microspheres (BioSphere Medical Inc.) suspended within physiological saline (0.1 ml) was given into the rat cardiac apex in the CME, CME + RES (50 mg/kg), and CME + RES (25 mg/kg) groups simultaneously but fast. After normal breathing and heart rate had returned, the suturation of the chest was gently performed and the tube of endotracheal was withdrawn. Following that, intraperitoneal delivery of 800,000 IU of penicillin was made. The rats placed within the sham group were injected with physiological saline (0.1 ml) in place of microspheres and underwent identical surgical and experimental procedures. Finally, 12 h following the operation, all of the rats in the four groups were sacrificed.
Evaluation of rat cardiac function
Cardiac function in the animals involved in experiments is frequently at its weakest, 12 h following the modeling of CME, according to prior investigations [32]. As a result, heart function was measured simultaneously in the current investigation. The left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS), left ventricular end-diastolic diameter (LVEDd), and left ventricular end-systolic diameter (LVESd) were all estimated by employing a 12 MHz transducer on a Hewlett Packard Sonos 7500 ultrasound device (Philips Technologies). In addition, the average of the above characteristics was estimated over three cardiac cycles, and the echocardiographic readings were taken separately by an expert.
Tissue collection and sample processing
All rats were given an intraperitoneal anesthetic overdose of pentobarbitone sodium (60 mg/kg) following 12 h of CME modeling and heart function measurements. Blood was taken from each rat through the abdominal aorta before being sacrificed to test serum myocardial damage markers. Following the cardiac arrest, the heart tissue was rapidly taken and split into three sections: apex, middle, and bottom parallel to the atrioventricular sulcus. The tips and centre sections were then frozen at –80°C for respective utilization in RT-qPCR and Western blot tests. At last, the bottom of the cardiac was fixed in paraffin and serially sliced into 4 μm slices for hematoxylin-basic fuchsin-picric acid (HBFP) and hematoxylin and eosin (H&E) staining.
Estimation of serum myocardial injury markers and inflammatory cytokines
Before sacrifice, blood specimens were taken from the abdominal aorta of rats in individual groups 12 h following the sham operation or CME induction. The levels of serum cardiac troponin I (cTnI), IL-1β, and IL-18 were then measured using a commonly utilized kit of enzyme-linked immunosorbent assay (ELISA) (Bio-Swamp Biological Technology Co., Ltd) in accordance with the guideline pre-defined by the manufacturer. In addition, the levels of creatine kinase myocardial band isoenzyme (CK-MB) and lactate dehydrogenase (LDH) were expressed using an automated biochemical analyzer (Olympus 5400; Olympus Ltd.).
ATP assay
Using the instructions given by the manufacturer, the level of ATP within the tissues of the heart was measured using a commercial ATP detection kit (Solarbio). ATP lysis buffer (1 ml) was used to mince and homogenize 0.1 g tissues from the center of the heart. The samples were then centrifugated for 10 min at 8,000× g at 4°C, followed by the collection of the supernatants for further analysis. Then, in a 96-well plate, 20 μl supernatant from each rat was added along with the ATP working solution, and the level of ATP was estimated by employing a microplate reader. The OD value was then determined at 340 nm. Finally, total ATP levels were expressed in nanomoles per milligram of protein.
Electron microscopic analysis of heart tissues
Twelve hours following the operation, the rats were sacrificed. The specimens of myocardial tissue were sliced into 1 mm3 sections and preserved overnight at 4°C with 3% glutaraldehyde. Phosphate-buffered saline (PBS) (pH = 7.4) was subsequently employed to rinse the pieces thrice before being post-fixed for two hours in 1% osmium tetroxide. The specimens were then rinsed multiple times with PBS before being dehydrated using a series of graded ethanol (50%–100%). Samples were then fixed in resin, followed by slicing into ultrathin sections (50 nm). Later they were stained using lead citrate and uranyl acetate inspected and imaged by implementing an electron microscope of Hitachi H-7650 (Hitachi).
Measurement of myocardial microinfarct size
HBFP staining was employed for the detection of myocardial microinfarct areas in this investigation. Normal myocardiocytes had their cytoplasm colored yellow and their nuclei labeled blue, whereas ischemia myocardium and erythrocytes had their cytoplasm and nuclei stained red. Five non-overlapping fields were haphazardly picked from each region to compute the infarction area by utilizing a pathological image analyzer of DMR-Q550 (Leica). The proportion of infarction area over the entire observed area was used to calculate the percentage of infarction size.
Immunofluorescence (IF) staining
Rats were sacrificed after 12 hours of CME modeling. IF staining was performed on sections with a thickness of 4 μm. The manufacturer's instructions for IF staining were followed. The slices were rinsed three times in PBS (pH 7.4) and blocked at the temperature of the room for half an hour in 3% bovine serum albumin. After blocking, segments were treated by taking advantage of primary antibodies during the night hours at 4°C. Segments were rinsed by applying PBS five times after incubating with primary antibodies and later treated using fluorescent secondary antibodies at room temperature for 50 min. This was followed by counterstaining of the nuclei for 7 min with 4′,6-diamidino-2-phenylindole. A fluorescent microscope was used to capture the photographs (Olympus Ltd.).
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from cardiac tissue by employing the TRIzol reagent (Invitrogen) as directed by the manufacturer, and the concentration was determined by making use of the NanoDrop (Thermo Fisher Scientific Inc.). After that, the PrimeScript RT reagent Kit with a gDNA Eraser (Perfect Real Time) was used to reverse transcribe mRNA into complementary DNA. Additionally, qRT-PCR was conducted on the ABI PRISM 7500 system (Applied Biosystems) using the TB Green Premix Ex Taq II (Tli RNaseH Plus) (TaKaRa). The 2–∆∆Ct method was subsequently employed for calculating the relative expression levels of the target genes. The endogenous control employed was glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Table 1 lists all primer sequences (TaKaRa).
Table 1.
Sequences of the used primers
| Gene | Primer sequence |
|---|---|
| NLRP3 | Forward: 5′-CTCGCATTGGTTCTGAGCTC-3′ |
| Reverse: 5′-AGTAAGGCCGGAATTCACCA-3′ | |
| TLR4 | Forward: 5′-TATCGGTGGTCAGTGTGCTT-3′ |
| Reverse: 5′-CTCGTTTCTCACCCAGTCCT-3′ | |
| GAPDH | Forward: 5′-TGTGAACGGATTTGGCCGTA-3′ |
| Reverse: 5′-GATGGTGATGGGTTTCCCGT-3′ |
NLRP3, nod-like receptor protein 3; TLR4, toll-like receptor 4; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western blot analysis
A lysis buffer was utilized to extract total protein from the cardiac sample, and the concentration was determined by making use of an assessment kit of bicinchoninic acid (PC0020; Solarbio). The separation of proteins of equivalent quality was fulfilled utilizing 10%–15% sodium dodecyl sulphate-polyacrylamide gel electrophoresis after the concentration was determined, and then electrophoretically transferred onto polyvinylidene difluoride membranes (Millipore). Following the blocking with 5% fat-free milk for 1 h at room temperature, primary antibodies were used to incubate the membranes overnight at 4°C. Primary antibodies as follows: TLR4 (sc-293072, 1:1,000; Santa Cruz Biotechnology), p-NF-κB p65 (#3033, 1:1,000; Cell Signaling Technology), MyD88 (ab219413, 1:1,000; Abcam), Caspase-1 P20 (sc-398715, 1:1,000; Santa Cruz Biotechnology), ASC (ab180799, 1:1,000; Abcam), NLRP3 (ab214185, 1:1,000; Abcam), GSDMD (#39754, 1:1,000; Cell Signaling Technology), mature-IL-18 (ab191860, 1:1,000; Abcam), mature-IL-1β (ab200478, 1:1,000; Abcam), GAPDH (ab9485, 1:10,000; Abcam). After incubating overnight, the membranes were rinsed 5 times at room temperature by utilizing a 1×TBST buffer solution before being treated for 2 h with horseradish peroxidase-labeled secondary antibodies. Ultimately, employing an advanced detection system of chemiluminescence (Pierce), the protein bands belonging to rats in individual groups were detected and the expression levels were assessed by employing the ImageJ computer program (National Institutes of Health).
Statistical analysis
The outcomes were explained as mean ± standard deviation (SD) implementing the SPSS version 23.0 program (IBM Co.) for statistical analysis. Furthermore, the Student's t-test was applied for statistical investigation of the two considered groups, while the One-way Analysis of Variance (ANOVA) was employed for comparisons of multiple-group, succeeded by the analysis of Student-Newman-Keuls post-hoc. Statistical significance was described as a p-value < 0.05.
RESULTS
Resveratrol improved cardiac function following CME
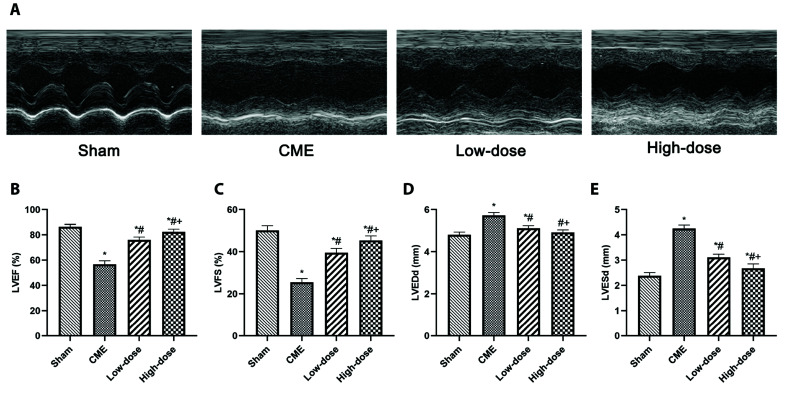
Fig. 1 shows the echocardiographic achievements of each group of rats. Compared to the sham group, the cardiac of CME group contractility was compromised, as evidenced by larger LVEDd and LVESd but lower LVFS and LVEF (p < 0.05). However, therapy with resveratrol significantly reduced cardiac dysfunction in a dose-dependent fashion, as demonstrated by low LVESd and LVEDd values but better LVFS and LVEF compared to the CME group (p < 0.05).
Fig. 1. Cardiac function-based indices of rats were measured by echocardiography.
(A) Representative M-mode images of each group. (B–E) LVEF, LVFS, LVEDd, and LVESd in each group (n = 8 per group). Low-dose: CME + low-dose RES (25 mg/kg), High-dose: CME + high-dose RES (50 mg/kg). Data were presented as the mean ± SD. LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening; LVEDd, left ventricular end-diastolic diameter; LVESd, left ventricular end-systolic diameter; CME, coronary microembolization; RES, resveratrol. *p < 0.05, compared with Sham group; #p < 0.05, compared with CME group; +p < 0.05, compared with Low-dose group.
Resveratrol diminished the serum levels of myocardial injury markers
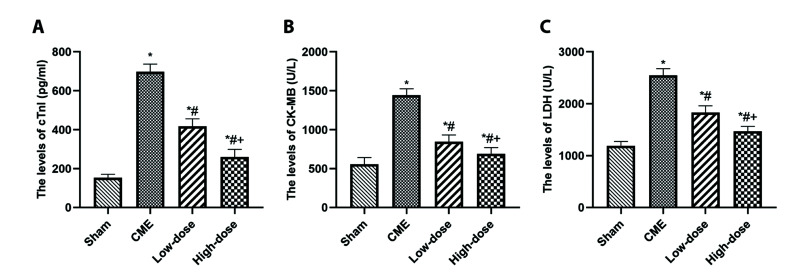
The blood concentrations of cTnI, CK-MB, and LDH were used to determine myocardial damage following CME induction (Fig. 2). The rats in the CME group had substantially greater concentrations of serums, namely cTnI, CK-MB, and LDH than those within the sham category, according to the findings. Pretreatment with resveratrol, on the other hand, significantly reduced myocardial injury following CME in a dose-dependent manner, as seen by lower concentrations of serum including cTnI, CK-MB, and LDH compared to the CME group (p < 0.05).
Fig. 2. Resveratrol reduced the serum levels of myocardial injury markers.
(A) cTnI levels in serum. (B) CK-MB levels in serum. (C) LDH levels in serum (n = 8 per group). Low-dose: CME + low-dose RES (25 mg/kg), High-dose: CME + high-dose RES (50 mg/kg). Data were presented as the mean ± SD. cTnI, cardiac troponin I; CK-MB, creatine kinase myocardial band isoenzyme; LDH, lactate dehydrogenase; CME, coronary microembolization; RES, resveratrol. *p < 0.05, compared with Sham group; #p < 0.05, compared with CME group; +p < 0.05, compared with Low-dose group.
CME histopathology
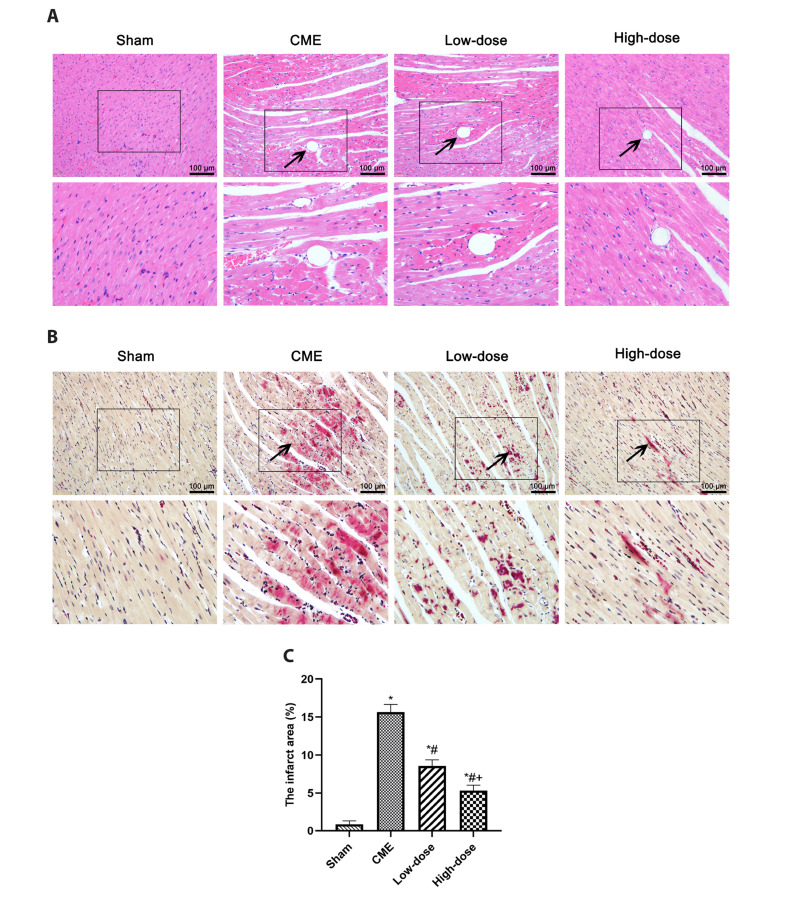
In the sham group, H&E staining revealed consistently aligned cardiac fibers with obvious staining and entire morphology (Fig. 3). The cytoplasm of the CME group was extensively stained by eosin after embolizing microspheres, and degeneration, loosening, odoema, hypertrophy, and also inflammatory cell infiltration were evident. Myocardial degeneration and odoema were reduced in the CME + RES (25 mg/kg) group, with fewer histomorphological anomalies and more consistent alignment. Furthermore, in the CME + RES (50 mg/kg) group, there was no myocardial degeneration or necrosis. The microinfarct lesions were more or less wedge-shaped, dispersed locally, and nontransmural, as revealed by HBFP staining (Fig. 3). They were predominantly found in the left ventricle and subendocardium. The infarct scale of the group of resveratrol administration was considerably tinier compared to the CME group (p < 0.05), suggesting that following CME induction in rats, the pre-exposure with resveratrol may have decreased the myocardial microinfarct zone. According to the findings, resveratrol pretreatment decreased the area of microinfarction after CME.
Fig. 3. Histopathological examination of myocardial tissues through H&E and HBFP staining.
(A) Representative H&E-stained myocardial sections from each group (×200 magnification; bar = 100 μm). The arrows indicate the microspheres. (B) Representative images of HBFP staining (×200 magnification; bar = 100 μm). The arrows indicate the microinfarct area. (C) The percentage of micro-infarct area in each group (n = 8 per group). Low-dose: CME + low-dose RES (25 mg/kg), High-dose: CME + high-dose RES (50 mg/kg). Data were presented as the mean ± SD. CME, coronary microembolization; RES, resveratrol. *p < 0.05, compared with Sham group; #p < 0.05, compared with CME group; +p < 0.05, compared with Low-dose group.
Pretreatment with resveratrol attenuated CME-induced mitochondrial injury
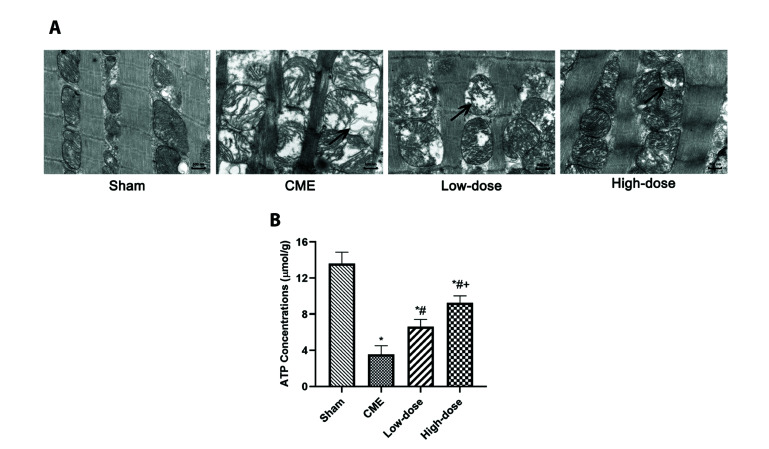
Because mitochondrial metabolism is a major source of ATP, we looked at ATP levels to see if they reflected mitochondrial function. After CME, ATP concentrations were shown to be significantly lower, which may be reversed by pretreatment with resveratrol (p < 0.05). Furthermore, transmission electron microscopy analysis of cardiac tissues revealed that the CME group's myocardial mitochondria had significantly vacuolated degeneration and ballooning. However, pretreatment with resveratrol significantly reduced the ultrastructural alterations seen in the CME group, while maintaining mitochondrial integrity (Fig. 4). This finding suggested that pretreatment with resveratrol can help restore mitochondrial function and mitigate mitochondrial damage.
Fig. 4. Resveratrol pretreatment attenuated CME-induced mitochondrial injury.
(A) Myocardial mitochondrial morphology observed by transmission electron microscopy (magnification ×60,000, scale bar = 500 nm). The black arrows indicate the typical vacuolated degeneration and enlarged mitochondria. (B) ATP concentrations of each group (n = 8 per group). Low-dose: CME + low-dose RES (25 mg/kg), High-dose: CME + high-dose RES (50 mg/kg). Data were presented as the mean ± SD. CME, coronary microembolization; RES, resveratrol. *p < 0.05, compared with Sham group; #p < 0.05, compared with CME group; +p < 0.05, compared with Low-dose group.
Resveratrol protects cardiomyocytes from CME-induced pyroptosis and inhibits NLRP3 inflammasome activation
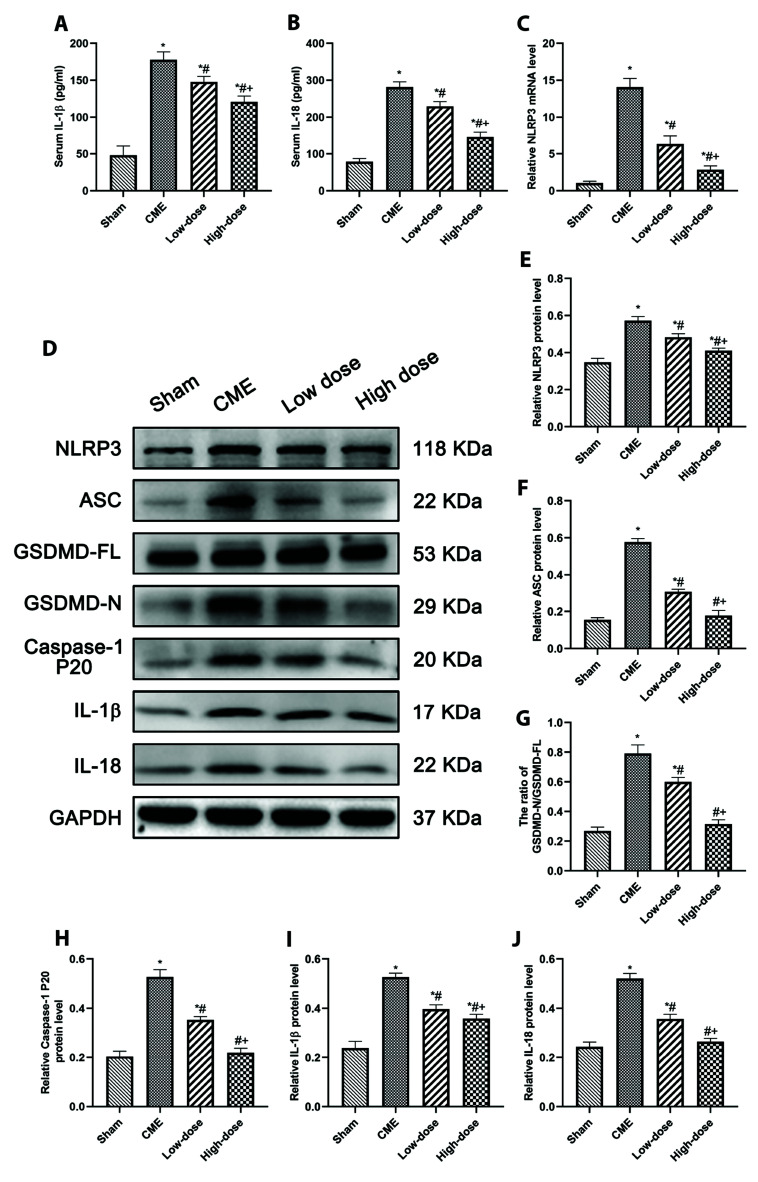
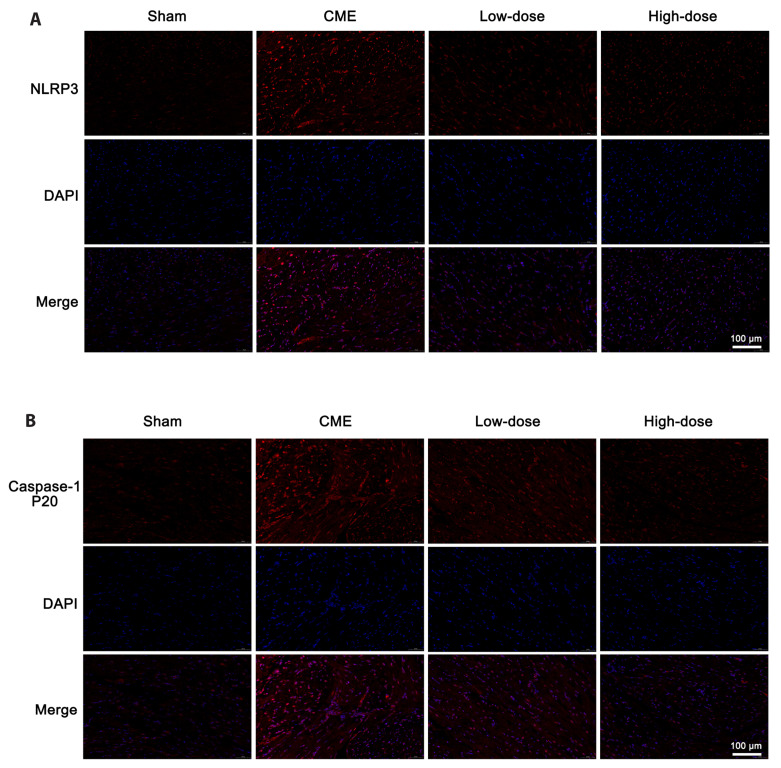
The effects of resveratrol on the expression of pyroptosis-associated molecules were studied to see how it affected myocardial pyroptosis following CME. The expression of IL-18 and IL-1β were substantially increased (p < 0.05) in the CME group, according to ELISA data (Fig. 5A, B). The CME group possessed a greater degree of mRNA expression of NLRP3 (p < 0.05) (Fig. 5C). Protein levels of pyroptosis-associated molecules also rose considerably (p < 0.05) (Fig. 5D). In addition, immunofluorescence results revealed that the expression of pyroptosis-associated molecules was increased in CME (Fig. 6). Notably, pretreatment with resveratrol greatly reduced these effects, indicating that resveratrol inhibits CME-induced pyroptotic cell death.
Fig. 5. Resveratrol attenuated CME-induced pyroptosis of cardiomyocytes.
(A, B) Serum levels of IL-1β and IL-18 in rats of each group (n = 8 per group). (C) mRNA levels of NLRP3 in each group (n = 8 per group). (D) Representative Western blot bands of NLRP3, ASC, caspase-1 p20, GSDMD-FL, GSDMD-N, IL-1β, and IL-18. (E–J) The relative expression levels of these proteins in each group. GAPDH was used for protein expression normalization (n = 3 per group). Low-dose: CME + low-dose RES (25 mg/kg), High-dose: CME + high-dose RES (50 mg/kg). Data were presented as the mean ± SD. IL, interleukin; NLRP3, nod-like receptor protein 3; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; CME, coronary microembolization; RES, resveratrol. *p < 0.05, compared with Sham group; #p < 0.05, compared with CME group; +p < 0.05, compared with Low-dose group.
Fig. 6. Immunofluorescence staining of NLRP3 and caspase-1 p20 in cardiac tissues.
(A) Representative NLRP3 immunofluorescence staining images of each group. (B) Representative caspase-1 p20 immunofluorescence staining images of each group (magnification ×400, scale bar = 100 μm). Low-dose: CME + low-dose RES (25 mg/kg), High-dose: CME + high-dose RES (50 mg/kg). NLRP3, nod-like receptor protein 3; CME, coronary microembolization; RES, resveratrol.
Resveratrol inhibits pyroptosis via the pathway of TLR4/MyD88/NF-κB
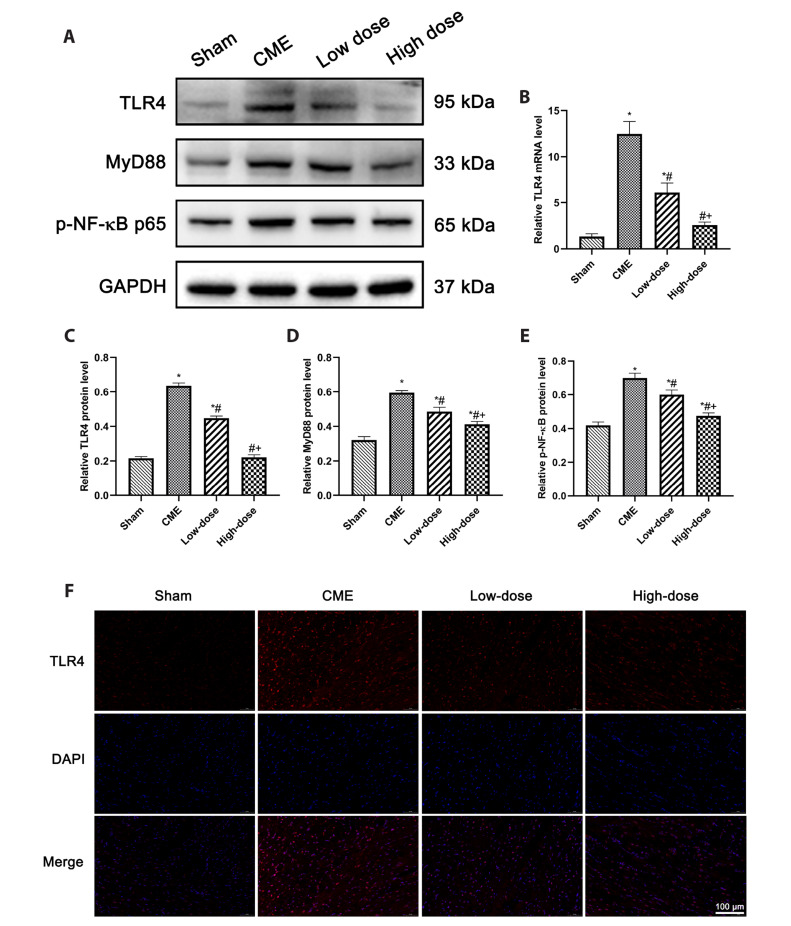
The levels of MyD88, p-NF-κB and TLR4 were measured to see if there was a link between resveratrol's suppression of cardiomyocyte pyroptosis induced through CME and the pathway of TLR4/MyD88/NF-κB. The protein levels of MyD88, p-NF-κB, and TLR4 were substantially higher within the CME group (p < 0.05) (Fig. 7A). TLR4 mRNA expression was observed to be higher (p < 0.05) in the CME group (Fig. 7B). TLR4 expression was also increased in CME, according to immunofluorescence data (Fig. 7F). Pretreatment with resveratrol dramatically reduced the expression levels of these compounds. These findings indicate that resveratrol pretreatment reduces CME-induced cardiomyocyte pyroptosis, possibly through modulating the signaling pathway of TLR4/MyD88/NF-κB.
Fig. 7. Resveratrol inhibited pyroptosis via the pathway of TLR4/MyD88/NF-κB.
(A) Representative Western blot bands of TLR4, MyD88, p-NF-κB p65. (B) The mRNA levels of TLR4 in each group (n = 8 per group). (C–E) Relative protein expression levels in each group. GAPDH was used for protein expression normalization (n = 3 per group). (F) Representative TLR4 immunofluorescence staining in each group (magnification ×400, scale bar = 100 μm). Low-dose: CME + low-dose RES (25 mg/kg), High-dose: CME + high-dose RES (50 mg/kg). Data were presented as the mean ± SD. TLR4, toll-like receptor 4; MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor-kappa B; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; CME, coronary microembolization; RES, resveratrol. *p < 0.05, compared with Sham group; #p < 0.05, compared with CME group; +p < 0.05, compared with Low-dose group.
DISCUSSION
Several new findings were made as a result of this investigation. To begin, we discovered that pyroptosis of cardiomyocyte is a critical pathophysiological episode in CME, leading to a significant loss of nonrenewable cardiomyocytes and subsequently cardiac failure. Secondly, resveratrol pretreatment might considerably enhance adenine nucleotide levels, shrink microinfarcts, and alleviate cardiac insufficiency. Resveratrol inhibited NLRP3 activation by blocking the signaling of TLR4/MyD88/NF-κB, which may be the mechanism. Furthermore, it inhibits pyroptotic cell death through decreasing the release of pro-inflammatory cytokines. Finally, our study provided the first evidence that resveratrol could prevent NLRP3 inflammasome-mediated cardiomyocyte pyroptosis during CME, potentially by preventing TLR4/MyD88/NF-κB signaling to reduce myocardial damage and CME-related cardiac dysfunction. As a result, pretreatment with resveratrol may be helpful in the management of myocardial injury caused by CME.
CME, quite distinctive from epicardial proximal vascular blockage, frequently occurs in unbalanced plaque rupture in ACS and after treatment with PCI. The area of the myocardium harmed by perfusion is not closely associated with left ventricular functional decline induced by CME [33]. This cannot be fully explained by inadequate local myocardial perfusion or minor infarcts. Other factors, besides lower blood flow, essentially contribute to myocardial injury after CME. The available evidence suggests that myocardial inflammatory response has an indispensable part in myocardial injury induced by CME. A number of animal investigations have revealed many inflammatory cell infiltrates near the CME-induced myocardial microinfarct, along with releasing inflammatory cytokines including TNF-α and IL-1β. This causes myocardial inflammation in the region that is necessary for gradual cardiac failure and progressive myocardial damage following CME [19]. Su et al. [20] discovered that cardiomyocyte pyroptosis, a kind of inflammatory programmed cell death mediated by the NLRP3 inflammasome, contributes to CME-induced cardiac injury. According to reports, NLRP3 stimulates pro-caspase-1 during the pathogenesis of CME by establishing an inflammatory complex with ASC, resulting in the death of cardiomyocytes. Following their cleavage by active caspase-1 to generate GSDMD-N, GSDMD, a pore-forming protein, undergoes activation, is transferred to the membrane of the cell, and subsequently brings about pyroptosis via triggering the release of mature interleukins form the membrane pores. The expression of pyroptosis-associated proteins (ASC, NLRP3, GSDMD, and Caspase-1 p20) and pro-inflammatory cytokines (IL-1β and IL-18) within the myocardium was considerably increased following CME in the current study and these findings are consistent with earlier research.
TLR4 was the foremost TLR-associated protein to be recognized, and it is found in the heart at the greatest levels. When the body is exposed to external stimuli, it first triggers the TLR4 expression and inherent immune response, then stimulates the obtained immunological response and activates NF-κB via the linker protein of MyD88 and the pathway of signal transduction. A number of endogenous ligands activate NF-κB via TLR4 receptors activation. As a result, pro-inflammatory factors, for instance, IL-1β and TNF-α, will be released. NF-κB is further activated by IL-1β and TNF-α, resulting in triggering the NLRP3 inflammatory bodies, the first amplification of the inflammatory trigger, and the initiation of an inflammatory chain reaction [34]. In earlier work, we discovered that the participation of CME in the inflammatory responses of the myocardium strongly triggered the signaling pathway of TLR4/MyD88/NF-κB, resulting in a loss of cardiac activity in rats and, as a result, myocardial damage. When the TLR4-specific inhibitor TAK-242 was employed, TLR4/MyD88/NF-κB was also significantly suppressed. Furthermore, NLRP3 inflammatory body activation was reduced, as was the expression of inflammatory elements including TNF-α, IL-18, and IL-1β, all of which have the potential of improving cardiac functions by lowering myocardial damage induced by CME [20]. Therefore, it is vital to research the role of the pathway of TLR4/Myd88/NF-κB in NLRP3 Inflammasome-mediated pyroptotic cell death in order to create medications that can efficiently minimize cardiomyocyte loss and improve the prognosis of CME patients.
Many plants, including peanuts, grapes, and Polygonum cuspidatum, contain resveratrol, which possesses anti-aging, antioxidation, anti-inflammatory, and anti-apoptosis characteristics, among others. On account of its extensive pharmacological attributes, diversified processes, and high levels of safety, it has the potential to be used in both cardiovascular and cerebrovascular illnesses [35]. Although there is no evidence of a link between resveratrol and cell pyroptosis, multiple investigations have shown that it has a protective influence on cardiovascular function. Resveratrol inhibits TLR4/NF-κB signaling, which reduces the inflammatory response generated by ischemia/reperfusion (I/R) damage, according to a prior study. Resveratrol inhibits neutrophil infiltration and TNF-α generation, reducing the inflammatory response generated by I/R damage [28]. In a prior investigation, resveratrol was found to protect against ischemia-induced cardiac injury in mice and hypoxia-induced NRCM damage in vitro via Sirt1/p53 regulation suppressing NLRP3-controlled inflammasome activation and NLRP3-mediated cell senescence [29]. We hypothesize that resveratrol inhibits CME-induced pyroptotic cell death since the core of pyroptosis is essentially inflammatory death. Furthermore, the participation of the pathway of TLR4/Myd88/NF-κB in NLRP3 Inflammasome-mediated pyroptosis cell death could be a possible mechanism.
After CME modeling, rats developed myocardial damage and left ventricular systolic dysfunction, as evidenced by increased cTnI, CK-MB, and LDH, as well as reduced LVEF and LVFS values along with elevated LVESd and LVEDd. The outcome suggests that the CME paradigm was successful in its implementation. Following that, we noticed that the expression of pyroptosis-related molecules was up-regulated in CME. We also looked at the expression of NLRP3, a critical protein involved in pyroptosis, and found that it was highly up-regulated in CME. This suggests that pyroptosis takes place in the course of CME pathogenesis. In the second study, we looked at how resveratrol pretreatment affected pyroptosis and heart function in CME rats. RES-pretreated rats manifested a lower degree of myocardial injury, relatively small infarct scale, enhanced function of cardiac, and diminished pyroptosis than the CME group, as evidenced by reduced levels of cTnI, CK-MB, and LDH, enhanced adenine nucleotides, elevated LVFS and LVEF, and diminished LVESd and LVEDd, and also downregulated expression of pyroptosis-related molecules. This shows that resveratrol reduces pyroptosis in myocardial cells and enhances cardiac function in CME animals. Finally, we looked at the chance of involvement TLR4/MyD88/NF-κB signaling pathway in resveratrol's protection against myocardial pyroptosis injury. We further discovered that resveratrol suppressed TLR4 expression, resulting in a decrease in the expression of TLR4's downstream target proteins. As a result, we conclude that resveratrol attenuates myocardial pyroptosis in CME-induced myocardial damage possibly by attenuating TLR4/MyD88/NF-κB signaling.
There are some drawbacks to this study. We started by inserting actual microembolic spheres into the coronary microvessels of rats to create a CME model. However, because this form of plastic microspheres lacks biological features, for instance, vascular, thrombotic, or inflammatory activity, the pathophysiological alterations in the CME model generated by this plastic material differ from the ones resulting from atherosclerotic plaque in the clinic. Secondly, the impact of resveratrol on CME-induced cardiomyocyte pyroptosis and its regulatory influence on the signaling pathway of TLR4/MyD88/NF-κB was investigated in the current research. However, using TLR4 inhibitors or agonists to investigate better the particular regulation mechanism of resveratrol on the pathway of TLR4/MyD88/NF-κB in CME is critical. Third, the molecular processes regulating cardiomyocyte pyroptosis are extremely complicated, and it is impossible to rule out the possibility that other atypical pyroptosis signaling pathways are implicated in CME-induced cardiac damage, which requires more investigation.
In conclusion, resveratrol reduces cardiomyocyte pyroptosis and improves the function of cardiac in CME rats, as revealed by the outcome of this study. The signaling pathway of TLR4/MyD88/NF-κB is involved in the underlying strategy, which could be linked to the activation of the NLRP3 Inflammasome. This could be one of the key mechanisms for using resveratrol as a preventive measure before PCI or as a therapy for ischemic heart disease to enhance prognosis. Overall, resveratrol is a promising treatment for ischemic heart disease that merits additional investigation.
ACKNOWLEDGEMENTS
None.
Funding Statement
FUNDING This study was supported by the National Natural Science Foundation of China (Grant No.81770346) and the Project for Innovative Research Team in Guangxi Natural Science Foundation (Grant No.2018GXNSFGA281006).
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Heusch G, Kleinbongard P, Böse D, Levkau B, Haude M, Schulz R, Erbel R. Coronary microembolization: from bedside to bench and back to bedside. Circulation. 2009;120:1822–1836. doi: 10.1161/CIRCULATIONAHA.109.888784. [DOI] [PubMed] [Google Scholar]
- 2.Bahrmann P, Werner GS, Heusch G, Ferrari M, Poerner TC, Voss A, Figulla HR. Detection of coronary microembolization by Doppler ultrasound in patients with stable angina pectoris undergoing elective percutaneous coronary interventions. Circulation. 2007;115:600–608. doi: 10.1161/CIRCULATIONAHA.106.660779. [DOI] [PubMed] [Google Scholar]
- 3.Su Q, Li L, Zhao J, Sun Y, Yang H. MiRNA expression profile of the myocardial tissue of pigs with coronary microembolization. Cell Physiol Biochem. 2017;43:1012–1024. doi: 10.1159/000481699. [DOI] [PubMed] [Google Scholar]
- 4.Su Q, Li L, Wang J, Zhou Y, Liu Y. Mechanism of programmed cell death factor 4/nuclear factor-κB signaling pathway in porcine coronary micro-embolization-induced cardiac dysfunction. Exp Biol Med (Maywood) 2015;240:1426–1433. doi: 10.1177/1535370215573400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thielmann M, Dörge H, Martin C, Belosjorow S, Schwanke U, van De Sand A, Konietzka I, Büchert A, Krüger A, Schulz R, Heusch G. Myocardial dysfunction with coronary microembolization: signal transduction through a sequence of nitric oxide, tumor necrosis factor-alpha, and sphingosine. Circ Res. 2002;90:807–813. doi: 10.1161/01.RES.0000014451.75415.36. [DOI] [PubMed] [Google Scholar]
- 6.Heusch G. The coronary circulation as a target of cardioprotection. Circ Res. 2016;118:1643–1658. doi: 10.1161/CIRCRESAHA.116.308640. [DOI] [PubMed] [Google Scholar]
- 7.Heusch G, Gersh BJ. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur Heart J. 2017;38:774–784. doi: 10.1093/eurheartj/ehw224. [DOI] [PubMed] [Google Scholar]
- 8.Samali A, Zhivotovsky B, Jones D, Nagata S, Orrenius S. Apoptosis: cell death defined by caspase activation. Cell Death Differ. 1999;6:495–496. doi: 10.1038/sj.cdd.4400520. [DOI] [PubMed] [Google Scholar]
- 9.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei Q, Yi T, Chen C. NF-κB-gasdermin D (GSDMD) axis couples oxidative stress and NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome-mediated cardiomyocyte pyroptosis following myocardial infarction. Med Sci Monit. 2018;24:6044–6052. doi: 10.12659/MSM.908529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng C, Duan F, Hu J, Luo B, Huang B, Lou X, Sun X, Li H, Zhang X, Yin S, Tan H. NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox Biol. 2020;34:101523. doi: 10.1016/j.redox.2020.101523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhaolin Z, Guohua L, Shiyuan W, Zuo W. Role of pyroptosis in cardiovascular disease. Cell Prolif. 2019;52:e12563. doi: 10.1111/cpr.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundberg AM, Ketelhuth DF, Johansson ME, Gerdes N, Liu S, Yamamoto M, Akira S, Hansson GK. Toll-like receptor 3 and 4 signalling through the TRIF and TRAM adaptors in haematopoietic cells promotes atherosclerosis. Cardiovasc Res. 2013;99:364–373. doi: 10.1093/cvr/cvt033. [DOI] [PubMed] [Google Scholar]
- 14.Lu M, Tang F, Zhang J, Luan A, Mei M, Xu C, Zhang S, Wang H, Maslov LN. Astragaloside IV attenuates injury caused by myocardial ischemia/reperfusion in rats via regulation of toll-like receptor 4/nuclear factor-κB signaling pathway. Phytother Res. 2015;29:599–606. doi: 10.1002/ptr.5297. [DOI] [PubMed] [Google Scholar]
- 15.Soraya H, Clanachan AS, Rameshrad M, Maleki-Dizaji N, Ghazi-Khansari M, Garjani A. Chronic treatment with metformin suppresses toll-like receptor 4 signaling and attenuates left ventricular dysfunction following myocardial infarction. Eur J Pharmacol. 2014;737:77–84. doi: 10.1016/j.ejphar.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Chimenti C, Verardo R, Scopelliti F, Grande C, Petrosillo N, Piselli P, De Paulis R, Frustaci A. Myocardial expression of Toll-like receptor 4 predicts the response to immunosuppressive therapy in patients with virus-negative chronic inflammatory cardiomyopathy. Eur J Heart Fail. 2017;19:915–925. doi: 10.1002/ejhf.796. [DOI] [PubMed] [Google Scholar]
- 17.Ma SR, Xie XW. NLRC5 deficiency promotes myocardial damage induced by high fat diet in mice through activating TLR4/NF-κB. Biomed Pharmacother. 2017;91:755–766. doi: 10.1016/j.biopha.2017.03.062. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Zhang J, Yu P, Chen M, Peng Q, Wang Z, Dong N. Remote ischaemic preconditioning and sevoflurane postconditioning synergistically protect rats from myocardial injury induced by ischemia and reperfusion partly via inhibition TLR4/MyD88/NF-κB signaling pathway. Cell Physiol Biochem. 2017;41:22–32. doi: 10.1159/000455815. [DOI] [PubMed] [Google Scholar]
- 19.Dörge H, Schulz R, Belosjorow S, Post H, van de Sand A, Konietzka I, Frede S, Hartung T, Vinten-Johansen J, Youker KA, Entman ML, Erbel R, Heusch G. Coronary microembolization: the role of TNF-alpha in contractile dysfunction. J Mol Cell Cardiol. 2002;34:51–62. doi: 10.1006/jmcc.2001.1489. [DOI] [PubMed] [Google Scholar]
- 20.Su Q, Li L, Sun Y, Yang H, Ye Z, Zhao J. Effects of the TLR4/Myd88/NF-κB signaling pathway on NLRP3 inflammasome in coronary microembolization-induced myocardial injury. Cell Physiol Biochem. 2018;47:1497–1508. doi: 10.1159/000490866. [DOI] [PubMed] [Google Scholar]
- 21.Wang XT, Lu YX, Sun YH, He WK, Liang JB, Li L. TAK-242 protects against apoptosis in coronary microembolization-induced myocardial injury in rats by suppressing TLR4/NF-κB signaling pathway. Cell Physiol Biochem. 2017;41:1675–1683. doi: 10.1159/000471248. [DOI] [PubMed] [Google Scholar]
- 22.Wiciński M, Socha M, Walczak M, Wódkiewicz E, Malinowski B, Rewerski S, Górski K, Pawlak-Osińska K. Beneficial effects of resveratrol administration-focus on potential biochemical mechanisms in cardiovascular conditions. Nutrients. 2018;10:1813. doi: 10.3390/nu10111813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Wang X, Zhang L, An H, Zao Z. Inhibitory effects of resveratrol on platelet activation induced by thromboxane A2 receptor agonist in human platelets. Am J Chin Med. 2011;39:145–159. doi: 10.1142/S0192415X11008713. [DOI] [PubMed] [Google Scholar]
- 25.Abbas AM. Cardioprotective effect of resveratrol analogue isorhapontigenin versus omega-3 fatty acids in isoproterenol-induced myocardial infarction in rats. J Physiol Biochem. 2016;72:469–484. doi: 10.1007/s13105-016-0494-4. [DOI] [PubMed] [Google Scholar]
- 26.Xu K, Liu XF, Ke ZQ, Yao Q, Guo S, Liu C. Resveratrol modulates apoptosis and autophagy induced by high glucose and palmitate in cardiac cells. Cell Physiol Biochem. 2018;46:2031–2040. doi: 10.1159/000489442. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Zhang Y, Zhu M, Zhang Q, Wang X, Wang Y, Zhang J, Li J, Yang L, Liu J, Liu F, Yang Y, Kang L, Shen Y, Qi Z. Resveratrol attenuates myocardial ischemia/reperfusion injury through up-regulation of vascular endothelial growth factor B. Free Radic Biol Med. 2016;101:1–9. doi: 10.1016/j.freeradbiomed.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Xie C, Zhuang J, Li H, Yao Y, Shao C, Wang H. Resveratrol attenuates inflammation in the rat heart subjected to ischemia-reperfusion: role of the TLR4/NF-κB signaling pathway. Mol Med Rep. 2015;11:1120–1126. doi: 10.3892/mmr.2014.2955. [DOI] [PubMed] [Google Scholar]
- 29.Feng H, Mou SQ, Li WJ, Zhang N, Zhou ZY, Ding W, Bian ZY, Liao HH. Resveratrol inhibits ischemia-induced myocardial senescence signals and NLRP3 inflammasome activation. Oxid Med Cell Longev. 2020;2020:2647807. doi: 10.1155/2020/2647807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong SW, Jung KH, Zheng HM, Lee HS, Suh JK, Park IS, Lee DH, Hong SS. The protective effect of resveratrol on dimethylnitrosamine-induced liver fibrosis in rats. Arch Pharm Res. 2010;33:601–609. doi: 10.1007/s12272-010-0415-y. [DOI] [PubMed] [Google Scholar]
- 31.Mao Q, Liang X, Wu Y, Lu Y. Resveratrol attenuates cardiomyocyte apoptosis in rats induced by coronary microembolization through SIRT1-mediated deacetylation of p53. J Cardiovasc Pharmacol Ther. 2019;24:551–558. doi: 10.1177/1074248419845916. [DOI] [PubMed] [Google Scholar]
- 32.Su Q, Lv X, Sun Y, Ye Z, Kong B, Qin Z. Role of TLR4/MyD88/NF-κB signaling pathway in coronary microembolization-induced myocardial injury prevented and treated with nicorandil. Biomed Pharmacother. 2018;106:776–784. doi: 10.1016/j.biopha.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Dörge H, Neumann T, Behrends M, Skyschally A, Schulz R, Kasper C, Erbel R, Heusch G. Perfusion-contraction mismatch with coronary microvascular obstruction: role of inflammation. Am J Physiol Heart Circ Physiol. 2000;279:H2587–H2592. doi: 10.1152/ajpheart.2000.279.6.H2587. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Du Q, Yang Y, Wang J, Dou S, Liu C, Duan J. The protective effect of Luteolin on myocardial ischemia/reperfusion (I/R) injury through TLR4/NF-κB/NLRP3 inflammasome pathway. Biomed Pharmacother. 2017;91:1042–1052. doi: 10.1016/j.biopha.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 35.Nakata R, Takahashi S, Inoue H. Recent advances in the study on resveratrol. Biol Pharm Bull. 2012;35:273–279. doi: 10.1248/bpb.35.273. [DOI] [PubMed] [Google Scholar]