Abstract
Viral encephalitis can lead to encephalopathy, epileptic activity, focal neurological deficits, and death. Prompt recognition and a high index of clinical suspicion can lead to early initiation of appropriate management. We describe an interesting case of a 61-year-old presenting with fever and altered mental status, diagnosed with numerous episodes of viral encephalitis caused by divergent and recurrent viruses. On his initial presentation, lumbar puncture revealed lymphocytic pleocytosis and positivity for Human Herpesvirus 6 (HHV-6), and he was treated with ganciclovir. On subsequent admissions, he was diagnosed with recurrent HHV-6 encephalitis as well as Herpes Simplex Virus 1 encephalitis and treated with ganciclovir, foscarnet and acyclovir. Despite prolonged courses of treatment and resolution of symptoms, he continued to have persistently high plasma viral loads of HHV-6, consistent with probable chromosomal integration. In this report, we emphasize the clinical pearl of chromosomally integrated HHV-6 that can present in a patient with persistently high plasma viral loads of HHV-6, that are non-responsive to treatment. Individuals with chromosomally integrated HHV-6 may be more susceptible to other viral infections.
Keywords: Encephalitis, Viral encephalitis, Herpes simplex virus, Human herpesvirus 6, HHV-6, HSV
Background
Viral encephalitis is a clinical syndrome which can lead to encephalopathy, epileptic activity, focal neurological deficits, and even death [1]. Although herpes simplex virus (HSV) type 1 is a common cause of sporadic viral encephalitis, other pathogens may be observed depending on the specific region or predisposing factors [2]. Advances in molecular biology and neuroimaging now allow for increased speed and accuracy of diagnosis [1]. Furthermore, prompt recognition and a high index of clinical suspicion may lead to early initiation of appropriate management. Herein, we describe an interesting case of a patient with numerous episodes of viral encephalitis caused by divergent and recurrent viruses.
Case description
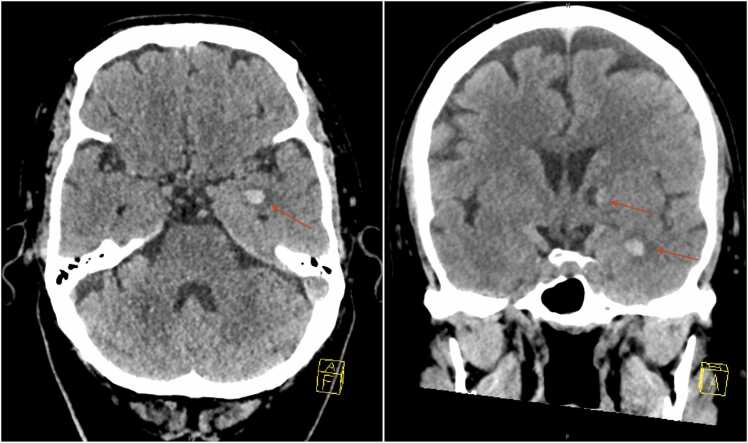
A 61-year-old man presented to the hospital in the context of fever and altered mental status. His past medical history was significant for hypertension and non-insulin dependent type 2 diabetes. On presentation, the patient was hemodynamically stable, but extremely drowsy and difficult to arouse. On physical examination he was not able to follow commands, had significant mixed aphasia with predominant word-finding deficits, and was unable to answer any orientation questions. His reflexes were intact including a downward contraction of the toes (Babinski absent). Cardiopulmonary exam was unremarkable; he had a regular heart rate and rhythm, no appreciated murmurs, and his lungs were clear to auscultation. Initial labs were significant for leukocytosis (12.0 k/uL with neutrophilic predominance; reference range 4.0–10.8 k/uL), hyponatremia (128 mmol/L; reference range: 137–145 mmol/L), hypokalemia (3.0 mmol/L; reference range: 3.4–4.5 mmol/L), hypochloremia (96), mild alkalosis (27), and an elevated serum creatinine (1.65 mg/dL; reference range: 0.6–1.1 mg/dL. A non-contrast computed tomography (CT) scan of the head showed 2 intracranial lesions: a 7 mm hemorrhagic lesion with surrounding edema in the left temporal lobe and a 9 mm mixed cystic and hyperdense lesion in the left internal capsule (Fig. 1). Magnetic resonance imaging (MRI) of the brain with and without contrast showed a lesion in the medial left temporal lobe with enhancement and edema, an underlying small intraparenchymal hemorrhage, and a remote left internal capsule lacunar infarct accounting for the hypodense lesion on seen on CT (Fig. 2). EEG demonstrated left temporal focal slowing and mild diffuse background slowing.
Fig. 1.
Non-contrast computed tomography (CT) scan of the head revealing two subcentimeter lesions in the brain (red arrows): A 7 mm hemorrhagic lesion with surrounding edema in the left temporal lobe, and a 9 mm mixed cystic and hypertense lesion in the left internal capsule.
Fig. 2.
Magnetic resonance imaging (MRI) of the brain, with and without contrast, revealing medial temporal lobe cortical enhancement and edema within underlying small intraparenchymal hemorrhage, and remote left internal capsule lacunar type infarction (red boxes).
Lumbar puncture revealed lymphocytic pleocytosis and positivity for Human Herpesvirus 6 (HHV-6). Herpes Simplex virus (HSV) 1 / 2 were negative. Additional CSF studies are presented in Table 1. Quantitative HHV-6 PCR was 6996 copies/mL in the cerebrospinal fluid (CSF). Serum HHV-6 was also noted to be elevated to 22,302 copies/mL. Notably, serum HHV-6 IgG was positive (1:40; reference range: <1:10) with a negative IgM (<1:20; reference range: <1:20). He was initiated on ganciclovir for a 21-day course for HHV-6 encephalitis. To monitor the viral response to treatment, serum HHV-6 DNA was rechecked 10 days after initiation of therapy; it had paradoxically increased to 3.3 million copies/mL. He was screened for other infectious diseases such as HIV and syphilis which were both negative. After his treatment course, he had significant improvement in his clinical status and resolution of delirium on discharge. The serum HHV-6 viral load was noted to decrease to 10,406 copies/mL (17 days after initiation of therapy).
Table 1.
Lumbar puncture diagnostic study results.
| CSF Studies | Admission 1 | Admission 2 | Normal Range |
|---|---|---|---|
| HHV-6: qualitative | positive | positive | |
| HHV-6 quantitative: copies/mL | 6996 | 5680 | |
| HSV ½: qualitative | – | positive | |
| Glucose (mg/dL) | 154 (H) | 97 (H) | (40–70) |
| Protein (mg/dL) | 62 (H) | 101 (H) | (15–45) |
| RBC per mm3 tube 1 | 656 (H) | 44 (H) | (0–1) |
| WBC per mm3: tube 1 | 22 (H) | 76 (H) | (0–5) |
| % Lymphocytes tube 1 | 64 % | 64 % | |
| RBC per mm3: tube 2 | 53 (H) | 26 (H) | (0–1) |
| WBC per mm3: tube 2 | 23 (H) | 74 (H) | (0–5) |
| % Lymphocytes tube 2 | 84 % | 57 % |
CSF: Cerebrospinal fluid; H: High; HHV-6: Human Herpesvirus 6; HSV: Herpes Simplex Virus; RBC: Red Blood Cells; WBC: White Blood Cells.
One month later, the patient represented to the hospital in the context of similar symptomatology and associated fever. During this second hospital admission, lumbar puncture revealed declining, albeit persistently present HHV-6 titers (5680 copies/mL; from 6996 copies/mL), but reactivity for HSV-1 (Table 1). He was treated with IV acyclovir for 3 weeks (10 mg/kg every 8 h). Serum HHV-6 DNA PCR was 267,166 copies/mL (from 10,406 copies/mL on discharge one month earlier) so he was started on IV foscarnet (2736 mg every 12 h). The acyclovir was stopped given foscarnet alone could be used to treat both viruses (HSV and HHV-6) and he completed an 11-day course of monotherapy with foscarnet. He demonstrated clinical improvement. Despite his clinical improvement, he continued to demonstrate persistent HHV-6 viremia (serum HHV-6 PCR of 568,994 copies/mL), and multidisciplinary shared decision-making between Infectious Disease, Internal Medicine, and the family elected to discontinue foscarnet in light of its presumed minimal benefit and potential toxicities. Since he had previously completed a 3-week course of ganciclovir and 11 days of foscarnet without significant improvement in the HHV-6 viral load, it was concluded that he was not able to clear the virus despite adequate treatment and had presumptive chromosomal HHV-6 integration.
Four months thereafter, the patient presented once again in the context of altered mental status. Magnetic Resonance Imaging (MRI) revealed an acute left cingulate gyrus stroke and subtotal resolution of left temporal hemorrhage. Lumbar puncture was once again performed and demonstrated lymphocytic pleocytosis. CSF was positive for HHV-6 (50,176 copies/mL; from 5680 copies/mL) and HSV1/2. More than 2 million copies/mL HHV-6 were noted in the serum. HSV-1 and 2 were not detected in serum by RT-PCR. He was again treated with 3 weeks IV acyclovir with improvement in his mental status on discharge. An appointment was made for him on discharge to follow-up in immunology clinic for further analysis of for any genetic immunodeficiencies.
Discussion
Encephalitis presents with altered mental status often in combination with other symptoms - fever, seizures, new focal neurologic deficits - and signs - CSF pleocytosis, parenchymal changes on brain imaging, or EEG findings consistent with encephalitis. In contrast, meningitis classically presents with signs of meningeal irritation - nuchal rigidity, headaches, nausea, blurry vision - yet intracranial viral infections will cause variable degrees of both parenchymal and meningeal inflammation, with notably overlapping symptoms. Therefore the term “meningoencephalitis” is often preferred [3], [4]. In the United States, 7 per 100,000 people each year are hospitalized for encephalitis with 20–50 % of the cases being attributed to viruses.
The most common findings on MRI for both HSV and HHV-6 is temporal lobe enhancement [3], [5]. EEG may be useful in showing evidence of encephalopathy, focal changes, and subclinical seizures. In our patient, in addition to his presentation suggestive of encephalitis, he had temporal lobe enhancement on his initial MRI and lumbar puncture findings consistent with a CNS infection.
There are no randomized controlled clinical trials for treatment of encephalitis caused by HHV-6, but case reports have described the use of ganciclovir (5 mg/kg every 12 h) or foscarnet (60 mg/kg every 8 h) for 3 weeks in bone marrow transplant recipients [6]. For HSV encephalitis, the recommended treatment is acyclovir 10 mg/kg every 8 h for 14–21 days [4]. Our case is unique in that it describes recurrent HSV encephalitis in an immunocompetent host with a chromosomally integrated HHV-6 virus.
HHV-6 is the only human herpesvirus that has been shown to become integrated into the telomere region of host chromosomes. In approximately 1 % of humans, the complete genome of the HHV-6 virus is integrated into every cell of the body including gametes, allowing this virus to be passed down from parent to child [7], [8]. Of note, this patient’s father also suffered from an intracerebral hemorrhage and what appears to be episodes of meningoencephalitis, highlighting the possibility of antenatal transmission. Chromosomal integration of HHV-6 has been previously defined as high concentrations of viral DNA in serum (> 3.5 log 10 copies/mL) or whole blood (> 6.0 log10 copies/mL) [7]. Chromosomal integration can also be shown through hair follicle analysis; although this is not necessary for the diagnosis because quantitative PCR testing provides a high enough level of certainty [9]. Hair follicle testing is something that could be useful in developing countries if blood sample analysis is not available [9]. Further confirmation of chromosomally integrated HHV-6 can be done by testing family members or by sequentially testing the patient in order to demonstrate the persistence of elevated levels of HHV-6 DNA [9]. Although, the clinical significance of chromosomally integrated HHV-6 is not completely understood, there is evidence that harboring this virus could put the host at a higher risk of infection. There was a study of 548 solid organ transplant recipients that suggested that those with chromosomally integrated HHV-6 were at an increased risk of bacterial infection as well as graft rejection [10]. Testing family members with the guidance of a genetic counselor would be beneficial given the clinical implications are not fully understood [9]. Immunocompetent individuals with HHV-6 viral loads in the order of thousands of copies in the CSF and millions of copies in the blood should raise concern for chromosomal integration of HHV-6 [11]. Indeed, the patient presented in this report demonstrated persistently elevated HHV-6 viral loads in the blood on the orders of millions and in the CSF on the order of thousands, consistent with prior findings of chromosomal integration. In addition, the persistence of the virus in blood for a period of 5 months, despite adequate therapy, as in this case, further supports chromosomal integration [9]. Although only a few case reports of immunocompetent individuals clinically diagnosed with HHV-6 encephalitis have been published, the detection of HHV-6 DNA in the CSF most likely represented chromosomal integration rather active infection [11].
HSV encephalitis, on the other hand, is the most common sporadic viral cause of encephalitis. It does not have a seasonal or geographic pattern and there is a bimodal distribution in age with peak incidence in the very young (up to 3 years old) and again in adults over 50 years old [4], [12]. The morbidity and mortality has significantly improved with the use of acyclovir in the 1980s [6]. There is limited data on recurrent HSV encephalitis, although the suspected mechanism is viral reactivation since HSV lays dormant in the sensory nerves after primary infection. Studies on recurrent HSV encephalitis in children have found multiple gene mutations (e.g. toll-like receptor 3 pathway gene mutations) linked to host susceptibility to recurrent infection, without compromising immunity to other pathogens [13]. One study evaluated clinical relapse in adults with HSV encephalitis and found that in those patients whom experience relapse, CSF was negative for HSV during the acute relapse; the mechanism was felt to be secondary to an immunologic/ inflammatory response to HSV in the peripheral blood [14].
Conclusion
In summary, it is crucial to quickly identify and treat viral encephalitis to prevent morbidity and mortality. This case report emphasizes the clinical pearl of chromosomally integrated HHV-6 virus that can present in a patient with persistently high plasma viral loads of HHV-6, that are non-responsive to treatment. Additionally, this study supports the idea that those with chromosomally integrated HHV-6 virus may be more susceptible to viral infection from other neurotropic viruses.
Ethical approval
N/A
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
CRediT authorship contribution statement
Claire Allen: Writing - Initial Draft, Data Collections. Shiavax J. Rao: Writing – review & editing. Kavneet Gill: Writing – review & editing. Marcos Wolff: Writing – review & editing. Christopher J. Haas: Writing – review & editing.
Conflict of interest
None.
References
- 1.Sonneville R., Jaquet P., Vellieux G., de Montmollin E., Visseaux B. Intensive care management of patients with viral encephalitis. Rev Neurol. 2022;178(1–2):48–56. doi: 10.1016/j.neurol.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Mailles A., Stahl J.P., Steering Committee and Investigators Group Infectious encephalitis in france in 2007: a national prospective study. Clin Infect Dis. 2009;49(12):1838–1847. doi: 10.1086/648419. [DOI] [PubMed] [Google Scholar]
- 3.Kumar R. Understanding and managing acute encephalitis. F1000Res. 2020;9:60. doi: 10.12688/f1000research.20634.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyler K.L. Acute viral encephalitis. N Engl J Med. 2018;379(6):557–566. doi: 10.1056/NEJMra1708714. [DOI] [PubMed] [Google Scholar]
- 5.Venkatesan A., Tunkel A.R., Bloch K.C., et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57(8):1114–1128. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tunkel A.R., Glaser C.A., Bloch K.C., et al. The management of encephalitis: clinical practice guidelines by the infectious diseases society of America. Clin Infect Dis. 2008;47(3):303–327. doi: 10.1086/589747. [DOI] [PubMed] [Google Scholar]
- 7.Ward K.N., Leong H.N., Thiruchelvam A.D., Atkinson C.E., Clark D.A. Human Herpesvirus 6 DNA levels in cerebrospinal fluid due to primary infection differ from those due to chromosomal viral integration and have implications for diagnosis of encephalitis. J Clin Microbiol. 2007;45(4):1298–1304. doi: 10.1128/JCM.02115-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward K.N., Hill J.A., Hubacek P., et al. Guidelines from the 2017 European Conference on Infections in Leukaemia for management of HHV-6 infection in patients with hematologic malignancies and after hematopoietic stem cell transplantation. Haematologica. 2019;104(11):2155–2163. doi: 10.3324/haematol.2019.223073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pellett P.E., Ablashi D.V., Ambros P.F., et al. Chromosomally integrated human herpesvirus 6: questions and answers. Rev Med Virol. 2012;22(3):144–155. doi: 10.1002/rmv.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S.O., Brown R.A., Razonable R.R. Clinical significance of pretransplant chromosomally integrated human herpesvirus-6 in liver transplant recipients. Transplantation. 2011;92(2):224–229. doi: 10.1097/TP.0b013e318222444a. [DOI] [PubMed] [Google Scholar]
- 11.Berzero G., Campanini G., Vegezzi E., et al. Human Herpesvirus 6 encephalitis in immunocompetent and immunocompromised hosts. Neurol - Neuroimmunol Neuroinflamm. 2021;8(2) doi: 10.1212/NXI.0000000000000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradshaw M.J., Venkatesan A. Herpes Simplex Virus-1 encephalitis in adults: pathophysiology, diagnosis, and management. Neurotherapeutics. 2016;13(3):493–508. doi: 10.1007/s13311-016-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsweed A., Alsuhibani M., Casanova J.L., Al-Hajjar S. Approach to recurrent Herpes Simplex Encephalitis in children. Int J Pediatr Adolesc Med. 2018;5(2):35–38. doi: 10.1016/j.ijpam.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sköldenberg B., Aurelius E., Hjalmarsson A., et al. Incidence and pathogenesis of clinical relapse after herpes simplex encephalitis in adults. J Neurol. 2006;253(2):163–170. doi: 10.1007/s00415-005-0941-6. [DOI] [PubMed] [Google Scholar]