Abstract
Sleep spindles are a signature feature of non-REM (NREM) sleep, with demonstrated relationships to sleep maintenance and learning and memory. Because PTSD is characterized by disturbances in sleep maintenance and in stress learning and memory, there is now a growing interest in examining the role of sleep spindles in the neurobiology of PTSD. This review provides an overview of methods for measuring and detecting sleep spindles as they pertain to human PTSD and stress research, presents a critical review of early findings examining sleep spindles in PTSD and stress neurobiology, and proposes several directions for future research. In doing so, this review underscores the extensive heterogeneity in sleep spindle measurement and detection methods, the wide range of spindle features that may be and have been examined, the many persisting unknowns about the clinical and functional relevance of those features, and the problems considering PTSD as a homogeneous group in between-group comparisons. This review also highlights the progress that has been made in this field and underscores the strong rationale for ongoing work in this area.
1. Introduction
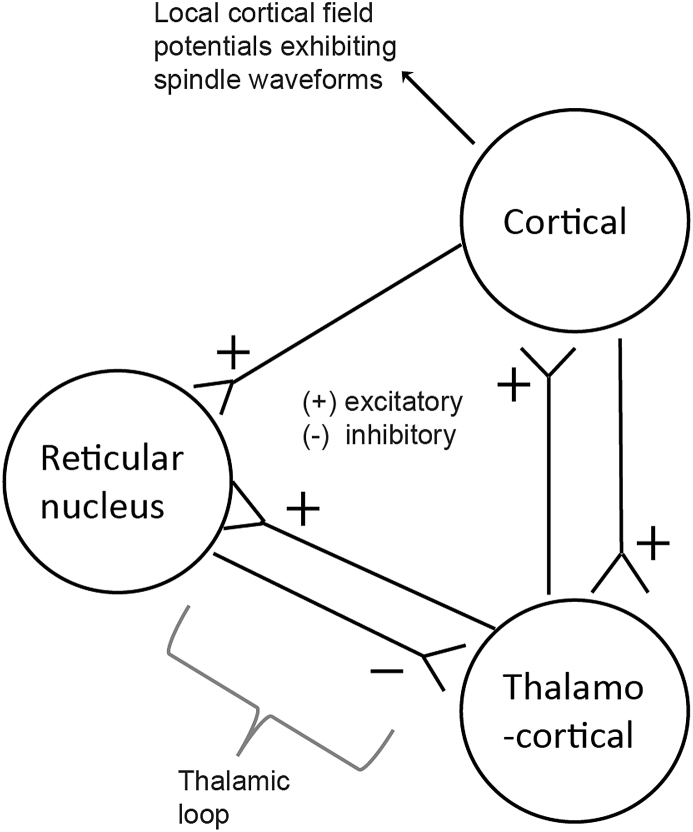
Sleep spindles are brain rhythms oscillating at approximately 11–16 Hz that are a defining feature of NREM, especially NREM stage 2 (N2) sleep (Fernandez and Luthi 2020). Sleep spindles derive their name from their signature appearance, waxing and waning as in the shape of a wool-spinning spindle, and are detectable via visible inspection of the scalp EEG signal. These rhythms reflect bursts of a cyclic interaction between cortical pyramidal neurons, thalamic reticular nucleus (TRN) neurons and thalamocortical (TC) neurons, involving TRN inhibition of TC cells, followed by re-bound firing of TC cells, leading to TC cell re-activation of TRN cells (Piantoni et al., 2016), Fig. 1). This forms an excitatory-inhibitory thalamic loop underlying the generation of spindle-like neuronal activity (Steriade 2003). The activity of this thalamic loop is heavily governed by projections from cortical to TC cells and shared reciprocal excitatory connections. Spindle oscillations generated by synchronous corticothalamic activity are measured at cortical layers in the local field potentials and are measured at the scalp surface using electroencephalography (EEG). Sleep spindles signal coordinated activity between the cortex, thalamus and hippocampus in the context of learning and memory (Staresina et al., 2015; Fernandez and Luthi 2020); their visibility at the EEG scalp level therefore renders sleep spindles incredibly appealing to study as an easily identifiable indicator of critical human brain activity. While there is compelling evidence that sleep spindles, or certain features thereof, are highly heritable and stable within individuals (Purcell et al., 2017), there is evidence that some sleep spindle characteristics, such as amplitude, duration and density, diminish as a function of age (Martin et al., 2013). There is also a burgeoning literature demonstrating that features of sleep spindles vary in a state-like fashion, such as in the context of learning, and that they may be associated with certain psychiatric conditions (Fernandez and Luthi 2020). In psychiatric disorders, they have been most extensively studied in schizophrenia (D'Agostino et al., 2018), but there is now early research examining their characteristics and function in the context of stress and stress disorder research (Dang-Vu et al., 2015; Beck et al., 2022; Denis et al., 2022), especially posttraumatic stress disorder (PTSD) (Wang et al., 2020a; Denis et al., 2021; van der Heijden et al., 2022).
Fig. 1.
Neural generators across the thalamocortical circuit that act as substrates for sleep spindles.
Published research provides an important rationale for studying the characteristics and functions of sleep spindles in PTSD. First, sleep disturbance is a core feature of PTSD, and a substantial body of evidence indicates that sleep plays a fundamental role in the development and maintenance of the disorder (see (Germain 2013, Richards et al., 2020) for reviews). While progress has been made in identifying objective sleep differences in PTSD relative to controls (Kobayashi et al., 2007; Zhang et al., 2019), sleep disturbance is a transdiagnostic phenomenon in psychopathology (Baglioni et al., 2016) and sleep biomarkers truly specific to PTSD or stress induced psychopathology are still lacking (Mellman 2019). Identifying biomarkers of abnormal sleep and their function in PTSD would be incredibly useful for purposes of targeted diagnosis, prevention, and treatment. Second, an older but significant body of literature indicates that sleep spindles play an important role in sleep continuity, or the maintenance of undisrupted sleep (Fernandez and Luthi 2020). Sleep spindle abnormalities might therefore be implicated in the subjective and objective sleep disruptions experienced in PTSD. However, the relevance of sleep spindles to sleep continuity problems in PTSD has yet to be systematically investigated. Last but not least, EEG sleep spindles are a surface indicator of learning and memory processes (Diekelmann and Born, 2010) and recent research even suggests a relationship specifically between spindle characteristics and declarative memory for negatively valent, emotional information (Kaestner et al., 2013). PTSD is a condition that revolves around a declarative and emotional (i.e., traumatic) memory, and is characterized by re-experiencing symptoms in the form of intrusive wake and sleep recall of lived traumatic experiences. Examination of the function of sleep spindles in a disorder in which memory is a core element is therefore highly indicated and could potentially uncover important sleep mechanisms linking stress exposure to psychopathology.
In light of the emerging literature on sleep spindles and PTSD and the strong theoretical rationale for this work, it appears timely to review what has been learned thus far on the characteristics and function of sleep spindles in PTSD, and to delineate avenues for future research. The latter may be particularly important because the number of spindle detection strategies and sleep spindle features that may be selected for study are numerous, and the possibility of broad exploration yielding spurious findings is therefore high. A mindful approach to measurement, hypothesis development and hypothesis testing, that is guided by the existing knowledge, is therefore critical to reduce the risk of non-reproducible findings and to make progress in the study of sleep spindles, stress, and PTSD.
2. What is a sleep spindle, and how is it detected?
The AASM visual scoring manual defines the sleep spindle as an EEG oscillation in the 11–16 Hz frequency band lasting 0.5–3 s, with a waxing and waning amplitude that stands out from the background EEG. It is one of the defining features of human NREM stage 2 (N2) sleep (Berry RB et al., 2018), although recent data suggest considerable inter-subject variability with the lower end of the spindle frequency even going down to 9Hz (Cox et al., 2017). Sleep spindles have traditionally been detected by visual scoring of sleep based on pattern recognition by the experienced human eye. However, it is well-established that within- and between-scorer reliability is far from perfect, indicating an important degree of measurement error (Warby et al., 2014; Lacourse et al., 2020). Spindles may be especially hard to visually detect when other rhythms which are higher in amplitude, such as slow oscillations and delta waves, occur simultaneously and obscure spindles’ classic appearance.
Automated computer algorithms are thus now often used to stage sleep and are increasingly used to identify sleep spindles for the purposes of research (Warby et al., 2014; Coppieters 't Wallant, Maquet et al. 2016). More recently, modern machine learning and signal processing approaches have sought to further improve the quality of the spindle detection methods (see Inset 1 for further details on recent developments in spindle detection). The strength of automated detection algorithms is that they allow for the rapid processing of large amounts of data and hopefully overcome some of the limitations of manual scoring of sleep. The weakness is that there is currently no gold standard for automated spindle detection, and that the spindles detected may vary widely across studies, which likely contributes to inconsistencies in the literature. Likewise, criteria used to build algorithms necessarily define thresholds for spindle features with important implications when comparing findings across clinical or demographic groups whose spindles may look different, or for comparing spindle change over time, even within a single night. A great advantage of human visual scoring is the adaptability of the human eye to between-subject variability in spindle features. For example, a human is more likely to detect spindles with lower amplitude in an older subject where automated methods may miss them because of thresholding effects. It has been recommended that automated spindle detection algorithms be tested against large datasets collected from a demographically and clinically heterogeneous subject population and previously visually scored through human consensus using crowdsourcing methods, such as modeled by Warby and colleagues (Warby et al., 2014; Lacourse et al., 2020). This may be the best way to determine how an algorithm performs within a specific subject population and for a question of interest. As far as we are aware, large open-source datasets with data from clinical populations, such as PTSD subjects, have not yet been created.
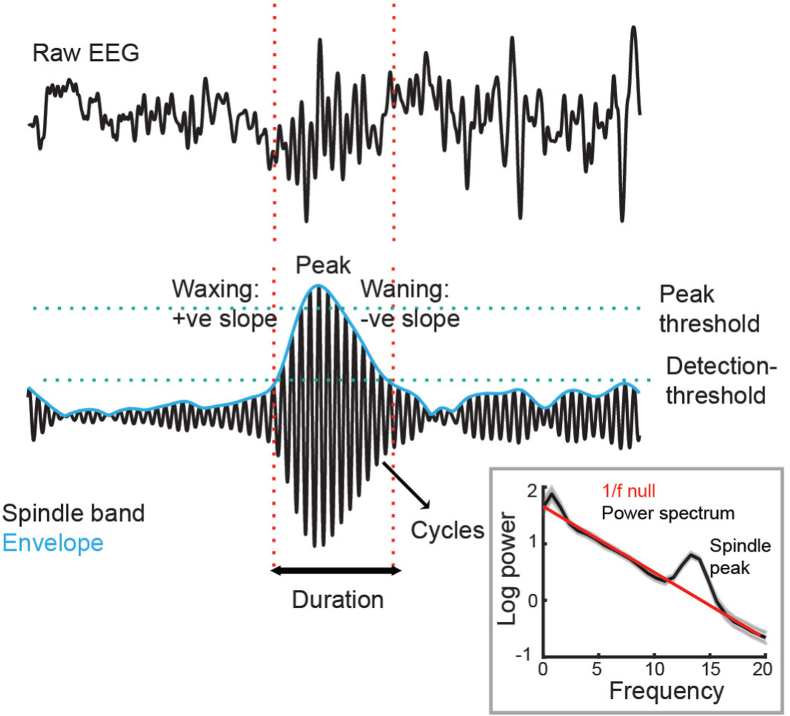
Inset 1.
Example of the detection of a spindle from raw EEG data. The various properties of the spindle both in terms of morphology, amplitude and frequency are detailed. In the inset, the power spectrum of this particular raw EEG segment is plotted against a null 1/f power law, and highlights an increase in spindle-specific power.
While a detailed description of the specifics of automated sleep detection algorithms is beyond the scope of this review (see (Coppieters 't Wallant, Maquet et al. 2016; Fernandez and Luthi 2020) for more extensive and technical reviews), a summary description may be useful for PTSD and stress researchers interested in studying this sleep rhythm and is outlined in Inset 1. The objective of most commonly used automated spindle detection methods is to detect a brief increase in the continuous spindle power (e.g., the strength of the 9–16 Hz signal) compared to background sleep, leading to the detection of a single discrete spindle event. However, in addition to studying spindles as discrete events, researchers occasionally look at the signal power in the spindle frequency band as a measure of sleep-specific information processing ((Mölle et al., 2011; Ramanathan et al., 2015; Lehmann et al., 2016); see Inset 1 for visualization of the continuous spindle oscillation and identification of discrete spindles therein). As a concrete example, researchers can examine the EEG signal power at a specific channel in sleep following learning versus a control sleep without performing spindle detection. Subsequently, if the signal power within typical spindle frequencies are greater in the experimental condition versus baseline, then inferences on spindle-specific information processing can be drawn.
2.1. Summary
In summary, sleep spindles may be detected via visual or computer-based automated scoring, and the resulting detected phenomena may differ widely across methods. Visually scored sleep spindles based on agreement between multiple experts may provide the best gold-standard for identifying the classic phenomena of interest, at least in N2 sleep, in which spindles may be less obscured by other signals (electrical noise, EEG slow/delta waves in N3 sleep). Automated strategies have multiple strengths, but typically rely on numerous arbitrary cut-offs impacting their sensitivity and specificity for true spindles. Their overall objective is typically to identify discrete events based on an increase in power satisfying specified amplitude, duration, shape and frequency criteria during a specified window. These differences should be taken into consideration when comparing findings across studies, and when interpreting between-group differences in spindle features.
3. What features of spindles are examined in stress and PTSD research?
The AASM definition and the detection of sleep spindles based on certain features underscores that frequency, duration, shape/morphology, and amplitude are typically the defining elements of the sleep spindle, and differences in these features are often used to examine group differences and/or predict behavioral outcomes. However, other spindle parameters, such as their density and cortical network dynamics have been studied in the context of PTSD and stress.
3.1. Frequency
Sleep spindle frequency generally refers to the frequency of the oscillations within a spindle event. Visual scoring typically aims to capture all events oscillating within the full range of frequencies from 11 to 16 Hz. Some researchers also extend the spindle bandwidth down to 9Hz (Cox et al., 2017). It should be noted that the lower frequencies fall well within the alpha frequency bandwidth (8–12 Hz), which risks confusing spindle activity with eyes-closed wake (i.e., brief arousals), especially in the posterior leads over occipital cortex. While this is outside the fast spindle frequency range in posterior leads (>12Hz), there is still the potential for falsely identifying alpha arousals as spindles given that alpha arousals are also momentary bursts of increased narrowband power (Cantero et al., 2002). This is most likely to occur when the roll-off associated with the spindle filtering process (say 12–16Hz) allows for bleed-through of lower frequency alpha activity and when detection criteria are purely focused on increases in power in the spindle band without taking into morphology characteristics of a spindle event (detailed in later sections). Care should thus be taken in designing the digital filter and ensuring robust spindle detection criteria. Of note, the spindle oscillation is not typically stationary for the duration of the spindle so that the term “frequency” often refers to the mean frequency of oscillations in a visually detected spindle, or alternatively, the mean of the sine wave frequencies with maximal power within the automatically detected spindle window. The latter frequency is therefore also defined as the “peak frequency” due to its association with a power maximum (See Inset 1, Inset 2 and below).
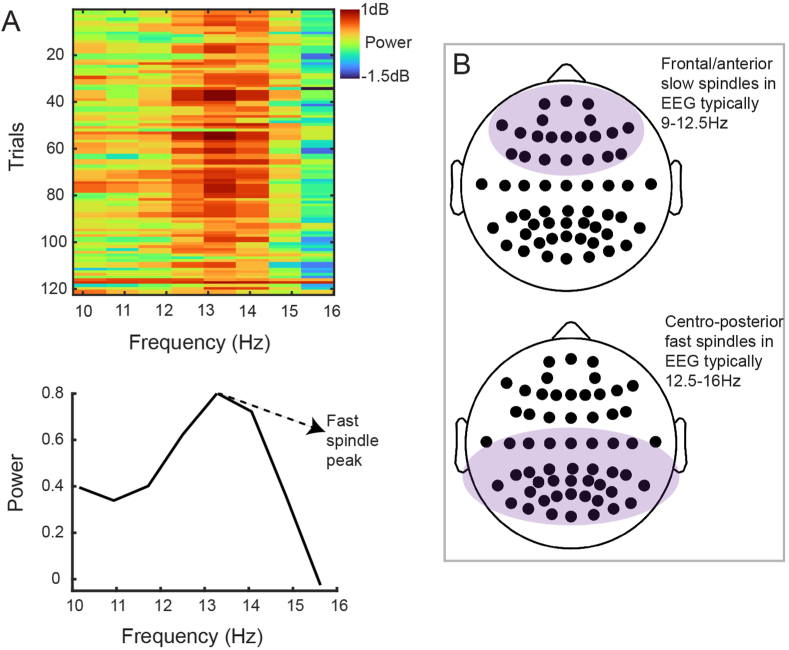
Inset 2.
A) Example of determining whether the spindles at a particular channel are fast or slow spindles, defined here with ranges of 9–12.5 Hz (slow) and 12.5–16 Hz (fast). The power at various frequencies within each trial where there was a general broadband increase in power within the spindle band are plotted (top). It can be seen that across trials, there was a consistent increase in power within the 12–14Hz frequency range (bottom). Averaging the power across trials reveals a consistent peak at around 13Hz, classifying spindles at this particular channel to be fast spindles. B) The general EEG topographical distribution (in a 64 channel EEG system with 58 cortical recording electrodes) of slow spindles (top) and fast spindles (bottom).
Increasingly, including in several studies of PTSD (Wang et al., 2020a, 2020b; Denis et al., 2021), researchers distinguish between fast and slow spindles because of evidence that they may be associated with different brain sources, with different scalp surface topographies and preponderance in distinct states of sleep (Fernandez and Luthi 2020). For instance, in healthy adults, slow spindles tend to occur over more frontal brain regions whereas fast spindles occur over more posterior brain regions. The proportions of slow vs. fast spindles also differ between N2 and N3 sleep (see Inset 2 for greater detail on the classification and biology of fast and slow spindles, since frequency is an important criterion studied in PTSD research).
Notwithstanding these important observations regarding spindle frequency in human sleep, it must be noted that the functional significance of this feature of sleep spindles, including the differentiation between slow and fast spindles, continues to be unclear. One view is that slow frontal spindles might correspond more to cortico-cortical dialogue with the prefrontal cortex in the human brain during sleep, whereas fast spindles might be biased towards thalamocortical communication (Mölle et al., 2011). However, this distinction is not binary and there are a number of factors that dictate spindle frequencies, including the rates of firing of TRN and TC neurons, the duration of inhibition prior to TC cell rebound firing (i.e. longer hyperpolarizing rates), and the neuromodulatory milieu affecting contributing neuronal networks. In addition, fast and slow spindles might differentially relate to demographic and clinical factors such as age, medications etc. (De Gennaro and Ferrara 2003). It is thus challenging to begin with solid hypotheses about the relationship of fast and slow spindles to clinical symptoms, behaviors or diagnosis. That being said, several recent studies have examined spindle frequency and peaks in sigma power in the context of PTSD ((Wang et al., 2020b; Denis et al., 2021), see Section 4 below).
3.2. Duration
Using crowdsourcing methods involving 47 experts visually scoring sleep EEG from 180 healthy subjects ranging in age from 18 to 76 years old, Lacourse, Warby and colleagues generated a gold-standard open-source dataset comprised of over 5000 spindles from N2 sleep (Lacourse et al., 2020). They found that spindle duration was on average 0.79 s (S.D. 0.14) in younger subjects and 0.75 s (0.16) in older subjects. These findings are consistent with prior findings from Warby et al., indicating a mean duration of 0.75 s (S.D. 0.27) in middle-aged to older adults (Warby et al., 2014) and within the same range of values in the analyses of another large scale sleep spindle dataset 11,630 subjects (Purcell et al., 2017). They found that 14% of detected spindles were shorter than the AASM defined duration of 0.5 s. Compared with this gold standard, measured spindle durations using automatic detection algorithms are highly variable, and depend on the combinations of amplitude, shape and/or minimum and maximum duration thresholds used for spindle detection. Thus, satisfaction of amplitude or shape requirements for a minimum of 500 ms or 400 ms (Mölle et al., 2002; Schabus et al., 2008, Staresina et al., 2015a; Purcell et al., 2017) risks non-detection of true spindles. On the flip side, satisfaction of duration criteria for up to 3 s could theoretically result in the false detection of a single spindle event when there may in fact be two or more. Analyses comparing spindle durations between groups or across timepoints should therefore consider the impacts of their spindle detection criteria during interpretation of findings. Likewise, differences in measurement methods should be considered when interpreting similarities and differences in findings across studies.
As with spindle frequency, the implications of spindle duration for functioning are still poorly understood. Some studies in humans (non-clinical subjects) have shown that increased spindle durations correlate with offline performance gains and learning (Morin et al., 2008, Schabus et al., 2008; Fogel and Smith 2011), although the exact mechanisms underlying this effect remain unclear given that spindle duration can be positively correlated to other spindle features such as spindle power. The reduced duration in conjunction with advancing age may be an indicator of reduced availability of neuronal populations to recruit in the spindle (Helfrich et al., 2018). The functional significance of an increased average duration in women as compared to men is also not well understood. Age-related changes and some preliminary hints of reductions in insomnia and autism may indicate reduced functioning of corticothalamic circuitry in producing spindles (Fernandez and Luthi 2020). Therefore, exploration of PTSD vs. control differences in spindle duration would be predominantly exploratory at this juncture.
3.3. Amplitude
Spindle amplitude is typically defined as the value of the peak amplitude of the visually detected spindle, or the peak amplitude of the spindle envelope in the automatically detected window. Data from the above-cited crowdsourcing study (Lacourse et al., 2020) reported an average visually-scored spindle amplitude of 31 (S.D. 7) in younger subjects and 26 (S.D. 7) in older subjects, with a similar difference in mean amplitude between women (32 (S.D. 8)) and men 27 (S.D. 6). These measurements were based on C3 spindles, where spindle amplitude is known to be the highest. The amplitude of spindles, similarly to the amplitude of electrical brain signals in general, is likely a reflection of engagement and synchrony in underlying neural sources of the spindle. Therefore, an increase in spindle amplitude could reflect greater activity in thalamoreticular and/or thalamocortical neurons contributing to spindling activity. It is also reflective of the signal to noise ratio inherent in neurophysiological recordings and is thus highly dependent on artifact correction methods. Here, examining peaks in the power spectrum within the spindle band against the background 1/f power law (see Inset 1) can be particularly useful. Moreover, it is important to recall that factors such as volume conduction (conduction of depth signals at the cortical surface through the skull and measured across the scalp surface) and distance of signal detection electrodes from intracranial sources have impacts on amplitude (Buzsaki et al., 2012) and can potentially introduce considerable variation in mean EEG spindle amplitudes across individuals (Hagemann et al., 2008). Furthermore, it is critical to note that most automated detection algorithms set specific requirements for amplitude, either in terms of absolute voltage or voltage relative to background. This may affect the sensitivity to detect true spindles given varying signal-to-noise ratios, and depending on the amplitude threshold there might be a bias to detect only spindles with the largest amplitude. This can potentially obfuscate group differences or changes across timepoints. As suggested earlier, the amplitude criterion tends to correlate with other criteria such as duration and density as well. To our knowledge, no studies have reported PTSD vs. control differences in spindle amplitude per se, although Van der Heiden and colleagues found that a measure combining amplitude features of waxing and waning spindle-band activity did predict PTSD symptoms (see below, (van der Heijden et al., 2022).
3.4. Morphology
The waxing and waning feature of the sleep spindle is thought to be due to the gradual recruitment of thalamocortical neurons into the synchronous oscillation, followed by gradual disengagement of neurons from the oscillating network. Automatic detection methods do not always explicitly account for the presence of the classic waxing and waning spindle shape, but many include criteria that are consistent with it. For instance, spindle detection methods require sigma power in the specified frequency bandwidth to rise to a detection threshold (such as 3 S.D. of all power across sleep) and then be above 1 S.D. for a specified duration on either side of the timepoint satisfying the detection threshold. Thus, indirectly the detection algorithm accounts for a rise in spindle power to a peak and then to a decay, all while satisfying certain duration constraints (Inset 1 and see also (Clemens et al., 2005; Andrillon et al., 2011; Clemens et al., 2011)). In addition, it is possible to include the number of peaks and troughs within the detected window as an additional criterion to be satisfied. Since the morphology of a true spindle consists of repeated peaks and troughs, including this as a criterion can protect against detection of transient power increases due to artifacts in the EEG signal that might be wrongfully detected as a spindle event. Using a novel strategy for spindle detection, a recent EEG sleep PTSD study explicitly detected spindles based on the waxing and waning of spindle-band amplitude (see (van der Heijden et al., 2022), and Section 4below). After computing the envelope of spindle band oscillations (see Inset 1), the authors then computed the slope of the envelope (slope in Inset 1, for example). A spindle peak was defined as the point where the slope switched from positive to negative, and a trough was defined as where the slope changed from negative to positive. The authors then generally defined spindles to be data between one trough to the next; this would necessarily include periods with the waxing (rise in power to peak) and waning (fall in power from peak) morphology of spindles (See PTSD Studies for further discussion, below). Studies investigating the morphology of spindles relative to PTSD are scarce, and little is known in terms of the functional implications of variability in this morphology (for example symmetry, kurtosis, etc.) with respect to PTSD.
3.5. Density
In contrast to the above features, reflecting characteristics of a single sleep spindle, spindle density refers to the frequency of occurrence of detected spindles per unit time, typically in units of spindles per minute, and typically measured separately for N2 and N3 spindles. Increases in spindle densities are thought to reflect the excitability of corticothalamic networks and have been heavily implicated in memory consolidation; specifically, the increased spindle densities in the sleep following a learning task or exposure to stimuli are thought to represent increased activation of the thalamocortical spindle network. Mechanistically, it has been proposed that such increased spindle activity can potentially induce intra-neuronal calcium increases and thus incentivize synaptic potentiation of neuronal assemblies (Rosanova and Ulrich 2005). Increased spindle density can thus lead to synaptic reinforcement in thalamocortical networks during learning (Eschenko et al., 2006). This increase in spindle density is a strong predictor of offline task-specific learning gains and is thus a robust indicator of sleep-specific memory processing (Gais et al., 2002; Clemens et al., 2005; Eschenko et al., 2006). Sleep spindle densities also tend to be higher in females than males and tend to diminish with age (Purcell et al., 2017). In large-scale studies of N2 spindles in non-clinical human samples, average spindle density hovers around 2 spindles per minute, but also demonstrates an asymmetrical distribution with rates up to 10 spindles per minute in some individuals (Warby et al., 2014; Purcell et al., 2017; Fernandez and Luthi 2020, Lacourse et al., 2020). Our preliminary research has evaluated the role of sleep spindle densities in PTSD; here our results indicate that sleep spindle density increases in sleep following stress and rather than serve to consolidate memories associated with stress, they may in fact differentially impact emotional information processing associate with stressors in high vs. low PTSD (See preliminary findings below, and (Natraj et al., 2022)). Early research is indeed suggestive that examination of sleep spindle density is one of the more promising avenues for exploration in research on sleep and stress/PTSD (see Section 5 below).
3.6. Cortical networks
Apart from evaluating spindle properties at individual channels, measurements at multiple cortical areas afforded by high density EEG channels can lead to the analysis of whole brain spindle networks. The goals of such approaches are to accurately uncover connectivity between spatially diverse EEG electrodes in the spindle band that is distinct from spurious connectivity due to the intrinsic noise in cortical measurements.
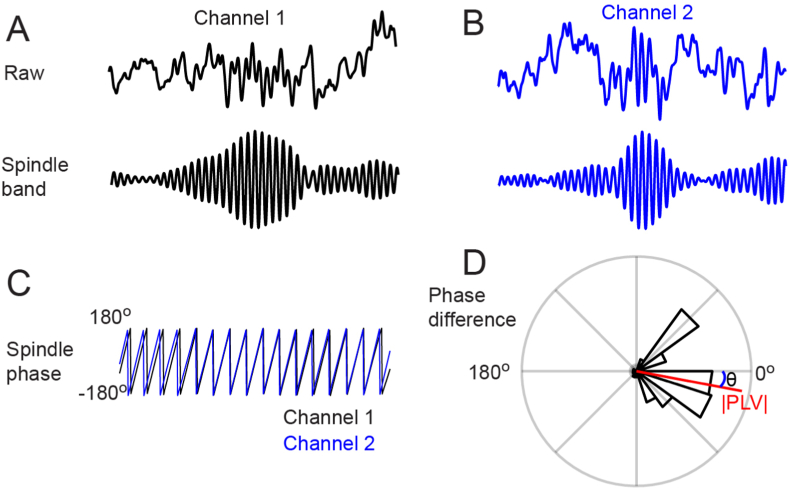
Here, most of the approaches involve analyzing the phasic consistency (pairwise) between electrodes’ spindle oscillations. A common method involves the measurement of coherence, or frequency-specific synchronization in activity, in the spindle band between any two channels (Nolte et al., 2004; Bastos and Schoffelen 2016). In the context of PTSD research, a related measure is the phase locking value, used by Wang et al. (Wang et al., 2020b). The phase locking value (PLV) evaluates the difference in oscillatory phase between two channels (See Inset 3), and proposes to uncover functional networks while taking into account volume conduction and measurement noise that can falsely affect connectivity analyses. The amplitude of the PLV denotes the strength of the phase coupling between any two channels while the angle, also referred to as the mean phase difference (MPD), denotes the lead/lag offset between the two channels. A depiction of the basic principle of phase based analyses is outlined in Inset 3. Further details and considerations pertaining to measurement of cortical network dynamics of sleep spindles, given their examination in PTSD, are provided in Inset 3.
Inset 3.
Example of evaluating spindle networks between channels using a phase-based analysis. A) The raw spindle-containing EEG segment at a particular channel (channel 1) (top) along with activity filtered within the spindle band (bottom). B) Similar raw (top) and spindle segments (bottom) in another EEG channel (channel 2) within the same time window when a spindle was detected on channel 1. C) The phase time series of each channel's spindle band-pass filtered oscillation, obtained here via the Hilbert transform. D) Evaluating the circular histogram of the phase difference between the two channels' phases in C) revealed that the phase differences were not random or uniformly spread over the unit circle; the mean of all these phase differences from the histogram is the phase locking value (PLV, red vector). Its magnitude or length is dependent on how in-phase the two channels' spindle oscillations are and its angle relative to 0° denotes the temporal offset (lead/lag) between the two channels' spindle oscillations. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Notwithstanding these compelling methods for examining whole brain sleep spindle dynamics, current limitations in our understanding of the underlying anatomical generators of such functionally coupled activity creates a barrier to interpretation of differences. Methodological problems related to volume conduction in particular, and general neurophysiological noise, also limit our confidence in the degree to which connectivity of signals detected at the scalp surface actually reflect synchrony of deeper networks.
3.7. Nesting
While spindles are a hallmark feature of N2 sleep, they can also co-occur with slow oscillations (SOs, <1.25Hz oscillations) in N3 sleep. SOs are large up and down states in brain wave activity that typically are global rhythms in slow wave sleep at the EEG level (though appear to be more local at the mesoscale electrocorticographic level (Nir et al., 2011)) and generally are a hallmark feature of slow wave sleep during N3. This co-occurrence of SOs and spindles, two cardinal NREM sleep rhythms, is called nesting. Typically, nesting is defined as the presence of a discrete spindle event within close proximity to the start of a SO (Mölle et al., 2002; Klinzing et al., 2016). In addition, rises in the background spindle power during a SO can also be used as indicators of nesting rather than the presence of spindles as discrete events. Research has shown that SO-nested spindles tend to increase during learning and memory consolidation and correlate with improvements in offline gains during sleep (Diekelmann and Born, 2010; Staresina et al., 2015; Latchoumane et al., 2017). This phenomenon of SO-spindle nesting, along with the temporal cooccurrence with hippocampal rhythms termed “ripples” (Clemens et al., 2011; Staresina et al., 2015; Latchoumane et al., 2017) is thought to reflect information transfer between brain systems during memory consolidation in slow wave deep sleep. Understanding the nesting of spindles to SOs in N3 sleep in relation to the processing of stressors in PTSD is a ripe avenue for research. To our knowledge, ours is the only lab that has preliminarily examined nesting in the context of sleep, stress and PTSD, with evidence that nesting increases in response to stress exposure, and differentially in trauma-exposed individuals with high vs. low PTSD symptoms (Natraj et al., 2022).
3.8. Summary
In summary, amplitude, duration, frequency, morphology, density, cortical dynamics, and nesting are features of spindles commonly examined in the literature. Despite an early understanding of physiological contributors to variability in these features, the functional significance of variability in most of these features is still poorly understood. This issue must therefore be considered when interpreting group differences in these features.
4. Sleep spindles in PTSD vs. controls: published findings to date
The published literature provides early evidence of differences between PTSD and controls in sleep spindle characteristics. Table 1 contains a summary description of study methods and findings. A pubmed search for articles with key words “spindles,” “PTSD”, “posttraumatic stress disorder,” was performed. Articles cited in the identified publications were reviewed to identify other articles that may have been missed using these focused search terms.
Table 1.
Brief Summary of Methods and Findings in Studies of Sleep Spindles in PTSD vs. Controls.
| Authors | Sample Characteristics | Sleep Comparisons | Spindle Detector | Slow and Fast Spindle Distinctions | NREM stage | Primary Hypotheses | Findings: PTSD vs. Control | Findings: Associations with Clinical Variables (post-hoc) |
|---|---|---|---|---|---|---|---|---|
| Dotan et al. (2008) | N = 30 (15 PTSD, 15 healthy control students) | 1 night of PSG (no adaptation night); in sleep lab | Visually Scored | N/A | N2 | N2 Spindle Density would be higher in PTSD relative to controls | No PTSD vs. Control differences in Spindle Density | SSRI use was associated with higher Spindle Density |
| Wang et al. (2020b) | N = 78 (31 PTSD, 47 controls) combat-exposed male veterans aged 18-50 | 2 consecutive nights of PSG, analyzed separately (no adaptation night); in sleep lab | Automated detection algorithm | Slow: 10–13 Hz | N2 and N3 | N/A: Exploratory analysis of group differences in spindle amplitude, duration, oscillatory frequency, and density using discovery and replication datasets | Slow spindle frequency was higher in PTSD vs. controls | Slow spindle oscillatory frequency was correlated with CAPS-based intrusive symptom score. This effect survived multiple comparison control. |
| Fast: 13–16 Hz | ||||||||
| Wang et al. (2020a) | N = 78 (31 PTSD, 47 controls) male combat-exposed veterans aged 18-50 | 2 consecutive nights of PSG, analyzed separately (no adaptation night); in sleep lab | Automated detection algorithm | Slow: 10–13 Hz | N2 and N3 | N/A: Exploratory analysis of group differences in measures of coherence, based on the Phase Locking Value (PLV) and the Mean Phase Difference (MPD) using discovery and replication datasets | No PTSD vs. control differences in PLV. MPD for slow spindles was higher in PTSD vs. controls. | MPD was negatively correlated with total CAPS score in the sample as a whole, although not in PTSD subjects alone. |
| Fast: 13–16 Hz | ||||||||
| Denis et al. (2021) | N = 97 (45 with PTSD, 52 controls) males and females, mean age 24, with recent trauma exposure | 1 night of PSG after an adaptation night, at home | Automated detection algorithm | Slow: Oscillations with peak frequency of 9–12.5 Hz; | N2 and N3 | NREM spindle frequency would be higher in PTSD relative to controls. | Fast spindle frequency was higher in PTSD vs. controls | No associations surviving multiple comparisons. |
| Fast: Oscillations with peak frequency between 12.5 and 16 Hz | ||||||||
| Van der Heijden et al. (2022) | N = 28 (14 with PTSD, 14 matched controls) police officers and war veterans, mean age 45, all trauma-exposed | 1 night of PSG during one of two nights in sleep lab (no formal adaptation night) | Proposed automated detector of spindle-shaped activity | N/A: spindle-shaped activity in 11–16 Hz bandwidth was detected; Right frontal electrode only | N2 | N/A: Examined differences between PTSD and controls in spindle activity across ranges of duration, frequency, amplitude. Amplitude-based measures of spindle activity were defined and calculated. | Spindle activity, based on a smaller difference between peak and trough amplitudes, was elevated in PTSD vs. controls and survived control for medication use. | A spindle activity index based on peak and waxing and waning amplitudes of spindle shaped fluctuations correlated with the clinical interview based intrusive memory score. |
Dotan and colleagues published the first study of which we are aware examining sleep spindles in PTSD (Dotan et al., 2008). They studied a sample of 15 subjects with PTSD and 15 healthy control students. The authors focused on visually scored N2 sleep spindle density and hypothesized that higher sleep spindle density in PTSD would explain some reports that PTSD was associated with higher arousal thresholds (“deeper” sleep) than that observed in controls. They found no difference in spindle density between PTSD and controls. Several methodological issues restrict the generalizability of these findings, including a small sample size, a single night of PSG, demographic differences between the PTSD and control groups, among others. Post-hoc analysis indicated that SSRI use was associated with a significant increase in N2 spindle density. This finding may serve as a reminder that medication use is critical to control for in such studies.
More recently, Wang and colleagues published a study examining sleep spindle features in 78 male combat-exposed veterans with PTSD (n = 31) and without PTSD (n = 47) using high-density EEG (Wang et al., 2020b). Participants were aged 18–50, were free of medications affecting sleep or daytime functioning, had no current severe or untreated depression, had no active post-concussive symptoms or active involvement in TBI treatment, and had no history of psychotic disorders. Each participant's sleep was studied for 2 consecutive nights in the laboratory, each involving an 8-h sleep opportunity, with night 1 and night 2 effects analyzed separately. They compared several sleep spindle features, including amplitude, duration, frequency and density between PTSD and controls, with the objective of identifying potential sleep spindle biomarkers for PTSD. Using a previously published automated spindle detection method, they analyzed oscillations in the 10–16 Hz frequency band, and examined slow spindles (here, 10–13 Hz) and fast spindles (here, 13–16 Hz) separately. They divided the full sample into discovery and replication subsamples, a method for enhancing confidence in exploratory findings by examining the reproducibility of findings from the first (discovery) sample in the second (replication) sample. They utilized several statistical methods to assess reproducibility across the two subsamples, to control for comparisons on multiple spindle features and types (fast vs. slow), and to correct for the potential effect of age on spindle features. The researchers' findings indicate that the oscillatory frequency of slow spindles is higher in PTSD than in matched controls. They also found a similar but weaker and less consistent finding suggestive of a higher oscillatory frequency in fast spindles in PTSD vs. controls. They performed several additional analyses to examine the robustness of their findings, including the analysis of N2 spindles alone, repeat analyses with removal of EEG arousals, re-analysis with an alternative spindle detection algorithm, and re-analysis using the full 10–16 Hz bandwidth. They report similar findings using these alterations. Linking their findings with PTSD symptom scores as measured by the clinician-administered PTSD scale (CAPS), they found that a positive correlation between slow spindle oscillatory frequency and intrusive symptoms of PTSD withstood multiple comparisons. The main interpretation associated with these findings was that higher slow spindle oscillatory frequency may reflect overall brain hyperarousal in PTSD.
Wang and colleagues published findings from a separate analysis on the same set of sleep data as described above, focused on spindle-band specific inter-channel synchronization of spindles (Wang et al., 2020a). They utilized the same sample, same spindle detection method, and same discovery and replication sub-samples as above. Based on the typical topographical distribution of fast and slow spindles, they used a single frontal channel (Fz) as a reference for slow spindles, and used a single parietal channel (Pz) as a reference for fast spindles for analysis of phase relationships across channels. Using the discovery dataset, they observed high phase locking value (PLV) in both PTSD and controls, indicating strong phase coherence across channels, and did not observe differences between groups for either fast or slow spindles. They did observe group differences in the mean phase difference (MPD) for slow spindles, indicating a lower MPD in PTSD relative to controls, which was similar across the 2 nights of data collection, but which did not survive control for multiple comparisons for the 64 channels. However, they did find a similar pattern of effects in the replication sample, and found that the dataset combining the discovery and replication samples yielded a significant group effect of MPD, focused on phase difference between spindles in left centro-parietal channels and the reference frontal channel, that survived multiple comparisons across 64 channels and also survived Bonferroni corrections for selected channel pairs within the region of interest. Linking this finding to PTSD symptoms, they explored relationships between MPD and CAPS score in the entire sample and found a negative correlation between MPD and CAPS score, suggesting that a larger phase difference was associated with a lower symptom burden. Notably, however, this effect was absent in PTSD subjects. Noting a trend negative correlation between MPD and CAPS hyperarousal symptoms in PTSD (not corrected for multiple comparisons), the authors proposed that the relatively lower MPD in PTSD could be a reflection of hyperarousal in thalamocortical circuits in PTSD.
Denis and colleagues recently published a study of trauma-exposed individuals with and without clinical-interview based PTSD (Denis et al., 2021). They reported sleep spindle findings on PSG data from 97 male and female participants with a mean age of 24 years and with a history of recent trauma (<2 years). Forty-five (45) met criteria for PTSD and 52 did not. The study excluded individuals with a number of psychiatric and/or medical conditions known to affect sleep spindles and/or sleep. Medication effects were not specifically examined. The researchers administered standard PSG, in subjects’ home environments, on one night preceded by an adaptation night. Their analyses were based on exploration of group (PTSD vs. control) differences in spindle peak frequency, density, amplitude and duration in both N2 and N3 sleep and in early and late NREM sleep, and correlations between these features and PTSD symptoms. Based on findings by Wang et al. described above (Wang et al., 2020b), their pre-registered a priori hypothesis stated that PTSD subjects would show higher frequency sleep spindles in NREM sleep compared to trauma-exposed controls. Here the authors defined slow spindles as those with a peak between 9 and 12.5 Hz, and fast spindles as those with a peak between 12.5 and 16 Hz. They observed that fast spindle peak frequency was slightly higher in PTSD relative to controls, although they did not observe such a finding for slow spindles. Additional exploratory analyses did not observe any findings that withstood multiple comparisons. They also did not observe relationships between spindle properties and PTSD symptoms in multiple-comparison-corrected analyses.
A recently published paper investigating spindles and PTSD proposed an alternative method of spindle detection involving fewer assumptions than typically applied in automated spindle detection methods, focused on the waxing and waning property of spindles (van der Heijden et al., 2022). They studied 14 police officers and war veterans with CAPS-IV-measured PTSD vs. 14 trauma-exposed controls. Exclusions included psychotic disorders, bipolar disorder, excessive recent substance use, a history of sleep disorder preceding PTSD diagnosis, and habitual sleep duration of <6 h per night and outside of the window of 10pm to 10am. Six participants with PTSD were on medications including SSRI's (5 subjects) and benzodiazepines (1 subject) for clinical reasons. Laboratory sleep PSG was collected on 1 of 2 nights spent in the sleep lab for all participants, with counterbalancing of data collection night (1st or 2nd) across clinical groups. They analyzed sigma band power in the 11–16 Hz frequency band collected from the right frontal electrode (F4), referenced to the average of the mastoids, during N2 sleep only. To detect spindle-band fluctuations, they examined the slope of the spindle envelope over a large enough moving window to detect peaks and troughs in spindle-band power based on the sign of the slope at each timepoint. Amplitude criteria were applied (min and max set at 5 and 35 , respectively) to ensure that spindling activity was distinguished from background neurophysiological noise. The result of this method is the measurement of discrete spindle-band fluctuations spanning the duration of N2 sleep. Because duration criteria were not applied, the authors note that a single visually scored spindle might therefore contain numerous such fluctuations. They then calculated duration, and three amplitude measures including peak amplitude (max absolute magnitude) and waxing and waning amplitudes (difference between the peak amplitude and the amplitude of the trough preceding and following the peak, respectively) for all fluctuations. They found that the mean peak amplitude of these fluctuations was higher in PTSD compared to controls, although this effect was no longer significant after controlling for medications. They also found that the difference between peaks and troughs was smaller in PTSD than in controls, and this effect did persist after controlling for medication effects. They explored group differences across the distribution of fluctuation durations and did not observe group differences. They also examined group effects on density of these fluctuations, and did not observe any differences. After observing PTSD effects on amplitude, they created an amplitude index, defined as the peak spindle amplitude divided by the average of the waxing and waning amplitudes. The authors found that this index was significantly correlated with higher intrusive (i.e., re-experiencing) symptom score on the CAPS, which was accounted for by a significant correlation with all the CAPS intrusive symptoms with the exception of nightmares. The authors concluded that this effect was indicative of the role of heightened spindle activity in aberrant memory processing in PTSD.
4.1. Summary and evaluation of published findings
These findings reflect important initials steps to examine spindle features and dynamics in PTSD vs controls, and to link these to specific symptoms of the disorder. Different spindle detection methods were utilized by different authors, and different features of spindles were examined. If there is a common signal between any two publications, it may be that spindle frequency may be increased in PTSD vs. controls, as suggested in two studies using different spindle detection methods (Wang et al., 2020b; Denis et al., 2021). However, while one group observed this effect in slow spindles and not fast spindles, the other observed this effect in fast spindles and not slow spindles. As stated above, because fast and slow spindles typically manifest preferentially in different stages of sleep and have different topographies, and may also have different neural generators and functions, the overlap in findings must be considered with caution. Understanding the exact frequency at which fast and slow spindles operate via power spectral analysis (Inset 1, Inset 2) can greatly aid in ensuring consistency in findings across research groups. Both research groups speculated that elevated spindle frequency may be an indicator of overall elevation in brain activation during NREM sleep. Replication of frequency-based findings in combination with greater understanding of the biological basis of spindle frequency are therefore indicated to substantiate these findings further. Additionally, greater clarity on the underlying neurobiological basis of brain “hyperarousal” in PTSD and PTSD sleep is needed to support the interpretation that PTSD hyperarousal promotes (or results from) higher frequency sleep spindles. Whether abnormal elevations of arousal neurotransmitters, possibly contributing to PTSD sleep disturbances (Richards et al., 2020), may promote increased spindle frequency in PTSD or in general may merit investigation. Furthermore, the utility of the frequency finding as a marker or as a factor contributing to behavioral and/or (brain) functional outcomes remains to be determined. As with PTSD research in general, much remains to be learned about the relationship of brain hyperarousal during either wake or sleep with the manifestation of intrusive symptoms.
Along with these frequency-based findings, other reported findings remain to be evaluated and/or replicated through future research. The findings reported by Wang and colleagues regarding spindle coherence are challenging to interpret. The authors observed significant coupling with very low phase offsets, raising the specter of common noise signals and volume conduction as a potential factor obfuscating real (larger) phase differences. This problem was noted by the authors, and because their methods were applied to both PTSD and control subjects, this issue may be less problematic, but the relevance of this finding to behavior and brain function nonetheless remains fairly opaque. For instance, increased phase-coupling between distant EEG electrodes might potentially indicate globally coordinated thalamocortical spindle activity and as such might be strong indicators of whole brain systems during memory consolidation given that sleep spindles are typically isolated cortical phenomena (Nir et al., 2011). There is also the potential that such coordinated activity can be linked to SOs which are known to have waves of propagating activity over the cortex (Massimini et al., 2004). Research in other psychiatric conditions such as schizophrenia have generally shown that reduced coherence can be a marker for impaired consolidation (Wamsley et al., 2012), however the neurophysiological relevance of such globally coordinated spindle networks with respect to PTSD is poorly understood and in its infancy of being researched. Nonetheless, the authors used rigorous methods and multiple strategies to support the robustness of their findings.
Only one study (Dotan et al.) examined the relevance of spindles to sleep continuity based on a priori hypotheses, despite the body of evidence indicating a role for sleep spindles in sleep maintenance (Dotan et al., 2008). These authors, however, reported no differences based on analysis of spindle density. Post-hoc analyses by Wang et al. indicated that fast spindle frequency tended to predict awakenings per sleep hour in PTSD patients, leading to a speculative link between higher spindle frequency and brain hyperarousal. No studies of which we are aware closely examined spindle counts or density with measures of arousals or microarousals in trauma-exposed or PTSD subjects.
Van der Heijden et al.’s findings point to a possible effect of PTSD (vs. trauma-exposed controls) on amplitude of N2 sigma-band fluctuations and found an association between a combined measure of peak amplitude and difference between peak and average adjacent trough amplitudes to predict intrusive symptoms. The methodology used by the authors, wherein spindles were identified by their morphological properties such as waxing and waning shape, has some advantages and disadvantages. An important advantage is that it provides a model-free approach for detecting spindles. A disadvantage is that it risks many false positives. The authors do apply an amplitude threshold to partially address the issue, but this cannot fully resolve the problem, and again highlights the challenge of standardizing methods given the diversity of the underlying cortical data and subject populations. Finally, the reduction of the PTSD effect on peak amplitude to trend when controlling for medications is problematic, especially given that medications were not controlled for in relationships analyzing the amplitude index to CAPS symptoms. However, the observation that the ratio of local sigma-band peaks and average trough positively predicts intrusive re-experiencing symptoms in PTSD is compelling, if replicated, given the strong evidence that spindle activity predicts declarative memory. As will be described in the next section, such research on spindles and memory has mostly focused on spindle density using a variety of spindle detection methods.
Van der Heijden et al. developed their hypotheses based on their prior exploratory findings indicating higher levels of frontal sigma power in NREM sleep in PTSD vs controls (de Boer et al., 2020), and in that report proposed that higher frontal sigma in PTSD might be related to exaggerated reactivation and consolidation of trauma memories. However, those results based on a predominantly male sample are not entirely consistent with other published findings. For example, in a larger study of objectively measured sleep in PTSD, Richards el. al. found that NREM sigma power was in fact significantly higher in trauma-exposed males without PTSD compared to males with PTSD, and found no difference between females with and without PTSD (Richards et al., 2013). (To note a potentially impactful difference, the latter analyses were based on analysis of the C3, rather than frontal electrodes as in the Van der Heijden analysis.) Woodward et al. also did not observe a NREM sigma power difference between PTSD and control subjects (Woodward et al., 2000). In exploratory analyses, however, they did observe a relationship between NREM sigma power and CAPS IV hyperarousal symptoms, which withstood multiple comparisons. Woodward and colleagues found this relationship counterintuitive, given the established correlation between sigma power and sleep spindles, and given that the authors associated sleep spindles with a sleep protective function. Overall, discrepancies in results could be attributed to methodological differences or to clinical differences in the underlying populations studied, and as such call for more investigation.
5. Sleep spindles and negative emotional learning: A function-specific avenue for studying sleep spindles and Stress/PTSD
One of the great challenges of scientific work in posttraumatic stress disorder is that it is an extremely heterogeneous disorder. It has been observed that, based on the current DSM criteria, there are 636,120 different ways to satisfy the PTSD diagnostic criteria based on a list of 21 symptoms segregated into 4 symptom categories (Galatzer-Levy and Bryant 2013). Diagnosis requires the presence of a minimum number of symptoms within each category, but the combinations of symptoms within categories and with symptoms in other categories are unrestricted. Mellman points out that the heterogeneity of the disorder has likely contributed to lack of consistency in objective sleep findings in PTSD vs. control comparisons (Mellman 2019). Clinical and demographic factors also may complicate results further (Zhang et al., 2019). This serious problem weighs heavily on science that seeks to examine group differences between individuals meeting criteria for a PTSD diagnosis from those who do not meet criteria. Combining this problem with the lack of standardized methods to detect spindles, and a lack of a clear understanding of the biological basis or function of many of the spindle features reviewed above, a potentially fruitful strategy may be to focus research on specific neurocognitive functions known to be correlated with specific spindle features and known to be relevant to PTSD. This would also address the issue that sleep spindles in one context may be adaptive (beneficial), but in another context may be problematic. For example, research has provided solid evidence that sleep spindles are sleep protective (Martin et al., 2013; Helfrich et al., 2018; Fernandez and Luthi 2020), such that an increase in spindle activity would potentially be a sign of more healthy sleep in PTSD. At the same time, if sleep spindle activity (broadly speaking) is an indicator of pathological over-consolidation of trauma (van der Heijden et al., 2022), then we have two opposing effects of the same brain behavior. Probing these functions directly, and ideally in participants with a trauma history and varying degrees of trauma-related psychopathology, may be the best way to better understand the role of spindles in stress and PTSD. The role of spindles in learning and memory is perhaps the domain in which sleep spindle science is currently most advanced. However, given that trauma information also carries emotional affect, extending the study of sleep spindles to the processing of emotionally salient (negative) information is highly relevant to PTSD, and has already begun.
Focusing on studies in humans uniquely, several labs, including our own, offer early findings hinting at the relevance of this approach. For example, Alger, Kensinger and Payne (Alger et al., 2018) studied the effect of sleep spindles on memory for negative emotional information in a sample of healthy adults aged 18–64. They used a previously published spindle detection algorithm to detect spindles (Ferrarelli et al., 2007), and used data from the frontal and central electrodes only. They found that all measured aspects of sleep spindle activity, including total number, mean duration, density and amplitude predicted recognition memory for previously viewed emotional image content. These findings were limited to N3 spindles, rather than N2 spindles. This finding might be consistent with the idea suggested by others (van der Heijden et al., 2022), that increased spindle activity could be associated with the over-consolidation of negative emotional information. However, Alger and colleagues did not report on the relationship between spindle features and neutral image memory to determine whether this relationship was unique to emotional image content. Thus, this finding might simply be consistent with research supporting the role of spindles in learning and memory more generally (Dudai et al., 2015). Kaestner and colleagues, however, used a within-subjects’ design to examine the relationship of pharmacologically enhanced sleep spindles with recall for negative valence, positive and neutral images, and found that zolpidem was associated with both increased sleep spindle density and increased memory accuracy for negative emotional content relative to a placebo sleep condition (Kaestner et al., 2013). Of note, however, while a correlation between spindle density and negative emotional memory accuracy was observed in one sample sub-group, it was not observed in the zolpidem group. More recently, Lehmann and colleagues reported compelling findings indicating that auditory cueing during post-learning NREM sleep, using words that had been paired with negative and neutral images during pre-sleep learning, was associated with improved memory for those pictures(Lehmann et al., 2016). They also reported that cueing associated with correct memory was associated with an increase in 12–15 Hz oscillatory power shortly after the cue (during sleep). While improvement in cued memory performance was positively associated with cue-induced spindle power in NREM for both negative and neutral memories, this effect was significantly stronger for negative images(Lehmann et al., 2016). These findings therefore directly link cue-associated spindles (measured via sigma power changes) with a subsequent emotional memory benefit. This finding is also consistent with evidence that sleep spindles are indicators of sleep-dependent replay of learning and the redistribution of memories from temporary (hippocampal) storage to (cortical) long-term memory storage (Diekelmann and Born 2010, Dudai et al., 2015). However, it should be noted that not all of these early studies consistently link sleep spindle activity, broadly speaking, or sleep spindle density with emotional memory effects. For example, Goder and colleagues observed a relationship between sleep spindle density and recognition accuracy for neutral, but not negative, images in a study of healthy subjects and schizophrenics (Goder et al., 2015). Denis and colleagues reported a negative correlation between nested spindles and emotional memory in healthy stressed, vs. non-stressed, laboratory subjects and found no association between N2 spindles and emotional memory in their sample(Denis et al., 2022).
Perhaps more directly relevant to PTSD, Kleim and colleagues examined the relationship between objective sleep variables and intrusive trauma memories after exposure to a laboratory analog film trauma in 18 healthy female subjects (Kleim et al., 2016). They found that a higher density of fast parietal N2 spindles was negatively associated with the frequency of intrusive memories of the lab trauma in the week following exposure. A non-significant negative correlation was similarly observed for frontal N2 slow spindles. They utilized a previously published spindle detection algorithm associated with learning-dependent increases in spindle density (Gais et al., 2002). Interestingly, the Kleim et al. finding is suggestive of a beneficial effect of sleep spindles (density) on stress information processing, rather than a maladaptive effect of sleep spindle activity (e.g, overconsolidation, as per van der Heijden et al.(van der Heijden et al., 2022)). Kleim's finding related to a clinically-relevant form of emotional memory underscores the importance of distinguishing between sleep effects on declarative emotional memory performance from sleep effects on the unsolicited, distressing memories that are characteristic of PTSD. It has been argued that it is in fact quite advantageous (in terms of future survival benefit) to have strong declarative recall for a stressful or traumatic event as long as the distressing emotional tone associated with the event can be dissipated, and there is some evidence indicating that sleep results in the enhancement of declarative memory along with the elimination of the emotional “tag” associated with that memory (Goldstein and Walker 2014, Ben Simon et al., 2020). To date, the focus of such theories has been on REM sleep, and there has been some controversy (Baran et al., 2012; Goldstein and Walker 2014). However, there is growing appreciation of the role of NREM sleep in healthy emotional information processing (Ben Simon et al., 2020).
Another clinically relevant study published by Azza and colleagues reported that sleep spindle density was associated with a greater reduction in the heartrate response to a negative autobiographical memory script presented both before sleep and after sleep in a study that involved an imagery reprocessing exercise prior to sleep (Azza et al., 2022). Data for 21 subjects, predominantly female, who completed a 90-min nap opportunity, were analyzed for spindle effects. Participants underwent a script-driven imagery session involving audio-guided imagining of neutral and distressing autobiographical scripts with continuous recording of ECG, followed by a rescripting session to modify the aversive autobiographical memory to render it less distressing. The researchers examined the relationship of N2 sleep spindle density and slow wave sleep with change in heartrate from pre-to post-nap script-driven imagery, using heartrate during the neutral memory exposure as a reference. This study's results indicate that higher N2 spindle density promotes emotion regulation, as indexed by a greater reduction (post-sleep vs. pre-sleep) in the difference in HR response to the emotional vs. neutral script. The authors interpreted this as a spindle density benefit for adaptive reconsolidation of “updated” autobiographical memory. While preliminary, these findings have direct implications for the study of sleep benefits on trauma-focused and nightmare treatments, such as CPT, PE and IRT. Together, the Kleim and Azza findings are suggestive that a higher density of N2 sleep spindles has a beneficial emotional information processing role.
Preliminary findings from our lab complement the findings of Kleim et al. and Azza et al. by providing compelling evidence that sleep spindle density affects emotional information processing. We used a nap protocol in 45 trauma-exposed men and women with varying PTSD symptom severity. Participants completed two laboratory nap visits after an adaptation nap, all separated by at least 6 days. A “stress” nap included a fear-potentiated startle protocol in the morning and an image viewing session, comprised of viewing a series of negative and neutral valence images, prior to the nap. The control nap was not preceded by stressful procedures. We found that the density of N2 sleep spindles in the stress nap increased relative to N2 sleep spindle density in the control nap. Furthermore, increased N2 sleep spindle density predicted a reduction in anxiety symptoms across the nap specifically in subjects with higher PTSD symptoms as assessed by a binary split of subjects into a high and low PTSD group based on their CAPS score. Additionally, this increase in N2 spindle density in the high PTSD group correlated with lower memory recall of negatively emotional images as compared to the low PTSD group (Natraj et al., 2022). These findings provide evidence that sleep spindle density increases with stress, in a manner analogous to that observed with learning in other modalities, and that sleep spindles may have a differential impact on processing in higher vs. lower PTSD individuals, which emphasizes memory consolidation in healthier subjects and emotion regulation in more symptomatic individuals.
5.1. Summary and comments
These studies provide early evidence that sleep spindles, especially density, may be involved in processing of emotionally salient information, which processing has important implications for understanding mechanisms in PTSD. There is a tension between theories that implicate sleep spindles in excess consolidation of emotional memory (e.g., van der Heijden et al. in PTSD, and others in non-clinical laboratory studies), and findings suggestive that sleep spindles may have an emotional information processing benefit (e.g., Kleim et al., Azza et al., and our preliminary research (Natraj et al., 2022)). Emotion processing in the clinical context in fact requires the retrieval of negative emotional memories, at least to some extent, and the infusion of those memories with new and therapeutic content. Therefore, the association of “emotional” with “maladaptive” in the context of PTSD and its treatment is not necessarily uniformly correct: a spindle association with emotional memory may be problematic in the context of PTSD development or chronic PTSD (over-consolidation) and may be adaptive during emotional information processing in healthy individuals or in the process of treatment (e.g., consolidation of “updated” emotional autobiographical memories). Our findings actually suggest that sleep spindle density following stress predicts emotion regulation, and not over-consolidation of memory, at least as reflected in memory accuracy. These early findings provide a fascinating template for future research that is highly clinically relevant.
6. Conclusions/future directions
This review provides an overview of the methods used to detect sleep spindles and measure sleep spindle features, presents the early findings examining sleep spindles in PTSD vs. controls, and proposes alternative strategies for understanding the relevance of sleep spindles to PTSD mechanisms, with a focus here on early research on emotional memory. Several important goals are to underscore the extensive heterogeneity in sleep spindle measurement and detection methods, the wide range of spindle features that may be and have been examined, the many persisting unknowns about the clinical and functional relevance of those features, and the problems with considering PTSD as a homogeneous group in between-group comparisons. Equally important goals are to emphasize the work that has been done, the possible consistencies in preliminary findings in PTSD vs. control comparisons, and the laboratory studies analyzing sleep spindles in relation to emotional learning and memory. There is a strong rationale for the latter inquiry based on well-established functions of sleep spindles in learning and memory and a clear relevance of negative emotional memory to PTSD. Of note, however: this focused review did not cover the full extent of published literature on spindles and stress more generally. For example, a still sparse body of studies report associations between sleep spindles and stress-related insomnia (Dang-Vu et al., 2015) and spindles as a potential indicator of stress-related sleep disturbance(Beck et al., 2022).
While not studied much to date, understanding the role of sleep spindles in sleep continuity, and the interacting effects of sleep spindles and other features of sleep on PTSD mechanisms, are possible avenues of future research. Sleep spindle activity in the vulnerable transitional zone between N2 and REM sleep, may be a particularly interesting with respect to the themes of sleep continuity and emotional memory. PTSD and sleep research has traditionally emphasized REM sleep effects on fear learning and extinction. Increasingly, exclusively REM-sleep-based hypotheses regarding sleep and emotional memory are proving unsatisfactory. Purcell and Manoac reported that a higher density of sleep spindles was associated with longer duration of REM sleep based on their analysis of sleep from 11,630 human subjects in a general population sample (Purcell et al., 2017). A recent study in narcoleptics found that spindle features predicted memory consolidation only when followed by REM sleep (Strauss et al., 2022). The interaction of sleep spindles in the transitional zone and subsequent REM sleep on emotional memory remains to be explored. A recent study of the spectral dynamics of NREM sleep in frequent nightmare sufferers specifically examined this transitional period and reported a moderate effect of frequent nightmares on high sigma-band power during that period (Blaskovich et al., 2020).
All in all, the above studies have established a compelling rationale for future research on sleep spindles, stress and PTSD and specifically focusing on the multifaceted nature of sleep spindles. It is clear that both clinical research in PTSD subjects and laboratory studies (ideally also including PTSD subjects) will be important for further progress. A major advantage of clinical studies of PTSD vs. trauma-exposed controls is the certainty regarding the presence of trauma exposure. Like all human laboratory studies seeking to understand mechanisms of PTSD development, laboratory experiments cannot ethically replicate the traumatic nature of stressors experienced in real life. It is therefore impossible to discern whether laboratory-based observations of laboratory stress responses reflect biological processes consistent with traumatic, vs. sub-acute, stressors. Longitudinal studies, ideally studying sleep prior to and after stress exposure, are rare but best suited to determine whether observed group effects precede or result from trauma exposure and/or PTSD.
Given the heterogeneity in spindle detection methods and its potential implications, it is advisable at this juncture to use previously published methods when testing hypotheses that replicate or directly build on prior findings. At the very least, providing a well-justified rationale for a change in strategy and comparing one's findings using both a new and published strategy, is recommended. As suggested by Warby and colleagues, it also may be worthwhile to test new computer-based detection strategies against the best visually-scored gold-standard, at least for N2 spindles. This may be even more advisable if a large database of spindles derived from a demographically diverse population and including both healthy and clinical samples is developed. At the same time, advances in machine learning (especially deep learning with artificial neural networks for spindle and sleep event detection (Chambon et al., 2019; Kulkarni et al., 2019)), advances in understanding the biology of sleep spindles and the relevance of sigma power fluctuations, and limitations of human scoring highlight that researchers should keep an eye open for novel developments in methodology that can most faithfully capture the neural phenomena of interest. It should be noted, however, that use of existing and/or novel methods will depend on each lab's resources, including availability of human expertise and computing resources, the public sharing of data and code, and the collaboration of experts with clinical and neuroimaging/signal processing backgrounds.
6.1. Inset 1: Common spindle detection methods
While there are many methods in the literature to detect spindles, the vast majority involve first band-pass filtering the raw EEG signal into the selected spindle frequency band (typically 11–16Hz) and subsequently computing the power in the spindle band. To compute power, a number of options exist, from computing smoothed power estimates via the root-mean square function (the square root of the mean of squares of spindle oscillation voltages within a fixed moving window), to estimating the envelope of the spindle waveform via the Hilbert transform (Le Van Quyen et al., 2001). After computing the power, a discrete spindle event is typically identified if the spindle power satisfies a number of user-defined criteria, such as an amplitude criterion (e.g., if peak power is greater than some cut-off value, say of all spindle band-specific power) (Andrillon et al., 2011); shape criteria (e.g., if the power around the peak rises and falls and surpasses another cut-off value, say 1 S.D. of all spindle power, during the rise and fall); duration criteria (e.g., if the sustained increase lasts between 500 ms and 3000 ms), and/or cycling criteria (e.g., the rhythm contains at least 3 cycles of spindle oscillations within the detected window). Alternative strategies exist. For example, an alternate method of capturing the waxing/waning characteristic of sleep spindles is to look at the rise and fall of the spindle power (RMS or Hilbert amplitude) by measuring the slope of the spindle amplitude; waxing and waning should give rise to a positive or negative slope on either side of the peak spindle power (van der Heijden et al., 2022). It is important however to note that the detection of spindles from the power spectrum can also be artificially induced by transient artifacts in the EEG signal which typically is reflected in broadband increase in power. Thus, it is critically important to run statistics on the individual power spectrum for each detected signal window in automated detection algorithms to ascertain that there is an actual significant increase in spindle band power. One particularly elegant analysis technique is statistically analyzing the power spectrum of the EEG signal and its deviation from the 1/f power law curve, wherein the power of the signal is inversely proportional to the frequency (See Inset 1 Figure, and (Voytek et al., 2015; Zhang et al., 2018; Donoghue et al., 2020)).
More recently, modern machine learning and signal processing approaches have sought to improve the quality of the spindle detection methods and include supervised methods (wherein the automated system is fed sample spindles scored by sleep experts and learns to detect these in new data (Lacourse et al., 2020) and unsupervised methods (where the system is directed to look for spindle patterns based on preset parameters (Souza et al., 2016). Newer and highly sophisticated deep learning methods are now leading to high-performance classification and prediction of spindles. While most spindle detection algorithms work at a single channel level, methods that work at multiple channels simultaneously also exist (Brunton et al., 2016).
6.2. Inset 2: Characteristics of fast and slow spindles
To ascertain the frequency of a detected spindle and classify it as fast or slow, the frequency that exhibits peak power within the detected spindle window is obtained from inspection of the power spectrum of the EEG signal in a sleep-stage dependent manner. For instance, EEG time-domain signals can be converted into the frequency domain using spectral transformations such as the Fast Fourier Transform or Wavelet Transform using windowed (segmented) epochs within each stage of sleep, from which the average power in each frequency can be computed and depicted by a power spectrum plot (Inset 2 Figure and see also (Babadi and Brown 2014, Prerau et al., 2017)). Peaks in power can be statistically quantified by contrasting them with the background 1/f power law (see Fig. 1) or contrasting them against non-sleep epochs. Using this method on depth EEG and MEG signals, it has been shown that frontal cortex tends to be dominated by slower spindle rhythms whereas posterior and occipital cortex tends to be dominated by faster spindle oscillations.
There is considerable inter-subject variability in terms of the frequency bandwidths delineating slow and fast spindles (Mölle et al., 2011; Cox et al., 2017). However, the distinct peak frequency of fast and slow spindles may be unique and stable within individuals while also being heritable, indicating that this feature of spindles contributes to a unique “spindle fingerprint”(Fernandez and Luthi 2020). Moreover, this differentiation of fast and slow spindles appears to be unique to the human cortex possibly driven by recent evolution of the frontal neocortex in humans that generates slow spindles, and not so certain in the animal neuroscience literature (Cox et al., 2017) where predominantly only fast spindles are to be found (Kim et al., 2019). Finally, it should be noted that thalamic neurons exhibit very similar oscillatory frequencies as measured at cortex (Steriade et al., 1993), suggestive of a potential mechanisms of information transfer between cortex and deeper brain regions via thalamocortical feedback loops.
There is also a notable sleep-cycle dependent evolution of spindles. Whereas spindles typically dominate earlier in sleep, especially in N2 sleep, this is more so for faster posterior spindles than slower frontal spindles. Slow frontal spindles are known to be present in N3 sleep as well, which is dominated by slow waves (Himanen et al., 2002; Cox et al., 2017). Slow spindles tend to oscillate at slightly slower frequencies in N3 than in stage 2 sleep. The exact mechanism underlying this phenomenon is unclear, possibly reflective of the sleep-stage dependent strength of thalamocortical hyperpolarization sequences. With respect to fast spindles in N3 sleep, the nesting of faster spindles with slow oscillations (oscillations with frequency <1.25Hz) is thought to reflect memory consolidation and learning (Morin et al., 2008; Tamaki et al., 2008; Staresina et al., 2015). This view is based on the nature of the temporal relationship of fast vs. slow spindles to slow oscillations as described in human and animal studies (Steriade 2003, Staresina et al., 2015).
6.3. Inset 3: Connectivity and phase-based analyses of spindles
A commonly used method to detect the connectivity across diverse brain regions is coherence (Nolte et al., 2004). Coherence is a complex number, whose magnitude depends on the level of synchrony between two signals at a specific frequency and whose phase information denotes the relative offset between two signals’ oscillations at that specific frequency. If there is perfect synchrony, then the magnitude of the coherence is 1; it is 0 for completely unrelated signals.
There are various extensions on this analysis. Notably, to account for the fact that volume conduction from a common cortical source or a common EEG reference can falsely give rise to high coherence values, Nolte et al. suggested methods to determine whether signals at different leads actually reflected a functional connection between source neuronal populations (Nolte et al., 2004). In intracranial recordings, for example, such measures have elegantly been used to map out hippocampal-amygdala connectivity underlying anxiety and mood variations (Kirkby et al., 2018).
The phase locking value is a related frequency-based measure (Lachaux et al., 1999) used to understand functional connectivity between diverse brain regions and alleviates some of the noise issues associated with coherence. It evaluates the circular distribution of phase differences between two electrode's signals and in general this procedure is thought to be more robust to issues such as electrical noise. Such phase based analyses and its extensions (Stam et al., 2007; Vinck et al., 2011) have been used by Laxminarayan, Wang and colleagues in understanding cortical spindle networks in PTSD to great effect (Laxminarayan, Wang et al., 2020; Wang et al., 2020a).
Here we summarize the crux of the phase-based analyses specific to spindle networks. In brief, this procedure involves extracting the phase time series (in radians or degrees) of each channel's spindle band activity (Inset 3 Fig. A and B) and subsequently evaluating the circular difference between any two channels' phase time series (Inset 3 Fig. C). The circular difference is used given that the phase information is cyclic from 0 to for the spindle signal. A circular histogram or distribution of the phase differences is then plotted (Inset 3 Fig. D) and the circular mean of all the phase differences is the phase locking value, a complex number. From this complex number, we can extract its absolute value which is its length and the angular argument which is the mean phase difference (Inset 3 Fig. D). The absolute value ranges from 0 (i.e., no synchrony) to 1 (i.e., perfect synchrony), meaning that the phase difference is perfectly stable across time. The angle of the complex PLV (termed the mean phase difference (MPD) in (Wang et al., 2020b)) denotes the lead or lag between the two signals of interest. Note that if two signals are not phase-coupled to each other at a specific frequency, then the distribution of phase differences will be uniformly distributed for all phases i.e., maximally random. As a result, the length of the PLV will be close to zero, denoting no coupling.
CRediT authorship contribution statement
Nikhilesh Natraj: literature review, Formal analysis, writing and generation of figures. Anne Richards: Conceptualization, literature review, Formal analysis, writing.
Declaration of competing interest
The authors Drs. Anne Richards and Nikhilesh Natraj have no conflicts of interest related to this review article to report.
Acknowledgements
Funding for this project was provided by the U.S. Department of Veterans Affairs through a VA Career Development Award to Dr. Anne Richards (5IK2CX000871-05).
References
- Alger S.E., Kensinger E.A., Payne J.D. Preferential consolidation of emotionally salient information during a nap is preserved in middle age. Neurobiol. Aging. 2018;68:34–47. doi: 10.1016/j.neurobiolaging.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrillon T., Nir Y., Staba R.J., Ferrarelli F., Cirelli C., Tononi G., Fried I. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J. Neurosci. 2011;31(49):17821–17834. doi: 10.1523/JNEUROSCI.2604-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azza Y., Wilhelm F.H., Seifritz E., Junghanns K., Kleim B., Wilhelm I. Sleep's role in updating aversive autobiographical memories. Transl. Psychiatry. 2022;12(1):117. doi: 10.1038/s41398-022-01878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babadi B., Brown E.N. A review of multitaper spectral analysis. IEEE (Inst. Electr. Electron. Eng.) Trans. Biomed. Eng. 2014;61(5):1555–1564. doi: 10.1109/TBME.2014.2311996. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Nanovska S., Regen W., Spiegelhalder K., Feige B., Nissen C., Reynolds C.F., Riemann D. Sleep and mental disorders: a meta-analysis of polysomnographic research. Psychol. Bull. 2016;142(9):969–990. doi: 10.1037/bul0000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran, B., Pace-Schott, E.F., Ericson, C., Spencer, R.M.C., 2012. Processing of emotional reactivity and emotional memory over sleep. J. Neurosci.. 2012 Jan 18;32(3):1035-42. [DOI] [PMC free article] [PubMed]
- Bastos A.M., Schoffelen J.-M. A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front. Syst. Neurosci. 2016;9:175. doi: 10.3389/fnsys.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J., Loretz E., Rasch B. Stress dynamically reduces sleep depth: temporal proximity to the stressor is crucial. Cerebr. Cortex. 2022;33(1):96–113. doi: 10.1093/cercor/bhac055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Simon E., Rossi A., Harvey A.G., Walker M.P. Overanxious and underslept. Nat. Human Behav. 2020;4(1):100–110. doi: 10.1038/s41562-019-0754-8. [DOI] [PubMed] [Google Scholar]
- Berry Rb A.C., Harding S.M., Lloyd R.M., Plante D.T., Quan S.F., et al. American Academy of Sleep Medicine; 2018. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.5. Darien, IL. [Google Scholar]
- Blaskovich B., Reichardt R., Gombos F., Spoormaker V.I., Simor P. Cortical hyperarousal in NREM sleep normalizes from pre- to post- REM periods in individuals with frequent nightmares. Sleep. 2020;43(1) doi: 10.1093/sleep/zsz201. [DOI] [PubMed] [Google Scholar]
- Brunton B.W., Johnson L.A., Ojemann J.G., Kutz J.N. Extracting spatial–temporal coherent patterns in large-scale neural recordings using dynamic mode decomposition. J. Neurosci. Methods. 2016;258:1–15. doi: 10.1016/j.jneumeth.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Buzsaki G., Anastassiou C.A., Koch C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012;13(6):407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero J.L., Atienza M., Salas R.M. Human alpha oscillations in wakefulness, drowsiness period, and REM sleep: different electroencephalographic phenomena within the alpha band. Neurophysiologie Clinique/Clinical Neurophysiol. 2002;32(1):54–71. doi: 10.1016/s0987-7053(01)00289-1. [DOI] [PubMed] [Google Scholar]
- Chambon S., Thorey V., Arnal P.J., Mignot E., Gramfort A. DOSED: a deep learning approach to detect multiple sleep micro-events in EEG signal. J. Neurosci. Methods. 2019;321:64–78. doi: 10.1016/j.jneumeth.2019.03.017. [DOI] [PubMed] [Google Scholar]
- Clemens Z., Fabo D., Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132(2):529–535. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Clemens Z., Mölle M., Erőss L., Jakus R., Rásonyi G., Halász P., Born J. Fine‐tuned coupling between human parahippocampal ripples and sleep spindles. Eur. J. Neurosci. 2011;33(3):511–520. doi: 10.1111/j.1460-9568.2010.07505.x. [DOI] [PubMed] [Google Scholar]
- Coppieters 't Wallant D., Maquet P., Phillips C. Neural Plast; 2016. Sleep Spindles as an Electrographic Element: Description and Automatic Detection Methods. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R., Schapiro A.C., Manoach D.S., Stickgold R. Individual differences in frequency and topography of slow and fast sleep spindles. Front. Hum. Neurosci. 2017;11:433. doi: 10.3389/fnhum.2017.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino A., Castelnovo A., Cavallotti S., Casetta C., Marcatili M., Gambini O., Canevini M., Tononi G., Riedner B., Ferrarelli F., Sarasso S. Sleep endophenotypes of schizophrenia: slow waves and sleep spindles in unaffected first-degree relatives. NPJ Schizophr. 2018;4(1):2. doi: 10.1038/s41537-018-0045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang-Vu T.T., Salimi A., Boucetta S., Wenzel K., O'Byrne J., Brandewinder M., Berthomier C., Gouin J.P. Sleep spindles predict stress-related increases in sleep disturbances. Front. Hum. Neurosci. 2015;9:68. doi: 10.3389/fnhum.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer M., Nijdam M.J., Jongedijk R.A., Bangel K.A., Olff M., Hofman W.F., Talamini L.M. The spectral fingerprint of sleep problems in post-traumatic stress disorder. Sleep. 2020;43(4) doi: 10.1093/sleep/zsz269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L., Ferrara M. Sleep spindles: an overview. Sleep Med. Rev. 2003;7(5):423–440. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- Denis D., Bottary R., Cunningham T.J., Zeng S., Daffre C., Oliver K.L., Moore K., Gazecki S., Kram Mendelsohn A., Martinez U., Gannon K., Lasko N.B., Pace-Schott E.F. Sleep power spectral density and spindles in PTSD and their relationship to symptom severity. Front. Psychiatr. 2021;12 doi: 10.3389/fpsyt.2021.766647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis D., Kim S.Y., Kark S.M., Daley R.T., Kensinger E.A., Payne J.D. Slow oscillation-spindle coupling is negatively associated with emotional memory formation following stress. Eur. J. Neurosci. 2022;55(9–10):2632–2650. doi: 10.1111/ejn.15132. [DOI] [PubMed] [Google Scholar]
- Diekelmann S., Born J. The memory function of sleep. Nat. Rev. Neurosci. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Donoghue T., Haller M., Peterson E.J., Varma P., Sebastian P., Gao R., Noto T., Lara A.H., Wallis J.D., Knight R.T. Parameterizing neural power spectra into periodic and aperiodic components. Nat. Neurosci. 2020;23(12):1655–1665. doi: 10.1038/s41593-020-00744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotan Y., Suraiya S., Pillar G. [Sleep spindles in post traumatic stress disorder: significant importance of selective serotonin reuptake inhibitors] Harefuah. 2008;147(10):763–767. 839-740. [PubMed] [Google Scholar]
- Dudai Y., Karni A., Born J. The consolidation and transformation of memory. Neuron. 2015;88(1):20–32. doi: 10.1016/j.neuron.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Eschenko O., Mölle M., Born J., Sara S.J. Elevated sleep spindle density after learning or after retrieval in rats. J. Neurosci. 2006;26(50):12914–12920. doi: 10.1523/JNEUROSCI.3175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez L.M.J., Luthi A. Sleep spindles: mechanisms and functions. Physiol. Rev. 2020;100(2):805–868. doi: 10.1152/physrev.00042.2018. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F., Huber R., Peterson M.J., Massimini M., Murphy M., Riedner B.A., Watson A., Bria P., Tononi G. Reduced sleep spindle activity in schizophrenia patients. Am. J. Psychiatr. 2007;164(3):483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- Fogel S.M., Smith C.T. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci. Biobehav. Rev. 2011;35(5):1154–1165. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Gais S., Molle M., Helms K., Born J. Learning-dependent increases in sleep spindle density. J. Neurosci. 2002;22(15):6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy I.R., Bryant R.A. 636,120 ways to have posttraumatic stress disorder. Perspect. Psychol. Sci. 2013;8(6):651–662. doi: 10.1177/1745691613504115. [DOI] [PubMed] [Google Scholar]
- Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am. J. Psychiatr. 2013;170(4):372–382. doi: 10.1176/appi.ajp.2012.12040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goder R., Graf A., Ballhausen F., Weinhold S., Baier P.C., Junghanns K., Prehn-Kristensen A. Impairment of sleep-related memory consolidation in schizophrenia: relevance of sleep spindles? Sleep Med. 2015;16(5):564–569. doi: 10.1016/j.sleep.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Goldstein A.N., Walker M.P. The role of sleep in emotional brain function. Annu. Rev. Clin. Psychol. 2014;10:679–708. doi: 10.1146/annurev-clinpsy-032813-153716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann D., Hewig J., Walter C., Naumann E. Skull thickness and magnitude of EEG alpha activity. Clin. Neurophysiol. 2008;119(6):1271–1280. doi: 10.1016/j.clinph.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Helfrich R.F., Mander B.A., Jagust W.J., Knight R.T., Walker M.P. Old brains come uncoupled in sleep: slow wave-spindle synchrony, brain atrophy, and forgetting. Neuron. 2018;97(1):221–230. doi: 10.1016/j.neuron.2017.11.020. e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen S.L., Virkkala J., Huhtala H., Hasan J. Spindle frequencies in sleep EEG show U‐shape within first four NREM sleep episodes. J. Sleep Res. 2002;11(1):35–42. doi: 10.1046/j.1365-2869.2002.00273.x. [DOI] [PubMed] [Google Scholar]
- Kaestner E.J., Wixted J.T., Mednick S.C. Pharmacologically increasing sleep spindles enhances recognition for negative and high-arousal memories. J. Cognit. Neurosci. 2013;25(10):1597–1610. doi: 10.1162/jocn_a_00433. [DOI] [PubMed] [Google Scholar]
- Kim J., Gulati T., Ganguly K. Competing roles of slow oscillations and delta waves in memory consolidation versus forgetting. Cell. 2019;179(2):514–526. doi: 10.1016/j.cell.2019.08.040. e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby L.A., Luongo F.J., Lee M.B., Nahum M., Van Vleet T.M., Rao V.R., Dawes H.E., Chang E.F., Sohal V.S. An amygdala-hippocampus subnetwork that encodes variation in human mood. Cell. 2018;175(6):1688–1700. doi: 10.1016/j.cell.2018.10.005. e1614. [DOI] [PubMed] [Google Scholar]
- Kleim B., Wysokowsky J., Schmid N., Seifritz E., Rasch B. Effects of sleep after experimental trauma on intrusive emotional memories. Sleep. 2016;39(12):2125–2132. doi: 10.5665/sleep.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinzing J.G., Mölle M., Weber F., Supp G., Hipp J.F., Engel A.K., Born J. Spindle activity phase-locked to sleep slow oscillations. Neuroimage. 2016;134:607–616. doi: 10.1016/j.neuroimage.2016.04.031. [DOI] [PubMed] [Google Scholar]
- Kobayashi I., Boarts J.M., Delahanty D.L. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44(4):660–669. doi: 10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- Kulkarni P.M., Xiao Z., Robinson E.J., Jami A.S., Zhang J., Zhou H., Henin S.E., Liu A.A., Osorio R.S., Wang J. A deep learning approach for real-time detection of sleep spindles. J. Neural. Eng. 2019;16(3) doi: 10.1088/1741-2552/ab0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux J.P., Rodriguez E., Martinerie J., Varela F.J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 1999;8(4):194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacourse K., Yetton B., Mednick S., Warby S.C. Massive online data annotation, crowdsourcing to generate high quality sleep spindle annotations from EEG data. Sci. Data. 2020;7(1):1–14. doi: 10.1038/s41597-020-0533-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchoumane C.-F.V., Ngo H.-V.V., Born J., Shin H.-S. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron. 2017;95(2):424–435. doi: 10.1016/j.neuron.2017.06.025. e426. [DOI] [PubMed] [Google Scholar]
- Laxminarayan S., Wang C., Ramakrishnan S., Oyama T., Cashmere J.D., Germain A., Reifman J. Alterations in sleep electroencephalography synchrony in combat-exposed veterans with post-traumatic stress disorder. Sleep. 2020;43(7):zsaa006. doi: 10.1093/sleep/zsaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Van Quyen M., Foucher J., Lachaux J.-P., Rodriguez E., Lutz A., Martinerie J., Varela F.J. Comparison of Hilbert transform and wavelet methods for the analysis of neuronal synchrony. J. Neurosci. Methods. 2001;111(2):83–98. doi: 10.1016/s0165-0270(01)00372-7. [DOI] [PubMed] [Google Scholar]
- Lehmann M., Schreiner T., Seifritz E., Rasch B. Emotional arousal modulates oscillatory correlates of targeted memory reactivation during NREM, but not REM sleep. Sci. Rep. 2016;6(1):1–13. doi: 10.1038/srep39229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N., Lafortune M., Godbout J., Barakat M., Robillard R., Poirier G., Bastien C., Carrier J. Topography of age-related changes in sleep spindles. Neurobiol. Aging. 2013;34(2):468–476. doi: 10.1016/j.neurobiolaging.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Massimini M., Huber R., Ferrarelli F., Hill S., Tononi G. The sleep slow oscillation as a traveling wave. J. Neurosci. 2004;24(31):6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman T.A. A new meta-analysis of sleep findings in PTSD, toward integration and coherence. Sleep Med. Rev. 2019;48 doi: 10.1016/j.smrv.2019.101220. [DOI] [PubMed] [Google Scholar]
- Mölle M., Bergmann T.O., Marshall L., Born J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep. 2011;34(10):1411–1421. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M., Marshall L., Gais S., Born J. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J. Neurosci. 2002;22(24):10941–10947. doi: 10.1523/JNEUROSCI.22-24-10941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin A., Doyon J., Dostie V., Barakat M., Tahar A.H., Korman M., Benali H., Karni A., Ungerleider L.G., Carrier J. Motor sequence learning increases sleep spindles and fast frequencies in post-training sleep. Sleep. 2008;31(8):1149–1156. [PMC free article] [PubMed] [Google Scholar]
- Natraj N., Neylan T.C., Yack L.M., Metzler T.J., Woodward S.H., Hubachek S.Q., Dukes C., Udapa N.S., Mathalon D.H., Richards A. bioRxiv; 2022. Sleep Spindles Favor Emotion Regulation over Memory Consolidation of Stressors in PTSD. [DOI] [PubMed] [Google Scholar]
- Nir Y., Staba R., Andrillon T., Vyazovskiy V., Cirelli C., Fried I., Tononi G. Regional slow waves and spindles in human sleep. Neuron. 2011;70(1):153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte G., Bai O., Wheaton L., Mari Z., Vorbach S., Hallett M. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin. Neurophysiol. 2004;115(10):2292–2307. doi: 10.1016/j.clinph.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Piantoni G., Halgren E., Cash S.S. The contribution of thalamocortical core and matrix pathways to sleep spindles. Neural Plast. 2016 doi: 10.1155/2016/3024342. PMID: 27144033. Hindawi Publishing Corporation Neural Plasticity Volume 2016, Article ID 3024342, 10 pages http://dx.doi.org/10.1155/2016/30243422016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prerau M.J., Brown R.E., Bianchi M.T., Ellenbogen J.M., Purdon P.L. Sleep neurophysiological dynamics through the lens of multitaper spectral analysis. Physiology. 2017;32(1):60–92. doi: 10.1152/physiol.00062.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S.M., Manoach D.S., Demanuele C., Cade B.E., Mariani S., Cox R., Panagiotaropoulou G., Saxena R., Pan J.Q., Smoller J.W., Redline S., Stickgold R. Characterizing sleep spindles in 11,630 individuals from the national sleep research resource. Nat. Commun. 2017;8 doi: 10.1038/ncomms15930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan D.S., Gulati T., Ganguly K. Sleep-dependent reactivation of ensembles in motor cortex promotes skill consolidation. PLoS Biol. 2015;13(9) doi: 10.1371/journal.pbio.1002263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A., Kanady J.C., Neylan T.C. Sleep disturbance in PTSD and other anxiety-related disorders: an updated review of clinical features, physiological characteristics, and psychological and neurobiological mechanisms. Neuropsychopharmacology. 2020;45(1):55–73. doi: 10.1038/s41386-019-0486-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A., Metzler T.J., Ruoff L.M., Inslicht S.S., Rao M., Talbot L.S., Neylan T.C. Sex differences in objective measures of sleep in post-traumatic stress disorder and healthy control subjects. J. Sleep Res. 2013;22(6):679–687. doi: 10.1111/jsr.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M., Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J. Neurosci. 2005;25(41):9398–9405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M., Hoedlmoser K., Pecherstorfer T., Anderer P., Gruber G., Parapatics S., Sauter C., Kloesch G., Klimesch W., Saletu B. Interindividual sleep spindle differences and their relation to learning-related enhancements. Brain Res. 2008;1191:127–135. doi: 10.1016/j.brainres.2007.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza R.T. F.d., Gerhardt G.J.L., Schönwald S.V., Rybarczyk-Filho J.L., Lemke N. Synchronization and propagation of global sleep spindles. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0151369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam C.J., Nolte G., Daffertshofer A. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum. Brain Mapp. 2007;28(11):1178–1193. doi: 10.1002/hbm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina B.P., Bergmann T.O., Bonnefond M., Van Der Meij R., Jensen O., Deuker L., Elger C.E., Axmacher N., Fell J. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat. Neurosci. 2015;18(11):1679–1686. doi: 10.1038/nn.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. The corticothalamic system in sleep. Front. Biosci. Landmark. 2003;8(4):878–899. doi: 10.2741/1043. [DOI] [PubMed] [Google Scholar]
- Steriade M., McCormick D.A., Sejnowski T.J. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Strauss M., Griffon L., Van Beers P., Elbaz M., Bouziotis J., Sauvet F., Chennaoui M., Leger D., Peigneux P. Order matters: sleep spindles contribute to memory consolidation only when followed by rapid-eye-movement sleep. Sleep. 2022;45(4) doi: 10.1093/sleep/zsac022. [DOI] [PubMed] [Google Scholar]
- Tamaki M., Matsuoka T., Nittono H., Hori T. Fast sleep spindle (13–15 Hz) activity correlates with sleep-dependent improvement in visuomotor performance. Sleep. 2008;31(2):204–211. doi: 10.1093/sleep/31.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden A.C., Hofman W.F., de Boer M., Nijdam M.J., van Marle H.J.F., Jongedijk R.A., Olff M., Talamini L.M. Sleep; 2022. Sleep Spindle Dynamics Suggest Over-consolidation in Post-traumatic Stress Disorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M., Oostenveld R., Van Wingerden M., Battaglia F., Pennartz C.M. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage. 2011;55(4):1548–1565. doi: 10.1016/j.neuroimage.2011.01.055. [DOI] [PubMed] [Google Scholar]
- Voytek B., Kramer M.A., Case J., Lepage K.Q., Tempesta Z.R., Knight R.T., Gazzaley A. Age-related changes in 1/f neural electrophysiological noise. J. Neurosci. 2015;35(38):13257–13265. doi: 10.1523/JNEUROSCI.2332-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley E.J., Tucker M.A., Shinn A.K., Ono K.E., McKinley S.K., Ely A.V., Goff D.C., Stickgold R., Manoach D.S. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biol. Psychiatr. 2012;71(2):154–161. doi: 10.1016/j.biopsych.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Laxminarayan S., Cashmere J.D., Germain A., Reifman J. Inter-channel phase differences during sleep spindles are altered in Veterans with PTSD. Neuroimage: Clinic. 2020;28 doi: 10.1016/j.nicl.2020.102390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Laxminarayan S., Ramakrishnan S., Dovzhenok A., Cashmere J.D., Germain A., Reifman J. Increased oscillatory frequency of sleep spindles in combat-exposed veteran men with post-traumatic stress disorder. Sleep. 2020;43(10) doi: 10.1093/sleep/zsaa064. [DOI] [PubMed] [Google Scholar]
- Warby S.C., Wendt S.L., Welinder P., Munk E.G., Carrillo O., Sorensen H.B., Jennum P., Peppard P.E., Perona P., Mignot E. Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nat. Methods. 2014;11(4):385–392. doi: 10.1038/nmeth.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward S.H., Murburg M.M., Bliwise D.L. PTSD-related hyperarousal assessed during sleep. Physiol. Behav. 2000;70(1–2):197–203. doi: 10.1016/s0031-9384(00)00271-7. [DOI] [PubMed] [Google Scholar]
- Zhang H., Watrous A.J., Patel A., Jacobs J. Theta and alpha oscillations are traveling waves in the human neocortex. Neuron. 2018;98(6):1269–1281. doi: 10.1016/j.neuron.2018.05.019. e1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Ren R., Sanford L.D., Yang L., Zhou J., Zhang J., Wing Y.K., Shi J., Lu L., Tang X. Sleep in posttraumatic stress disorder: a systematic review and meta-analysis of polysomnographic findings. Sleep Med. Rev. 2019;48 doi: 10.1016/j.smrv.2019.08.004. [DOI] [PubMed] [Google Scholar]