Summary
The adult rodent subventricular zone (SVZ) generates neural stem cells (NSCs) throughout life that migrate to the olfactory bulbs (OBs) and differentiate into olfactory interneurons. Few SVZ NSCs generate oligodendrocyte precursor cells (OPCs). We investigated how neurogliogenesis is regulated during aging in mice and in a non-human primate (NHP) model, the gray mouse lemur. In both species, neuronal commitment decreased with age, while OPC generation and myelin content unexpectedly increased. In the OBs, more tyrosine hydroxylase interneurons in old mice, but fewer in lemurs, marked a surprising interspecies difference that could relate to our observation of a continuous ventricle in lemurs. In the corpus callosum, aging promoted maturation of OPCs into mature oligodendrocytes in mice but blocked it in lemurs. The present study highlights similarities and dissimilarities between rodents and NHPs, revealing that NHPs are a more relevant model than mice to study the evolution of biomarkers of aging.
Keywords: neurogenesis, oligodendrogenesis, myelin, subventricular zone, aging, olfaction, rodents, non-human primates
Introduction
Adult neural stem cells (NSCs), localized within the rodent subventricular zone (SVZ), produce neuronal and glial cells throughout life. Adult SVZ NSCs give rise mainly to young immature neurons, neuroblasts, which migrate tangentially through the rostral migratory stream (RMS) toward the olfactory bulbs (OBs) (Lim and Alvarez-Buylla, 2016; Ming and Song, 2011). In the OBs, neuroblasts differentiate into mature and functional dopaminergic and GABAergic interneurons (Lepousez et al., 2013; Lois and Alvarez-Buylla, 1993, 1994). Furthermore, under physiological conditions, few SVZ NSCs generate oligodendrocyte precursor cells (OPCs) (Menn et al., 2006; Suzuki and Goldman, 2003) capable of migrating toward the corpus callosum, where they differentiate into myelinating oligodendrocytes. After a demyelinating insult, SVZ OPCs can participate to myelin repair (Keirstead and Blakemore, 1999; Nait-Oumesmar et al., 1999; Remaud et al., 2017; Xing et al., 2014).
In rodents, several studies have demonstrated an age-related decline in proliferation and neuroblast formation (Enwere et al., 2004; Luo et al., 2006; Piccin et al., 2014; Shook et al., 2012), reducing the supply of newborn olfactory interneurons (Bouab et al., 2011; Capilla-Gonzalez et al., 2013; Daynac et al., 2016; Shuboni-mulligan et al., 2019). The decline in SVZ neurogenesis over the course of life has also been observed in some primates, including humans. In both, SVZ neurogenesis declines rapidly after birth (Bergmann et al., 2012; Gil-Perotin et al., 2009; Pencea et al., 2001; Sanai et al., 2011; Sawamoto et al., 2011; Wang et al., 2011). Moreover, although the production of new SVZ OPCs seems to be preserved throughout life (Capilla-Gonzalez et al., 2013), little is known about whether the capacity of NSCs to commit toward a glial fate is preserved in old rodents and non-human primates (NHPs). Likewise, the age-related effects on NHP olfactory neuronal circuits are unknown, although the integration of new olfactory interneurons decreases by half from the earliest stages of life in the marmoset, a monkey (Akter et al., 2020), and appears to be negligible in humans (Bergmann et al., 2012).
In view of the strong similarities between humans and NHPs and their disparity with rodents, further studies on NHPs are required to develop more translational strategies to address the lack of knowledge on age-related neurodegenerative diseases in humans. An emerging basal NHP model is the gray mouse lemur (Microcebus murinus), a Malagasy prosimian NHP. Lemurs are genetically closely related to humans (estimated 91% nucleotide identity between human and lemur orthologs) and have roughly half the genetic distance between mouse and human (Margulies et al., 2007). Moreover, lemurs present more advantages compared with other NHPs in terms of size, rapid reproduction, lifespan, and relatively large litters (Ezran et al., 2017). Considering its size and weight, the lemur has a relatively long life expectancy, four times longer than mammals with an equivalent body mass (reviewed in Languille et al., 2012), suggesting that lemurs could provide further knowledge on aging processes (Ezran et al., 2017; Languille et al., 2012; Perret, 1997; Pifferi et al., 2014; Rahman et al., 2017). The lemur shows features identical to human cerebral aging (reviewed in Languille et al., 2012) and spontaneously develops age-related deficits, such as (1) biological rhythm disorders, including abnormal sleep/wake rhythms (Cayetanot et al., 2005); (2) hormonal secretion disruption (Aujard et al., 2001; Perret and Aujard, 2006; Pifferi et al., 2014); (3) cognitive decline, including reduced executive function and spatial memory (Chaudron et al., 2021; Languille et al., 2012, 2015; Picq et al., 2012); as well as (4) Alzheimer disease-related neuropathologies (Laurijssens et al., 2013). Finally, neurogenesis has been recently described in the lemur SVZ and OB (Royo et al., 2018). Therefore, NHPs are models complementary to rodents (as observed previously by Pellegrino et al., 2018) and have significant translational potential for studying human aging.
The overall objective of this study is to extend our knowledge on NSC fate commitment in a NHP as a function of aging in comparison with rodents. To this end, we evaluated the age-related effects on the (1) neuro-gliogenesis balance in the mouse and lemur SVZ, (2) interneuronal networks in the OBs as well as olfactory function, and (3) myelination of the corpus callosum. Our work demonstrated fundamental differences between NHPs and rodents that underscore the advantage of the lemur in aging studies, making this model particularly interesting for understanding the mechanisms underlying myelin and neurodegenerative diseases and potential treatments.
Results
Aging favored SVZ oligodendrogenesis at the expense of SVZ neurogenesis
We first investigated the impact of aging on NSC determination in the dorsal SVZ of young, middle-aged, and aged (2, 12, and 18 months old respectively) male mice using markers for neuronal (doublecortin or DCX+) and oligodendroglial (OLIG2+) precursors (Figure 1A). As expected (Capilla-Gonzalez et al., 2013), the density of DCX+ neuroblasts decreased as a function of age by more than half in 18-month-old mice compared with young adults (Bonferroni test, p < 0.05; Figure 1B). In contrast, the density of OLIG2+ OPCs was significantly higher in middle-aged and aged mice (Bonferroni test; p = 0.002 and p = 0.012, respectively; Figure 1C) compared with young mice. Accordingly, the neuron/glia ratio shifted from ±0.9/0.1 in young adults to ±0.7/0.3 in old mice (Bonferroni test, p < 0.0001; Figure 1G).
Figure 1.
Aging affected the neurogenesis/oligodendrogenesis balance in vivo similarly in mice and lemurs
(A) DCX+ neuroblasts and OLIG2+ OPCs within the dorsal SVZ of 2- and 18-month-old male mice.
(B and C) The neuroblast density (B) is reduced, whereas the OPC density (C) is increased in the SVZ in middle-aged and aged mice compared with young mice.
(D) DCX+ neuroblasts and OLIG2+ OPCs within the distal SVZ of 3- and 9-year-old lemurs.
(E and F) Neuroblast (E) and OPC (F) densities are negatively and positively correlated, respectively, with the animals’ age.
(G) The ratio of DCX+ neuroblasts versus OLIG2+ OPCs in the SVZ is reduced in aged animals compared with young mice and lemurs.
Scale bars, 100 μm. Mice: n = 10, one-way ANOVA followed by Bonferroni test; lemurs: n = 6–10, Spearman correlation. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. LV, lateral ventricle.
In parallel, we analyzed the age-related effect on neuro-gliogenesis in the lemur distal SVZ (Figure 1D). As in mice, the density of DCX+ neuroblasts gradually decreased during aging (Spearman correlation, p = 0.023; Figure 1E), whereas the generation of OLIG2+ OPCs was more than 2-fold higher in 9-year-old compared with 3-year-old animals (Spearman correlation, p = 0.002; Figure 1F). In 3-year-old lemurs, more than 80% of committed cells were DCX+ neuroblasts, and only 20% were OPCs, but this ratio gradually shifted to ±0.5/0.5 in 9-year-old animals (Bonferroni test, p < 0.0001; Figure 1G). Hence, the distal SVZ output was predominantly neurogenic in the young adult and became undifferentiated in the aged lemur; i.e., no preference for generating more neuroblasts than OPCs. Therefore, aging reduced SVZ neurogenesis, while OPC genesis became more prominent in mice and in lemurs.
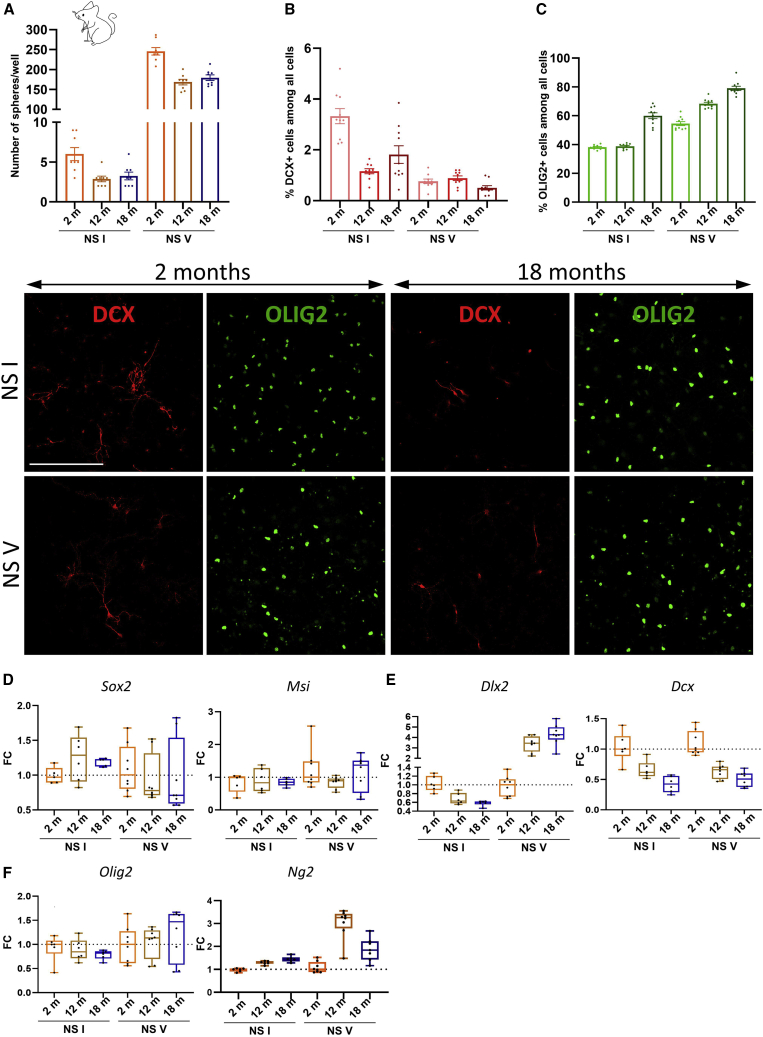
Then, we compared the in vitro self-renewal potential of young versus aged mice SVZ NSCs using the well-established neurosphere model. Please note that the neurosphere experiment was carried out only once and, thus, did not allow us to perform statistical tests. To expand specifically the pool of NSCs at the expense of committed progenitors, neurospheres can be serially subcultured (passaged several times) (Azari et al., 2010). Floating neurospheres were imaged to determine neurosphere numbers per well (Figure 2A) over serial clonal passaging from primary neurospheres (NS I) to NSC-enriched neurospheres (NS V). Aged SVZ cells generated approximately 50% fewer NS Is than young SVZ cells. After five passages, the number of NS Vs generated from middle-aged and aged SVZ NSCs was lower than those from young SVZ NSCs. These data confirm that aged NSCs have lower proliferation and self-renewal capacities (Ahlenius et al., 2009; Enwere et al., 2004; Piccin et al., 2014).
Figure 2.
In vitro proliferation and determination of young versus aging SVZ NSCs
(A) NS I formation is not affected by aging, while SVZ NSCs derived from aged mice generate fewer neurospheres after 5 passages (N = 8 wells per group).
(B) DCX+ neuroblast generation derived from middle-aged and aged NS Is decreased. The proportion of DCX+ neuroblasts after 5 passages is not affected by aging (N = 30 images per group) but seemed to be decreased in 2-month-old mice.
(C) OLIG2+ OPC densities derived from aged NS Is and NS Vs are increased. The enrichment in NSCs increased OPC formation in the three age groups (N = 30 images per group).
(D) Gene expression levels in primary (NS I) and enriched neurospheres (NS V) generated from young versus aged SVZ NSCs.
(D–F) Gene expression analysis of (D) NSC/progenitor markers (Sox2 and Msi), (E) neuronal progenitor (Dlx2) and neuroblast (Dcx) markers, and (F) oligodendrocyte markers (Olig2 and Ng2).
N = 5–8 wells per group. Scale bars, 100 μm.
NS Is and NS Vs were then dissociated and plated without growth factors during 5 days, and percentages of DCX+ neuroblasts (Figure 2B) and OLIG2+ OPCs (Figure 2C) were quantified. NS Is derived from 12- and 18-month-old mice generated 67% and 42% fewer neuroblasts than young adult mice. However, after five passages, neuroblast generation is not altered by age (Figure 2B). While the proportion of neuroblasts was unchanged between passages I and V for middle-aged and aged SVZ NSCs, NSC enrichment was associated with a 77% drop in the proportion of DCX+ neuroblasts in 2-month-old mice (Figure 2B). In parallel, NS Is derived from 18-month-old mice generated 50% more OLIG2+ OPCs compared with the other two age groups (Figure 2C). Moreover, NS Vs generated from 12- and 18-month-old mice produced, respectively, 30% and 45% more OPCs than those from young adult mice. Interestingly, NSC enrichment favored OPC production in all three age groups. Together, our in vivo and in vitro experimental approaches demonstrate that aged SVZ NSCs commit increasingly to the glial lineage, unlike young NSCs, which generate predominantly neuroblasts.
Last, we characterized the molecular signature changes that underlie this progressive determination of NSCs toward a glial fate. To this end, we investigated gene expression profiles using RNA extracted from neurospheres, using primers for NSCs/progenitors (Sox2 and Msi; Figure 2D), neuronal precursor cells (Dlx2 and Dcx; Figure 2E), and OPCs (Olig2 and Ng2; Figure 2F) by qRT-PCR. No effects of aging were observed in expression of Sox2 and Msi. In NS Is derived from old mice, we found potentially decreased Dlx2 and Dcx expression. Decreased Dcx expression as a function of aging was also observed in NS Vs. Surprisingly, Dlx2 expression seemed upregulated in aged NS Vs. Concerning OPCs, although Olig2 expression was not modified with aging, Ng2 was apparently upregulated in NS Is and NS Vs, suggesting a pro-gliogenic effect of aging. Altogether, these results suggest that aged NSCs have intrinsic capacity to generate more OPCs than neuronal progenitors compared with young NSCs.
Anatomical description of the gray mouse lemur continuous ventricle
The anatomical structure of adult lemur ventricular walls was examined at three levels of the rostro-caudal axis: anterior (Figure S1A), median (Figure S1B), and posterior (Figure S1C). At the anterior level, a cavity corresponding to the lateral ventricle (LV) is observed. This cavity elongates and forms a dorsolateral extension between the corpus callosum and the striatum at the median level. At the posterior level, the ventricular wall is subdivided into two distinct areas: a narrow space between the corpus callosum and the striatum in which the two opposite ventricular walls are very close to each other or collapsed (dorsal part) and a ventricular space containing cerebrospinal fluid (distal part).
This particular anatomical organization of the lemur SVZ (Figure S1D) suggests a different organization of the RMS. In the adult lemur, the morphology of the RMS-like pathway was first examined using brain sagittal sections stained with DAPI nuclear dye (Figure S1E). As for other NHPs, the LV of lemurs extends toward the OBs in a continuous tube-like shape (Curtis et al., 2007; Gil-Perotin et al., 2009; Kam et al., 2009; Wang et al., 2011). Consequently, this continuous ventricle begins at the ventral part of the LV , grows rostro-dorsally along the rostrum of the corpus callosum, takes a latero-rostro-ventral turn to traverse the striatum, and enters the olfactory tract. Finally, the continuous ventricle terminates in the anterior nucleus of the OB and forms an olfactory ventricle in the granular cell layer, the innermost layer of the OB.
DCX-expressing neuroblasts were found throughout the RMS-like pathway, organized around the continuous ventricle (Figure S1F). Neuroblasts formed chain-like structures and exhibited a heterogeneous morphology depending on their location in the RMS, notably in terms of shape and length of processes. At the edge of the LVs, neuroblasts have an immature morphology with spherical cell bodies (frame F1), while they adopt a fusiform and unipolar extension toward the middle of the RMS (frame F2). In the olfactory tract and OBs, neuroblasts had the same morphological characteristics as mature neurons (i.e., an elongated cell body and long processes; bipolar form, frame F3), suggesting that neuroblast migration and/or maturation processes occur in the adult lemur forebrain.
Age-related effects on the cellular organization of the RMS
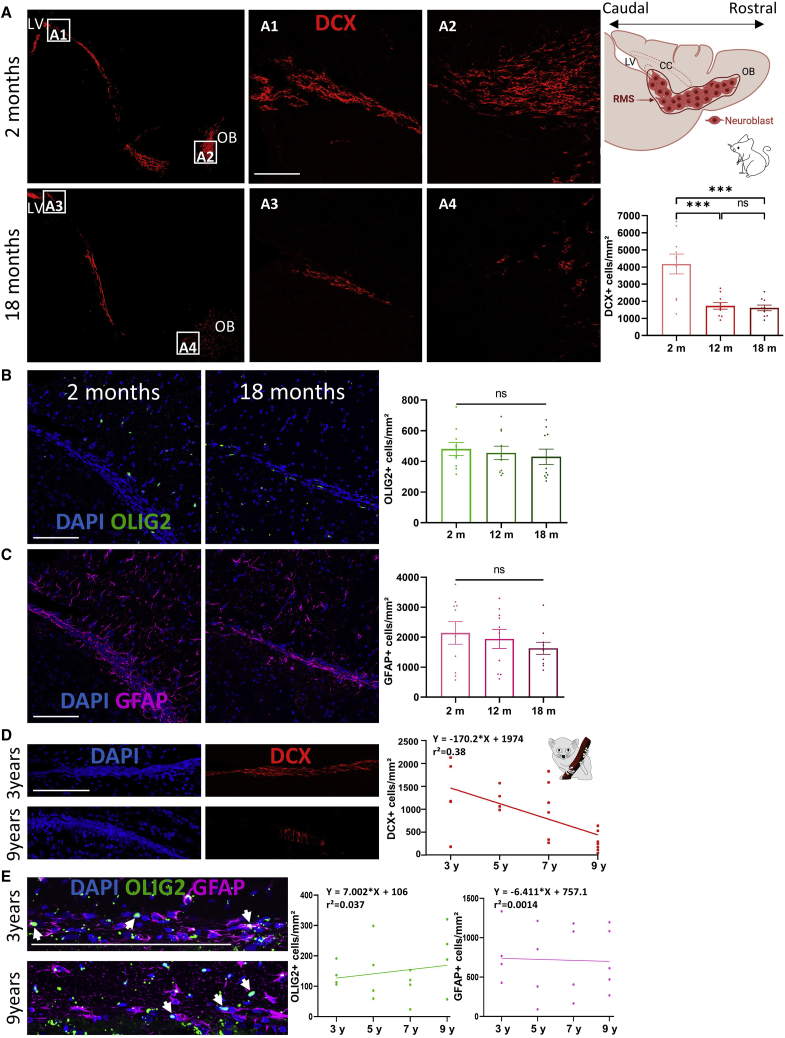
To determine whether the decrease in SVZ neurogenesis during aging alters the amount of migrating neuronal precursors, we quantified DCX+ neuroblasts along the entire RMS in both species (Figure 3). As expected, the density of DCX+ neuroblasts sharply decreased with age, by approximately 60% in middle-aged and aged mice compared with young adults (Bonferroni test; p = 0.005 and 0.0002, respectively; Figure 3A). Glial cells, particularly astrocytes, provide a substrate for neuroblast migration and survival throughout the RMS (Gengatharan et al., 2016). We therefore quantified glial cells in the RMS and showed that the densities of OLIG2+ OPCs (ANOVA test, p > 0.05; Figure 3B) and GFAP+ (glial fibrillary acidic protein) astrocytes (ANOVA test, p > 0.05; Figure 3C) were not modified during aging.
Figure 3.
Aging decreased the density of neuroblasts throughout the RMS in both species
(A) DCX+ neuroblasts along the entire RMS of 2- and 18-month-old mice (left boxes). The insets show the migratory chain of neuroblasts localized at the beginning (A1 and A3, near the LV) and the end of the RMS (A2 and A4, near the OB). On the right is a schematic sagittal representation of neuroblast migratory chains along the RMS. The neuroblast density is reduced in 12- and 18-month-old mice.
(B and C) The OPC (B) and astrocyte (C) densities were equivalent in the three age groups.
(D) A negative correlation was found between the neuroblast density and the age of the lemurs.
(E) The astrocyte and OPC densities are unaffected during aging in the lemur RMS.
Scale bars, 100 μm (A–D) and 50 μm (E). White arrows point to OLIG2+ OPCs. Mice: n = 10, one-way ANOVA followed by Bonferroni test; lemurs: n = 4–7, Spearman correlation. ∗∗∗p < 0.001. OB, olfactory bulb.
Interestingly, we observed that neuroblasts derived from old lemurs did not form migratory chains and were often found isolated. A negative correlation between the density of DCX+ neuroblasts and the animals’ age was observed, showing a 70% reduction of neuroblasts located in the RMS of 9-year-old lemurs compared with young adults (Spearman correlation, p = 0.0013; Figure 3D). As in mice, the two populations of glial cells present in the RMS are unaffected by aging (Spearman correlation, p > 0.05; Figure 3E). These results strongly indicate that, as in mice, aging alters the generation of neuroblasts and their migration/survival within the RMS, most likely decreasing the pool of neuroblasts that serves the formation of new olfactory interneurons.
Dopaminergic interneurons are the only subpopulation affected as a function of age in both species
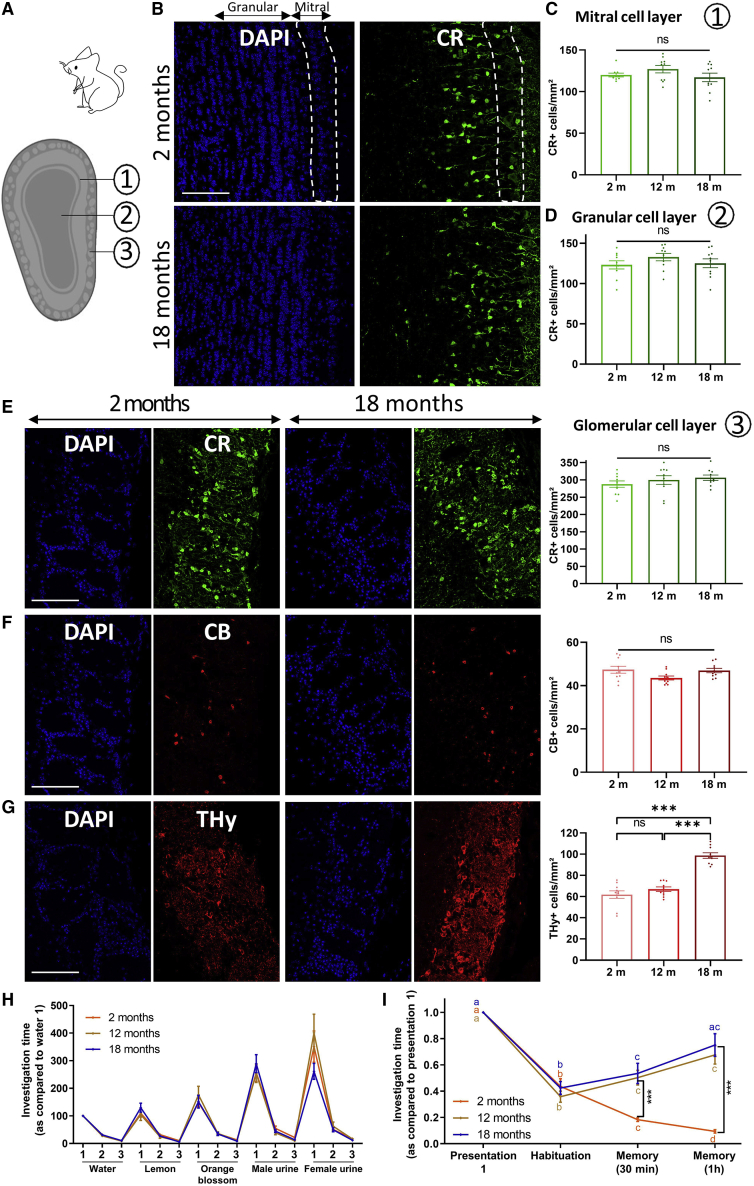
In the OBs, SVZ-derived neuroblasts differentiate into granular and periglomerular interneurons that migrate to the granular/mitral and glomerular cell layer, respectively (Alvarez-Buylla et al., 2001; Lim and Alvarez-Buylla, 2016; Figures 4A and 5A). Therefore, we analyzed the different OB interneurons, Calretinin (CR), Calbindin (CB), and tyrosine hydroxylase (THy) interneurons. The density of CR+ interneurons was not modified in the mitral or in the granular cell layers in aged mice (ANOVA test, p > 0.05; Figures 4B–4D) and lemurs (Spearman correlation, p > 0.05; Figures 5B–5D). The glomerular cell layer, the outermost OB layer, is composed of the three interneuron populations (Lim and Alvarez-Buylla, 2016). Again, the density of CR+ and CB+ interneurons was unaffected by aging in mice (ANOVA test, p > 0.05; Figures 4E and 4F) and lemurs (Spearman correlation, p > 0.05; Figures 5E and 5F). In contrast, aging affected THy+ dopaminergic interneurons but in an opposite manner in both species; their density was higher in 18-month-old mice (Bonferroni test, p < 0.0001; Figure 4G), whereas a 50% loss was observed in older lemurs compared with young adults (Spearman correlation, p < 0.0001; Figure 5G). These data demonstrate that the age-dependent decrease of SVZ neurogenesis does not impact GABAergic interneuron populations in either species. Interestingly, only the density of dopaminergic neurons was modified with aging, but differently in both species.
Figure 4.
OB organization throughout life and olfactory behavior analysis in mice
(A) Schematic of the three OB layers in mice.
(B) Calretinin+ (CR) interneurons in the mitral and granular cell layers of 2- and 18-month-old mice.
(C and D) The density of CR+ interneurons in the mitral and granular cell layers was equivalent in the three age groups.
(E and F) The densities of CR+ (E) and CB+ (F) interneurons are equivalent in the three age groups in the glomerular cell layer.
(G) The density of THy+ interneurons is increased in 18-month-old mice.
(H) Aged mice were able to discriminate odors, as observed by the reduced investigation time during three consecutive presentations of the same odor and increased investigation time when a novel odor was presented.
(I) The short-term olfactory memory is impaired in middle-aged and aged mice, as shown by the increased investigation time 30 min and 1 h after the previous presentation. Asterisks mark differences between groups at one time point and letters between time points within one group.
Scale bars, 100 μm. n = 10, one-way ANOVA followed by Bonferroni test, ∗∗∗p < 0.001.
Figure 5.
OB organization throughout life in lemurs
(A) Schematic of the three OB layers in lemurs.
(B) CR interneurons in the mitral and granular cell layers of 3- and 9-year-old lemurs.
(C and D) No correlation was found between the density of CR+ interneurons and the animals’ age in the mitral and granular cell layer groups.
(E and F) The densities of CR+ (E) and CB+ (F) interneurons were not affected by aging in the glomerular cell layer.
(G) A negative correlation was observed between the THy+ interneuron density and the animals’ age.
Scale bars, 100 μm. n = 5–9, Spearman correlation.
Rodent olfactory memory is impaired during aging
Then, we assessed the age-related effects on odor discrimination and memorization abilities in mice using the habituation-dishabituation and the short-term olfactory memory test (Arbuckle et al., 2015; Lazarini et al., 2009). First, aging did not impair the ability to discriminate easily distinguishable non-social and social odors, as observed by the decreased investigation time after three consecutive presentations of the same odor, or the increase when a novel odor was presented (Bonferroni test, p < 0.05; Figure 4H).
In the short-term olfactory memory test, the investigation time of young adults was significantly reduced over the four successive odor presentations (Bonferroni test, p < 0.05; Figure 4I). In middle-aged and aged mice, the investigation time decreased with the two first presentations (Bonferroni test, p < 0.0001). However, after 30-min and 1-h intervals (for both time points: Bonferroni test, p < 0.05), the investigation time in 12- and 18-month-old mice increased significantly and was almost as long as the first presentation for the older mice (Bonferroni test, p > 0.05). This shows that middle-aged and aged mice are less able to memorize easily distinguishable odors. Consequently, both groups spent significantly more time investigating the odor during the memorization phase compared with young adults (Bonferroni test, p < 0.001). Together, these results show that decreased SVZ neurogenesis during aging is strongly associated with impairment of short-term olfactory memory.
Aging favored recrudescence and maturation of oligodendrocytes in the corpus callosum of both species
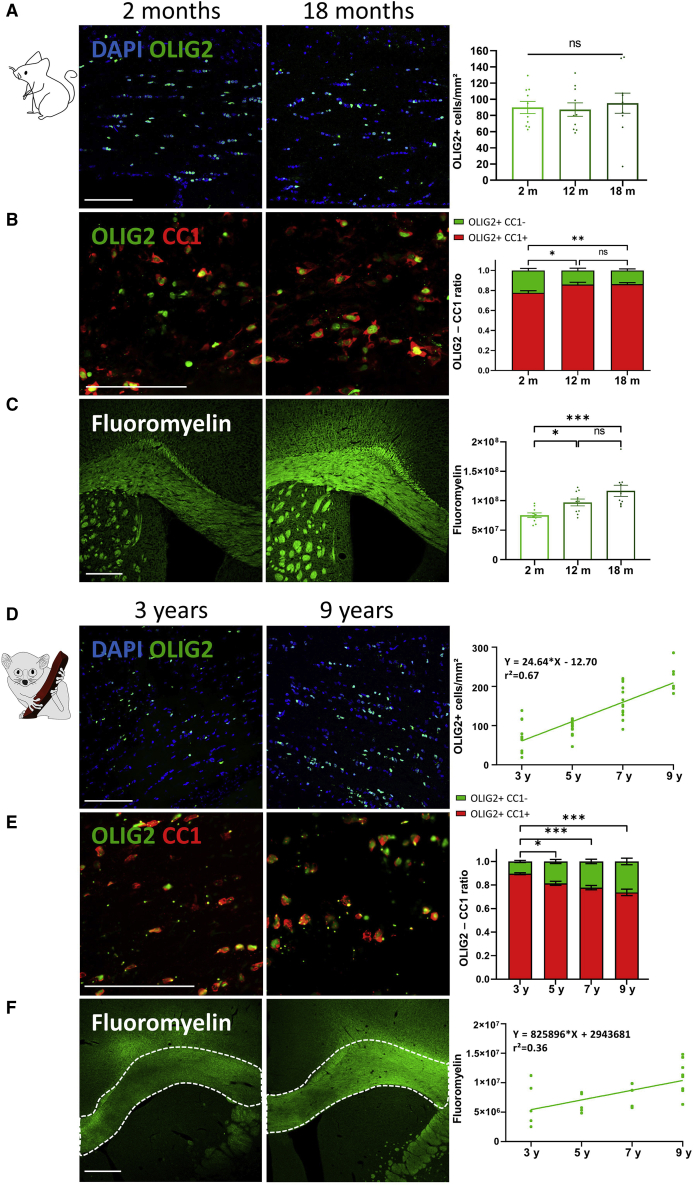
Because aging increased SVZ OPC generation in mice and lemurs, we hypothesized that the OPC density in the corpus callosum would also change with age (Figure 6). In mice, the corpus callosum surface (ANOVA test, p > 0.05; data not shown) and the density of OLIG2+ parenchymal OPCs (pOPCs) (ANOVA test, p > 0.05; Figure 6A) were not modified during aging, suggesting that this oligodendroglial population is maintained throughout life. Next, we evaluated whether aging affected oligodendroglial lineage progression and maturation. We immunostained the protein adenomatous polyposis coli (APC or CC1), which labels mature myelinating oligodendrocytes and evaluated the proportion of pOPCs (OLIG2+ CC1−) versus mature oligodendrocytes (OLIG2+ CC1+; Figure 6B). Unexpectedly, we found a 10% increase in the proportion of mature oligodendrocytes in middle-aged and aged mice and, consequently, a 10% decrease in pOPC proportion (Bonferroni test, p < 0.05), suggesting that aging promotes generation of mature oligodendrocytes in the mouse corpus callosum close to the LVs. In line with these results, we visualized myelin content in the corpus callosum using the FluoroMyelin Green fluorescent myelin stain (Figure 6C). FluoroMyelin staining intensity drastically increased in 12- and 18-month-old mice compared with young adults (Dunn’s test, p = 0.05 and 0.0009, respectively), suggesting progressive myelination of the corpus callosum throughout aging.
Figure 6.
Aging promoted oligodendrocyte generation and myelin content in the corpus callosum
(A and B) In mice, OLIG2+ pOPCs are unaffected by age (A), whereas the ratio of OLIG2+CC1+ mature oligodendrocytes versus OLIG2+CC1− pOPCs (B) showed an increased proportion of mature oligodendrocytes in aged mice.
(C) The FluoroMyelin integrative density is progressively increased as a function of age.
(D) In lemurs, the OLIG2+ pOPC density is progressively increased as a function of age.
(E) The ratio of OLIG2+CC1+ mature oligodendrocytes versus OLIG2+CC1− pOPCs shows a decreased proportion of mature oligodendrocytes in aged lemurs.
(F) A positive correlation between the FluoroMyelin integrative density and the animals’ age was observed in the corpus callosum.
Scale bars, 100 μm and 300 μm (C–F). Mice: n = 10, one-way ANOVA followed by Bonferroni test; lemurs: n = 6–10, Spearman correlation. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Similar to mice, the corpus callosum area of lemurs remained unchanged during aging (Spearman correlation, p = 0.27; data not shown), whereas the density of OLIG2+ OPCs was more than 2-fold higher in 9-year-old lemurs compared with young adults (Spearman correlation, p < 0.0001; Figure 6D). In contrast to mice, a 20% decrease in the proportion of mature oligodendrocytes in aged lemurs was found (Dunn’s test, p < 0.05; Figure 6E), suggesting that aging blocks oligodendrocytes at the precursor stage in the lemur corpus callosum. Next, the FluoroMyelin signal intensity progressively increased during adult life and was twice as high in 9-year-old animals compared with young adults (Spearman correlation, p = 0.0008; Figure 6F), suggesting that the corpus callosum of lemurs is also gradually myelinated during aging.
In parallel, we analyzed myelin content in another white matter area, the striatum, in mouse and lemur models (Figure S3). In both species, FluoroMyelin integrative density was not altered during aging (Dunn’s test and Spearman correlation, p > 0.05), suggesting that OPCs generated during aging populate predominantly the corpus callosum and not another white matter area to produce mature oligodendrocytes.
Existence of olfactory neurogenesis in the lemur, sensitive to aging
In the lemur OBs, the presence of a ventricular cavity strongly suggests the existence of olfactory neurogenesis. We therefore identified the presence of SOX2-expressing neural stem/progenitor cells (NSPCs) in the lemur OBs (Figure S2A). As expected, NSPCs were detected in the ventricular wall (Figure S2B) but also in the three layers of the OB (Figures S2E–S2G). Surprisingly, the olfactory ventricular surface gradually decreased during aging, by approximatively 70% in 9-year-old animals compared with young animals (Spearman correlation, p = 0.0015; Figure S2C). SOX2-expressing NSPC densities in the ventricular border of the OB were similar between the different age groups (data not shown). However, because the olfactory ventricular surface was significantly smaller in older animals, absolute numbers of SOX2+ NSPCs progressively decreased during aging (Spearman correlation, p = 0.04; Figure S2D). We also found an age-related decline in the density of SOX2+ NSPCs in the three layers of the OB: the glomerular (Spearman correlation, p = 0.0004; Figure S2E), granular (p < 0.0001; Figure S2F), and mitral cell layers (p = 0.037; Figure S2G). Like SVZ neurogenesis, olfactory neurogenesis also appears to be sensitive to aging in the lemur.
Discussion
Age-related effects on SVZ-OB neurogenesis have been extensively studied in rodents. They clearly show a decrease in the generation of SVZ-derived neuroblasts during aging (Enwere et al., 2004; Luo et al., 2006; Piccin et al., 2014; Shook et al., 2012). However, little is known about these processes in primates, especially in humans, in whom postnatal SVZ neurogenesis is still debated. In contrast to rodents, for which neuronal production continues throughout adulthood, NHP species, closer to humans, are a more suitable model to assess the aging processes. Use of NHPs will provide a better understanding of how the mammalian brain evolved and, thus, to determine the species most suited to study the mechanisms of human brain plasticity. For this purpose, we used the most basal NHP, the gray mouse lemur. Lemurs span about half the genetic distance between mouse and human (Margulies et al., 2007). Furthermore, age-related brain alterations in lemurs are comparable with those described for human neurodegenerative pathologies (Languille et al., 2012; Bons et al., 2006). Thus, the lemur model represents an attractive bridge between rodents and humans to highlight the evolution of the cellular and molecular mechanisms impacted by aging.
Age-dependent physiological decline in SVZ neurogenesis
By combining in vivo and in vitro approaches, we found a limited self-renewal potential of aged NSCs and a significant decrease in the production of SVZ-derived neuroblasts during aging in mice and lemurs. Interestingly, DCX+ neuroblast density in the lemur SVZ and RMS is significantly lower than in the mouse; 70% and 60%, respectively. This result strongly suggests that, despite the drastic reduction in neuroblast generation during the NHP postnatal period (Gil-Perotin et al., 2009; Pencea et al., 2001; Sawamoto et al., 2011), the lemur SVZ continues to produce neuroblasts throughout adult life, albeit at a lower level. Furthermore, we also demonstrated an age-related decline in neuroblast density in the RMS of mice and lemurs, which could be a consequence of (1) fewer neuroblasts generated in the SVZ and/or (2) impaired neuroblast migration (Capilla-Gonzalez et al., 2013; Shuboni-mulligan et al., 2019), thus decreasing the supply in newly formed olfactory interneurons. However, this decrease is not a consequence of astrocyte scaffold deterioration during aging. For instance, the age-related changes in SVZ neurogenesis in marmosets results in a 50% reduction of newly formed olfactory interneurons (Akter et al., 2020). Likewise, the integration of newborn olfactory neurons into the human pre-existing neuronal networks seems to be negligible (Bergmann et al., 2012; Wang et al., 2011).
Generation of the three olfactory interneuron populations depends on a spatiotemporally regulated transcriptional code along the dorso-latero-ventral SVZ (Alvarez-Buylla et al., 2008; Bovetti et al., 2007; Kohwi et al., 2007). Granular and periglomerular GABAergic interneurons are generated from the ventral SVZ (Merkle et al., 2007), while THy+ interneurons are largely derived from the dorsal SVZ (Kohwi et al., 2007; Merkle et al., 2007; Young et al., 2007). In our study, we showed that, in both species, GABAergic interneuron populations remain stable throughout life, as observed previously in older mice (Mirich et al., 2002). However, the dopaminergic interneuron population changed during aging, but in a different way depending on the species. In mice, although aging negatively regulates SVZ neurogenesis and, hence, the supply of new olfactory interneurons, the density of dopaminergic interneurons increased over the course of aging, suggesting that the continuous supply of newly formed neurons throughout life is mainly derived from the dorsal SVZ. These SVZ-derived newborn neurons mostly give rise to dopaminergic interneurons, leading to a progressive increase in the size of this interneuronal population. In contrast, the dopaminergic interneuron population in the lemur OBs decreased concomitantly with the drop in SVZ neurogenesis in old animals. This discrepancy between both mammalian species may imply different temporal apoptotic windows. In rodents, apoptosis occurs mainly during neuroblasts migration along the RMS (Platel et al., 2019), while in marmosets, it appears during olfactory neuronal maturation (Akter et al., 2020). Assuming that cerebral plasticity processes in lemurs could be similar to marmosets, a gradual decrease in the dopaminergic interneuronal population size during aging could be explained by an increase in THy+ interneuron apoptosis. Moreover, the majority of neuroblasts generated in the murine SVZ migrate to the OBs, while a much larger part of human and NHP SVZ neuroblasts, whose function is still unknown, also migrates to the neocortex (Bernier et al., 2002; Gould et al., 1999), ventral medial prefrontal cortex (Paredes et al., 2016; Sanai et al., 2004), amygdala and piriform cortex (Bernier et al., 2002), and striatum (Bédard et al., 2006; Ernst et al., 2014). Therefore, we hypothesize that fewer neuroblasts populate the NHP OBs compared to rodents, which could also explain why THy+ interneurons decreased in number during aging in lemurs.
The functional contribution of olfactory interneuronal populations is partially unknown. Only the role of dopaminergic neurons in odor detection and discrimination is currently established (Cave and Baker, 2009). In rodents, selective ablation of dopaminergic neurons by 6-hydroxydopamine injection (Zhang et al., 2019), the dopaminergic D2 receptor (Escanilla et al., 2009; Tillerson et al., 2006), or the dopaminergic transporter (Tillerson et al., 2006) strongly impairs odor discrimination abilities. Likewise, the olfactory preference of rodents was altered following downregulation of dopaminergic neurons and was physiologically rescued by the continuous supply of SVZ-derived newborn neurons (Lazarini et al., 2014). In parallel, increased densities of dopaminergic neurons by odor enrichment (Bonzano et al., 2014) or overexpression of two cell cycle regulators, Cdk4/CyclinD1 (Bragado Alonso et al., 2019), that increased proliferation of SVZ progenitors, thus improved odor discrimination performance without affecting GABAergic neuronal populations. Together, these results highlight the fundamental role of dopaminergic interneurons, the first relay of olfactory information, in neuronal plasticity mechanisms and adaptation of the olfactory system. In our model of physiological aging, we demonstrated a specific increase in dopaminergic interneuronal density in the glomerular cell layer of 18-month-old mice. The functional significance of this upregulation over the course of aging could maintain and ensure a fine-tuning of the olfactory discrimination in aged mice, necessary for their survival. Thus, the significant decrease in the population of dopaminergic interneurons in lemurs agrees with the age-related decline of olfactory discrimination capacities described previously in this species (Aujard and Némoz-Bertholet, 2004). Likewise, olfactory capacity in smell identification tests was strongly altered during aging in humans (Ship et al., 1996; Stevens and Dadarwala, 1993). Further work is necessary to determine which olfactory behaviors in NHPs change during aging. In parallel, we demonstrated that the short-term olfactory memory is reduced in old mice. Olfactory memory appears to be correlated with the number of newborn olfactory neurons generated throughout life (Breton-Provencher et al., 2009; Nicolis di Robilant et al., 2019; Rochefort et al., 2002). In fact, the study by Breton-Provencher et al. (2009) shows that adult SVZ neurogenesis is important to maintain proper OB network function and that newborn olfactory interneurons are involved in some odor-related tasks, including short-term olfactory memory. In this study, SVZ neurogenesis was abolished by an antimitotic treatment (cytosine arabinoside or AraC) that removed mitotic cells, thereby disrupting short-term olfactory memory and the odor detection threshold without altering odor discrimination or long-term olfactory memory. Furthermore, AraC treatment did not affect the total number of olfactory interneurons, dendrite length, or spine density, demonstrating that the pre-existing network of interneurons is not altered by decreased SVZ neurogenesis. However, ablation of SVZ neurogenesis reduced the inhibitory synapses received by mitral cells, their frequency of spontaneous inhibitory postsynaptic currents (IPSCs), and their synchronized activity. Taken together, these changes in the mitral cell network altered the short-term olfactory memory in AraC-treated mice. Thus, the age-related decline in SVZ neurogenesis could induce the same pattern in olfactory interneuron networks, similar to what happens when neurogenesis is abolished.
NSC fate is increasingly oriented to a glial fate during aging
Several studies have shown that SVZ oligodendrogenesis is maintained during aging in rodents (Bouab et al., 2011; Capilla-Gonzalez et al., 2013) and humans (Bergmann et al., 2012; Ernst et al., 2014; Weissleder et al., 2016) and can be increased in some pathological conditions, notably after a demyelinating insult (Nait-Oumesmar et al., 2007). Furthermore, myelination of the corpus callosum occurs gradually after birth in marmosets (Akter et al., 2020) and humans (Yeung et al., 2014). In our study, SVZ NSCs in older mice and lemurs had a higher ability to produce SVZ OPCs compared with those in young animals. More precisely, although the majority (about 70%) of NSCs in aged mice preferentially generated neuroblasts, a 20% increase in OPC production was observed during aging. In lemurs, half of the NSCs derived from aged animals committed to a glial fate (i.e., around 20% more OPCs generated compared with mice), indicating that NHP NSCs are more prone to generate OPCs than rodent NSCs as a function of age. The neuronal or oligodendroglial identity is intrinsically defined at the NSC stage (Adlaf et al., 2016; Menn et al., 2006; Ortega et al., 2013; Parras et al., 2004; Poiana et al., 2020), strongly suggesting that SVZ NSCs are unipotent. However, whether and how aging affects intrinsic properties of NSCs that govern their fate is not yet understood. The gene expression profile of aged mouse SVZ NSCs share transcriptional signatures of glial precursor cells (Dawson et al., 2003). Indeed, we found that they expressed higher levels of Ng2 mRNA than young NSCs together with lower Dcx expression, indicating that NSCs with a gliogenic profile could be selected or survive better during aging, thus producing more OPCs at the expense of neuronal progenitors. Along the same line, NSCs cultured from aged mouse SVZs became increasingly engaged in glial fate choice. However, Dlx2 was more expressed in middle-aged and aged mice, suggesting that aging downregulated Dcx expression and SVZ neuroblast density by slowing NSPC determination toward a neuronal fate. We also provide evidence that NSC enrichment, independent of age, promotes oligodendrogenesis and favors NSC reprogramming.
The adult mammalian brain is composed of two distinct OPC populations, i.e. SVZ OPCs continuously generated throughout adult life from SVZ NSCs, and resident pOPCs, which are produced during development and remain quiescent in the adult parenchyma (Psachoulia et al., 2009; Tokumoto et al., 2017). Under pathological conditions, in some animal models of demyelinating diseases, SVZ OPCs migrate toward the corpus callosum, where they differentiate into mature myelinating oligodendrocytes, allowing myelin repair in mice and humans (Brousse et al., 2015; Jablonska et al., 2010; Nait-Oumesmar et al., 1999; Remaud et al., 2017; Xing et al., 2014). In parallel to the increased production of SVZ OPCs during aging, the proportion of mature myelinating oligodendrocytes in the corpus callosum, just above the LVs, increased in old mice, showing that aging favors the differentiation and maturation of OPCs in this white matter area. Unexpectedly, while the total oligodendrocyte density in the lemur corpus callosum had more than doubled during aging, the majority were stuck in the precursor stage. This result is consistent with a recent study showing (1) a reduced number of new myelinating oligodendrocytes in the corpus callosum of rhesus monkeys during aging and (2) a decreased differentiation potential into mature oligodendrocytes from OPCs that are blocked at the progenitor stage (Dimovasili et al., 2022).
Because most pOPCs fail to differentiate into new myelinating oligodendrocytes (Chang et al., 2000; Wolswijk, 1998; Xing et al., 2014), the gradual enhancement of mature oligodendrocytes observed during aging in the mouse corpus callosum could be linked to the rise of SVZ OPCs in the aged SVZ. Although aging prevents maturation of OPCs in lemurs, unlike in mice, it was associated with increased myelin content (as seen using FluoroMyelin) in both species and could reflect an improved ability of SVZ OPCs to differentiate into functional oligodendrocytes capable to myelinate new axons or strengthen pre-existing connections. Indeed, several studies demonstrated that, after a demyelinating insult, oligodendrocytes derived from rodent SVZ OPCs produce myelin of normal thickness, whereas pOPCs generate thinner myelin sheaths, leading to incomplete remyelination. Therefore, these observations indicate that SVZ-derived oligodendrocytes are more efficient to functionally repair myelin than pOPCs in response to a demyelinating insult in the corpus callosum (Brousse et al., 2015; Remaud et al., 2017; Xing et al., 2014). Understanding how aging preferentially regulates SVZ NSCs toward an oligodendroglial fate to produce more OPCs throughout life could open new avenues to enhance endogenous myelin repair in multiple sclerosis and other demyelinating diseases.
In humans, evidence of a potential age-related decline in myelin content is controversial and requires longitudinal studies. Interestingly, oligodendrocyte numbers and myelin content increase progressively after birth and reach a plateau at middle age (Lynn et al., 2021; Yeung et al., 2014). Thus, myelination of the corpus callosum occurs rapidly in early life and remains relatively stable with aging. These results contradict previous studies showing age-related microstructural changes in the corpus callosum, probably caused by insufficient myelin synthesis, transport dysfunction, and/or demyelination (Branzoli et al., 2016; Faizy et al., 2018). In NHPs, only two studies have investigated myelination of the corpus callosum during aging, notably in the rhesus macaque (Peters and Sethares, 2002) and the common marmoset (Phillips et al., 2019). In both species, a thinner myelin sheath was observed with age, without axonal degeneration. Indeed, fewer than 0.5% of the axons showed age-related alterations (Peters and Sethares, 2002). Therefore, the increased proportion of mature oligodendrocytes in aged mice, coupled with higher expression of FluoroMyelin in the corpus callosum of mice and lemurs, suggest (1) sustained myelin formation in older individuals, (2) limited or no myelin degradation in the corpus callosum, and (3) a constant or even increased supply of new SVZ-derived OPCs.
Does a continuous ventricle play a role in maintaining olfactory neurogenesis?
Neuroanatomical analysis of the SVZ-OB axis in lemurs has shown the presence of a continuous ventricle that extends from the LVs to the OBs. Interestingly, the olfactory ventricular cavity is predominantly composed of SOX2+ progenitors, suggesting an active olfactory neurogenesis. Moreover, this OB neurogenesis is sensitive to aging since the SOX2+ progenitor density was reduced in old lemurs (Figure S2). This ventricular extension could favor the continued supply of various molecules and hormones important for proper brain development and functioning and for local control of proliferation and differentiation of olfactory progenitors.
This ventricular extension has been previously described in rabbits (Luzzati et al., 2003), sheep (Brus et al., 2013), opossums (Tepper et al., 2021), wild dogs (Chengetanai et al., 2020) and some NHPs, such as macaques, rhesus monkeys, and common marmosets (Gil-Perotin et al., 2009; Sawamoto et al., 2011; Wang et al., 2011). Intriguingly, all of these species are photoperiodic animals whose main physiological functions, including reproduction, are controlled by day length (Butruille et al., 2021). In adult humans, several studies have also demonstrated the presence of a continuous ventricle along a cerebrospinal fluid-filled migratory stream (Curtis et al., 2007; Kam et al., 2009; Sawamoto et al., 2006). However, the physiological function of this ventricular extension is not yet known. Surprisingly, proliferating cells were found along the continuous ventricle in the adult human forebrain, indicating neurogenic processes (Kam et al., 2009). Therefore, adult human OBs constitute another potential source of NSCs capable of generating new neurons (Alizadeh et al., 2017; Durante et al., 2020; Liu and Martin, 2003; Pagano et al., 2000). However, the function of the SVZ-OB axis is still debated in humans because of the rapid decline in neuroblast production after birth (Sanai et al., 2011; Wang et al., 2011). Thus, the absence of postnatal human SVZ neurogenesis could be compensated by neurogenesis occuring in the OBs that would take over the production of new neurons after birth. Surprisingly, human OB NSCs grafted into the injured central nervous system do not develop tumors and thereby differ from embryonic NSCs, commonly used for transplants, and, thus, might be better candidates for combatting neurodegenerative diseases (Marei et al., 2015).
Similarities and dissimilarities between rodents and NHPs
Analysis of NSC fate during aging in mice and lemurs revealed key differences that may explain the dissimilarities between rodents and humans. First, while the SVZ remains pro-neurogenic in aged mice, it generates as many neuroblasts as OPCs in lemurs. Thus, the NHP SVZ loses its preferential capacity to generate new neurons during aging. Second, in mice, aging promotes maturation of OPCs into mature oligodendrocytes in the corpus callosum, which could confer a better protection against demyelinating insults. In contrast, aging blocks oligodendrocyte differentiation at the OPC stage in the lemur, preventing their maturation, which could alter myelin production, as observed previously in humans (Branzoli et al., 2016; Faizy et al., 2018). Third, only the dopaminergic population is sensitive to aging, but in different ways, depending on the species, leading to preserved olfactory discrimination in mice but an altered one in lemurs. Fourth, the presence of a continuous ventricle in the lemur suggests active olfactory neurogenesis in NHPs, also sensitive to aging. In conclusion, use of NHPs could be crucial to develop translational strategies to circumvent the technical problems encountered in humans and to increase our knowledge about age-related neurodegenerative diseases in humans.
Experimental procedures
Resource availability
Corresponding authors
Sylvie Remaud sremaud@mnhn.fr; and Lucile Butruille lucile.butruille@mnhn.fr.
Materials availability
This study did not generate any unique reagents.
Animals
Two, 12 and 18 months-old C57BL/6J wild-type male mice were purchased from Janvier (Le Genest-Saint-Isle, France) and kept in ventilated cages under a 12:12 h light-dark cycle. Chow and water were available ad libitum. Male and female lemurs (M. murinus) 3–9 years old were born and raised in the laboratory colony of UMR 7179 (CNRS/MNHN, France, license approval A91.114.1). Animals were maintained in cages equipped with branches and wooden nests at constant temperature (25°C–27°C) and humidity (55%). Lemurs were exposed to the winter-like short-day-length photoperiod (10:14 h light-dark cycle). In the Brunoy colony, the animal median age was estimated to be 4.9 years for females and 5.7 years for males (Languille et al., 2012). Therefore, the senescence of animals was established from 6 years, associated with increased energy loss (body mass, resting metabolism) (Djelti et al., 2016; Perret, 1997; Pifferi et al., 2018). All experiments were approved by the Comité d’éthique Cuvier (n° 68) ethical board (authorization 22530-2019100917156614 and 13064-2018011615434702) and performed in strict accordance with European Directive 2010/63/EU.
Tissue preparation
Mice and lemurs were anesthetized with pentobarbital (130 mg/kg and 50 mg/kg respectively, Centravet) and perfused intracardially with 4% paraformaldehyde in 1× PBS (0.1 M, pH 7.4). 24 h after fixation, brains were cryoprotected in 30% sucrose in 1× PBS at 4°C. Brains were then cut in half along the median line, and blocks were embedded in OCT (Sakura), frozen, and stored at −80°C. Right hemispheres were cut coronally and left hemispheres sagittally. Mouse tissues were cut at 12 μm on a cryostat (Leica), directly mounted onto Superfrost Plus glass slides (Thermo Fisher Scientific), and stored at −80°C. For lemurs, 12-μm-thick coronal sections of the OBs were directly mounted onto Superfrost Plus glass slides, and floating 30-μm-thick coronal and sagittal sections were stored at −20°C in cryoprotectant solution.
Immunohistochemistry
All reagents are listed in Table S1. The procedure is described in the supplemental information.
Neurosphere culture and differentiation
The procedure is described in the supplemental information. This experiment was performed only once.
Imaging and quantification
Images were acquired using a Leica TCS-SP5 confocal microscope under 400× magnification. The in vivo acquisitions were done on single snap images (1,024 × 1,024 pixels) for mice and on Max Intensity Z projections of 5-μm stack images for lemurs. To analyze the neuron-glia ratio, coronal sections of the dorsal SVZ (5–8 sections per mouse) and distal SVZ (3–5 sections per lemur) were acquired. To quantify oligodendrocytes in the corpus callosum, three images were acquired per section (5–8 sections per mouse and 3–5 sections per lemur). To analyze the migration of neuroblasts and glial cells from the SVZ to the OBs, the entire structure of the RMS was imaged using sagittal sections (3–5 sections per animal). The entire RMS-like pathway of lemurs stained with DAPI was acquired under 100× magnification. Finally, to quantify neuronal populations in the OBs of both species and SOX2+ progenitors in the OBs of lemurs, 4 regions of interest (ROIs) were imaged per coronal section (5 sections per animal). Cell quantification was performed with FIJI software, using the cell counter plugin. The cell density (number/mm2) was calculated by counting the number of immuno-positive cells per area.
For the myelination assay, the entire corpus callosum and striatum stained with FluoroMyelin was acquired under 100× magnification. Three to five sections were imaged per animal the same day, with the same fluorescent excitation for all samples. The FluoroMyelin integrative density was automatically measured with FIJI software in the entire corpus callosum and in 10 ROIs for the striatum.
The in vitro acquisitions were done on Max Intensity Z projections of 4-μm stack images. Ten pictures (370 × 370 mm2) per well from each condition were taken, and the number of DCX+ and OLIG2+ cells was divided by the total number of DAPI nuclei present in the image.
RNA extraction and qRT-PCR
The procedure is described in the supplemental information.
Olfactory behaviors
The procedure is described in the supplemental information.
Statistical analysis
Statistical analyses were performed with GraphPad Prism8 (GraphPad, San Diego, CA, USA) and StatXact8 (Cytel) software. Data were first subjected to D’Agostino and Pearson normality tests. For mice, comparisons of the three groups of age were done using a one-way ANOVA test followed by Bonferroni post hoc test in case of significance or a Kruskal-Wallis test followed by Dunn’s post hoc test. A two-way repeated-measures ANOVA followed by a Bonferroni post hoc test was used to analyze the olfactory behavioral tests. For qRT-PCR data, Dixon’s Q test was used for identification and rejection of outliers, and a Kruskal-Wallis test was used to compare the three age groups. For lemurs, data were analyzed using computed non-parametric Spearman correlation to assess whether a positive or negative correlation existed between the different age groups and the marker studied. “n” refers to the number of animals for the mouse study, and “N” refers to the number of sections for the lemur study (the number of animals was too small to perform statistics). Differences were considered statistically significant when p < 0.05. Values are presented in the histograms and expressed as mean ± standard error of the mean (SEM) or as boxplots with medians and minimum and maximum values. Correlation results are shown as linear regression.
Author contributions
S.R., F.P., and L.B. conceived and designed the study. A.S. performed and analyzed the qPCR experiment. L.B. performed and analyzed the IHC experiment. K.Á. analyzed IHC data obtained from the lemur SVZ and RMS. L.B. and A.S. performed the in vitro experiments. L.B. performed the behavioral studies. L.B. collected, statistically analyzed, and interpreted the final data. L.B. made the figures and wrote the manuscript together with P.V., S.R., F.P., and B.A.D., who conceptualized and supervised the experiments. All authors read and approved the final manuscript.
Acknowledgments
We thank S. Sosinsky and F. Uridat for excellent animal care. We also thank the ImagoSeine platform of the Institute Jacques Monod, Université René Descartes for imaging advice. This work was supported by the CNRS and EU H2020 contract Thyrage (grant 666869).
Conflict of interests
The authors declare no competing interests.
Published: January 19, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2022.12.015.
Contributor Information
Lucile Butruille, Email: lucile.butruille@mnhn.fr.
Sylvie Remaud, Email: sremaud@mnhn.fr.
Supplemental information
Data and code availability
No large datasets and new code were generated in this study.
References
- Adlaf E.W., Mitchell-Dick A., Kuo C.T. Discerning Neurogenic vs. Non-Neurogenic Postnatal Lateral Ventricular Astrocytes via Activity-Dependent Input. Front. Neurosci. 2016;10:111. doi: 10.3389/fnins.2016.00111. https://www.frontiersin.org/article/10.3389/fnins.2016.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlenius H., Visan V., Kokaia M., Lindvall O., Kokaia Z. Neural Stem and Progenitor Cells Retain Their Potential for Proliferation and Differentiation into Functional Neurons Despite Lower Number in Aged Brain. J. Neurosci. 2009;29:4408–4419. doi: 10.1523/JNEUROSCI.6003-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter M., Kaneko N., Herranz-Pérez V., Nakamura S., Oishi H., García-Verdugo J.M., Sawamoto K. Dynamic changes in the neurogenic potential in the ventricular–subventricular zone of common marmoset during postnatal brain development. Cerebr. Cortex. 2020;30:4092–4109. doi: 10.1093/cercor/bhaa031. [DOI] [PubMed] [Google Scholar]
- Alizadeh R., Hassanzadeh G., Joghataei M.T., Soleimani M., Moradi F., Mohammadpour S., Ghorbani J., Safavi A., Sarbishegi M., Pirhajati Mahabadi V., Alizadeh L., Hadjighassem M. In vitro differentiation of neural stem cells derived from human olfactory bulb into dopaminergic-like neurons. Eur. J. Neurosci. 2017;45:773–784. doi: 10.1111/ejn.13504. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Kohwi M., Nguyen T.M., Merkle F.T. The Heterogeneity of Adult Neural Stem Cells and the Emerging Complexity of Their Niche. Cold Spring Harb. Symp. Quant. Biol. 2008;73:357–365. doi: 10.1101/sqb.2008.73.019. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla, Arturo, García-Verdugo J.M., Tramontin A.D. A unified hypothesis on the lineage of neural stem cells. Nat. Rev. Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Arbuckle E.P., Smith G.D., Gomez M.C., Lugo J.N. Testing for odor discrimination and habituation in mice. J. Vis. Exp. 2015;99 doi: 10.3791/52615. e52615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujard F., Dkhissi-Benyahya O., Fournier I., Claustrat B., Schilling A., Cooper H.M., Perret M. Artificially accelerated aging by shortened photoperiod alters early gene expression (Fos) in the suprachiasmatic nucleus and sulfatoxymelatonin excretion in a small primate, Microcebus murinus. Neurosci. 2001;105:403–412. doi: 10.1016/S0306-4522(01)00202-0. [DOI] [PubMed] [Google Scholar]
- Aujard F., Némoz-Bertholet F. Response to urinary volatiles and chemosensory function decline with age in a prosimian primate. Physiol. Behav. 2004;81:639–644. doi: 10.1016/j.physbeh.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Azari H., Rahman M., Sharififar S., Reynolds B.A. Isolation and expansion of the adult mouse neural stem cells using the neurosphere assay. J. Vis. Exp. 2010;45:2393. doi: 10.3791/2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard A., Gravel C., Parent A. Chemical characterization of newly generated neurons in the striatum of adult primates. Exp. Brain Res. 2006;170:501–512. doi: 10.1007/s00221-005-0233-5. [DOI] [PubMed] [Google Scholar]
- Bergmann O., Liebl J., Bernard S., Alkass K., Yeung M.S.Y., Steier P., Kutschera W., Johnson L., Landén M., Druid H., Spalding K.L., Frisén J. The Age of Olfactory Bulb Neurons in Humans. Neuron. 2012;74:634–639. doi: 10.1016/j.neuron.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Bernier P.J., Bédard A., Vinet J., Lévesque M., Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc. Natl. Acad. Sci. USA. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bons N., Rieger F., Prudhomme D., Fisher A., Krause K.H. Microcebus murinus: a useful primate model for human cerebral aging and Alzheimer's disease? Genes Brain Behav. 2006;5:120–130. doi: 10.1111/j.1601-183X.2005.00149.x. [DOI] [PubMed] [Google Scholar]
- Bonzano S., Bovetti S., Fasolo A., Peretto P., De Marchis S. Odour enrichment increases adult-born dopaminergic neurons in the mouse olfactory bulb. Eur. J. Neurosci. 2014;40:3450–3457. doi: 10.1111/ejn.12724. [DOI] [PubMed] [Google Scholar]
- Bouab M., Paliouras G.N., Aumont A., Fernandes K.J.L. Aging of the subventricular zone neural stem cell niche : evidence for quiescence-associated changes between early and mid- adulthood. NSC. 2011;173:135–149. doi: 10.1016/j.neuroscience.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Bovetti S., Peretto P., Fasolo A., De Marchis S. Spatio-temporal specification of olfactory bulb interneurons. J. Mol. Histol. 2007;38:563–569. doi: 10.1007/s10735-007-9111-8. [DOI] [PubMed] [Google Scholar]
- Bragado Alonso S., Reinert J.K., Marichal N., Massalini S., Berninger B., Kuner T., Calegari F. An increase in neural stem cells and olfactory bulb adult neurogenesis improves discrimination of highly similar odorants. EMBO J. 2019;38:e98791. doi: 10.15252/embj.201798791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branzoli F., Ercan E., Valabrègue R., Wood E.T., Buijs M., Webb A., Ronen I. Differentiating between axonal damage and demyelination in healthy aging by combining diffusion- tensor imaging and diffusion-weighted spectroscopy in the human corpus callosum at 7 T. Neurobiol. Aging. 2016;47:210–217. doi: 10.1016/j.neurobiolaging.2016.07.022. [DOI] [PubMed] [Google Scholar]
- Breton-Provencher V., Lemasson M., Peralta M.R., Saghatelyan A. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J. Neurosci. 2009;29:15245–15257. doi: 10.1523/JNEUROSCI.3606-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brousse B., Magalon K., Durbec P., Cayre M. Region and dynamic specificities of adult neural stem cells and oligodendrocyte precursors in myelin regeneration in the mouse brain. Biol. Open. 2015;4:980–992. doi: 10.1242/bio.012773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brus M., Meurisse M., Gheusi G., Keller M., Lledo P.M., Lévy F. Dynamics of olfactory and hippocampal neurogenesis in adult sheep. J. Comp. Neurol. 2013;521:169–188. doi: 10.1002/cne.23169. [DOI] [PubMed] [Google Scholar]
- Butruille L., Vancamp P., Demeneix B.A., Remaud S. Thyroid hormone regulation of adult neural stem cell fate: A comparative analysis between rodents and primates. Vitam. Horm. 2021;116:133–192. doi: 10.1016/bs.vh.2021.02.009. [DOI] [PubMed] [Google Scholar]
- Capilla-Gonzalez V., Cebrian-silla A., Guerrero-cazares H., Garcia-verdugo J.M., Quiñones-Hinojosa A. The generation of oligodendroglial cells is preserved in the rostral migratory stream during aging. Front. Cell. Neurosci. 2013;7:147. doi: 10.3389/fncel.2013.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave J.W., Baker H. Dopamine systems in the forebrain. Adv. Exp. Med. Biol. 2009;651:15–35. doi: 10.1007/978-1-4419-0322-8_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayetanot F., Van Someren E.J.W., Perret M., Aujard F. Shortened Seasonal Photoperiodic Cycles Accelerate Aging of the Diurnal and Circadian Locomotor Activity Rhythms in a Primate. J. Biol. Rhythms. 2005;20:461–469. doi: 10.1177/0748730405279174. [DOI] [PubMed] [Google Scholar]
- Chang A., Nishiyama A., Peterson J., Prineas J., Trapp B.D. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J. Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudron Y., Pifferi F., Aujard F. Overview of age-related changes in psychomotor and cognitive functions in a prosimian primate, the lemur (Microcebus murinus): Recent advances in risk factors and antiaging interventions. Am. J. Primatol. 2021;83 doi: 10.1002/ajp.23337. [DOI] [PubMed] [Google Scholar]
- Chengetanai S., Bhagwandin A., Bertelsen M.F., Hård T., Hof P.R., Spocter M.A., Manger P.R. The brain of the African wild dog. II. The olfactory system. J. Comp. Neurol. 2020;528:3285–3304. doi: 10.1002/cne.25007. [DOI] [PubMed] [Google Scholar]
- Curtis M.A., Kam M., Nannmark U., Anderson M.F., Axell M.Z., Wikkelso C., Holtås S., van Roon-Mom W.M.C., Björk-Eriksson T., Nordborg C., et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- Dawson M.R.L., Polito A., Levine J.M., Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 2003;24:476–488. doi: 10.1016/S1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Daynac M., Morizur L., Chicheportiche A. Age-related neurogenesis decline in the subventricular zone is associated with specific cell cycle regulation changes in activated neural stem cells. Sci. Rep. 2016;6 doi: 10.1038/srep21505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimovasili C., Fair A.E., Garza I.R., Batterman K.V., Mortazavi F., Moore T.L., Rosene D.L. Aging compromises oligodendrocyte precursor cell maturation and efficient remyelination in the monkey brain. Geroscience. 2022 doi: 10.1007/s11357-022-00621-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djelti F., Dhenain M., Terrien J., Picq J.-L., Hardy I., Champeval D., Perret M., Schenker E., Epelbaum J., Aujard F. Impaired fasting blood glucose is associated to cognitive impairment and cerebral atrophy in middle-aged non-human primates. Aging. 2016;9:173–186. doi: 10.18632/aging.101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante M.A., Kurtenbach S., Sargi Z.B., Harbour J.W., Choi R., Kurtenbach S., Goss G.M., Matsunami H., Goldstein B.J. Single-cell analysis of olfactory neurogenesis and differentiation in adult humans. Nat. Neurosci. 2020;23:323–326. doi: 10.1038/s41593-020-0587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwere E., Shingo T., Gregg C., Fujikawa H., Ohta S., Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst A., Alkass K., Bernard S., Salehpour M., Perl S., Tisdale J., Possnert G., Druid H., Frisén J. Neurogenesis in the Striatum of the Adult Human Brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Escanilla O., Yuhas C., Marzan D., Linster C. Dopaminergic modulation of olfactory bulb processing affects odor discrimination learning in rats. Behav. Neurosci. 2009;123:828–833. doi: 10.1037/a0015855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezran C., Karanewsky C.J., Pendleton J.L., Sholtz A., Krasnow M.R., Willick J., Razafindrakoto A., Zohdy S., Albertelli M.A., Krasnow M.A. The Mouse Lemur, a Genetic Model Organism for Primate Biology, Behavior, and Health. Genetics. 2017;206:651–664. doi: 10.1534/genetics.116.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faizy T.D., Kumar D., Broocks G., Thaler C., Flottmann F., Leischner H., Kutzner D., Hewera S., Dotzauer D., Stellmann J.P., Reddy R., Fiehler J., Sedlacik J., Gellißen S. Age- Related Measurements of the Myelin Water Fraction derived from 3D multi-echo GRASE reflect Myelin Content of the Cerebral White Matter. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-33112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengatharan A., Bammann R.R., Saghatelyan A. The role of astrocytes in the generation, migration, and integration of new neurons in the adult olfactory bulb. Front. Neurosci. 2016;10:149. doi: 10.3389/fnins.2016.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Perotin S., Duran-Moreno M., Belzunegui S., Luquin M.R., Garcia-Verdugo J.M. Ultrastructure of the subventricular zone in Macaca fascicularis and evidence of a mouse-like migratory stream. J. Comp. Neurol. 2009;514:533–554. doi: 10.1002/cne.22026. [DOI] [PubMed] [Google Scholar]
- Gould E., Reeves A.J., Fallah M., Tanapat P., Gross C.G., Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc. Natl. Acad. Sci. USA. 1999;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonska B., Aguirre A., Raymond M., Szabo G., Kitabatake Y., Sailor K.A., Ming G.-L., Song H., Gallo V. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat. Neurosci. 2010;13:541–550. doi: 10.1038/nn.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam M., Curtis M.A., McGlashan S.R., Connor B., Nannmark U., Faull R.L.M. The cellular composition and morphological organization of the rostral migratory stream in the adult human brain. J. Chem. Neuroanat. 2009;37:196–205. doi: 10.1016/j.jchemneu.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Keirstead H.S., Blakemore W.F. The role of oligodendrocytes and oligodendrocyte progenitors in CNS remyelination. Adv. Exp. Med. Biol. 1999;468:183–197. doi: 10.1007/978-1-4615-4685-6_15. [DOI] [PubMed] [Google Scholar]
- Kohwi M., Petryniak M.A., Long J.E., Ekker M., Obata K., Yanagawa Y., Rubenstein J.L.R., Alvarez-Buylla A. A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1- and Dlx5/6-expressing progenitors. J. Neurosci. 2007;27:6878–6891. doi: 10.1523/JNEUROSCI.0254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languille S., Blanc S., Blin O., Canale C.I., Dal-Pan A., Devau G., Dhenain M., Dorieux O., Epelbaum J., Gomez D., et al. The grey mouse lemur: a non-human primate model for ageing studies. Ageing Res. Rev. 2012;11:150–162. doi: 10.1016/j.arr.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Languille S., Liévin-Bazin A., Picq J.-L., Louis C., Dix S., De Barry J., Blin O., Richardson J., Bordet R., Schenker E., et al. Deficits of psychomotor and mnesic functions across aging in mouse lemur primates. Front. Behav. Neurosci. 2015;8:446. doi: 10.3389/fnbeh.2014.00446. https://www.frontiersin.org/article/10.3389/fnbeh.2014.00446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurijssens B., Aujard F., Rahman A. Animal models of Alzheimer’s disease and drug development. Drug Discov. Today Technol. 2013;10:319–327. doi: 10.1016/j.ddtec.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Lazarini F., Mouthon M.A., Gheusi G., de Chaumont F., Olivo-Marin J.C., Lamarque S., Abrous D.N., Boussin F.D., Lledo P.M. Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PLoS One. 2009;4:e7017. doi: 10.1371/journal.pone.0007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarini F., Gabellec M.-M., Moigneu C., de Chaumont F., Olivo-Marin J.-C., Lledo P.-M. Adult Neurogenesis Restores Dopaminergic Neuronal Loss in the Olfactory Bulb. J. Neurosci. 2014;34:14430–14442. doi: 10.1523/JNEUROSCI.5366-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepousez G., Valley M.T., Lledo P.-M. The Impact of Adult Neurogenesis on Olfactory Bulb Circuits and Computations. Annu. Rev. Physiol. 2013;75:339–363. doi: 10.1146/annurev-physiol-030212-183731. [DOI] [PubMed] [Google Scholar]
- Lim D.A., Alvarez-Buylla A. The adult ventricular–subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb. Perspect. Biol. 2016;8:a018820. doi: 10.1101/cshperspect.a018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Martin L.J. Olfactory bulb core is a rich source of neural progenitor and stem cells in adult rodent and human. J. Comp. Neurol. 2003;459:368–391. doi: 10.1002/cne.10664. [DOI] [PubMed] [Google Scholar]
- Lois C., Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc. Natl. Acad. Sci. USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C., Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Luo J., Daniels S.B., Lennington J.B., Notti R.Q., Conover J.C. The aging neurogenic subventricular zone. Aging Cell. 2006;5:139–152. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Luzzati F., Peretto P., Aimar P., Ponti G., Fasolo A., Bonfanti L. Glia-independent chains of neuroblasts through the subcortical parenchyma of the adult rabbit brain. Proc. Natl. Acad. Sci. USA. 2003;100:13036–13041. doi: 10.1073/pnas.1735482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn J.D., Anand C., Arshad M., Homayouni R., Rosenberg D.R., Ofen N., Raz N., Stanley J.A. Microstructure of Human Corpus Callosum across the Lifespan: Regional Variations in Axon Caliber, Density, and Myelin Content. Cerebral Cortex. 2021;31:1032–1045. doi: 10.1093/cercor/bhaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marei H.E.S., Lashen S., Farag A., Althani A., Afifi N., A.,A.-E., Rezk S., Pallini R., Casalbore P., Cenciarelli C. Human olfactory bulb neural stem cells mitigate movement disorders in a rat model of Parkinson’s disease. J. Cell. Physiol. 2015;230:1614–1629. doi: 10.1002/jcp.24909. [DOI] [PubMed] [Google Scholar]
- Margulies E.H., Cooper G.M., Asimenos G., Thomas D.J., Dewey C.N., Siepel A., Birney E., Keefe D., Schwartz A.S., Hou M., Taylor J., Nikolaev S., Montoya-Burgos J.I., Löytynoja A., Whelan S., Pardi F., Massingham T., Brown J.B., Bickel P., Marra M.A. Analyses of deep mammalian sequence alignments and constraint predictions for 1% of the human genome. Genome Res. 2007;17:760–774. doi: 10.1101/gr.6034307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B., Garcia-Verdugo J.M., Yaschine C., Gonzalez-Perez O., Rowitch D., Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle F.T., Mirzadeh Z., Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Ming G., Song H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirich J.M., Williams N.C., Berlau D.J., Brunjes P.C. Comparative study of aging in the mouse olfactory bulb. J. Comp. Neurol. 2002;454:361–372. doi: 10.1002/cne.10426. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B., Decker L., Lachapelle F., Avellana-Adalid V., Bachelin C., Baron-Van Evercooren A. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur. J. Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B., Picard-Riera N., Kerninon C., Decker L., Seilhean D., Höglinger G.U., Hirsch E.C., Reynolds R., Baron-Van Evercooren A. Activation of the subventricular zone in multiple sclerosis: Evidence for early glial progenitors. Proc. Natl. Acad. Sci. USA. 2007;104:4694–4699. doi: 10.1073/pnas.0606835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolis di Robilant V., Scardigli R., Strimpakos G., Tirone F., Middei S., Scopa C., De Bardi M., Battistini L., Saraulli D., Farioli Vecchioli S. Running-Activated Neural Stem Cells Enhance Subventricular Neurogenesis and Improve Olfactory Behavior in p21 Knockout Mice. Mol. Neurobiol. 2019;56:7534–7556. doi: 10.1007/s12035-019-1590-6. [DOI] [PubMed] [Google Scholar]
- Ortega F., Gascón S., Masserdotti G., Deshpande A., Simon C., Fischer J., Dimou L., Chichung Lie D., Schroeder T., Berninger B. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat. Cell Biol. 2013;15:602–613. doi: 10.1038/ncb2736. [DOI] [PubMed] [Google Scholar]
- Pagano S.F., Impagnatiello F., Girelli M., Cova L., Grioni E., Onofri M., Cavallaro M., Etteri S., Vitello F., Giombini S., Solero C.L., Parati E.A., Parati E. Isolation and Characterization of Neural Stem Cells from the Adult Human Olfactory Bulb. Stem Cells. 2000;18:295–300. doi: 10.1634/stemcells.18-4-295. PMID: 10924096. [DOI] [PubMed] [Google Scholar]
- Paredes M.F., James D., Gil-Perotin S., Kim H., Cotter J.A., Ng C., Sandoval K., Rowitch D.H., Xu D., McQuillen P.S., Garcia-Verdugo J.M., Huang E.J., Alvarez-Buylla A. Extensive migration of young neurons into the infant human frontal lobe. Science. 2016;354:aaf7073. doi: 10.1126/science.aaf7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parras C.M., Galli R., Britz O., Soares S., Galichet C., Battiste J., Johnson J.E., Nakafuku M., Vescovi A., Guillemot F. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino G., Trubert C., Terrien J., Pifferi F., Leroy D., Loyens A., Migaud M., Baroncini M., Maurage C.-A., Fontaine C., Prévot V., Sharif A. A comparative study of the neural stem cell niche in the adult hypothalamus of human, mouse, rat and lemur (Microcebus murinus) J. Comp. Neurol. 2018;526:1419–1443. doi: 10.1002/cne.24376. [DOI] [PubMed] [Google Scholar]
- Pencea V., Bingaman K.D., Freedman L.J., Luskin M.B. Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp. Neurol. 2001;172:1–16. doi: 10.1006/exnr.2001.7768. [DOI] [PubMed] [Google Scholar]
- Perret M. Change in Photoperiodic Cycle Affects Life Span in a Prosimian Primate (Microcebus murinus. J. Biol. Rhythms. 1997;12:136–145. doi: 10.1177/074873049701200205. [DOI] [PubMed] [Google Scholar]
- Perret M., Aujard F. Vieillissement et rythmes biologiques chez les primates. Med. Sci. (Paris) 2006;22:279–283. doi: 10.1051/medsci/2006223279. [DOI] [PubMed] [Google Scholar]
- Peters A., Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J. Comp. Neurol. 2002;442:277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- Phillips K.A., Watson C.M., Bearman A., Knippenberg A.R., Adams J., Ross C., Tardif S.D. Age-related changes in myelin of axons of the corpus callosum and cognitive decline in common marmosets. Am. J. Primatol. 2019;81 doi: 10.1002/ajp.22949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccin D., Tufford A., Morshead C.M. Neurobiology of Aging Neural stem and progenitor cells in the aged subependyma are activated by the young niche. Neurobiol. Aging. 2014;35:1669–1679. doi: 10.1016/j.neurobiolaging.2014.01.026. [DOI] [PubMed] [Google Scholar]
- Picq J.-L., Aujard F., Volk A., Dhenain M. Age-related cerebral atrophy in nonhuman primates predicts cognitive impairments. Neurobiol. Aging. 2012;33:1096–1109. doi: 10.1016/j.neurobiolaging.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifferi F., Aujard F., Perret M. [Is the biological clock central to the aging process? Studies in a non-human primate] Biologie aujourd’hui. 2014;208:281–287. doi: 10.1051/jbio/2015003. [DOI] [PubMed] [Google Scholar]
- Pifferi F., Terrien J., Marchal J., Dal-Pan A., Djelti F., Hardy I., Chahory S., Cordonnier N., Desquilbet L., Hurion M., Zahariev A., Chery I., Zizzari P., Perret M., Epelbaum J., Blanc S., Picq J.-L., Dhenain M., Aujard F. Caloric restriction increases lifespan but affects brain integrity in grey mouse lemur primates. Commun. Biol. 2018;1:30. doi: 10.1038/s42003-018-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel J.-C., Angelova A., Bugeon S., Wallace J., Ganay T., Chudotvorova I., Deloulme J.-C., Béclin C., Tiveron M.-C., Coré N., et al. Neuronal integration in the adult mouse olfactory bulb is a non-selective addition process. Elife. 2019;8:e44830. doi: 10.7554/eLife.44830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiana G., Gioia R., Sineri S., Cardarelli S., Lupo G., Cacci E. Transcriptional regulation of adult neural stem/progenitor cells: tales from the subventricular zone YR - 2020/10/1. Neural Regen. Res. 2020;15:1773–1783. doi: 10.4103/1673-5374.280301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psachoulia K., Jamen F., Young K.M., Richardson W.D. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009;5:57–67. doi: 10.1017/S1740925X09990354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A., Lamberty Y., Schenker E., Cella M., Languille S., Bordet R., Richardson J., Pifferi F., Aujard F. Effects of acute administration of donepezil or memantine on sleep-deprivation- induced spatial memory deficit in young and aged non-human primate grey mouse lemurs (Microcebus murinus) PLOS ONE. 2017;12:e0184822. doi: 10.1371/journal.pone.0184822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaud S., Ortiz F.C., Perret-Jeanneret M., Aigrot M.S., Gothié J.D., Fekete C., Kvárta-Papp Z., Gereben B., Langui D., Lubetzki C., et al. Transient hypothyroidism favors oligodendrocyte generation providing functional remyelination in the adult mouse brain. Elife. 2017;6:e29996. doi: 10.7554/eLife.29996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C., Gheusi G., Vincent J.D., Lledo P.M. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J. Neurosci. 2002;22:2679–2689. doi: 10.1523/jneurosci.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo J., Villain N., Champeval D., Del Gallo F., Bertini G., Aujard F., Pifferi F. Effects of n- 3 polyunsaturated fatty acid supplementation on cognitive functions, electrocortical activity and neurogenesis in a non-human primate, the grey mouse lemur (Microcebus murinus) Behav. Brain Res. 2018;347:394–407. doi: 10.1016/j.bbr.2018.02.029. [DOI] [PubMed] [Google Scholar]
- Sanai N., Tramontin A.D., Quiñones-Hinojosa A., Barbaro N.M., Gupta N., Kunwar S., Lawton M.T., McDermott M.W., Parsa A.T., Manuel-García Verdugo J., et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Sanai N., Nguyen T., Ihrie R.A., Mirzadeh Z., Tsai H.-H., Wong M., Gupta N., Berger M.S., Huang E., Garcia-Verdugo J.-M., et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto K., Wichterle H., Gonzalez-Perez O., Cholfin J.A., Yamada M., Spassky N., Murcia N.S., Garcia-Verdugo J.M., Marin O., Rubenstein J.L.R., et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Sawamoto K., Hirota Y., Alfaro-Cervello C., Soriano-Navarro M., He X., Hayakawa-Yano Y., Yamada M., Hikishima K., Tabata H., Iwanami A., et al. Cellular composition and organization of the subventricular zone and rostral migratory stream in the adult and neonatal common marmoset brain. J. Comp. Neurol. 2011;519:690–713. doi: 10.1002/cne.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ship J.A., Pearson J.D., Cruise L.J., Brant L.J., Metter E.J. Longitudinal Changes in Smell Identification. J. Gerontol. 1996;51A:M86–M91. doi: 10.1093/gerona/51a.2.m86. [DOI] [PubMed] [Google Scholar]
- Shook B.A., Manz D.H., Peters J.J., Kang S., Conover J.C. Spatiotemporal changes to the subventricular zone stem cell pool through aging. J. Neurosci. 2012;32:6947–6956. doi: 10.1523/JNEUROSCI.5987-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuboni-mulligan D.D., Chakravarty S., Mallett C.L., Wolf A.M., Dmitriev P.M., Forton S.M., Shapiro E.M. NeuroImage In vivo serial MRI of age-dependent neural progenitor cell migration in the rat brain. NeuroImage. 2019;199:153–159. doi: 10.1016/j.neuroimage.2019.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J.C., Dadarwala A.D. Variability of olfactory threshold and its role in assessment of aging. Percept. & Psychophys. 1993;54:296–302. doi: 10.3758/BF03205264. [DOI] [PubMed] [Google Scholar]
- Suzuki S.O., Goldman J.E. Multiple Cell Populations in the Early Postnatal Subventricular Zone Take Distinct Migratory Pathways: A Dynamic Study of Glial and Neuronal Progenitor Migration. J. Neurosci. 2003;23:4240–4250. doi: 10.1523/JNEUROSCI.23-10-04240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper B., Koguc-Sobolewska P., Jaslan K., Turlejski K., Bartkowska K., Djavadian R. Impaired olfactory neurogenesis affects the performance of olfactory-guided behavior in aged female opossums. Sci. Rep. 2021;11:4418. doi: 10.1038/s41598-021-83834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillerson J.L., Caudle W.M., Parent J.M., Gong C., Schallert T., Miller G.W. Olfactory discrimination deficits in mice lacking the dopamine transporter or the D2 dopamine receptor. Behav. Brain Res. 2006;172:97–105. doi: 10.1016/j.bbr.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Tokumoto Y., Tamaki S., Kabe Y., Takubo K., Suematsu M. Quiescence of adult oligodendrocyte precursor cells requires thyroid hormone and hypoxia to activate Runx1. Sci. Rep. 2017;7:1019. doi: 10.1038/s41598-017-01023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Liu F., Liu Y.-Y., Zhao C.-H., You Y., Wang L., Zhang J., Wei B., Ma T., Zhang Q., et al. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 2011;21:1534–1550. doi: 10.1038/cr.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissleder C., Fung S.J., Wong M.W., Barry G., Double K.L., Halliday G.M., Webster M.J., Weickert C.S. Decline in Proliferation and Immature Neuron Markers in the Human Subependymal Zone during Aging: Relationship to EGF- and FGF-Related Transcripts. Front. Aging Neurosci. 2016;8:274. doi: 10.3389/fnagi.2016.00274. https://www.frontiersin.org/article/10.3389/fnagi.2016.00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J. Neurosci. 1998;18:601–609. doi: 10.1523/JNEUROSCI.18-02-00601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y.L., Röth P.T., Stratton J.A.S., Chuang B.H.A., Danne J., Ellis S.L., Ng S.W., Kilpatrick T.J., Merson T.D. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J. Neurosci. 2014;34:14128–14146. doi: 10.1523/JNEUROSCI.3491-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung M.S.Y., Zdunek S., Bergmann O., Bernard S., Salehpour M., Alkass K., Perl S., Tisdale J., Possnert G., Brundin L., et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159:766–774. doi: 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Young K.M., Fogarty M., Kessaris N., Richardson W.D. Subventricular Zone Stem Cells Are Heterogeneous with Respect to Their Embryonic Origins and Neurogenic Fates in the Adult Olfactory Bulb. J. Neurosci. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Sun C., Shao Y., Zhou Z., Hou Y., Li A. Partial depletion of dopaminergic neurons in the substantia nigra impairs olfaction and alters neural activity in the olfactory bulb. Sci. Rep. 2019;9:254. doi: 10.1038/s41598-018-36538-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No large datasets and new code were generated in this study.