Abstract
Background:
Melanoma aetiology has been proposed to have two pathways – determined by naevi and type of sun exposure – and related to the anatomical site where melanoma develops.
Objectives:
We examined associations with melanoma by anatomical site for a comprehensive set of risk factors including pigmentary and naevus phenotypes, ultraviolet radiation exposure, and polygenic risk.
Methods:
We analysed harmonised data from 2,617 people with incident first invasive melanoma and 975 healthy controls recruited through two population-based case-control studies in Australia and the United Kingdom. Questionnaire data were collected by interview using a single protocol, and pathway-specific polygenic risk scores were derived from DNA samples. We estimated adjusted odds ratios (ORs) using unconditional logistic regression that compared melanoma cases at each anatomical site with all controls.
Results:
Comparing case with control participants, there were stronger associations for many versus no naevi for melanomas on the trunk, upper and lower limbs than on the head and neck (P-heterogeneity <0.001). Very fair skin (vs. olive/brown skin) was more weakly related to melanoma on the trunk than to melanomas at other sites (P-heterogeneity=0.04). There was no significant difference by anatomical site for polygenic risk. Increased weekday sun exposure was positively associated with melanoma on the head and neck but not on other sites.
Conclusions:
We found evidence of aetiological heterogeneity for melanoma, supporting the dual pathway hypothesis. These findings enhance understanding of risk factors for melanoma and can guide prevention and skin examination education and practices.
Keywords: melanoma, aetiology, heterogeneity, population-based, risk factor, case-control study, anatomical site
INTRODUCTION
Cutaneous melanoma incidence is increasing in many countries with populations of predominantly European origin, despite improvements in prevention.1,2 Most of the risk for melanoma is driven by intensity and pattern of sun exposure, host factors like pigmentary phenotypes, propensity to develop naevi, genetic susceptibility and the complex association among these factors.3–6 The aetiology of melanoma is also indicated by the anatomical site on which it develops,7,8 with two main biological pathways proposed.9–12 The first of these is a naevus pathway which is initiated by early-life sun exposure to epidermal melanocytes, promoted by intermittent sun exposure or host factors, and is predominant on areas less exposed to sun (e.g., trunk) and in younger individuals. The second is a chronic (more continuous) sun exposure pathway, predominant in sun-sensitive and older people, in which sun damage progressively accumulates on areas of skin that are habitually exposed (e.g., head and neck).12–14 A third pathway involving increased germline telomere length has been implicated through genetic studies,15 but its association with pigmentation and naevus count is thus far largely undescribed.
Most epidemiological studies that have examined this hypothesis have focused on the association of sun exposure (or a proxy such as solar elastosis) with melanoma risk stratified by anatomical site.14,16,17 Few studies have examined associations of other risk factors by anatomical site, such as pigmentary and naevus characteristics, despite their strong associations with melanoma risk and the importance of host characteristics in the dual pathway hypothesis.3,6,12,18–22 Most of these studies have been case-only designs with small sample sizes and limited statistical power, have captured data for only a few risk factors, or have been limited to one sex.3,14,23 Therefore, we aimed to examine associations with melanoma by anatomical site for a comprehensive set of risk factors including pigmentary and naevus characteristics (measured both phenotypically and genetically using polygenic risk scores (PRS)) and ultraviolet radiation (UV) exposure using two population-based studies from Australia and the United Kingdom (UK).
MATERIALS AND METHODS
We analysed data from 3,592 participants, including 2,617 people with newly diagnosed melanoma (cases) and 975 people without melanoma (controls). Participants were recruited through the Australian Melanoma Family Study, which is a multi-centre population-based case-control study, and through the Leeds (United Kingdom) population-based case-control study (Leeds Melanoma Case-Control Study). A detailed description of the study designs and data collections for these two studies has been given previously.6 Identical questionnaires and assessment measures were applied across the study sites. Approval to conduct this study was obtained from the ethics committees of the coordinating centres and cancer registries in Australia, and from the UK Multi-Centre Research Ethics Committee and the Patient Information Advisory Group. All participants provided written, informed consent.
Study subjects
For the Australian Melanoma Family Study, 629 individuals residing within Queensland, New South Wales and Victoria who had histopathologically-confirmed first primary invasive cutaneous melanoma diagnosed between 1st July 2000 and 31st December 2002 at ages 18–39 years were included.24 They were recruited through population-based cancer registries and participation was 54%. Age, sex and city frequency matched population controls (n=240) were recruited through electoral rolls (registration to vote is compulsory for adult Australian citizens) and were frequency matched to cases by age (within 5 years) and sex using proportional random sampling; participation was 23% of those eligible. Eligible spouse/partner or friend controls (n=295) were nominated by case participants as a potential control; 80% of those nominated consented to participated. They were ineligible if they had a previous invasive or in situ melanoma.
For the Leeds case-control study, cases were aged 18–82 years with histopathologically-confirmed first primary invasive melanoma, living in a geographically defined area of Yorkshire and the Northern region of the UK (67% participation). Between September 2000 and June 2003, all people with invasive melanoma were included and from July 2003 to September 2011, only cases with Breslow thickness ≥0.75mm were included. Age and sex frequency matched population-based controls identified as not having cancer were recruited from general practices (55% participation).
Data collection
Details of the data collection are described in the Supplementary file.
Statistical analysis
All pigmentary and naevus phenotype variables were analysed as categorical variables. Sun exposure and PRS were analysed as continuous variables. Missing exposure values were excluded from the relevant analysis.
Adjusted odds ratios (OR), approximating the relative risk,25 and 95% confidence intervals (CI) for melanoma were calculated using unconditional logistic regression models fit separately for each anatomical site (head and neck, trunk, lower limbs, upper limbs) and compared with all controls. Thus, unlike case-only analyses where one anatomical site is used as a reference group for the other sites, in this analysis the cases from each site were compared to the single control group, and the reference category for each exposure corresponded to the lowest exposure level or darkest phenotype. For continuous measures of sun exposure, the ORs were calculated per 1-hr increase in sun exposure per day. For continuous measures of PRS, the ORs were calculated per 1 standard deviation increase in PRS. We adjusted regression models for age (continuous), sex, and city of recruitment, and for the PRS we additionally adjusted for self-reported ethnicity. We also further adjusted UV exposure associations for pigmentary and naevus phenotype characteristics, and vice versa. Population controls and spouse/partner/friend controls were combined into one control group for this analysis, as we have previously shown that associations for standard risk factors were similar when either control group or both groups were used.24
To examine potential interaction between pigmentary phenotypes and sex, we fit additional site-specific models including main effects and interaction terms. To test whether the associations for risk factors differed by anatomical subtype, we calculated p-values for aetiological heterogeneity as described by Zabor and Begg26 using the R package “riskclustr”.27,28 Data were analysed using R version 3.5. Statistical significance was assessed using a two-sided threshold p<0.05. P-values were not adjusted for multiple testing as we had clearly defined hypotheses informed by prior research.29,30 We reported the study according to STROBE guidelines for observational studies.
RESULTS
Analysis dataset
Of the 629 Australian cases and 535 controls, 25 cases and 65 controls were excluded from this analysis because of missing anatomical site (cases), presence of CDKN2A mutation (as genetic factors in this analysis focus on polygenic risk), non-European ancestry or age over 45 years (partner/friend controls). This resulted in 604 Australian cases and 470 controls for analysis. In the Leeds study, 2,184 cases and 513 controls were recruited, from which 171 cases and 8 controls were excluded due to either missing or rare anatomical site, presence of CDKN2A mutation, or missing data for some exposures (a shorter questionnaire was used after 2007 when only cases were being recruited), resulting in 2,013 cases and 505 controls for analysis. Combined, a total of 2,617 cases and 975 controls were included in the analysis.
Participant characteristics
The characteristics of the pooled study sample are presented in Table 1 and stratified by study in Supplementary Tables 1 (Australia) and 2 (Leeds). Melanoma most commonly occurred on the trunk (35%) and lower limbs (34%), followed by the upper limbs (20%) and the head and neck (11%). Compared with males, females had a higher frequency of melanomas on the upper and lower limbs (M:F ratio 1:1.8 and 1:3.3 respectively), while the opposite was true for trunk and head and neck melanomas (M:F ratio 1:0.68 and 1:0.83 respectively). The proportion of melanomas occurring in those aged 70 and older was higher for head and neck (21%) than for any other site (9–10%; χ2 p < 0.001). Family history of melanoma in a first degree relative was more common for cases with melanoma on the upper limb or trunk (10%) compared with other anatomical sites (5–8%; χ2 p < 0.001).
Table 1:
Characteristics of melanoma cases and controls in the pooled Australian Melanoma Family Study and Leeds Melanoma Case-Control Study
| Controls n=975 (%) | Cases (N = 2,617) | |||||
|---|---|---|---|---|---|---|
| Head and Neck n=289 (%) | Trunk n=910 (%) | Upper-Limb n=525 (%) | Lower-Limb n=893 (%) | |||
| Study | ||||||
| Leeds | 505 (51.8) | 207 (71.6) | 699 (76.8) | 397 (75.6) | 710 (79.5) | |
| Australia | 470 (48.2) | 82 (28.4) | 211 (23.2) | 128 (28.4) | 183 (20.5) | |
| Sex | ||||||
| Male | 406 (41.6) | 158 (54.7) | 541 (59.5) | 187 (35.6) | 208 (23.3) | |
| Female | 569 (58.4) | 131 (45.3) | 369 (40.6) | 338 (64.4) | 685 (76.7) | |
| Age at diagnosis/interview (years) | ||||||
| 18–29 | 101 (10.4) | 47 (16.3) | 104 (11.4) | 46 (8.8) | 89 (10.0) | |
| 30–39 | 350 (35.9) | 63 (21.8) | 217 (23.9) | 137 (26.1) | 220 (24.6) | |
| 40–49 | 163 (16.8) | 19 (6.6) | 124 (13.6) | 88 (16.8) | 139 (15.6) | |
| 50–59 | 131 (13.4) | 42 (14.5) | 184 (20.2) | 87 (16.6) | 172 (19.3) | |
| 60–69 | 133 (13.6) | 58 (20.1) | 197 (21.7) | 117 (22.3) | 186 (20.8) | |
| ≥70 | 97 (9.9) | 60 (20.8) | 84 (9.2) | 50 (9.5) | 87 (9.7) | |
| Ethnic background | ||||||
| English | 740 (75.9) | 259 (89.6) | 775 (85.4) | 458 (87.6) | 784 (87.8) | |
| Scottish, Irish, Welsh | 39 (4.0) | 12 (4.2) | 50 (5.5) | 19 (3.6) | 51 (5.7) | |
| Other Northern European | 29 (3.0) | 2 (0.7) | 15 (1.7) | 6 (1.2) | 9 (1.0) | |
| Southern European | 10 (1.0) | 0 (0.0) | 6 (0.7) | 1 (0.2) | 1 (0.1) | |
| Eastern European | 132 (13.5) | 11 (3.8) | 48 (5.3) | 28 (5.4) | 38 (4.3) | |
| Mixed/Other European | 25 (2.6) | 5 (1.7) | 14 (1.5) | 11 (2.1) | 10 (1.1) | |
| Family history of melanoma (in a first-degree relative) | ||||||
| No | 919 (94.3) | 274 (94.8) | 815 (89.7) | 472 (89.9) | 821 (91.9) | |
| Yes | 56 (5.7) | 15 (5.2) | 94 (10.3) | 53 (10.1) | 72 (8.1) | |
Pigmentary and naevus phenotypic characteristics
The associations between key pigmentary phenotypic characteristics and melanoma by anatomical site are presented in Figure 1 for the pooled analysis, and separately for Australia and Leeds in Supplementary Tables 3 and 4. In the pooled analysis, increased naevus density was associated with higher odds of melanoma for all sites, but the strength of the association differed by anatomical site (P-heterogeneity <0.001). The association of naevi was stronger for melanoma on the trunk and upper limbs (OR for many compared with no naevi =6.86, 95%CI 4.45–10.59 and 6.11, 95%CI 3.62–10.31, respectively) and lower limbs (OR=4.70, 95%CI 3.03–7.3) than head and neck melanoma (OR=1.85, 95%CI 1.05–3.26).
Figure 1:
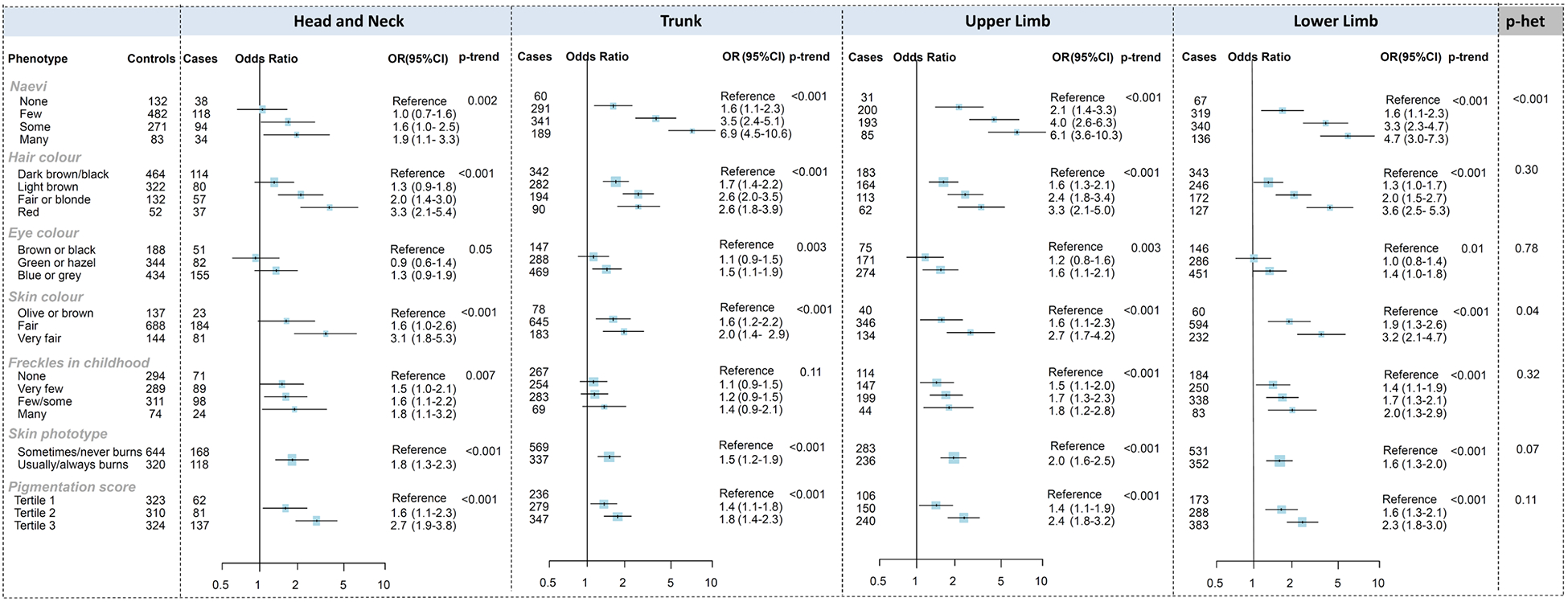
Associations between melanoma and naevus and pigmentation phenotypes, stratified by anatomical site, in the pooled Australian Melanoma Family Study and Leeds Melanoma Case-Control Study
Odds ratios (OR) were calculated using logistic regression models that compared melanoma cases at each anatomical site to all controls. Models were adjusted for age (continuous), sex, city of recruitment.
The association of skin colour also differed by site (P-heterogeneity=0.04), with very fair skin being more weakly related to melanoma on the trunk (OR=2.0, 95%CI 1.4–2.9 compared with olive or brown skin) than on other sites (ORs 2.7–3.2). When examined separately by study, the association with skin colour appeared stronger for melanoma on the head and neck in the Leeds study (OR=3.6 for very fair skin), and for melanoma on the lower limbs in the Australian study (OR=4.4).
Red or blonde hair, blue or grey eye colour, increasing number of freckles in childhood, propensity to sunburn, skin phototype and pigmentation score were associated with increased odds of melanoma for all sites, with no significant heterogeneity among the different sites in the pooled analysis (P-heterogeneity >0.05). When examined separately by study, sun-sensitive skin (skin phototype) was more weakly related to melanoma on the trunk in the Leeds study and in the Australian study pigmentation score was more strongly related to head and neck melanoma (both P-heterogeneity=0.02).
The associations did not materially changed when the pooled results were adjusted by UV exposures (Supplementary Table 5).
Given the sex differences in the development of melanoma at different anatomical sites, we examined whether the association of phenotypic characteristics with melanoma risk was modified by sex, separately for each anatomical site (Supplementary Table 6). The OR for freckles in childhood, comparing many to none, was higher for females compared with males for melanomas on the head and neck (ratio of ORs=3.4, 95%CI 1.1–10.8) and for melanomas on the trunk (ratio of ORs=2.8, 95%CI 1.3–6.3). Potential interactions with sex were also present for the association of red hair with melanomas on the head and neck (stronger association in females), and the association of naevi with melanomas on the lower limb (weaker association in females).
Ultraviolet radiation (UV) exposure
The associations between UV exposures and melanoma by anatomical site are presented in Figure 3 for the pooled multivariable analysis, and separately for Australia and Leeds in Supplementary Tables 7 and 8. In the pooled analysis, increased weekday sun exposure was associated with head and neck melanoma (for 1 hour/day increase in exposure, OR=1.2, 95%CI 1.1–1.4) but there was no significant heterogeneity by site (P-heterogeneity=0.43). Summer holiday sun exposure was associated with reduced risk of melanoma on the lower limbs and trunk, and weekend sun exposure was associated with reduced risk of melanoma on the lower limbs, but there was no significant heterogeneity by site.
Figure 3:
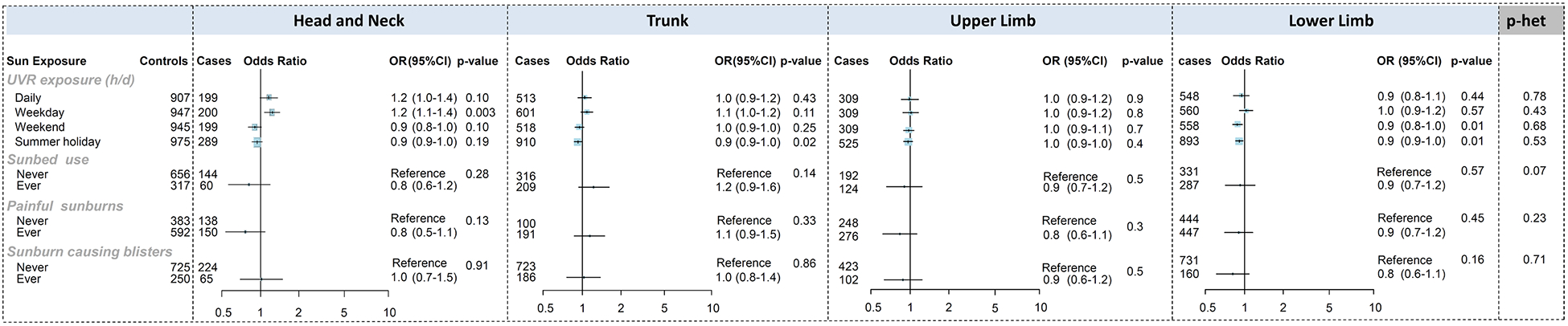
Associations between melanoma and ultraviolet radiation exposures, stratified by anatomical site, in the pooled Australian Melanoma Family Study and Leeds Melanoma Case-Control study
Odds ratios (OR) were calculated using logistic regression models that compared melanoma cases at each anatomical site to all controls. Models were adjusted for age (continuous), sex, city of recruitment and phenotypic characteristics: naevi, hair colour, eye colour, skin colour, freckles in childhood, skin phototype.
For continuous measures of sun exposure, the ORs were calculated per 1-hour increase in sun exposure per day and heterogeneity p-values were computed using variables categorised into tertiles.
There was borderline-significant heterogeneity by site (p=0.07) for sunbed use, which had a stronger association with melanoma on the trunk (Figure 3); this association with the trunk was more apparent in the Australian study (OR=1.8, 95%CI 1.1–2.9; Supplementary Table 7). There was no association with sunburns at any site in the pooled analysis (Figure 3). Increased risk of melanoma on the trunk was associated with painful sunburns in Leeds and blistering sunburns in Australia, although there was no significant heterogeneity by site (Supplementary Tables 7 and 8). Painful sunburns were associated with reduced risk of melanoma for all sites except the trunk in Australia.
Some risk estimates changed after adjustment for pigmentation and naevus phenotypic characteristics (Supplementary Tables 7 and 8); the inverse associations between sun exposure during weekends and summer holidays and melanoma risk were partly attenuated, associations with sunburns were mostly strengthened, and with sunbed use were mostly unchanged.
Genetic risk factors
Polygenic risk scores (PRS) were used to examine the risk of melanoma across different anatomical sites conferred by common genomic variants in several biological pathways important to melanoma development (pigmentation, naevus, and telomere/other pathways) (Figure 2 for the pooled analysis and separately for Australia and Leeds in Supplementary Tables 9 and 10). Associations with melanoma were strongest for the pigmentation pathway PRS, with more than 3-fold higher odds per SD increase of melanoma across all anatomical sites without evidence of heterogeneity (P-heterogeneity=0.14). Similarly, the telomere/other pathway PRS was consistently associated with melanoma at all anatomical sites. The naevus pathway PRS had a statistically significant association only with upper limb melanoma (OR per SD=1.9, 95%CI 1.2–3.0) and a borderline association with trunk melanoma (OR=1.4, 95%CI 0.97–2.1) but there was no evidence of heterogeneity. For head and neck melanoma, the pooled OR associated with the naevus pathway PRS was 1.3, but it appeared to differ by between Australia (OR=0.6) and Leeds (OR=2.0) (Cochran’s Q p=0.046). A PRS combining all genetic variants indicated an approximate 3-fold increased odds of melanoma, with no evidence of heterogeneity by anatomical site. The associations did not materially changed when the pooled results were adjusted by UV exposures (Supplementary Table 11).
Figure 2:
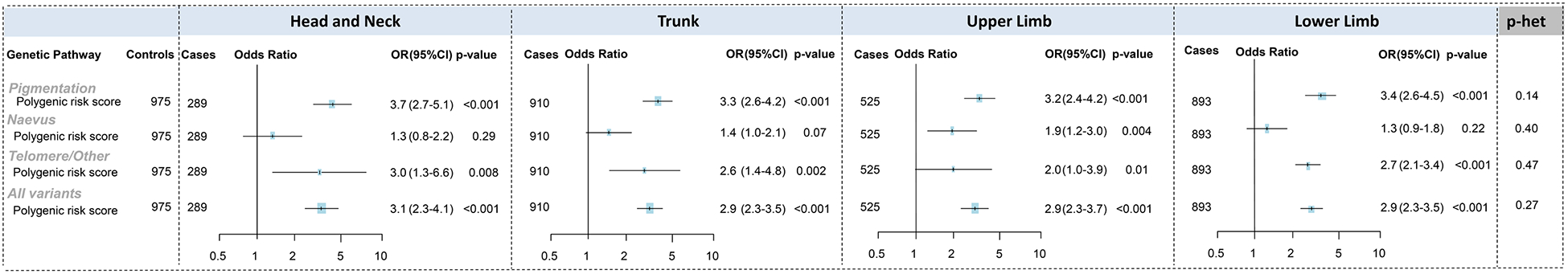
Associations between melanoma and genetic pathway scores, stratified by anatomical site, in the pooled Australian Melanoma Family Study and Leeds Melanoma Case-Control Study
Odds ratios (OR) were calculated using logistic regression models that compared melanoma cases at each anatomical site to all controls. Models were adjusted for age (continuous), sex, city of recruitment. ORs were calculated per 1 standard deviation increase in PRS and heterogeneity p-values were computed using variables categorised into tertiles.
DISCUSSION
To shed light on the aetiological heterogeneity of melanoma, we analysed a harmonised dataset of two population-based case-control studies in Australia and the United Kingdom to characterise risk factors for cutaneous melanoma according to anatomical site. Several of our findings are consistent with the dual pathway hypothesis, which proposes that there is heterogeneity in the aetiological pathways to melanoma such that risk of melanomas on the trunk is determined by propensity to form naevi (which are both genetically determined and caused by early-life sun exposure6,31–33) and intermittent sun exposure, whereas melanoma on the head and neck is more likely to be caused by chronic (more continuous) sun exposure.11,12,14,16,34 Consistent with this, we found that number of naevi was more strongly associated with trunk melanoma than head and neck melanoma. Increased weekday sun exposure (a proxy for occupational sun exposure) was associated with head and neck melanoma, which has been reported by other studies7,16,17 although not consistently.35 This positive association with head and neck melanoma was more apparent for the Leeds study than the Australian study, which may be due to the older age distribution of the Leeds study than the Australian study, as melanomas associated with chronic UV exposure are more common among older ages.36 Sunbed use and sunburns, considered intermittent exposures, appeared more strongly associated with trunk melanoma, which is also consistent with the dual pathways hypothesis and other studies,37,38 although there was no statistical evidence for heterogeneity and the risk estimates differed between studies.
Evidence for melanoma on the limbs is less clear, but one recent study suggested that lower-limb melanoma, like trunk melanoma, may tend to arise via a naevus-related pathway whereas upper-limb melanoma, like head and neck melanomas, may tend to arise via the sun damage pathway.23 In contrast with this suggestion, we found the strongest associations with naevi for upper-limb melanoma and trunk melanoma, although the association of naevi with melanomas on the lower limb was weaker for females than males.
In addition to number of naevi, the other risk factor with heterogeneity by anatomical site in our study was skin colour. In particular, the increased risk associated with very fair skin was weaker for melanoma on the trunk than for other sites, though this difference was smaller than for number of naevi. A similar difference by site was also observed in a previous meta-analysis.7 Taken together, our results do not support a clear classification of upper- and lower-limb melanoma into the two pathways indicated by melanoma on the trunk and head or neck. Instead, they suggest that both pathways may be important for development of melanoma on the limbs.
While certain findings based on phenotypic risk factors showed clear support for aetiological heterogeneity by anatomical site, we did not find clear evidence of heterogeneity in associations with polygenic risk scores quantifying genetic pathways for pigmentation, naevi, and telomere/other biological processes. Unlike the phenotypic naevus and skin colour variables, the naevus and pigmentation pathway PRS ORs were similar between melanoma on the head/neck and on the trunk. Despite naevi being one of the strongest risk factors for melanoma,39 a naevus-pathway PRS has a relatively weak overall association with melanoma risk.20 This discrepancy may be because our current naevus PRS captures only a small proportion of the total variation in naevus phenotypes.40
Contrary to previous studies,4,7,17,41 we did not observe positive associations between melanoma risk and sunburn at all anatomical sites, nor with measures of recreational sun exposure. The inverse association with weekend and summer holidays and melanoma risk has been previously reported for the Leeds study and was hypothesized to be mediated by photoadaptation or higher vitamin D levels.22 They also observed a stronger association with melanoma for sunburns after the age of 20.22 Interestingly, painful sunburns (but not sunburns causing blisters) were inversely associated with melanoma risk on all sites except the trunk in the Australian study, and this was stronger after adjustment for phenotypic characteristics. Sun sensitivity may modify or confound this association,42 and we previously showed that the association with sunburn was modified by host factors because a positive association was only observed in people who tended to tan rather than burn and in people who had few nevi.43 We observed null associations with total sun exposure at all sites. A meta-analysis by Chang et al also found mostly null associations at different body sites except for an increased risk of melanoma on the limbs at low latitudes.17
Key strengths of our study are its size and comprehensive genetic and phenotypic risk factor measures, which allowed detailed analysis by anatomical sites of melanoma, and which was achieved by pooling two population-based case-control studies that used the same measures for data collection. The approach of pooling these data sources was supported by our previous finding that the associations between melanoma and self-reported pigmentary and naevus phenotypes were similar across countries.6 We also examined the associations by site in each study separately, although these sub-group analyses had limited statistical power.
The younger age of participants in the Australian study (<40 years at diagnosis) is a limitation for the study of divergent pathways of melanoma, particularly for the UV-related exposures. However, since ambient sun exposure varies greatly between Australia and Leeds, at any given age the cumulative dose of UV exposure is expected to be higher for Australia than the UK. The main focus of our analysis was on pigmentary and naevus characteristics, as fewer studies have examined associations of these risk factors by anatomical site. Other limitations of our study include the lack of detailed pathological information, as some studies have suggested that the presence of solar elastosis and naeval remnants influence aetiologically distinct subtypes.8,44 When using self-reported risk factors people tend to underestimate their naevus counts and pigmentation;45 although associations with melanoma have been shown to be very similar for most self-reported and clinically-assessed risk factors.6 Measurement error, recall bias and selection bias may have also influenced the observed sun exposure associations. Participation was higher for cases than controls. Sun exposure is a widely known risk factor for melanoma, and controls with high sun exposure may have been more interested to participate in the study, which would lead to inverse associations. Personal lifetime sun exposure is also a complex behaviour to measure,46 and non-differential measurement error usually biases the result towards the null.47 We previously showed stronger associations of childhood total sun exposure and sunburn with melanoma risk when exposure level was recalled concordantly by participants and their parents.43 We had lower numbers of controls than cases in our analysis. Most previous studies have conducted a case-only analysis, however including controls produces risk estimates that are more easily communicated to the public and comparable with other risk factor studies. The Leeds study recruited people with thicker melanomas (≥0.75mm) in the later years of the study, but stratification of our results by this factor did not materially alter the results.
In conclusion, in our analysis by anatomical site we found evidence of aetiological heterogeneity for melanoma, supporting the dual pathway hypothesis. The evidence was strongest for naevus phenotype measures, but weaker for pigmentary phenotype, sun exposure and genetically-measured risk factors. These findings promote a better understanding of melanoma development. They may also be helpful for guiding skin examination education and practices, for example by highlighting to patients and clinicians which areas of the body may require closer or more regular examination, according to their risk factor profile.
Supplementary Material
What’s already known about this topic?
Two main biological pathways have been proposed for the aetiology of melanoma – determined by naevi and type of sun exposure and related to the anatomical site at which melanoma develops.
Risk factors for melanoma may differ by anatomical site, but analyses are often limited by study sample size and most have focussed on sun exposure.
What does this study add?
An examination of a comprehensive set of risk factors for melanoma by anatomical site, using a harmonised dataset from two population-based studies with 3,592 participants.
The presence of increased numbers of naevi was more strongly associated with melanomas on the trunk and limbs than on the head and neck.
Very fair skin was more weakly related to melanoma on the trunk than on other sites.
The association of pathway-specific polygenic risk scores with melanoma did not differ by anatomical site.
Acknowledgments:
We thank Dr. Emily Zabor for developing and helping us implement the “riskclustr” R package to quantify aetiological heterogeneity. We gratefully acknowledge all of the participants, the work and dedication of the research coordinators, interviewers, examiners and data management staff. Emma Northwood and Martin Drummond assisted with the harmonisation of data across the studies and Caro Badcock assisted with data preparation. For the Australian Melanoma Family Study, this included Judith Maskiell, Jackie Arbuckle, Steven Columbus, Michaela Lang, Helen Rodais, Caroline Ellis (The University of Melbourne, Melbourne, Australia); Carol El Hayek, Lynne Morgan, Joanne Roland, Emma Tyler, Jodi Barton, Caroline Watts, Lesley Porter (Westmead Institute for Medical Research, The University of Sydney, Sydney, Australia); Jodie Jetann, Megan Ferguson, Michelle Hillcoat, Kellie Holland, Pamela Saunders, Joan Roberts and Sheree Tait (Viertel Centre for Research in Cancer Control, Cancer Council Queensland, Brisbane, Australia). In the Leeds Melanoma Study, recruitment was facilitated by the UK National Cancer Research Network. Patricia Mack and Kate Gamble collected data for the studies. Paul King carried out data entry. We are extremely grateful to Birute Karpavicius, Susan Leake, Susan Haynes, Elaine Fitzgibbon, and the many clinicians and research staff who assisted with recruiting participants to the studies, and to the pathologists who assisted with the melanoma samples.
Abbreviations:
- CI
confidence interval
- OR
odds ratio
- RR
relative risk
- UK
United Kingdom
Footnotes
Conflict of interest disclosures: none
Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization.
Funding:
This work was supported by the National Health and Medical Research Council of Australia (NHMRC) (project grants 566946, 107359, 211172 and program grant number 402761); the Cancer Council New South Wales (project grant 77/00, 06/10), the Cancer Council Victoria and the Cancer Council Queensland (project grant 371); the US National Institutes of Health (NIH RO1 grant CA83115 to Genomel (www.genomel.org)); Cancer Research UK (Project Grant C8216/A6129 and Programme awards C588/A4994, C588/A10589 and C588/A19167); NHMRC Career Development Fellowship (#1147843) to AEC; and in part by the Intramural Research Program of the National Cancer Institute, the National Institutes of Health, the Division of Cancer Epidemiology and Genetics.
REFERENCES
- 1.Glazer AM, Winkelmann RR, Farberg AS et al. Analysis of Trends in US Melanoma Incidence and Mortality. JAMA Dermatol 2017; 153:225–6. [DOI] [PubMed] [Google Scholar]
- 2.Karimkhani C, Green AC, Nijsten T et al. The global burden of melanoma: results from the Global Burden of Disease Study 2015. Br J Dermatol 2017; 177:134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandini S, Sera F, Cattaruzza MS et al. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer 2005; 41:2040–59. [DOI] [PubMed] [Google Scholar]
- 4.Gandini S, Sera F, Cattaruzza MS et al. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer 2005; 41:45–60. [DOI] [PubMed] [Google Scholar]
- 5.Gandini S, Sera F, Cattaruzza MS et al. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer 2005; 41:28–44. [DOI] [PubMed] [Google Scholar]
- 6.Cust AE, Drummond M, Bishop DT et al. Associations of pigmentary and naevus phenotype with melanoma risk in two populations with comparable ancestry but contrasting levels of ambient sun exposure. J Eur Acad Dermatol Venereol 2019; 33:1874–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caini S, Gandini S, Sera F et al. Meta-analysis of risk factors for cutaneous melanoma according to anatomical site and clinico-pathological variant. Eur J Cancer 2009; 45:3054–63. [DOI] [PubMed] [Google Scholar]
- 8.Mauguen A, Zabor EC, Thomas NE et al. Defining Cancer Subtypes With Distinctive Etiologic Profiles: An Application to the Epidemiology of Melanoma. J Am Stat Assoc 2017; 112:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holman CD, Armstrong BK, Heenan PJ. A theory of the etiology and pathogenesis of human cutaneous malignant melanoma. J Natl Cancer Inst 1983; 71:651–6. [PubMed] [Google Scholar]
- 10.Green A A theory of site distribution of melanomas: Queensland, Australia. Cancer Causes Control 1992; 3:513–6. [DOI] [PubMed] [Google Scholar]
- 11.Whiteman DC, Watt P, Purdie DM et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst 2003; 95:806–12. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong BK, Cust AE. Sun exposure and skin cancer, and the puzzle of cutaneous melanoma: A perspective on Fears et al. Mathematical models of age and ultraviolet effects on the incidence of skin cancer among whites in the United States. American Journal of Epidemiology 1977; 105: 420–427. [DOI] [PubMed] [Google Scholar]; Cancer Epidemiol 2017; 48:147–56. [DOI] [PubMed] [Google Scholar]
- 13.Whiteman DC. Testing the divergent pathway hypothesis for melanoma: recent findings and future challenges. Expert Rev Anticancer Ther 2010; 10:615–8. [DOI] [PubMed] [Google Scholar]
- 14.Siskind V, Whiteman DC, Aitken JF et al. An analysis of risk factors for cutaneous melanoma by anatomical site (Australia). Cancer Causes Control 2005; 16:193–9. [DOI] [PubMed] [Google Scholar]
- 15.Iles MM, Bishop DT, Taylor JC et al. The effect on melanoma risk of genes previously associated with telomere length. J Natl Cancer Inst 2014; 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiteman DC, Stickley M, Watt P et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol 2006; 24:3172–7. [DOI] [PubMed] [Google Scholar]
- 17.Chang YM, Barrett JH, Bishop DT et al. Sun exposure and melanoma risk at different latitudes: a pooled analysis of 5700 cases and 7216 controls. Int J Epidemiol 2009; 38:814–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts MR, Asgari MM, Toland AE. Genome-wide association studies and polygenic risk scores for skin cancer: clinically useful yet? Br J Dermatol 2019; 181:1146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu F, Chen TH, Pfeiffer RM et al. Combining common genetic variants and non-genetic risk factors to predict risk of cutaneous melanoma. Hum Mol Genet 2018; 27:4145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cust AE, Drummond M, Kanetsky PA et al. Assessing the incremental contribution of common genomic variants to melanoma risk prediction in two population-based studies. J Invest Dermatol 2018; 138:2617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Law MH, Bishop DT, Lee JE et al. Genome-wide meta-analysis identifies five new susceptibility loci for cutaneous malignant melanoma. Nat Genet 2015; 47:987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newton-Bishop JA, Chang YM, Elliott F et al. Relationship between sun exposure and melanoma risk for tumours in different body sites in a large case-control study in a temperate climate. Eur J Cancer 2011; 47:732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghiasvand R, Robsahm TE, Green AC et al. Association of Phenotypic Characteristics and UV Radiation Exposure With Risk of Melanoma on Different Body Sites. JAMA Dermatol 2019; 155:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cust AE, Schmid H, Maskiell JA et al. Population-based, case-control-family design to investigate genetic and environmental influences on melanoma risk: Australian Melanoma Family Study. Am J Epidemiol 2009; 170:1541–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breslow NE. Statistics in epidemiology: the case-control study. J Am Stat Assoc 1996; 91:14–28. [DOI] [PubMed] [Google Scholar]
- 26.Zabor EC, Begg CB. A comparison of statistical methods for the study of etiologic heterogeneity. Stat Med 2017; 36:4050–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zabor EC. riskclustr: Functions to Study Etiologic Heterogeneity. The Journal of Open Source Software 2019; 4:1269, 10.21105/joss.01269. [DOI] [Google Scholar]
- 28.Zabor EC. Tutorial: Test for etiologic heterogeneity in a case-control study. In. 2019.
- 29.Althouse AD. Adjust for Multiple Comparisons? It’s Not That Simple. Ann Thorac Surg 2016; 101:1644–5. [DOI] [PubMed] [Google Scholar]
- 30.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1:43–6. [PubMed] [Google Scholar]
- 31.Bataille V, Snieder H, MacGregor AJ et al. Genetics of risk factors for melanoma: an adult twin study of nevi and freckles. J Natl Cancer Inst 2000; 92:457–63. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Duffy DL, McClenahan P et al. Heritability of naevus patterns in an adult twin cohort from the Brisbane Twin Registry: a cross-sectional study. Br J Dermatol 2016; 174:356–63. [DOI] [PubMed] [Google Scholar]
- 33.Wachsmuth RC, Gaut RM, Barrett JH et al. Heritability and gene-environment interactions for melanocytic nevus density examined in a U.K. adolescent twin study. J Invest Dermatol 2001; 117:348–52. [DOI] [PubMed] [Google Scholar]
- 34.Cho E, Rosner BA, Colditz GA. Risk factors for melanoma by body site. Cancer Epidemiol Biomarkers Prev 2005; 14:1241–4. [DOI] [PubMed] [Google Scholar]
- 35.Vuong K, McGeechan K, Armstrong BK et al. Occupational sun exposure and risk of melanoma according to anatomical site. Int J Cancer 2014; 134:2735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guitera P, Collgros H, Madronio CM et al. The steadily growing problem of lentigo maligna and lentigo maligna melanoma in Australia: Population-based data on diagnosis and management. Australas J Dermatol 2019; 60:118–25. [DOI] [PubMed] [Google Scholar]
- 37.Lazovich D, Isaksson Vogel R, Weinstock MA et al. Association Between Indoor Tanning and Melanoma in Younger Men and Women. JAMA Dermatol 2016; 152:268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Clair MZ, Cockburn MG. Tanning bed use and melanoma: Establishing risk and improving prevention interventions. Prev Med Rep 2016; 3:139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olsen CM, Carroll HJ, Whiteman DC. Estimating the attributable fraction for cancer: A meta-analysis of nevi and melanoma. Cancer Prev Res (Phila) 2010; 3:233–45. [DOI] [PubMed] [Google Scholar]
- 40.Duffy DL, Zhu G, Li X et al. Novel pleiotropic risk loci for melanoma and nevus density implicate multiple biological pathways. Nat Commun 2018; 9:4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olsen CM, Zens MS, Green AC et al. Biologic markers of sun exposure and melanoma risk in women: Pooled case-control analysis. Int J Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dennis LK, Vanbeek MJ, Beane Freeman LE et al. Sunburns and risk of cutaneous melanoma: does age matter? A comprehensive meta-analysis. Ann Epidemiol 2008; 18:614–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cust AE, Jenkins MA, Goumas C et al. Early-life sun exposure and risk of melanoma before age 40 years. Cancer Causes Control 2011; 22:885–97. [DOI] [PubMed] [Google Scholar]
- 44.Kvaskoff M, Pandeya N, Green AC et al. Solar elastosis and cutaneous melanoma: a site-specific analysis. Int J Cancer 2015; 136:2900–11. [DOI] [PubMed] [Google Scholar]
- 45.Cust AE, Pickles KM, Goumas C et al. Accuracy of self-reported nevus and pigmentation phenotype compared with clinical assessment in a population-based study of young Australian adults. Cancer Epidemiol Biomarkers Prev 2015; 24:736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kricker A, Vajdic CM, Armstrong BK. Reliability and validity of a telephone questionnaire for estimating lifetime personal sun exposure in epidemiologic studies. Cancer Epidemiol Biomarkers Prev 2005; 14:2427–32. [DOI] [PubMed] [Google Scholar]
- 47.Wacholder S, Hartge P, Lubin JH et al. Non-differential misclassification and bias towards the null: a clarification. Occup Environ Med 1995; 52:557–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


