Abstract
Purpose
This report aimed to present a case of corneal fibrosis with prolonged atopic blepharitis caused by psychological resistance to steroid treatment.
Observations
A 49-year-old woman presented with atopic dermatitis and a history of panic attack and autism spectrum disorder. The upper and lower eyelid margins of her right eye became adherent, and the eyelid remained closed for several years due to refusal of steroid treatment and aggravation of blepharitis. During the initial examination, a lesion with elevated white opacity on the corneal surface was observed. Subsequently, superficial keratectomy was performed. The histopathological findings were indicative of corneal keloid.
Conclusions and Importance
Persistent atopic ocular surface inflammation and prolonged eyelid closure resulted in the formation of a corneal keloid.
Keywords: Corneal keloid, Atopic blepharitis, Corneal fibrosis, Keratectomy
1. Introduction
Corneal keloids are a rare ophthalmic disease characterized by focal white elevated lesions on the cornea.1 These lesions are opaque and can be vascular or nonvascular, with well-defined borders and varying thickness and size.1 Corneal keloids have been reported to form in patients without a history of surgery or corneal trauma, although most cases occur months to years after trauma or surgery.1,8, 9, 10, 11 Definitive diagnosis of corneal keloids requires biopsy and histopathology and is characterized by dysplasia of the corneal epithelium and hyperplasia of collagen fibers.1,4,9, 10, 11 Here, we report a case of corneal keloid that might have developed after prolonged eyelid closure and been maintained due to severe atopic blepharitis (see Table 1).
Table 1.
Clinical and pathological features of corneal keloids.
| Features of corneal keloids |
|---|
| Clinical findings |
|
|
|
|
|
| There was gradual expansion beyond the wound after trauma over months to years. |
| Pathological findings |
|
|
|
|
|
|
|
| Onset triggers |
|
|
|
|
|
| * PRK: Photo-refractive keratectomy |
2. Case report
A 49-year-old woman with vision loss in the right eye for 3 years was referred to our hospital. The patient had atopic dermatitis, and the disease in her eyelid worsened approximately 10 years ago. She refused steroid treatment because of a strong fear of steroids. The patient said that the upper and lower eyelid margins of her right eye were fused, and the eyelids had remained closed for several years because of atopic conjunctivitis and blepharitis. She also said that her eyelid dermatitis improved with steroid-free atopic treatment (eyelid hygiene and chiropractic treatment of autonomic imbalance) during the past 3 years. She became aware of a loss of vision in her right eye and noticed that it was cloudy when she looked at the mirror with the open right eye. Notably, she has been diagnosed with atopic dermatitis since childhood and has been attending a psychosomatic medicine clinic for a panic disorder and autism. She underwent right cataract surgery and right posterior capsulotomy 9 years ago and a second posterior capsulotomy 8 years ago. Her best corrective visual acuity was 20/30 in the left eye, and light perception was present in the right eye. Furthermore, there was an elevated white opaque lesion on the central corneal surface of the right eye with vascular invasion and a dislocated intraocular lens (IOL) in the anterior chamber (Figs. 1–). Anterior segment 3-dimensional optical coherence tomography (AS-OCT) scan revealed areas of high intensity from the corneal surface to the superficial layer of stroma, with an increased central corneal thickness of 1570 μm (Fig. 1, Fig. 2). B-mode ultrasonography did not identify abnormal findings in the posterior segment.
Fig. 1.
Initial examination findings
Ophthalmologic examination findings of a 49-year-old woman's right eye
1) An elevated white opaque lesion was observed on the central corneal surface.
2) Anterior segment optical coherence tomography (AS-OCT) scan demonstrated areas of hyperintensity from the corneal surface to the superficial layer of stroma and a dislocated intraocular lens (IOL).
Fig. 2.
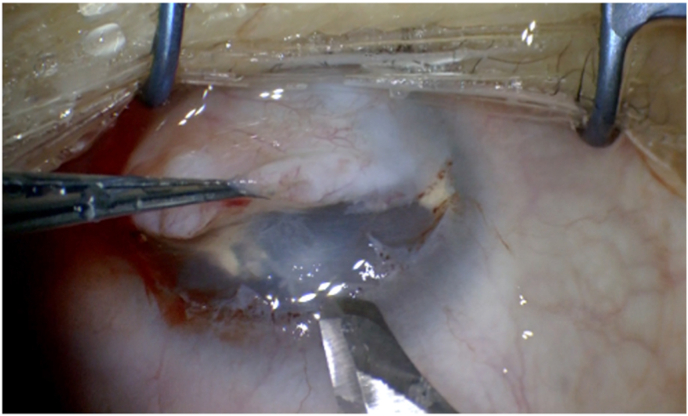
Surgical Treatment
Most of the fibrotic tissue was easily detached using superficial keratectomy. Superficial keratectomy enabled easy detaching of most of the fibrotic tissue.
Superficial keratectomy was performed on the right eye. Major portion of the lesion was easily detached in the Bowman's layer; however, a small portion was infiltrating into the stroma (Fig. 2).
On histopathologic examination, the majority of the excised tissue comprised thick collagen-rich tissue, with a mildly thickened stratified squamous epithelium in the superficial layer. Capillaries were formed in the collagen fibers under the epithelium, and plasma cell and lymphocyte infiltrates were seen surrounding the vessels, suggesting that the tissue was different from the original corneal stroma (Fig. 3 a, b, c). In addition, immunohistochemical staining was performed using anti-vimentin and anti-alpha smooth muscle actin (αSMA) antibodies. Both stains diffusely highlighted the stroma in the keloid lesion (Fig. 3 e, f). The patient was diagnosed with a corneal keloid based on clinical, histopathological, and immunohistochemical findings.
Fig. 3.
Histopathological and immunohistochemical findings
a. Hematoxylin and eosin (HE) staining revealed a mildly thickened superficial epithelium (*) and plasma cell and lymphocyte infiltration surrounding capillaries formed in the superficial layers of the stroma (→). b. Periodic acid-Schiff staining revealed no Bowman's layer between the epithelium and stromal tissue. c. Azan staining demonstrated thick collagen fibrils in the stromal tissue. d. HE staining. e. Vimentin staining was diffusely positive in the stromal tissue, predominantly in the anterior stroma, highlighting fibroblasts and neovascular endothelial cells. f. Alpha-SMA staining was diffusely positive in the stromal tissue, predominantly in the anterior stroma, highlighting myofibroblasts and neovascular endothelial cells.
After carefully explaining to the patient that topical steroid treatment is safer than systemic administration and obtaining her consent, postoperative eye drops containing a 0.1% betamethasone sodium phosphate ophthalmic solution were administered until the third postoperative day; subsequently, 0.1% fluorometholone ophthalmic solution was administered until 1 month after surgery, and this was then switched to tacrolimus ophthalmic solution. Consequently, the corneal transparency improved, and the anterior chamber could be observed, but the IOL had dislocated into the anterior chamber with the completely cloudy lens capsule, and the fundus was not assessable (Fig. 4). Approximately 1 month after the surgery, IOL removal and vitrectomy were performed, and the fundus became observable, but the optic nerve papilla was pale. Postoperative best visual acuity was 20/300, intra ocular pressure was low-teen, and the residual visual field was limited. A specular microscopy could not detect endothelial cells. Finally, the patient was considered to have severe visual dysfunction due to glaucoma and bullous keratopathy. Epithelialization was achieved 2 months after the surgery, but subepithelial corneal opacification was observed at a follow-up visit after 5 months of the surgery. The lesion led to the development of an elevated opacity at another follow-up visit after 15 months of the surgery. Eventually, the patient was considered to have recurrent corneal keloid because the elevated lesion was found to be increasing in size at the final visit of 15 months after the surgery (Fig. 5). The best postoperative corrected visual acuity in the right eye was 20/300, and the final corrected visual acuity was 20/2000.
Fig. 4.
Findings at 1-month after surgery
One month after the superficial keratectomy, the IOL was dislocated into the anterior chamber with the lens capsule.
Fig. 5.
Findings at 15 months after surgery
Fifteen months after the superficial keratectomy, the patient was diagnosed with recurrent corneal keloids owing to the development of an elevated corneal opacity.
3. Discussion
Corneal keloids usually produce localized white elevated lesions on the corneal surface.1 Although congenital cases have been reported, and some cases are associated with specific genetic syndromes, such as Lowe's syndrome,3,6,7 most corneal keloids occur after trauma, surgery, or keratitis.1 Differential diagnosis with similar white elevated lesions include hypertrophic scars, Salzmann's nodular degeneration, limbal dermoids, sclerocornea, myxomas, congenital glaucoma with corneal edema, and metabolic diseases, such as Peters anomaly, fibrous histiocytomas, and mucopolysaccharidosis.1,5,9
Histopathological findings of corneal keloids are characterized by a normal or thickened and keratinized epithelium, fragmented or absent Bowman's membrane, and proliferated collagen fibers.1,4,9,10 Based on immunohistochemical examination of the present case, positive staining with αSMA and vimentin should be expected in cases of corneal keloids. Notably, the positive αSMA staining is correlated with myofibroblasts and the smooth muscle walls of the limited vasculature. Furthermore, αSMA immunostaining is characteristically positive in corneal keloids, but it is negative in the normal stroma.16 In contrast, vimentin staining is positive for fibroblasts as well as vascular endothelial, smooth muscle, and other cells.15, 16, 17 In corneal keloids, vimentin staining is nonspecific as is positive for both the lesion and normal corneal stroma.16 In such cases, the diagnosis is established based on the patient's history and clinical and histopathological findings.
In the present case, Salzmann's nodular degeneration and hypertrophic scarring were considered as differential diagnoses. In Salzmann's nodular degeneration, the lesions are multiple and elevated occurring after ocular surface diseases, such as keratitis or vernal keratoconjunctivitis.12 However, the pathology of this case where there was a single large opacity with thickened overlying epithelium.
Additionally, a post-traumatic hypertrophic scar was suspected if the severe ocular abrasion was considered to be similar to trauma. Corneal keloid lesions occur months to years after trauma and expand over time, extending beyond the boundaries of the trauma, whereas hypertrophic scars appear immediately after trauma and do not expand beyond the initial boundaries.4,5 In this case, the patient's history makes a hypertrophic scar unlikely.
Before 9 years, the patient underwent cataract surgery and Nd-YAG laser capsulotomy. We speculated that the IOL was dislocated to anterior chamber after rubbing eye lid and caused corneal edema. Moreover, this pathologic change may have caused stimulation of cytokines releasing in corneal stroma.
The pathogenesis of corneal keloids remains unknown, but several hypotheses have been proposed. Mejia et al. reported that cytokines released from the damaged epithelium might prevent normal corneal growth and differentiation, influencing fibroblasts.9 Furthermore, it has been reported that in patients with atopic keratoconjunctivitis, damage to the corneal epithelium triggered by ocular abrasion or other factors stimulates fibroblasts in the corneal stroma to express chemokines and adhesion molecules in the stroma through cytokines, such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and matrix metalloproteinase (MMP)-13, in the tear fluid.13,14
In the present case, the patient suffered from severe atopic blepharitis that resulted in her eyelid being closed for a prolonged period. The patient's difficulty in opening the eyelids may have been caused by blepharoconjunctivitis or an epithelial disorder. Mechanical trauma caused by eye rubbing would have resulted in corneal epithelial damage, and atopic keratoconjunctivitis might have led to chronic inflammation and corneal keloid formation as a result of cytokine-mediated fibroblast proliferation. Alternatively, inflammation due to prolonged atopic dermatitis may have affected the corneal surface.
Depending on the location, size, and depth of the corneal keloid, treatments, other than conservative treatment, have been reported; these include superficial keratectomy, lamellar keratoplasty, deep anterior lamellar keratoplasty, penetrating keratoplasty, and keratoplasty combined with amniotic membrane transplantation,2 but recurrence is often observed.
In the present case, the remaining visual field was minimal, and the patient did not wish to undergo corneal transplantation.
4. Conclusions
This is a report of a specific case of corneal keloid following prolonged eyelid closure and eye rubbing due to severe atopic blepharitis. Prolonged eyelid closure may have resulted in prolonged ocular surface inflammation, and trauma due to ocular abrasion may have caused the fibrotic corneal hyperplasia.
Patient consent
Written informed consent was obtained from the patient.
Funding
This study received no funding or grant support.
Authorship
R.M., T.T., and T.M. contributed to the design and conduct of the study; R.M., T.T., R.A., and S.Y. contributed to the collection, management, analysis, and interpretation of data; and T.T., Y.A., S·Y., and T.M. contributed to the preparation, review, and approval of the manuscript.
Declaration of competing interest
The authors have no financial disclosures.
Acknowledgements
None.
References
- 1.Vanathi M., et al. Corneal keloid. Ocul Surf. 2008;6:186–197. doi: 10.1016/s1542-0124(12)70179-9. [DOI] [PubMed] [Google Scholar]
- 2.Lee H.K., et al. Corneal keloid: four case reports of clinicopathological features and surgical outcome. BMC Ophthalmol. 2016;16:198. doi: 10.1186/s12886-016-0372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esquenazi S., et al. Corneal keloid in Lowe syndrome. J Pediatr Ophthalmol Strabismus. 2005;42:308–310. doi: 10.3928/0191-3913-20050901-16. [DOI] [PubMed] [Google Scholar]
- 4.Bakhtiari P., et al. Corneal keloid: report of natural history and outcome of surgical management in two cases. Cornea. 2013;32:1621–1624. doi: 10.1097/ICO.0b013e3182a73a10. [DOI] [PubMed] [Google Scholar]
- 5.Gupta J., et al. Diagnosis, management, and histopathological characteristics of corneal keloid: a case series and literature review. Asia Pac J Ophthalmol (Phila) 2016;5:354–359. doi: 10.1097/APO.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 6.Cibis G.W., et al. Corneal keloid in Lowe's syndrome. Arch Ophthalmol. 1982;100:1795–1799. doi: 10.1001/archopht.1982.01030040775013. [DOI] [PubMed] [Google Scholar]
- 7.McElvanney A.M., et al. Corneal keloid:aetiology and management. in Loweʼs syndrome. Eye. 1995;9:375–376. doi: 10.1038/eye.1995.75. [DOI] [PubMed] [Google Scholar]
- 8.Holbach L.M., et al. Bilateral keloid-like myofibroblastic proliferations of the cornea in children. Ophthalmology. 1990;97:1188–1193. doi: 10.1016/s0161-6420(90)32437-5. [DOI] [PubMed] [Google Scholar]
- 9.Mejía L.F., et al. Clinical, surgical, and histopathologic characteristics of corneal keloid. Cornea. 2001;20:421–424. doi: 10.1097/00003226-200105000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Jung J.J., et al. Giant corneal keloid: case report and review of the literature. Cornea. 2010;29:1455–1458. doi: 10.1097/ICO.0b013e3181d83858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuda K., et al. Long-term follow-up after lamellar keratoplasty in a patient with bilateral idiopathic corneal keloid. Cornea. 2011 Dec;30:1491–1494. doi: 10.1097/ICO.0b013e31822018f2. [DOI] [PubMed] [Google Scholar]
- 12.Maharana P.K., et al. Salzmann's nodular degeneration. Ocul Surf. 2016 Jan;14:20–30. doi: 10.1016/j.jtos.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Greiner J.V., et al. Effects of eye rubbing on the conjunctiva as a model of ocular inflammation. Am J Ophthalmol. 1985 Jul 15;100:45–50. doi: 10.1016/s0002-9394(14)74981-5. [DOI] [PubMed] [Google Scholar]
- 14.Balasubramanian S.A., et al. Effects of eye rubbing on the levels of protease, protease activity and cytokines in tears: relevance in keratoconus. Clin Exp Optom. 2013;96:214–218. doi: 10.1111/cxo.12038. [DOI] [PubMed] [Google Scholar]
- 15.Miyamoto R., et al. A rare case of corneal keloid occurred 30 years after pterygium surgery and 3 years after cataract surgery. Am J Ophthalmol Case Rep. 2020 Sep 7;20 doi: 10.1016/j.ajoc.2020.100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palko J.R., et al. Corneal keloid presenting forty years after penetrating injury: case report and literature review. Surv Ophthalmol. Sep-Oct 2019;64:700–706. doi: 10.1016/j.survophthal.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Alkatan H.M., et al. Healed corneal ulcer with keloid formation. Saudi J Ophthalmol. 2012 Apr;26:245–248. doi: 10.1016/j.sjopt.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]