Introduction
Photodynamic therapy (PDT) aims to destroy targeted abnormal cells with the use of a photosensitizer that selectively accumulates in cancerous cells and metabolizes into protoporphyrin IX (PpIX) during incubation. The skin lesions are then exposed to a light source at a specific wavelength based on the absorption spectrum of PpIX.1 This results in a phototoxic reaction leading to the apoptosis of targeted cells.2
Two main photosensitizers are used: 5-aminlevulinic-acid and methyl-aminolevulinic (MAL). MAL is described as more lipophilic with deeper penetration of the skin and higher stability than 5-aminlevulinic-acid.3,4 MAL-PDT could possibly be more effective for the treatment of deep skin lesions.3
PDT is used in numerous non-melanoma skin cancers5,6 and in non-oncological diseases such as infectious or inflammatory pathologies.
A handful of studies have shown promising results of PDT in the treatment of early-stage mycosis fungoides.7,8 However, little data regarding PDT in cutaneous B-cell lymphomas (CBCL) have been published.9 We report a case series of 4 patients with marginal zone lymphoma (MZL)-type CBCL treated by PDT.
Patients and methods
Four patients with MZL-type CBCL were treated with MAL-PDT. The patients’ characteristics are shown in Table I. The mean age was 51 years, ranging from 27 to 64. The sex ratio was 1 (2 females and 2 males). All the patients had multiple target skin lesions ranging from 5 to 7 thus PDT was preferred over surgical excision or radiotherapy. The diagnosis was established in all patients by routine histopathology and immunophenotyping on skin biopsy samples by a trained pathologist, with experience in cutaneous lymphomas. The patients were staged and confirmed to have skin limited diseases. Half of them had received at least 1 treatment prior to our study. The treatments were topical high potency corticosteroids (patient 2 and patient 3) and rituximab (patient 2 and patient 3). Both patients previously treated by rituximab had received 8 infusions prior to PDT.
Table I.
Clinical characteristics of patients
| Patient no | Age | Gender | Prior treatment lines | Number of skin lesions treated |
|---|---|---|---|---|
| 1 | 64 y old | F | None | 5 |
| 2 | 55 y old | M | Rituximab, clobetasol | 7 |
| 3 | 61 y old | M | Rituximab, clobetasol | 5 |
| 4 | 27 y old | F | None | 7 |
We performed a micro-needle abrasion of the skin lesions by using a dermaroller to enhance drug penetration on the targeted skin lesions. We immediately applied a thin layer of MAL 168 mg/g 0.5 to 1 cm beyond the skin lesions.10 MAL was applied under light occlusive dressing for 2 and a half hours.
We then removed the dressing and the excess topical MAL and carried out illumination for 7 minutes and 30 seconds with the AKTILITE© device (Galderma) with a light dose of 37 J/cm2.11
Every patient received multiple sessions: patient 1 and patient 2 were treated initially every 2 weeks and patient 3 and patient 4 every 4 weeks. Treatment was carried out until 6 illuminations were completed. Early discontinuation was possible in case of a clinical response confirmed by histopathology.
Results
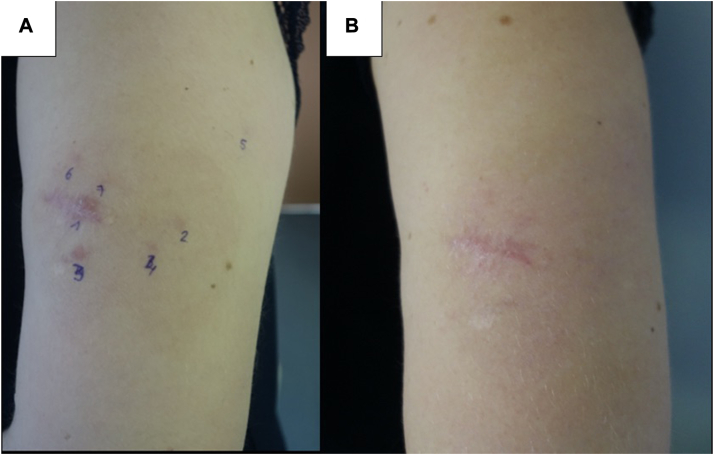
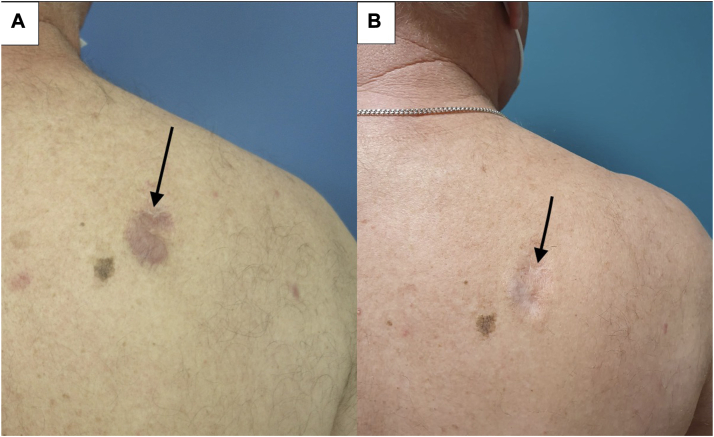
PDT showed effectiveness in all 4 patients of varying degrees, with both partial and complete responses in 1 or several lesions. Out of the 4 patients treated, 2 patients (50%) experienced clinical and histological remission of all treated skin lesions (patient 2 and patient 4) after 5 to 6 MAL-PDT sessions (Figs 2, A and B, and 3, A and B). Patient 3 showed clinical response, however a skin biopsy demonstrated histological evidence of persistent disease (Fig 4, A and B). Patient 1 had a mixed response, with complete resolution of 3 lesions (1 of which later relapsed) and stable disease in 2 others (Fig 1, A and B). During treatment and follow-up no new lesions were observed. The results are summarized in Table II.
Fig 2.
Cutaneous B-cell lymphoma of patient n° 2: (A) before the third illumination. B, Reevaluation 16 months after the last illumination (arrows pointing the skin lesion).
Fig 3.
Cutaneous B-cell lymphoma of patient n° 4: (A) before second illumination. B, One month after fifth illumination.
Fig 4.
Cutaneous B-cell lymphoma of patient n° 3 (arrows pointing the skin lesion): (A) Before second illumination. B, during follow-up showing clinical response.
Fig 1.
Cutaneous B-cell lymphoma of patient n° 1 (arrows pointing the skin lesion): (A) before second illumination. B, Before sixth illumination.
Table II.
Results to treatment
| Patient no | Number of PDT sessions | Response to treatment | Duration of response |
|---|---|---|---|
| 1 | 9 | 2 complete clinical response (Fig 1, A and B) 1 complete clinical response followed by tumor recurrence 2 partial clinical response |
12 and a half months |
| 2 | 5 | Complete clinical and histological response | 16 and a half months ongoing response |
| 3 | 6 | Complete clinical response with histological evidence of persistent disease (Fig 2, A and B) | 16 months ongoing clinical response |
| 4 | 5 | Complete clinical and histological response (Fig 4, A and B) | 3 and a half months |
PDT, Photodynamic therapy.
The tolerance of treatment was moderate for each patient. Patient 1, however, had early termination during 2 sessions due to pain. Average pain scale was 4.15 ranging from 2 to 9 (based on a Visual Analog Scale score 0 to 10).
Discussion
Several treatment options are available for CBCL, including radiotherapy, high potency topical corticosterois, local excision, intralesional steroids, topical imiquimod, and rituximab.2 Our study supports PDT as a potential treatment option for MZL-type CBCL.
Previous studies suggest effectiveness of PDT for the treatment of localized cutaneous T-cell lymphomas such as mycosis fungoides7,8 which led us to believe it could be a plausible treatment for MZL-type CBCL. The mechanism is based on an accumulation of PpIX in the lymphocytic infiltrate of CBCL after topical use of a photosensitizer7 and apoptosis of targeted cells during illumination.
A novel aspect of our treatment includes, a micro-needle abrasion of the skin lesions, with the use of a dermaroller, to improve efficacy by enhancing drug penetration as atypical lymphocytes are present in deep skin layers.10,12,13 Photosensitizers such as 5-aminlevulinic-acid poorly penetrate intact skin13 and therefore poorly metabolize to PpIX in the deeper dermis. Microneedling of the skin causes abrasion of the stratum corneum by creating micropores and increases penetration of topical drugs to the dermal layer.3,12,14 The use of microneedles prior to the application of photosensitizers has shown higher PpIX production.15
By increasing skin penetration of photosensitizers, it could boost the efficacy of PDT, lead to a reduction in dose and a decrease in potential side effects.16
To our knowledge, only 1 other study reports the efficacy of PDT in CBCL (1 follicle center, 2 MZL). Our 4 patients therefore add substantially to the current literature on this topic.9
We had close follow-up of our patients for a median of 14.25 months. Recurrence after treatment was seen in 1 patient: patient 4 had local recurrence of 3 lesions measuring 1 mm each, 3 and a half months after the last PDT illumination. PDT, similar to local treatments, like topical corticosteroids or imiquimod, treats visible lesions and does not prevent the risk of distant relapse. In some cases, efficacy of PDT can be seen after several months of treatment, as we observed in patient 3 who showed clinical response 4 months after the last PDT illumination. This highlights the need to pursue follow-up after treatment so as not to overlook delayed effectiveness of treatment as well as disease recurrence.
The limits of our study include the small number of patients, the variable illumination protocols based on the absence of codified protocols. Additionally, spontaneous remission of CBCL can be seen, therefore creating a possible confirmation bias. Moreover, 2 patients had a prior treatment by several cycles of rituximab which can take time to show effectiveness and could have altered presumed efficacy of PDT.
In our 4 cases, PDT was an effective treatment with mild and short-term side effects and could be considered a treatment option for patients with multiple lesions of MZL-type CBCL who fail topical therapy and prefer conservative treatment.
Conclusion
PDT seems to be an interesting therapeutic option for localized MZL-type CBCL. Microneedles could increase the efficacy of treatment by enhancing drug penetration. However, illumination protocols are not codified. Further studies with larger series of patients are needed to validate this treatment and establish an optimal illumination scheme. Clinical examination must be pursued as recurrence of skin lesions have been seen.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: We have declared our research to the CNIL (request number 1189).
Patient consent: Consent for the publication of recognizable patient photographs or other identifiable material was obtained by the authors and included at the time of article submission to the journal stating that all patients gave consent with the understanding that this information may be publicly available.
References
- 1.Bartosińska J., Szczepanik-Kułak P., Raczkiewicz D., et al. Topical photodynamic therapy with different forms of 5-aminolevulinic acid in the treatment of actinic keratosis. Pharmaceutics. 2022;14:346. doi: 10.3390/pharmaceutics14020346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang C.C.V., Ramelyte E., Dummer R. Innovative therapeutic approaches in primary cutaneous B cell lymphoma. Front Oncol. 2020;10:1163. doi: 10.3389/fonc.2020.01163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Champeau M., Vignoud S., Mortier L., Mordon S. Photodynamic therapy for skin cancer: how to enhance drug penetration? J Photochem Photobiol B. 2019;197:111544. doi: 10.1016/j.jphotobiol.2019.111544. [DOI] [PubMed] [Google Scholar]
- 4.Lee C.-N., Hsu R., Chen H., Wong T.-W. Daylight photodynamic therapy: an update. Molecules. 2020;25:5195. doi: 10.3390/molecules25215195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piccolo D., Kostaki D. Photodynamic therapy activated by intense pulsed light in the treatment of nonmelanoma skin cancer. Biomedicines. 2018;6:18. doi: 10.3390/biomedicines6010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalka K., Merk H., Mukhtar H. Photodynamic therapy in dermatology. J Am Acad Dermatol. 2000;42:389–413. doi: 10.1016/S0190-9622(00)90209-3. [DOI] [PubMed] [Google Scholar]
- 7.Wolf P., Fink-Puches R., Cerroni L., Kerl H. Photodynamic therapy for mycosis fungoides after topical photosensitization with 5-aminolevulinic acid. J Am Acad Dermatol. 1994;31:678–680. doi: 10.1016/S0190-9622(08)81742-2. [DOI] [PubMed] [Google Scholar]
- 8.Quéreux G., Brocard A., Saint-Jean M., et al. Photodynamic therapy with methyl-aminolevulinic acid for paucilesional mycosis fungoides: a prospective open study and review of the literature. J Am Acad Dermatol. 2013;69:890–897. doi: 10.1016/j.jaad.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 9.Mori M., Campolmi P., Mavilia L., Rossi R., Cappugi P., Pimpinelli N. Topical photodynamic therapy for primary cutaneous B-cell lymphoma: a pilot study. J Am Acad Dermatol. 2006;54:524–526. doi: 10.1016/j.jaad.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Shi L., Wang H., Chen K., et al. Chinese guidelines on the clinical application of 5-aminolevulinic acid-based photodynamic therapy in dermatology (2021 edition) Photodiagnosis Photodynamic Ther. 2021;35:102340. doi: 10.1016/j.pdpdt.2021.102340. [DOI] [PubMed] [Google Scholar]
- 11.Wulf H.C., Heerfordt I.M., Philipsen P.A. How much protoporphyrin IX must be activated to obtain full efficacy of methyl aminolevulinate photodynamic therapy? Implication for treatment modifications. Pharmaceuticals. 2021;14:333. doi: 10.3390/ph14040333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikolajewska P., Donnelly R.F., Garland M.J., et al. Microneedle pre-treatment of human skin improves 5-aminolevulininc acid (ALA)- and 5-aminolevulinic acid methyl ester (MAL)-induced PpIX production for topical photodynamic therapy without increase in pain or erythema. Pharm Res. 2010;27:2213–2220. doi: 10.1007/s11095-010-0227-2. [DOI] [PubMed] [Google Scholar]
- 13.Tsai J.-C., Chen I.-H., Wong T.-W., Lo Y.-L. In vitro/in vivo correlations between transdermal delivery of 5-aminolaevulinic acid and cutaneous protoporphyrin IX accumulation and effect of formulation. Br J Dermatol. 2002;146:853–862. doi: 10.1046/j.1365-2133.2002.04715.x. [DOI] [PubMed] [Google Scholar]
- 14.Li D., Hu D., Xu H., et al. Progress and perspective of microneedle system for anti-cancer drug delivery. Biomaterials. 2021;264:120410. doi: 10.1016/j.biomaterials.2020.120410. [DOI] [PubMed] [Google Scholar]
- 15.Gracielli Sousa R.P., de Menezes P.F.C., Fujita A.K.L., et al. Microneedles rollers as a potential device to increase ALA diffusion and PpIX production: evaluations by wide-field fluorescence imaging and fluorescence spectroscopy. Proc SPIE - Int Soc Opt Eng. 2014;8926:892614. doi: 10.1117/12.2040618. [DOI] [Google Scholar]
- 16.Kulkarni D., Damiri F., Rojekar S., et al. Recent advancements in microneedle technology for multifaceted biomedical applications. Pharmaceutics. 2022;14:1097. doi: 10.3390/pharmaceutics14051097. [DOI] [PMC free article] [PubMed] [Google Scholar]