Abstract
Purpose
To develop and evaluate a low-volume contrast media protocol for thoracoabdominal CT angiography (CTA) with photon-counting detector (PCD) CT.
Materials and Methods
This prospective study included consecutive participants (April–September 2021) who underwent CTA with PCD CT of the thoracoabdominal aorta and previous CTA with energy-integrating detector (EID) CT at equal radiation doses. In PCD CT, virtual monoenergetic images (VMI) were reconstructed in 5-keV intervals from 40 to 60 keV. Attenuation of the aorta, image noise, and contrast-to-noise ratio (CNR) were measured, and subjective image quality was rated by two independent readers. In the first group of participants, the same contrast media protocol was used for both scans. CNR gain in PCD CT compared with EID CT served as the reference for contrast media volume reduction in the second group. Noninferiority analysis was used to test noninferior image quality of the low-volume contrast media protocol with PCD CT.
Results
The study included 100 participants (mean age, 75 years ± 8 [SD]; 83 men). In the first group (n = 40), VMI at 50 keV provided the best trade-off between objective and subjective image quality, achieving 25% higher CNR compared with EID CT. Contrast media volume in the second group (n = 60) was reduced by 25% (52.5 mL). Mean differences in CNR and subjective image quality between EID CT and PCD CT at 50 keV were above the predefined boundaries of noninferiority (−0.54 [95% CI: −1.71, 0.62] and −0.36 [95% CI: −0.41, −0.31], respectively).
Conclusion
CTA of the aorta with PCD CT was associated with higher CNR, which was translated into a low-volume contrast media protocol demonstrating noninferior image quality compared with EID CT at the same radiation dose.
Keywords: CT Angiography, CT-Spectral, Vascular, Aorta, Contrast Agents–Intravenous, Technology Assessment
© RSNA, 2023
See also the commentary by Dundas and Leipsic in this issue.
Keywords: CT Angiography, CT-Spectral, Vascular, Aorta, Contrast Agents–Intravenous, Technology Assessment
Summary
Photon-counting detector CT angiography of the thoracoabdominal aorta using virtual monoenergetic images at 50 keV allowed for 25% contrast media volume reduction and demonstrated noninferior image quality compared with energy-integrating detector CT.
Key Points
■ Angiography of the aorta using photon-counting detector (PCD) CT showed higher objective and equivalent subjective image quality compared with energy-integrating detector (EID) CT at an equal contrast media volume and radiation dose (at 50 keV, contrast-to-noise ratio, 25.1 ± 9.7 vs 18.8 ± 7.4 and overall subjective image quality score, 4.65 ± 0.28 vs 4.76 ± 0.33, respectively).
■ PCD CT with low-energy virtual monoenergetic images (VMI) at 50 keV showed the best trade-off between objective and subjective image quality (contrast-to-noise ratio is highest at 40 keV and lowest at 60 keV [31.0 ± 11.7 and 20.2 ± 7.8, respectively], and image noise score is lowest at 40 keV and highest at 60 keV [3.25 ± 0.54 and 4.53 ± 0.60, respectively]) compared with EID CT for imaging of the thoracoabdominal aorta.
■ VMI at 50 keV from PCD CT with reduced contrast media volume yielded noninferior image quality compared with EID CT, with objective and subjective image quality being above the predefined boundaries of noninferiority (−0.54 [95% CI: −1.71, 0.62] and −0.36 [95% CI: −0.41, −0.31], respectively).
Introduction
CT angiography (CTA) of the aorta is the reference standard imaging modality for the diagnosis of aortic disease and for follow-up after endovascular intervention and surgery (1,2). Patients with this condition often require lifelong imaging surveillance and are therefore exposed to repetitive contrast media administration. Unfortunately, many patients of this usually elderly population have comorbidities such as chronic kidney disease, diabetes, or arterial hypertension, which are all risk factors for contrast-induced acute kidney injury (CI-AKI) (3–5).
The true risk for CI-AKI is still a matter of debate. Large-scale propensity score–matched retrospective studies have indicated that the CI-AKI risk has been overestimated in the past (6), and current literature suggests that risk appears negligible for patients with an estimated glomerular filtration rate (eGFR) of greater than 45 mL/min/1.73 m2. However, a certain level of risk for CI-AKI appears to remain at an eGFR of less than 45 mL/min/1.73 m2 (6–8). Given the high prevalence of risk factors and repetitive administration of iodinated contrast media in this patient population, CI-AKI risk may be clinically relevant. As the volume of iodinated contrast media represents an independent risk factor for CI-AKI, reduction and optimization of contrast media dose should be aimed for at each contrast-enhanced CT examination (9,10). Two further aspects justify efforts to reduce contrast media dosages. First, because of the COVID-19 pandemic, disruption of the pharmaceutical supply chain led to a contrast media shortage (11). Second, reports indicate emerging environmental concerns regarding the negative ecological effects of iodinated contrast media in surface and drinking water (12).
Various contrast media volume reduction strategies for CTA of the aorta have been described, such as low-tube-voltage scanning (13–15), individualized tube voltage–adapted contrast media protocols (16), and low-energy virtual monoenergetic images (VMI) from energy-integrating detector (EID) CT operated in the dual-energy mode (17–20). All these options mainly aim to improve the contrast-to-noise ratio (CNR) in contrast-enhanced vessels, allowing lower amounts of administered contrast media.
Recent advancements led to the introduction of photon-counting detector (PCD) CT (21,22). In contrast to conventional EID CT, PCD CT converts incoming photons directly to electrical signals; thus, each photon can be counted, and the energy associated with each one can be measured (22). PCD CT offers several advantages compared with EID CT, including higher image contrast, lower image noise, higher detector efficiency, and constant availability of spectral information, which enables the reconstruction of spectrally based VMI with each scan (22–24).
In a recent study, Euler et al (24) demonstrated higher objective and subjective image quality in PCD CTA of the aorta compared with the latest-generation EID CT using identical contrast media volume at a matched radiation dose. That study also reported a CNR increase between 18% and 29%, depending on the energy level of VMI. Such a gain in CNR could be translated into a lower contrast media volume at CTA of the aorta with PCD CT using low-energy VMI while maintaining image quality, although this was not performed in that study (24).
The purpose of this study was to develop and evaluate a low-volume contrast media protocol for thoracoabdominal CTA with PCD CT.
Materials and Methods
Study Participants
This prospective, single-center study was approved by our institutional review board and local ethics committee; written informed consent was obtained. Between April and September 2021, consecutive participants who had undergone clinically indicated CTA of the thoracoabdominal aorta with PCD CT were screened for study inclusion using the following criteria: older than 18 years and previously underwent thoracoabdominal CTA with an EID CT scanner. Scans performed between April and May 2021 were assigned to group 1, and scans performed between June and September 2021 were assigned to group 2. Exclusion criteria were as follows: electrocardiographically gated CTA, an interval between EID CT and PCD CT greater than 5 years, body mass index (BMI) change greater than 3 kg/m2, and effective diameter (ED) change greater than 20 mm between scans (Fig 1 and Table 1). ED was calculated by measuring the anteroposterior diameter and lateral diameter at the level of the origin of the celiac trunk using the following formula: ED = √(AD·LD), where AD is the anteroposterior diameter, and LD is the lateral diameter.
Figure 1:

Study flowchart of participant selection based on inclusion and exclusion (dark gray) criteria. BMI = body mass index, CTA = CT angiography, ECG = electrocardiography, ED = effective diameter, PCD = photon-counting detector.
Table 1:
Participant Characteristics and Radiation Dose Estimates
EID CT Scans
Scans were performed with a third-generation 192-section dual-source CT scanner (SOMATOM Force; Siemens Healthcare) in the single-source mode. Scan and reconstruction parameters are listed in Table 2. Bolus tracking was used for scan initiation. A circular region of interest (ROI) was placed in the ascending aorta, and after attenuation in the ROI reached a threshold of 100 HU at 120 kVp, the scan was initiated with a delay of 16 seconds.
Table 2:
Scan and Reconstruction Parameters
PCD CT Scans
Scans were performed with a first-generation dual-source PCD CT scanner (NAEOTOM Alpha; Siemens Healthcare) equipped with two cadmium telluride detectors and operated in single-source mode. Scan and reconstruction parameters are listed in Table 2. To achieve intraindividual dose neutrality between scans, the volume CT dose index (CTDIvol) from the previous EID CT scan of each participant was used as the reference, and the tube current–time product of the actual PCD CT scan was adapted to match the reference CTDIvol. The same bolus tracking technique was used as described above.
Contrast Media Protocol
For all EID CT scans and group 1 PCD CT scans, our institutional triphasic contrast media protocol with 70 mL of contrast media (370 mg of iodine/mL, iopromide [Ultravist; Bayer Healthcare]) was used. A bolus of 40 mL of iodinated contrast media followed by a 60-mL 1:1 mixture of contrast media and saline solution and a saline flush of 30 mL was injected into an antecubital vein at a flow rate of 4 mL/sec. Total injection time was 32.5 seconds.
Based on the results of group 1 (see below), contrast media volume in the second group was reduced by 25%, resulting in a total contrast media volume of 52.5 mL. A bolus of 30 mL of iodinated contrast media followed by a 45-mL 1:1 mixture of contrast media and saline solution and a 22.5-mL saline flush was injected into an antecubital vein. To maintain the total time of injection, the injection rate was lowered by 25% to 3 mL/sec.
Radiation Dose Estimates
The CTDIvol and dose-length product from both scans were retrieved from the picture archiving and communication system. Size-specific dose estimates for each scan were calculated by multiplying CTDIvol with a conversion factor based on the ED (25).
Objective Image Quality
Objective image quality was assessed by a board-certified radiologist (K.H.) with 10 years of experience in cardiovascular radiology. In each participant and reconstruction, circular ROIs were drawn into the lumen of the aorta as large as possible at the following locations while carefully avoiding vessel walls and plaques: ascending aorta at the level of the pulmonary trunk, aortic arch, descending aorta at the level of the left atrium, and abdominal aorta at the level of the origin of the celiac trunk, and left common iliac artery (13,24). Mean vascular attenuation (in Hounsfield units) was calculated by averaging the five measurements. Mean psoas muscle attenuation and mean image noise were measured by placing a total of four circular ROIs in four different locations in the psoas muscle, preferably two on the left side and two on the right side. ROIs in the psoas muscle were drawn as large as possible while carefully avoiding fatty streaks or vessels within the muscle. Noise was defined as the SD of attenuation within the ROI. CNR was calculated as follows: CNR = (mean vascular attenuation – mean attenuation of psoas muscle)/mean noise.
Subjective Image Quality
The subjective image quality of all scans was independently rated by a board-certified radiologist (M.E.) with 10 years of experience in cardiovascular radiology (reader 1) and a radiology resident (V.M.) with 3 years of experience in cardiovascular radiology (reader 2). Readers were blinded to scanner type, VMI energy level, and contrast media protocol. Five training cases were used for practice, which were not included in the study.
Subjective image quality was assessed using three criteria that have been previously described (24): image noise, vessel attenuation, and vessel sharpness. The following five-point scale was used for each criterion: 5 = excellent, 4 = good, 3 = moderate, 2 = poor, and 1 = nondiagnostic. Finally, an overall subjective image quality score was determined as the average value of the three scores.
Statistical Analysis
Descriptive statistics included means ± SDs for continuous variables and frequencies and percentages for categorical variables. To assess similarities in participant characteristics and radiation doses between EID CT and PCD CT, standardized mean differences (SMDs) were calculated using the correlation-corrected SD of both measurements (26). The SMD is an effect size measure that is used to show similarity (27). SMD values less than 0.1 were interpreted as a negligible difference between EID CT and PCD CT (27).
The goal of the analysis of group 1 was to find the optimal energy level for VMI from PCD CT and to quantify the gain of CNR. In the analysis of group 1, ratings of qualitative outcomes were treated as continuous to simplify numerical comparisons between the energy levels of VMI and to make qualitative outcomes more directly comparable to the quantitative outcome. In the analysis of group 2, the individual ratings of qualitative outcomes were treated as ordinal and the overall subjective image score as continuous. Individual ratings of qualitative outcomes of the two raters were compared by weighted Cohen κ with weights as squared distances from the diagonal, which corresponds to the intraclass correlation coefficient. The overall subjective image score was compared between the two raters by an intraclass correlation coefficient of the average random raters type (in the ICC function of the psych package in R software). This corresponds to the intraclass correlation type ICC (2, k) (28).
Selection of the optimal energy level for VMI from PCD CT was based on the balancing of effect sizes in consensus by three radiologists (K.H., M.E., and V.M.).
For group 2, quantitative outcomes and overall scores of qualitative outcomes were analyzed with mean difference measures with 95% Wald CIs and were compared with a noninferiority boundary. Based on the literature and clinical experience, a noninferiority boundary of −5 was predefined for CNR, and a noninferiority boundary of −2 was predefined for the overall score of qualitative outcomes, which corresponds to a change from excellent to moderate or from good to poor in all outcomes (18). All analyses were carried out with R (R Core Team [2022]. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing; https://www.R-project.org/).
Results
Participant Characteristics
In total, 100 participants were included (83 men) (Fig 1). Mean age at the time of PCD CT (second scan) was 75 years ± 8. Mean eGFR at the time of PCD CT was 64.0 mL/min/1.73 m2 ± 21.1. An eGFR less than 45 mL/min/1.73 m2 was observed in 22 participants (22%). Mean time interval between the two scans was 21 months ± 14.
Similar values were observed in BMI, ED, CTDIvol, and size-specific dose estimates between groups (all, SMD < 0.1) (Table 1). Median tube voltage for EID CT for the first and second groups was 90 kVp (range, 70–120 kVp) and 90 kVp (range, 70–100 kVp), respectively. Indications for CTA of the aorta were follow-up after endovascular intervention and surgery (n = 80), follow-up of aortic dissection (n = 10), follow-up of untreated aortic aneurysm (n = 4), and other indications (n = 6).
Group 1
Objective image quality.— For PCD CT, highest attenuation, image noise, and CNR values were observed in VMI at 40 keV, with decreasing values at higher energy VMI. Overall lowest attenuation, image noise, and CNR values were observed in images from EID CT. All measurements for each reconstruction are reported in Table 3.
Table 3:
Objective Image Quality Measurements of EID CT and VMI from PCD CT
Subjective image quality.— Images from EID CT achieved the highest ratings for subjective image noise, vessel attenuation, and vessel sharpness, with an overall image quality score of 4.76 ± 0.33. For VMI from PCD CT, highest scores were given to VMI at 60 keV, with decreasing scores toward lower energy levels. Overall subjective image scores of EID CT were similar to those of VMI at 50, 55, and 60 keV from PCD CT. Detailed results are provided in Table 4.
Table 4:
Subjective Image Quality Scores for EID CT and VMI from PCD CT for Group 1
Selection of optimal VMI energy level for CTA of the aorta.— As illustrated in Figures 2 and 3, diverging objective and subjective image quality were observed for different VMI energies, with the highest objective and lowest subjective image quality at 40 keV and lowest objective and highest subjective image quality at 60 keV. In consensus, VMI at 50 keV represented the best trade-off between objective and subjective image quality. Hence, 50 keV was selected as the ideal energy of VMI for CTA of the aorta and was used as the reference for group 2. Mean CNR of VMI at 50 keV was 25% higher compared with CNR of EID CT.
Figure 2:
Contrast-to-noise ratio (CNR) and subjective image quality scores of photon-counting detector CT examinations for group 1 at each kiloelectron volt level. Note the diverging trends in objective and subjective image quality, with the highest objective and lowest subjective image quality at 40 keV and the lowest objective and highest subjective image quality at 60 keV. Scores are based on a five-point Likert scale: 5 = excellent, 4 = good, 3 = moderate, 2 = poor, 1 = nondiagnostic.
Figure 3:
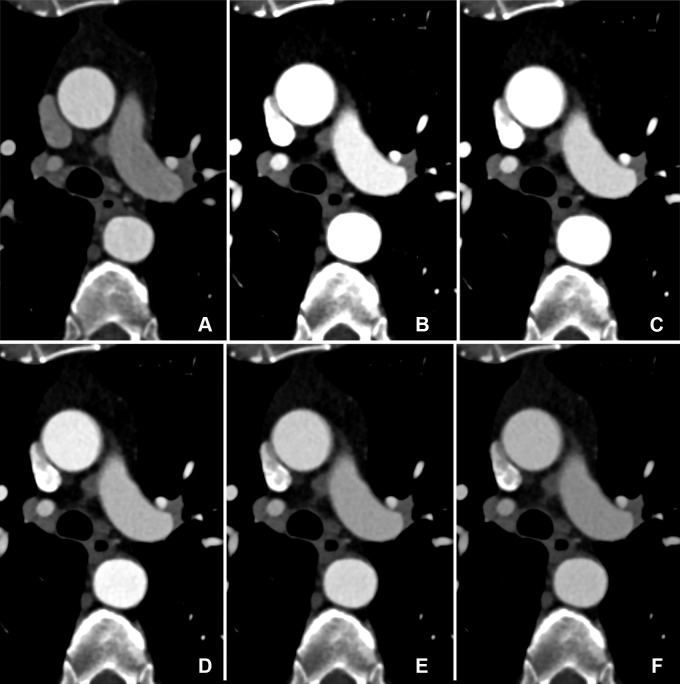
Comparison of image quality between EID CT and PCD CT using identical contrast media protocol and matched radiation dose. Transverse CTA images show the thoracic aorta at the level of the pulmonary trunk in a 62-year-old man in group 1, shown using identical window settings (window center: 350 HU, window width: 1000 HU). (A) Images from scans performed with a third-generation dual-source CT system with EIDs. Images were reconstructed with a section thickness of 2 mm. The automated tube voltage selection algorithm selected 80 kVp. BMI, effective diameter, CTDIvol, and SSDE were 24.2 kg/m2, 293 mm, 3.02 mGy, and 3.87 mGy, respectively. (B–F) Images from PCD CT with VMI at energy levels from 40 to 60 keV (B: 40 keV, C: 45 keV, D: 50 keV, E: 55 keV, F: 60 keV). Time interval between scans was 11 months. Mean BMI, effective diameter, CTDIvol, and SSDE at the time of the second scan were 25.3 kg/m2, 295 mm, 3.05 mGy, and 3.90 mGy, respectively. Both scans were performed with the same contrast media protocol (volume, 70 mL). Note the higher vessel attenuation at lower energy levels for PCD CT. BMI = body mass index, CTA = CT angiography, CTDIvol = volume CT dose index, EID = energy-integrating detector, PCD = photon-counting detector, SSDE = size-specific dose estimate, VMI = virtual monoenergetic images.
Group 2
Objective image quality.— Mean CNR of EID CT and VMI at 50 keV from PCD CT were nearly identical (19.6 ± 6.1 and 19.0 ± 5.6, respectively). Mean difference of CNR between EID CT and VMI at 50 keV from PCD CT was −0.54, with the entire CI of CNR difference (95% CI: −1.71, 0.62) above the predefined boundary of noninferiority of −5. Mean difference of image noise between EID CT and VMI at 50 keV from PCD CT was 6.96 HU (95% CI: 6.29, 7.63). Means ± SDs for attenuation of the aorta, psoas attenuation, image noise, and CNR for EID CT and VMI at 50 keV from PCD CT are reported in Table 3.
Subjective image quality.— Good to excellent agreement was found between the two raters for qualitative ratings (Cohen κ, 0.94 for image noise and 0.6 for vessel sharpness; for vessel attenuation, no Cohen κ could be calculated because rater 2 [V.M.] rated all images with a score of 5). Excellent agreement was found for the overall subjective quality scores (intraclass correlation coefficient, 0.95). Hence, the rating of reader 1 (M.E.) was used for the final analysis of ordinal outcomes.
Scores for image noise, vessel attenuation, vessel sharpness, and overall subjective image quality are shown in Figure 4. Between EID CT and VMI at 50 keV from PCD CT, we found no evidence of a difference in image scores for vessel attenuation and vessel sharpness, with a median difference of 0 (IQR, 0–0) for both scores. Lower image scores for image noise were observed for VMI at 50 keV from PCD CT compared with EID CT, with a median difference of −1 (IQR, −1 to −1). Overall quality scores were slightly lower for VMI at 50 keV from PCD CT compared with EID CT (mean difference, −0.36). The entire CI (95% CI: −0.41, −0.31) of the difference of overall quality score was above the predefined boundary of noninferiority of −2 (Fig 5).
Figure 4:
Subjective image quality scores of reader 1 for group 2. Alluvial diagram with vertical stacked bar charts of the subjective image scores from EID CT with standard contrast media volume and PCD CT at 50 keV VMI with reduced contrast media volume are shown. The blue and gray curved lines between columns represent the image score change for each individual participant. Interpretation of scores: 5 = excellent, 4 = good, 3 = moderate. EID = energy-integrating detector, PCD = photon-counting detector, VMI = virtual monoenergetic images.
Figure 5:
Comparison of image quality between EID CT with standard contrast media protocol and PCD CT with low-volume contrast media protocol using a matched radiation dose. Transverse and three-dimensional cinematic rendered images from thoracoabdominal CTA in a 71-year-old woman in group 2 are shown. (A–C) Images from third-generation EID CT with automated tube voltage selection of 90 kVp. BMI, effective diameter, CTDIvol, and SSDE were 23.7 kg/m2, 278 mm, 3.98 mGy, and 5.25 mGy, respectively; 70 mL of contrast media was used. (D–F) Images from PCD CT with reduced contrast media volume of 52.5 mL and VMI at 50 keV. Time interval between scans was 6 months. Mean BMI, effective diameter, CTDIvol, and SSDE at the time of the second scan were 24.2 kg/m2, 282 mm, 3.99 mGy, and 5.27 mGy, respectively. Mean contrast-to-noise ratio for EID CT and PCD CT were 17.2 and 17.9, respectively. BMI = body mass index, CTA = CT angiography, CTDIvol = volumetric CT dose index, EID = energy-integrating detector, PCD = photon-counting detector, SSDE = size-specific dose estimate, VMI = virtual monoenergetic images.
Discussion
Patients requiring repetitive CTA examinations of the aorta are at potential risk of developing CI-AKI, which is reflected by the high prevalence of 22% of participants in our study having an eGFR less than 45 mL/min/1.73 m2. This study evaluated and demonstrated the potential of PCD CT to reduce contrast media volume for CTA of the aorta at a noninferior image quality compared with EID CT. In the first study group, we demonstrated the improved image quality of low-energy VMI from PCD CT compared with those from EID CT using an identical contrast media protocol and matched radiation dose. Based on these results, a low-volume contrast media protocol with a 25% volume reduction was used for participants in the second study group. Here, compared with EID CT, VMI at 50 keV from PCD CT had noninferior objective and subjective image quality, with mean differences in CNR and subjective image quality between EID CT and PCD CT at 50 keV being above the predefined boundaries of noninferiority (−0.54 [95% CI: −1.71, 0.62] and −0.36 [95% CI: −0.41, −0.31], respectively), illustrating the potential of PCD CT to reduce contrast media exposure to potentially susceptible patients.
The results of group 1 are compatible with those of previous studies evaluating the image quality of thoracoabdominal CTA using low-energy VMI from PCD CT (24) or EID CT operated in the dual-energy mode (29,30). A recent study demonstrated superior image quality in low-energy VMI from PCD CT compared with EID CT (24) and proposed VMI at 45–50 keV as the best trade-off between objective and subjective image quality. Comparable results have been reported for EID CT operated in the dual-energy mode, proposing 50–60 keV as the ideal energy level (29). In our study, VMI at 40 keV achieved the highest objective image quality, which is explained by the proximity of this energy level to the K edge of iodine at 33.2 keV, resulting in a disproportionally higher increase of vascular attenuation compared with the increase of image noise. However, the higher image noise at lower kiloelectron volt levels resulted in lower subjective image noise and vessel sharpness ratings. Interestingly, almost similar subjective vascular attenuation was observed among the different energy levels, with ratings ranging between 5.0 for VMI at 40 keV and 4.82 for VMI at 60 keV. Overall, the highest subjective image quality rating was awarded to VMI at 50–60 keV, and therefore, we propose VMI at 50 keV as the best trade-off in terms of objective and subjective image quality.
In group 2 of our study, we reduced the contrast media volume by 25%. In previous studies, contrast media reductions ranging from 20% to 70% were reported with low-energy VMI from EID CT, without deteriorating image quality (19,31–34). Similar results were reported in studies evaluating low-tube-voltage scanning techniques for contrast media volume reduction ranging between 20% and 60%, mostly at a tube voltage of 80 kVp (13–16). The following reasons might explain the rather low relative contrast media volume reduction in our study. First, the above-mentioned studies used single-energy CT with a tube voltage of 120 kVp as the reference, while in our study, automated tube voltage selection optimized for vascular imaging was used, resulting in a median tube voltage of 90 kVp. Second, our standard contrast media protocol with 70 mL of contrast media was already optimized and relatively low, not allowing higher contrast media volume savings. The amount of iodine in our reduced contrast media protocol (19.4 g of iodine) was similar to the amount reported in the literature, ranging between 13 and 24 g of iodine (13–17,19,31–33). Third, radiation doses from previous studies were relatively high (CTDIvol between 11.7 and 23.3 mGy) (13,19,31,33), while the mean CTDIvol in our study was only 4.9 mGy. Fourth, the average BMI (27 kg/m2) in our study sample was higher than that in the literature, especially in studies evaluating low-tube-voltage techniques for contrast media volume reduction (BMI of 22–24 kg/m2) (13–15).
The following study limitations must be acknowledged. First, neither the diagnostic accuracy nor the diagnostic value was evaluated. Second, the study included a rather small sample with a small proportion of women. Third, we only compared the image quality of EID CT operated in single-energy mode with PCD CT. Further studies are needed to compare the image quality between EID CT operated in the dual-energy mode and PCD CT. Fourth, the role of iterative reconstruction was not assessed in detail. In our study, we used the emerging technique quantum iterative reconstruction (QIR), which was specifically developed for PCD CT (34). Further studies are necessary to evaluate the influence of QIR on image quality in comparison with traditional iterative reconstruction techniques from EID CT. Moreover, the impact of the chosen strength level for QIR also needs further investigation. A recent study assessed the improvement of image quality at different strength levels of QIR in contrast-enhanced abdominal PCD CT, in which the highest image quality was observed for QIR at a strength level of 4 (34). In our study, we used QIR at a strength level of 3. By using higher strength levels, image noise might be further decreased, permitting even higher contrast media volume reduction. Fifth, image quality was assessed on a five-point Likert scale and analyzed as if it were continuous, as mainly scores of 4 and 5 were present. Finally, we used the same amount of contrast media for all participants, independent of individual body weight or BMI. Individualized patient-specific low-volume contrast media protocols might allow for further contrast media dose reduction (16).
In conclusion, PCD CTA of the aorta demonstrated higher objective and subjective image quality compared with third-generation EID CT at equal contrast media volume and matched radiation dose. We propose VMI at 50 keV as the ideal energy level for VMI reconstructions with PCD CTA, representing the best trade-off between objective and subjective image quality. By translating the gain of image quality into contrast media volume reduction, we were able to demonstrate noninferior image quality from PCD CTA of the thoracoabdominal aorta using VMI at 50 keV and using a low-volume contrast media protocol.
V.M. supported by a Young Talents in Clinical Research grant From the Swiss Academy of Medical Sciences and Gottfried and Julia Bangerter-Rhyner Foundation.
Data sharing: Data generated or analyzed during the study are available from the corresponding author by request.
Disclosures of conflicts of interest: K.H. No relevant relationships. V.M. No relevant relationships. M.E. Payment for speakers bureaus from Siemens Healthineers. L.J. No relevant relationships. M.H. No relevant relationships. S.R. No relevant relationships. B.Z. No relevant relationships. A.K. No relevant relationships. K.M. No relevant relationships. A.E. No relevant relationships. H.A. Institutional grants from Bayer, Canon, Guerbet, and Siemens; payment for speakers bureaus from Siemens.
Abbreviations:
- BMI
- body mass index
- CI-AKI
- contrast-induced acute kidney injury
- CNR
- contrast-to-noise ratio
- CTA
- CT angiography
- CTDIvol
- volume CT dose index
- ED
- effective diameter
- eGFR
- estimated glomerular filtration rate
- EID
- energy-integrating detector
- PCD
- photon-counting detector
- QIR
- quantum iterative reconstruction
- ROI
- region of interest
- SMD
- standardized mean difference
- VMI
- virtual monoenergetic images
References
- 1. Rubin GD , Leipsic J , Joseph Schoepf U , Fleischmann D , Napel S . CT angiography after 20 years: a transformation in cardiovascular disease characterization continues to advance . Radiology 2014. ; 271 ( 3 ): 633 – 652 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fleischmann D , Afifi RO , Casanegra AI , et al . Imaging and surveillance of chronic aortic dissection: a scientific statement from the American Heart Association . Circ Cardiovasc Imaging 2022. ; 15 ( 3 ): e000075 . [DOI] [PubMed] [Google Scholar]
- 3. Moos SI , van Vemde DNH , Stoker J , Bipat S . Contrast induced nephropathy in patients undergoing intravenous (IV) contrast enhanced computed tomography (CECT) and the relationship with risk factors: a meta-analysis . Eur J Radiol 2013. ; 82 ( 9 ): e387 – e399 . [DOI] [PubMed] [Google Scholar]
- 4. Saratzis A , Bath MF , Harrison S , et al . Long-term renal function after endovascular aneurysm repair . Clin J Am Soc Nephrol 2015. ; 10 ( 11 ): 1930 – 1936 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sohrabi S , Wheatcroft S , Barth JH , et al . Cardiovascular risk in patients with small and medium abdominal aortic aneurysms, and no history of cardiovascular disease . Br J Surg 2014. ; 101 ( 10 ): 1238 – 1243 . [DOI] [PubMed] [Google Scholar]
- 6. Davenport MS , Khalatbari S , Cohan RH , Dillman JR , Myles JD , Ellis JH . Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate . Radiology 2013. ; 268 ( 3 ): 719 – 728 . [DOI] [PubMed] [Google Scholar]
- 7. Davenport MS , Perazella MA , Yee J , et al . Use of intravenous iodinated contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation . Radiology 2020. ; 294 ( 3 ): 660 – 668 . [DOI] [PubMed] [Google Scholar]
- 8. McDonald JS , McDonald RJ , Carter RE , Katzberg RW , Kallmes DF , Williamson EE . Risk of intravenous contrast material-mediated acute kidney injury: a propensity score-matched study stratified by baseline-estimated glomerular filtration rate . Radiology 2014. ; 271 ( 1 ): 65 – 73 . [DOI] [PubMed] [Google Scholar]
- 9. Nie Z , Liu Y , Wang C , Sun G , Chen G , Lu Z . Safe limits of contrast media for contrast-induced nephropathy: a multicenter prospective cohort study . front med (lausanne) 2021. ; 8 : 701062 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barrett BJ , Parfrey PS . Clinical Practice. Preventing nephropathy induced by contrast medium . N Engl J med 2006. ; 354 ( 4 ): 379 – 386 . [DOI] [PubMed] [Google Scholar]
- 11. Grist TM , Canon CL , Fishman EK , Kohi MP , Mossa-Basha M . Short-, mid-, and long-term strategies to manage the shortage of iohexol . Radiology 2022. ; 304 ( 2 ): 289 – 293 . [DOI] [PubMed] [Google Scholar]
- 12. Dekker HM , Stroomberg GJ , Prokop M . Tackling the increasing contamination of the water supply by iodinated contrast media . Insights Imaging 2022. ; 13 ( 1 ): 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen CM , Chu SY , Hsu MY , Liao YL , Tsai HY . Low-tube-voltage (80 kVp) CT aortography using 320-row volume CT with adaptive iterative reconstruction: lower contrast medium and radiation dose . Eur Radiol 2014. ; 24 ( 2 ): 460 – 468 . [DOI] [PubMed] [Google Scholar]
- 14. Kanematsu M , Goshima S , Miyoshi T , et al . Whole-body CT angiography with low tube voltage and low-concentration contrast material to reduce radiation dose and iodine load . AJR Am J Roentgenol 2014. ; 202 ( 1 ): W106 – W116 . [DOI] [PubMed] [Google Scholar]
- 15. Wei L , Li S , Gao Q , Liu Y , Ma X . Use of low tube voltage and low contrast agent concentration yields good image quality for aortic CT angiography . Clin Radiol 2016. ; 71 ( 12 ): 1313.e5 – 1313.e10 . [DOI] [PubMed] [Google Scholar]
- 16. Higashigaito K , Schmid T , Puippe G , et al . CT angiography of the aorta: prospective evaluation of individualized low-volume contrast media protocols . Radiology 2016. ; 280 ( 3 ): 960 – 968 . [DOI] [PubMed] [Google Scholar]
- 17. Patino M , Parakh A , Lo GC , et al . Virtual monochromatic dual-energy aortoiliac CT angiography with reduced iodine dose: a prospective randomized study . AJR Am J Roentgenol 2019. ; 212 ( 2 ): 467 – 474 . [DOI] [PubMed] [Google Scholar]
- 18. Euler A , Taslimi T , Eberhard M , et al . Computed tomography angiography of the aorta-optimization of automatic tube voltage selection settings to reduce radiation dose or contrast medium in a prospective randomized trial . Invest Radiol 2021. ; 56 ( 5 ): 283 – 291 . [DOI] [PubMed] [Google Scholar]
- 19. Shuman WP , O’Malley RB , Busey JM , Ramos MM , Koprowicz KM . Prospective comparison of dual-energy CT aortography using 70% reduced iodine dose versus single-energy CT aortography using standard iodine dose in the same patient . Abdom Radiol (NY) 2017. ; 42 ( 3 ): 759 – 765 . [DOI] [PubMed] [Google Scholar]
- 20. Noda Y , Nakamura F , Kawai N , et al . Optimized bolus threshold for dual-energy CT angiography with monoenergetic images: a randomized clinical trial . Radiology 2021. ; 300 ( 3 ): 615 – 623 . [DOI] [PubMed] [Google Scholar]
- 21. Rajendran K , Petersilka M , Henning A , et al . First clinical photon-counting detector CT system: technical evaluation . Radiology 2022. ; 303 ( 1 ): 130 – 138 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Willemink MJ , Persson M , Pourmorteza A , Pelc NJ , Fleischmann D . Photon-counting CT: technical principles and clinical prospects . Radiology 2018. ; 289 ( 2 ): 293 – 312 . [DOI] [PubMed] [Google Scholar]
- 23. Higashigaito K , Euler A , Eberhard M , Flohr TG , Schmidt B , Alkadhi H . Contrast-enhanced abdominal CT with clinical photon-counting detector CT: assessment of image quality and comparison with energy-integrating detector CT . Acad Radiol 2022. ; 29 ( 5 ): 689 – 697 . [DOI] [PubMed] [Google Scholar]
- 24. Euler A , Higashigaito K , Mergen V , et al . High-pitch photon-counting detector computed tomography angiography of the aorta: intraindividual comparison to energy-integrating detector computed tomography at equal radiation dose . Invest Radiol 2022. ; 57 ( 2 ): 115 – 121 . [DOI] [PubMed] [Google Scholar]
- 25. Boone JM , Strauss K , Cody DD . Size-specific dose estimates (SSDE) in pediatric and adult body CT examinations . American Association of Physicists in Medicine; ; 2011. . [Google Scholar]
- 26. Lakens D . Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs . Front Psychol 2013. ; 4 : 863 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Austin PC . An introduction to propensity score methods for reducing the effects of confounding in observational studies . Multivariate Behav Res 2011. ; 46 ( 3 ): 399 – 424 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shrout PE , Fleiss JL . Intraclass correlations: uses in assessing rater reliability . Psychol Bull 1979. ; 86 ( 2 ): 420 – 428 . [DOI] [PubMed] [Google Scholar]
- 29. Martin SS , Albrecht MH , Wichmann JL , et al . Value of a noise-optimized virtual monoenergetic reconstruction technique in dual-energy CT for planning of transcatheter aortic valve replacement . Eur Radiol 2017. ; 27 ( 2 ): 705 – 714 . [DOI] [PubMed] [Google Scholar]
- 30. Albrecht MH , Trommer J , Wichmann JL , et al . Comprehensive comparison of virtual monoenergetic and linearly blended reconstruction techniques in third-generation dual-source dual-energy computed tomography angiography of the thorax and abdomen . Invest Radiol 2016. ; 51 ( 9 ): 582 – 590 . [DOI] [PubMed] [Google Scholar]
- 31. Noda Y , Nakamura F , Yasuda N , et al . Advantages and disadvantages of single-source dual-energy whole-body CT angiography with 50% reduced iodine dose at 40 keV reconstruction . Br J Radiol 2021. ; 94 ( 1121 ): 20201276 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Agrawal MD , Oliveira GR , Kalva SP , Pinho DF , Arellano RS , Sahani DV . Prospective comparison of reduced-iodine-dose virtual monochromatic imaging dataset from dual-energy CT angiography with standard-iodine-dose single-energy CT angiography for abdominal aortic aneurysm . AJR Am J Roentgenol 2016. ; 207 ( 6 ): W125 – W132 . [DOI] [PubMed] [Google Scholar]
- 33. Shuman WP , Chan KT , Busey JM , Mitsumori LM , Koprowicz KM . Dual-energy CT aortography with 50% reduced iodine dose versus single-energy CT aortography with standard iodine dose . Acad Radiol 2016. ; 23 ( 5 ): 611 – 618 . [DOI] [PubMed] [Google Scholar]
- 34. Sartoretti T , Landsmann A , Nakhostin D , et al . Quantum iterative reconstruction for abdominal photon-counting detector CT improves image quality . Radiology 2022. ; 303 ( 2 ): 339 – 348 . [DOI] [PubMed] [Google Scholar]