Abstract
Purpose
To apply Doppler US (DUS)–gated fetal cardiac cine MRI in clinical routine and investigate diagnostic performance in complex congenital heart disease (CHD) compared with that of fetal echocardiography.
Materials and Methods
In this prospective study (May 2021 to March 2022), women with fetuses with CHD underwent fetal echocardiography and DUS-gated fetal cardiac MRI on the same day. For MRI, balanced steady-state free precession cine images were acquired in the axial and optional sagittal and/or coronal orientations. Overall image quality was assessed on a four-point Likert scale (from 1 = nondiagnostic to 4 = good image quality). The presence of abnormalities in 20 fetal cardiovascular features was independently assessed by using both modalities. The reference standard was postnatal examination results. Differences in sensitivities and specificities were determined by using a random-effects model.
Results
The study included 23 participants (mean age, 32 years ± 5 [SD]; mean gestational age, 36 weeks ± 1). Fetal cardiac MRI was completed in all participants. The median overall image quality of DUS-gated cine images was 3 (IQR, 2.5–4). In 21 of 23 participants (91%), underlying CHD was correctly assessed by using fetal cardiac MRI. In one case, the correct diagnosis was made by using MRI only (situs inversus and congenitally corrected transposition of the great arteries). Sensitivities (91.8% [95% CI: 85.7, 95.1] vs 93.6% [95% CI: 88.8, 96.2]; P = .53) and specificities (99.9% [95% CI: 99.2, 100] vs 99.9% [95% CI: 99.5, 100]; P > .99) for the detection of abnormal cardiovascular features were comparable between MRI and echocardiography, respectively.
Conclusion
Using DUS-gated fetal cine cardiac MRI resulted in performance comparable with that of using fetal echocardiography for diagnosing complex fetal CHD.
Keywords: Pediatrics, MR-Fetal (Fetal MRI), Cardiac, Heart, Congenital, Fetal Imaging, Cardiac MRI, Prenatal, Congenital Heart Disease
Clinical trial registration no. NCT05066399
Supplemental material is available for this article.
© RSNA, 2023
See also the commentary by Biko and Fogel in this issue.
Keywords: Pediatrics, MR-Fetal (Fetal MRI), Cardiac, Heart, Congenital, Fetal Imaging, Cardiac MRI, Prenatal, Congenital Heart Disease
Summary
The use of fetal cardiac MRI with Doppler US–gated cine imaging in clinical routine enabled high-quality delineation of fetal cardiovascular anatomy and diagnostic performance comparable with that of echocardiography in complex congenital heart disease.
Key Points
■ In a prospective study of 23 female participants with fetuses with congenital heart disease, the detectability of abnormal cardiovascular features between Doppler US (DUS)–gated fetal cardiac MRI and fetal echocardiography in late gestation was comparable (sensitivity: 91.8% vs 93.6%, P = .53; specificity: 99.9% vs 99.9%; P > .99).
■ Median overall image quality of DUS-gated cardiac cine MRI on a four-point Likert scale was 3 (IQR, 2.5–4), with moderate artifacts due to fetal movement, respiratory motion, and/or pulsation.
Introduction
With an incidence of six to nine per 1000 live births, congenital heart disease (CHD) represents a major proportion of congenital anomalies, ranging from asymptomatic malformations to severe heart defects (1,2). Detailed anatomic assessment for comprehensive prenatal diagnosis of CHD is necessary for adequate parental counseling, postnatal therapy planning, and outcome assessment (3,4). Although initial suspicion of CHD typically occurs during routine midtrimester US screening, detailed fetal echocardiography represents the primary diagnostic tool for accurate characterization of congenital heart defects (5). Because several malformations might be missed at the second trimester, targeted assessment is required even in the advanced stages of pregnancy (6). However, echocardiography can be limited because of maternal or fetal factors (eg, obesity, anterior placenta, or oligohydramnios) or, especially in advanced gestation, poor acoustic windows influenced by fetal position or bone calcification (7). Thus, fetal MRI has emerged as an adjunct imaging modality for the evaluation of noncardiac malformations, such as diaphragmatic hernia or congenital brain abnormalities (8–10). However, performing diagnostic evaluation of CHD by using fetal MRI is challenging because of the small size of fetal cardiovascular structures, short cardiac cycle, and different sources of motion artifacts (eg, fetal heartbeat, fetal movements, and maternal breathing) (11). Fetal electrocardiography is not available for direct gating in cardiac MRI. Indirect gating methods, such as metric-optimized gating or self-gating strategies, have been applied (12–15) to allow for cine imaging of the fetal heart, but time-consuming postprocessing routines and the need for additional software limit their widespread use in clinical routine. Recently, a direct gating method using Doppler US (DUS) has been applied to fetal cardiac MRI (16,17). With this method, retrospectively gated cardiac MRI of the fetal heart can be performed in a manner similar to electrocardiographically gated MRI in adults, with immediate image reconstruction on a scanner console. However, the clinical usefulness of fetal cardiac cine MRI with DUS gating for the diagnosis of various complex CHDs has not yet been investigated. This prospective study aims to evaluate the feasibility of DUS-gated fetal cardiac cine MRI in clinical routine and to investigate diagnostic performance in complex CHD in comparison with that of fetal echocardiography.
Materials and Methods
This prospective study was approved by the institutional review board (application no. 523/21), and all participants gave written informed consent. The study was registered in the clinical trials database of the U.S. National Library of Medicine (ClinicalTrials.gov identifier: NCT05066399).
Study Participants
Women whose fetuses were suspected to have CHD, determined with US screening during the second-trimester anatomy scan, were referred to the Department of Obstetrics and Prenatal Medicine of the University Hospital Bonn for dedicated fetal assessment including fetal echocardiography and counseling. Patients were invited to participate in this study and were consecutively included from May 2021 to March 2022. Exclusion criteria were general contraindications for MRI and gestational age less than the third trimester.
Fetal Echocardiography
All participants underwent dedicated fetal echocardiography (Voluson E10, GE Healthcare; or Aplio i900, Canon Medial Systems) performed by using a segmental approach with standardized anatomic planes incorporating pulsed-wave and color Doppler imaging. Multifrequency, two-dimensional curved-array probes (2–5, 3–9 MHz) were used for fetal echocardiographic examinations. Examinations and image analysis were performed analogously to fetal cardiac MRI by one board-certified perinatologist (A.G.) and one pediatric cardiologist (U.H.) with 20 and 31 years of fetal echocardiography experience, respectively.
Fetal Cardiac MRI
Fetal cardiac MRI was performed with a 3-T clinical MRI system (Philips Ingenia Elition 3.0T X; Philips Healthcare) on the same day as fetal echocardiography. The maximum specific absorption rate was limited to 2.0 W/kg. An MRI-compatible DUS transducer (smart-sync; Northh Medical) was used for direct fetal cardiac gating (16,17). Participants were scanned while in the left lateral position. Prior to DUS transducer application, an MRI survey scan was performed to determine the fetal heart position in utero. The DUS transducer was then centered on the fetal heart and slowly moved until a stable gating signal was derived. Care was taken to avoid potentially interfering structures, such as large placental vessels within the Doppler acoustic window. The DUS transducer was fastened with an elastic cardiotocography belt on the maternal abdomen. In some participants, a foam wedge was placed between the belt and the transducer to keep the acoustic window at a correct angle toward the fetal heart. A 32-channel torso coil was used for signal reception without directly contacting the DUS transducer.
Standard single-shot T2-weighted turbo spin-echo images were obtained to visualize gross fetal anatomy and for planning purposes. For assessing fetal cardiovascular anatomy, retrospectively DUS-gated balanced steady-state free precession cine images were continuously obtained in axial orientation covering the whole heart, including the supra-aortic branches. The acquisition of cine stacks was repeated in cases of distinct artifacts due to drastic fetal movements or unstable DUS signals. Cine images in sagittal or coronal orientation were only obtained when findings on axial images necessitated additional anatomic information (eg, aortic isthmus assessment). Scan parameters were as follows: field of view, 254 × 254 mm; repetition time, 3.3 msec; echo time, 1.65 msec; flip angle, 65°; parallel imaging factor, two; acquired voxel size, 1.72 × 1.42 × 4.0 mm3 (reconstructed voxel size, 99 × 0.99 × 4.00 mm3) with no intersection gaps; scan duration per section, 13 seconds; temporal resolution, 21.6 msec; and reconstructed cardiac phases, 25. Images were acquired during an end-expiratory breath hold. In women with limited breath-holding capacity, images were acquired during free breathing with two signal averages.
Image Quality Assessment of Fetal Cardiac MRI
Fetal cardiac cine MR images were analyzed by two readers (J.A.L. [reader 1] and C.H. [reader 2], with 9 and 17 years of cardiac MRI experience in CHD, respectively) using a standard Digital Imaging and Communications in Medicine viewer (DeepUnity R20; Dedalus Healthcare Systems). Image quality and artifact levels (eg, breathing, movement, pulsation, or dielectric shielding artifacts) of final cine images were qualitatively assessed using an overall image quality score, which was based on a four-point Likert scale (1 = nondiagnostic image quality with a high degree of artifacts, 2 = poor image quality with distinct artifacts, 3 = moderate image quality with few artifacts, and 4 = good image quality without artifacts). Exemplary cine images to demonstrate the Likert scale are provided (Fig S1).
Additionally, a delineability score was assessed for the following cardiovascular structures: ventricles (including septum and papillary muscles), atria (including septum and foramen ovale), atrioventricular valves, semilunar valves, great arteries, systemic veins, and pulmonary veins (including venoatrial connections). Delineability assessment was also based on a four-point Likert scale (1 = structure not visible, 2 = structure is poorly delineated from surrounding structures, 3 = structure is moderately delineated from surrounding structures, and 4 = structure is well delineated from surrounding structures).
The ratings of reader 1 are given in the Results section. For inter- and intrareader reproducibility measurements, image quality assessment was performed independently by the two readers and was repeated by the first reader 2 weeks after initial rating.
Image Analysis and Diagnostic Performance for Fetal Cardiac MRI and Fetal Echocardiography
To compare the diagnostic performance of fetal cardiac MRI and echocardiography, potential abnormalities of 20 fetal cardiovascular features (eg, situs, cardiac position, atrioventricular connections, right ventricle, aortic valve, and aortic arch; for all features, see the study evaluation form [Fig S2]) were assessed for each modality by using a binary scoring system (abnormality present or not present). Both the MRI and echocardiography studies were read in consensus by two readers (J.A.L. and C.H.). Readers were blinded to the results of the other imaging modality but were not blinded to referral diagnosis. After feature assessment, a separate CHD diagnosis was made for each modality.
Following delivery, all neonates were examined within 48 hours by pediatric cardiologists using transthoracic echocardiography. In addition, further postnatal feature assessment for the presence of abnormalities in the outlined features as conducted with follow-up echocardiography, CT, and/or surgery reports served as the reference standard against which the findings from fetal cardiac MRI and fetal echocardiography were tested. Anatomic variants were considered to be abnormal (ie, aberrant right subclavian artery).
Statistical Analysis
SPSS Statistics software (version 26; IBM) was used for statistical analysis. Continuous variables are summarized as means ± SDs or as medians and IQRs. Dichotomous variables are summarized as percentages to absolute frequencies. Differences in sensitivity and specificity for the classification of cardiovascular features between echocardiography and cardiac MRI were determined by using a random-effects model fitted to account for data dependencies resulting from the clustering of features within each fetus. Intra- and interrater reproducibility of image quality evaluation were assessed by using intraclass correlation coefficient (ICC) estimates. ICC estimates and their 95% CIs were based on a single-measure, two-way mixed (consistency) model. The level of statistical significance was set to a P value less than .05.
Results
Participant and Fetal Characteristics
After the exclusion of two participants from the initial cohort because of an unstable trigger signal (n = 1) and claustrophobia (n = 1), a total of 23 participants (mean age, 32 years ± 5) in the third trimester of pregnancy (mean gestational age, 36 weeks ± 1; range, 32–37 weeks) were included in the study (Fig 1). Risk factors for CHD development were present in 11 of 23 participants (48%). The mean interval between fetal MRI and delivery was 24 days ± 15. The mean gestational age at delivery was 39 weeks ± 2. All neonates were admitted to the neonatal intensive care unit immediately after birth. One neonate (fetus 2) died during follow-up because of intracranial hemorrhage. Clinical characteristics are summarized in Table 1.
Figure 1:

Study flow diagram. CHD = congenital heart disease.
Table 1:
Clinical Characteristics of Study Sample

Feasibility of Fetal Cardiac MRI
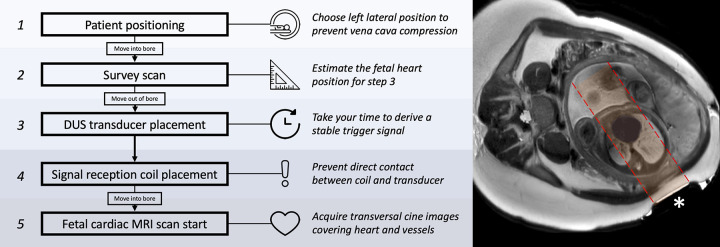
Left lateral positioning and DUS transducer application were well tolerated by all participants. Once the position of the fetal heart was identified on survey images, DUS transducer application and derivation of a stable gating signal were achieved within 10 minutes ± 4. The mean fetal heart rate was 136 beats per minute ± 10. The examination time including the time for DUS transducer application was 45 minutes ± 13. Transient signal loss of more than 3 seconds because of maternal breathing or fetal movement was noticed in 11 of 23 women (48%), resulting in premature termination of cine acquisitions. Image acquisitions had to be repeated in these participants once the trigger signal was stable again. DUS transducer repositioning was necessary for two of 23 participants (9%). In one case, the heart shifted out of the acoustic window because of substantial fetal movement, whereas in the other case, the transducer was dislocated because of a lack of fixation. A continuous axial cine stack was successfully acquired in all participants, and 21 of 23 (91%) examinations were performed by using the breath-hold technique. In 18 of 23 participants (78%), additional cine images in the sagittal (14 of 23 [61%]) or coronal (11 of 23 [48%]) orientation were obtained. A dedicated workflow for DUS-gated fetal cardiac MRI is illustrated in Figure 2.
Figure 2:
Fetal cardiac MRI workflow used in this study. An axial single-shot T2-weighted turbo spin-echo planning or survey image at fetal heart level (right) shows the Doppler US (DUS) transducer (*) placed on the maternal abdomen. The acoustic window (highlighted in red) is correctly positioned toward the fetal heart, providing a stable trigger signal.
Image Quality of Fetal Cardiac MRI
The median overall image quality score was 3 (IQR, 2.5–4). Artifacts that resulted in image quality impairment were related to fetal movement (six of 23 [26%]), maternal respiratory motion (five of 23 [22%]), and/or pulsation (three of 23 [13%]). There was no degradation of image quality related to dielectric shielding artifacts or to the DUS transducer itself (ie, off-resonance artifacts). Intra- and interobserver reproducibility of the overall image quality scores were good (intraobserver ICC, 0.89 [95% CI: 0.74, 0.95]; interobserver ICC, 0.79 [95% CI: 0.51, 0.91]). The median delineability scores were 4 (IQR, 3–4) for the ventricles, 4 (IQR, 3–4) for the atria, 3 (IQR, 3–4) for the great arteries, 3 (IQR, 3–4) for the systemic veins, 3 (IQR, 2.5–3.5) for the pulmonary veins, 3 (IQR, 2–4) for the atrioventricular valves, and 2 (IQR, 2–3) for the semilunar valves (Fig 3). Intra- and interobserver reproducibility for delineability scores were excellent and good, respectively (intraobserver ICC, 0.91 [95% CI: 0.87, 0.93]; interobserver ICC, 0.80 [95% CI: 0.73, 0.86]).
Figure 3:

Bar plot shows the distribution of ratings for the overall image quality score and delineability scores for different cardiovascular structures. The four-point Likert scale ratings were defined as follows: For the overall image quality score, 1 = nondiagnostic image quality with high degree of artifacts, 2 = poor image quality with distinct artifacts, 3 = moderate image quality with few artifacts, and 4 = good image quality without artifacts. For the delineability scores, 1 = structure not visible, 2 = structure is poorly delineated from surrounding structures, 3 = structure is moderately delineated from surrounding structures, and 4 = structure is well delineated from surrounding structures.
Diagnostic Performance of Fetal Cardiac MRI
According to the postnatal reference standard, 27.6% (127 of 460) of cardiovascular features in all fetuses were abnormal. Fetal cardiac MRI yielded a sensitivity of 91.8% (112 of 127; 95% CI: 85.7, 95.1) and a specificity of 99.9% (330 of 333; 95% CI: 99.2, 100) for detecting abnormal cardiovascular features. Fetal echocardiography had a sensitivity of 93.6% (115 of 127; 95% CI: 88.8, 96.2) and a specificity of 99.9% (331 of 333; 95% CI: 99.5, 100). We found no evidence of differences in sensitivity (P = .53) or specificity (P > .99) between the two modalities. Most fetuses had a combination of different complex malformations, and postnatal reports confirmed a wide spectrum of underlying CHD (Fig 4, Movie 1). Table 2 summarizes all diagnoses. Apart from the cardiovascular system, no abnormalities of other organ systems were present in any fetus. Compared with the postnatal reference standard, the CHD diagnosis enabled by fetal cardiac MRI as well as by fetal echocardiography was correct in 21 of 23 examinations (91%). For one fetus (fetus 10), the presence of tricuspid atresia was erroneously assumed from findings from both modalities independently, and postnatal diagnosis revealed two atrioventricular valves with crossed inflow of both valves predominantly into the left ventricle, leading to the diagnosis of a double-inlet left ventricle with a crisscross arrangement of the inflow tracts. In another fetus (fetus 8), situs inversus with levocardia and congenitally corrected transposition of the great arteries was correctly suspected on the basis of MRI findings, but venoatrial connections and ventricular inversion could not be identified with echocardiography, leading to the incorrect diagnosis of a dextro-transposition of the great arteries with regular orifices of the systemic veins on the right side and of the pulmonary veins on the left side (Fig 5). In another fetus (fetus 16), the ventricles could not be assigned correctly using MRI in contrast to using echocardiography; postnatal findings revealed atrioventricular discordance and tricuspid atresia, resulting in the diagnosis of a congenitally corrected transposition of the great arteries instead of a dextro-transposition and mitral atresia as determined by using MRI (Fig 6, Movie 2).
Figure 4:
Images in a 35-year-old woman at a gestational age of 36 weeks 0 days with dextro-transposition of the great arteries of the fetus (fetus 5). Balanced steady-state free precession cardiac cine MR images in (A–C) axial and (D) sagittal views reveal (A) atrioventricular concordance but (B–D) ventriculoarterial discordance, with the aorta arising from the right ventricle (RV) and the main pulmonary artery (MPA) arising from the left ventricle (LV). (A) The interatrial communication is given via the nonrestrictive foramen ovale. The complete axial cine imaging stack is provided (Movie 1). (E, F) Corresponding echocardiographic images also show (E) a wide foramen ovale and (F) ventriculoarterial discordance with the pulmonary artery arising from the LV. DA = ductus arteriosus, DAO = descending aorta, LA = left atrium, LPA = left pulmonary artery, LVOT = LV outflow tract, PV = pulmonary valve, RA = right atrium, RPA = right pulmonary artery, SVC = superior vena cava.
Table 2:
Overview of Study Participants with Missed and Differing Findings from Fetal Echocardiography and Fetal Cardiac MRI Compared with Postnatal Final Diagnosis
Figure 5:
Images in a 30-year-old woman at a gestational age of 34 weeks 5 days with complex congenital heart disease of the fetus (fetus 8). (A–C) Axial and (D) coronal balanced steady-state free precession cine images demonstrate congenitally corrected transposition of the great arteries in complete situs inversus with isolated levocardia. (A) The pulmonary veins join the right-sided left atrium (LA), and the systemic veins are connected to the left-sided right atrium (RA). The LA (which lies anteriorly because of the levocardia) is connected to the right ventricle (RV), and the RA (which lies posteriorly because of the levocardia) is connected to the left ventricle (LV) (atrioventricular discordance). Note that in complete situs inversus with isolated levocardia, the LA and the LV would have both been positioned anteriorly, which is not the case here because of the congenitally corrected transposition of the great arteries (LV lies posteriorly). (B) The aorta arises from the RV, and the main pulmonary artery (MPA) arises from the LV (ventriculoarterial discordance). (C) The right-sided aortic arch and (D) the left-sided hepatic vein confluence indicate situs inversus. The complete axial cine imaging stack is provided (Movie 2). LSVC = left superior vena cava, PV = pulmonary valve.
Figure 6:
Images in a 29-year-old woman at a gestational age of 33 weeks 6 days with congenitally corrected transposition of the great arteries with tricuspid atresia and a hypoplastic right ventricle (RV) of the fetus (fetus 16). (A–C) Axial balanced steady-state free precession cine images show a univentricular atrioventricular junction with a normally sized ventricle on the right side and a hypoplastic ventricle on the left side communicating via the large ventricular septal defect (VSD, *). (B) Overriding the VSD, the main pulmonary artery (MPA) arises mainly from the right-sided ventricle. These findings were incorrectly defined as a double-outlet RV with dextro-malpositioned great arteries and mitral atresia with a hypoplastic left ventricle (LV). (D) The corresponding echocardiographic image demonstrates an LV shape and septophobic atrioventricular valve chordae of the normally sized ventricle, resulting in the correct diagnosis of a congenitally corrected transposition of the great arteries with tricuspid atresia and a hypoplastic RV on the left side. (B, C) Other findings included an aberrant right subclavian artery (ARSA) and a persistent left superior vena cava (LSVC), which could be clearly depicted with MRI. CCA = common carotid artery, LPA = left pulmonary artery, LSA = left subclavian artery, LVOT = LV outflow tract, MV = mitral valve, PV = pulmonary valve, RA = right atrium.
Movie 1:
Balanced steady-state free precession cine images in axial orientation of a fetus with dextro-transposition of the great arteries (fetus 5).
Movie 2:
Balanced steady-state free precession cine images in axial orientation of a fetus with congenitally corrected transposition of the great arteries in complete situs inversus with levocardia (fetus 8).
Discussion
Cardiac MRI is an important imaging modality for the diagnosis and management of pediatric and adult CHD (18). A DUS-based transducer enables direct gating of cardiac MRI sequences for assessment of the fetal heart (16,17). We showed that DUS-gated fetal cardiac MRI can be implemented in clinical routine and that it was able to reliably enable the diagnosis of complex forms of CHD. The diagnostic performance of fetal cardiac MRI for the detection of abnormal cardiovascular features was comparable with that of fetal echocardiography (sensitivity, 91.8% vs 93.6% [P = .53]; specificity, 99.9% vs 99.9% [P > .99]). DUS-gated fetal cardiac cine MRI provided diagnostic image quality (median image quality score, 3 [IQR, 2.5–4]) and may serve as an adjunct diagnostic tool for the assessment of CHD in late pregnancy.
Although early dynamic imaging of the fetal heart with real-time sequences suffered from limited spatial and temporal resolution to fully resolve the fetal cardiac cycle, different techniques have been developed to overcome the lack of electrocardiographic gating, including metric-optimized gating and self-gating strategies. However, these techniques often require time-consuming postprocessing steps with offline reconstruction of images, which limits quality assessment and scan adjustments of acquired cine images during the scan (14,19–22). Furthermore, these gating techniques might also limit temporal and spatial resolution, which is a major drawback of real-time cine imaging (22). With DUS gating, the DUS signal of the fetal heartbeat is directly recorded and transmitted to the physiologic unit of the MRI system. This DUS signal can be used as a gating signal for cine or phase-contrast flow sequences with high temporospatial resolution. To compensate for fetal motion artifacts, strategies for both in-plane and through-plane motion compensation, as well as acceleration techniques using parallel imaging or compressed sensing, have been applied (11,23–27). In addition to standard two-dimensional imaging used in most studies, the use of a three-dimensional data set reconstructed from overlapping two-dimensional images combined with motion-correction algorithms allows for better visualization of the fetal vasculature (28,29). Although these technical advancements including DUS gating have been studied in fetuses with CHD and have enabled clinically useful information to be added to echocardiographic findings to influence clinical decision-making (30–32), our study provides a direct comparison to fetal echocardiography for previously defined anatomic features by using a prospective study design.
As in fetal echocardiography, most cardiovascular abnormalities can be visualized on standard axial four-chamber views with increasing sensitivity when combined with slightly angled outflow tract views (6,33); therefore, we primarily chose an axial cine imaging approach for the assessment of cardiovascular structures. Axial cardiac cine images offer a convenient tool for the assessment of cardiovascular anatomy and can be easily planned. Heart-specific views (ie, short-axis view) usually require additional time-consuming planning sequences or the use of real-time planning, which might be inconvenient and unpractical in cases of fetal movements. We also chose a breath-holding approach for cine image acquisition, which was well tolerated by pregnant participants. This approach generally improved image quality, but breathing artifacts were still found to substantially degrade image quality in some participants.
It is important to note that the mean gestational age in the current study was 36 weeks ± 1, and fetal cardiac MRI might be challenging at earlier gestational ages, as fetuses are more mobile and cardiovascular structures are even smaller. Cine MRI imaging earlier in gestation might therefore require improved motion compensation and a higher spatial resolution, which would decrease the temporal resolution of segmented cine images, if image acquisition should fit into a breath hold. Another cardiac MRI study that investigated 31 fetuses with CHD at a gestational age of 31 weeks ± 5 with a more static steady-state free precession sequence reported a sensitivity of 77.5% for the detection of cardiovascular anomalies with MRI (34). The higher sensitivity for the detection of abnormal cardiovascular features found in our study might be explained by either the higher gestational age or the implementation of cine images, which may facilitate the traceability of structures across multiple sections and allow for indirect estimation of flow characteristics.
Our study shows that fetal cardiac MRI can provide additional diagnostic value compared with fetal echocardiography by being able to depict diagnostic findings that were previously unknown, such as a persistent left vena cava superior in fetus 22. Therefore, it may be considered an adjunct diagnostic tool in cases of nondiagnostic echocardiographic image quality or inconsistent echocardiographic results. Additionally, it may be possible to analyze gross anatomy more objectively with MRI, which could be particularly important in patients with heterotaxy syndrome, a disorder often associated with CHD. Other congenital disorders (ie, brain abnormalities) may also be investigated during the MRI examination. However, diagnostic confidence when using MRI to assess smaller cardiac structures like semilunar valves may be reduced, as reflected by the lower delineability scores for valves.
Our study had limitations. Readers were not blinded to the referral diagnosis, which might have contributed to observer bias. However, fetal MRI and echocardiography are specialized diagnostic tests that are usually performed because of suspected pathologic conditions. Therefore, the study design reflects a real-world clinical setting. In view of the small sample size, further validation is needed to evaluate the diagnostic accuracy of using fetal cardiac MRI to classify different CHD subtypes and to identify subgroups that may benefit in terms of counseling or postnatal care planning. Finally, flow measurements were not obtained in our study, and such measurements could provide additional hemodynamic information.
In conclusion, our results demonstrate that DUS-gated fetal cardiac MRI can be implemented in a routine clinical setting. Fetal cardiac MRI may enable reliable diagnosis of even complex forms of CHD, with a sensitivity and specificity comparable with those of fetal echocardiography. Especially in late pregnancy, fetal cardiac MRI might become an additional diagnostic tool for CHD assessment (ie, in cases of inconclusive fetal echocardiographic results) and further broaden the diagnostic applications of cardiac MRI.
Authors declared no funding for this work.
Data sharing: Data generated or analyzed during the study are available from the corresponding author by request.
Disclosures of conflicts of interest: T.M.V. No relevant relationships. C.H. No relevant relationships. S.Z. Salary from Philips. C.K. Employee of Philips, the manufacturer of the MRI system used in this study. A.I. Grant to author’s university from BONFOR and DFG. C.C.P. Educational grant from Guerbet to author’s institution; advisory board consulting fees from Guerbet to author; payment for speakers bureaus from Guerbet and Julius Zorn to author; support from Guerbet and Julius Zorn for attending meetings and/or travel. D.K. No relevant relationships. B.F. No relevant relationships. B.S. No relevant relationships. U.A. Consulting fees from Bayer Healthcare; payment from Siemens Healthineers for speakers bureau; support from Siemens Healthineers for attending meetings and/or travel. F.K. No relevant relationships. U.H. Leadership or fiduciary role in German Society of Pediatric Cardiology. A.G. No relevant relationships. J.A.L. Received payments for lectures from Philips Healthcare and for activities related to the scientific advisory board for Bayer Healthcare.
Abbreviations:
- CHD
- congenital heart disease
- DUS
- Doppler US
- ICC
- intraclass correlation coefficient
References
- 1. van der Linde D , Konings EEM , Slager MA , et al . Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis . J Am Coll Cardiol 2011. ; 58 ( 21 ): 2241 – 2247 . [DOI] [PubMed] [Google Scholar]
- 2. Hoffman JIE , Kaplan S . The incidence of congenital heart disease . J Am Coll Cardiol 2002. ; 39 ( 12 ): 1890 – 1900 . [DOI] [PubMed] [Google Scholar]
- 3. Holland BJ , Myers JA , Woods CR Jr . Prenatal diagnosis of critical congenital heart disease reduces risk of death from cardiovascular compromise prior to planned neonatal cardiac surgery: a meta-analysis . Ultrasound Obstet Gynecol 2015. ; 45 ( 6 ): 631 – 638 . [DOI] [PubMed] [Google Scholar]
- 4. Franklin O , Burch M , Manning N , Sleeman K , Gould S , Archer N . Prenatal diagnosis of coarctation of the aorta improves survival and reduces morbidity . Br Heart J 2002. ; 87 ( 1 ): 67 – 69 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donofrio MT , Moon-Grady AJ , Hornberger LK , et al . Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association . Circulation 2014. ; 129 ( 21 ): 2183 – 2242 . [Published correction appears in Circulation 2014;129(21):e512.] [DOI] [PubMed] [Google Scholar]
- 6. Zhang YF , Zeng XL , Zhao EF , Lu HW . Diagnostic value of fetal echocardiography for congenital heart disease: a systematic review and meta-analysis . Medicine (Baltimore) 2015. ; 94 ( 42 ): e1759 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wielandner A , Mlczoch E , Prayer D , Berger-Kulemann V . Potential of magnetic resonance for imaging the fetal heart . Semin Fetal Neonatal Med 2013. ; 18 ( 5 ): 286 – 297 . [DOI] [PubMed] [Google Scholar]
- 8. Recio Rodríguez M , Martínez de Vega V , Cano Alonso R , Carrascoso Arranz J , Martínez Ten P , Pérez Pedregosa J . MR imaging of thoracic abnormalities in the fetus . RadioGraphics 2012. ; 32 ( 7 ): E305 – E321 . [DOI] [PubMed] [Google Scholar]
- 9. Furey EA , Bailey AA , Twickler DM . Fetal MR imaging of gastrointestinal abnormalities . RadioGraphics 2016. ; 36 ( 3 ): 904 – 917 . [DOI] [PubMed] [Google Scholar]
- 10. Jarvis DA , Griffiths PD . Current state of MRI of the fetal brain in utero . J Magn Reson Imaging 2019. ; 49 ( 3 ): 632 – 646 . [DOI] [PubMed] [Google Scholar]
- 11. Roy CW , van Amerom JFP , Marini D , Seed M , Macgowan CK . Fetal cardiac MRI: a review of technical advancements . Top Magn Reson Imaging 2019. ; 28 ( 5 ): 235 – 244 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jansz MS , Seed M , van Amerom JFP , et al . Metric optimized gating for fetal cardiac MRI . Magn Reson Med 2010. ; 64 ( 5 ): 1304 – 1314 . [DOI] [PubMed] [Google Scholar]
- 13. Yamamura J , Frisch M , Ecker H , et al . Self-gating MR imaging of the fetal heart: comparison with real cardiac triggering . Eur Radiol 2011. ; 21 ( 1 ): 142 – 149 . [DOI] [PubMed] [Google Scholar]
- 14. Roy CW , Seed M , van Amerom JFP , et al . Dynamic imaging of the fetal heart using metric optimized gating . Magn Reson Med 2013. ; 70 ( 6 ): 1598 – 1607 . [DOI] [PubMed] [Google Scholar]
- 15. Chaptinel J , Yerly J , Mivelaz Y , et al . Fetal cardiac cine magnetic resonance imaging in utero . Sci Rep 2017. ; 7 ( 1 ): 15540 . [Published correction appears in Sci Rep 2018;8(1):7886.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kording F , Schoennagel BP , de Sousa MT , et al . Evaluation of a portable Doppler ultrasound gating device for fetal cardiac MR imaging: initial results at 1.5T and 3T . Magn Reson Med Sci 2018. ; 17 ( 4 ): 308 – 317 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kording F , Yamamura J , de Sousa MT , et al . Dynamic fetal cardiovascular magnetic resonance imaging using Doppler ultrasound gating . J Cardiovasc Magn Reson 2018. ; 20 ( 1 ): 17 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fratz S , Chung T , Greil GF , et al . Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease . J Cardiovasc Magn Reson 2013. ; 15 ( 1 ): 51 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gorincour G , Bourlière-Najean B , Bonello B , et al . Feasibility of fetal cardiac magnetic resonance imaging: preliminary experience . Ultrasound Obstet Gynecol 2007. ; 29 ( 1 ): 105 – 108 . [DOI] [PubMed] [Google Scholar]
- 20. Manganaro L , Savelli S , Di Maurizio M , et al . Potential role of fetal cardiac evaluation with magnetic resonance imaging: preliminary experience . Prenat Diagn 2008. ; 28 ( 2 ): 148 – 156 . [DOI] [PubMed] [Google Scholar]
- 21. Roy CW , Seed M , Macgowan CK . Accelerated MRI of the fetal heart using compressed sensing and metric optimized gating . Magn Reson Med 2017. ; 77 ( 6 ): 2125 – 2135 . [DOI] [PubMed] [Google Scholar]
- 22. Haris K , Hedström E , Bidhult S , et al . Self-gated fetal cardiac MRI with tiny golden angle iGRASP: a feasibility study . J Magn Reson Imaging 2017. ; 46 ( 1 ): 207 – 217 . [DOI] [PubMed] [Google Scholar]
- 23. Malamateniou C , Malik SJ , Counsell SJ , et al . Motion-compensation techniques in neonatal and fetal MR imaging . AJNR Am J Neuroradiol 2013. ; 34 ( 6 ): 1124 – 1136 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haris K , Hedström E , Kording F , et al . Free-breathing fetal cardiac MRI with Doppler ultrasound gating, compressed sensing, and motion compensation . J Magn Reson Imaging 2020. ; 51 ( 1 ): 260 – 272 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roy CW , Seed M , Kingdom JC , Macgowan CK . Motion compensated cine CMR of the fetal heart using radial undersampling and compressed sensing . J Cardiovasc Magn Reson 2017. ; 19 ( 1 ): 29 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Amerom JFP , Lloyd DFA , Price AN , et al . Fetal cardiac cine imaging using highly accelerated dynamic MRI with retrospective motion correction and outlier rejection . Magn Reson Med 2018. ; 79 ( 1 ): 327 – 338 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Amerom JFP , Lloyd DFA , Deprez M , et al . Fetal whole-heart 4D imaging using motion-corrected multi-planar real-time MRI . Magn Reson Med 2019. ; 82 ( 3 ): 1055 – 1072 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lloyd DFA , Pushparajah K , Simpson JM , et al . Three-dimensional visualisation of the fetal heart using prenatal MRI with motion-corrected slice-volume registration: a prospective, single-centre cohort study . Lancet 2019. ; 393 ( 10181 ): 1619 – 1627 . [Published correction appears in Lancet 2022;399(10335):1606.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lloyd DFA , van Poppel MPM , Pushparajah K , et al . Analysis of 3-dimensional arch anatomy, vascular flow, and postnatal outcome in cases of suspected coarctation of the aorta using fetal cardiac magnetic resonance imaging . Circ Cardiovasc Imaging 2021. ; 14 ( 7 ): e012411 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tavares de Sousa M , Hecher K , Kording F , et al . Fetal dynamic magnetic resonance imaging using Doppler ultrasound gating for the assessment of the aortic isthmus: a feasibility study . Acta Obstet Gynecol Scand 2021. ; 100 ( 1 ): 67 – 73 . [DOI] [PubMed] [Google Scholar]
- 31. Tavares de Sousa M , Hecher K , Yamamura J , et al . Dynamic fetal cardiac magnetic resonance imaging in four-chamber view using Doppler ultrasound gating in normal fetal heart and in congenital heart disease: comparison with fetal echocardiography . Ultrasound Obstet Gynecol 2019. ; 53 ( 5 ): 669 – 675 . [DOI] [PubMed] [Google Scholar]
- 32. Ryd D , Fricke K , Bhat M , Arleen H , Liuba P , Hedström E . Utility of fetal cardiovascular magnetic resonance for prenatal diagnosis of complex congenital heart defects . JAMA Netw Open 2021. ; 4 ( 3 ): e213538 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cardiac screening examination of the fetus: guidelines for performing the ‘basic’ and ‘extended basic’ cardiac scan . Ultrasound Obstet Gynecol 2006. ; 27 ( 1 ): 107 – 113 . [DOI] [PubMed] [Google Scholar]
- 34. Goncalves LF , Lindblade CL , Cornejo P , Patel MC , McLaughlin ES , Bardo DME . Contribution of fetal magnetic resonance imaging in fetuses with congenital heart disease . Pediatr Radiol 2022. ; 52 ( 3 ): 513 – 526 . [DOI] [PubMed] [Google Scholar]