Abstract
Background:
Reports suggest a potential association between coronavirus disease 2019 (COVID-19) vaccines and acute central nervous system (CNS) inflammation.
Objective:
The main objective of this study is to describe features of acute CNS inflammation following COVID-19 vaccination.
Methods:
A retrospective observational cohort study was performed at the BARLO MS Centre in Toronto, Canada. Clinicians reported acute CNS inflammatory events within 60 days after a COVID-19 vaccine from March 2021 to August 2022. Clinical characteristics were evaluated.
Results:
Thirty-eight patients (median age 39 (range: 20–82) years; 60.5% female) presented within 0–55 (median 15) days of a receiving a COVID-19 vaccine and were diagnosed with relapsing remitting multiple sclerosis (MS) (n = 16), post-vaccine transverse myelitis (n = 7), clinically isolated syndrome (n = 5), MS relapse (n = 4), tumefactive demyelination (n = 2), myelin oligodendrocyte glycoprotein antibody disease (n = 1), neuromyelitis optica spectrum disorder (n = 1), chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (n = 1) and primary autoimmune cerebellar ataxia (n = 1). Twenty-two received acute treatment and 21 started disease-modifying therapy. Sixteen received subsequent COVID-19 vaccination, of which 87.5% had no new or worsening neurological symptoms.
Conclusion:
To our knowledge, this is the largest study describing acute CNS inflammation after COVID-19 vaccination. We could not determine whether the number of inflammatory events was higher than expected.
Keywords: COVID-19, vaccination, demyelinating diseases, neuroinflammatory diseases, multiple sclerosis, central nervous system
Introduction
Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) became available in late 2020 and remains a critical measure to control the coronavirus disease 2019 (COVID-19) pandemic. 1 Four COVID-19 vaccines are authorized for use in Canada and include Pfizer-BioN-Tech (BNT162b2), Moderna (mRNA-1273), Oxford-AstraZeneca (ChAdOx1S/nCoV-19, AZD1222) and Johnson & Johnson/Janssen (JNJ-78436735). 2 Although the vaccines differ in their mode of delivery, all of them use the viral spike protein as an immunogen. 3 Each vaccine has been shown to be remarkably effective and safe.4,5
Upon global uptake of COVID-19 vaccines, a number of reports have described various presentations of acute central nervous system (CNS) inflammation in individuals shortly after vaccination. This includes new onset multiple sclerosis (MS),6–8 MS relapse,6,9 transverse myelitis (TM),4,10–13 myelin oligodendrocyte glycoprotein antibody disease (MOGAD),14,15 neuromyelitis optica spectrum disorder (NMOSD),6,16–18 acute disseminated encephalomyelitis (ADEM),19–21 leucine-rich glioma-inactivated protein 1 (LGI1) antibody encephalitis, 22 and seronegative autoimmune limbic encephalitis. 23
Vaccination has long been speculated to increase the risk of CNS inflammatory events, most notably demyelinating diseases such as ADEM. 24 A large case–control study published in 2014 suggested that vaccination of any type may accelerate the transition from subclinical to overt demyelinating disease, but a longer-term association between vaccination and demyelinating disease was not found, arguing against a causal association. 25 More recently, a disproportionality analysis using the World Health Organization’s pharmacovigilance database observed a weak association between the Pfizer-BioN-Tech, Moderna and Oxford-AstraZeneca vaccines and CNS demyelinating disease, but this risk was low and similar to that of other viral vaccines. 26
Several questions remain about the relationship between COVID-19 vaccines and acute CNS inflammation. There is a need to define the clinical spectrum of acute CNS inflammatory events occurring in temporal relation to COVID-19 vaccination and to assess outcomes in response to immunotherapy. It is also unclear whether subsequent vaccination against COVID-19 is associated with recurrent adverse events in this population. We report 38 patients with or without pre-existing neuroinflammatory disease who experienced an acute CNS inflammatory event within 60 days of receiving at least one dose of a COVID-19 vaccine. We describe their clinical, laboratory and imaging features, as well as their treatment outcomes and response to subsequent vaccination against COVID-19.
Materials and methods
Study design, setting and participants
We performed a retrospective observational cohort study using clinical data from the BARLO MS Centre, a tertiary care centre in Toronto, Ontario, Canada. Patients were identified by their treating clinician for inclusion in the study. Eligible patients were age 18 years or older and experienced an acute CNS inflammatory event within 60 days of receiving at least one COVID-19 vaccine from March 2021 to August 2022. The 60-day range was chosen based on reports of acute CNS inflammation occurring mostly within 30 days and less commonly up to 90 days after vaccination.27,28 Patients with or without pre-existing neuroinflammatory disease were included. Data of interest were retrospectively obtained from patients’ electronic health records and included only information already collected in clinical practice. Patients underwent investigation, received treatment and were seen in follow-up at the discretion of their treating clinician and not for the purpose of the study. The study was approved by the Unity Health Toronto Research Ethics Board with a waiver of informed consent.
Exposures and outcomes
We collected demographic data including age at symptom onset and sex. The type and dose of COVID-19 vaccine received most recently prior to symptom onset was recorded. Information was collected on the type and duration of acute treatment, the continuation or initiation of disease-modifying therapy (DMT) and whether a subsequent COVID-19 vaccine was administered at any time after the acute CNS inflammatory event.
Outcome data included the number of days between vaccination and symptom onset. Neurological symptoms and corresponding neurological syndromes were evaluated. We recorded the duration of follow-up and diagnosis at last follow-up. Neurological and functional disability was measured using the expanded disability status scale (EDSS) at symptom nadir and at last follow-up. The EDSS was either documented by the examiner at the time of the clinical visit or retrospectively calculated by the authors based on the available clinical documentation. Clinical outcome was based on reported symptoms and neurological examination at last follow-up and defined as ‘return to baseline’, ‘partial recovery’ or ‘worsening’. Subsequent vaccination against COVID-19 was documented and patient charts were reviewed for symptom recrudescence or disease relapse.
Radiological data included magnetic resonance imaging (MRI) brain and spinal cord results. Patients seen at the BARLO MS Centre are geographically dispersed across the province of Ontario, and neuroimaging was sometimes obtained at an outside hospital, with results made available to the treating clinician. Therefore, MRI protocols varied among patients in the study. Images were reviewed directly by study personnel. T2 and fluid-attenuated inversion recovery (FLAIR) sequences were examined for new or enlarging T2 hyperintense lesions in the brain and spinal cord. T1 sequences with gadolinium were evaluated for enhancing lesions in the brain and spinal cord. T1 sequences were reviewed for T1 ‘black hole’ lesions in the brain. All abnormal lesions were included, regardless of whether they were located in regions that meet criteria for MS as outlined in the 2017 McDonald Criteria. 29 Repeat MRI brain and spinal cord images were reviewed if available and compared to the initial studies. ‘Stable’ was defined as no change in the number or size of lesions, ‘improvement’ was defined as a decrease in the number or size of lesions, and ‘worsening’ was defined as an increase in the number or size of lesions.
Laboratory data comprised antibody positivity for MOG and aquaporin 4 (AQP4) IgG in serum. Cerebrospinal fluid (CSF) results were reviewed for white blood cell (WBC) count, WBC differential, red blood cell (RBC) count, glucose, protein, oligoclonal bands and IgG index.
Statistical analysis
Descriptive statistics were reported as frequency counts and percentages for categorical variables and as median and range for continuous variables.
Results
Thirty-eight patients were identified for inclusion in the study. The median age at symptom onset was 39 years (range 20–82) and 23 were female (60.5%, ratio 1.5:1). Four (10.5%) patients had a pre-existing diagnosis of relapsing remitting multiple sclerosis (RRMS), and three of them were on DMT at the time of receiving a COVID-19 vaccine. One (2.6%) patient had a retrospective history of neurological symptoms in keeping with an MS relapse but did not have a known diagnosis at the time of receiving a COVID-19 vaccine. One (2.6%) patient had a pre-existing diagnosis of overlap syndrome with rheumatoid arthritis and systemic lupus erythematosus. The remaining 32 (84.2%) patients had no known history of neuroinflammatory or autoimmune disease. Prior to symptom onset, individuals received the Pfizer-BioN-Tech (n = 26), Moderna (n = 10), Oxford-AstraZeneca (n = 1) or unknown (n = 1) COVID-19 vaccines. Although the Johnson & Johnson/Janssen COVID-19 vaccine was available in Ontario at the time of the study, it was not commonly used and we did not encounter any individuals who received this vaccine.
Within 0 to 55 days (median 15) of receiving either the first (n = 13), second (n = 16), third (n = 7) or fourth (n = 2) vaccine dose, patients developed a variety of neurologic symptoms that localized to the CNS. This included numbness (n = 25), weakness (n = 19), sphincter dysfunction (n = 8), vision loss (n = 6), ataxia (n = 6), dysarthria (n = 5), diplopia (n = 1), aphasia (n = 1) and vertigo (n = 1). Median EDSS at symptom nadir was 2.5 (range 0–7). The predominant clinical syndrome was TM (n = 24), followed by hemispheric (n = 7), optic neuritis (ON) (n = 6), brainstem (n = 4), cerebellar (n = 3) and thalamic (n = 1). Six (15.8%) patients had a multifocal presentation, with neurological symptoms localizing to more than one region of the CNS. Of those with a multifocal presentation, 5 (83.3%) had corresponding lesions on neuroimaging. Demographic and clinical characteristics are summarized in Table 1a. Given that most cases reported in the literature presented within 30 days of receiving a COVID-19 vaccination, we re-examined the demographic and clinical characteristics of our cohort, limiting the time window from 60 to 30 days (Table 1b). The demographic and clinical characteristics were similar for the 30 day group (n = 24) when compared to the cohort as a whole (n = 38).
Table 1.
| (a) Demographic and clinical characteristics of patients presenting within 60 days of COVID-19 vaccination | Patients with CNS inflammatory event, n = 38 |
|---|---|
| Age at symptom onset, median years (range) | 39 (20–82) |
| Female, n (%) | 23 (60.5) |
| Pre-existing immune-mediated disease, n (%) | |
| RRMS | 4 (10.5) |
| Retrospective MS relapse | 1 (2.6) |
| Overlap syndrome (RA/SLE) | 1 (2.6) |
| None | 32 (84.2) |
| Vaccine received, n (%) | |
| Pfizer-BioN-Tech | 26 (68.4) |
| Moderna | 10 (26.3) |
| Oxford-AstraZeneca | 1 (2.6) |
| Unknown | 1 (2.6) |
| Symptom onset following, n (%) | |
| First dose | 13 (34.2) |
| Second dose | 16 (42.1) |
| Third dose | 7 (18.4) |
| Fourth dose | 2 (5.3) |
| Time of symptom onset post-vaccine, median days (range) | 15 (0-55) |
| Presenting symptoms, n (%) | |
| Numbness | 25 (65.8) |
| Weakness | 19 (50.0) |
| Sphincter dysfunction | 8 (21.1) |
| Vision loss | 6 (15.8) |
| Ataxia | 6 (15.8) |
| Dysarthria | 5 (13.2) |
| Diplopia | 1 (2.6) |
| Aphasia | 1 (2.6) |
| Vertigo | 1 (2.6) |
| EDSS at symptom nadir, median (range) | 2.5 (0–7) |
| Clinical syndrome, n (%) | |
| Transverse myelitis | 24 (63.2) |
| Hemispheric | 7 (18.4) |
| Optic neuritis | 6 (15.8) |
| Brainstem | 4 (10.5) |
| Cerebellar | 3 (7.9) |
| Thalamic | 1 (2.6) |
| Multifocal presentation, n (%) | 6 (15.8) |
| (b) Demographic and clinical characteristics of patients presenting within 30 days of COVID-19 vaccination. | Patients with CNS inflammatory event, n = 24 |
| Age at symptom onset, median years (range) | 44 (20–82) |
| Female, n (%) | 15 (62.5) |
| Pre-existing immune-mediated disease, n (%) | |
| RRMS | 3 (12.5) |
| Retrospective MS relapse | 1 (4.2) |
| Overlap syndrome (RA/SLE) | 1 (4.2) |
| None | 19 (79.2) |
| Vaccine received, n (%) | |
| Pfizer-BioN-Tech | 16 (66.7) |
| Moderna | 6 (25.0) |
| (b) Demographic and clinical characteristics of patients presenting within 30 days of COVID-19 vaccination. | Patients with CNS inflammatory event, n = 24 |
| Oxford-AstraZeneca | 1 (4.2) |
| Unknown | 1 (4.2) |
| Symptom onset following, n (%) | |
| First dose | 11 (45.8) |
| Second dose | 9 (37.5) |
| Third dose | 4 (16.7) |
| Time of symptom onset post-vaccine, median days (range) | 7 (0–30) |
| Presenting symptoms, n (%) | |
| Numbness | 18 (75.0) |
| Weakness | 10 (41.7) |
| Sphincter dysfunction | 6 (25.0) |
| Vision loss | 4 (16.7) |
| Dysarthria | 4 (6.7) |
| Ataxia | 2 (8.3) |
| Vertigo | 1 (4.2) |
| EDSS at symptom nadir, median (range) | 2.5 (0–7) |
| Clinical syndrome, n (%) | |
| Transverse myelitis | 16 (66.7) |
| Hemispheric | 4 (16.7) |
| Optic neuritis | 4 (16.7) |
| Brainstem | 3 (12.5) |
| Cerebellar | 2 (8.3) |
| Multifocal presentation, n (%) | 4 (16.7) |
Abbreviations: CNS: central nervous system; RRMS: relapsing remitting multiple sclerosis; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus; EDSS: expanded disability status scale.
Thirty-six patients underwent MRI brain (Table 2). Twenty-two (61.1%) had T2 hyperintense lesions. This included all four patients with pre-existing RRMS, who had new lesions when compared to previous MRI brain. Twenty-seven patients had an MRI brain with contrast, and 10 (37.0%) had enhancing lesions. T1 sequences were available for review in 30 patients, and 11 (36.7%) had T1 hypointense ‘black hole’ lesions, including 3 with pre-existing RRMS and 8 with no prior history (6 new diagnosis of RRMS, 1 CIS, 1 MOGAD). Thirty-five patients underwent MRI spinal cord. T2 hyperintense lesions were observed in 27 (77.1%). This included 2 patients with pre-existing RRMS, who had new lesions when compared to previous MRI spinal cord. Two (7.4%) patients had a longitudinally extensive spinal cord lesion (1 NMOSD and 1 post-vaccine TM) and 3 (11.1%) had a lesion involving the conus medullaris (1 MS relapse, 1 CIS and 1 post-vaccine TM). Twenty-six patients had an MRI spinal cord with contrast and 9 (34.6%) had enhancing lesions. One patient with a final diagnosis of primary autoimmune cerebellar ataxia (PACA) did not have any T2 hyperintense lesions on MRI brain or spinal cord, but was found to have mild cerebellar atrophy. Figure 1 shows representative MRI images.
Table 2.
Neuroradiological findings.
| Patients with CNS inflammatory event, n = 38 a | |
|---|---|
| MRI brain, n (%) | |
| Normal | 13/36 (36.1) |
| T2 hyperintense lesions | 22/36 (61.1) |
| Enhancing lesions | 10/27 (37.0) |
| T1 “black hole” lesions | 11/30 (36.7) |
| Cerebellar atrophy | 1/36 (2.8) |
| MRI spine, n (%) | |
| Normal | 8/35 (22.9) |
| T2 hyperintense lesions | 27/35 (77.1) |
| LETM | 2/27 (7.4) |
| Lesion of the conus medullaris | 3/27 (11.1) |
| Enhancing lesions | 9/26 (34.6) |
| Time of repeat MRI from symptom onset, median days (range) | 180 (39–405) |
| Repeat MRI, n (%) | |
| Improvement | 10/28 (35.7) |
| Stable | 9/28 (32.1) |
| Worsening | 9/28 (32.1) |
Abbreviations: CNS = central nervous system; MRI = magnetic resonance imaging; FLAIR = fluid-attenuated inversion recovery; LETM = longitudinally extensive transverse myelitis.
Imaging not available for all patients, with sample size shown for each variable based on available imaging.
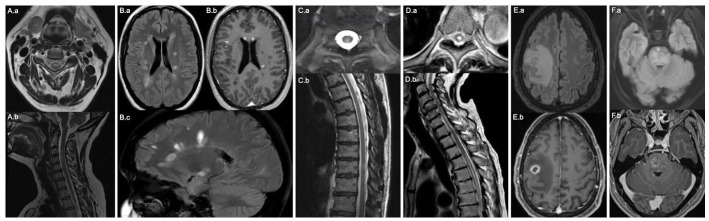
Figure 1.
Select MRI brain and spinal cord images of patients with acute CNS inflammatory events following COVID-19 vaccination. (A) Post-vaccine transverse myelitis. (A.a) Axial and (A.b) Sagittal T2 of the cervical spine reveal an intramedullary T2 hyperintense lesion at the level of C3–C4. (B) New onset relapsing remitting MS. (B.a) Axial and (B.c) Sagittal FLAIR reveal multiple ovoid T2 hyperintense lesions in the supratentorial white matter, including periventricular and juxtacortical involvement. (B.b) Axial T1 post-contrast shows enhancement of several lesions, as well as multiple T1 black hole lesions. (C) Myelin oligodendrocyte glycoprotein antibody disease. (C.a) Axial and (C.b) Sagittal T2 of the thoracic spine reveal an intramedullary T2 hyperintense lesion at the level of T10. (D) Neuromyelitis optica spectrum disorder. (D.a) Axial T2 of the thoracic spine at the level of T6 and (D.b) Sagittal T2 of the cervical and thoracic spine reveal a longitudinally extensive intramedullary T2 hyperintense lesion from T3–T4 to T9–T10 (thoracic spine only visualized to T8). (E) Post-vaccine tumefactive demyelination. (E.a) Axial FLAIR reveals a right frontal mass lesion with vasogenic edema and (E.b) Axial T1 post-contrast reveals associated ring-enhancement. (F) Chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids. (F.a) Axial FLAIR reveals diffuse, patchy increased T2 signal in the pons and (F.b) Axial T1 post-contrast reveals associated nodular enhancement.
Serology for MOG IgG was performed in 33 patients, and 2 (6.1%) were positive (1 with unknown titre, 1 with high titre). The patient with unknown titre MOG IgG had clinical and radiologic features in keeping with a diagnosis of MOGAD. The patient with high titre MOG IgG had a pre-existing diagnosis of RRMS and no prior testing for MOG IgG. Clinical and radiologic features were in keeping with an MS relapse, rather than a new diagnosis of MOGAD. Serology for AQP4 IgG was performed in 34 patients, and 2 (5.9%) were positive (1 with high titre, 1 with weak titre). The patient with high titre AQP4 IgG had clinical and radiologic features in keeping with a diagnosis of NMOSD. 18 The patient with weak titre AQP4 IgG presented with short segment TM and was positive for MOG IgG (unknown titre as indicated above). This patient received a diagnosis of MOGAD and repeat AQP4 IgG was negative.
Twenty patients underwent a lumbar puncture for CSF analysis (Table 3). This revealed a lymphocytic pleocytosis (WBC > 5 per mm3 with lymphocytic predominance) in 8 (40.0%). All 20 (100.0%) patients had normal CSF glucose (>60% serum glucose). Eight (40.0%) had elevated CSF protein (>0.45 g/L), but only 5 (25.0%) had elevated CSF protein when adjusted for age. 30 Oligoclonal bands were tested in 17 patients and 7 (41.2%) had CSF specific oligoclonal bands (3 new diagnosis of RRMS, 3 post-vaccine TM, 1 MS relapse). CSF IgG index was assessed in 15 patients and elevated in 3 (20.0%).
Table 3.
Serologic and cerebral spinal fluid results.
| Patients with CNS inflammatory event, n = 38 a | |
|---|---|
| Serologic results, n (%) | |
| Positive MOG IgG | 2/33 (6.1) |
| Positive AQP4 IgG | 2/34 (5.9) |
| CSF results, n (%) | |
| Elevated WBC (WBC > 5 per mm3) | 8/20 (40.0) |
| Lymphocytic pleocytosis (WBC > 5 per mm3 with lymphocytic predominance) | 8/20 (40.0) |
| Elevated protein (>0.45 g/L) | 8/20 (40.0) |
| Elevated protein (adjusted for age) | 5/20 (25.0) |
| Normal glucose (>60% serum glucose) | 20/20 (100.0) |
| CSF specific oligoclonal bands | 7/17 (41.2) |
| Elevated IgG index (>70) | 3/15 (20.0) |
Abbreviations: CNS = central nervous system; MOG = myelin oligodendrocyte glycoprotein; IgG = immunoglobulin G; AQP4 = aquaporin 4; CSF = cerebral spinal fluid; WBC = white blood cell.
Serum/CSF results not available for all patients, with sample size shown for each variable based on available data.
Patients were followed for a median of 299 days (range 6–473). Diagnoses at last available follow-up were new onset RRMS (n = 16), post-vaccine TM (n = 7), clinically isolated syndrome (CIS) (n = 5), MS relapse (n = 4), post-vaccine tumefactive demyelination (n = 2), MOGAD (n = 1), NMOSD (n = 1), PACA (n = 1) and chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids (CLIPPERS) (n = 1). All four patients with pre-existing RRMS had an MS relapse. Twenty-two (57.9%) received acute treatment with intravenous (IV) methylprednisolone (n = 16), high-dose pulse oral prednisone (n = 5) or oral dexamethasone (n = 1). The remaining 16 (42.1%) did not receive acute treatment. Of those who received acute treatment, eight had an inadequate response and went on to receive plasma exchange therapy (PLEX) (n = 5), repeat IV methylprednisolone (n = 2) or IV immunoglobulin (n = 1). Sixteen (42.1%) patients completed a tapering course of oral prednisone. At the time of last follow-up, 78.9% had partial recovery (n = 30), 18.4% returned to baseline (n = 7) and 2.6% had worsening (n = 1). The median follow-up EDSS score was 2 (range 0–6.5) and was performed at a median of 141 (range 6–473) days from symptom onset.
At the time of last follow-up, 21 (55.3%) were on DMT. This included three patients with pre-existing RRMS, who were switched to a different DMT (glatiramer acetate to ofatumumab (n = 1), interferon beta-1a to ocrelizumab (n = 1) and ocrelizumab to cladribine (n = 1)) and 17 with a new diagnosis of MS/CIS who were started on a range of DMTs (Table 4). The patient with PACA was started on cyclophosphamide. One patient with NMOSD was initially started on mycophenolate mofetil, but this was discontinued due to oral ulcers. At the time of last follow-up, the patient was waiting to start rituximab. The remaining 16 (42.1%) did not start long-term treatment (7 post-vaccine TM, 3 CIS, 2 tumefactive demyelination, 1 RRMS, 1 MS relapse, 1 MOGAD and 1 CLIPPERS). Table 4 outlines diagnosis, treatment and outcome data.
Table 4.
Diagnosis, treatment and outcome.
| Patients with CNS inflammatory event, n = 38 | |
|---|---|
| Duration of follow up, median days (range) | 299 (6–473) |
| Diagnosis at last follow up, n (%) | |
| RRMS | 16 (42.1) |
| Post-vaccine TM | 7 (18.4) |
| CIS | 5 (13.2) |
| MS relapse | 4 (10.5) |
| Post-vaccine tumefactive demyelination | 2 (5.3) |
| MOGAD | 1 (2.6) |
| NMOSD | 1 (2.6) |
| PACA | 1 (2.6) |
| CLIPPERS | 1 (2.6) |
| Acute treatment 1, n (%) | |
| IV methylprednisolone | 16 (42.1) |
| High dose pulse PO prednisone | 5 (13.2) |
| PO dexamethasone | 1 (2.6) |
| None | 16 (42.1) |
| Acute treatment 2, n (%) | |
| PO prednisone taper | 16 (42.1) |
| PLEX | 5 (13.2) |
| IV methylprednisolone | 2 (5.3) |
| IVIG | 1 (2.6) |
| None | 14 (36.8) |
| EDSS at last follow up, median (range) | 2 (0–6.5) |
| Time of last EDSS from symptom onset, median days (range) | 141 (6–473) |
| Outcome at last follow up, n (%) | |
| Partial recovery | 30 (78.9) |
| Baseline | 7 (18.4) |
| Worsening | 1 (2.6) |
| DMT by diagnosis at last follow up, n (%) | |
| RRMS | |
| Ocrelizumab | 4/16 (25.0) |
| Dimethyl fumarate | 4/16 (25.0) |
| Ofatumumab | 3/16 (18.8) |
| Glatiramer acetate | 2/16 (12.5) |
| Natalizumab | 1/16 (6.3) |
| Unknown | 1/16 (6.3) |
| None | 1/16 (6.3) |
| Post-vaccine TM | |
| None | 7/7 (100.0) |
| CIS | |
| Glatiramer acetate | 2/5 (40.0) |
| None | 3/5 (60.0) |
| MS relapse | |
| Ofatumumab | 1/4 (25.0) |
| Cladribine | 1/4 (25.0) |
| Waiting to start ocrelizumab | 1/4 (25.0) |
| None | 1/4 (25.0) |
| Post-vaccine tumefactive demyelination | |
| None | 2/2 (100.0) |
| MOGAD | |
| None | 1/1 (100.0) |
| NMOSD | |
| Waiting to start rituximab | 1/1 (100.0) |
| PACA | |
| Cyclophosphamide | 1/1 (100.0) |
| CLIPPERS | |
| None | 1/1 (100.0) |
Abbreviations: CNS = central nervous system; RRMS = relapsing remitting multiple sclerosis; TM = transverse myelitis; CIS = clinically isolated syndrome; MOGAD = myelin oligodendrocyte glycoprotein antibody disease; NMOSD = neuromyelitis optica spectrum disorder; PACA = primary autoimmune cerebellar ataxia; CLIPPERS = chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids; IV = intravenous; PO = by mouth; PLEX = plasmapheresis; IVIG = intravenous immunoglobulin; EDSS = expanded disability status scale; DMT = disease-modifying therapy.
Repeat MRI brain and/or spinal cord was performed in 28 patients at a median of 180 days (range 39–405) from symptom onset (Table 2). Compared to the initial MRI, 35.7% had improvement (n = 10), 32.1% had worsening (n = 9), and 32.1% had stable lesion burden (n = 9).
Data on subsequent vaccination were available for 34 patients (Table 5). Sixteen (47.1%) went on to have subsequent COVID-19 vaccination (10 had one additional vaccine and 6 had two additional vaccines) (12 RRMS, 2 CIS, 1 MOGAD and 1 tumefactive demyelination). Following subsequent vaccination, 14 (87.5%) had no adverse events and 2 (12.5%) experienced new or recurrent neurological symptoms (both had new diagnosis of RRMS after a prior vaccine). The first patient developed focal numbness of the fifth digit 21 days after a second vaccine dose. It was unclear if this represented a relapse, symptom recrudescence or focal nerve injury, but given the mild nature of symptoms the patient did not require acute therapy. The second patient experienced recurrent right eye pain, vision loss, bilateral leg weakness, bilateral foot numbness and cognitive disturbance 2 days after receiving a second vaccine dose. Repeat MRI did not reveal radiologic evidence of disease activity, and this event was suspected to be symptom recrudescence, rather than a new demyelinating attack.
Table 5.
Subsequent COVID-19 vaccination.
| Patients with CNS inflammatory event, n = 38 a | |
|---|---|
| Received subsequent COVID-19 vaccine, n (%) | |
| Yes | 16/34 (47.1) |
| No | 18/34 (52.9) |
| Outcome following subsequent COVID-19 vaccine, n (%) | |
| No adverse events | 14/16 (87.5) |
| New or recurrent neurological symptoms | 2/16 (12.5) |
| Symptom recrudescence | 1/16 (6.3) |
| Unknown | 1/16 (6.3) |
Abbreviations: CNS = central nervous system.
Not available for all patients, with sample size shown for each variable based on available data.
Discussion
We identified 38 patients with acute CNS inflammation following COVID-19 vaccination. To our knowledge, this represents the largest cohort study to date describing clinical, radiologic, laboratory and outcome data. Our study revealed a female predominance and median age of 39 years, which is similar to previous reports.6,7 Unlike previous studies, our cohort included only a handful of patients with pre-existing immune-mediated disease. 27 Although our inclusion criteria allowed the identification of patients presenting with neurological symptoms up to 60 days following COVID-19 vaccination, patients in our cohort presented after a median of only 15 days. The Pfizer-BioN-Tech vaccine was the most common vaccine received prior to symptom onset, followed by Moderna and AstraZeneca. This likely reflects differences in the availability of COVID-19 vaccines in Ontario at the time of the study and does not imply that CNS inflammatory events are more likely to occur with the Pfizer-BioN-Tech vaccine. 2
Numbness and weakness were the most common symptoms in our cohort, and TM was the predominant clinical syndrome. It is unclear if post-vaccination CNS inflammatory events are more likely to affect the spinal cord than the brain or if inflammatory events involving the spinal cord are more likely to result in symptoms that bring patients to medical attention. Our cohort had a median follow-up duration of 299 days. The most common diagnosis at last follow-up was new onset RRMS, followed by post-vaccine TM, CIS, MS relapse, post-vaccine tumefactive demyelination, MOGAD, NMOSD, PACA and CLIPPERS. Although CLIPPERS has been reported following influenza vaccination, this is the first report of CLIPPERS following COVID-19 vaccination that we are aware of. 31 To our knowledge, this is also the first report of PACA following COVID-19 vaccination; however, vaccination against several agents has been known to precede the development of other cerebellar disorders, namely paediatric acute cerebellar ataxia. 32 Patients in our cohort generally responded well to high-dose oral or IV steroids; however, some required additional acute treatment. At the time of last follow-up, most patients had partial recovery, with some returning to baseline and only a couple with worsening symptoms. Just over half of the patients started DMT, which was determined by their diagnosis and disease severity.
We assessed the effects of subsequent COVID-19 vaccination in our cohort, which was given to patients with a range of diagnoses including RRMS, CIS, MOGAD and tumefactive demyelination. Although two patients with RRMS experienced new or recurrent neurological symptoms, there was no definitive new inflammatory activity associated with subsequent vaccination. This reflects existing data demonstrating that COVID-19 vaccination is safe in MS and MOGAD, with no increased risk of relapse activity following vaccination compared to other time periods.33,34 None of the patients with post-vaccine TM received subsequent vaccination in this study. Given the absence of features to suggest MS or other neuroinflammatory disease in this group, it was felt that an isolated TM was more likely to be related to the vaccine, which may have prevented patients from receiving subsequent vaccination in clinical practice.
There are limitations to our study. This was a retrospective observational study at a single centre. We cannot determine whether the rate of CNS inflammatory events was higher than expected. The rate of COVID-19 vaccination in the eligible general population was high during the time of the cases, with 90.5% having received one dose, 87.0% having received two doses, and 51.0% having received three doses in Ontario by 15 September 2022. 35 The BARLO MS Centre is a tertiary referral centre, and we could not capture an accurate denominator for the cases in this study. Furthermore, we could not accurately compare the incidence of cases in our study to the usual incidence of cases seen at our centre prior to March 2021 due to several factors that have increased referral rates over time. Importantly, our study cannot determine a causal relationship between the COVID-19 vaccines and CNS inflammatory events. The presence of T1 black hole lesions in six patients with a new diagnosis of RRMS and one with CIS suggests that these individuals may have had pre-existing clinically silent disease activity. Similarly, the COVID-19 vaccine may have unmasked previously silent disease in patients with a new diagnosis of RRMS or CIS who met dissemination in space criteria on MRI but did not have a multifocal clinical presentation. This hypothesis is supported by prior studies suggesting that in general, vaccines may accelerate the transition from subclinical to overt demyelinating disease without a longer-term or causal association 25 and that COVID-19 vaccines only have a weak association with CNS demyelinating diseases, with a low risk similar to other viral vaccines. 26 The BARLO MS Centre evaluates patients from a diverse population across Toronto and Ontario. However, data were not readily available on race, ethnicity or socioeconomic status, which may limit generalizability. The inclusion of patients presenting within 60 days after vaccination may underestimate or overestimate the number of individuals with a CNS inflammatory event that is temporally related to the vaccine.
In summary, we present 38 cases of acute CNS inflammation following COVID-19 vaccination. Clinical, laboratory and imaging features were heterogenous, and most patients had a favourable outcome. Repeat COVID-19 vaccination was not associated with recurrent CNS inflammatory events in our cohort. Our study could not determine whether the rate of CNS inflammatory events was higher than expected, and large prospective controlled studies are needed to evaluate for a relationship between COVID-19 vaccines and acute CNS inflammation. Currently, it seems that the benefits of vaccination against COVID-19 outweigh the risk of developing CNS inflammatory disease.
Acknowledgments
The authors thank the BARLO MS Centre and its clinicians for the identification of study participants and access to clinical records.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iDs: Sydney Lee  https://orcid.org/0000-0002-8591-2062
https://orcid.org/0000-0002-8591-2062
Kristen M Krysko  https://orcid.org/0000-0003-0090-597X
https://orcid.org/0000-0003-0090-597X
Contributor Information
Sydney Lee, Department of Medicine, University of Toronto, St. Michael’s Hospital, Toronto, ON, Canada.
Alexandra Muccilli, Division of Neurology, Department of Medicine, BARLO MS Centre, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada.
Raphael Schneider, Division of Neurology, Department of Medicine, BARLO MS Centre, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada Li Ka Shing Knowledge Institute, Toronto, ON, Canada.
Daniel Selchen, Division of Neurology, Department of Medicine, BARLO MS Centre, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada.
Kristen M Krysko, Division of Neurology, Department of Medicine, BARLO MS Centre, St. Michael’s Hospital, University of Toronto, Toronto, ON, Canada Li Ka Shing Knowledge Institute, Toronto, ON, Canada.
References
- 1.Watson OJ, Barnsley G, Toor J, et al. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect Dis 2022; 22(9): 1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Government of Canada. Vaccines for COVID-19: Free vaccines are available for everyone in Canada, https://www.canada.ca/en/public-health/services/diseases/coronavirus-disease-covid-19/vaccines.html (2022, accessed 14 September 2022).
- 3.Heinz FX, Stiasny K.Profiles of current COVID-19 vaccines. Wien Klin Wochenschr 2021; 133(7–8): 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pormohammad A, Zarei M, Ghorbani S, et al. Efficacy and safety of COVID-19 vaccines: A systematic review and meta-analysis of randomized clinical trials. Vaccines 2021; 9: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khayat-Khoei M, Bhattacharyya S, Katz J, et al. COVID-19 mRNA vaccination leading to CNS inflammation: A case series. J Neurol 2022; 269: 1093–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toljan K, Amin M, Kunchok A, et al. New diagnosis of multiple sclerosis in the setting of mRNA COVID-19 vaccine exposure. J Neuroimmunol 2022; 362: 577785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havla J, Schultz Y, Zimmermann H, et al. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J Neurol 2022; 269(1): 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maniscalco GT, Manzo V, Di Battista ME, et al. Severe multiple sclerosis relapse after COVID-19 vaccination: A case report. Front Neurol 2021; 12: 721502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Román GC, Gracia F, Torres A, et al. Acute transverse myelitis (ATM): Clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222). Front Immunol 2021; 12: 653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tahir N, Koorapati G, Prasad S, et al. SARS-CoV-2 vaccination-induced transverse myelitis. Cureus 2021; 13(7): 16624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pagenkopf C, Südmeyer M.A case of longitudinally extensive transverse myelitis following vaccination against COVID-19. J Neuroimmunol 2021; 358: 577606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao JJ, Tseng HP, Lin CL, et al. Acute transverse myelitis following COVID-19 vaccination. Vaccines 2021; 9: 1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mumoli L, Vescio V, Pirritano D, et al. ADEM anti-MOG antibody-positive after SARS-CoV2 vaccination. Neurol Sci 2022; 43: 763–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escolà JK, Deuschl C, Junker A, et al. MOG antibody–associated encephalomyelitis mimicking bacterial meningomyelitis following ChAdOx1 nCoV-19 vaccination: A case report. Ther Adv Neurol Disord 2022; 15: 1070684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anamnart C, Tisavipat N, Owattanapanich W, et al. Newly diagnosed neuromyelitis optica spectrum disorders following vaccination: Case report and systematic review. Mult Scler Relat Disord 2022; 58: 103414. [DOI] [PubMed] [Google Scholar]
- 17.Badrawi N, Kumar N, Albastaki U.Post COVID-19 vaccination neuromyelitis optica spectrum disorder: Case report & MRI findings. Radiol Case Rep 2021; 16(12): 3864–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuntz S, Saab G, Schneider R.Antibody-positive neuromyelitis optica spectrum disorder after second COVID-19 vaccination: A case report. SN Compr Clin Med 2022; 4(1): 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao L, Ren L.Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: A case report. Acta Neurol Belg 2022; 122(3): 793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rinaldi V, Bellucci G, Romano A, et al. ADEM after ChAdOx1 nCoV-19 vaccine: A case report. Mult Scler 2022; 28(7): 1151–1154. [DOI] [PubMed] [Google Scholar]
- 21.Nagaratnam SA, Ferdi AC, Leaney J, et al. Acute disseminated encephalomyelitis with bilateral optic neuritis following ChAdOx1 COVID-19 vaccination. BMC Neurol 2022; 22: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zlotnik Y, Gadoth A, Abu-Salameh I, et al. Case report: Anti-LGI1 encephalitis following COVID-19 vaccination. Front Immunol 2022; 12: 813487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaulen LD, Doubrovinskaia S, Mooshage C, et al. Neurological autoimmune diseases following vaccinations against SARS-CoV-2: A case series. Eur J Neurol 2022; 29(2): 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pohl D, Alper G, van Haren K, et al. Acute disseminated encephalomyelitis. Neurology 2016; 87: S38–S45. [DOI] [PubMed] [Google Scholar]
- 25.Langer-Gould A, Qian L, Tartof SY, et al. Vaccines and the risk of multiple sclerosis and other central nervous system demyelinating diseases. JAMA Neurol 2014; 71(12): 1506–1513. [DOI] [PubMed] [Google Scholar]
- 26.Kim JE, Park J, Song TJ.A disproportionality analysis for the association of central nervous system demyelinating diseases with COVID-19 vaccination using the World Health Organization pharmacovigilance database. Mult Scler 2022; 28(13): 2112–2123. [DOI] [PubMed] [Google Scholar]
- 27.Ismail II, Salama S. A systematic review of cases of CNS demyelination following COVID-19 vaccination. J Neuroimmunol 2022; 362: 577765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agmon-Levin N, Kivity S, Szyper-Kravitz M, et al. Transverse myelitis and vaccines: A multi-analysis. Lupus 2009; 18(13): 1198–1204. [DOI] [PubMed] [Google Scholar]
- 29.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 30.Breiner A, Moher D, Brooks J, et al. Adult CSF total protein upper reference limits should be age-partitioned and significantly higher than 0.45 g/L: A systematic review. J Neurol 2019; 266(3): 616–624. [DOI] [PubMed] [Google Scholar]
- 31.Saénz-Silva J, Ordinola Navarro A.CLIPPERS syndrome after vaccination. An unusual ASIA presentation. Reumatol Clin. Epub ahead of print 4 December 2021. DOI: 10.1016/j.reuma.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Connolly AM, Dodson WE, Prensky AL, et al. Course and outcome of acute cerebellar ataxia. Ann Neurol 1994; 35(6): 673–679. [DOI] [PubMed] [Google Scholar]
- 33.Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021. Mult Scler 2021; 27(6): 864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dinoto A, Sechi E, Ferrari S, et al. Risk of disease relapse following COVID-19 vaccination in patients with AQP4-IgG-positive NMOSD and MOGAD. Mult Scler Relat Disord 2022; 58: 103424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.COVID-19 Tracker Canada. Ontario vaccination data, https://covid19tracker.ca/provincevac.html?p=ON (2022, accessed 14 September 2022).