Abstract
Hexavalent chromium, toxic heavy metal, among the top-rated environmental contaminants, is declared a potent endocrine disruptor in humans and animals. The present study was planned to find harmful effects on the reproductive system caused by Cr (VI) and the ameliorative effect of Nigella sativa and Nigella sativa-mediated AgNP on male mice (Mus musculus). In the present study, known infertility medicine, clomiphene citrate is also used as a positive control. The main objective of the present study was to assess the ameliorative potential of oral administration of a dose of 50 mg/kg BW clomiphene citrate (control), AgNP via chemical synthesis, Nigella sativa seed extract, and Nigella sativa-mediated AgNP against the Cr (VI) at the dose of 1.5 mg/kg BW from K2Cr2O7 orally induced toxicity over eight weeks on the reproductive performance of male albino mice. Nigella sativa mediated AgNPs were characterized by UV, SEM, FTIR, and XRD. The histological analysis, smear study, antioxidant capacity test, and hormone analysis were conducted by blood samples of albino mice. Cr exposed groups showed a significant decrease in sperm head breadth (5.29 ± 0.54 µ) and length (19.54 ± 1.18 µ), middle piece length, tail length, LH (1.65 ± 0.15 ng/mL), testosterone (2.63 ± 0.29 ng/mL), SOD (61.40 ± 2.48 mmol/mL), CAT (87.40 ± 6.01 mmol/mL), GSH (1.54 ± 0.09 µmol/mL), and no of spermatogonia (1.22 ± 0.25), and spermatocytes (2.33 ± 0.943). However, FSH level (160.00 ± 4.98 ng/mL), seminiferous tubule CSA (1094.69 ± 49.76 mm2), size of spermatogonia (41.30 ± 1.24 µ), and spermatocytes (26.07 ± 1.34 µ) were significantly increased. Administration of Nigella sativa and Nigella sativa-mediated AgNPs reduced the toxicity.
Keywords: Hexavalent chromium, Nigella sativa, Testes, Histopathology, Micrometry, Antioxidant potential
Abbreviations: ROS, Reactive oxygen species; Cr (VI), Hexavalent chromium; CC, Clomiphene citrate; FSHR, Follicle stimulating hormone receptor; Ar, Androgen receptor; SC, Sertoli cell; SOD, Superoxide dismutase; THQ, Thymohydroquinone; DTQ, Dithymoquinone; THY, Thymol; TQ, Thymoquinone; AgNP, Silver Nanoparticles; K2Cr2O7, Potassium dichromate; GSH, Glutathione; CAT, Catalase; GSI, Gonadosomatic index; NS, Nigella sativa; CSA, Cross sectional area; ST, Seminiferous tubule; LCs, Leydig's cells; LHR, Luteinizing hormone receptor; PRLR, Prolactin receptor; StAR, Steroidogenic acute regulatory
1. Introduction
Chromium is the twenty-first most abundant element existing in nature (Genchi et al., 2021) with an average concentration of 93.4 mg/kg in the Earth’s crust (Al-Faili & Al-Jumaily, 2022). Cr (III) is used as a micronutrient to improve health conditions, however, Cr (VI) is mutagenic and classified group 1 carcinogenic species (DesMarias & Costa, 2019). Chromium is associated with reproductive abnormalities including decreased spermatogenesis, and cellular degeneration in seminiferous tubules, especially the outer layer. The loss of weight in many parts was observed including the seminal vesicle, testis, and preputial gland leading to decrease in fertility (Navin & Aruldhas, 2021). Chromium (VI) promotes the generation of ROS in the cell that triggers the breakage of DNA double-strand, which results in a cascade of apoptosis (Lv et al., 2018) along with a global increase in H3K9me3 and H3K27me3 (Lv et al., 2018). As testis is enclosed in a membrane that is highly enriched with poly-unsaturated fatty acid so vulnerable to oxidative stress (Castellini et al., 2019). Oxidative damage affects the permeability of this polyunsaturated fatty acid membrane causing alteration in function (Rawat et al., 2021).
The DNA damage induced by chromium may lead to the repair of DNA followed by DNA replication or the initiation of a signal for apoptosis leading to programmed death of the cell when DNA damage is not repairable or beyond the cell's capability (Handa & Jindal, 2020). In males, hormonal balance is altered by excessive use of Cr as it disrupts the steroidogenic machinery, and hypothalamus-hypophysial-testicular axis, affecting the spermatogenesis and sperm maturation process, thus potential agent for male infertility (Navin & Aruldhas, 2021). The blood-testis barrier is also affected by Cr (VI) altering the transcriptional expression of tight junction signaling molecules including tight junction protein 1(TJP1), occluding (Ocln), and vimentin (Vim) (Navin & Aruldhas, 2021) and represses the receptors in a cell like a follicle stimulating hormone receptor (Fshr) and androgen receptor (Ar) that play important role in maturation and functioning of Sertoli cell (Shobana et al., 2020). Chromium promotes the synthesis of cholesterol in the liver by upregulation of enzymes involved in cholesterol synthesis leading to the accumulation of cholesterol. Chromium also provides a compensatory mechanism of protein and carbohydrate depletion as fatty acid synthesis increases along with the stimulation of isocitrate dehydrogenase and promoting lipogenesis in liver tissues (Mohammed & Abd-Elwahab, 2020).
Clomiphene citrate (CC) is a demanding modulator for estrogen receptors that improve the release of gonadotrophin from the pituitary gland while blocking the negative feedback to the hypothalamus (Guo et al., 2020). Thus CC is used to treat male hypogonadism and infertility (Herzog, Nguyen, Soubra, & Hellstrom, 2020) by elevating the level of FSH and LH (Ameli et al., 2019). In young males, hypogonadism is treated with clomiphene citrate which improves the deficiency of serum testosterone without any interruption in spermatogenesis (Habous et al., 2018). In testosterone therapy, exogenous testosterone is administered that improves testosterone level in serum in men, however, it is not effective for retaining the spermatogenesis process in seminiferous tubules (Ameli et al., 2019). However, according to already reported data the efficacy of CC in men has been mixed (Guo et al., 2020).
Medicinal plants are nowadays in demand. Different plant parts can be used for drug synthesis due to the presence of (Jamshidi-Kia, Lorigooini, & Amini-Khoei, 2018) phenols and flavonoids (Shahbazi et al., 2018) the secondary metabolites, and the largest group of phytochemicals with antioxidant properties (Tungmunnithum et al., 2018). Flavonoids are diverse compounds, potent agents that can interfere with cellular metabolism and proliferation and hence can control the progression of cancer. As flavonoids are natural plant products, can be used as natural sources of mild antioxidants in diet to maintain health (Selamoglu, 2017a, Selamoglu, 2017b, Selamoglu, 2018).
Long before the world of modern medicine knew about Nigella sativa (NS), Holly Prophet Hazrat Muhammad SAAW had already highlighted and recommended Nigella sativa seeds to treat disease (Sapitri & Khaeruman, 2021). Their seeds contain volatile oil, thymohydroquinone (THQ), dithymoquinone (DTQ), thymol (THY), thymoquinone (TQ), 4-terpineol, sesquiterpene longifolene, p-cymene and carvacrol (Samarghandian et al., 2018).
Nanotechnology is one of the most powerful and promising techniques of the 21st century (Joy, 2020). Chemically synthesized silver nanoparticles (AgNPs) are responsible for the lower level of testosterone in male rats (Parang & Moghadamnia, 2018). Recently, the green synthesis of nanomaterials are more points of interest by using biological organisms such as microbes, plants, animals, and fungi are eco-friendly substitutes with low cost and mild conditions (Jyoti et al., 2021). In plants, phytochemicals are used as reducing and stabilizing agents to synthesize nanoparticles (Siddiqi & Husen, 2020).
In our society infertility has become a major issue with detrimental effects on individual, social and economic contexts. Chemotherapy may induce oxidative damage that’s why herbal therapy (Nigella sativa seed extract) and nanotechnology (AgNP biosynthesized by Nigella sativa) are used in the present study to investigate their ameliorative potential against the toxic effect of Cr (VI) on male reductive performance.
2. Materials and methods
2.1. Ethical approval
For this study protocol was approved by the institutional bioethical committee of the Government College University, Lahore with No. GCU-IIB-364 dated 06–10–2020. The subject for this study was albino mice who were given chromium, and Nigella sativa was used as a mitigating agent.
2.2. Chemicals
The tested chemical potassium dichromate with a purity of 99 % was purchased from Merck (Merck, Darmstad, Germany). Nigella sativa seeds were used in the present study. All other chemicals used in the experiment were of analytical grade. The stock solutions were prepared with distilled water. All working solutions were freshly prepared from stock solution with distilled water.
2.3. Animals and their maintenance
Sixty male albino mice of 2 to 3 months old, weighed 25 to 30 g, reproduced at Animal's House (for mice) of The Department of Zoology, GC University Lahore was used. All treatments and mice rearing was carried out in line with the guidelines of the Institutional Bioethical Committee, GC University Lahore (Punjab, Pakistan). Mice were kept group-wise in cages and allowed to acclimatize for two weeks in the animal house before the start of an experiment. The animals were kept at 23 ± 2 °C, 45–50 % humidity and 12 hrs. dark-light cycle. The animals received feed and water under controlled conditions (ad-labitum). Mice were subjected to standard for 8 weeks containing soya protein, calcium carbonate, cellulose, potassium citrate, potassium phosphate, vitamin mix, and mineral mix, macronutrients protein 14 % per gram, fat 11 % per gram, carbohydrate 63 % per gram and fiber 5 % per gram.
2.4. Preparation of chromium solution
A stock solution of chromium 1000 ppm was prepared by dissolving and accurately weighed 2.828 g of K2Cr2O7 (99.9 %) of analytical grade in 1000 mL of distilled water (Joshi, Menon, & Joshi, 2019). 1.5 mg/kg BW dose was prepared from the stock solution.
2.5. Preparation of dose of clomiphene citrate
A clomiphene citrate tablet of 50 mg was purchased from Clinix medicine, Choburji, Lahore, Pakistan. The tablet was ground in pestle mortar and dissolved in distilled water to prepare a dose of 50 mg/kg BW having a concentration of 1.6 mg per 0.2 mL for 30 g weight.
2.6. Preparation of kalonji (Nigella sativa) seed extract
The extraction of kalonji seeds was done according to the method described by (Singh et al., 2019) and (Habib & Choudhry, 2021) with few modifications. About 500 g of Nigella sativa seeds were purchased from the local market from Akabari Store Lahore, Pakistan, washed with water, dried at 25–30 °C, and ground into a fine powder via an electric grinder. Ten g powder was mixed with 100 mL ethanol and shacked in an electric shaker for 24 hrs. The extract was filtered by using Whatman paper 1 and evaporated into rotavapour. The thick black paste was stored for further use at 4 °C. The extract was dissolved in distilled water before giving it to mice at the dose of 50 mg/kg BW had a concentration of 1.6 mg per 0.2 mL for 30 g weight (Babar et al., 2018).
2.7. Chemical synthesis of silver nanoparticles
In the chemical synthesis of AgNP, silver nitrate (AgNO3) and trisodium citrate were used according to the procedure described below. Silver nitrate solution 50 mL with the concentration of 0.001 M was heated at 90 °C for 5 min. 5 mL of 1 % trisodium citrate was added drop in AgNO3 solution. The mixture was stirred and heated on a hot plate until a pale yellow color appeared. This color change was due to the reduction of Ag + ions and the formation of AgNPs. The nanoparticles were dried in an electric oven and stored at 4 °C for further use (Rashid et al., 2013, Yerragopu et al., 2020). The AgNPs were dissolved in distilled water before giving it to mice at the dose of 50 mg/kg BW had a concentration of 1.6 mg per 0.2 mL for 30 g weight. The dose was selected from the already published article with slight modifications.
2.8. Green synthesis of silver nanoparticles
Kalonji seed 5 g powder was dissolved into 100 mL of distilled water under continuous stirring. The mixture was turned to boil for 20 min at 100 °C. Then the mixture was left to cooled and filtered with Whatman paper 1 and stocked at 4 °C for the next level of processing. To prepare AgNPs, AgNO3 solution with concentration 0.1 mM was prepared. Silver nitrate solution 100 mL was mixed with Nigella sativa seed extract 10 mL by using a stirrer for 10 min. Then, the mixture was warmed up for 120 min at 80 °C and finally kept in dark for 24 hrs at 25 °C. The color of the mixture turned light brown to dark brown due to reduced silver (Ag) ions. The further mixture was centrifuged for 10 min at 12000 rpm. Later thrice washed with ethanol followed by washing of pellets with distilled water thrice to remove plant impurities. Nanoparticles were dried at 70 °C for 12 hrs by placing them in an oven. Finally, AgNPs were collected and stored at 4 °C for further use (Chand et al., 2021).
The AgNPs were dissolved in distilled water before giving it to mice at the dose of 50 mg/kg BW had a concentration of 1.6 mg per 0.2 mL for 30 g weight. The dose was selected from the already published article with slight modifications (Hamad, Shnawa, Jalil, & Ahmed, 2022).
2.9. Characterization of AgNPs (Nigella sativa)
The reduction of silver ions was detected and documented by using a UV–vis spectrophotometer (TECHCOMP (Shimdzu, Japan), in the range from 300 to 700 nm. Fourier transform infrared spectroscopy (FTIR) at a resolution of 4 cm − 1, in the range of 400 to 4000 cm − 1 at 25 °C by utilizing Bruker instrument (Model NO. ALPHA-P) to detect biomolecules and functional groups. The shape and size of AgNPs were examined by SEM JEOL-JSM 6480. The X-ray diffraction patterns were observed by utilizing XRD-D8 Discover, Bruker, Germany (Almatroudi et al., 2020).
2.10. In vivo dosing and experimental design for animals
Albino mice (Mus musculus) were distributed into 12 groups of 5 mice randomly each as follows:
Group 1: control (Cont), ad-labitum drinking water (60 days).
Group 2: Chromium (Cr), 1.5 mg/kg BW, orally from K2Cr2O7 once a day (60 days).
Group 3: AgNP, chemically synthesized AgNPs 50 mg/kg BW, orally once a day (60 days).
Group 4: Clomiphene citrate (CC) 50 mg/kg BW, orally once a day (60 days).
Group 5: NS, Kalonji extract 50 mg/kg BW, orally once a day (60 days).
Group 6: NS + NP, kalonji mediated AgNPs 50 mg/kg BW, orally once a day (60 days).
Group 7: CC (P), Cr 1.5 mg/kg BW, orally from K2Cr2O7 + CC 50 mg/kg BW, orally once a day (60 days).
Group 8: NS (P), Cr 1.5 mg/kg BW, orally from K2Cr2O7 + NS extract 50 mg/kg BW, orally once a day (60 days).
Group 9: NS + NP (P), Cr 1.5 mg/kg BW, orally from K2Cr2O7 + kalonji mediated AgNPs 50 mg/kg BW, orally once a day (60 days).
Group 10: CC (T), Cr 1.5 mg/kg, orally from K2Cr2O7 (30 days) continued by CC 50 mg/kg BW, orally once a day (30 days).
Group 11: NS (T), Cr 1.5 mg/kg BW, orally from K2Cr2O7 (30 days) continued by NS extract 50 mg/kg BW, orally once a day (30 days).
Group 12: NS + NP (T), 1.5 mg/kg BW, orally from K2Cr2O7 (30 days) continued by kalonji mediated AgNPs 50 mg/kg BW, orally once a day (30 days) (Supplementary Fig. 1).
2.11. Collection of samples
To collect testes, the mice were euthanized by deep anesthesia on day 60. Both testes were retracted back, into the visceral chamber and detached intact through an incision in the middle of the abdomen. Testes were collected for histopathology and micrometry analysis. The blood sample was collected by venipuncture from the jugular vein on the 61st day of the experiment. The blood sample was shifted in a 3 mL ImuMed vacutainer coated with EDTA and stored at 4 °C for biochemical analysis (Hussain et al., 2020).
To analyze biochemical parameters, blood samples were centrifuged at room temperature at 2000 rpm for 10 min, for serum separation. The supernatant was stored at − 20 °C until analysis.
2.12. General observations
Behavioral changes in mice among all groups were observed and noted on regular basis. Every animal in the study was weighed every week and dose administration was regulated according to their body weight. Weight of reproductive organs including testes, seminal vesicle, and epididymis was noted.
2.13. Preparations of testicular smear
To study sperm morphology testis was cut out into two horizontal parts and quietly crushed with the blunt rod of glass on a plain glass slide. Two to three drops of normal saline were added followed by thorough mixing. This material was used to prepare smears followed by Hematoxylin & Eosin (H & E) staining (Ahmad et al., 2012).
2.14. Sperm micrometry
Photographs of 10 intact, morphologically normal spermatozoa were randomly chosen, from testis smears from every experimental group and were captured at 100 × (oil immersion) with a 5 megapixel (MP) digital camera automatically fixed on a binocular microscope (XSZ 107BN). Different aspects of the spermatozoa including breadth and length of the head, middle piece length, and tail length were measured in Corel DRAW X7 (64-Bit) graphics software via digital images at 100 x. For calibration, the stage micrometer of digital images, captured on the digital and optical specifications selected for photographs of sperms were utilized (Ahmad et al., 2012).
2.15. Histopathology of testes
Testes were fixed in 10 % formalin fixative, later proceeding for de-hydration, embedding in wax, and section cutting in an established way. Histological testicular sections of 6 µ were secured on a manual microtome. These sections were shifted on albumin-coated clear glass slides following H & E staining. Digital images of the testis's histological sections at 10 X and 40 X were obtained to observe the histopathology of testes and to collect histometric/ micrometric data (Ahmad et al., 2012).
2.16. Micrometry
For micrometric measurements from digital photographs at 10 X, randomly chosen nearly round seminiferous tubules (100 cells per group) were traced from each group. To measure the seminiferous tubule cross-sectional area (CSA), the cross-section of the lumen, size of spermatogonium, and spermatocyte gauged measurement of diameter were measured through two square points in every case. The following formula was used to calculate the cross section area (CSA) in each case:
Ten seminiferous tubules and 10 cells were randomly chosen from each group. Results were expressed as square millimeters (mm2) (Ahmad et al., 2012).
2.17. Spermatic cords
Spermatic cords for successive generations from spermatogonia to spermatocytes from 10 spermatic cords were counted in randomly selected seminiferous tubules.
2.18. Hormonal analysis
Quantitative determination of FSH, LH, and testosterone ng/mL level of serum was measured by commercially available diagnostic kits as described in Table 1.
Table 1.
Layout for kits used in the current study.
| Sr No | Biochemical parameter | Specification of Kit | Reference |
|---|---|---|---|
| 1 | Follicle stimulating hormone (FSH) | Immunodiagnostic reagents and Elisa Kit test (DiaMetra kits, Via Giustozzi, Italy | (El‐Demerdash et al. 2019) |
| 2 | Luteinizing hormone (LH) | ELISA(enzyme-linked immunosorbent assay | (Ismael et al., 2017) |
| 3 | Testosterone | enzyme-linked immunosorbent assays (ELISA, Dialab GmbH-testosterone, Austria) | (El‐Demerdash et al. 2019) |
| 4 | Super oxide dismutase (SOD) | Diagnostic kit by RANDOX (Randox Laboratories ltd., Crumlin, Country Antrim, UK | (Dworzański et al., 2020) |
| 5 | Catalase (CAT) | CAT diagnostic (Egypt) | (El-Sebaey et al., 2019) |
| 6 | Reduced glutathione (GSH) | GSH diagnostic (Egypt) | (El-Sebaey et al., 2019) |
2.19. Evaluation of oxidative stress
Superoxide dismutase (SOD), catalase (CAT), and reduced glutathione (GSH) were measured by commercially available diagnostic kits as described in Table 1.
2.20. Gonadosomatic index (GSI)
Gonadosomatic index (GSI = testicular weight/body weight × 100) is the ratio of the testicular weight of mice to the total weight of the body that is the measure of the energy reserves of an animal. For the calculation of GSI, firstly, the weight of testes of mice was calculated and then divided by the body weight of mice.
2.21. Statistical analysis
Statistical data were presented as mean ± SEM, later analyzed statistically by one-way ANOVA. Version 5.0 of GraphPad Prism for windows (GraphPad Software, San Diego, CA, USA) was utilized for analysis. The significant values of P ≤ 0.05 were considered significant.
3. Results
3.1. Characterization of nanoparticles
3.1.1. UV visible spectrum analysis of Nigella sativa mediated silver nanoparticles
The absorption peaks of Nigella sativa mediated AgNPs were recorded through (UV–Vis spectrometer) in the range of 420–480 nm instead of 380–450 nm in chemical synthesis and 278 nm which indicates the formation of nanoparticles as shown in Supplementary Fig. 2.
3.1.2. Scanning electron microscopy analysis of Nigella sativa mediated silver nanoparticles
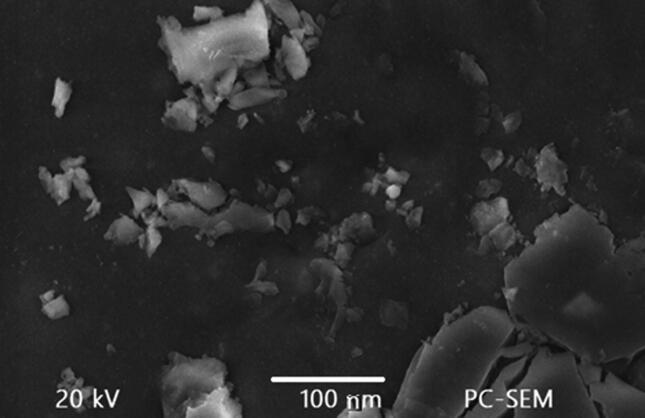
Morphology of Nigella sativa mediated AgNPs was confirmed by scanning electron microscopy. Spherical shaped with some angular morphology often agglomerate in small aggregation with an average 15 nm the size range in Fig. 1.
Fig. 1.
Scanning Electron Microscopy analysis of Nigella sativa silver AgNPs.
3.1.3. Fourier transformed infrared spectroscopy spectra of Nigella sativa mediated silver nanoparticles
The FTIR spectra were observed between ranges of 800 to 4000. cm−1 Nigella sativa seed extract showed the spectral bands at 3274, 3010, 1806, 1590, 1514, 1156, 1053 and 874 cm−1. Whereas conjugation of Ag + ions with Nigella sativa seed extract shifted the intensity of bands at 3306, 2998, 1783, 1577, 1509, 1389, and 1192 cm−1 respectively. These results indicated that Ag + ions did not alter significantly structure of Nigella sativa seed extract by the formation of silver nanoparticles (Supplementary Fig. 3). The peaks observed at 3274 (Nigella sativa seed extract) shifted to 3306 cm−1 (Nigella sativa AgNP) absorption band containing stretching vibrations of OH from water, ketones, aldehydes and hydroperoxides, peak observed at 3010 cm−1 represent the stretching vibrations of cis bond of C C shifted to 2998 cm−1 (Nigella sativa AgNP) represents the region of asymmetric CH bond stretching of the alkanes functional groups, peaks observed at 1806 cm−1 shifted to 1783 cm−1 represent the region of double bonds (C O) stretching of stretching vibrations of the carbonyl group of triglyceride esters functional groups and peak detected at 1590 cm−1 represent C—C stretch of phenyl ring shifted at 1577 cm−1 represent quinoid ring, peak observed at 1514 cm−1 represent vibration of aromatic ring C C shifted to 1509 cm−1 (Supplementary Fig. 4). The bands of vibrations observed in FTIR spectra indicate presence of flavonoids and alkaloids in Nigella sativa. So it is assumed that the proteins and other biomolecules play a vital role in stabilizing, capping, and reducing silver ions into a silver nanoparticle.
3.1.4. X-ray diffraction analysis of Nigella sativa mediated silver nanoparticles
The XRD analysis has executed the determination of crystal structure and size distribution. The XRD data represent four peaks at 2θ values from the range of 20 to 80 of 28.18°, 31.37°, 43.36° and 50.14° correspond to (1 1 1), (2 0 0), (2 2 0), and (3 1 1) respectively, confirming the angular structure of nanoparticles. The other small peaks show the crystal structure of silver ions. The spectrum was compared with JCPD No. 04–0783 (Fig. 2).
Fig. 2.
X-ray Diffraction analysis of Nigella sativa AgNPs.
3.2. General observations
The morphometric analysis including the weight of the body in experimental animals, seminal vesicle, epididymis, and GSI is represented in Table 2. Initial and final body weight was compared, no significant difference was found in initial body weight. However, in final body weight AgNP and CC treated groups showed significant decrease (Table 2). No significant difference was observed in weight of testes, seminal vesicles and epididymis between Cr and treatment groups. GSI in Cr treated group was significantly decreased (0.005 ± 0.0004 g) in comparison to the control (0.009 ± 0.00035 g). In CC, NS and NS + NP gonadosomatic index was significantly increased in comparison to Cr treated group (P ≤ 0.05) (Table 2).
Table 2.
The comparison between animal body weight, gonadosomatic index (GSI), the weight of seminal vesicle, and epididymis, among all the treatment groups.
| Groups |
Parameters |
|||||
|---|---|---|---|---|---|---|
| Initial body weight (g) | Final body weight (g) | Testes weight (g) | Gonadosomatic index (g) | Seminal vesicle weight (g) | Epididymis weight (g) | |
| Cont | 33.75 ± 0.85 | 38.5 ± 1.3 | 0.33 ± 0.01 | 0.009 ± 0.0003 | 0.28 ± 0.006 | 0.146 ± 0.014 |
| Cr | 30.75 ± 1.31 | 25.5 ± 0.9 | 0.12 ± 0.007 | 0.005 ± 0.0004aa | 0.30 ± 0.04 | 0.2 ± 0.02 |
| AgNP | 27 ± 1.08 | 35 ± 1.29bb | 0.27 ± 0.02 | 0.008 ± 0.0007 | 0.2 ± 0.03 | 0.16 ± 0.002 |
| CC | 33.2 ± 0.75 | 37 ± 1.7 cc | 0.29 ± 0.038 | 0.008 ± 0.001 ccc | 0.18 ± 0.030 | 0.19 ± 0.02 |
| NS | 36.50 ± 0.50 | 25.5 ± 0.6 | 0.2 ± 0.007 | 0.009 ± 0.0003dd | 0.2 ± 0.019 | 0.15 ± 0.01 |
| NS + NP | 34.00 ± 2.48 | 38.25 ± 2.93 | 0.310 ± 0.04 | 0.008 ± 0.0006 e | 0.302 ± 0.01 | 0.19 ± 0.003 |
| CC (P) | 34.25 ± 0.853 | 30.75 ± 0.95 | 0.22 ± 0.03 | 0.007 ± 0.0009 | 0.21 ± 0.035 | 0.15 ± 0.01 |
| NS (P) | 30.5 ± 0.65 | 31.4 ± 0.69 | 0.21 ± 0.010 | 0.007 ± 0.0003 | 0.22 ± 0.006 | 0.09 ± 0.013aa |
| NS + NP (P) | 35.75 ± 2.02 | 35.50 ± 2.25 | 0.210 ± 0.018 | 0.0059 ± 0.0003 | 0.2325 ± 0.041 | 0.10 ± 0.013a |
| CC (T) | 32.25 ± 2.62 | 29.75 ± 2.72 | 0.25 ± 0.03 | 0.008 ± 0.0007 | 0.225 ± 0.02 | 0.18 ± 0.01 |
| NS (T) | 29.25 ± 1.65 | 34.00 ± 0.71 | 0.237 ± 0.032 | 0.007 ± 0.001 | 0.2210 ± 0.042 | 0.1678 ± 0.022 |
| NS + NP (T) | 36.00 ± 2.48 | 39.00 ± 3.44 | 0.237 ± 0.015 | 0.0061 ± 0.0003 | 0.2975 ± 0.046 | 0.1630 ± 0.0268 |
Cont (Control); Cr (chromium treated); AgNP (chemically synthesized silver nanoparticles), CC (clomiphene citrate treated); NS (Nigella sativa extract treated); NS + NP’ (Nigella sativa nanoparticles treated); P (prevention); T (treatment).
*Different alphabets show significant difference between Cr treated group and treatment groups (Results represented in terms of mean ± SEM. P ≤ 0.05).
Statistical icons: a, b, c, d, e, f, g, h, i, j, k = P ≤ 0.05, aa, bb, cc, dd, ee, ff, gg, hh, ii, jj, kk = P ≤ 0.01, aaa, bbb, ccc, ddd, eee, fff, ggg, hhh, iii, jjj, kkk = P ≤ 0.001.
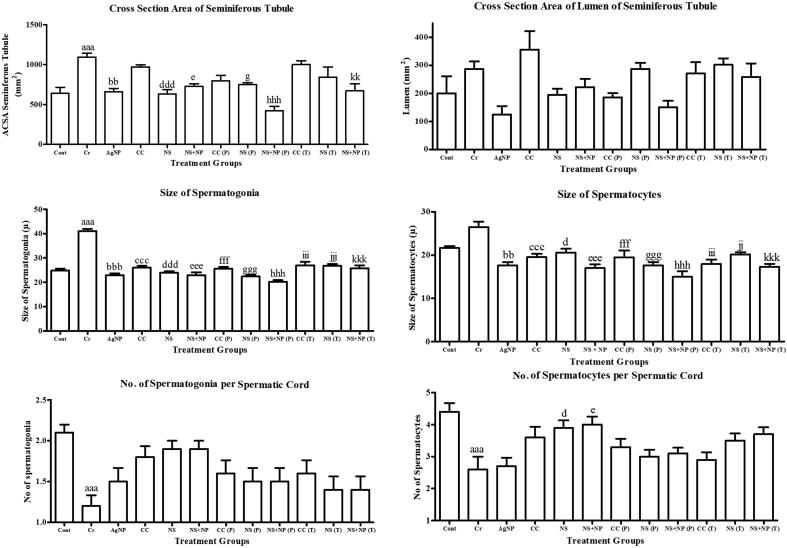
3.3. Micrometry and spermatic cord
The micrometric analysis of testes includes measurement of cross section area (CSA) of the seminiferous tubule, CSA of lumen size, size of spermatogonia, and spermatocytes, head breadth, head length, middle piece length, tail length, and spermatic cords in Fig. 3. A significant increase was observed in CSA of the seminiferous tubule in all Cr treated animals (1094.69 ± 49.76 mm2) in comparison to control group (640.36 ± 73.38 mm2). However, in kalonji, kalonji mediated AgNP, clomiphene citrate and AgNP treated groups, there was no significant difference in comparison to control. The administration of kalonji seed extract and kalonji-mediated AgNPs at a dose rate of 50 mg/kg BW significantly decreased the cross section area of the seminiferous tubule in NS (P): 750.74 ± 22.74 mm2; NS + NS (P): 423.96 ± 55.64 mm2 and NS + NS (T): 670.33 ± 91.22 mm2. However in CC (P) and CC (T) found no significant difference (Fig. 3). In CSA of lumen of seminiferous tubule no significant deference was observed among all study groups.
Fig. 3.
Micrometric Analysis of Testes in experimental groups, Cont (Control); Cr (chromium treated); AgNP (chemically synthesized silver nanoparticles), CC (clomiphene citrate treated); NS (Nigella sativa extract treated); NS + NP’ (Nigella sativa nanoparticles treated); P (prevention); T (treatment). Different alphabets shows significant difference between Cr treated group and treatment groups (Results represented in terms of mean ± SEM. P ≤ 0.05). Statistical icons: a, b, c, d, e, f, g, h, i, j, k = P ≤ 0.05, aa, bb, cc, dd, ee, ff, gg, hh, ii, jj, kk = P ≤ 0.01, aaa, bbb, ccc, ddd, eee, fff, ggg, hhh, iii, jjj, kkk = P ≤ 0.001.
A significant increase was observed in the size of spermatogonia in all Cr treated animals (41.30 ± 1.24 µ) in comparison to the control group (24.59 ± 1.18 µ). In clomiphene citrate AgNP, kalonji and kalonji mediated AgNP treated groups, there was no significant difference in comparison to control. With administration of clomiphene citrate, kalonji seed extract and kalonji mediated AgNPs at dose rate of 50 mg/kg BW significantly decreased the size of spermatogonia in CC (P): 25.54 ± 0.9 µ; NS (P): 22.24 ± 0.60 µ; NS + NS (P): 20.12 ± 0.57 µ; CC (T): (27.39 ± 3.26 µ); NS (T): 26.66 ± 1.11 µ and NS + NS (T): 25.43 ± 0.74 µ (P ≤ 0.05) (Fig. 3).
The size of spermatocytes was significantly changed in Cr treated animals (26.07 ± 1.34 µ) in comparison to the control group (21.82 ± 0.78 µ). In the AgNP, clomiphene citrate kalonji and kaloni mediated AgNP treated group, there was no significant difference in comparison to control 19.72 ± 1.11 µ. With the administration of clomiphene citrate, kalonji seed extract and kalonji mediated AgNPs at a dose rate of 50 mg/kg BW significantly decreased the size of spermatocytes in treatment groups in CC (P): 19.26 ± 0.71 µ; NS (P): 17.83 ± 1.36 µ; NS + NS (P): 15.22 ± 0.76 µ; CC (T): 17.97 ± 0.4 µ; NS (T): 20.18 ± 0.61 µ and NS + NS (T): 17.29 ± 1.68 µ (P ≤ 0.05) (Fig. 3). The number of spermatogonia and spermatocytes per cord were significantly decreased in Cr treated group (1.22 ± 0.25, 2.33 ± 0.943) in comparison to control (2.0 ± 0.00, 4.22 ± 0.629) respectively, however no significant difference was observed in all other study groups in comparison to Cr treated group (Fig. 3).
The sperm size was measured by micrometric analysis of head breadth, head length, middle piece length, and tail length (Fig. 4). A significant decrease was observed in the head breadth of sperms in all Cr treated animals (5.29 ± 0.54 µ) in comparison to the control group (19.15 ± 0.99 µ). In AgNP, clomiphene citrate, kalonji and kalonji mediated AgNP treated groups, no significant difference was observed in the head breadth of sperms in comparison to control (17.56 ± 0.18 µ). With the administration of kalonji seed extract and kalonji mediated AgNPs at a dose rate of 50 mg/kg BW significantly improved the head breadth of sperms in treatment groups in CC (P): 15.7 ± 0.5 µ; NS (P): 19.13 ± 0.68 µ; NS + NS (P): 18.34 ± 0.72 µ; CC (T): 15.5 ± 0.9 µ; NS (T): 21.97 ± 0.94 µ and NS + NS (T): 20.12 ± 0.75 µ (P ≤ 0.05) (Fig. 4).
Fig. 4.
Analysis of Sperm size measurement in experimental groups Cont (Control); Cr (chromium treated); AgNP (chemically synthesized silver nanoparticles), CC (clomiphene citrate treated); NS (Nigella sativa extract treated); NS + NP’ (Nigella sativa nanoparticles treated); P (prevention); T (treatment). Different alphabets shows significant difference between Cr treated group and treatment groups (Results represented in terms of mean ± SEM. P ≤ 0.05). Statistical icons: a, b, c, d, e, f, g, h, i, j, k = P ≤ 0.05, aa, bb, cc, dd, ee, ff, gg, hh, ii, jj, kk = P ≤ 0.01, aaa, bbb, ccc, ddd, eee, fff, ggg, hhh, iii, jjj, kkk = P ≤ 0.001.
The head length of sperms was also affected in Cr exposed animals (19.54 ± 1.18 µ) in comparison to the control group (51.82 ± 2.08 µ). In the AgNP, clomiphene citrate, kalonji and kalonji mediated AgNP treated groups, no significant difference was observed in head length of sperms observed in comparison to the control. With the administration of kalonji seed extract and kalonji mediated AgNPs at a dose rate of 50 mg/kg BW significantly improved the head length of sperms in treatment groups in CC (P):38.93 ± 2.18 µ; NS (P): 45.25 ± 1.49 µ; NS + NS (P): 45.43 ± 1.34 µ; CC (T): 48.42 ± 2.45 µ; NS (T): 50.61 ± 1.22 µ and NS + NS (T): 53.61 ± 1.25 µ (P ≤ 0.05) (Fig. 4).
The middle piece and tail length were drastically reduced in Cr treated animals (0.00 ± 0.00 µ) in comparison to the control group (75.97 ± 4.77 µ). With the administration of clomiphene citrate, kalonji seed extract and kalonji mediated AgNPs at a dose rate of 50 mg/kg B.W. significantly improved the middle piece length of sperms in treatment groups in CC (P): 42.32 ± 3.58 µ; NS (P): 53.25 ± 1.65 µ; NS + NS (P): 54.65 ± 1.67 µ; CC (T): 37.34 ± 4.09 µ; NS (T): 74.62 ± 2.06 µ and NS + NS (T): 57.18 ± 0.89 µ (P ≤ 0.05) (Fig. 4).
Tail length was drastically reduced in Cr treated animals (0.00 ± 0.00 µ) in comparison to the control group (157.17 ± 23.43 µ). With the administration of clomiphene citrate, kalonji seed extract and kalonji mediated AgNPs at a dose rate of 50 mg/kg BW significantly improved the tail length of sperms in treatment groups in CC (P): 134.57 ± 8.72 µ; NS (P): 199.00 ± 7.45 µ; NS + NS (P): 229.74 ± 7.25 µ; CC (T): 114.87 ± 5.82 µ; NS (T): 119.87 ± 3.26 µ and NS + NS (T): 220.14 ± 5.46 µ (P ≤ 0.05) (Fig. 4).
3.4. Testicular histology
The histological section of testes for the control group depicted the intact structure of seminiferous tubules encircled by interstitial tissue. In seminiferous tubules, spermatogonia were arranged with the basement membrane, in successive layers exhibiting zone for mitosis, followed by spermatocytes concentric layer, while the core area carried spermatozoa in different stages present in spermiogenesis zone. The lumen part of the tubule was occupied with mature spermatozoa. The same layout was obvious in CC, NS, NS + NP, NS (P), NS (T), NS + NP (P), and NS + NP (T) groups however NS and NS + NP results were better. In the AgNP (chemical synthesis) treated group interstitial spaces were widened indicating loss of Leydig cells along with disrupted spermatic cords indicating loss of spermatogenesis partially. In contrary conditions chromium, exposed groups showed various histological changes including necrosis, disruption of spermatic cords, obliterations of interstitial cells, the decline in Leydig tissue, and absence of mature spermatozoa. Fig. 5 showed a significant decline in the number of spermatogonia, present on the basement membrane leaving spaces in the seminiferous tubule. Widened intestinal spaces and the involution of spermatozoa were the pronounced histopathological finding of chromium exposure and same results were observed in CC (P) and CC (T) treated groups (Supplementary Fig. 5).
Fig. 5.
Histopathology of Testis (40 × ). A: control; B: Cr; C: AgNP D: CC; E: NS; F: NS + NP; G: CC (P); H: NS(P); I: N + NP (P); J: CC (T); K; NS (T); L: N + NP (T) treated groups. Yellow arrow: spermatogonium (mitosis zone), white arrow: spermatocyte (meiosis zone), green: spermiogenesis (transformation zone), red arrow: interstitial space, purple arrow: widened interstitial space.
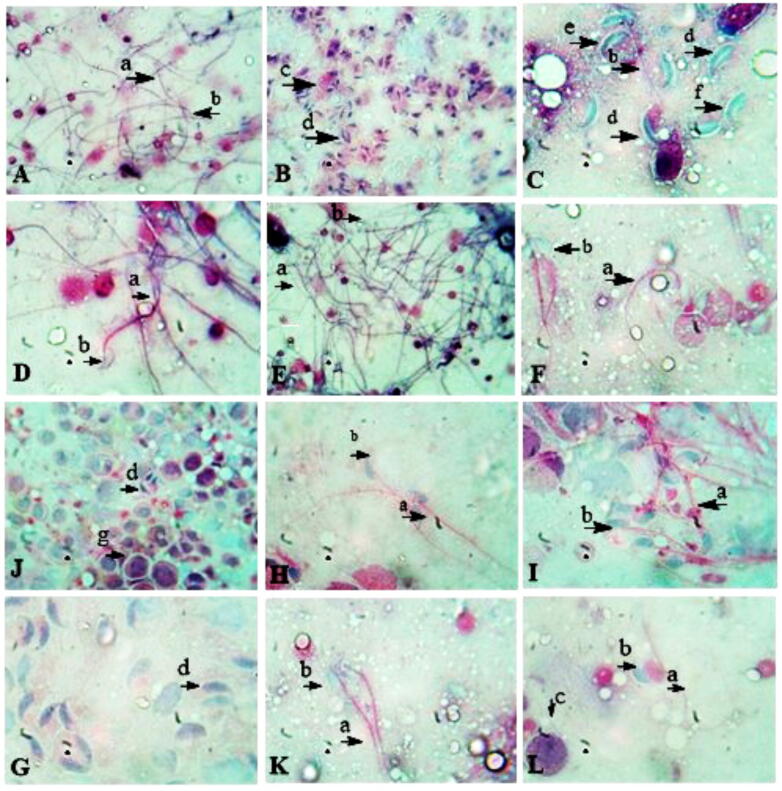
3.5. Testicular smear
Testicular smear micrographs illustrated that albino mice exposed to Cr (VI) showed acaudate sperms, with smaller heads. Sperm maturation was incomplete whereas macrophages were seen, an overall significant decrease in spermatogenesis. Mature sperms were not seen. In clomiphene citrate, kalonji and kalonji mediated AgNPs treated groups mature sperms with regular head and tail were visible. In AgNP via chemical synthesis treated group majority of the sperms were acaudate however some sperms were tailed with abnormal heads including double heads and with sickle shape and wavy structure. Macrophages and double-headed sperms were also seen. In prevention and treatment groups spermatogenesis was restored significantly except in CC (P) and CC (T) treated groups. Sperm with normal tails and heads were visible in micrographs (Fig. 6).
Fig. 6.
The photomicrographs of sperm morphology in Control and other groups (Eosin stain, x 100) A: control; B: Cr; C: AgNP D: CC; D; E: NS; F: NS + NP; G: CC (P); H: NS (P); I: N + NP (P); J: CC (T); K; NS (T); L: N + NP (T) treated groups. a: sperm tail, b: sperm head, c: macrophage, d: tailless acaudate sperms, e: double-headed sperm, f: wavy headed.
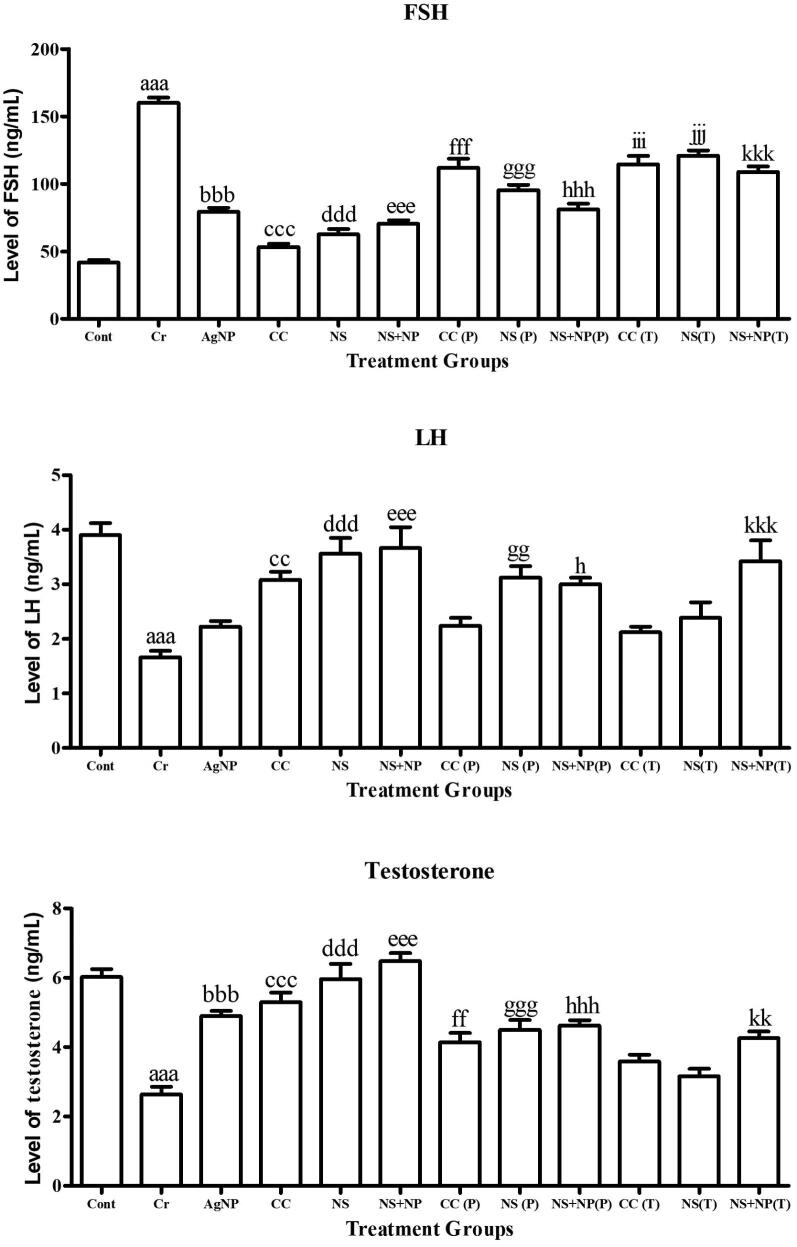
3.6. Hormone analysis
The result of hormones including FSH, LH, and testosterone quantification among all groups are shown in Fig. 6. A significant increase was observed in the level of FSH in all Cr treated animals (160.00 ± 4.98 ng/mL) in comparison to the control group (41.25 ± 2.56 ng/mL). In AgNP treated group, the level of FSH was significantly increased in comparison to the control (79.2 ± 3.9 ng/mL) whereas in the clomiphene citrate, kalonji and kalonji mediated AgNP treated group there was no significant difference in comparison to the control group. With administration of kalonji seed extract and kalonji mediated AgNPs at dose rate of 50 mg/kg BW significantly decreased level of FSH in treatment groups CC (P): 111.8 ± 8.17 ng/mL; NS (P): 95.50 ± 4.99 ng/mL; NS + NS (P): 81.00 ± 5.45 ng/mL; CC (T): 114.43 ± 8.08 ng/mL; NS (T): 121.00 ± 4.92 ng/mL and NS + NS (T): 109.00 ± 5.07 ng/mL (P ≤ 0.05) (Fig. 7).
Fig. 7.
Analysis of FSH, LH, and testosterone measurement in experimental groups Cont (Control); Cr (chromium treated); AgNP (chemically synthesized silver nanoparticles), CC (clomiphene citrate treated); NS (Nigella sativa extract treated); NS + NP’ (Nigella sativa nanoparticles treated); P (prevention); T (treatment). Different alphabets shows significant difference between Cr treated group and treatment groups (Results represented in terms of mean ± SEM. P ≤ 0.05). Statistical icons: a, b, c, d, e, f, g, h, i, j, k = P ≤ 0.05, aa, bb, cc, dd, ee, ff, gg, hh, ii, jj, kk = P ≤ 0.01, aaa, bbb, ccc, ddd, eee, fff, ggg, hhh, iii, jjj, kkk = P ≤ 0.001.
A significant decrease was observed in the level of LH in all Cr treated animals (1.65 ± 0.15 ng/mL) in comparison to the control group (3.89 ± 0.28 ng/mL). In AgNP treated group, the level of LH was significantly decreased in comparison to control (2.2 ± 0.1 ng/mL). With administration of clomiphene citrate, kalonji seed extract and kalonji mediated AgNPs at dose rate of 50 mg/kg BW significantly increased level of LH in treatment groups NS (P): 3.13 ± 0.27 ng/mL; NS + NS (P): 3.05 ± 0.20 ng/mL and NS + NS (T) (3.43 ± 0.5 ng/mL) whereas in CC (P): 2.24 ± 0.14 ng/mL; CC (T):2.12 ± 0.11 ng/mL and NS (T): 2.38 ± 0.27 ng/mL no significant difference was observed in comparison to Cr treated group (P ≤ 0.05) (Fig. 7).
A significant decline was observed in the level of testosterone in all Cr treated animals (2.63 ± 0.29 ng/mL) in comparison to the control group (6.05 ± 0.3 ng/mL). In AgNP, clomiphene citrate, kalonji and kalonji mediated AgNP treated group, the level of testosterone was not significantly different in comparison to the control. With administration of clomiphene citrate, kalonji seed extract and kalonji mediated AgNPs at dose rate of 50 mg/kg BW significantly increased the level of testosterone in treatment groups CC (P): 4.14 ± 0.35 ng/mL; NS (P): 4.50 ± 0.36 ng/mL; NS + NS (P): 4.63 ± 0.21 ng/mL; CC (T): 3.58 ± 0.17 ng/mL; NS (T): 3.13 ± 0.27 ng/mL and NS + NS (T): 4.23 ± 0.24 ng/mL (P ≤ 0.05) (Fig. 7).
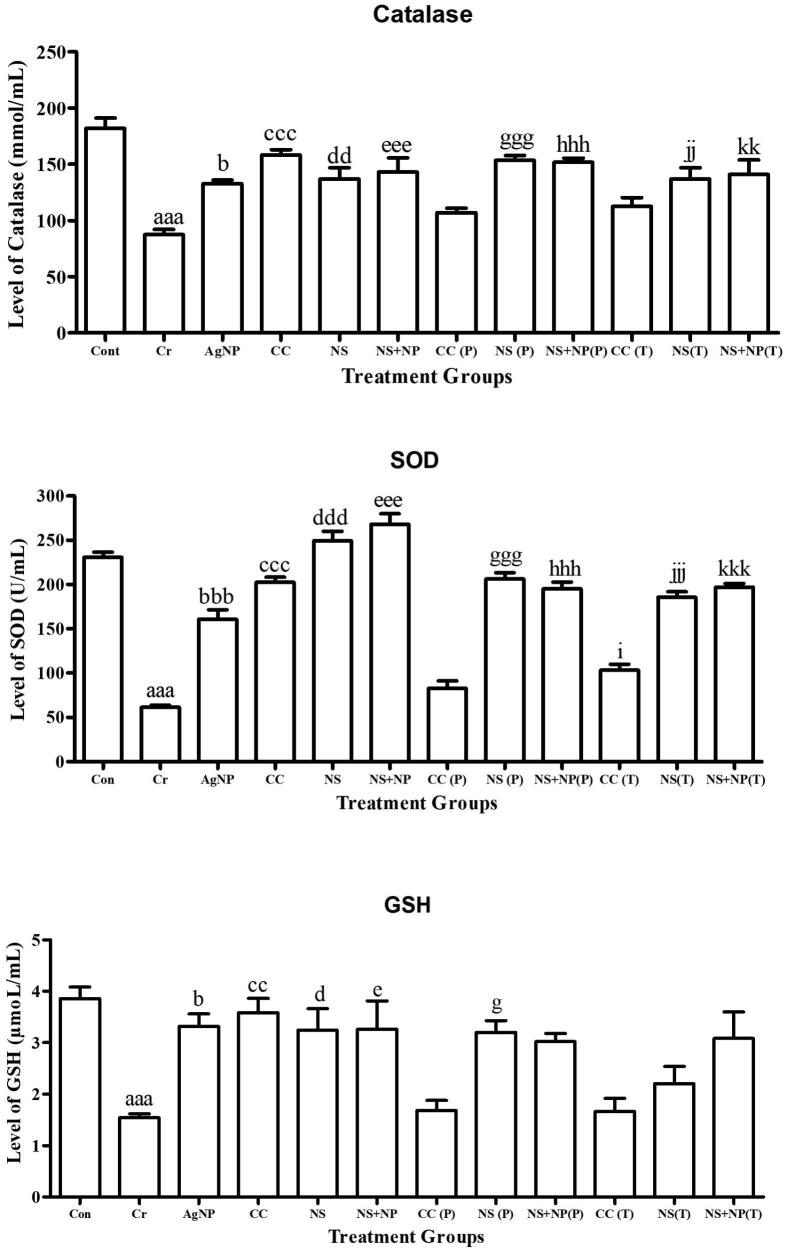
3.7. Antioxidant activity
A significant reduction was observed in antioxidant activity of CAT, SOD, and GSH in serum (P < 0.05) in chromium treated groups. On the other hand administration of NS and NS + NP showed significant increase as compared to Cr intoxicated group (Fig. 8).
Fig. 8.
Analysis of CAT, SOD, and GSH measurement in experimental groups Cont (Control); Cr (chromium treated); AgNP (chemically synthesized silver nanoparticles), CC (clomiphene citrate treated); NS (Nigella sativa extract treated); NS + NP’ (Nigella sativa nanoparticles treated); P (prevention); T (treatment). Different alphabets shows significant difference between Cr treated group and treatment groups (Results represented in terms of mean ± SEM. P ≤ 0.05). Statistical icons: a, b, c, d, e, f, g, h, i, j, k = P ≤ 0.05, aa, bb, cc, dd, ee, ff, gg, hh, ii, jj, kk = P ≤ 0.01, aaa, bbb, ccc, ddd, eee, fff, ggg, hhh, iii, jjj, kkk = P ≤ 0.001.
A significant decline was observed in the level of CAT in all Cr treated animals (87.40 ± 6.01 mmol/mL) in comparison to the control group (182.00 ± 10.68 mmol/mL). In AgNP, the level of CAT showed a significant decline (132.8 ± 2.43 mmol/mL) in comparison to the control group. With administration kalonji seed extract and kalonji mediated AgNPs at dose rate of 50 mg/kg BW significantly improved the level of CAT in treatment groups NS (P): 153.60 ± 5.02 mmol/mL; NS + NS (P): 151.80 ± 3.66 mmol/mL; NS (T): 137.00 ± 9.54 mmol/mL and NS + NS (T): 141.00 ± 16.46 mmol/mL. However, there was no significant difference was observed in CC (P): 107.00 ± 5.15 mmol/mL and CC (T): 112.8 ± 8.32 mmol/mL groups in comparison to Cr treated group (P ≤ 0.05) (Fig. 8).
A significant decline was observed in the level of SOD in all Cr treated animals (61.40 ± 2.48 mmol/mL) in comparison to the control group (230.80 ± 4.96 mmol/mL). In the clomiphene citrate, kalonji and kalonji mediated AgNP treated group, the level of SOD was not significantly different in comparison to the control whereas in AgNP treated group showed a significant decline (162.0 ± 13.7 mmol/mL) in comparison to the control group. With administration of kalonji seed extract and kalonji mediated AgNPs at dose rate of 50 mg/kg BW significantly improved the level of SOD in treatment groups NS (P) (206.20 ± 9.07 mmol/mL), NS + NS (P) (195.20 ± 7.68 mmol/mL), NS (T) (185.40 ± 8.41 mmol/mL) and NS + NS (T) (197.00 ± 5.28 mmol/mL). However, in CC (P): 82.8 ± 10.54 mmol/mL and CC (T): 103.4 ± 8.44 mmol/mL group no significant difference was observed in comparison to Cr treated groups (P ≤ 0.05) (Fig. 8).
A significant decrease was observed in the level of GSH in all Cr treated animals (1.54 ± 0.09 µmol/mL) in comparison to the control group (3.85 ± 0.25 µmol/mL). In the AgNP treated group, the level of GSH was significantly decreased (3.31 ± 0.31 µmol/mL) in comparison to the control group. With administration of kalonji seed extract and kalonji mediated AgNPs at dose rate of 50 mg/kg BW significantly decreased the level of GSH in treatment groups NS (P): 3.2 ± 0.18 µmol/mL; NS + NS (P): 3.02 ± 0.09 µmol/mL; NS (T): (2.2 ± 0.39 µmol/mL and NS + NS (T): 3.08 ± 0.66 µmol/mL. However, in CC (P): 1.68 ± 0.25 µmol/mL and CC (T): 1.66 ± 0.39 µmol/mL no significant difference was found in comparison to Cr treated group (P ≤ 0.05) (Fig. 8).
4. Discussion
The pilot study was designed to examine the effects of Cr (VI) on testes and amelioration by Nigella sativa and Nigella sativa mediated AgNPs. The clomiphene citrate used as standard drug to find out their impact on fertility. No sufficient data exist related to this study that’s why it is arduous to compare our findings with already reported data. Our study indicated that Cr (VI) caused excessive production of ROS in testicular tissue hence decreased spermatogenesis, destroyed the architecture of seminiferous tubules, defected germ cells, and altered the antioxidants (SOD, CAT, and GSH) and hormone level (FSH, LH, and testosterone). However, there was no significant difference in animal weight and body organ weight. Chemically synthesized AgNP also exerted toxic effects on male reproductive performance as compared to biosynthesized AgNP which showed improved spermatogenesis, hormone level, and antioxidant activity. Clomiphene citrate exposure showed normal spermatogenesis, sperm, and seminiferous tubule structure.
At present Cr (VI) caused hazardous effects via the production of oxidative stress and disrupts spermatogenesis (Pereira, Oliveira, Oliveira, Pereira, & Alves, 2021) spermiogenesis (Jurkowska, Kratz, Sawicka, & Piwowar, 2019), steroidogenesis (Navin & Aruldhas, 2021), and altered the expression of antioxidant enzymes related to male reproduction such as SOD, CAT, and GSH. Chromium induced abnormalities in sperm structures (Abd et al., 2020) including tailless heads (acaudate sperms), (Abd Elhafeez, 2019), and decreased motility (Abd et al., 2020). During spermiogenesis, degeneration of flagella component, axoneme affects sperm motility whereas degeneration in mitochondria disrupts energy supply (ATP), effect on sperm motility (El-Samad et al., 2021). Testes are organs with low oxygen levels and a very effective antioxidant defense system to defend both interstitial cells required for steroidogenesis and developing germ cells during spermatogenesis. ROS are produced in testes by macrophages and defective germ cells including head, middle piece, or tail defects (Pereira et al., 2021). Cr (V) also promotes the synthesis of ROS resulting in toxicity (Wakeel et al., 2020), and induces damage to cellular proteins, lipids and DNA. ROS decrease sperm viability by attack on spermatozoa membrane (Marzec-Wróblewska et al., 2019).
Nigella sativa active component thymoquinone (TQ) with good antioxidant properties improves the quality of spermatozoa, decreases defected sperms (Gur et al., 2021) by improving sperm producing mother cells (Wani et al., 2022), sperm motility by maintaining energy pathways in aerobic respiration result in improved fertility (Inanc et al., 2022). Chemically synthesized silver nanoparticles exerted a negative effect on spermatogenesis whereas green synthesis by Nigella sativa exerted positive effects on spermatogenesis. As AgNP groups showed defected sperms whereas NS + NP groups showed improved sperms structure in both treatment and prevention groups.
In the present study chromium induced atrophy in seminiferous tubule (ST), disorganization in testicular architecture, disruption of germinal epithelium, and necrosis of Leydig's cells (LCs) (Tahir et al., 2017). In seminiferous tubule diameter was increased due to aggregation of undifferentiated cells and spermatogenic arrest, wider interstitial spaces were observed due to loss of SC, and LCs and decreased number of germ cells was found (Andleeb et al., 2018). The cellular phospholipid and mitochondrial membrane permeability are maintained by redox balance (Lin et al., 2022). Overproduction of free radicals alters lipid metabolism by significantly decreasing sugars and diverging the acetyl CoA from glycolysis product to beta-oxidation of lipids. The intermediate products in this mechanism divert sugar into another pathway that produces cholesterol. Excessive production of cholesterol leads to seminiferous tubule vacuolation and ultimately sloughing of cells consequently dislodged spermatids in the lumen of seminiferous tubule, consequently CSA of seminiferous tubules was increased (Tahir et al., 2017). The clomiphene citrate prevention and treatment groups showed no significant difference with compare to chromium exposed group.
Chromium causes aging of LCs, as well as alters p450 system in steroid synthesis cells, hence decreasing the testosterone level, (Pereira et al., 2021). AS LCs are triggered by LH for the production of testosterone whereas FSH triggers SCs synergistically to testosterone, and produce regulatory molecules required for spermatogenesis (Alghamdi, 2020). Cr (VI) lowers the LH level in contrast to an elevation of the FSH level (Igharo et al., 2018, Zheng et al., 2018). Hexavalent chromium converts into trivalent chromium when entering the cell through the membrane, repressing luteinizing hormone receptor (LHR) and prolactin receptor (PRLR), attenuating transport of cholesterol by steroidogenic acute regulatory (StAR) protein, and repressing activity of various steroidogenic enzymes, due to diminished LHR promote resistance in testes to LH. SCs support spermatogenesis, chromium induces resistance in Sertoli cell’s FSHR to FSH result in reduced synthesis of steroidogenesis controlling paracrine factors including androgin binding protein (ABP), inhibin and activin, leading to hypoandrogenism (Navin & Aruldhas, 2021). ROS induces alteration in fatty acids and converts them into toxic lipids that may damage cell components (Mohammed & Abd-Elwahab, 2020). In the present study, AgNP showed a significant increase in FSH in comparison to control, showed a decline in the level of LH in contrast to control groups. However testosterone level was not significantly different in comparison to control. The clomiphene citrate prevention and treatment groups showed no significant difference with compare to chromium exposed group.
In the present study, LCs and SCs were regenerated by Nigella sativa especially due to TQ. Administration of NS the active component TQ improved histopathology of seminiferous tubules (Inanc et al., 2022). The unsaturated fatty acids and TQ play a key role in NS to improve steroidogenesis and spermatogenesis resulting in maintained hormone levels (Inanc et al., 2022). In AgNP group seminiferous tubules were damaged whereas in the clomiphene citrate treated group spermatogenesis was up to normal indicating a good fertility drug.
In testicular tissue, superoxide anion (O2 − ), hydroxyl radicals (OH), and H2O2 are the main reactive oxygen species that target the reproductive system in males actively (Pereira et al., 2021). SCs and LCs play important role in antioxidant balance (Orta Yilmaz et al., 2020). The principal antioxidant enzymes involved in the reproductive system of males include CAT and SOD accompany by non-enzymatic antioxidants like GSH. To maintain homeostasis oxidative stress and the antioxidant system must be balanced by SCs and LCs failure to that disrupt complex signaling pathways leading to selective germ cell death (Pereira et al., 2021). CAT converts H2O2 into oxygen and water (Chen et al., 2018), and the level was decreased by chromium (Karaulov et al., 2019). SOD converts O2•− into H2O2 (Huang et al., 2020), decreased level was observed by chromium treated groups (Karaulov et al., 2019). GSH is needed in the degradation of H2O2 by glutathione peroxidase (Huang et al., 2020). H2O2 and O2 − formed in the metabolic pathway are reduced by GSH. GSH makes random conjugates with toxic electrophiles, which are more soluble in water and metabolized into mercapturic acid and excreted out into bile (Lumlerdkij, 2018), found to decrease by chromium (Handa & Jindal, 2021). The thymoquinone component of Nigella sativa played role in maintaining free radical balance and improved levels of GSH, SOD, and CAT in serum (Danaei et al., 2019). Cr (VI) caused damage to spermatogenesis (Pereira et al., 2021, Zheng et al., 2018) spermatogonial cells (SSCs) were affected (Lv et al., 2018). The number of spermatogonia and spermatocytes was decreased by chromium (Mustofa and Mulyati, 2021, Navin and Aruldhas, 2021, Tahir et al., 2017) NS and NS + NP improved the spermatogonia and spermatocytes but not significantly. Cr (VI) exposure increased in size of spermatogonium and spermatocytes and NS, the active component TQ decreased the size significantly. In AgNP treated group, showed a significant increased level of CAT whereas the increased level of SOD indicated oxidative stress condition. However, in the clomiphene citrate treated group antioxidant activity was not significantly different from that control indicating oxidative balance condition. However clomiphene citrate prevention and treatment groups showed no significant different with compare to chromium exposed group.
5. Conclusion
The present study reported adverse effects of Cr (VI) on reproductive performance in males by inducing oxidative stress. Administration of Nigella sativa extract and Nigella sativa mediated AgNPs reduced the oxidative stress to an appreciable extent. Clomiphene citrate is a standard drug that improves reproductive health in males but doesn’t have any potential to ameliorate the toxic effects of chromium (VI). However, Nigella sativa is a natural product that improves reproductive performance in male and also ameliorate toxic effects induced by Cr (VI) due to its active components. It is better to eat black seeds and black seed mediated AgNP to treat infertility.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2023.103570.
Contributor Information
Tooba Nauroze, Email: tooba.nauroze@ue.edu.pk.
Shaukat Ali, Email: dr.shaukatli@gcu.edu.pk.
Lubna Kanwal, Email: lubna.kanwal@uo.edu.pk.
Tafail Akbar Mughal, Email: dr_tafail@wuajk.edu.pk.
Shagufta Andleeb, Email: drshagufta@ue.edu.pk.
Chaman Ara, Email: chaman.zool@pu.edu.pk.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary Fig. 1.
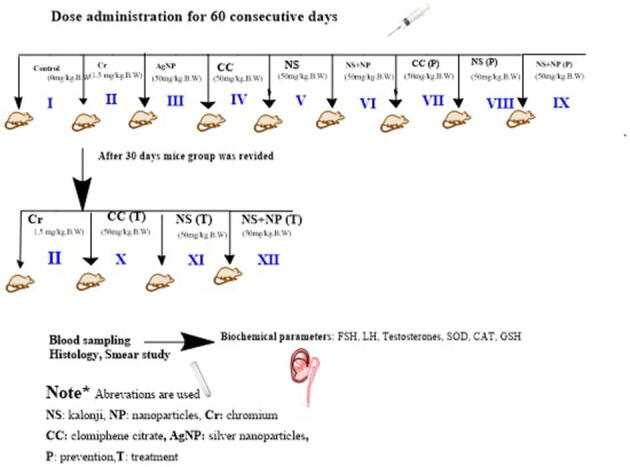
Experimental scheme of study
Supplementary Fig. 2.

UV- visible spectrum analysis of Nigella sativa silver AgNPs
Supplementary Fig. 3.

Fourier Transformed Infrared Spectroscopy analysis of Nigella sativa seed extract
Supplementary Fig. 4.
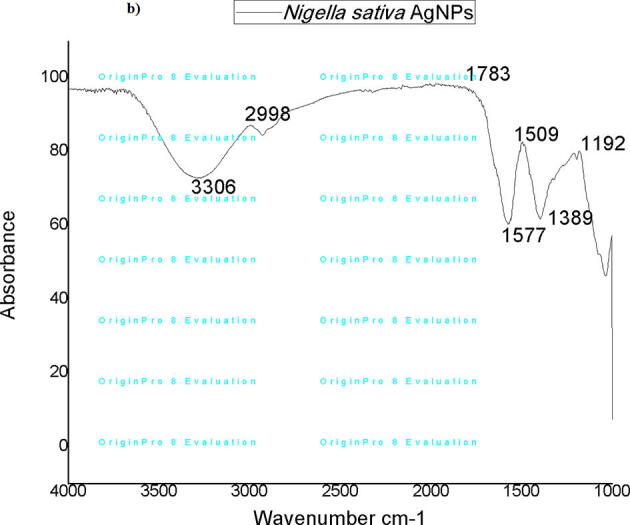
Fourier Transformed Infrared Spectroscopy analysis of Nigella sativa AgNPs
Supplementary Fig. 5.

Selected region of the histological section of chromium treated testis exhibiting histopathology (40×). Yellow arrow: basement membrane cell; red arrow: spermatogonium; orange arrow: spermatocyte; blue arrow: spermatozoa (sperm head only); purple arrow: wide luminal space of the seminiferous tubule; black arrow: Leydig cells necrosis leading to hollow interstitial space
References
- Abd H.H., Ahmed H.A., Mutar T.F. Moringa oleifera leaves extract modulates toxicity, sperms alterations, oxidative stress, and testicular damage induced by tramadol in male rats. Toxicol. Res. 2020;9(2):101–106. doi: 10.1093/toxres/tfaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd Elhafeez, E., 2019. Effects of Cadmium and/or Chromium on reproductive organs and semen profiles of male albino rats. Mansoura Veterinary Medical Journal, 20(3), 14-18
- Ahmad K.R., Nauroze T., Raees K., Abbas T., Kanwal M.A., Noor S., Jabeen S. Protective role of jambul (Syzygium cumini) fruit-pulp extract against fluoride-induced toxicity in mice testis: a histopathological study. Fluoride. 2012;45(3 Pt 2):281–289. [Google Scholar]
- Al-Faili J.O., Al-Jumaily H.A. Environmental geochemistry and bioavailability and health effects of chromium in topsoil and cauliflower plant in Kirkuk, Northern Iraq. Iraqi Geol. J. 2022:176–188. [Google Scholar]
- Alghamdi S.A. Effect of Nigella sativa and Foeniculum vulgare seeds extracts on male mice exposed to carbendazim. Saudi J. Biol. Sci. 2020;27(10):2521–2530. doi: 10.1016/j.sjbs.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almatroudi A., Khadri H., Azam M., Rahmani A.H., Khaleefah A., Khaleefah F. Antibacterial, Antibiofilm and anticancer activity of biologically synthesized silver nanoparticles using seed extract of Nigella sativa. Processes. 2020;8(4):388. [Google Scholar]
- Ameli M., Moghimian M., Saeb F., Bashtani E., Shokoohi M., Salimnejad R. The effect of clomiphene citrate and human chorionic gonadotropin on the expression of CatSper1, CatSper2, LHCGR, and SF1 genes, as well as the structural changes in testicular tissue of adult rats. Mol. Reprod. Dev. 2019;86(6):738–748. doi: 10.1002/mrd.23151. [DOI] [PubMed] [Google Scholar]
- Andleeb S., Mahmood T., Khalid A., Akrim F., Fatima H. Hexavalent +-chromium induces testicular dysfunction in small Indian mongoose (Herpestes javanicus) inhabiting tanneries area of Kasur District, Pakistan. Ecotoxicol. Environ. Saf. 2018;148:1001–1009. [Google Scholar]
- Babar Z.U., Azad A.K., Sulaiman W.M.A.W., Uddin J., Labu Z.K. Neuroprotective properties of Nigella sativa (L.) seeds extract in Sprague Dawley rats models. Dhaka Univ. J. Pharm. Sci. 2018;17(1):113–121. [Google Scholar]
- Castellini C., Mattioli S., Signorini C., Cotozzolo E., Noto D., Moretti E. Effect of Dietary Source on Rabbit Male Reproduction; Oxidative medicine and cellular longevity: 2019. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand K., Jiao C., Lakhan M.N., Shah A.H., Kumar V., Fouad D.E. Green synthesis, characterization and photocatalytic activity of silver nanoparticles synthesized with Nigella Sativa seed extract. Chem. Phys. Lett. 2021;763 [Google Scholar]
- Chen L., Zhang J., Zhu Y., Zhang Y. Interaction of chromium (III) or chromium (VI) with catalase and its effect on the structure and function of catalase: an in vitro study. Food Chem. 2018;244:378–385. doi: 10.1016/j.foodchem.2017.10.062. [DOI] [PubMed] [Google Scholar]
- Danaei G.H., Memar B., Ataee R., Karami M. Protective effect of thymoquinone, the main component of Nigella Sativa, against diazinon cardio-toxicity in rats. Drug Chem. Toxicol. 2019;42(6):585–591. doi: 10.1080/01480545.2018.1454459. [DOI] [PubMed] [Google Scholar]
- DesMarias T.L., Costa M. Mechanisms of chromium-induced toxicity. Curr. Opin. Toxicol. 2019;14:1–7. doi: 10.1016/j.cotox.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworzański J., Strycharz-Dudziak M., Kliszczewska E., Kiełczykowska M., Dworzańska A., Drop B. Glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity in patients with diabetes mellitus type 2 infected with Epstein-Barr virus. PLoS One. 2020;15(3):e0230374. doi: 10.1371/journal.pone.0230374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Demerdash F.M., Jebur A.B., Nasr H.M., Hamid H.M. Modulatory effect of Turnera diffusa against testicular toxicity induced by fenitrothion and/or hexavalent chromium in rats. Environ. Toxicol. 2019;34(3):330–339. doi: 10.1002/tox.22688. [DOI] [PubMed] [Google Scholar]
- El-Samad L.M., El-Ashram S., Kheirallah D.A., Abdul-Aziz K.K., Toto N.A., Mokhamer E.H.M. Relative gene expression, micronuclei formation, and ultrastructure alterations induced by heavy metal contamination in Pimelia latreillei (Coleoptera: Tenebrionidae) in an urban-industrial area of Alexandria, Egypt. Plos One. 2021;16(6):e0253238. doi: 10.1371/journal.pone.0253238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sebaey A.M., Abdelhamid F.M., Abdalla O.A. Protective effects of garlic extract against hematological alterations, immunosuppression, hepatic oxidative stress, and renal damage induced by cyclophosphamide in rats. Environ. Sci. Pollut. Res. 2019;26(15):15559–15572. doi: 10.1007/s11356-019-04993-7. [DOI] [PubMed] [Google Scholar]
- Genchi G., Lauria G., Catalano A., Carocci A., Sinicropi M.S. The double face of metals: the intriguing case of chromium. Appl. Sci. 2021;11(2):638. [Google Scholar]
- Guo D.P., Zlatev D.V., Li S., Baker L.C., Eisenberg M.L. Demographics, usage patterns, and safety of male users of clomiphene in the United States. World J. Men's Health. 2020;38(2):220. doi: 10.5534/wjmh.190028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur F.M., Timurkaan S., Taskin E., Guven C., Gur H.E., Senturk M. Thymoquinone improves testicular damage and sperm quality in experimentally varicocele-induced adolescent rats. Andrologia. 2021;53(5):e14033. doi: 10.1111/and.14033. [DOI] [PubMed] [Google Scholar]
- Habib, N., Choudhry, S., 2021. HPLC Quantification of Thymoquinone Extracted from Nigella sativa L. (Ranunculaceae) Seeds and Antibacterial Activity of Its Extracts against Bacillus Species. Evidence-Based Complementary and Alternative Medicine, 2021, 1–11. [DOI] [PMC free article] [PubMed]
- Habous M., Giona S., Tealab A., Aziz M., Williamson B., Nassar M. Clomiphene citrate and human chorionic gonadotropin are both effective in restoring testosterone in hypogonadism: a short-course randomized study. BJU Int. 2018;122(5):889–897. doi: 10.1111/bju.14401. [DOI] [PubMed] [Google Scholar]
- Hamad S.M., Shnawa B.H., Jalil P.J., Ahmed M.H. Assessment of the therapeutic efficacy of silver nanoparticles against secondary cystic echinococcosis in BALB/c mice. Surfaces. 2022;5(1):91–112. [Google Scholar]
- Handa K., Jindal R. Genotoxicity induced by hexavalent chromium leading to eryptosis in Ctenopharyngodon idellus. Chemosphere. 2020;247 doi: 10.1016/j.chemosphere.2020.125967. [DOI] [PubMed] [Google Scholar]
- Handa K., Jindal R. Estimating the hepatotoxic impact of hexavalent chromium on Ctenopharyngodon idellus through a multi-biomarker study. Environ. Adv. 2021;5 [Google Scholar]
- Herzog B.J., Nguyen H.M.T., Soubra A., Hellstrom W.J. Clomiphene citrate for male hypogonadism and infertility: an updated review. Androgens: Clin. Res. Therap. 2020;1(1):62–69. [Google Scholar]
- Huang Z., Chen Y., Zhang Y. Mitochondrial reactive oxygen species cause major oxidative mitochondrial DNA damages and repair pathways. J. Biosci. 2020;45(1):1–17. [PubMed] [Google Scholar]
- Hussain S., Ali S., Mumtaz S., Shakir H.A., Ahmad F., Tahir H.M. Dose and duration-dependent toxicological evaluation of lead acetate in chicks. Environ. Sci. Pollut. Res. 2020;27(13):15149–15164. doi: 10.1007/s11356-020-08016-8. [DOI] [PubMed] [Google Scholar]
- Igharo O.G., Anetor J.I., Osibanjo O., Osadolor H.B., Odazie E.C., Uche Z.C. Endocrine disrupting metals lead to alteration in the gonadal hormone levels in Nigerian e-waste workers. Univ. Med. 2018;37(1):65–74. [Google Scholar]
- Inanc, M. E., Güngör, Ş., Avdatek, F., Yeni, D., Gülhan, M. F., Olğaç, K. T., 2022. Thymoquinone Improves Motility, Plasma Membrane and DNA Integrity of Frozen‐Thawed Ram Semen. Andrologia, 54, e-14547. [DOI] [PubMed]
- Ismael, Z. K., AL-Anbari, L. A., Mossa, H. A., 2017. Relationship of FSH, LH, DHEA and testosterone levels in serum with sperm function parameters in infertile men. J. Pharma. Sci. 9(11), 2056–2061
- Jamshidi-Kia F., Lorigooini Z., Amini-Khoei H. Medicinal plants: past history and future perspective. J. Herbmed. Pharmacol. 2018;7(1) [Google Scholar]
- Joshi N., Menon P., Joshi A. Effect of chromium on germination in some crops of India. J. Agric. Sci. Bot. 2019;3(1):1–5. [Google Scholar]
- Joy B. Emerging Technologies: Ethics, Law and Governance. Routledge; 47–63: 2020. Why the future doesn’t need us: Our most powerful 21st-century technologies-robotics, genetic engineering, and nanotech-are threatening to make humans an endangered species. [Google Scholar]
- Jurkowska K., Kratz E., Sawicka E., Piwowar A. The impact of metalloestrogens on the physiology of male reproductive health as a current problem of the XXI century. J. Physiol. Pharmacol. 2019;70(3):337–355. doi: 10.26402/jpp.2019.3.02. [DOI] [PubMed] [Google Scholar]
- Jyoti K., Arora D., Fekete G., Lendvai L., Dogossy G., Singh T. Antibacterial and anti-inflammatory activities of Cassia fistula fungal broth-capped silver nanoparticles. Mater. Technol. 2021;36(14):883–893. [Google Scholar]
- Karaulov A.V., Renieri E., Smolyagin A.I., Mikhaylova I.V., Stadnikov A., Begun D. Long-term effects of chromium on morphological and immunological parameters of Wistar rats. Food Chem. Toxicol. 2019;133 doi: 10.1016/j.fct.2019.110748. [DOI] [PubMed] [Google Scholar]
- Lin Y.-X., Xu H.-J., Yin G.-K., Zhou Y.-C., Lu X.-X., Xin X. Dynamic changes in membrane lipid metabolism and antioxidant defense during soybean (Glycine max L. Merr.) seed aging. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.908949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumlerdkij N. UCL (University College London); 2018. Thai traditional medicine as a source for cancer prevention: from local concepts to the discovery of potential chemopreventive extracts. [Google Scholar]
- Lv Y., Zhang P., Guo J., Zhu Z., Li X., Xu D. Melatonin protects mouse spermatogonial stem cells against hexavalent chromium-induced apoptosis and epigenetic histone modification. Toxicol. Appl. Pharmacol. 2018;340:30–38. doi: 10.1016/j.taap.2017.12.017. [DOI] [PubMed] [Google Scholar]
- Marzec-Wróblewska U., Kamiński P., Łakota P., Szymański M., Wasilow K., Ludwikowski G. Human sperm characteristics with regard to cobalt, chromium, and lead in semen and activity of catalase in seminal plasma. Biol. Trace Elem. Res. 2019;188(2):251–260. doi: 10.1007/s12011-018-1416-9. [DOI] [PubMed] [Google Scholar]
- Mohammed A., Abd-Elwahab W. The potential protective role of vitamin e and selenium against sub-chronic toxicity of hexavalent chromium on the testis of adult male albino rats. Ain Shams J. Forensic Med. Clin. Toxicol. 2020;34(1):22–33. [Google Scholar]
- Mustofa W.W.I., Mulyati D.K.M.S. Administration of the α-tocopherol for repairing testicle histological damage in rats exposed to dioxin. Thai J. Vet. Med. 2021;51(2):293–301. [Google Scholar]
- Navin, A. K., Aruldhas, M. M., 2021. Hexavalent Chromium and Male Reproduction: An Update. Proc. Zool. Soc. 74, 617–633.
- Orta Yilmaz B., Yildizbayrak N., Erkan M. Sodium arsenite-induced detriment of cell function in Leydig and Sertoli cells: the potential relation of oxidative damage and antioxidant defense system. Drug Chem. Toxicol. 2020;43(5):479–487. doi: 10.1080/01480545.2018.1505902. [DOI] [PubMed] [Google Scholar]
- Parang Z., Moghadamnia D. Synthesis of silver-cobalt nanoparticles by chemical reduction method and its effects on serum levels of thyroid hormones in adult male rats. Nanomed. Res. J. 2018;3(4):236–244. [Google Scholar]
- Pereira, S. C., Oliveira, P. F., Oliveira, S. R., Pereira, M. d. L., Alves, M. G., 2021. Impact of environmental and lifestyle use of chromium on male fertility: focus on antioxidant activity and oxidative stress. Antioxidants 10(9), 1365. [DOI] [PMC free article] [PubMed]
- Rashid M.U., Bhuiyan M.K.H., Quayum M.E. Synthesis of silver nano particles (Ag-NPs) and their uses for quantitative analysis of vitamin C tablets. Dhaka Univ. J. Pharma. Sci. 2013;12(1):29–33. [Google Scholar]
- Rawat N., Singla-Pareek S.L., Pareek A. Membrane dynamics during individual and combined abiotic stresses in plants and tools to study the same. Physiol. Plant. 2021;171(4):653–676. doi: 10.1111/ppl.13217. [DOI] [PubMed] [Google Scholar]
- Samarghandian, S., Farkhondeh, T., Samini, F., 2018. A review on possible therapeutic effect of Nigella sativa and thymoquinone in neurodegenerative diseases. NS Neurol. Disord. Drug Targets 17(6), 412-420. [DOI] [PubMed]
- Sapitri P., Khaeruman B. Takhrij and Syarah Hadith of chemistry: the role of black seed in fighting disease. Emerg.: J. Edu. Discov. Lifelong Learn. (EJEDL) 2021;2(05):16–21. [Google Scholar]
- Selamoglu Z. The natural products and healthy life. J. Traditional Med. Clin. Naturo. 2018;7(2):1–2. [Google Scholar]
- Selamoglu, Z., 2017a. Biotechnological approaches on anticancer activity of flavonoids-mini review. Modern Approaches to Drug Design. 1(2). MADD.000510.
- Selamoglu, Z., 2017b. Polyphenolic Compounds in Human Health with Pharmacological Properties. J. Traditional Med. Clin. Naturo. 6(4), 137.
- Shahbazi H., Hashemi Gahruie H., Golmakani M.T., Eskandari M.H., Movahedi M. Effect of medicinal plant type and concentration on physicochemical, antioxidant, antimicrobial, and sensorial properties of kombucha. Food Sci. Nutr. 2018;6(8):2568–2577. doi: 10.1002/fsn3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobana N., Kumar M.K., Navin A.K., Akbarsha M.A., Aruldhas M.M. Prenatal exposure to excess chromium attenuates transcription factors regulating expression of androgen and follicle stimulating hormone receptors in Sertoli cells of prepuberal rats. Chem. Biol. Interact. 2020;328 doi: 10.1016/j.cbi.2020.109188. [DOI] [PubMed] [Google Scholar]
- Siddiqi K.S., Husen A. Current status of plant metabolite-based fabrication of copper/copper oxide nanoparticles and their applications: a review. Biomater. Res. 2020;24(1):1–15. doi: 10.1186/s40824-020-00188-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V., Gupta A., Verma U.P., Mishra T., Pal M. An evaluation of the efficacy of ethanolic extract of Nigella sativa L. (Kalonji) on the clinical parameters of moderate-to-severe gingivitis: a split-mouth clinical study. Ayu. 2019;40(3):152. doi: 10.4103/ayu.AYU_68_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir, A., Khawaja, A., Qaisar, F., Sajjad, A., 2017. Effects of chromium on testes and protective role of mulberry. JSZMC. 8(3), 1200–1204.
- Tungmunnithum D., Thongboonyou A., Pholboon A., Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines. 2018;5(3):93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeel A., Xu M., Gan Y. Chromium-induced reactive oxygen species accumulation by altering the enzymatic antioxidant system and associated cytotoxic, genotoxic, ultrastructural, and photosynthetic changes in plants. Int. J. Mol. Sci. 2020;21(3):728. doi: 10.3390/ijms21030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani J.A., Tsagkaris C., Majid S., Ganie M.A., Akhter R., Ahmad S.B. Black Seeds (Nigella sativa) Elsevier; 2022. Therapeutic effects of Nigella sativa on hormonal dysfunctions; pp. 217–238. [Google Scholar]
- Yerragopu P.S., Hiregoudar S., Nidoni U., Ramappa K., Sreenivas A., Doddagoudar S. Chemical synthesis of silver nanoparticles using tri-sodium citrate, stability study and their characterization. Int. Res. J. Pure Appl. Chem. 2020;21(3):37–50. [Google Scholar]
- Zheng W., Ge F., Wu K., Chen X., Li X., Chen Y. In utero exposure to hexavalent chromium disrupts rat fetal testis development. Toxicol. Lett. 2018;299:201–209. doi: 10.1016/j.toxlet.2018.10.010. [DOI] [PubMed] [Google Scholar]