Graphical abstract
Keywords: ST elevation myocardial infarction, Takotsubo syndrome, Electrocardiography
Abstract
Background
Electrocardiography (ECG) on admission is similar in ST elevation myocardial infarction (STEMI) and Takotsubo syndrome (TTS). ECG on admission has been extensively investigated and compared between STEMI and TTS, however, only a few studies have compared temporal ECG. Our aim was to compare ECG in anterior STEMI versus female TTS from admission to day 30.
Methods
Adult patients with anterior STEMI or TTS treated at Sahlgrenska University Hospital (Gothenburg, Sweden) from December 2019 to June 2022 were prospectively enrolled. Baseline characteristics, clinical variables and ECGs from admission to day 30 were analyzed. Using a mixed effects model, we compared temporal ECG between female patients with anterior STEMI or TTS, as well as between female and male patients with anterior STEMI.
Results
A total of 101 anterior STEMI patients (31 female, 70 male) and 34 TTS patients (29 female, 5 male) were included. The temporal pattern of T wave inversion was similar between female anterior STEMI and female TTS, as well as between female and male anterior STEMI. ST elevation was more common, whereas QT prolongation was less common, in anterior STEMI compared with TTS. Q wave pathology was more similar between female anterior STEMI and female TTS than between female and male anterior STEMI.
Conclusions
The pattern of T wave inversion and Q wave pathology from admission to day 30 was similar in female patients with anterior STEMI and female patients with TTS. Temporal ECG in female patients with TTS may be interpreted as following a “transient ischemic” pattern.
1. Introduction
ST elevation myocardial infarction (STEMI) and Takotsubo syndrome (TTS) are acute cardiac conditions with similar presenting symptoms and non-invasive test results. Whereas STEMI is caused by an acute coronary occlusion, TTS is associated with severe emotional or physical stress. Several electrocardiographic (ECG) criteria has been proposed to distinguish between acute myocardial infarction (AMI) and TTS, none of which can reliably differentiate the two conditions to avoid coronary angiography to exclude coronary occlusion. [1], [2], [3], [4], [5].
ECG on admission is particularly similar in anterior STEMI and TTS [5]. According to previous literature, the most common ECG changes on admission in TTS are ST elevation and T wave inversion; and the most common ECG changes in the sub-acute phase are T wave inversion and QT prolongation. Conversely, ST depression (in particular reciprocal ST depression) and pathological Q waves on admission are less common [3], [5]. Although T wave inversion has been regarded as a hallmark ECG change in TTS [2], [6], [7], [8], it is not included in the majority of ECG criteria used to differentiate TTS from STEMI on admission. Instead, presence of reciprocal ST depression or ST depression per se, and lead-specific combinations of ST elevations are commonly used in these criteria [9], [10], [11], [12], [13], [14]. Post-admission, the temporal evolution of ECG changes in TTS has been described and compared to ischemic cardiac conditions [8], [10], [15], [16]. However, prospective studies with temporal head-to-head comparisons of ECG in STEMI specifically versus TTS are lacking.
The aim of the present study was to prospectively investigate the temporal pattern of 1) T wave inversion and 2) ST elevation, Q wave pathology or QT prolongation, with head-to-head comparisons among patients with anterior STEMI and female patients with TTS.
2. Methods
Study cohort. We prospectively enrolled adult patients (≥18 years old) with anterior STEMI or TTS who were admitted to Sahlgrenska University Hospital (Gothenburg, Sweden) from December 2019 to June 2022. We obtained informed consent from all patients before enrollment in the study. Exclusion criteria were pacemaker rhythm at admission, prior acute myocardial infarction, known pre-existing persistent regional myocardial dysfunction and expected inability to comply with study protocol. Patients were stratified by sex, and 12 lead ECGs were obtained in-hospital at admission (day 0), day 1 (24 ± 6 h), day 2 (48 ± 12 h), day 3 (72 ± 12 h) and at follow-up day 7 ± 48 h, day 14 ± 48 h and day 30 ± 48 h. Time of symptom onset was obtained from an interview with patients according to a pre-defined questionnaire. Baseline characteristics were obtained from patients’ and/or their medical charts, and results from diagnostic work-up and clinical variables were registered consecutively as patients were enrolled.
All 12-lead ECGs were recorded at a paper speed of 50 mm/s and an amplification of 10 mm/mV. ST segment deviation was measured manually (to the nearest 0.5 mm) from the isoelectric line to the J-point for ST elevation, and to 60 ms after the J-point for ST depression. T wave inversions were measured manually from the isoelectric line to nadir to the nearest 0.5 mm. For heart rate, PR interval, QRS duration, QRS axis and QT time; electronically derived values were registered. The corrected QT interval (QTc) was calculated using Bazzet’s formula [17]. All ECGs were analyzed and validated by two different physicians.
Endpoints and definitions. The primary objective of this study was to compare temporal ECG between female anterior STEMI and female TTS, and the secondary objective was to compare temporal ECG between female and male patients with anterior STEMI. The primary endpoints were the prevalence of T wave inversion, the maximum single lead T wave inversion and the average sum of all T wave inversions over time from admission to day 30. The secondary endpoints were the prevalence of ST elevation, long QTc or pathological Q waves over time from admission to day 30. All STEMI and TTS diagnoses were confirmed and defined according to European Society of Cardiology (ESC) criteria [18], [19] and all patients underwent coronary angiography. Anterior STEMI was defined as STEMI with culprit lesion in the left anterior descending artery (LAD) or any of its branches. Long QTc was defined as QTc time > 450 ms in males and > 460 ms in females. All ECG definitions are summarized in Supplementary Table 1.
Statistical analysis. Variables are presented as mean ± standard deviations, median and interquartile range, or percentages for categorical variables. Categorical variables were compared using Fischer’s Exact test and continuous variables were compared using T-test for normally distributed variables and Mann-Whitney U Test for non-normally distributed variables.
We used a mixed effects model (lme4 package, R) to compare the primary and secondary endpoints between females with anterior STEMI and females with TTS, as well as between female and male patients with anterior STEMI, from admission (day 0) to day 30. Disease type (anterior STEMI or TTS) or sex and ECG day were included as fixed effects in all models. Before fitting the final models, possible interaction between disease type or sex and ECG day (day 0, 1, 2, 3, 7, 14, 30) was assessed. Patient identification number was included as random effect in all models to account for the repeated measures design of the study. Model A included disease type and sex as main fixed effect and ECG day as covariate. Model B included (in addition to the variables in Model A) age, hypertension, diabetes, chronic obstructive pulmonary disease and pre-admission treatment with beta-blockers as covariates. Model C was a sensitivity model with the same variables as model A, but only including patients with complete ECG data (i.e. no missing ECGs) at day 0, 7 or 30 (N = 82: 23 female anterior STEMI, 42 male anterior STEMI, 17 female TTS). All statistical analyses were performed using RStudio version 1.4.1717. The level of significance was set at p < 0.05.
This study complies with the declaration of Helsinki and was approved by the Swedish Ethical Review Authority (registration number 2020–06257). Individual informed consent was obtained from all patients.
3. Results
Baseline characteristics and presenting symptoms. The study cohort consisted of 31 female patients with anterior STEMI, 70 male patients with anterior STEMI, 29 female patients with TTS and 5 male patients with TTS (Table 1). Among all patients with TTS, 74 % (25/34) had typical TTS (i.e. apical type); and among female patients with TTS, 72 % (21/29) had typical TTS. The remaining cases of TTS were atypical (midventricular, inverted, focal, biventricular). Baseline characteristics were similar between groups but female patients with anterior STEMI or TTS were on average older than male patients with anterior STEMI or TTS. Patients with anterior STEMI presented with lower heart rate, had a lower prevalence of prior or current cancer and higher left ventricular ejection fraction (LVEF), compared with female TTS. NTproBNP, and minutes from symptom onset to ECG, were lowest in male anterior STEMI, intermediate in female anterior STEMI and highest in female TTS. The most pronounced differences between male patients with TTS and male patients with anterior STEMI were that male patients with TTS hade lower BMI and a lower proportion of smokers.
Table 1.
Baseline characteristics and presenting symptoms.
| Takotsubo |
Anterior STEMI |
SMD* | SMD** | SMD*** | Missing (%) | |||
|---|---|---|---|---|---|---|---|---|
| Female N = 29 |
Male N = 5 |
Female N = 31 |
Male N = 70 |
|||||
| Age, years | 68 ± 9.8 | 50 ± 28 | 72 ± 12 | 64 ± 10 | 0.32 | 0.73 | 0.72 | 0.7 |
| Home status independent† % (n/N) | 93 % (27/29) | 100 % (5/5) | 97 % (30/31) | 100 % (69/69) | 0.17 | 0.26 | <0.001 | 0.7 |
| BMI, kg/m2 | 26 (22 – 29) | 23 (22 – 31) | 26 (23 – 29) | 27 (26 – 29) | 0.042 | 0.24 | 0.41 | 0.7 |
| Current smoking % (n/N) | 18 % (5/28) | 0 % (0/5) | 36 % (11/31) | 23 % (16/70) | 0.41 | 0.28 | 0.78 | 0.7 |
| Diabetes | 7.1 % (2/28) | 25 % (1/4) | 3.2 % (1/31) | 14 % (10/70) | 0.18 | 0.40 | 0.27 | 1.5 |
| Hypertension | 45 % (13/29) | 20 % (1/5) | 40 % (12/30) | 37 % (26/70) | 0.098 | 0.059 | 0.37 | 0.7 |
| Hyperlipidemia | 14 % (4/29) | 20 % (1/5) | 6.5 % (2/31) | 18 % (12/68) | 0.25 | 0.35 | 0.053 | 1.5 |
| Atrial fibrillation | 6.9 % (2/29) | 0 % (0/5) | 3.3 % (1/30) | 1.4 % (1/69) | 0.16 | 0.12 | 0.17 | 1.5 |
| Previous PCI | 0 % (0/29) | 0 % (0/5) | 0 % (0/30) | 2.9 % (2/70) | <0.001 | 0.24 | 0.24 | 0.7 |
| Prior stroke | 6.9 % (2/29) | 0 % (0/5) | 0 % (0/31) | 4.3 % (3/70) | 0.39 | 0.30 | 0.30 | 0 |
| Peripheral artery disease | 6.9 % (2/29) | 0 % (0/5) | 3.2 % (1/31) | 2.9 % (2/70) | 0.17 | 0.021 | 0.24 | 0 |
| COPD | 14 % (4/29) | 0 % (0/5) | 9.7 % (3/31) | 1.4 % (1/70) | 0.13 | 0.37 | 0.17 | 0 |
| Chronic renal disease‡ | 0 % (0/29) | 0 % (0/5) | 0 % (0/30) | 2.9 % (2/69) | <0.001 | 0.24 | 0.25 | 1.5 |
| Prior or current cancer | 14 % (4/29) | 0 % (0/5) | 0 % (0/31) | 2.9 % (2/70) | 0.57 | 0.24 | 0.24 | 0 |
| Beta-blocker treatment | 0 % (0/22) | 0 % (0/3) | 0 % (0/25) | 0 % (0/63) | <0.001 | <0.001 | <0.001 | 16 |
| ACEi or ARB treatment | 0 % (0/18) | 0 % (0/3) | 4.5 % (1/22) | 0 % (0/60) | 0.31 | 0.31 | <0.001 | 23 |
| Calcium inhibitor | 0 % (0/26) | 0 % (0/4) | 0 % (0/28) | 0 % (0/61) | <0.001 | <0.001 | <0.001 | 11 |
| Presenting symptoms and signs on admission | ||||||||
| Chest pain | 44 % (12/27) | 40 % (2/5) | 93 % (28/30) | 94 % (62/66) | 1.2 | 0.025 | 1.4 | 5.2 |
| Heart rate | 100 ± 24 | 94 ± 22 | 80 ± 18 | 79 ± 22 | 1.0 | 0.051 | 0.66 | 0.7 |
| Systolic blood pressure | 130 ± 32 | 141 ± 27 | 140 ± 22 | 140 ± 24 | 0.15 | 0.14 | 0.026 | 0.7 |
| Diastolic blood pressure | 87 ± (17) | 92 ± 26 | 81 ± 15 | 87 ± 17 | 0.36 | 0.38 | 0.20 | 3.0 |
| Acute Heart Failure§ | 45 % (13/29) | 40 % (2/5) | 20 % (6/30) | 16 % (11/70) | 0.55 | 0.11 | 0.64 | 0.7 |
| Killip class 3 or 4# | 24 % (7/29) | 20 % (1/5) | 10 % (3/30) | 5.7 % (4/70) | 0.38 | 0.16 | 0.49 | 0.7 |
| Killip class 4 | 10 % (3/29) | 0 % (0/5) | 3.3 % (1/30) | 1.4 % (1/70) | 0.28 | 0.13 | 0.17 | 0.7 |
| LVEF % | 35 (30 – 45) | 40 (30 – 45) | 45 (40 – 50) | 45 (40 – 50) | 0.82 | 0.093 | 0.67 | 16 |
| Troponin T | 440 (120 – 1700) | 500 (460 – 1600) | 370 (103 – 600) | 625 (140 – 2600) | 0.60 | 0.18 | 0.28 | 30 |
| NTproBNP | 2300 (1200 – 4200) | 1240 (970 – 1730) | 534 (220 – 2400) | 135 (78 – 680) | 0.53 | 0.39 | 0.19 | 27 |
| Oxygen administered | 41 % (12/29) | 40 % (2/5) | 23 % (7/30) | 17 % (12/70) | 0.39 | 0.16 | 0.60 | 0.7 |
| Minutes from symptom to admission ECG (day 0) | 127 (41 – 250) | 350 (240 – 2767) | 99 (54 – 150) | 50 (29 – 100) | 0.25 | 0.25 | 0.74 | 12 |
STEMI = ST elevation myocardial infarction; SMD = standardized mean difference; BMI = body mass index; PCI = percutaneous coronary intervention; COPD = chronic obstructive pulmonary disease; ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; LVEF = left ventricular ejection fraction.
*Takotsubo female versus anterior STEMI female; **Anterior STEMI, female vs male; ***Takotsubo male versus anterior STEMI male.
†Independent = independent living with no domestic service or home health care; ‡glomerular filtration rate < 60 ml/kg/min; §Killip Class 2, 3 or 4; #Killip class 3 = pulmonary edema, Killip class 4 = cardiogenic shock.
Electrocardiographic findings. Detailed information regarding times between ECG recordings (Day 0, 1, 2, 3, 7, 14 and 30) and the investigated ECG changes are summarized in Supplementary Table 2.
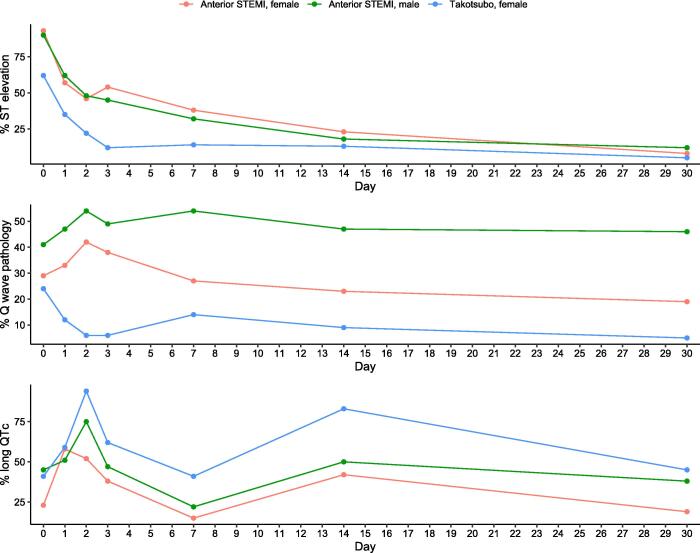
There were no differences regarding Q wave pathology, the maximum single lead T wave inversion, the average T wave inversion per lead or T inversion per se between female anterior STEMI and female TTS. The odds of having ST elevation was higher, whereas the odds of having long QTc was lower, in female anterior STEMI compared with female TTS (Table 2, Fig. 1, Fig. 2).
Table 2.
ECG changes in females with anterior STEMI versus females with Takotsubo syndrome.
| Model A* |
Model B† |
Model C‡ |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95 % CI) | P-value | P interaction§ | OR (95 % CI) | P-value | P interaction§ | OR (95 % CI) | P-value | P interaction§ | |
|
ECG variable, female patients |
|||||||||
| T wave inversion (%) | 0.81 (0.34 – 1.9) | 0.63 | 0.71 | 1.1 (0.48 – 2.6) | 0.80 | 0.75 | 1.1 (0.43 – 3.0) | 0.80 | 0.60 |
| Maximum single lead T wave inversion (mm) | 0.96 (0.39 – 2.4) | 0.92 | 0.97 | 1.1 (0.42 – 2.9) | 0.85 | 0.96 | 0.83 (0.31 – 2.2) | 0.71 | 0.82 |
| Average T wave inversion per lead (mm) | 0.79 (0.57 – 1.1) | 0.14 | 0.39 | 0.79 (0.57 – 1.1) | 0.17 | 0.48 | 0.78 (0.55 – 1.1) | 0.18 | 0.71 |
| ST elevation (%) | 8.1 (1.6 – 41) | 0.012 | 0.34 | 5.7 (1.0 – 32) | 0.045 | 0.40 | 9.3 (1.7 – 50) | 0.0091 | 0.23 |
| Q wave pathology (%) | 5.0 (1.0 – 25) | 0.050 | 0.21 | 2.8 (0.57 – 14) | 0.21 | 0.35 | 2.6 (0.50 – 13) | 0.26 | 0.52 |
| Long QTc (%) | 0.22 (0.082 – 0.60) | 0.0031 | 0.23 | 0.27 (0.099 – 0.74) | 0.010 | 0.085 | 0.18 (0.054 – 0.61) | 0.0057 | 0.93 |
STEMI = ST elevation myocardial infarction; OR = odds ratio; CI = confidence interval.
*No adjustment for baseline characteristics; †Adjusted for age, hypertension, diabetes, chronic obstructive pulmonary disease and pre-admission treatment with beta-blockers; ‡Patients with complete ECG day 0, 7 and 30; §Interaction between ECG day and group (disease).
Fig. 1.
T wave inversion, maximum single lead T wave inversion and average T wave inversion per lead in female patients with Takotsubo syndrome or anterior STEMI.
Fig. 2.
ST elevation, Q wave pathology and long QTc in female patients with Takotsubo syndrome or anterior STEMI.
When analyzing female versus male patients with anterior STEMI, there were no significant differences regarding T wave inversion, the maximum single lead T wave inversion, the average T wave inversion per lead, ST elevation or long QTc. However, the odds of Q wave pathology was lower in female versus male patients with anterior STEMI (Supplementary Table 3, Fig. 1, Fig. 2).
The temporal dynamic pattern of T wave inversions, the maximum single lead T wave inversion, the average T wave inversion per lead and long QTc was similar in all three groups. These ECG changes reach a first peak at day 2, decreased at day 7, increased to a second peak at day 14 and slowly decreased towards day 30.
Supplementary Fig. 1 displays the temporal pattern of ST elevation, T wave inversion, long QTc and Q wave pathology (within each disease group) in female and male patients with anterior STEMI, as well as in female patients with TTS. The temporal dynamic pattern of ST elevation in relation to T wave inversion and long QTc displayed a similar pattern in anterior STEMI and female TTS. Between day 0 and 2 in anterior STEMI, and between day 0 and 1 in female TTS, ST elevation markedly decreased to < 50 % with development of T wave inversion and long QTc in the majority of patients. However, in patients with ST elevation on admission (89 anterior STEMI, 18 female TTS), median days to resolution of ST elevation was longer in anterior STEMI compared with female TTS (2.0 [IQR 1.0 – 4.0] days vs 1.0 [IQR 1.0 – 2.0] days; p = 0.043).
Between day 0 and day 2, Q wave pathology increased in patients with anterior STEMI. However, Q wave pathology decreased towards day 30 in female patients with anterior STEMI (from day 2) and in female patients with TTS, whereas Q wave pathology remained unchanged in male patients with anterior STEMI.
Supplementary Figs. 2 and 3 summarizes the temporal pattern of ECG changes for TTS patients presenting with or without ST elevation. In univariable analysis, comparing TTS patients with ST elevation on admission to those without, we found no significant differences regarding T wave inversion, ST elevation (after day 0), Q wave pathology or long QTc (Supplementary Table 4). All anterior STEMI patients except one (female) received stent/s in the culprit vessel. Among anterior STEMI patients who received stents, only two patients (both female) had no-reflow (defined as TIMI flow < 2 post revascularization). The results regarding the primary objective were consistent when excluding the three female anterior STEMI patients (one who did not receive stent, two with no-reflow) who were not successfully revascularized (Supplementary Table 5).
4. Discussion
The major finding in the present study was that the prevalence of T wave inversion, the maximum single lead T wave inversion, as well as the average T wave inversion per ECG lead was similar in patients with anterior STEMI compared to female patients with TTS. Furthermore, both TTS and anterior STEMI demonstrated a dynamic “biphasic” temporal pattern of T wave inversion. That is, T wave inversion increased day 0 – 2, decreased day 2 – 7, and increased again day 7 – 14. Both conditions showed persistent T wave inversion, with 94 % of patients with anterior STEMI and 85 % of female patients with TTS having T wave inversion at day 30. This is an important finding; since widespread T wave inversions, deep T wave inversions, persistent T wave inversion, as well as biphasic temporal pattern of T wave inversion; has previously been described as ECG changes typical for TTS [5], [6], [7], [8], [10].
A major challenge when comparing temporal ECG changes in STEMI versus TTS is the time from symptom onset to first ECG. Two previous studies, with temporal head-to-head comparisons of ECG changes in TTS versus acute coronary syndrome (ACS) and AMI respectively, showed more and deeper T waves in TTS [15], [16]. However, in these previous studies, patients were included within 12 and 24 h of symptom onset; whereas most patients in the present study had a first ECG recorded within 3 h of symptom onset. Since T wave inversion develops early in both STEMI and TTS, delayed ECG at presentation in any of the groups will result in unsynchronized comparisons. Delayed admission ECG in the TTS groups is a possible explanation for the observed higher prevalence and magnitude of T wave inversion at presentation in these previous studies.
Another finding was that Q wave pathology in female patients with anterior STEMI was more similar to female patients with TTS than male patients with anterior STEMI. The small sample size may explain the lack of difference between female anterior STEMI and female TTS. The difference in Q wave pathology between female and male patients with anterior STEMI could be explained by larger infarct size in male patients, which may be supported by the lower Troponin T (TnT) in female compared with male patients with anterior STEMI (median TnT 370 vs 625) observed in the present study. Previous research has demonstrated smaller infarct size in female patients with AMI compared with male patients, despite more advanced age and more comorbidities among females [20], [21], [22]. Also, women develop IHD on average 7–10 years later than men, which is probably related to the cardioprotective effect of estrogen [19], [23].
Transient pathological Q waves in TTS have been attributed to reversible myocardial stunning rather than myocardial infarction [9], [24], and the predominance of postmenopausal women in TTS [2] may indicate a higher propensity of myocardial stunning in females than in males. It is known that acute ischemia with fast restoration of blood flow can cause myocardial stunning [25] and therefore pathological Q waves in both STEMI and TTS may be explained by myocardial stunning to some degree. The decrease of pathological Q waves from day 2 to 30 observed in female anterior STEMI, but not in male anterior STEMI, may hypothetically be explained by a larger proportion of myocardial stunning in relation to myocardial infarction in females than in males.
In the present study, long QTc was common in female TTS, increasing and decreasing in parallel with T wave inversions, which is in accordance with previous research [5]. Although more prevalent in TTS, long QTc was observed at some point in a majority of patients with anterior STEMI, also following the pattern of T wave inversions. Some authors have described this pattern of dynamic QT prolongation/T wave inversion as equal to Wellen’s ECG pattern [26], which previously has been viewed as unfavorable because of the link to sub-occlusive LAD stenosis [27]. However, later studies have suggested transient QT prolongation/T wave inversion as a marker of stunned viable myocardium in AMI patients recovering after reperfusion [28], [29]. Wellen’s ECG pattern per se has been attributed to myocardial edema [26], and CMR studies have shown myocardial edema in both STEMI and TTS. Also, ischemic T-wave abnormalities in the setting of non-ST elevation ACS have previously been associated with myocardial edema [30], [31]. Theoretically, myocardial edema may be caused by ischemia with post-ischemic stunning in both STEMI and TTS [26], [31]. Furthermore, a purely stunned myocardium, with electrically active myocardial edema (as in TTS) resulting in prolonged depolarization; as opposed to partly electrically inactive necrosis (as in STEMI); could explain the higher prevalence of long QTc in female TTS compared with anterior STEMI observed in previous research [5], as well as in the present study. Therefore, as opposed to Wellen’s ECG pattern due to untreated LAD stenosis, this ECG pattern may be favorable in re-perfused STEMI and TTS [28], [29], [32].
All TTS patients in the present study did not have ST elevation on admission ECG, which is in accordance with previous literature [5]. Therefore, ST elevation was more common in female STEMI compared with female TTS. In the pre-specified objective of this study, we considered comparing STEMI specifically (as opposed to also including non-STEMI patients) to TTS as the most appropriate approach. Whereas STEMI is caused by complete coronary artery occlusion (with ST elevation as the electrophysiological manifestation of transmural ischemia) the pathophysiology of non-STEMI is much more heterogenous; and the pathophysiology of TTS is incompletely understood [1], [2]. However, ischemic ST elevation may be dynamic, due to e.g. treatment/intervention, intermittent occlusive stenosis or transient vascular spasm, and previous evidence supports that ST elevation is the earliest ECG-change in TTS [1], [24]. Is this setting, both STEMI and TTS are characterized by transient ST elevation (after PCI in STEMI, spontaneous in TTS), supporting the choice of comparing STEMI specifically to TTS.
There is near agreement that stress-induced catecholamine excess plays a central role in the development of TTS [33], [34], and that the downstream mechanical dysfunction probably is a form of myocardial stunning [2], [34], [35]. However, there are different theories regarding the link between catecholamine excess and myocardial stunning. The main theories are direct catecholamine effect/toxicity on the cardiomyocyte and/or microvascular dysfunction/spasm [34], [35]. Regardless the pathophysiology of TTS per se, the present study indicates that temporal ECG changes in anterior STEMI and TTS may be more similar than previously thought. However, with anterior STEMI as the pathophysiological reference, temporal ECG in TTS in the present study could be interpreted as following a “transient ischemic” pattern. The longer duration of ST elevation in anterior STEMI could be explained by longer period of ischemia in anterior STEMI compared with female TTS. The higher prevalence of Q waves; and lower prevalence of long QTc; could be explained by higher proportion of myocardial necrosis in relation to post-ischemic myocardial stunning/edema in anterior STEMI compared with female TTS [9], [24], [25], [28], [29].
Another “transient ischemic” condition is Prinzmetal angina (also called variant angina or vasospastic angina) which is characterized by chest discomfort and ischemic ECG changes at rest attributed to coronary artery spasm [36]. Whereas Prinzmetal angina is attributed to spasm in large epicardial arteries, TTS has been suggested to arise from severe and prolonged microvascular spasm [37]. In fact, several previous studies have found evidence of microvascular dysfunction in TTS [34], [38], [39], [40], and microvascular dysfunction was observed only in TTS when compared with STEMI [41]. Other research has suggested a continuous spectrum between TTS and Prinzmetal angina, in which typical apical form TTS is suggested to arise from narrowing of all the mid- and distal branches of LAD [42]. Interestingly, we have previously demonstrated that ECG in TTS was particularly similar to STEMI with occlusion in LAD or any of its branches [4].
A major strength of the present study compared to previous studies [4], [9], [11], [12], [13], [14], [16] is the prospective study design. This allowed us to obtain admission ECGs with similar time from symptom onset for all patients, as well as temporal ECGs at specific time-points that were similar across all three groups. Another important strength of our study is that we did not include patients with previous myocardial infarction or previous regional myocardial dysfunction of any cause. Therefore, the ECGs obtained in the present study were not affected by previous cardiac disease. To the best of our knowledge, this is the first prospective head-to-head comparison of temporal ECG changes in anterior STEMI specifically compared with TTS.
Limitations. Although we had almost no missing ECG on admission, the study is limited by a varying degree of missing ECG at follow-up (18–29 % depending on day). However, we used a mixed effects model which is capable of handling missing data under the missing at random assumption [43]. Also, we included a sensitivity model with no missing ECG data, which showed results in line with the models including the full cohort. Furthermore, the study is limited by small number of TTS patients. However, the size of the TTS cohort is similar to previous studies of temporal ECG in ischemic conditions versus TTS, and including the STEMI group this is the largest cohort to date with temporal head-to-head comparisons of ECG. Although our study provides the most synchronized comparison of temporal ECG in STEMI versus TTS to date, the time from symptom onset to admission ECG was shorter in STEMI compared with TTS. Since ST elevation is an early ECG change in TTS [24], earlier ECGs may have resulted in a higher proportion of ST elevation in the TTS group. However, we found no differences of temporal ECG changes for female TTS patients with ST elevation on admission compared to those without (although this particular analysis should be interpreted with some caution due to the small sample size and the risk for statistical type 2 error). Lastly, because of the expected low number of male patients with TTS (due to the known rarity of TTS in males), the primary objective of the present study was to compare female anterior STEMI to female TTS; which makes our results mainly applicable to female patients with anterior STEMI or TTS.
In conclusion, the prevalence of T wave inversions, the maximum single lead T wave inversion and the average T wave inversion per lead from admission to day 30 was similar in female patients with anterior STEMI and female patients with TTS. Q wave pathology was more similar between female patients with anterior STEMI and TTS than between female and male patients with anterior STEMI. With anterior STEMI as the pathophysiological reference, temporal ECG in female patients with TTS may be interpreted as following a “transient ischemic” pattern.
Funding: This work was supported by the Swedish Heart-Lung Foundation [20180555] and the Swedish Society of Medical Research [181015].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2023.101187.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) Eur Heart J. 2018;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 2.Ghadri J.-R., Wittstein I.S., Prasad A., et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur Heart J. 2018;39:2032–2046. doi: 10.1093/eurheartj/ehy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mir T., Prakash P., Sattar Y., et al. Takotsubo syndrome vs anterior STEMI electrocardiography; a meta-analysis and systematic review. Expert Rev Cardiovasc Ther. 2020;18:819–825. doi: 10.1080/14779072.2020.1813027. [DOI] [PubMed] [Google Scholar]
- 4.Zeijlon R., Chamat J., Le V., et al. ECG differences and ECG predictors in patients presenting with ST segment elevation due to myocardial infarction versus takotsubo syndrome. IJC Heart & Vasculature. 2022;40 doi: 10.1016/j.ijcha.2022.101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghadri J.R., Wittstein I.S., Prasad A., et al. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic Workup, Outcome, and Management. Eur Heart J. 2018;39:2047–2062. doi: 10.1093/eurheartj/ehy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitsuma W., Kodama M., Ito M., et al. Serial electrocardiographic findings in women with Takotsubo cardiomyopathy. Am J Cardiol. 2007;100:106–109. doi: 10.1016/j.amjcard.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 7.Bennett J., Ferdinande B., Kayaert P., et al. Time course of electrocardiographic changes in transient left ventricular ballooning syndrome. Int J Cardiol. 2013;169:276–280. doi: 10.1016/j.ijcard.2013.08.126. [DOI] [PubMed] [Google Scholar]
- 8.Scally C., Choo W., Rudd A., et al. The early dynamic of ECG in Takotsubo syndrome presenting with ST-elevation: A comparison with age and gender-matched ST-elevation myocardial infarction. Int J Cardiol. 2020;320:7–11. doi: 10.1016/j.ijcard.2020.07.025. [DOI] [PubMed] [Google Scholar]
- 9.Frangieh A.H., Obeid S., Ghadri J.R., et al. ECG Criteria to Differentiate Between Takotsubo (Stress) Cardiomyopathy and Myocardial Infarction. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Looi J.L., Wong C.W., Lee M., Khan A., Webster M., Kerr A.J. Usefulness of ECG to differentiate Takotsubo cardiomyopathy from acute coronary syndrome. Int J Cardiol. 2015;199:132–140. doi: 10.1016/j.ijcard.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 11.Mugnai G., Pasqualin G., Benfari G., et al. Acute electrocardiographic differences between Takotsubo cardiomyopathy and anterior ST elevation myocardial infarction. J Electrocardiol. 2015;48:79–85. doi: 10.1016/j.jelectrocard.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Vervaat F.E., Christensen T.E., Smeijers L., et al. Is it possible to differentiate between Takotsubo cardiomyopathy and acute anterior ST-elevation myocardial infarction? J Electrocardiol. 2015;48:512–519. doi: 10.1016/j.jelectrocard.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Kosuge M., Ebina T., Hibi K., et al. Simple and accurate electrocardiographic criteria to differentiate takotsubo cardiomyopathy from anterior acute myocardial infarction. J Am Coll Cardiol. 2010;55:2514–2516. doi: 10.1016/j.jacc.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 14.Johnson N.P., Chavez J.F., Mosley W.J., 2nd, Flaherty J.D., Fox J.M. Performance of electrocardiographic criteria to differentiate Takotsubo cardiomyopathy from acute anterior ST elevation myocardial infarction. Int J Cardiol. 2013;164:345–348. doi: 10.1016/j.ijcard.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 15.Guerra F., Rrapaj E., Pongetti G., et al. Differences and similarities of repolarization patterns during hospitalization for Takotsubo cardiomyopathy and acute coronary syndrome. Am J Cardiol. 2013;112:1720–1724. doi: 10.1016/j.amjcard.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 16.Kurisu S., Inoue I., Kawagoe T., et al. Time course of electrocardiographic changes in patients with tako-tsubo syndrome: comparison with acute myocardial infarction with minimal enzymatic release. Circ J. 2004;68:77–81. doi: 10.1253/circj.68.77. [DOI] [PubMed] [Google Scholar]
- 17.Bazett H.C. AN ANALYSIS OF THE TIME-RELATIONS OF ELECTROCARDIOGRAMS. Annals of noninvasive electrocardiology. 1997;2:177–194. [Google Scholar]
- 18.Lyon A.R., Bossone E., Schneider B., et al. Current state of knowledge on Takotsubo syndrome: a Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18:8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 19.Ibanez B., James S., Agewall S., et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2017;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 20.Ng V.G., Mori K., Costa R.A., et al. Impact of gender on infarct size, ST-segment resolution, myocardial blush and clinical outcomes after primary stenting for acute myocardial infarction: Substudy from the EMERALD trial. Int J Cardiol. 2016;207:269–276. doi: 10.1016/j.ijcard.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 21.De Luca G., Parodi G., Sciagrà R., et al. Relation of gender to infarct size in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty. Am J Cardiol. 2013;111:936–940. doi: 10.1016/j.amjcard.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Nordlund D., Engblom H., Bonnet J.-L., et al. Gender but not diabetes, hypertension or smoking affects infarct evolution in ST-elevation myocardial infarction patients – data from the CHILL-MI, MITOCARE and SOCCER trials. BMC Cardiovasc Disord. 2019;19:161. doi: 10.1186/s12872-019-1139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosengren A., Wallentin L., Gitt A.K., Behar S., Battler A., Hasdai D. Sex, age, and clinical presentation of acute coronary syndromes. Eur Heart J. 2004;25:663–670. doi: 10.1016/j.ehj.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Kosuge M., Kimura K. Electrocardiographic findings of takotsubo cardiomyopathy as compared with those of anterior acute myocardial infarction. J Electrocardiol. 2014;47:684–689. doi: 10.1016/j.jelectrocard.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Vaidya Y, Cavanaugh SM, Dhamoon AS. Myocardial Stunning and Hibernation. StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2022, StatPearls Publishing LLC.; 2022. [PubMed]
- 26.Migliore F., Zorzi A., Marra M.P., et al. Myocardial edema underlies dynamic T-wave inversion (Wellens' ECG pattern) in patients with reversible left ventricular dysfunction. Heart Rhythm. 2011;8:1629–1634. doi: 10.1016/j.hrthm.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 27.de Zwaan C., Bär F.W.H.M., Wellens H.J.J. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J. 1982;103:730–736. doi: 10.1016/0002-8703(82)90480-x. [DOI] [PubMed] [Google Scholar]
- 28.Obayashi T., Tokunaga T., Iiizumi T., Shiigai T., Hiroe M., Marumo F. Transient QT interval prolongation with inverted T waves indicates myocardial salvage on dual radionuclide single-photon emission computed tomography in acute anterior myocardial infarction. Jpn Circ J. 2001;65:7–10. doi: 10.1253/jcj.65.7. [DOI] [PubMed] [Google Scholar]
- 29.Ieva R., Casavecchia G., Gravina M., et al. Prolonged QT and myocardium recovery after primary PCI: a cMRI study. Eur J Clin Invest. 2016;46:873–879. doi: 10.1111/eci.12670. [DOI] [PubMed] [Google Scholar]
- 30.Cardona A., Zareba K.M., Nagaraja H.N., et al. T-Wave Abnormality as Electrocardiographic Signature of Myocardial Edema in Non-ST-Elevation Acute Coronary Syndromes. J Am Heart Assoc. 2018;7(3) doi: 10.1161/JAHA.117.007118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrick D., Haig C., Ahmed N., et al. Temporal Evolution of Myocardial Hemorrhage and Edema in Patients After Acute ST-Segment Elevation Myocardial Infarction: Pathophysiological Insights and Clinical Implications. J Am Heart Assoc. 2016 Feb 23;5(2):e002834. doi: 10.1161/JAHA.115.002834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renkin J., Wijns W., Ladha Z., Col J. Reversal of segmental hypokinesis by coronary angioplasty in patients with unstable angina, persistent T wave inversion, and left anterior descending coronary artery stenosis. Additional evidence for myocardial stunning in humans. 1990;82:913–921. doi: 10.1161/01.cir.82.3.913. [DOI] [PubMed] [Google Scholar]
- 33.Wittstein I.S., Thiemann D.R., Lima J.A., et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 34.Pelliccia F., Kaski J.C., Crea F., Camici P.G. Pathophysiology of Takotsubo Syndrome. Circulation. 2017;135:2426–2441. doi: 10.1161/CIRCULATIONAHA.116.027121. [DOI] [PubMed] [Google Scholar]
- 35.Lyon A.R., Citro R., Schneider B., et al. Pathophysiology of Takotsubo Syndrome: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;77:902–921. doi: 10.1016/j.jacc.2020.10.060. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez Ziccardi M, Hatcher JD. Prinzmetal Angina. StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2022, StatPearls Publishing LLC.; 2022. [PubMed]
- 37.Lüscher T.F., Templin C. Is takotsubo syndrome a microvascular acute coronary syndrome? Towards of a new definition. Eur Heart J. 2016;37:2816–2820. doi: 10.1093/eurheartj/ehw057. [DOI] [PubMed] [Google Scholar]
- 38.Crea F., Camici P.G., Bairey Merz C.N. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–1111. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rigo F., Sicari R., Citro R., Ossena G., Buja P., Picano E. Diffuse, marked, reversible impairment in coronary microcirculation in stress cardiomyopathy: A Doppler transthoracic echo study. Annals of medicine (Helsinki). 2009;41:462–470. doi: 10.1080/07853890903022793. [DOI] [PubMed] [Google Scholar]
- 40.Patel S.M., Lerman A., Lennon R.J., Prasad A. Impaired coronary microvascular reactivity in women with apical ballooning syndrome (Takotsubo/stress cardiomyopathy) Eur Heart J Acute Cardiovasc Care. 2013;2:147–152. doi: 10.1177/2048872613475891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galiuto L., De Caterina A.R., Porfidia A., et al. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in Apical Ballooning or Tako-Tsubo Syndrome. Eur Heart J. 2010;31:1319–1327. doi: 10.1093/eurheartj/ehq039. [DOI] [PubMed] [Google Scholar]
- 42.Angelini P. Takotsubo cardiomyopathy: what is behind the octopus trap? Tex Heart Inst J. 2010;37:85–87. [PMC free article] [PubMed] [Google Scholar]
- 43.Detry M.A., Ma Y. Analyzing Repeated Measurements Using Mixed Models. JAMA. 2016;315:407–408. doi: 10.1001/jama.2015.19394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.