Highlights
-
•
Disease-free survival was not different when comparing laparoscopic versus open surgery.
-
•
Overall survival at 2 years in patients with stage I–II high-risk endometrial cancer was similar.
-
•
Overall survival at 4 years in patients with stage I–II high-risk endometrial cancer was similar.
Keywords: Endometrial cancer, Gynecologic surgical procedures, Perioperative complications, Surgical oncology, Surgical procedures
Abstract
Objective
Compare the perioperative outcomes and disease-free survival between minimally invasive and open surgery in women with stage I–II high-risk endometrial cancer.
Methods
A retrospective, cohort study was performed involving twenty-four centers from Argentina. Patients with grade 3 endometrioid, serous, clear cell, undifferentiated carcinoma or carcinosarcoma who underwent hysterectomy, bilateral salpingo-oophorectomy, and staging between January 2010–2018 were included. Cox hazard regression analysis and Kaplan-Meier curves evaluated the association of surgical technique with survival.
Results
Of 343 eligible patients, 214 (62 %) underwent open surgery and 129 (38 %) underwent laparoscopic surgery. No significant differences were seen between the two groups with respect to greater or equal grade III Clavien-Dindo postoperative complications (11 % in the open surgery group vs 9 % minimally invasive surgery group; P = 0.34) Minimally invasive surgery was not associated with worse disease-free survival at four years (79.14 % [95 % CI 69.42– 86.08] vs 78.80 % [95 % CI 70.61–84.96]), (p = 0.25), even after creating a Cox proportional model (hazard ratio [HR] 1.08 95 % CI 0.63–1.84); (p = 0.76).
Conclusion
There was no difference between postoperative complications nor oncologic outcomes comparing minimally invasive and open surgery among patients with high-risk endometrial cancer.
1. Introduction
Endometrial cancer is the most common gynecologic malignancy in the developed world and the second most common in developing countries. The incidence of endometrial cancer in Argentina is around 7.6 per 100,000 inhabitants per year, while the mortality rate is 2.3 (Sung et al., 2021, [Internet]. Accessed Sep 29, 2022, https://gco.iarc.fr/today/data/factsheets/populations/32-argentina-fact-sheets.pdf.). The estimated annual incidence of carcinoma of the uterine corpus is 320,000 cases. Most of these are early-stage, low-grade tumors, traditionally classified as Type 1 tumors. Nevertheless, there are less frequent histopathologic types, known as Type 2 tumors, considered to be more aggressive variants of malignant epithelial tumors, with a higher incidence of extra uterine disease at presentation, poorer prognosis, and, consequently, higher risk (Bokhman, 1983).
International guidelines currently recommend minimally invasive surgery (MIS) in those patients with apparent uterine-confined disease, including patients with high-risk endometrial carcinoma (Concin et al., 2021, National Comprehensive Cancer Network. Uterine neoplasms (version 1.2022). Accessed Mar 25, 2022, https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf). Numerous studies report better perioperative outcomes with this approach rather than with laparotomy (Kornblith et al., 2009, Walker et al., 2009). Considering the oncological outcomes, prospective and randomized trials have proven the safety of MIS in low-grade, early-stage tumors (LAP2, LACE) (Walker et al., 2009, Janda et al., 2017), but there are few reports evaluating its role in the management of high-grade uterine malignancies (Fader et al., 2016 Dec).
The higher rates of locoregional recurrence and mortality in patients undergoing minimally invasive radical hysterectomy for cervical malignancies have raised concern regarding the association between the surgical approach chosen and oncological outcomes in patients with high-risk endometrial cancer. The aim of this study was to compare the perioperative and oncological outcomes in women with stage I–II high-risk endometrial cancer who were operated by MIS vs laparotomy.
2. Methods
2.1. Study population
A retrospective, cohort study was performed involving twenty-four Argentinean centers: Hospital Italiano de Buenos Aires, Hospital CEMIC, Sanatorio Allende, Hospital Provincial Neuquén, Hospital Español, Hospital Marie Curie, Hospital Italiano Córdoba, Hospital Britanico, Instituto de oncología Angel Roffo, Hospital Central Mendoza, Hospital Privado Rosario, Hospital Italiano Rosario, Hospital Clemente Álvarez, Grupo OSECAC, Clínica del Niño y la Madre Mar del Plata, Hospital José María Penna, Hospital Nacional de Clínicas de Córdoba, Hospital San Martín de Paraná, Hospital Alemán, Hospital Zonal Carlos Bocalandro, Hospital Privado de Córdoba, Hospital Policial Churruca, Hospital José M. Cullen, Hospital Universitario de Maternidad y Neonatología de la Ciudad de Córdoba. Institutional review board approval was obtained at all participating sites.
Women with a pathologic diagnosis of a grade 3 endometrioid carcinoma, uterine papillary serous carcinoma (USC), clear cell carcinoma, carcinosarcoma, dedifferentiated and undifferentiated endometrial tumor were identified from the surgical pathology databases from the years 2010–2018. The inclusion criteria were patients older than 25 years, operated by laparoscopy or laparotomy with disease limited to the uterus in the final pathology report, certainly stage I–II, according to the International Federation of Gynecology and Obstetrics (FIGO) 2018 (Amant et al., 2018 Oct). A pre-operative impression of apparent early-stage disease by exam and imaging was required (computed tomography or magnetic resonance imaging of the abdomen and pelvis and chest radiograph or chest computed tomography). At least 24 months of follow-up was requested after surgery. Exclusion criteria were patients with synchronous tumors, and patients treated during a relapse but who had not received their primary treatment at their institution, and patients operated with a robotic approach.
Perioperative results were mainly based on the following variables: duration of surgery in minutes, hospital stay in days, use of uterine manipulator, and perioperative complications according to the Clavien-Dindo Score.
All patients underwent pelvic lymph node dissection regardless of whether there was sentinel lymph node assessment. Para-aortic lymph node dissection was performed at the surgeon’s discretion. A simple hysterectomy was performed in stage II cases. Patients were followed every 3 months for the first 2 years and every 6 months from years 3–5 after completion of treatment with routine pelvic examinations. Imaging studies were obtained if there was suspicion of disease recurrence.
Patients could be observed after surgery or received adjuvant therapy according to the treating physician's discretion, which included one of the following: platinum/taxane based chemotherapy, radiation therapy, or a combination of chemotherapy and radiation.
2.2. Data collection
The collection and audit of the quality of the data was carried out in two phases. First, the data were extracted by a coordinator, collected in the Research Electronic Data Capture (REDCap) software, and audited by the researcher from each participating center. Subsequently, a central verification of the information was carried out in the Data Analysis Unit of the Hospital Italiano de Buenos Aires, comparing the medical records with the REDCap data. Clinical information, final pathology reports, the need for adjuvant treatment, recurrence rate, and vital status were included.
2.3. Statistical analysis
Disease-free survival (DFS) was established as the time elapsed between the event of relapse or death from the date of surgery, in accordance with other published studies (Janda et al., 2017, Fader et al., 2012, Segarra-Vidal et al., 2021, Gao and Zhang, 2015) Overall survival (OS) was defined as the time elapsed from the date of the surgical procedure, to the date of the last visit or the date of death. The relapse event was interpreted as the presence of disease by images or histological biopsy after having completed a primary treatment. Continuous variables were described as mean with their respective standard deviation if they had a normal distribution, or as a median if the distribution was skewed with their respective interquartile ranges 25–75 % (IQR) and were compared using a Student's t-test or a Mann-Whitney test, respectively. Categorical variables were reported as number or percentage and were compared using the Chi-square test. The DFS and OS were estimated at 2 and 4 years, with their respective confidence intervals, using the Kaplan-Meier method. A Cox proportional hazard model was constructed to analyze the presence of possible confounders in the estimation of DFS and OS. Factors included in the model were age, stage, histology, type of surgery (laparoscopy-open), adjuvant treatment (observation/other, radiation therapy, chemotherapy +/−radiation). Crude and adjusted Hazard Ratio of each predictor with its confidence interval was presented. Two-tailed p-values < 0.05 were considered statistically significant. STATA version 13 software was used for statistical analysis.
3. Results
Of the 530 patients with high-risk endometrial cancer FIGO stage IA, IB,II treated in the participating institutions within the study period, 343 patients met the inclusion criteria (Fig. 1 A). Of these, 214 (62 %) underwent open surgery, while 129 (38 %) underwent a minimally invasive approach. Of the 24 centers that provided patients for this study, 12 (50 %) corresponded to public and governmental institutions and the other 12 (50 %) belonged to the private healthcare system (Fig. 1 B).
Fig. 1.
A-Flowchart of patient selection. B- Cities that contributed patients.
After analyzing demographic characteristics of patients in both groups, we noticed that, of the total number of patients treated with a minimally invasive approach, 102 (79 %) were treated in Buenos Aires. In addition to this, more than 90 % of this minimally invasive subgroup were managed in private institutions.
The mean (standard deviation) age in the MIS group was 65 years (11.63) vs 64 (10.63) in the open surgery group, while the mean body mass index was 27.05 (24.31) vs 29.05 (25.35), respectively. There were no significant differences between the two groups with respect to the histologic subtype (Table 1).
Table 1.
Demographic characteristics.
| Variable | Open (n = 214) | MIS (n = 129) | P |
|---|---|---|---|
| Age (SD) | 64.4 (10.63) | 65.44 (11.96) | 0.42 |
| BMI | 29 (25, 35) | 27 (24, 31) | 0.025 |
| Public healths institutions | 79 (36.9) | 2 (1.6) | <0.001 |
| Private Institutions | 135 (63.1) | 127 (98.4) | <0.001 |
| Histology | |||
| Serous | 73 (34.1) | 55 (42.6) | 0.54 |
| Endometrioid G3 | 75 (35.0) | 37 (28.7) | |
| Clear Cell | 27 (12.6) | 19 (14.7) | |
| Carcinosarcoma | 27 (12.6) | 15 (11.6) | |
| Undifferentiated | 7 (3.3) | 3 (2.3) | |
SD, standard deviation, BMI, body mass index, MRI, magnetic resonance imaging, CT, computed tomography.
Data are median (interquartile range) or n (%) unless otherwise specified.
In all the patients who were treated minimally invasively, a uterine manipulator was used. The open surgery group presented more patients with stage II according to the FIGO 2009 classification compared with the MIS group (n = 39 [18.22 %] vs 7 [5.43 %]). The were no differences between median [interquartile range] duration of surgery in both groups (199 min [150, 240] vs 180 min [120, 240], P = 0.08); estimated blood loss was lower in the MIS group compared with the open surgery group (150 mL [80–250] vs 430 mL [270–650], P < 0.01). No difference was evident when analyzing the number of pelvic and para-aortic lymphadenectomies in both groups. A total of 8 (6 %) cases were converted from laparoscopy to laparotomy (3 due to the impossibility to remove the uterus through the vagina, 3 due to intraoperative complications, and 2 due to technical issues). A total of 20 (6 %) intraoperative complications were reported, including 5 vascular lesions (4 in the MIS and 1 in the laparotomy group), 8 bladder injuries (4 in the in each group), and 7 intestinal lacerations (2 in the MIS group and 5 in the laparotomy group). No significant differences were seen between the two groups with respect to greater or equal grade III Clavien-Dindo postoperative complications (11 % in the open surgery group vs 9 % minimally invasive surgery group; P = 0.34) (Table 2).
Table 2.
Clinical characteristics.
| Variable | Open (n = 214) | MIS (n = 129) | P |
|---|---|---|---|
| FIGO classification n, (%) | |||
| IA | 89 (41.6) | 73 (56.6) | <0.01 |
| IB | 86 (40.2) | 49 (38.0) | |
| II | 39 (18.2) | 7 (5.4) | |
| Surgery time (min) | 199 (150–240) | 180 (120, 240) | 0.08 |
| Estimated blood loss (mL) | 350 (280–600) | 150 (70–240) | <0.01 |
| Standard pelvic lymphadenectomy | 141 (66.9) | 90 (70.0) | 0.49 |
| Standard paraaortic lymphadenectomy | 79 (36.9) | 52 (40.3) | 0.55 |
| Sentinel node mapping | 8 (3.7) | 28 (21.7) | <0.01 |
| Adjuvant therapy | 138 (64.5) | 99 (76.7) | 0.01 |
| Postoperative complications* | 11 (5.1) | 9 (7.0) | 0.34 |
| Hospital length of stay (days) | 4 (2–7) | 2 (1–4) | <0.01 |
| Chemotherapy | 98 (45.8) | 53 (41.1) | 0.39 |
| Pelvic radiotherapy | 21 (9.8) | 10 (7.8) | 0.51 |
| Both chemotherapy and pelvic radiotherapy | 21 (9.8) | 32 (24.8) | <0.01 |
| Lymphatic vascular invasion | 47 (22.0) | 51 (39.5) | <0.01 |
* Grade III or higher Clavien-Dindo classification.
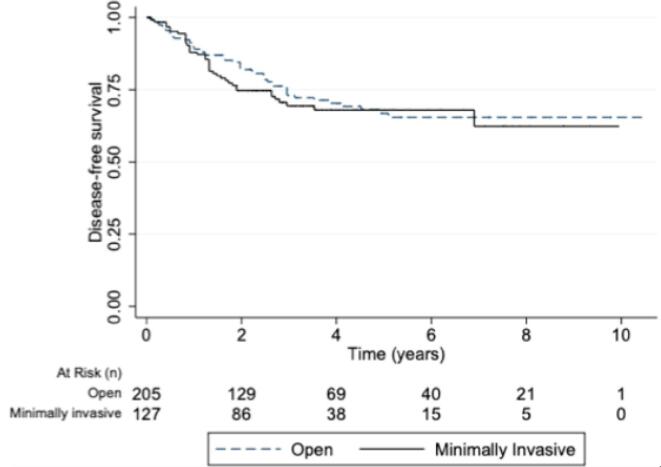
The Median follow-up time was 40 months (range: 5–160 months). Patients in the minimally invasive group were more likely to receive at least one adjuvant treatment after surgery (138 [64.49 %]) vs 99 [76.74 %], P < 0.001). When evaluating the type of adjuvant treatment received, the MIS group received a higher percentage of a combined treatment (chemotherapy plus radiotherapy) compared to the laparotomy group (32 [24.8 %]) vs 21 [9,8%], P < 0.001). When comparing the results in terms of survival, the median [interquartile range] disease-free survival time was 31.56 months (16.27–59.40 months) in the open surgery group and 33.99 months (19.56– 52.63 months) in the minimally invasive group (P = 0.77) (Fig. 2) Disease-free survival at 2 years was 74.11 % (95 % CI 65.25–81.03) in the minimally invasive surgery group and 82.02 % (95 % CI 75.50–89.96) in the open surgery group. At 4 years, disease-free survival was 67.06 % (95 % CI 57.23–75.11) in the minimally invasive surgery group compared with 69.21 % (95 % CI 60.96–76.06) in the open surgery group (p = 0.55) (Table 3). Patients undergoing minimally invasive surgery had a similar rate of overall survival compared to those undergoing open surgery at 2 years (90.63 % [95 % CI 83.71–94.70] vs 91.81 % [95 % CI 86.54–95.08]), and at 4 years (79.14 % [95 % CI 69.42–86.08] vs 78.80 % [95 % CI 70.61–84.96]), (p = 0.25).
Fig. 2.
Kaplan-Meier curves for disease-free survival (A), overall survival (B).
Table 3.
Survival outcomes for minimally invasive surgery compared with open surgery.
| Variable | Open (n = 214) | MIS (n = 129) | P |
|---|---|---|---|
| Disease-free survival | |||
| 2 y | 82.02 % (75.50–86.96) | 74.11 % (65.25–81.03) | 0.55 |
| 4 y | 69.21 % (60.96–76.06) | 67.06 % (57.23–75.11) | |
| Overall survival | |||
| 2 y | 91.81 % (86.54–95.08) | 90.63 % (83.71–94.70) | 0.25 |
| 4 y | 78.80 % (70.61–84.96) | 79.14 % (69.42–86.08) | |
| Recurrence | 52 (25 %) | 36 (28 %) | 0.48 |
| Location of recurrence | |||
| Vaginal cuff | 9 (17 %) | 3 (8 %) | 0.31 |
| Pelvic | 11(21 %) | 6 (17 %) | |
| Distant | 32 (62 %) | 27 (78 %) | |
| Months to recurrence | 31(4.92–96.84) | 33 (9.84–79.92) | 0.53 |
When analyzing the results in terms of DFS and OS according to whether the patient underwent the treatment, private vs state, no differences were found.
After creating a Cox proportional model, the type of surgical approach was not associated with an increased risk of recurrence (hazard ratio [HR] 1.08 95 % CI 0.63–1.84); (p = 0.76), adjusted by age, BMI, FIGO stage, adjuvant treatment received and histological cell type (Table 4). Finally, we found no evidence of association between DFS and type of surgical approach (adjusted HR 1.35; 95 % CI, 0.67–2.37).
Table 4.
Cox multivariable regression for progression-free survival-surgical approach.
| Variable | Adjusted hazards ratio (95 % CI) | P |
|---|---|---|
| Surgery (MIS vs laparotomy) | 1.08 (0.63–1.84) | 0.76 |
| Age | 1.04 (1.02–1.07) | <0.01 |
| BMI | 1.00 (0.96–1.04) | 0.98 |
| FIGO stage | ||
| IA vs IB | 1.48 (0.85–2.58) | 0.56 |
| IB vs II | 2.16 (1.03–4.50) | 0.04 |
| Adjuvant treatment received | 0.68 (0.40–1.17) | 0.17 |
| Private vs public coverage | 0.95 (0.49–1.83) | 0.89 |
| Histology | ||
| Serous | 0.61 (0.29–1.29) | 0.19 |
| Endometrioid G3 | 0.64 (0.29–1.42) | 0.28 |
| Clear Cell | 0.51 (0.26–0.99) | 0.04 |
| Carcinosarcoma | 0.69 (0.35–1.49) | 0.22 |
4. Discussion
In this retrospective multicenter study, we compared the outcomes of minimally invasive surgery vs open surgery in the treatment of 342 patients with high-risk endometrial cancer, FIGO stages I and II. After comparing in terms of treatment performed and oncological results between both groups, we were able to confirm that minimally invasive surgery was not significantly associated with worse disease-free survival compared with open surgery (hazard ratio [HR] 1.08 95 % CI 0.63–1.84; p = 0.76). As well, patients undergoing minimally invasive surgery had a similar rate of overall survival at 2 years (90.63 % [95 % CI 83.71–94.70] vs 91.81 % [95 % CI 86.54–95.08, p = 0.25) and at 4 years (79.14 % [95 % CI 69.42– 86.08] vs 78.80 % [95 % CI 70.61–84.96] p = 0.31), when compared with laparotomy procedures (Table 3).
It should be noted that patients in the MIS group had a lower percentage of FIGO stage II tumors (5.43 % vs 18.22 %). These patients also underwent at least one adjuvant treatment in 77 % of the cases, while in the laparotomy group only in 65 % of the cases (p = 0.01). However, when the type of surgical approach was adjusted for FIGO stage and adjuvant treatment received, the laparotomy group did not present worse outcomes in terms of DFS or OS (Table 4).
The use of minimally invasive surgery in the treatment of endometrial cancer was established as a standard based on two randomized studies. First, the Gynecologic Oncology Group LAP-2 trial, which randomized more than 2600 patients with endometrial cancer to open surgery or laparoscopy. This study reported fewer postoperative adverse events, shorter hospital stay, less pain, earlier resumption of daily activities, and more improved quality of life, than the open surgery group, along with a similar estimated 5-year OS (89.8 %) (Walker et al., 2012). Secondly, the LACE trial included 760 patients randomized to both surgical approaches, and like the LAP-2 trial, presented similar DFS and OS (Janda et al., 2017). These studies focused primarily on patients with relatively low-risk type I endometrial cancer. In the LACE trial <4 % of the patients had high-risk tumors, while in the GOG LAP 2 <20 % belonged to this subgroup. Furthermore, with a very low percentage of type II endometrial cancer cases as the rarer and more aggressive histotype, this cancer often has been excluded or represented only marginally in randomized trials. Therefore, these randomized studies give us insufficient data regarding the best approach for high-risk endometrial tumors.
Based on these findings, several authors have questioned the safety of laparoscopic surgery in this setting. In 2012, Fader et al. published a retrospective evaluation of patients with high-risk endometrial cancer identified between 1999 and 2009 in tumor registries and surgical pathology databases, treated in several US academic centers. 383 patients were included, 191 underwent laparotomy and 192 MIS. In addition to confirming shorter hospital stays and fewer complications in favor of MIS, the authors concluded that both approaches had similar DFS (Three-year DFS 81.5 % vs 83.6 % [p = 0.58]) (Fader et al., 2012 Aug).
Martin Koskas et al. reported a cohort of patients with high-risk endometrial cancer (114 patients operated by MIS, vs 114 by laparotomy). Groups were comparable for stage, body mass index, histology and adjuvant therapies. With a median follow-up time of 49 months, DFS and OS were not significantly different between the surgical cohorts. In multivariable analysis, both higher stage (hazard ratio [HR] 2.2) and histology (HR 4.9) were associated with DFS in contrast to surgical procedure (HR 0.9) (Koskas et al., 2016).
Recently, Blanca Segarra et al, published a comparative study between both approaches in two high-volume centers in the United States. It included patients with high-risk endometrial tumors, 263 (42 %) underwent minimally invasive surgery and 363 (58 %) underwent open surgery. In the matched cohort, minimally invasive surgery was not associated with worse disease-free survival (hazard ratio [HR] 0.85, 95 % CI 0.63–1.16; P = 0.30), overall survival (HR 1.04, 95 % CI 0.73–1.48, P = 0.81), or recurrence rate (HR 0.99; 95 % CI 0.69–1.44; P5.99) compared with open surgery. Interestingly, it is one of the first studies that mentions no difference in oncological outcome even with the use of uterine manipulator (Segarra-Vidal et al., 2021).
Our results were similar to those published to date in the retrospective literature, demonstrating that both minimally invasive and open surgery are adequate and safe for the management of high-risk endometrial tumors. However, we consider that the evidence is still weak, since it is only based on retrospective reviews. Our research has several limitations, mainly referring to its retrospective design, which increases information bias. In addition, the incorporation of many centers with high and low volume patients prevents a strict control of important variables at diagnosis, at surgical treatment, and at follow-up. Furthermore, 52 % of the patients treated with MIS were treated in a single center, and the adjuvant treatments applied were very varied.
The strengths of the study lie in that it is the first with the incorporation of 24 hospital centers in Argentina, with a significant number of patients evaluated with adequate follow-up (40 months [range: 5–160]). In addition, only patients with disease limited to the uterus were evaluated, unlike almost all published reports, which include patients with more advanced stages.
There are currently good levels of evidence from clinical trials that support the safety of a minimally invasive approach for low risk endometrial cancer. According to this study, MIS is also safe and feasible in high risk endometrial tumors. Prospective and randomized studies are needed to confirm these findings.
Financial support
No financial support was received for this study.
Author contribution
All the authors have contributed to the preparation of this article (design, planning, conduct, data analysis, and manuscript writing).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We wish to thank Natalia Zeff, Lara Vargas, Soledad Gasparini, Juan Carlos Stalinger, Graciela Horton, Sebastian Irico, Julian Diguilmi, Angeles Nico, Martin Riege, Juan Taverna, Florencia Camer, Mariano Leanza, Tomas Ramilo, Romina Cardozo, Jorgelina Ponce Traverso, Natalia Melchiorre, D'Imperio Nicolas, Gemma Aguil, Laura Bergamin and Agustina Simonielo for their valuable input in this study.
Synopsis: In this Argentine multicenter cohort study, stage I–II high-risk endometrial cancer treated by laparoscopic or laparotomy approach has similar perioperative results and disease free survival.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2023.101147.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Amant F., Mirza M.R., Koskas M., Creutzberg C.L. Cancer of the corpus uteri. Int. J. Gynaecol. Obstet. 2018;143(Suppl. 2):37–50. doi: 10.1002/ijgo.12612. [DOI] [PubMed] [Google Scholar]
- Bokhman J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983;15(1):10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- Concin N., Matias-Guiu X., Vergote I., Cibula D., Mirza M.R., Marnitz S., et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer. 2021;31(1):12–39. doi: 10.1136/ijgc-2020-002230. [DOI] [PubMed] [Google Scholar]
- Fader A.N., Seamon L.G., Escobar P.F., Frasure H.E., Havrilesky L.A., Zanotti K.M., et al. Minimally invasive surgery versus laparotomy in women with high grade endometrial cancer: a multi-site study performed at high volume cancer centers. Gynecol. Oncol. 2012;126(2):180–185. doi: 10.1016/j.ygyno.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Fader A.N., Java J., Tenney M., Ricci S., Gunderson C.C., Temkin S.M., et al. Impact of histology and surgical approach on survival among women with early-stage, high-grade uterine cancer: an NRG Oncology/Gynecologic Oncology Group ancillary analysis. Gynecol. Oncol. 2016;143(3):460–465. doi: 10.1016/j.ygyno.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Zhang Z. Laparoscopy versus laparotomy in the treatment of high-risk endometrial cancer: a propensity score matching analysis. Medicine. 2015;94(30):e1245. doi: 10.1097/MD.0000000000001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda M., Gebski V., Davies L.C., Forder P., Brand A., Hogg R., et al. Effect of total laparoscopic hysterectomy vs total abdominal hysterectomy on disease-free survival among women with stage I endometrial cancer: a randomized clinical trial. J. Am. Med. Assoc. 2017;317(12):1224–1233. doi: 10.1001/jama.2017.2068. [DOI] [PubMed] [Google Scholar]
- Kornblith A.B., Huang H.Q., Walker J.L., Spirtos N.M., Rotmensch J., Cella D. Quality of life of patients with endometrial cancer undergoing laparoscopic international federation of gynecology and obstetrics staging compared with laparotomy: a Gynecologic Oncology Group study. J. Clin. Oncol. 2009;27(32):5337–5342. doi: 10.1200/JCO.2009.22.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskas M., Jozwiak M., Fournier M., Vergote I., Trum H., Lok C., et al. Long-term oncological safety of minimally invasive surgery in high-risk endometrial cancer. Eur. J. Cancer. 2016;65:185–191. doi: 10.1016/j.ejca.2016.07.001. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. Uterine neoplasms (version 1.2022). Accessed Mar 25, 2022, https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf.
- Segarra-Vidal B., Dinoi G., Zorrilla-Vaca A., Mariani A., Student V., Garcia N.A., et al. Minimally invasive compared with open hysterectomy in high-risk endometrial cancer. Obstet. Gynecol. 2021;138(6):828–837. doi: 10.1097/AOG.0000000000004606. [DOI] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Walker J.L., Piedmonte M.R., Spirtos N.M., Eisenkop S.M., Schlaerth J.B., Mannel R.S., et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J. Clin. Oncol. 2009;27(32):5331–5336. doi: 10.1200/JCO.2009.22.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J.L., Piedmonte M.R., Spirtos N.M., Eisenkop S.M., Schlaerth J.B., Mannel R.S., et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J. Clin. Oncol. 2012;30(7):695–700. doi: 10.1200/JCO.2011.38.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Internet]. Accessed Sep 29, 2022. https://gco.iarc.fr/today/data/factsheets/populations/32-argentina-fact-sheets.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.