Abstract
People living with the human immunodeficiency virus (HIV) have an elevated risk of opioid misuse due to both prescriptions for HIV-associated chronic pain and because injection drug use remains a primary mode of HIV transmission. HIV pathogenesis is characterized by chronic immune activation and microbial dysbiosis, and translocation across the gut barrier exacerbating inflammation. Despite the high rate of co-occurrence, little is known about the microbiome during chronic opioid use in the context of HIV and combination antiretroviral therapy (cART). We recently demonstrated the reduction of the CD4 + T-cell reservoir in lymphoid tissues but increased in microglia/macrophage reservoirs in CNS by using morphinetreated, simian immunodeficiency virus (SIV)-infected rhesus macaques with viremia suppressed by cART. To understand whether morphine may perturb the gut-brain axis, fecal samples were collected at necropsy, DNA isolated, and 16S rRNA sequenced and changes of the microbiome analyzed. We found that morphine treatment led to dysbiosis, primarily characterized by expansion of Bacteroidetes, particularly Prevotellaceae, at the expense of Firmicutes and other members of healthy microbial communities resulting in a lower α-diversity. Of the many genera in Prevotellaceae, the differences between the saline and morphine group were primarily due to a higher relative abundance of Prevotella_9, the taxa most similar to Prevotella copri, an inflammatory pathobiont in the human microbiome. These findings reinforce previous research showing that opioid abuse is associated with dysbiosis, therefore, warranting additional future research to elucidate the complex interaction between the host and opioid abuse during HIV and SIV infection.
Keywords: Opioids, Simian immunodeficiency virus, Microbiome, Combination antiretroviral Therapy, Macaque models
Introduction
New HIV infections continue to climb globally despite increasing rates of combination antiretroviral therapies (cART) and increased measures to decrease transmission. Globally, approximately 38 million people are living with HIV (PLWH)(UNAIDS 2019). Of these, 1.2 million are in the United States. In addition to a high HIV rate, there is also an opioid epidemic caused by the overuse of opioid medications and illicit drug use. HIV-associated chronic pain is a common symptom for PLWH, with perhaps over 50% of this population being treated with either short- or long-acting opioid prescriptions (Larue et al. 1997; Miaskowski et al. 2011). Chronic pain rates are even more significant for HIV + injection drug users (Del Borgo et al. 2001).
Further, up to 7% of HIV infections are the result of injection drug use, and injection opioid use, in particular, has remained a significant mode of HIV transmission during the ongoing epidemic in the US (CDC; Williams and Bisaga 2016). Considerable progress has been made in treating people with HIV, including developing highly effective and combination antiretroviral therapies (cART), which have eased much of the pathology associated with HIV allowing most individuals infected with HIV to live long healthy lives. Additionally, advancements in cART have allowed for the development of pre-exposure prophylaxis (PrEP) to prevent infection in high-risk individuals and preventing HIV transmission with the current understanding that individuals with cART-suppressed viral plasma viral loads are unable to transmit the virus, or at least the risk is negligible (undetectable = untransmittable)(Brault et al. 2019; Chou et al. 2019; Cohen et al. 2011; Rodger et al. 2019). Despite these improvements, HIV continues to be a problem with a continued increase in infections globally, and still no vaccine is available. Additionally, PLWH experience a continued elevated risk of pathology associated with chronic inflammation, including an increased risk of HIV-Associated Neurocognitive Disorders (HAND)(Clifford and Ances 2013; Thurman et al. 2020).
The cause of chronic inflammation during HIV infection is multi-fold. First, despite the effectiveness of cART in reducing the continued replication of the intact virus, low-level replication continues in viral reservoirs. These cells evade immune detection and cause viral rebound following cART interruption (Vanhamel et al. 2019). Similarly, even if competent virus replication is blocked, continued low-level transcription and translation of viral genes such as tat and gp120 also elicit a pro-inflammatory immune response(Imamichi et al. 2020). Further, cART cannot fully restore the damage caused by acute infection, including changes to the immune system and disruption of the gut barrier with continued impairment even during long-term cART (Crakes and Jiang 2019). Finally, despite significant immune reconstitution, the gut microbiome is significantly altered following HIV infection, even though cART can partially restore gut homeostasis(Li et al. 2016). The combination of chronic dysbiosis, incomplete immune reconstitution, and damaged gut epithelial barrier allow for the translocation of bacteria and bacterial byproducts, including the cell wall component of gram-negative bacteria lipopolysaccharide (LPS). When LPS enters the bloodstream, it activates the CD14/TLR4/MD2 receptor complex, which leads to a chronic pro-inflammatory response.
Additionally, the gram-negative (LPS-producing) Bacteroidete family Prevotelleaceae has specifically been shown to increase during HIV infection across demographics, particularly the species Prevotella copri (Armstrong et al. 2018; Dillon et al. 2014; Kaur et al. 2018). Because of the link between HIV, microbial dysbiosis, and immune dysregulation, it has been suggested that the expansion of Prevotella drives inflammation and loss of epithelial barrier function. It has further been suggested that Prevotella can increase HIV transmission risk by priming the intestinal milieu for a pro-inflammatory response(Armstrong et al. 2018; Kelley et al. 2017; Li et al. 2019), further complicating the interaction between HIV infection and Prevotella-driven dysbiosis. Additionally, human studies suggest that Prevotella replaces butyrate-producing bacteria (BPB) in HIV infection. Butyrate, a short-chain fatty acid (SCFA), is an essential microbial bi-product for healthy gut function. The replacement of BPB with Prevotella is associated with increased CD4 + and CD8 + T cell activation and inflammatory markers(Dillon et al. 2017; Donohoe et al. 2011). SIV infection has been associated with an expansion of other Bacteroidetes and total Bacteroidetes, along with a higher Bacteroidete:Firmicute (B:F) Ratio (Allers et al. 2020; Siddiqui et al. 2020). This higher abundance of Bacteroidetes is associated with loss of CCR5 + and total CD4 + T cells in the colonic mucosa at peak viral load (Allers et al. 2020). However, SIV-infection itself does not explicitly increase Prevotella, although cART-treatment following SIV infection does (Blum et al. 2020).
Similar to HIV, opioid use has been tethered to gut dysfunction and dysbiosis. Clinical studies with patients using substance abuse (heroin, ephedrine, and methamphetamine) have found an increase in Thauera, Paracoccus, and Prevotella compared with healthy controls(Xu et al. 2017). In murine models, short-term morphine administration has been linked to decreased α-diversity, characterized primarily by decreases to Bacteroidales leading to bile salt metabolism changes. Further, models of opioid tolerance have demonstrated that Bifidobacterium and Lactobacillus’s losses, accompanied by increases in Prevotellaceae, Allobaculum, and Peptostreptococcaceae, were associated with increased intestinal permeability and microbial translocation(Zhang et al. 2019). In this model, germ-free mice and antibiotic treatment could attenuate morphine tolerance (Wang et al. 2018). A similar study found morphine-dependent mice had lower Bacteroidetes and Firmicutes and increased Proteobacteria(Kang et al. 2017). There is limited understanding of opioid use in the context of HIV. However, to date, there is some indication that opioids potentiate changes caused by dysbiosis in acute SIV infection with SIV-infected, morphine-treated macaques having lower α-diversity than macaques with SIV infection or morphine treatment alone. Morphine treatment in macaques was also associated with lower Steptococcaceae and Ruminococcaceae (Sindberg et al. 2019).
Recently, our laboratories demonstrated for the first time that morphine administration differentially modulates the viral reservoir in diverse immune compartments, including an increase in the CD11b + microglia/macrophage reservoir in the central nervous system (CNS) and a decrease in CD4 + cells in the peripheral blood, the lymph nodes, and rectal biopsies (Acharya et al. 2020). Because of the differential viral reservoir in the gut and CNS compartments, we wanted to determine if morphine may perturb the gut-brain axis. The microbiome is a crucial component, and we hypothesized that microbial homeostasis might be disrupted during chronic opioid abuse during SIV infection even with cART administration. To test this hypothesis, we collected feces at necropsy from the same animals included in this previous study, isolated DNA, and performed rRNA gene sequencing and analysis to understand the dysbiosis and its role gut-brain axis.
Results
Changes and Trends in Relative Abundance and Operational Taxonomic Units
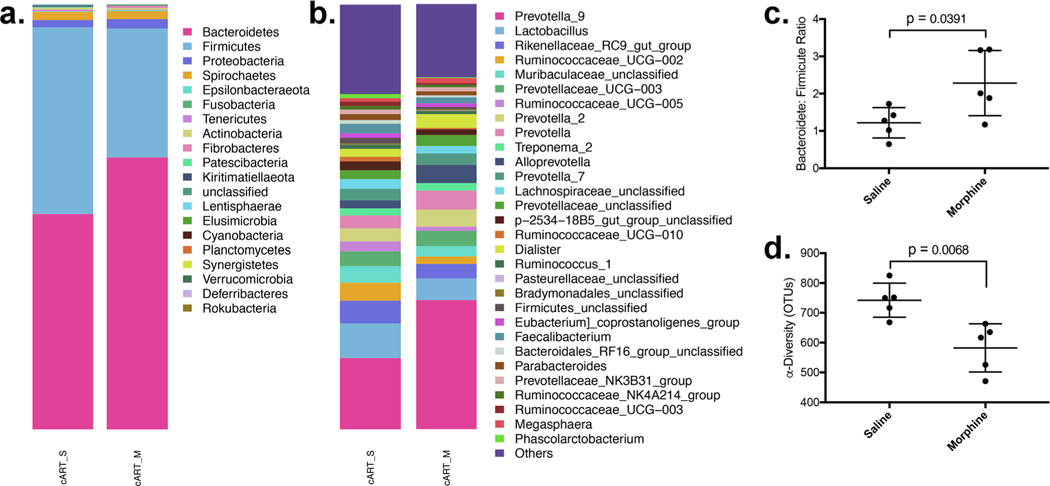
To determine differences in the microbial diversity and relative abundance of different taxonomic groups caused by chronic opioid administration during lentiviral infection, SIV-infected macaques were used to model HIV infection in humans. To model opioid abuse, macaques were administered a gradually increasing daily intramuscular (IM) injection of morphine leading up to a twice-daily 6 mg/kg by the end of two weeks. 6 mg/kg was then maintained throughout the remainder of the study(Acharya et al. 2020). Most PLWH currently have their viral replication suppressed with cART. To recapitulate this in our model, daily cART injections were administered to all animals in this study(Acharya et al. 2020). Saline/morphine and cART administration was continued until 45 weeks, and animals were necropsied, and feces samples were collected, fecal DNA isolated, and 16S rRNA sequencing performed. The mean relative abundance of Bacteroidetes was 50.6% in the saline-treated and 63.9% in the morphine group, while the abundance of firmicutes was 44.0% with saline and 30.4% with morphine (Fig. 1a). This led to an 82.9% (p = 0.0391) higher B:F ratio in the morphine group (1.15 vs 2.11) (Fig. 1c). This expansion of Bacteroidetes was accompanied by a lower mean total count of operational taxonomic units (OTUs), a measure of α-diversity, from 742 compared to 582, a 21.6% lower count in the morphine group (p = 0.0079) (Fig. 1d). Shannon diversity was lower in the morphine group, with an average of 7.55 compared to 7.94.
Fig. 1.
Morphine administration exacerbates dysbiosis in cART-treated macaques: Morphine administration was associated with significant changes to the relative abundance a phyla with b the specific genus Prevotella_9_Unclassified being expanded. c The bacteroidete: firmicute ratio was significantly higher in the morphine-administered group, which was accompanied by a considerably lower alpha-diversity, as measured by total unique operational taxonomic units (OTUs)
Prevotellaceae Replaces Ruminococcaceae and Other Diverse Bacteria Taxa
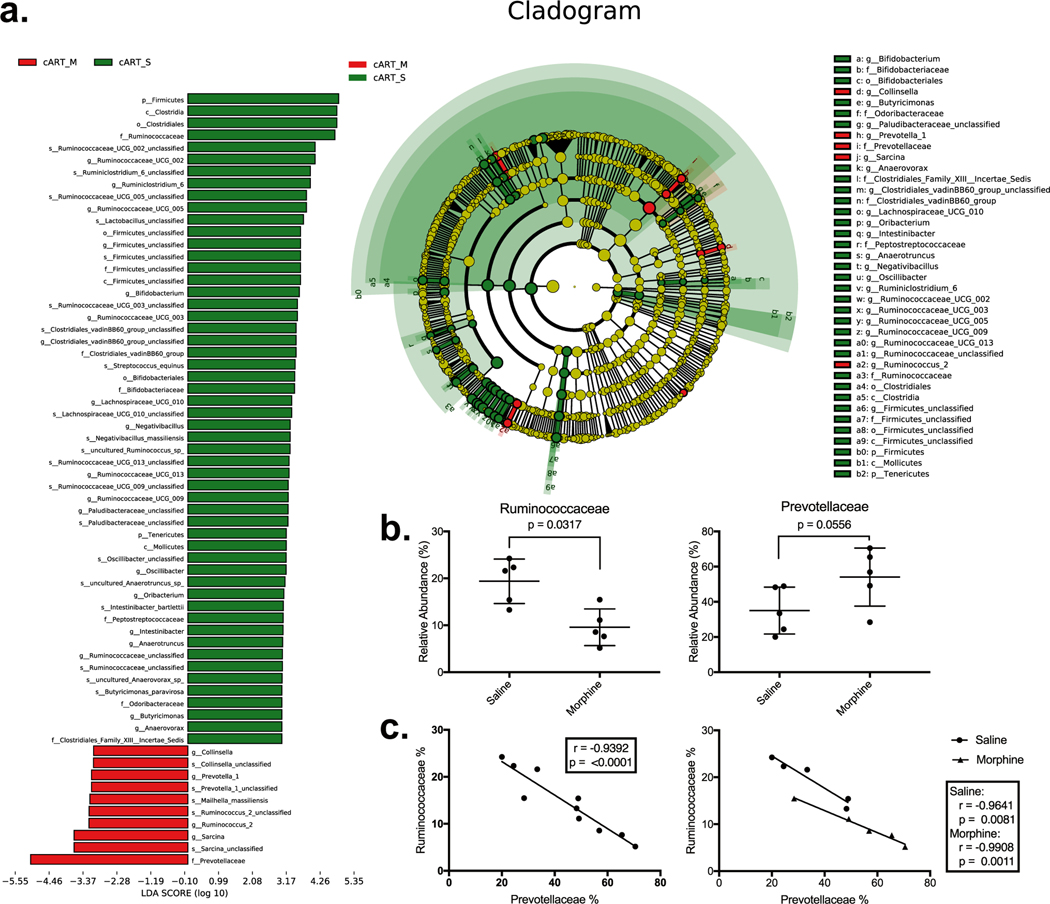
The above data suggested that changes that typically occur during HIV infection, including a high B:F ratio, may also be seen in our model of opioid abuse. To better understand these specific taxonomic changes, we analyzed changes to the top 20 bacterial families (not shown). We performed a LEfSE cladogram analysis to determine significant differences between our experimental groups. In each analysis, the families of Prevotellaceae and Ruminococcaceae were most different from the LEfSE, including several higher and lower taxa containing each family member (Fig. 2a). After pinpointing these two families as driving the change to the B: F ratio, Mann–Whitney U tests were performed. The relative abundance of Prevotellaceae was expanded in the morphine-treated group from 35.02% to 54.09%, although this was not statically different (p = 0.0556). A concomitant decrease in Ruminococcaceae accompanied this higher relative abundance of Prevotellaceae. The association between these differences was then tested using linear regression, which showed a direct negative correlation between the two families (r = −0.9392, p = < 0.0001) (Fig. 2c). This association was stronger when the two experimental groups were separated as determined by higher r values. The saline grouping had an r = −0.9641 (p = 0.0081) and the morphine group having an r = −0.09908 (p = 0.0011).
Fig. 2.
Prevotellaceae replaces Ruminococcaceae: a An LEfSe analysis showed significant differences between the morphine and saline controls. Many of the taxonomic differences contained or were contained by the two largest families in our analysis Prevotellaceae and Ruminococcaceae. b The relative abundance of Ruminococcaceae was lower in the morphine-treated group (p = 0.0317) and trended higher (p = 0.0556). c Linear regression analysis gave a Pearson correlation r = (p = < 0.0001) which was maintained at r = −0.9641 (p = 0.0081) and r = −0.9908 (p = 0.011) for the saline and morphine groups separated, respectively
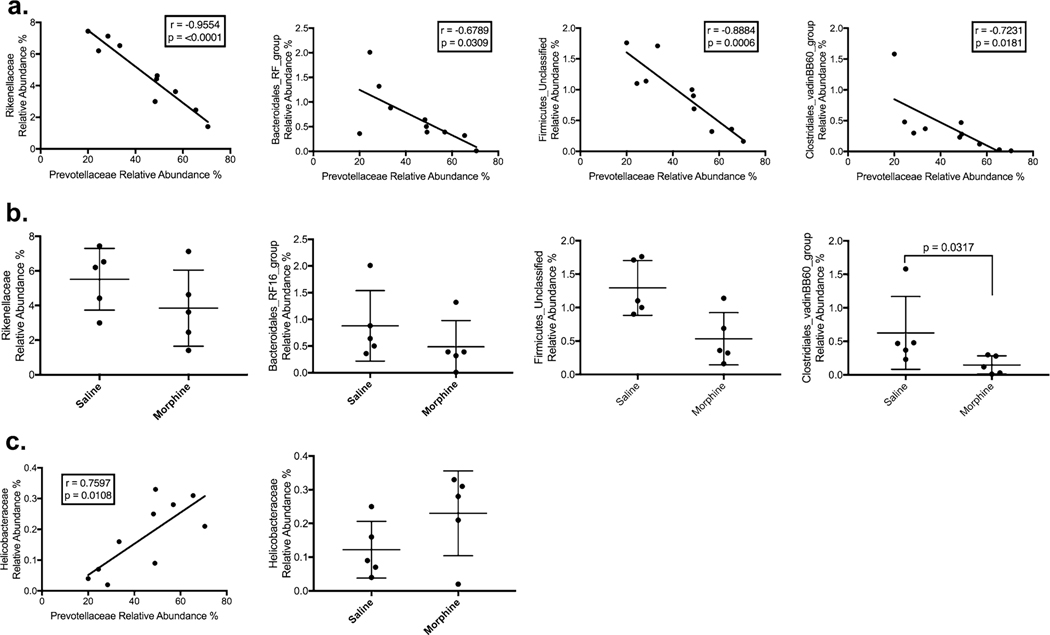
Differences correlating with changes to Prevotellaceae were not limited to decreases to Ruminococcaceae. Two other firmicute families were also negatively correlated with Prevotellaceae: Firmicutes_Unclassified (r = −0.8884, p = 0.0006) and Clostridales_vadinBB60_group (r = −0.7231, p = 0.0181). Several other diverse families of bacteria were also negatively associated with Prevotellaceae, including Rikenellaceae (r = −0.9554, p = < 0.0001), Spirochaetaceae (r = −0.6918, p = 0.0267), p-2534–18B5_gut_group (r = −0.7296, p = 0.0166), Bacteroidales_RF16_ group (r = −0.6789, p = 0.0309), and Paludibacteraceae (r = −0.693, p = 0.0263) (Fig. 3a, Table 1). Together, of the top 20 bacterial families, Prevotellaceae replaced members of three phyla: Firmicutes, Spirochetes, and other Bacteroidetes. Prevotellaceae was positively correlated with an expansion in Helicobacteraceae (r = 0.7597, p = 0.0108), a proteobacteria family associated with colitis in captive macaques (Fig. 3c).
Fig. 3.
Correlations between Prevotellaceae with families other than Ruminococcaceae. a Rikenellaceae, Firmicutes_Unclassified, Bacteroidales_RF16_group, and Clostridiales_vadinBB60_group were negatively correlated Prevotellaceae b Only Clostridiales_vadinBB60_group was statistically different between saline- and morphine-treated groups. c Helicobacteraceae was positively associated with Prevotellaceae
Table 1.
Expansion of Prevotellaceae replaces other bacterial families. The 20 most abundant families were compared with Prevotellaceae using linear regression. Listed are the slope, Pearson coefficient, and p value. Also included is the mean relative abundance between the saline and morphine groups with a p-value. Only Ruminococcaceae and Clostridales_vadinBB60_group were statistically different between the saline and morphine groups
| Taxonomic Groups |
Relative Abundance (Means) |
Correlations with Prevotellaceae |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Phylum | Order | Family | Slope | Morphine | P Value | Slope | R Squared | r | P Value |
|
| |||||||||
| Firmicute | Clostridales | Ruminococcaceae | 19.38 | 9.58 | 0.0317 | −0.357 ± 0.04615 | 0.8821 | −0.9392 | < 0.0001 |
| Bacteroidete | Bacteroidales | Rikenellaceae | 5.52 | 3.85 | 0.3095 | −0.1147 ± 0.01253 | 0.9128 | −0.9554 | < 0.0001 |
| Spirochaete | Spirochaetia | Spirochaetaceae | 1.92 | 1.86 | 0.4206 | −0.06702 ± 0.02473 | 0.4786 | −0.6918 | 0.0267 |
| Bacteroidete | p-2534–18B5_gut_group | 2.09 | 1.33 | 0.3095 | −0.09722 ± 0.03222 | 0.5323 | −0.7296 | 0.0166 | |
| Firmicute | Firmicutes_Unclassified | 1.29 | 0.53 | 0.0556 | −0.02817 ± 0.005146 | 0.7893 | −0.8884 | 0.0006 | |
| Bacteroidete | Bacteroidales | Bacteroidales_RF16_group | 0.88 | 0.48 | 0.2063 | −0.02298 ± 0.008787 | 0.4609 | −0.6789 | 0.0309 |
| Proteobacteria | Campylobacterales | Helicobacteraceae | 0.12 | 0.23 | 0.2222 | 0.005076 ± 0.001536 | 0.5771 | 0.7597 | 0.0108 |
| Bacteroidete | Bacteroidales | Paludibacteraceae | 1.23 | 0.38 | 0.2857 | −0.04528 ± 0.01665 | 0.4803 | −0.693 | 0.0263 |
| Firmicute | Clostridales | Clostridales_vadinBB60_group | 0.63 | 0.15 | 0.0317 | −0.01877 ± 0.006339 | 0.5229 | −0.7231 | 0.0181 |
Expansion of Prevotellaceae Primarily due to Prevotella_9
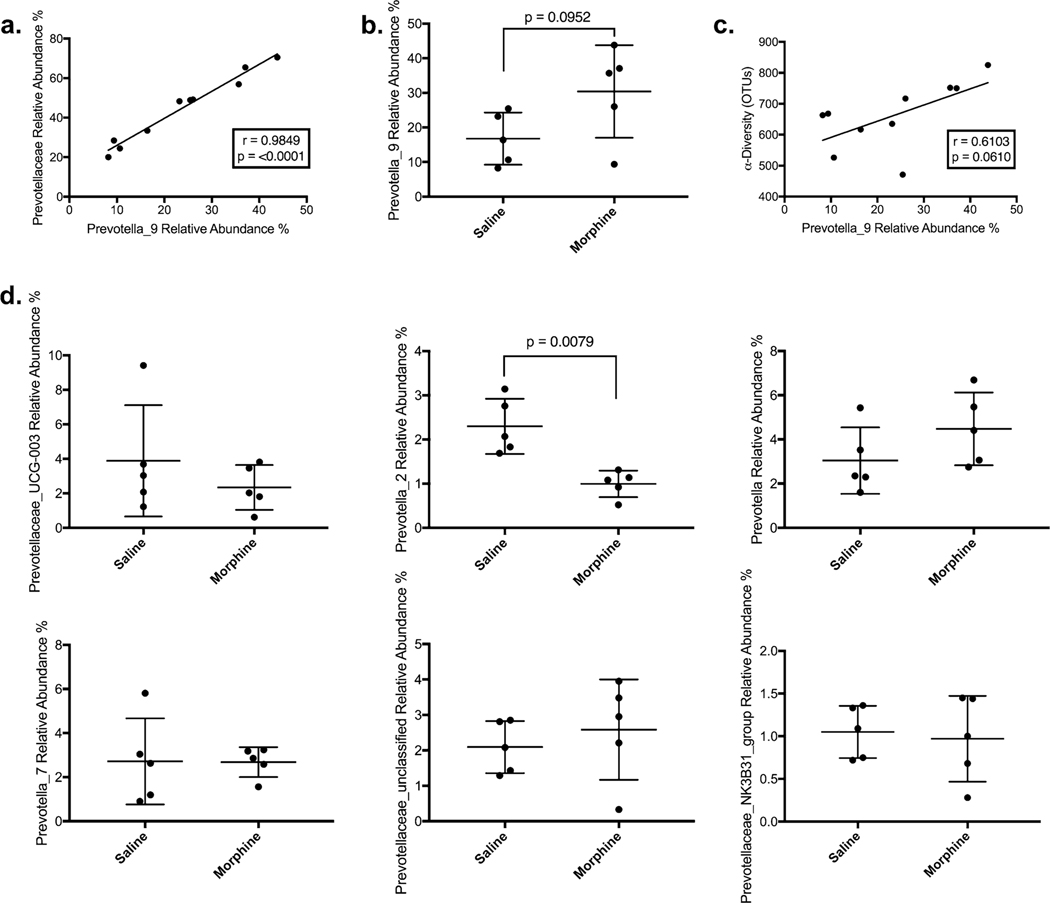
The relative abundance of Prevotellaceae was strongly correlated with the specific genus Prevotella_9 (r = 0.9849, p = < 0.0001), the group of bacteria with the closest homology with human pathobiont Prevotella copri (Fig. 4a). Most (71%) of the difference between Prevotellaceae in the morphine and saline groups was due to higher levels of Prevotella_9, the taxa with the closest homology with the human pathobiont Provetella copri with a trend toward a higher relative abundance in the morphine group of 30.4% compared with 16.8% (NS) (Fig. 4b). There was also a positive trend (r = 0.6103, p = 0.0610) with Prevotella_9 and α-diversity (Fig. 4c). There was no similar association or increase in any of the largest Prevotellaceae genera (Fig. 4d).
Fig. 4.
Expansion of Prevotellaceae and Associated Dysbiosis is Primarily Due to Prevotella_9: a Expansion of Prevtoellaceae are strongly correlated with the genus Prevotella_9. b There is a trend in higher Prevotella_9 relative abundance in the morphine treated group, and c there is a trend between Prevotella_9 and α-diversity, as measured by OTUs. Of the other genera measured in our 16S rRNA sequencing, only Prevotella_2 is statistically significant and is lower in the morphine-treated group suggesting Prevotella_9 expansion is unique in the Prevotellaceae family
Morphine may Reduce Butyrate-producing Bacteria, but this is not due to the Loss of Ruminococcaceae
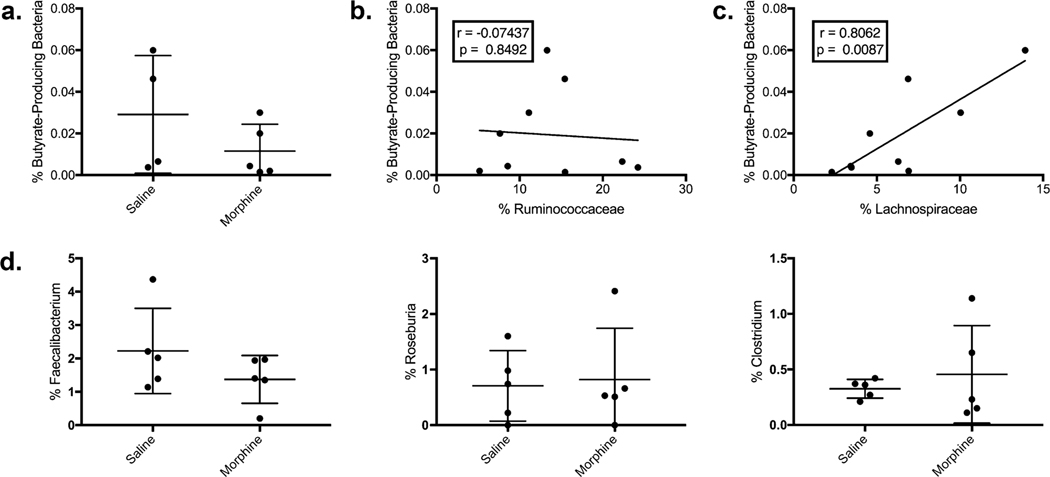
Because Ruminococcocaceae is known as one of the two families of bacteria that metabolize dietary fiber to produce the SCFA butyrate, we sought to determine if reductions in Ruminococcaceae may be sources of pathogenicity by reducing the total number of butyrate-producing bacteria. This was done using a method described elsewhere. Due to horizontal gene transfer, taxonomic classification alone is insufficient for determining their relative abundance (Dillon et al. 2017; Louis and Flint 2007). Briefly, qPCR was performed using degenerate primers to determine the total number of Butyrl CoA-CoA Transferase and 16S rRNA in the microbiome to get a simple relative abundance of bacteria carrying the critical enzyme in butyrate production. Our analysis shows a trend toward a decreased relative abundance of BPB in the morphine-treated group. The average percent BPB is 0.029% in the saline group and 0.012%, a difference of 60.3%. However, this trend was not significant (p = 0.2857) (Fig. 5a). Next, a linear regression analysis was performed to determine if Ruminococcaceae was correlated with BPB. No correlation was found, but an additional analysis was performed on Lachnospiraceae, the second family that produces most butyrate in the microbiome. Linear regression indicated a significant positive Pearson correlation coefficient (r = 0.7917, p = 0.0037) (Fig. 5c), indicating that the measure of butyrate-producing bacteria was more closely associated with Lachnospiraceae, a finding corroborated by other sources. To further test our result, BPB was not reduced. We analyzed three of the most abundant BPB genera (Faecalibacterium, Roseburia, and Clostridium) found no statistical difference between the two groups (Fig. 5d).
Fig. 5.
Loss of Ruminococcaceae was not associated with loss of total butyrate-producing bacteria (BPB): a To test whether the total relative abundance of BPB was statistically different between saline and morphine treatments, the relative abundance of Butyrl CoA-CoA Transferase genes was quantified and compared to the total 16S rRNA genes in isolated fecal DNA. b The relative abundance of BPB was not correlated with Ruminococcaceae in linear regression analysis but c was associated with Lachnospiraceae (r =0.8062, p = 0.0037), which was not statistically different in between groups in our analysis. d Morphine administration was not associated with significant changes in the largest BPB genera
Discussion
Both HIV and opioid use cause rapid and persistent dysbiosis. Despite these known disruptions, little work has been done to characterize microbial changes in long-term models of these conditions when they co-occur. To determine these changes, we performed 16S rRNA sequencing after long-term morphine and cART administrated macaques. We found that morphine administration was associated with greater dysbiosis as indicated by a high B: F ratio and lower α-diversity than the saline group. This dysbiosis was driven by an expansion of Prevotellaceae, primarily Prevotella_9, at the expense of critical firmicutes, especially the largest family Ruminococcaceae. However, other significant families including Rikenellaceae, Firmicutes_Unclassified, Bacteroidales_RF16_group, and Clostridiales_vadinBB60_group. Despite the lower level of Ruminococcaceae in the morphine-treated group, the number of Ruminococcaceae was not statistically significantly associated with a lower relative abundance of butyrate-producing bacteria.
Our findings indicate that morphine administration is associated with dysbiosis, even during cART administration, measured by an increase in the B:F ratio and decreased α-diversity with decreased unique total operational taxonomic units. α-diversity, in particular, has been used as a measure of overall microbial richness and is associated with better health outcomes. Because we are using rhesus macaques as a model, our 16S rRNA analysis has low specificity for undescribed bacteria. However, Prevotella_9_Unclassified shares significant homology with P. copri, the species most increased during HIV pathogenesis and associated with worse prognosis (Kaur et al. 2018). In HIV-negative individuals, traditional diets higher in fiber are typically associated with increased Prevotella or the “Prevotella enterotype” as opposed to Western diets higher in animal proteins commonly associated with higher levels of Bacteroides(Arumugam et al. 2011; Costea et al. 2018; Kovatcheva-Datchary et al. 2015; Makki et al. 2018). Despite the high fiber diets being associated with better overall health outcomes, elevated Prevotella is frequently related to the risk of specific inflammatory diseases, including rheumatoid arthritis and colitis (Iljazovic et al. 2020; Maeda and Takeda 2019). Because Prevotella has been associated with these conditions and colitis, which like HIV, is associated with changes to mucosal immune responses, a better understanding of the complex interaction between the host and Prevotella is needed to understand its contribution to HIV pathologies, including HAND. Multiple studies have already tied the expansion of Prevotella with clinical outcomes during HIV infection (Kaur et al. 2018).
In our data, the increase in the B:F ratio was primarily driven by the expansion of Prevotellaceae at the phylum Firmicutes’ cost, as seen in our LEfSE analysis and correlation plots. This loss was caused mostly by a decrease in the total number of Rumionococcaceae, a large family of bacteria including many butyrate-producing bacteria. Indeed, in a linear regression analysis, increases in the relative abundance of Prevotellaceae were coupled with a net loss in Ruminococcaceae in our study. In comparison to Prevotellaceae, significantly less work has been done to understand the impact of lost Ruminococcaceae in PLWH. However, one study was able to show that their loss, particularly the loss of Faecalibacterium prausnitzi in PLWH, was inversely correlated with inflammation/immune activation markers (Dubourg et al. 2016). Faecalibacterium is one of the primary sources of butyrate production in the human microbiome, and butyrate produced by F. prauznitzii, in particular, has been shown to ameliorate experimental colitis in mice by modulating Th17 and Treg differentiation by targeting HDAC1 (Zhou et al. 2018). Our findings are corroborated by recent reports in an HIV-infection of the humanized mice model. Both Lachnospiraceae and Ruminococcaceae, the prominent families contributing to butyrate production, were depleted with morphine administration and correlated with loss of gut epithelial integrity (Meng et al. 2020). Additional bacterial families were negatively correlated with Prevotellaceae. These include both other bacteroidete and firmicute families, both of which are also important for SCFA synthesis. The correlations between Prevotella expansion and the loss of specific firmicute families have not been well described in the context of HIV or SIV, despite each change being characterized in different studies. A possible unifying model for these close correlations has been proposed in murine DSS-induced colitis. IL-17F knockout reduces colitis risk and symptoms by decreasing antimicrobial peptide expression, allowing Clostridium to expand (Tang et al. 2018). This expansion or supplementation with exogenous Clostridium then increases Tregs differentiation and Betadefensins expression, which further inhibited Prevotella growth. While it is known that plasma IL-17F is increased during HIV pathogenesis, which could drive a decrease in butyrate-producing bacteria and increase Prevotella, further work is required to better characterize this microbiota-gut immune interaction during dysbiosis (Salem et al. 2013).
Finally, to better understand changes to BPB, we utilized a well-established assay to quantify the relative abundance of bacteria carrying the Butyrl CoA Transferase gene, the rate-limiting step in butyrate synthesis. This was necessary because the ability to catabolize dietary fiber into butyrate has spread through horizontal transfer across various microbial taxa. Therefore, determining bacterial species identity is inadequate to characterize butyrate-producing potential, especially in the poorly characterized macaque microbiome. Using this BPB assay, we found a trend toward a lower relative abundance of BPB in the morphine-administered group. However, using linear regression, it became clear that this was not correlated with Ruminococcaceae. It was still instead correlated with Lachnospiraceae, the family containing Roseburia, a genus responsible for most butyrate production in humans and strongly correlated with HIV progression. Despite this, our analysis was unable to find significant differences between the saline- and morphine-treated groups. As it becomes clear that BPB is an important biomarker and modulator of disease progression in HIV infection, a better understanding of this is warranted in future studies, including studies with larger sample sizes.
Our previous report demonstrated that morphine administration in cART-treated macaques differentially modulated the establishment of viral reservoirs (Acharya et al. 2020). We have shown that a decrease in the inducible SIV reservoir in the peripheral blood and lymph nodes and an increase in the CD11b + macrophages and microglia in the CNS. Due to this difference, we reasoned whether the gut-brain axis is also impacted my opioid dependence. Our data demonstrate that morphine administration is associated with Prevotella-dominated dysbiosis. The co-occurrence of each difference with morphine administration offers some indication that dysbiosis and viral reservoir establishment may be linked, whether by modulation of immune cells by opioid administration or by a more complex interaction between the immune system and microbial composition. However, additional studies are warranted to better elucidate the interactions between microbial composition and SIV pathogenesis.
One of the major limitations of our study is the comparison between two groups during necropsy. However, many previous reports on dysbiosis during SIV infection have provided single time points as well (Blum et al. 2020; Siddiqui et al. 2020). Ideally, though, changes in individual microbiomes would be tracked throughout morphine administration, SIV-infection, and cART treatment. These changes should be recorded in conjunction with immune responses, including peripheral lymphocyte dynamics and plasma biomarkers. Further, our study focused on the fecal microbiome, which can differ substantially from the epithelium-associated microbiome across the gastrointestinal tract. The relationship between the two should be further analyzed. An additional limitation of this study was the small sample size. While we see a trend in decreased butyrate-producing bacteria with morphine treatment, it is not statistically significant. Future large-scale studies are warranted to characterize better these functional bacteria groups and changes to actual microbial metabolites, including butyrate and other SCFAs.
Conclusions
In this study, we report the first-time differences between the gut microbiome in chronic morphine-treated macaques when infected with SIV and treated with cART. This model closely recapitulates the sequence of events seen in HIV-infected humans, where the goal is invariably suppressing viral replication. Here we report significant Prevotella-dominated dysbiosis as measured by both α-diversity and B:F ratio, reinforcing previous findings that opioid administration is associated with similar microbial changes. Given that both elevated Prevotella and dysbiosis generally have been associated with increased inflammation and worse clinical prognosis, additional work is warranted to further elucidate the complex hostmicrobiome interactions in the context of HIV and opioid abuse. Because microbial dysbiosis is associated with opioid tolerance, the findings presented here and elaborated in future studies will help develop targets for future therapeutic interventions.
Methods
Animal Care and Ethical Statement
Ten out-bred, Indian-origin rhesus macaques (Macaca mulatta) were housed at the primate facilities in the Department of Comparative Medicine at the University of Nebraska Medical Center (Omaha, NE, USA) in compliance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals. The temperature was maintained at 72F with a 12-h light/dark cycle and monitored for distress and disease by veterinarians and animal care staff. Monkeys were fed monkey chow daily, supplemented with fresh fruits and vegetables, and had access to freshwater and libitum. This study was approved by the UNMC Institutional Animal Care and Use Committee (IACUC) and Institutional Biosafety Committee (IBC) under protocols outlined in ‘16–073–07-FC’ titled “The effect of cART and drug of abuse on the establishment of CNS viral reservoirs.” UNMC has been accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Chronic Morphine Administration
The 10 animals included in this study were randomly divided into two groups: one group treated with saline (n = 5) and the other treated with morphine (n = 5). Morphine was administered twice daily with a gradually increasing dose for the first two weeks to recapitulate tolerance with the amount given, eventually reaching 6 mg/kg given twice a day. This twice-daily morphine administration was maintained at 6 mg/kg for seven more weeks before SIV inoculation and then continued for the remainder of the experiment as described previously (Acharya et al. 2020).
Infection with SIV
After seven weeks at the 6 mg/kg dosage of saline/morphine, all macaques were intravenously inoculated with 200 TCID50 of SIVmac251 (viral stock was obtained from Dr. Mahesh Mohan from Tulane National Primate Research Center). To confirm infection two weeks post-inoculation, RNA was isolated from plasma isolated using a QIAamp Viral RNA mini kit (QIAGEN Germantown, MD, USA; Cat# 52,906) following manufacturer’s instructions. SIV RNA copies in the isolated RNA was quantified using quantitative-reverse transcription-PCR (qRT-PCR) with TaqMan RNA-to-Ct 1-Step Kit (Thermo Fisher Scientific, MA; Cat#4,392,938) and Applied Biosystems QuantStudio 3 Real-Time PCR System (Applied Biosystems, Waltham, MA, USA) as previously described with primers and probes for SIV gag: SIVGAGF:5′ −GTCTGCGTCATCTGGTGCATTC −3′; SIVGAGR:5′ −CACTAGGTGTCTCTGCACTATCTG TTTTG −3′ and SIVP:5′-/6-FAM/ CTTCCTCAG/ZEN/TGTGTTTCACTTTCTCTTCTGCG/3IABkFQ/ −3 (Acharya et al. 2020).
Combination Antiretroviral Treatment
To suppress viral replication, a combination of 40 mg/mL Emtricitabine (FTC), 20 mg/mL Tenofovir (TFV), and 2.5 mg/mL Dolutegravir (DTG) were dissolved in 15% Kleptose HPB (Roquette, parenteral grade) (w/w) in 0.1 N NaOH. Five weeks after inoculation with SIV, daily intramuscular cART injections were initiated for all animals and continued until the end of the study. Viral suppression was confirmed within eight weeks of cART initiation in all animals using the qRT-PCR method described above (Acharya et al. 2020).
DNA Isolation and Microbiome Analysis
Fecal samples from all macaques were collected at necropsy and frozen in a −80 °C deep freezer. Once all samples were collected, they were thawed, and DNA was isolated using the spin column chromatography-based Stool DNA Isolation Kit (Norgen Biotek Corp.; Product # 27,600), following the recommended manufacturer instructions. Isolated fecal DNA samples were quantified using a GE SimpliNano spectrophotometer, and 100 ng of DNA were shipped on dry ice to LC Sciences, LLC (Houston, TX, USA) for 16S rRNA sequencing. A dual-zone 16S rDNA fragment amplification strategy was used with universal primers and sequenced with the Illumina MiSeq platform. Data analysis was performed by LC Sciences, including data output statistics, sequence clustering into operational taxonomic units (OTU), diversity analysis, species classification, and abundance analysis.
Quantification of Butyrate-Producing Bacteria
The relative abundance of bacteria with butyryl-coenzyme A (CoA) CoA transferase genes was quantified using a method described elsewhere (Dillon et al. 2017; Louis and Flint 2007). A ratio of Butyrl CoA-CoA transferase to the total number of 16S rRNA gene copies was calculated using qRT-PCR with PowerUp SYBR Green Master Mix (Applied Biosystems; Product #A25742) and Applied Biosystems QuantStudio 3 Real-Time PCR System. The number of total of bacteria genome copies in 0.5 ng DNA was quantified using universal primers for the highly conserved 16S rRNA as described (Salonen et al. 2010): 16S rRNA Fwd: 5′-TCCTACGGGAGGCAGCAGT-3′; 16SrRNA Rev: 5′-GGACTACCAGGGTATCTAATCCTGTT-3′. The total number of Butyrl CoA-CoA transferase was quantified using degenerate primers with 25 ng DNA (Louis and Flint 2007): Butyrl Fwd: 5′-GCIGAICATTTCACITGGAAYWSITGGCAYATG-3′;
Butyrl Rev: 5′-CCTGCCTTTGCAATRTCIACRAANGC-3’.
Statistical Analysis
Statistical analyses were performed with GraphPad PRISM 7 software. Mann–Whitney U Tests were performed to compare differences between specific bacterial taxa between the saline- and morphine-treated groups and compare the B: F ratio and α-diversity as defined by the number of unique operational taxonomic units, and the relative abundance of butyrate-producing bacteria. P-values were reported and determined to be significant at p ≤ 0.05. Pearson Correlation and Linear regression analysis was performed to compare the relative abundance of the top 20 most abundant bacteria families compared to Prevotellaceae. Pearson correlation coefficient, r, was determined and reported for each analysis.
Acknowledgments
We would like to thank the veterinarians and staff of the University of Nebraska Medical Center, Comparative Medicine department for housing and assisting with all animal procedures. We would also like to acknowledge all the research personnel from Drs. Buch, Fox, and Byrareddy laboratories on the macaque studies. We thank Dr. Mehdi Bidokhti and Emelind Rodriguez for inventorying various macaque samples. We would also like to thank Dr. Ernest Chivero for helpful discussions regarding our analysis. This research was funded by the NIH, National Institute of Drug Abuse Grant R01DA043164 to HSF, SB, and SNB. The graphical abstract was created with BioRender.com.
Footnotes
Declarations
Conflict of interest Authors declare no conflicts of interest relevant to the content of this article.
References
- Acharya A et al. (2020) Chronic morphine administration differentially modulates viral reservoirs in SIVmac251 infected rhesus macaque model. J Virol. 01657–01620. 10.1128/JVI.01657-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allers K et al. (2020) The colonic mucosa-associated microbiome in SIV infection: shift towards Bacteroidetes coincides with mucosal CD4+ T cell depletion and enterocyte damage. Sci Rep 10:10887. 10.1038/s41598-020-67843-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong AJS et al. (2018) An exploration of Prevotella-rich microbiomes in HIV and men who have sex with men Microbiome 6:198. 10.1186/s40168-018-0580-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M et al. (2011) Enterotypes of the human gut microbiome Nature 473:174–180. 10.1038/nature09944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum FC et al. (2020) Microbial Dysbiosis During Simian Immunodeficiency Virus Infection is Partially Reverted with Combination Anti-retroviral Therapy Scientific Reports 10:6387. 10.1038/s41598-020-63196-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault MA, Spiegelman D, Hargreaves J, Nash D, Vermund SH (2019) Treatment as Prevention: Concepts and Challenges for Reducing HIV Incidence. J Acquir Immune Defic Syndr (1999) 82(2):S104–s112. 10.1097/qai.0000000000002168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Diagnoses of HIV infection in the United States and dependent areas. 2020 [Google Scholar]
- Chou R et al. (2019) Preexposure Prophylaxis for the Prevention of HIV Infection: Evidence Report and Systematic Review for the US Preventive Services Task Force Jama 321:2214–2230. 10.1001/jama.2019.2591 [DOI] [PubMed] [Google Scholar]
- Clifford DB, Ances BM (2013) HIV-associated neurocognitive disorder. Lancet Infect Dis 13:976–986. 10.1016/S1473-3099(13)70269-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS et al. (2011) Prevention of HIV-1 Infection with early antiretroviral therapy. N Engl J Med 365:493–505. 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costea PI et al. (2018) Enterotypes in the landscape of gut microbial community composition. Nat Microbiol 3:8–16. 10.1038/s41564-017-0072-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crakes KR, Jiang G (2019) Gut microbiome alterations during HIV/SIV infection: implications for HIV Cure. Front Microbiol 10. 10.3389/fmicb.2019.01104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Borgo C, Izzi I, Chiarotti F, Del Forno A, Moscati AM, Cornacchione E, Fantoni M (2001) Multidimensional aspects of pain in HIV-infected individuals. AIDS Patient Care STDS 15:95–102. 10.1089/108729101300003690 [DOI] [PubMed] [Google Scholar]
- Dillon SM et al. (2017) Low abundance of colonic butyrate-producing bacteria in HIV infection is associated with microbial translocation and immune activation. AIDS (London, England) 31:511–521. 10.1097/qad.0000000000001366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SM et al. (2014) An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol 7:983–994. 10.1038/mi.2013.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe DR, Garge N, Zhang X, Sun W, O’Connell TM, Bunger MK, Bultman SJ (2011) The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab 13:517–526. 10.1016/j.cmet.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubourg G et al. (2016) Gut microbiota associated with HIV infection is significantly enriched in bacteria tolerant to oxygen BMJ Open Gastroenterol 3:e000080–e000080. 10.1136/bmjgast-2016-000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iljazovic A et al. (2020) Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation Mucosal. Immunol. 10.1038/s41385-020-0296-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamichi H et al. (2020) Defective HIV-1 proviruses produce viral proteins. Proc Natl Acad Sci 117:3704. 10.1073/pnas.1917876117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M et al. (2017) The effect of gut microbiome on tolerance to morphine mediated antinociception in mice Scientific reports 7:42658. 10.1038/srep42658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur US et al. (2018) High Abundance of genus Prevotella in the gut of perinatally HIV-infected children is associated with IP-10 levels despite therapy. Sci Rep 8:17679. 10.1038/s41598-018-35877-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley CF et al. (2017) The rectal mucosa and condomless receptive anal intercourse in HIV-negative MSM: implications for HIV transmission and prevention Mucosal immunology 10:996–1007. 10.1038/mi.2016.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovatcheva-Datchary P et al. (2015) Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab 22:971–982. 10.1016/j.cmet.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Larue F, Fontaine A, Colleau SM (1997) Underestimation and under-treatment of pain in HIV disease: multicentre study BMJ (Clinical research ed) 314:23–28. 10.1136/bmj.314.7073.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Armstrong A, Neff CP, Shaffer M, Lozupone CA, Palmer BE (2016) Complexities of Gut Microbiome Dysbiosis in the Context of HIV Infection and Antiretroviral Therapy. Clin Pharmacol Ther 99:600–611. 10.1002/cpt.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX et al. (2019) Gut microbiota from high-risk men who have sex with men drive immune activation in gnotobiotic mice and in vitro HIV infection. PLoS Pathog 15:e1007611. 10.1371/journal.ppat.1007611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P, Flint HJ (2007) Development of a semiquantitative degenerate real-time PCR-based assay for estimation of numbers of butyryl-coenzyme a (CoA) CoA transferase genes in complex bacterial samples. Appl Environ Microbiol 73:2009. 10.1128/AEM.02561-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Takeda K (2019) Host–microbiota interactions in rheumatoid arthritis. Exp Mol Med 51:1–6. 10.1038/s12276-019-0283-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makki K, Deehan EC, Walter J, Bäckhed F (2018) The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 23:705–715. 10.1016/j.chom.2018.05.012 [DOI] [PubMed] [Google Scholar]
- Meng J et al. (2020) Opioids impair intestinal epithelial repair in HIV-infected humanized mice. Front Immunol 10. 10.3389/fimmu.2019.02999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Penko JM, Guzman D, Mattson JE, Bangsberg DR, Kushel MB (2011) Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with HIV infection. The Journal of Pain : Official Journal of the American Pain Society 12:1004–1016. 10.1016/j.jpain.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger AJ et al. (2019) Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet (London, England) 393:2428–2438. 10.1016/s0140-6736(19)30418-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem M, Ronit A, Gaardbo JC, Lund TT, Ullum H, Gerstoft J, Nielsen SD (2013) Altered balance between IL-17A– and IL-17F–producing Th17 cells in HIV-infected patients. JAIDS J Acquir Immune Defic Syndr 63 [DOI] [PubMed] [Google Scholar]
- Salonen A et al. (2010) Comparative analysis of fecal DNA extraction methods with phylogenetic microarray: Effective recovery of bacterial and archaeal DNA using mechanical cell lysis. J Microbiol Methods 81:127–134. 10.1016/j.mimet.2010.02.007 [DOI] [PubMed] [Google Scholar]
- Siddiqui S, Bao D, Doyle-Meyers L, Dufour J, Wu Y, Liu YZ, Ling B (2020) Alterations of the gut bacterial microbiota in rhesus macaques with SIV infection and on short- or long-term antiretroviral therapy Sci Rep 10:19056. 10.1038/s41598-020-76145-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindberg GM et al. (2019) Morphine Potentiates Dysbiotic Microbial and Metabolic Shifts in Acute SIV Infection. J Neuroimmune Pharmacol 14:200–214. 10.1007/s11481-018-9805-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C et al. (2018) Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing Treg cells through modifica- tion of the intestinal microbiota. Nat Immunol 19:755–765. 10.1038/s41590-018-0134-y [DOI] [PubMed] [Google Scholar]
- Thurman M, Johnson S, Acharya A, Pallikkuth S, Mahesh M, Byrareddy SN (2020) Biomarkers of Activation and Inflammation to Track Disparity in Chronological and Physiological Age of People Living With HIV on Combination Antiretroviral Therapy Front Immunol 11:583934. 10.3389/fimmu.2020.583934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS (2019) Global HIV & AIDS statistics — 2019 fact sheet. Accessed February 29, 2020 2020 [Google Scholar]
- Vanhamel J, Bruggemans A, Debyser Z (2019) Establishment of latent HIV-1 reservoirs: what do we really know? J Virus Erad 5:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Meng J, Zhang L, Johnson T, Chen C, Roy S (2018) Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model Scientific reports 8:3596. 10.1038/s41598-018-21915-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Bisaga A (2016) From AIDS to Opioids - How to Combat an Epidemic. N Engl J Med 375:813–815. 10.1056/NEJMp1604223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y et al. (2017) Bacterial diversity of intestinal microbiota in patients with substance use disorders revealed by 16S rRNA gene deep sequencing. Sci Rep 7:3628 10.1038/s41598-017-03706-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L et al. (2019) Morphine tolerance is attenuated in germfree mice and reversed by probiotics, implicating the role of gut microbiome. Proc Natl Acad Sci 116:13523 10.1073/pnas.1901182116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L et al. (2018) Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1 Inflammatory bowel diseases 24:1926–1940 10.1093/ibd/izy182 [DOI] [PubMed] [Google Scholar]