Abstract
An experimental group of one-day-old chicken from a commercial hatchery was given a defined mixture of 7 gut anaerobes. The next day the chicks were inoculated by an APEC strain O78:H4-ST117 resistant to ciprofloxacin, alongside with the control group and monitored for 4 wk after the inoculation for the presence of the colonizing strains and ciprofloxacin-resistant E. coli. Significant reduction of colonization rates in the first 2 wk was recorded in the experimental group for the numbers of ciprofloxacin-resistant E. coli. The results show that colonization of chicken by defined anaerobic mixtures may provide a decisive protection during the critical period of the chicken intestinal microflora development.
Key words: competitive exclusion, colonization, chicken, avian pathogenic Escherichia coli (APEC), probiotics
INTRODUCTION
The importance of colibacillosis as a paramount economic and public health issue is well recognized. E. coli strains that are associated with colibacillosis have been termed APEC, avian pathogenic E. coli, and usually belong to certain serotypes and are characteristic by the presence of specific virulence genes. APEC are thought to form a specific pathotype within the group of extraintestinal pathogenic E. coli (ExPEC). Unfortunately, the immense diversity of these strains, many of which lack typical traits, complicate the precise definition of the pathotype. Recent studies confirm the importance of both secondary infections caused by very diverse commensal-like strains and the occurrence of adapted virulent lineages, often with presumable zoonotic potential (Mehat et al., 2021). In addition, virulent lineages may spread vertically through the production pyramid from breeder farms to production flocks via hatcheries (Projahn et al., 2017).
Intervention measures therefore pose a challenge. Use of antibiotics only supports the emergence and spread of resistant strains. In addition, the pressure to limit or completely ban antimicrobials in food animals may result in substantial losses in production farms and, on the other hand, calls for a discussion on different, more sustainable means of production. The principle of competitive exclusion (CE) represents one of the most promising strategies for reducing infectious pressure and transmission of pathogenic strains. Since its introduction in 1973 (Rantala and Nurmi, 1973), commercial products such as Aviguard and Broilact have been developed and their efficacy confirmed not only against Salmonella sp., but also against E. coli producing extended-spectrum beta-lactamases (ESBL) (Methner et al., 2019) and pathogenic E. coli in broilers (Hofacre et al., 2002).
Induction of "mature" intestinal microflora facilitates the development of the intestinal mucosal immune system and increases resistance to Salmonella infection (Crhanova et al., 2011). Notably, complex, often only partially defined, mixtures of intestinal anaerobic bacteria or cecal transplants have proven to be much more effective than a single species probiotic or a limited combination of several traditional probiotic strains, most of which are incapable of long-term colonization. Traditional probiotics mainly include lactobacilli, but their effects are often questionable (Yan et al., 2017; Li et al., 2021). The application of strict anaerobes, mainly from the phylum Bacteroidetes therefore makes more sense than administering facultative anaerobes and spore-forming anaerobes of which the environment is an abundant source (Kubasova et al., 2019a; Kubasova et al., 2021; Karasova et al., 2022).
The purpose of this study was to evaluate the effect of a complex mixture of defined intestinal anaerobes on cecal colonization by ciprofloxacin-resistant strain APEC ST117-O78:H4, a representative of one of the most prevalent risk lineages in poultry industry (Ronco et al., 2017).
MATERIAL AND METHODS
Experimental Animals
Ross 308 meat hybrid chickens were used for the experiment. The one-day-old chickens were delivered from a local commercial hatchery, originated from one parent farm and were formed by a mixture of both sexes.
Housing Conditions and Care
The experimental and control groups, each consisting of 28 chicks, were placed in two separate rooms on sawdust bedding, near the heating lamp. The commercial feed for given age was provided ad libitum. Water with the addition of a vitamins and organic acids (Acidomide, dosage according to the manufacturer's recommendations) was also administered ad libitum. A 12-h light regime was followed and the ambient temperature was recorded regularly.
Probiotic Mixture and Colonization E. coli Strain
The probiotic mixture consisted of 7 bacterial species including Bacteroides caecicola, Bacteroides plebeius, Megasphaera stantonii, Megamonas hypermegale, Megamonas funiformis, Phascolarctobacterium faecium, and Sutterella massiliensis. These strains were inoculated into 100 mL of Wilkins-Chalgren nutrient broth and incubated in anaerobic cabinet at 37°C in atmosphere consisting of 85% N2, 10% CO2, and 5% H2. After 48-h-fermentation, 0.1 mL of complex bacterial culture was used for oral inoculation of each chick in experimental group. An aliquot of 1 mL of the complex bacterial culture was saved and used for DNA purification to determine ratios of each of the strains in the inoculum.
E. coli 587 (serotype O78:H4, ST117) was used for challenge. This strain, representing an important APEC line, had been analyzed by whole-genome sequencing previously (Papouskova et al., 2020). The experimental strain was cultured in BHI at 37°C for 18 h and the culture was diluted with sterile buffered saline to a concentration of 105 CFU/mL as verified by cultivation. Each chicken was gavaged with 0.1 mL of inoculum, that is, with a dose of 104 CFU.
The Course of the Experiment
The experiment lasted 4 wk. On d 1 of life, chicks in the experimental group were orally inoculated with 0.1 mL of gut anaerobes. On the day 2, both groups received a suspension of ciprofloxacin-resistant strain E. coli 587. On d 7, 14, 21, and 28 of life, 7 chickens were randomly selected from each group, weighed and euthanized by carbon dioxide inhalation. Pathomorphological changes were recorded at the necropsy and the ceca of all chickens were aseptically excised.
Verification of Colonization Rate by Probiotic Mixture
Samples of the administered probiotic mixture and cecal contents were stored at −20°C. DNA from cecal samples was extracted using QIAamp Stool kit according to the manufacturer's instructions (Qiagen, Germany). Based on known genomic sequences, strain-specific real-time PCRs were designed and the real-time PCR in SybrGreen format was performed exactly as described previously (Kollarcikova et al, 2020).
Verification of Colonization Rate by Challenge APEC Strain
A serial decimal dilutions of cecal contents with sterile buffered saline were plated on Levine EMB agar (Thermo Fischer Diagnostics, Dardilly, France) supplemented with 1 μg/mL of ciprofloxacin (Sigma Aldrich, Prague, Czech Republic). After 18 hours of incubation at 37°C, colonies with a typical metallic sheen were counted and the presence of E. coli O78 was verified by slide agglutination with specific antisera according to the manufacturer's recommendations (Denka-Seiken Co., Ltd., Japan). The presence of the experimental strain was verified by slide agglutination on at least two plates with sufficient growth, and according to the highest dilution at which strain O78 was still detected, a colonization score of + (dilutions 10−1–10−2), ++ (dilutions 10−3–10−4), and +++ (dilutions 10−5–10−6, i.e., the highest), was assigned for easier result presentation.
Statistical Analysis
The obtained experimental data (cfu.g-1) were log10 transformed, and the mean values and standard deviations were calculated. Factorial ANOVA followed by Tuckey post hoc test was used to assess the effect of control/experimental group and chicken age on number of bacteria in the gut. P < 0.05 was considered as significant in all tests. Statistical analyses were performed using Statistica, version 13 (TIBCO Software Inc.).
RESULTS AND DISCUSSION
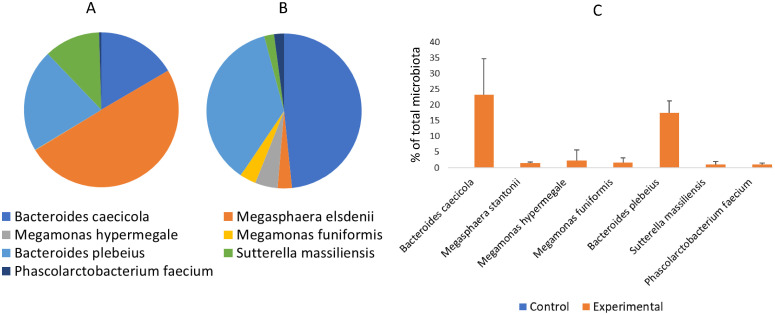
The aim of the experiment was to verify the effect of application of the probiotic mixture to one-day-old chicks on their health, performance and resistance to colonization by a defined APEC strain. Probiotic mixture consisted of 7 different species, though because the strains were co-fermented in a single culture medium, their representation was not identical. Instead, the inoculum was dominated by Bacteroides caecicola, Bacteroides plebeius, Megasphaera stantonii and Sutterella massiliensis while Megamonas hypermegale, Megamonas funiformis, and Phascolarctobacterium faecium were present at lower than 0.2% representation (Figure 1A). As expected, all the strains efficiently colonized chicken cecum though the composition of the inoculum only partially affected cecal colonization (Kubasova et al., 2019b). Bacteroides caecicola and Bacterioides plebeius dominated also in the cecum but Megasphaera stantonii was less abundant in the cecum than in the inoculum (Figure 1B). Moreover, minority species from the inoculum formed more than 1% of total microbiota in the cecum in vivo. Altogether, the used probiotic strains formed 48% of total microbiota on average ranging from 32.4 to 55.9% in individual chicks. All of the probiotic strains were absent from microbiota of control chicks (Figure 1C).
Figure 1.
Microbiota composition in the inoculum and caeca of inoculated and control chicks. Real time PCR was used to quantify probiotic strains in complex populations. The inoculum was dominated by Bacteroides caecicola, Bacteroides plebeius, Megasphaera stantonii and Sutterella massiliensis (Panel A). Ratios calculated only for the probiotic strains showed that composition of the inoculum only partially influenced caecal colonisation (Panel B). Used probiotic strains were completely absent from the caeca of control chicks (Panel C).
No mortality was recorded during the experiment and the chickens showed no visible pathomorphological changes at necropsy. No effect of the probiotic mixture on the weight of the chickens was observed; the mean weight remained slightly higher in the experimental group, but this difference was not statistically significant (Table 1).
Table 1.
Body weight of chickens and number of E. coli in the caecum of chickens in control (CTRL) and experimental (Exp) group.
| Body weight (g) |
E. coli counts (log CFU/g of caecum)a |
Experimental strain detection (number of positive chicken, colonization rateb) |
||||
|---|---|---|---|---|---|---|
| Week | Exp | CTRL | Exp | CTRL | Exp | CTRL |
| 1 | 126.4 ± 8.2 | 111.0 ± 17.8 | 6.38 ± 0.45* | 8.29 ± 0.70 | ND | 7/7 (+++) |
| 2 | 306.4 ± 90.9 | 262.3 ± 61.9 | 6.04 ± 0.47* | 7.83 ± 0.84 | ND | 7/7 (+++) |
| 3 | 743.6 ± 184.5 | 728.1 ± 88.0 | 6.58 ± 0.70 | 6.46 ± 0.89 | ND | 3/7 (++) |
| 4 | 849.0 ± 193.6 | 704.1 ± 51.9 | 5.10 ± 1.23 | 6.08 ± 1.17 | 1/7 (+) | 4/7 (++) |
Significantly different from control group of the same age by ANOVA with post hoc Tuckey test.
E. coli growing on a medium with ciprofloxacin.
Experimental strain detected in 10−6 to 10−5 dilution (+++), 10−4 to 10−3 dilution (++), 10−2 to 10−1 dilution (+), ND – not detected in any dilution.
In the first 2 wk, statistically significant differences in the numbers of all CIP-resistant E. coli between the control and experimental groups were recorded (Table 1). In the control group, the numbers of CIP-resistant E. coli reached values of 107 to 108 CFU/g, in the experimental group these were approximately 2 logs of magnitude lower. These data also show that that the competitive exclusion effect was not specific against the pathogenic strain but was general, decreasing the colonization of all E. coli clones present also in the control chickens. In older chickens (3-wk and 4-wk-old), the numbers of E. coli in the gut were significantly lower than in younger chickens, probably due to natural maturation and stabilization of the intestinal ecosystem, and these no longer differed between the control and experimental groups (Table 1).
The results of the quantitative cultivation of cecal contents were complicated by the presence of E. coli clones resistant to ciprofloxacin present also in the control chickens. The chickens must have been colonized by these strains either in the hatchery, during transport or shortly after placement in the animal house. CFU numbers varied by about two logs of magnitude in first two weeks, a result comparable to the effects of some single species probiotics, such as lactobacilli (Edens et al., 1997). Since the colonization by field E. coli strains probably occurred before the application of the probiotic mixture, a certain therapeutic effect was recorded, although further experimental work would be needed to confirm the higher efficacy of the probiotic mixture if applied directly in the hatchery. The results can also be compared with direct challenge with septicemic strain O78:H80 after application of a commercial competitive exclusion product (Aviguard), which led to a reduction in cecal colonization of 2 to 3 logs on d 7 and 14 (Hofacre et al., 2002) and studies testing the effect of the same product on ESBL/AmpC colonization producing E. coli strains (a decrease of 2–4 logs depending on the challenge strain dose) (Methner et al., 2019).
The experimental and control groups differed significantly in colonization by the experimental E. coli. The experimental strain was not detected in the experimental group when culturing the cecal contents with an exception of a single sample, which showed positive agglutination only at the lowest dilution; the experimental strain persisted in low intestinal numbers in this chicken at the wk 4. In contrast, in the control group in the first 2 wk, the experimental strain was found in all chickens up to a dilution of 105 to 106 CFU/g of cecal content (Table 1). In the third and fourth week, the O78 strain was recorded in about half of the chickens in the control group (in 3 and 4 chickens out of 7 tested, respectively). Thus, the probiotic mixture prevented colonization of the chickens in the experimental group by the APEC below a detectable level, whereas in the control group there was a massive and 100% colonization, which lasted for at least 14 d in all chickens. From the third week onwards, the differences between the groups were insignificant, but there was a significant decrease in E. coli colonization compared to previous weeks. The time course corresponds to the natural development of the intestinal microflora of commercially reared chickens, as previously described (Videnska et al., 2014), characterized by massive colonization by Proteobacteria in the first week and their gradual replacement by Firmicutes after the second week of life. The intestines of newly hatched chickens are susceptible to colonization by environmental bacteria, including potential pathogens, and the first hours of life determine the development of the intestinal ecosystem for next weeks. The first week is critical in terms of broiler chicken mortality, it is a period of extreme susceptibility to enteric and secondary systemic infections such as colibacillosis and salmonellosis (Crhanova et al., 2011). In contrast, chickens hatched in contact with adults very quickly develop "mature" intestinal microflora and associated resistance (Kubasova et al., 2019a). Although a time-limited effect can be expected after a single application of probiotics, colonization with complex microflora can be a way to ensure a substantial level of protection for commercial chickens in the most critical period. A satisfactory effect can be expected only 1) with early application of the mixture, before colonization of the intestine by bacteria from the environment; administration on the fattening farm comes too late; 2) when using complex mixtures, that is, commercial CE products, cecal content or own anaerobic mixtures, rather than classical probiotics such as lactobacilli, the effects of which are questionable.
ACKNOWLEDGMENTS
Special thanks to Sona Krmickova and Vera Novakova for their help during experiments.
This experiment has been supported by projects QK22020066 of the Czech Ministry of Agriculture and CEITEC/Literak/ITA 2021 by the Veterinary University Brno. Authors thank to Research Infrastructure RECETOX RI (No LM2018121) financed by the Ministry of Education, Youth and Sports, and Operational Programme Research, Development and Innovation – project CETOCOEN EXCELLENCE (No CZ.02.1.01/0.0/0.0/17_043/0009632) for supportive background.
ETHIC STATEMENT: The experiment was reviewed and approved by the committee for ethics in animal testing, Czech Ministry of Education, Youth and Sports, No. MSMT-19246/2021-3.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- Crhanova M., Hradecka H., Faldynova M., Matulova M., Havlickova H., Sisak F., Rychlik I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect. Immun. 2011;79:2755–2763. doi: 10.1128/IAI.01375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edens F.W., Parkhurst C.R., Casas I.A., Dobrogosz W.J. Principles of ex ovo competitive exclusion and in ovo administration of Lactobacillus reuteri. Poult. Sci. 1997;76:179–196. doi: 10.1093/ps/76.1.179. [DOI] [PubMed] [Google Scholar]
- Hofacre C.L., Johnson A.C., Kelly B.J., Froyman R. Effect of a commercial competitive exclusion culture on reduction of colonization of an antibiotic-resistant pathogenic Escherichia coli in day-old broiler chickens. Avian Dis. 2002;46:198–202. doi: 10.1637/0005-2086(2002)046[0198:EOACCE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Karasova D., Faldynova M., Matiasovicova J., Sebkova A., Crhanova M., Kubasova T., Seidlerova Z., Prikrylova H., Volf J., Zeman M., Babak V., Juricova H., Rajova J., Vlasatikova L., Rysavka P., Rychlik I. Host species adaptation of obligate gut anaerobes is dependent on their environmental survival. Microorganisms. 2022;10:1085. doi: 10.3390/microorganisms10061085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollarcikova M., Faldynova M., Matiasovicova J., Jahodarova E., Kubasova T., Seidlerova Z., Babak V., Videnska P., Cizek A., Rychlik I. Different Bacteroides species colonise human and chicken intestinal tract. Microorganisms. 2020;8:1483. doi: 10.3390/microorganisms8101483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubasova T., Kollarcikova M., Crhanova M., Karasova D., Cejkova D., Sebkova A., Matiasovicova J., Faldynova M., Pokorna A., Cizek A., Rychlik I. Contact with adult hen affects development of caecal microbiota in newly hatched chicks. PLoS One. 2019;14 doi: 10.1371/journal.pone.0212446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubasova T., Kollarcikova M., Crhanova M., Karasova D., Cejkova D., Sebkova A., Matiasovicova J., Faldynova M., Sisak F., Babak V., Pokorna A., Cizek A., Rychlik I. Gut anaerobes capable of chicken caecum colonisation. Microorganisms. 2019;7:597. doi: 10.3390/microorganisms7120597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubasova T., Seidlerova Z., Rychlik I. Ecological adaptations of gut microbiota members and their consequences for use as a new generation of probiotics. Int. J. Mol. Sci. 2021;22:5471. doi: 10.3390/ijms22115471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Castañeda C.D., Miotto J., McDaniel C., Kiess A.S., Zhang L. Effects of in ovo probiotic administration on the incidence of avian pathogenic Escherichia coli in broilers and an evaluation on its virulence and antimicrobial resistance properties. Poult. Sci. 2021;1003 doi: 10.1016/j.psj.2020.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehat J.W., van Vliet A.H.M., La Ragione R.M. The Avian Pathogenic Escherichia coli (APEC) pathotype is comprised of multiple distinct, independent genotypes. Avian Pathol. 2021;50:402–416. doi: 10.1080/03079457.2021.1915960. [DOI] [PubMed] [Google Scholar]
- Methner U., Friese A., Rösler U. Competitive exclusion: a tool to combat extended-spectrum β-lactamase-producing Escherichia coli strains in chickens. Res. Vet. Sci. 2019;123:124–128. doi: 10.1016/j.rvsc.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Papouskova A., Masarikova M., Valcek A., Senk D., Cejkova D., Jahodarova E., Cizek A. Genomic analysis of Escherichia coli strains isolated from diseased chicken in the Czech Republic. BMC Vet. Res. 2020;16:189. doi: 10.1186/s12917-020-02407-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Projahn M., Daehre K., Roesler U., Friese A. Extended-spectrum-beta-lactamase- and plasmid-encoded cephamycinase-producing enterobacteria in the broiler hatchery as a potential mode of pseudo-vertical transmission. J. Appl. Environ. Microbiol. 2017;83 doi: 10.1128/AEM.02364-16. e02364-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantala M., Nurmi E. Prevention of the growth of Salmonella Infantis in chicks by the flora of the alimentary tract of chickens. Br. Poult. Sci. 1973;14:627–630. doi: 10.1080/00071667308416073. [DOI] [PubMed] [Google Scholar]
- Ronco T., Stegger M., Olsen R.H., Sekse C., Nordstoga A.B., Pohjanvirta T., Lilje B., Lyhs U., Andersen P.S., Pedersen K. Spread of avian pathogenic Escherichia coli ST117 O78:H4 in Nordic broiler production. BMC Genomics. 2017;18:13. doi: 10.1186/s12864-016-3415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnska P., Sedlar K., Lukac M., Faldynova M., Gerzova L., Cejkova D., Sisak F., Rychlik I. Succession and replacement of bacterial populations in the caecum of egg laying hens over their whole life. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W., Sun C., Yuan J., Yang N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci. Rep. 2017;7:45308. doi: 10.1038/srep45308. [DOI] [PMC free article] [PubMed] [Google Scholar]