Abstract
Intestinal mucosa injury and loss of weight gain are unavoidable while using live vaccine strain to prevent chicken coccidiosis. In this study, recombinant Lactococcus lactis NZ3900/pNZ8149-IL-4-IL-2, expressing the fusion protein of chicken IL-4 and IL-2, was constructed using food-grade NICE expression system, trying to develop a possible oral immune adjuvant to enhance the immune effect of the live vaccine against chicken coccidiosis and minimize its adverse effects. Chickens were given different doses of recombinant L. lactis together with the live vaccine, then experimently attacked with coccidia virulent strains. Results showed that weight gains of co-immunization groups, given both 1 × 109 or 1 × 1010 CFU recombinant L. lactis and the live vaccine, were significantly higher than the vaccine-only group (P<0.05), while intestinal lesion scores of duodenum, jejunum, and cecum were significantly lower than the vaccine-only group (P<0.05), so was the oocyst shedding. The anticoccidial indexes (ACI) of the co-immunized groups given 1 × 109 and 1 × 1010 CFU recombinant L. lactis were 187.85 and 193.33, respectively, higher than 174.61 of the vaccine-only group. In addition, chickens in co-immunization groups gained more body weight than the vaccine-only group before being challenged with the virulent strains (P<0.05). All the results indicated that the constructed recombinant L. lactis NZ3900/ pNZ8149-IL-4-IL-2 exhibited an immune synergistic function to coccidiosis live vaccine, and could alleviate its adverse effect affecting weight gain. The application of the recombinant L. lactis showed the potency to lift the anticoccidial efficiency of the live vaccine from a medium level to a high level.
Key words: recombinant L. lactis NZ3900/pNZ8149-IL-4-IL-2, coccidiosis live vaccine, immune adjuvant, immune synergism
INTRODUCTION
Coccidiosis is an extremely serious intracellular protozoan parasitic disease caused by Eimeria parasitized on chicken intestine. The global economic losses caused by coccidia infection (chicken death, reduced productivity, and drug costs) reached 10.4 billion pounds every yr (Blake et al., 2020). In recent years, the use of Eimeria precocious attenuated strains as a live vaccine to inoculate chickens instead of chemical drugs to prevent and control coccidiosis has attracted extensive attention (Chapman and Jeffers, 2014; Price et al., 2015; Fatoba and Adeleke, 2018). Coccidia attenuated vaccine can not only avoid the drug resistance caused by repeated drug use, but also solve the problems of drug residues in chicken products. However, based on its good immune effect, it also caused slight damage to intestinal mucosa and delayed weight gain of infected chickens. Therefore, it is necessary to develop a new immune adjuvant that is effective, stable, inexpensive, available in large quantities, and free of drug resistance to improve the immune efficacy of Eimeria vaccines and reduce the adverse effect of limiting weight gain.
Cytokine adjuvants can strengthen the body's response to antigens by regulating cellular and humoral immunity (Decker and Safdar, 2011). In the immune regulation of Eimeria infection, cytokines are one of the most important effector and messenger molecules, and recombinant cytokines can be used to relieve intestinal lesions caused by Eimeria. Both cytokines interleukin-2 (IL-2) and interleukin-4 (IL-4) are major mediators of adaptive immune responses (Wen et al., 2016), and have been used as vaccine adjuvants to stimulate the cell-mediated immune response (T-helper type 1 cells, Th1) and drive a favorable humoral response (T-helper type 2 cells, Th2; Kopf et al., 1993; Song et al., 2013; Ranasinghe et al., 2014).
The nisin-controlled expression system (NICE) is a multifunctional and tightly controlled gene expression system based on the automatic regulation mechanism of nisin, it is also the most effective food grade inducible expression system at present. The L. lactis NZ3900/pNZ8149 expression system is an important and widely studied NICE as a live carrier for the delivery of heterologous proteins, which can induce mucosal and systemic immune responses after oral administration (Berlec et al., 2018). Plasmid pNZ8149 uses the Lac F gene rather than a resistance gene as a selectable marker, L. lactis NZ3900 lacks the lac F gene. Once the plasmid pNZ8149 containing lac F gene was transformed into NZ3900, it acquired the ability to grow with lactose, thus positive clones can be screened out. The inducer is nisin, nontoxic and harmless, the whole NZ3900/pNZ8149 expression system does not involve any antibiotics and be of biological safety (Wang et al., 2020).
In this study, a recombinant L. lactis NZ3900/PNZ8149-IL-4-IL-2 was constructed to express and secrete chicken IL-4 and IL-2 fusion proteins as cytokine adjuvant. The effect of the expressed IL-4-IL-2 fusion protein on the in vitro proliferation of spleen lymphocytes was evaluated by MTT assay. Chickens were orally administrated with recombinant L. lactis NZ3900/PNZ8149-IL-4-IL-2 adjuvant and coccidiosis vaccine, the synergistic effect of the recombinant L. lactis adjuvant on coccidiosis vaccine was evaluated by measuring the weight gain, intestinal lesions, bloody stool, and oocyst output of chickens. The aim is to provide a scientific basis for the development of a safe and oral live carrier adjuvant to improve the immune efficacy of live Eimeria vaccine, so as to find an economic, effective, and safe way to prevent and control chicken coccidiosis.
MATERIALS AND METHODS
Coccidial Vaccine Strains and Challenge Strains
The trivalent live vaccine with 4 precocious strains (E. tenella, E. acervulina and 2 strains of E. maxima) and the counterpart challenge virulent strains used in the current study were provided by the Laboratory of Veterinary Pathology (Shanxi Agricultural University, Shanxi, China).
Animals
Ninety 4-day-old specific pathogen-free (SPF) chicks were provided by Beijing Meri Avigon Laboratory Animal Technology Co., Ltd. (Beijing, China). The chicks were raised under strict pathogen-free conditions. The study was approved by the Animal Experiment Committee of Shanxi Agricultural University (the ethics number that allows animal experimentation: SXAU-EAW-2021C.ZD.01101401).
Bacterial Strain, Plasmid, and Growth Conditions
The L. lactis NZ3900 and plasmid pNZ8149 were from NIZO Food Research in the Netherlands. L. lactis were cultured in M17 medium (Solarbio, Beijing) supplemented with 5 g / L glucose (GM17) at 30°C.
Construction of Recombinant L. Lactis
The gene sequences AJ621249.1 and AF00631 encoding chicken IL-4 and IL-2 were obtained in the GenBank database, the codons were then optimized according to the codons preference of L. lactis. In the downstream of the gene sequences, the termination codon TGA of chicken IL-4 gene and TAA of IL-2 gene were removed, and a linker peptide was added between the 2 gene sequences, forming the IL-4-linker-IL-2 fusion gene. The signal peptide usp45 was added to the 5 ‘end of the fusion gene sequence. The restriction site Nco I (CCATGG) was introduced into the N-terminal of the sequence, the 6 × His tag sequence (CATCATCATCATCATCAT) and restriction site Sac I (GAGCTC) were introduced into the C-terminal of the sequence in turn (Figure 1). Sangon Biotech (Shanghai) Co., Ltd. was entrusted to synthesize the above fusion sequence, which were then inserted into the plasmid pNZ8149 and a new plasmid pNZ8149-IL-4-IL-2 was constructed.
Figure 1.
Recombinant plasmid pNZ8149-IL-4-IL-2 model.
The plasmid pNZ8149-IL-4-IL-2 was transformed into the competent cells of L. lactis NZ3900 by electroporation, and the recombinant positive colonies were screened through Eliker selective solid medium, colony PCR and sequencing technique according to the handbook from MoBiTec company (Germany). BGI Tech Solutions Co., Ltd. (Shenzhen, China) was entrusted to sequence the target genes in the positive clones, the target gene sequence must be identical to the designed gene sequence. The positive clones were cultured and induced with nisin at a final concentration of 30 ng/mL for 5 h to express IL-4-IL-2 fusion protein, the supernatant was collected and identified by Western blotting. The target protein in the supernatant reacted with mouse anti-6 × His-tag antibody diluted at 1:5,000 and goat-anti-mouse IgG secondary antibody diluted at 1:5,000. The positive L. lactis that can express and secrete IL-4-IL-2 fusion protein was named recombinant L. lactis NZ3900/pNZ8149-IL-4-IL-2. The Chicken Interleukin 4 (IL-4) ELISA Kit (Shanghai Jianglai Industrial Limited by Share Ltd., China) and Chicken Interleukin 2 (IL-2) ELISA Kit were used to detect the protein content of IL-4 and IL-2 in three batches of supernatants.
Activity of the Expressed Products
Chicken splenic lymphocytes were isolated with chicken spleen lymphocyte isolation solution kit (Solarbio, Beijing, China) and cultured in RPMI1640 medium (Hyclone, Taigu, Jinzhong, China), co-cultured with Con A at a final concentration of 20 μg/mL or LPS at a final concentration of 5 μg/mL for 24 h. Cells were collected by centrifugation (1,000 r/min, 5 min) and diluted to 5.0 × 106 cells /mL with RPMI1640 medium, and co-cultured with NZ3900/pNZ8149-IL-4-IL-2 culture supernatant. The proliferative activity of the expressed products by NZ3900/pNZ8149-IL-4-IL-2 on chicken spleen lymphocytes was detected by MTT colorimetry after co-cultivation for 48 h.
Immuno Synergistic of the Recombinant L. Lactis on Coccidiosis Live Vaccine
Ninety 4-day-old SPF chicks with similar body weight were randomly divided into 9 groups of 10 chicks each: the blank control group (C1), the vaccine-only group (C2), the challenge-only group (C3), 5 co-immune groups of NZ3900/pNZ8149-IL-4-IL-2 + vaccine (T1, T2, T3, T4, T5) and the co-immune group of no-load NZ3900/pNZ8149 + vaccine (T6). Chicks were weighed and orally inoculated with coccidiosis vaccine and different doses of recombinant L. lactis at 4 and 14 days old, respectively. All chicks were weighed again and given Eimeria virulent strains orally at 28 days old. Chicken feces and survivals were observed continuously, and blood stool scores were recorded on the 5th day after the challenge of the virulent strains. The feces were collected to observe the oocyst shedding, chicks were weighed on an empty stomach, and the lesion scores of duodenum, jejunum, and cecum were recorded while autopsy on the 7th day after the challenge of the virulent strains. The survival rate, oocyst output, average weight gain, relative weight gain rate average intestinal lesion score, and the anticoccidial index were calculated. The strains and doses of vaccine and the adjuvant in each group were shown in Table 1.
Table 1.
Groups and treatment.
| Primary immunization at 4 days old |
Secondary immunization at 14 days old |
Challenge |
|||||
|---|---|---|---|---|---|---|---|
| Group | Vaccine (dosage) | Adjuvant L.lactis | Adjuant dose (CFU) | Vaccine (dosage) | Adjuvant L.lactis | Adjuant dose (CFU) | Virulent strains (dosage) |
| T1 | 1 | NZ3900/pNZ8149-IL-4-IL-2 | 1 × 107 | 2 | NZ3900/pNZ8149-IL-4-IL-2 | 1 × 107 | 1 |
| T2 | 1 | 1 × 108 | 2 | 1 × 108 | 1 | ||
| T3 | 1 | 1 × 109 | 2 | 1 × 109 | 1 | ||
| T4 | 1 | 1 × 1010 | 2 | 1 × 1010 | 1 | ||
| T5 | 1 | 1 × 1011 | 2 | 1 × 1011 | 1 | ||
| T6 | 1 | No-load NZ3900/pNZ8149 | 1 × 1011 | 2 | No-load NZ3900/pNZ8149 | 1 × 1011 | 1 |
| C1 | 0 | PBS | 0 | 0 | PBS | 0 | 0 |
| C2 | 1 | PBS | 0 | 2 | PBS | 0 | 1 |
| C3 | 0 | PBS | 0 | 0 | PBS | 0 | 0 |
Note: The number of oocysts per dosage of vaccine was 1,000 oocysts of E. tenella precocious strain, 400 oocysts of E. acervulina precocious strain, and 100 oocysts of E. maxima Shanxi precocious strain and Shandong precocious strain respectively.
The number of oocysts per dosage of Eimeria virulent strains for challenge was 4.00 × 104 oocysts of E. tenella virulent strain, 20.00 × 104 oocysts of E. acervulina virulent strain, and 15.00 × 104 oocysts of E. maxima Shanxi virulent strain and Shandong virulent strain respectively.
Changes of Weight Gain
Average weight gain = (total final weight of chickens in each group - total initial weight of chickens in each group) / number of chickens per group.
Relative weight gain (RWG) = (average weight gain of immunized or infected chickens/average weight gain of blank control chickens) × 100%.
Bloody Stool Score
It was measured within 24 h after the occurrence of bloody stool, around the 5th day after giving the virulent strains. The score ranged from 0 to 4. A score of 0 indicated normal feces without hemorrhage; 1 indicated 1 to 25% hemorrhage in the feces; the score of 2 indicated 26 to 50% hemorrhage in the feces; 3 indicated 51 to 75% hemorrhage in the feces; and a score of 4 indicated 76 to 100% hemorrhage in the feces (Youn and Noh, 2001).
Scoring Criteria for Intestinal Lesions
The lesions of duodenum, jejunum and cecum were scored according to the method reported by Johnson & Reid on the 7th day after the virulent strains challenged (Johnson and Reid, 1970).
Lesion index = (average lesion score in duodenum + average lesion score in jejunum + average lesion score in cecum) / 3 × 10.
Oocyst Output
Chicken feces of each group were collected on the 6th and 7th days after challenged by virulent strains, and the oocysts were counted by McMaster method and blood cell count method. The oocysts index ranged from 0 to 40: A score of 0 indicated the oocyst counts (oocysts per gram of feces) were 0-0.1 × 106; a score of 1 indicated the oocyst counts were 0.11 × 106 -1 × 106; a score of 10 indicated the oocyst counts were 1.1 × 106 -5 × 106; a score of 20 indicated the oocyst counts were 5.1 × 106 -10 × 106; a score of 40 indicated the oocyst counts were more than 10.1 × 106 (Hodgson, 1970).
Reduction rate of oocyst = (oocysts output in the challenge group - oocysts output in the immune group) / oocysts output in the challenge group × 100%.
The standard of Vaccine Immunity Effect and Virulence of Virulent Strains
For chickens challenged with Eimeria virulent strains only, the mortality rate should be 40 to 60%, the lesion scores of duodenum and cecum should be ≥ 3, and the lesion scores of jejunum should be ≥ 2 in at least 9 chickens.
The immune efficacy standard of vaccine only or vaccine and adjuvant co-immunization strategy for effective resistance to coccidiosis is—the mortality rate of chickens should be ≤ 10.0%, and the lesion scores of duodenum, jejunum, and cecum should be ≤ 1 in at least 8 chickens.
Anticoccidial Index
The anticoccidiosis index (ACI) = (survival rate + relative weight gain) - (lesion index + oocyst index)
ACI ≥180 was considered the coccidia vaccine and adjuvant has a high efficiency; 160≤ ACI <180 was considered a medium efficiency; ACI <160 was considered that it does not possess anticoccidiosis effects (De Pablos et al., 2010; Song et al., 2020).
Statistical Analysis
All quantitative data were analyzed by ANOVA in a SPSS 19.0 (SPSS Inc., Chicago, IL) and expressed as mean ± SEM. A P-value of < 0.05 was considered as significant, a P-value of < 0.05 was considered as insignificant.
RESULTS
Construction of the Recombinant L. lactis NZ3900/pNZ8149-IL-4-IL-2
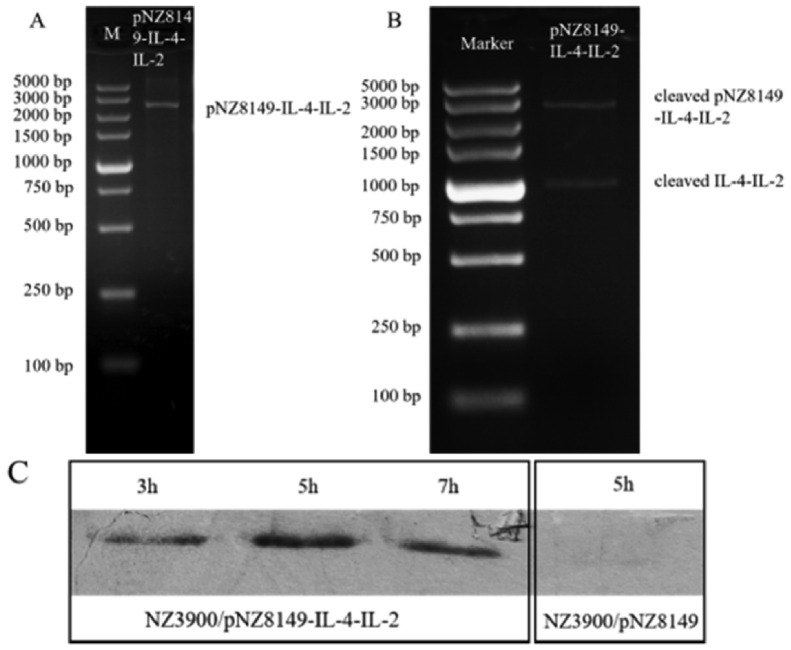
Nco I and Sac I were used to release the target fragment about 993 bp from the constructed plasmid pNZ8149-IL-4-IL-2. The sequencing result showed it was exactly the expected sequence. Western blotting analysis showed that L. lactis NZ3900/pNZ8149-IL-4-IL-2 induced by nisin secreted the His-tagged product into the medium, with the expected size of 36 KD of IL-4-IL-2 fusion protein, indicating that the food-grade L. lactis NZ3900/pNZ8149-IL-4-IL-2, could express and secrete IL-4-IL-2 fusion protein, was successfully constructed (Figure 2). The content of IL-4 and IL-2 fusion protein was 50 to 90 pg/mL (n = 3).
Figure 2.
Electropherogram of double-digested products of pNZ8149-IL-4-IL-2 and Western blot of recombinant L. lactis expression products. (A) Electropherogram of pNZ8149-IL-4-IL-2. (B) Electropherogram of double-digested products of pNZ8149-IL-4-IL-2. (C) Western blot of the expression products of recombinant L. lactis induced by nisin at 3, 5, and 7 h, respectively.
Activity of the Expressed Product to Promote Lymphocyte Proliferation
The stimulation indexes of the induced product of NZ3900/pNZ8149-IL-4-IL-2 to the proliferation of chicken spleen lymphocytes stimulated by Con A and LPS were significantly higher than that of blank control group and no-load L. lactis NZ3900/pNZ8149. This meant that the fusion protein IL-4-IL-2 expressed by the constructed recombinant L. lactis NZ3900/pNZ8149-IL-4-IL-2 had the biological activity of promoting the proliferation of chicken spleen lymphocytes in vitro (Figure 3).
Figure 3.
The SI of the expression product of recombinant L. lactis on chicken spleen lymphocytes.
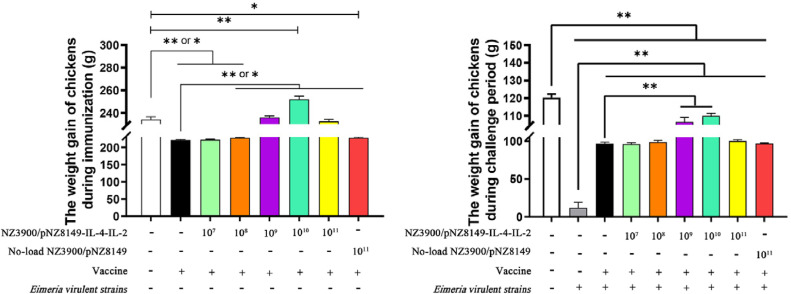
Promoting Effect of Recombinant L. lactis on Weight Gain During Immunization
During the immunization period (Aged 4 d–28 d), the weight gain of vaccine-only group was significantly lower than that of blank control group (P < 0.05). The weight gain of chickens in 1 × 108, 1 × 109, 1 × 1010, and 1 × 1011 CFU NZ3900/pNZ8149-IL-4-IL-2 + vaccine co-immunization groups were significantly higher than that in vaccine-only group (P < 0.05). The weight gain in 1 × 1010 CFU NZ3900/pNZ8149-IL-4-IL-2 + vaccine co-immunization group was significantly higher than that of blank control group (P < 0.05), and the weight gain in no-load NZ3900/pNZ8149 + vaccine co-immunization group was significantly lower than that of the blank control group (P < 0.05; Figure 4).
Figure 4.
The body weight gain in chickens.
Immune Synergistic Effect of Recombinant L. lactis on Coccidiosis Live Vaccine
Bloody Stool Score
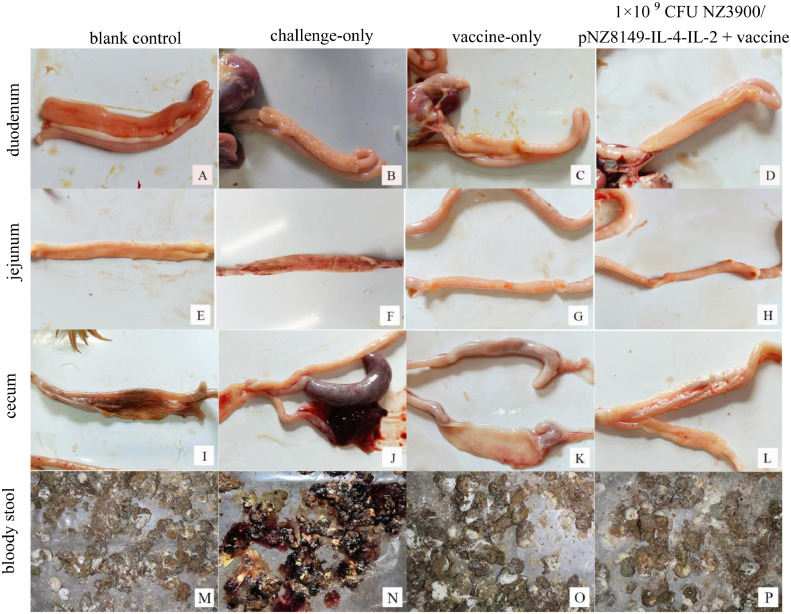
Chickens only challenged with virulent strains discharged bloody feces, and the bloody stool score was recorded as 3 on the 5th day after being challenged. No bloody stool was discharged in other groups (Figure 5).
Figure 5.
Intestinal lesion and bloody stool in chickens.
Weight Gain During Challenge
During the challenge period (Aged 28 d–35 d), the weight gain of the chickens in challenge-only group was significantly lower than that in blank control group and all immunized groups (P < 0.05). The weight gain of chickens in 1 × 109 and 1 × 1010 CFU NZ3900/pNZ8149-IL-4-IL-2 + vaccine co-immunization groups were significantly higher than that in vaccine-only group (P < 0.05), but significantly lower than that in blank control group (P < 0.05; Figure 4).
Intestinal Lesion Score
No visible lesions caused by coccidia were observed in the duodenum, jejunum, and cecum of blank control group (Figures 5A, 5E, 5I), and the lesion scores of the above intestines of challenge-only group were significantly higher than those of blank control group and all immunized groups 7 days after being challenged with virulent strains (P < 0.05; Figures 6 and 5B, 5F, 5J).
Figure 6.
The intestinal lesion score and distribution.
For the duodenum and the jejunum, the lesion scores of chickens in 1 × 109, 1 × 1010, 1 × 1011 CFU NZ3900/pNZ8149-IL-4-IL-2 + vaccine co-immunization groups were significantly lower than that in vaccine-only group (P < 0.05), but the differences were not significant compared with blank control group, nor were the differences between these groups (P > 0.05; Figures 6 and 5A, 5C, 5D, 5E, 5G, 5H).
For the cecum, the lesion scores of all recombinant L. lactis + vaccine co-immunization groups were significantly lower than that of vaccine-only group (P < 0.05). Except 1 × 107 CFU NZ3900/pNZ8149-IL-4-IL-2 + vaccine co-immunization group, there was no significant difference in the lesions scores between all other recombinant L. lactis + vaccine co-immunization groups and blank control group (P > 0.05; Figures 6 and 5I, 5K, 5L).
Oocyst Output
The oocyst output in chicken feces in challenge-only group was the biggest, followed by vaccine-only group, and NZ3900/pNZ8149-IL-4-IL-2 + vaccine co-immunization groups decreased with the increase of the oral dose of recombinant L. lactis. Compared with challenge-only group, the reduction rates of oocyst outputs of 1 × 109 and 1 × 1010 CFU NZ3900/pNZ8149-IL-4-IL-2 + vaccine co-immunization groups were 97.21% and 97.00%, respectively. The reduction rates of oocyst in the co-immunization groups with other doses of recombinant L. lactis adjuvant and vaccine ranged from 94.14% to 95.59%, which were higher than 93.39% in the vaccine-only group (Table 2).
Table 2.
Coccidia oocyst output in chicken feces of each group.
| Group | Adjuvant L. lactis | Dosage of adjuvant L. lactis (CFU) | Number of oocysts per gram of feces | Oocyst output in each group (104) | Reduction rate of oocyst (%) |
|---|---|---|---|---|---|
| T1 | NZ3900/pNZ8149-IL-4-IL-2 | 1 × 107 | 80,000 | 10,816.8 | 94.14 |
| T2 | 1 × 108 | 70,000 | 10,379.6 | 94.38 | |
| T3 | 1 × 109 | 63,300 | 8,141.6 | 95.59 | |
| T4 | 1 × 1010 | 53,500 | 5,155.3 | 97.21 | |
| T5 | 1 × 1011 | 42,720 | 5,534.4 | 97.00 | |
| T6 | No-load NZ3900/pNZ8149 | 1 × 1011 | 70,000 | 10,606.4 | 94.25 |
| C2 | PBS | 0 | 100,000 | 12,195.0 | 93.39 |
| C3 | PBS | 0 | 1,500,000 | 184,590.0 | - |
The Immunity Effect and ACI
The duodenal and cecal lesion scores of 10 chickens in challenge-only group were ≥ 3, and the jejunal lesion scores were ≥ 2, four chickens died, which met the virulence requirements of Eimeria virulent strains. All chickens in vaccine-only group and adjuvant + vaccine co immunization groups survived, and the lesion scores of duodenum, jejunum, and cecum were ≤ 1, indicating that the immune strategy of recombinant L. lactis adjuvant + vaccine used in this study could effectively resist chicken coccidiosis (Figure 6).
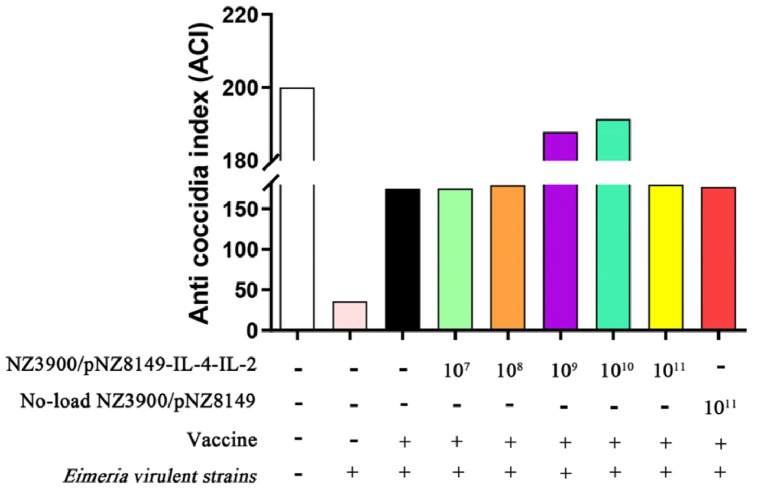
The ACI was calculated from survival rate, relative weight gain, lesion index, and oocyst index (Table 3). The ACI of 1 × 109 CFU and 1 × 1010 CFU NZ3900/pNZ8149-IL-4-IL-2 + vaccine co-immunization groups were 187.85 and 191.33, respectively, and the ACI of other co-immunization groups ranged from 174.84 to 179.98. It showed that the co-immunization to chicken with 1 × 109 CFU or 1 × 1010 CFU recombinant L. lactis adjuvant and vaccine could enhance the anti coccidial effect of chicken coccidiosis live vaccine from medium effect to high efficiency (Figure 7).
Table 3.
Survival rate, relative weight gain, lesion index, and oocyst index of chickens in each group.
| Group | Survival rate (%) | Relative weight gain (%) | Lesion index | Oocyst index |
|---|---|---|---|---|
| T1 | 100 | 79.84 | 5.00 | 0 |
| T2 | 100 | 82.14 | 3.00 | 0 |
| T3 | 100 | 88.51 | 0.66 | 0 |
| T4 | 100 | 91.33 | 0.00 | 0 |
| T5 | 100 | 83.31 | 3.33 | 0 |
| T6 | 100 | 80.52 | 3.66 | 0 |
| C1 | 100 | 100.00 | 0.00 | 0 |
| C2 | 100 | 81.27 | 6.66 | 0 |
| C3 | 60 | 9.79 | 33.00 | 1 |
Figure 7.
ACI of vaccine and recombinant L. lactis co-immunization.
DISCUSSION
IL-2 and IL-4 are secreted by T lymphocytes and B lymphocytes. They are the main cytokines involved in the adaptive immune response. The protein expression of IL-2 and IL-4 will increase in chickens when infected with different Eimeria strains (Cornelissen et al., 2009). IL-2 is a typical T cell growth factor, which induces the proliferation and differentiation of T cells to maintain the immune balance between helper T cells and effector T cells after antigen stimulation (Vazquez et al., 1986). In addition, high levels of IL-2 promote the proliferation of natural killer cells and stimulate B cell differentiation and antibody production (Sharma and Das, 2018). IL-4 stimulates B cells to proliferate and differentiate into plasma cells, induces B cells to convert Ig E and produce MHC-II molecules (Pene et al., 1988). In this study, the food grade recombinant L. lactis which can express and secrete IL-4-IL-2 fusion protein was constructed to explore its immune synergistic effect as an immune adjuvant.
It is difficult for conventional L. lactis expression system to secrete exogenous protein to the outside of the cell. So the signal peptide usp45 was added to the 5′ end of the codon-optimized IL-4-IL-2 gene sequence, which has a positive N-terminal, a cleavage C-terminal and a central hydrophobic region to guide the C-terminal linked protein across the cell wall. This not only prevents the target protein from being degraded by intracellular proteases, but also ensures that the expressed IL-4-IL-2 fusion protein can be secreted into the medium (Dong et al., 2015). The L. lactis NZ3900 / pNZ8149 expression system uses lac F gene instead of antibiotic gene as screening gene, antibiotics are not used throughout the whole process, which not only avoids the drift of resistance genes, but also reduces the risk of antibiotic residues in eggs and chicken, and reduces the production of microbecide resistance in chicken (Cao et al., 2020; Wang et al., 2021). The inducer nisin is a natural antibacterial peptide, which is nontoxic and safe to humans and animals. It can inhibit the growth of harmful bacteria, parasites, and viruses, promote the proliferation of beneficial bacteria, enhance the secretion of anti-inflammatory factors, inhibit the effect of pro-inflammatory factors, and promote the growth of broilers (Kieronczyk et al., 2020). It used as feed additive and has been licensed for food production in more than 60 countries and regions around the world (Shin et al., 2016). In this study, the constructed recombinant L. lactis NZ3900 / pNZ8149-IL-4-IL-2 induced by nisin to produce and secrete IL-4-IL-2 fusion protein, which can promote the proliferation of chicken spleen lymphocytes. All these make it possible to develope a new microbe type of food grade, safe, and efficient oral immune adjuvant against chicken coccidiosis.
During the immunization period, the weight gain of chickens only immunized with trivalent live vaccine of 4 Eimeria precocious strains was significantly lower than that of chickens not vaccinated (Figure 4). Although the pathogenicity of Eimeria precocious strains were much lower than that of their parent strains, they can still do harm to chicken intestinal mucosa by regulating the apoptosis and autophagy of intestinal epithelial cells (Zhang et al., 2019), thus hindering the digestion and absorption of nutrients and reducing weight gain. The weight gains of chickens co-immunized with vaccine and 1 × 108-1 × 1011 CFU NZ3900/pNZ8149-IL-4-IL-2 or 1 × 1011 CFU no-load NZ3900/pNZ8149 were significantly higher than vaccine-only group. So co-immunizing chickens with recombinant L. lactis could alleviate the reduced weight gain caused by Eimeria live vaccine.
Immunizing susceptible chickens with attenuated Eimeria live vaccine could prevent bloody stool and death caused by Eimeria virulent strains, to some extent alleviate the weight gain inhibition and intestinal lesions of infected chickens, and reduce oocyst output. In this study, the ACI of vaccine-only group was 176.41, the vaccine only had a medium protective effect on susceptible chickens. Co-immunization of susceptible chickens with recombinant L. lactis NZ3900/pNZ8149-IL-4-IL-2 and live vaccine could further increase the weight gain of chickens, palliate intestinal lesions, and decrease the oocyst output, especially at doses of 1 × 109 CFU and 1 × 1010 CFU. The weight gains of these 2 dose groups were significantly higher than that of the vaccine-only chickens, the intestinal lesion scores were almost the same as the uninfected healthy chickens, the reduction rates of oocyst outputs were above 97.0%, and the ACI reached to 187.85 and 191.33, suggesting highly effective against coccidiosis.
It has been reported that using recombinant plasmids pcDNA-IL-4 and pcDNA-IL-2 in combination as an adjuvant, co-immunizing piglets with the DNA vaccine pcDNA-ORF5 could significantly increase the level of specific anti-PRRSV IgG antibodies, promote the proliferation of specific T lymphocytes, increase the proportion of CD4+ and CD8+ T lymphocytes, and up-regulate the expression of IFN- γ (Tang et al., 2014). Chen embeded the recombinant plasmid co-expressing porcine IL-2 and IL-4/6 fusion protein in chitosan material as an immune adjuvant, and co-immunized pigs with porcine circovirus-2 (PCV-2) vaccine, which could improve the growth and weight gain of piglets, increase the serum level of IgG2a, CD4+ and CD8+ T lymphocytes. At the same time, increase the expression levels of IL-2, IL-4, IL-6, IL-15, TLR-2, TLR-7, Bcl-2, TNF-α, CD45 and STATs (STAT1, STAT2, STAT3, STAT4) genes (Chen et al., 2018). In earlier studies, we found that recombinant chicken IL-4-IL-2 gene adjuvant (pCI-chIL-4-chIL-2-EGFP) enhance the immune effect of coccidiosis live vaccine (Cui et al., 2022), make it highly effective against coccidiosis by upregulating the expression of IL-2, IL-4, TNF-α, and IFN-γ, and promoting the activation of T, B lymphocytes and APC cells in the intestinal tract and immune organs of chicken. In addition, L. lactis itself can promote the production of IgA in chickens to activate mucosal immunity (Ma et al., 2017; Sha et al., 2020), which further ensures the immunomodulatory effect of L. lactis NZ3900/pNZ8149-IL-4-IL-2, and makes the co-immunization of L. lactis NZ3900/pNZ8149-IL-4-IL-2 and live vaccine become a reliable strategy to prevent chicken coccidiosis.
CONCLUSIONS
Recombinant L.lactis NZ3900/ pNZ8149-IL-4-IL-2 exhibited an immune synergistic function to coccidiosis live vaccine, and could alleviate its adverse effect affecting weight gain. And lift the anticoccidial efficiency of the live vaccine from a medium level to a high level.
ACKNOWLEDGMENTS
This work was financially supported by Shanxi Province Basic Research Program, China (grant no. 20210302124495), the National Natural Science Foundation of China (grant no.31972647), Graduate education innovation project of Shanxi Province in 2021,China (grant no. 2021Y316), College of Veterinary Medicine Scientific research innovation project, Shanxi Agricultural University (grant no. DY-Q002), Financial reward for doctoral graduates and researchers from Shanxi Province to work in Shanxi, China (grant no. SXBYKY2021041).
Authors' contribution: Long-long Zheng carried out most of the experiments, wrote the manuscript, and should be considered as primary author. Nai-rui Huo and Ming-xue Zheng critically revised the manuscript and the experiment design. Li Zhang, Fan Tan, Chen Wang, Xiaoling Lv, Rui Bai helped with the experiment. All the authors read and approved the final version of the manuscript.
DISCLOSURES
No conflict of interest.
REFERENCES
- Berlec A., Skrlec K., Kocjan J., Olenic M., Strukelj B. Single plasmid systems for inducible dual protein expression and for CRISPR-Cas9/CRISPRi gene regulation in lactic acid bacterium Lactococcus lactis. Sci. Rep. 2018;8:1009. doi: 10.1038/s41598-018-19402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D.P., Knox J., Dehaeck B., Huntington B., Rathinam T., Ravipati V., Ayoade S., Gilbert W., Adebambo A.O., Jatau I.D., Raman M., Parker D., Rushton J., Tomley F.M. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020;51:115. doi: 10.1186/s13567-020-00837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W.Y., Dong M., Hu Z.Y., Wu J., Li Y.C., Xu H.D. Recombinant Lactococcus lactis NZ3900 expressing bioactive human FGF21 reduced body weight of Db/Db mice through the activity of brown adipose tissue. Benef. Microbes. 2020;11:67–78. doi: 10.3920/BM2019.0093. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Jeffers T.K. Vaccination of chickens against coccidiosis ameliorates drug resistance in commercial poultry production. Int. J. Parasitol. Drugs Drug. Resist. 2014;4:214–217. doi: 10.1016/j.ijpddr.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Song T., Xiao Y.L., Wan X., Yang L., Li J., Zeng G., Fang P., Wang Z.Z., Gao R. Enhancement of immune response of piglets to PCV-2 vaccine by porcine IL-2 and fusion IL-4/6 gene entrapped in chitosan nanoparticles. Res. Vet. Sci. 2018;117:224–232. doi: 10.1016/j.rvsc.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Cornelissen J.B., Swinkels W.J., Boersma W.A., Rebel J.M. Host response to simultaneous infections with Eimeria acervulina, maxima and tenella: a cumulation of single responses. Vet. Parasitol. 2009;162:58–66. doi: 10.1016/j.vetpar.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Cui K.L., Hao F.F., Zheng M.X., Lv X.L., Gong X., Zheng L.L., Xu T., Bai R. Synergistic effect of chicken IL-4 and IL-2 recombinant fusion gene adjuvant on chicken coccidia live vaccine. Heilongjiang Anim. Sci. Vet. Med. 2022;09:18–22. [Google Scholar]
- De Pablos L.M., dos Santos M.F., Montero E., Garcia-Granados A., Parra A., Osuna A. Anticoccidial activity of maslinic acid against infection with Eimeria tenella in chickens. Parasitol. Res. 2010;107:601–604. doi: 10.1007/s00436-010-1901-3. [DOI] [PubMed] [Google Scholar]
- Decker W.K., Safdar A. Cytokine adjuvants for vaccine therapy of neoplastic and infectious disease. Cytokine Growth Factor Rev. 2011;22:177–187. doi: 10.1016/j.cytogfr.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Dong Z., Zhang J., Li H., Du G., Chen J., Lee B. Codon and Propeptide Optimizations to Improve the Food-grade Expression of Bile Salt Hydrolase in Lactococcus lactis. Protein Pept. Lett. 2015;22:727–735. doi: 10.2174/0929866522666150610094829. [DOI] [PubMed] [Google Scholar]
- Fatoba A.J., Adeleke M.A. Diagnosis and control of chicken coccidiosis: a recent update. J. Parasit. Dis. 2018;42:483–493. doi: 10.1007/s12639-018-1048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson J.N. Coccidiosis: oocyst counting technique for coccidiostat evaluation. Exp. Parasitol. 1970;28:99–102. doi: 10.1016/0014-4894(70)90073-1. [DOI] [PubMed] [Google Scholar]
- Johnson J., Reid W.M. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Kieronczyk B., Rawski M., Mikolajczak Z., Swiatkiewicz S., Jozefiak D. Nisin as a novel feed additive: the effects on gut microbial modulation and activity, histological parameters, and growth performance of broiler chickens. Animals (Basel) 2020;10:101–115. doi: 10.3390/ani10010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M., Gros G.Le, Bachmann M., Lamers M.C., Bluethmann H., Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- Ma C., Zhang L., Gao M., Ma D. Construction of Lactococcus lactis expressing secreted and anchored Eimeria tenella 3-1E protein and comparison of protective immunity against homologous challenge. Exp. Parasitol. 2017;178:14–20. doi: 10.1016/j.exppara.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Pene J., Rousset F., Briere F., Chretien I., Paliard X., Banchereau J., Spits H., De Vries J.E. IgE production by normal human B cells induced by alloreactive T cell clones is mediated by IL-4 and suppressed by IFN-gamma. J. Immunol. 1988;141:1218–1224. [PubMed] [Google Scholar]
- Price K.R., Freeman M., Van-Heerden K., Barta J.R. Shedding of live Eimeria vaccine progeny is delayed in chicks with delayed access to feed after vaccination. Vet. Parasitol. 2015;208:242–245. doi: 10.1016/j.vetpar.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Ranasinghe C., Trivedi S., Wijesundara D.K., Jackson R.J. IL-4 and IL-13 receptors: roles in immunity and powerful vaccine adjuvants. Cytokine Growth Factor Rev. 2014;25:437–442. doi: 10.1016/j.cytogfr.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Sha Z., Shang H., Miao Y., Huang J., Niu X., Chen R., Hu L., Huang H., Wei K., Zhu R. Recombinant Lactococcus Lactis Expressing M1-HA2 fusion protein provides protective mucosal immunity against H9N2 avian influenza virus in chickens. Front. Vet. Sci. 2020;7:153. doi: 10.3389/fvets.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Das A. IL-2 mediates NK cell proliferation but not hyperactivity. Immunol. Res. 2018;66:151–157. doi: 10.1007/s12026-017-8982-3. [DOI] [PubMed] [Google Scholar]
- Shin J.M., Gwak J.W., Kamarajan P., Fenno J.C., Rickard A.H., Kapila Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2016;120:1449–1465. doi: 10.1111/jam.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H., Qiu B., Yan R., Xu L., Song X., Li X. The protective efficacy of chimeric SO7/IL-2 DNA vaccine against coccidiosis in chickens. Res. Vet. Sci. 2013;94:562–567. doi: 10.1016/j.rvsc.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Song X., Li Y., Chen S., Jia R., Huang Y., Zou Y., Li L., Zhao X., Yin Z. Anticoccidial effect of herbal powder “Shi Ying Zi” in chickens infected with Eimeria tenella. Animals (Basel) 2020;10:1484–1499. doi: 10.3390/ani10091484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Liu J., Li C., Zhang H., Ma P., Luo X., Zeng Z., Hong N., Liu X., Wang B., Wang F., Gan Z., Hao F. Positive effects of porcine IL-2 and IL-4 on virus-specific immune responses induced by the porcine reproductive and respiratory syndrome virus (PRRSV) ORF5 DNA vaccine in swine. J. Vet. Sci. 2014;15:99–109. doi: 10.4142/jvs.2014.15.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez A., Gerard J.P., Olive D., Auffredou M.T., Dugas B., Karray S., Delfraissy J.F., Galanaud P. Different human B cell subsets respond to interleukin 2 and to a high molecular weight B cell growth factor (BCGF) Eur. J. Immunol. 1986;16:1503–1507. doi: 10.1002/eji.1830161206. [DOI] [PubMed] [Google Scholar]
- Wang C., Zhou H., Guo F., Yang B., Su X., Lin J., Xu F. Oral immunization of chickens with Lactococcus lactis expressing cjaA temporarily reduces campylobacter jejuni colonization. Foodborne Pathog. Dis. 2020;17:366–372. doi: 10.1089/fpd.2019.2727. [DOI] [PubMed] [Google Scholar]
- Wang T., Wang Z., Mi J., Wang W., Li K., Qi X., Gao Y., Gao L., Liu C., Zhang Y., Pan Q., Wang X., Cui H. Recombinant avian beta-defensin produced by food-grade lactococcus as a novel and potent immunological enhancer adjuvant for avian vaccine. Probiotics Antimicrob. Proteins. 2021;13:1833–1846. doi: 10.1007/s12602-021-09847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Q., Xiong W., He J., Zhang S., Du X., Liu S., Wang J., Zhou M., Ma L. Fusion cytokine IL-2-GMCSF enhances anticancer immune responses through promoting cell-cell interactions. J. Transl. Med. 2016;14:41. doi: 10.1186/s12967-016-0799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn H.J., Noh J.W. Screening of the anticoccidial effects of herb extracts against Eimeria tenella. Vet. Parasitol. 2001;96:257–263. doi: 10.1016/s0304-4017(01)00385-5. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zheng M.X., Xi R., Xu Z.Y., Zhang X.S., Zheng L.L., Bai R., Mi C.L., Hao F.F., Feng Y.P. Comparison of the host cells apoptosis induced by precocious strains and virulent strains of Eimeria tenella. Poult. Sci. 2019;98:4384–4390. doi: 10.3382/ps/pez218. [DOI] [PubMed] [Google Scholar]