Summary
Background
Healthcare workers (HCWs) have been disproportionally affected by COVID-19. We investigated factors associated with two- and three-dose COVID-19 vaccine uptake and SARS-CoV-2 seropositivity among 1504 HCWs enrolled (19 February-7 May 2021) in a prospective COVID-19 vaccine effectiveness cohort in Albania through a secondary analysis.
Methods
We collected sociodemographic, occupational, health, prior SARS-CoV-2 infection, and COVID-19 vaccination data from all HCWs at enrollment. Vaccination status was assessed weekly through June 2022. A serum sample was collected from all participants at enrollment and tested for anti-spike SARS-CoV-2 antibodies. We analyzed HCWs characteristics and outcomes using multivariable logistic regression.
Findings
By 11 June 2022, 1337 (88.9%) HCWs had received two COVID-19 vaccine doses, of whom 255 (19.1%) received a booster. Factors significantly associated with receiving three doses (adjusted odds ratio (aOR), 95% CIs) were being ≥35 years (35–44 years: 1.76 (1.05–2.97); 45–54 years: 3.11 (1.92–5.05); ≥55 years: 3.38 (2.04–5.59)) and vaccinated against influenza (1.78; 1.20–2.64). Booster dose receipt was lower among females (0.58; 0.41–0.81), previously infected (0.67; 0.48–0.93), nurses and midwives (0.31; 0.22–0.45), and support staff (0.19; 0.11–0.32). Overall 1076 (72%) were SARS-CoV-2 seropositive at enrollment. Nurses and midwifes (1.45; 1.05–2.02), support staff (1.57; 1.03–2.41), and HCWs performing aerosol-generating procedures (AGPs) (1.40; 1.01–1.94) had higher odds of being seropositive, while smokers had reduced odds (0.55; 0.40–0.75).
Interpretation
In a large cohort of Albanian HCWs, COVID-19 vaccine booster dose uptake was very low, particularly among younger, female, and non-physician HCWs, despite evidence demonstrating the added benefit of boosters in preventing infection and severe disease. Reasons behind these disparities should be explored to develop targeted strategies in order to promote uptake in this critical population. SARS-CoV-2 seroprevalence was higher among non-physicians and HCWs performing APGs. A better understanding of the factors contributing to these differences is needed to inform interventions that could reduce infections in the future.
Funding
This study was funded by the Task Force for Global Health (US Centers for Disease Control (CDC) cooperative agreement # NU51IP000873) and the World Health Organization, Regional Office for Europe.
Keywords: Health care workers, COVID-19, Vaccination, Risk, SARS-CoV-2, Seropositivity
Research in context.
Evidence before this study
Health care workers (HCWs) are at increased risk for SARS-CoV-2 infection and are a priority group for both primary series and booster COVID-19 vaccination. However, in eastern Europe, COVID-19 vaccination uptake among HCWs has been moderate for primary series vaccine, and very low for booster dose. We searched Pubmed database for peer-reviewed articles on factors associated with COVID-19 vaccine uptake and SARS-CoV-2 infection among HCWs, published until October 1, 2022. Evidence on determinants of COVID-19 vaccination among HCWs largely comes from studies that investigated intention to vaccinate, rather than actual COVID-19 vaccine uptake, and generally suggests that male and older HCWs, physicians, and those vaccinated against influenza are more likely to accept vaccination. We identified only one study that evaluated factors associated with uptake of COVID-19 booster doses among HCWs. Globally, significant variation in SARS-CoV-2 seroprevalence among HCWs in the first year of the COVID-19 pandemic has been reported. Three global meta-analyses that included seroprevalence studies conducted within the first year of the pandemic estimated a pooled SARS-CoV-2 seroprevalence rate between 7% and 9% among HCWs. A number of studies have identified an association between gender, job role and socio-economic status, in addition to availability and quality of personal protective equipment, and the risk of SARS-CoV-2 infection in HCWs. However, findings are inconsistent, suggesting that risk factors for COVID-19 may be highly contextual and country-specific.
Added value of this study
To our knowledge, this is the first study to investigate factors associated with both COVID-19 primary series and booster vaccination uptake among health care workers in Europe. Among hospital-based HCWs working at three large hospitals in Albania, we found an overall low uptake of COVID-19 booster dose and lower uptake of COVID-19 vaccine (primary series and booster dose) among female and younger HCWs, and among nurses and non-clinical staff (booster dose only). Almost three-quarters of HCWs had serological evidence of SARS-CoV-2 infection within the first year of the pandemic, prior to receiving COVID-19 vaccine, considerably higher than rates found in other studies in Europe. Nurses and support staff, and HCWs performing aerosol-generating procedures were more likely to be SARS-CoV-2-seropositive.
Implications of all the available evidence
Our study identified demographic and occupational groups within the health workforce that remain unvaccinated more than a year after the roll out COVID-19 vaccines. Some of these groups overlap with groups of HCWs that appeared to be at higher risk of SARS-CoV-2 infection. A detailed understanding of the gaps in COVID-19 vaccination among HCWs is key for designing effective interventions aimed to increase vaccine uptake. The finding of higher SARS-CoV-2 seroprevalence among certain HCWs in Albania one year into the pandemic provides important evidence for policymakers for further investigation into the reasons for disparate infection rates to inform appropriate measures, including promotion of vaccination, in order to reduce work-related SARS-CoV-2 infections in anticipation of future waves of COVID-19.
Introduction
Healthcare workers (HCWs) are at increased risk of SARS-CoV-2 infection due to occupational exposure and have suffered considerable morbidity and mortality during the pandemic.1, 2, 3, 4 The World Health Organization (WHO) estimates that from January 2020 to May 2021 between 80,000 and 160,000 HCWs died from COVID-19 globally.2 For these reasons and concerns about the risk of onward transmission of SARS-CoV-2 from HCWs to patients, HCWs were among the first groups prioritized to receive COVID-19 vaccine in many countries.5
Whereas COVID-19 vaccine uptake has generally been high among HCWs in many high-income settings, uptake has varied in low- and middle-income countries (LMICs). In the WHO European Region, at the end of June 2022, more than 80% of HCWs in high income countries (HICs) had received a complete primary COVID-19 vaccine series and 51% an additional dose (booster) compared with 33% and 8%, respectively, of HCWs in upper MICs.6 Identifying which groups among HCWs have remained unvaccinated, and the reasons behind these lower vaccination rates, is critical for informing policies and interventions to increase uptake in this high priority group. Yet, data on COVID-19 immunizations among HCWs has largely focused on studies on intention to vaccinate rather than assessments of actual vaccination uptake after vaccines became available.7, 8, 9
In addition, while seroprevalence and factors associated with SARS-CoV-2 infection among HCWs have been widely described in HICs in the WHO European Region, limited data has been reported from MICs in Eastern Europe10, 11, 12, 13; a region that has experienced a disproportionately high burden of COVID-19.14 Understanding the burden of and risk factors for SARS-CoV-2 infection among HCWs remains important for guiding infection prevention and control measures.
In Albania, an upper MIC of 2.87 million people in Europe, COVID-19 vaccination began on 11 January 2021, using the Pfizer-BioNTech mRNA vaccine. HCWs were among the initial groups prioritized for vaccination by the Albanian National Technical Advisory Group for Immunization (NITAG).15 On 15 October 2021, the NITAG additionally recommended a booster dose for all HCWs six months following a primary series. Between June 2021 and January 2022, COVID-19 vaccination was mandatory for HCWs, with the implication that unvaccinated staff could not enter their workplace or receive a salary. Following vaccine introduction, Albania experienced three waves of COVID-19. The first occurred from January to April 2021, when the alpha variant was predominant. From July 2021 through the end of the year there was a similarly large increase in cases with the delta variant predominating. Finally, from January to March 2022 there was a substantial wave driven by circulation of the omicron variant (unpublished data, Albania Institute of Public Health).16
We used data from a prospective cohort study of COVID-19 vaccine effectiveness among HCWs in Albania17 to evaluate factors associated with uptake of COVID-19 vaccine primary series and booster dose between February 2021 and June 2022, and factors associated with SARS-CoV-2 infection prior to vaccination.
Methods
Study design and population
This study is a secondary analysis of data from the prospective cohort study “COVE-AL” in Albania to evaluate COVID-19 vaccine effectiveness in preventing SARS-CoV-2 infection among HCWs. Details of the cohort study have been previously published.18
In early 2021, all employees at three publicly funded hospitals in Albania (Durres, Fier, and Tirana) who were eligible for vaccination were invited to participate in the study, regardless of their intention to receive COVID-19 vaccine or known previous infections. Together the hospitals employ 3740 staff (2021 data), corresponding to one-third of all hospital-based HCWs in Albania. Recruitment to the study was performed by publicizing information within the three hospitals by email, word of mouth, flyers, and social media. In addition, study staff approached HCWs at various busy points in each of the hospitals.17 At enrollment, participants completed a survey, which included questions on demographics, personal health information, date of prior confirmed SARS-CoV-2 infection (RT-PCR, antigen rapid diagnostic test, or serology), occupation, performance of aerosol generating procedures (APGs), influenza vaccination during the 2020/2021 season, and COVID-19 vaccine history. During the study, a weekly questionnaire was administered to all participants to reassess COVID-19 vaccination status, including date of vaccine receipt.17 Vaccination status was verified using the national integrated immunization electronic system (IIS) and the family care physician's web-based system (E-vizita) in order to ensure completeness and validity of the vaccination data. In case of any discrepancies between the self-reported vaccination and IIS or E-vizita, such as the date of vaccination, we relied on the data from the national databases. However, for vaccinations received abroad that had not been registered in IIS or E-vizita, we used information reported by the participants.
At enrollment, we collected a serological sample from each participant, as previously described.18 Sera were tested for total IgG and IgM antibodies to SARS-CoV-2 spike protein using WANTAI SARS-CoV-2 Ab ELISA (WANTAI BioPharm, Beijing, China).19 Cut-off values were determined according to manufacturer guidelines.
Study data were uploaded and stored in the REDCap system (Research Electronic Data Capture, Vanderbilt University, Nashville, Tennessee, USA).20 For the present analysis, we used data collected through 11 June 2022.
Statistical analysis
We conducted uni- and multivariable logistic regression analyses to identify 1) independent factors associated with a) completing at least a primary (two-dose) COVID-19 vaccine series and b) receiving a third dose (among those receiving a primary series), and 2) in a separate analysis, factors associated with testing positive at study enrollment for total IgG and IgM antibodies against the SARS-CoV-2 spike protein. Our primary exposures of interest in both analyses were age, gender, any (self-reported) pre-existing chronic condition (including cardiac disease, hypertension, diabetes), body mass index (BMI), profession, smoking habits, household size, patient care responsibilities, hospital of employment, and receipt of seasonal influenza vaccination during the 2020/2021 season. In the vaccine analysis, we also included self-reported laboratory-confirmed SARS-CoV-2 infection and serological status (antibodies to SARS-CoV-2 spike protein at enrollment) as exposure variables.
We included all variables associated with the outcome in the univariate analyses at a significance level of P < .20 in the multivariable analysis. We used backwards stepwise elimination to remove covariates that were not significantly associated (P > .05) with the outcome in the multivariable analysis. Variables with the smallest effect were removed first. To assess the significance of each variable removed, we conducted a likelihood ratio test after each change. Only variables that significantly contributed to explain the outcome (P < .05) were retained in the main model.
We used the Mantel-Haenszel test for homogeneity to investigate possible effect modification of age on the association between gender and vaccine receipt, and gender and serological status. An interaction between gender and age-group was included in the multivariable analyses if the term was significant (P < .05). We computed crude and adjusted odds ratios (OR and aOR) with 95% confidence interval (CI) for each analysis.
The one sample z-test of differences in proportion was used to compare key demographic and occupational characteristics of HCWs in our cohort with all HCWs employed at the hospital at the start of the study.
For the seropositivity analysis, in order to ensure that seropositivity reflected antibodies acquired through natural infection rather than vaccination, we only included participants who had a serum sample collected either prior to receipt of their first COVID-19 vaccine or within 5 days after receiving their first vaccine dose. Individuals who had received only one COVID-19 vaccine dose by 11 June 2022 were excluded from the analyses on factors associated with COVID-19 vaccination.
All analyses were performed with Stata 10.0 (StataCorp, College Station, TX, USA).
Ethics
The study was approved by the Ethical Review Boards of WHO (reference number CERC.0097A) and the Albanian Institute of Public Health (reference number 156). This activity was determined to meet the requirements of public health surveillance as defined in 45 CFR 46.102(l) (2) (CDC reference number 0900f3eb81ce0ede). This study has been registered with ClinicalTrials.gov (NCT04811391). All participants provided written informed consent.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or development of the manuscript.
Results
During 19 February 2021–7 May 2021, 1504 HCWs enrolled in the study, corresponding to 40% of all HCWs in the three hospitals (Supplemental Table S1). Overall, age-groups were similar between the hospital population and the cohort. A higher proportion of females and physicians were included in the study compared with the overall workforce. However, absolute differences in proportions were relatively small (7%, or less).
Participants were mostly female [1132; (78.5%)] and the median age was 44 years (range 22–71 years, IQR; 33–53 years). Most participants were employed at Tirana University Hospital [942 (62.6%)] followed by Durres Regional Hospital [300 (20.0%)] and Fier Regional Hospital [262 (17.4%)]. HCWs included nurses and midwives [714 (47.5%); of whom 691 were nurses], physicians [305 (20.3%)], administrative and auxiliary staff [290 (19.3%)], and janitors and food workers (support staff) [195 (13.0%)]. Nearly all participants reported to have some patient contact at work [1434 (95.4%)]. In total, 535 participants (35.6%) reported having had a laboratory-confirmed SARS-CoV-2 infection prior to enrollment, and 420 (27.3%) reported receiving influenza vaccination in the 2020/2021 season.
Predictors of COVID-19 vaccination
By 11 June, 2022 (the cut-off date for analysis), 1337 (88.9%) participants had received at least a primary COVID-19 vaccine series (1082 (71.9%) received two doses, and 255 (16.9%) received three doses), 52 (3.5%) had received one dose, while 115 (7.7%) remained unvaccinated (Supplemental Table S2). During the 18 month study period, 68 participants (4.5%) dropped out or were lost to follow-up, of whom 17 (25%) were not vaccinated, 7 (11.3%) had received one dose, 37 (54.4%) two doses, and 7 (11.3%) three doses. A minority [11 (16.2%)] left the study within the first 9 months.
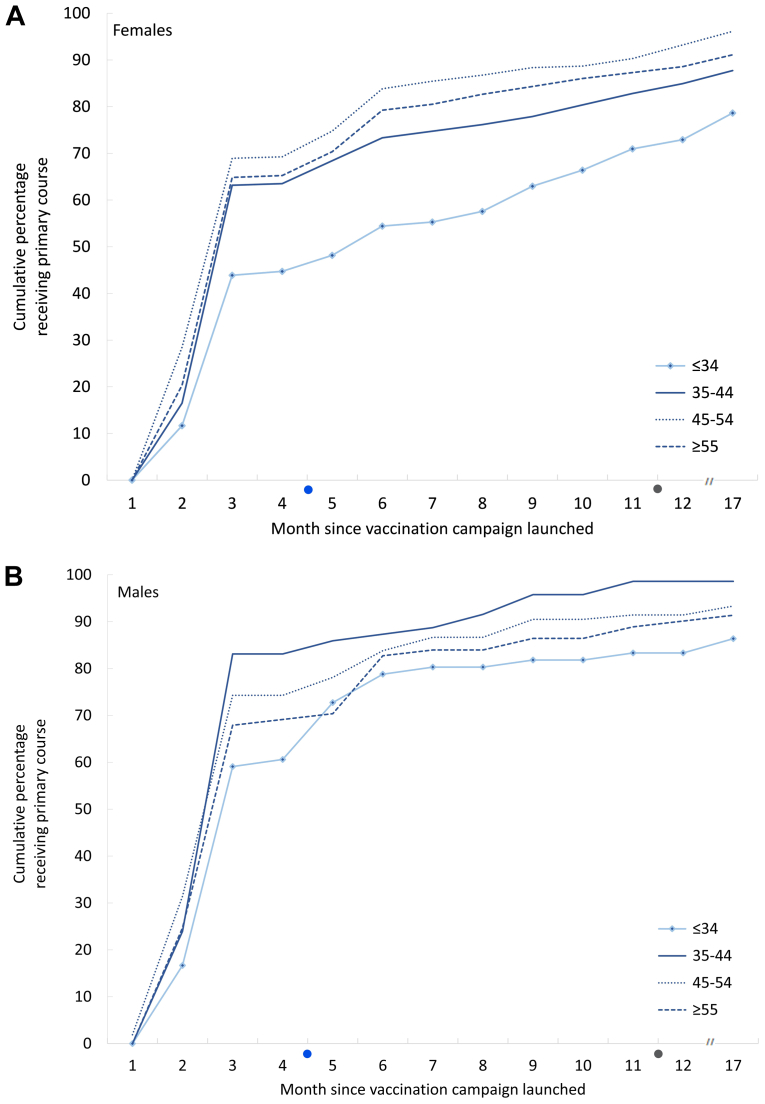
For the primary vaccine series, most participants were vaccinated with the Pfizer-BioNTech vaccine (87.9%) followed by Oxford-AstraZeneca (11.3%). Less than 1% received CoronaVac or Moderna. Completion of the primary (two-dose) vaccination series increased faster among male HCWs compared to female HCWs (Fig. 1). Uptake among female HCWs progressed most slowly in the age-group <35 years.
Fig. 1.
Monthly cumulative percent of health care workers receiving second dose of COVID-19 vaccine by gender and age-group (years), January 2021–June 2022, Albania. On the x-axis; blue dot = time when COVID-19 vaccination became mandatory for health care workers (June 2021), and grey dot = time when mandatory COVID-19 vaccination requirement was lifted (January 2022).
Factors associated with receiving a COVID-19 primary vaccine course
In the univariable analysis, factors most strongly associated with receiving at least a primary vaccine series were being ≥35 years, smoking (current and former), working in Fier Hospital, having direct contact with patients, caring for patients ≥65 years, while previous infection with SARS-CoV-2 and female sex was associated with a lower uptake (Table 1). We found no interaction between age and sex (χ2 = 4.55, P = .208).
Table 1.
Characteristics associated with uptake of at least two-doses of COVID-19 vaccine among health care workers in Albania, June 2022a.
| Characteristics N = 1452 | COVID-19 vaccination status |
Unadjusted Odds Ratio (OR) |
Adjusted Odds Ratio (aOR) |
|||
|---|---|---|---|---|---|---|
| Not vaccinated N = 115 (%) | Vaccinated two doses N = 1337 (%) | OR (95% CI) | p-value | aOR (95% CI) | p-value | |
| Age group (years) | – | – | – | – | – | – |
| ≤34 (n = 390) | 57 (14·6) | 333 (85·4) | Reference | – | Reference | – |
| 35–44 (n = 346) | 26 (7.5) | 320 (92.5) | 2.11 (1.29–3.43) | 0.003 | 2.07 (1.26–3.41) | 0.004 |
| 45–54 (n = 408) | 13 (3.2) | 395 (96.8) | 5.20 (2.80–9.67) | <0.001 | 4.62 (2.47–8.67) | <0.001 |
| ≥55 (n = 308) | 19 (6.2) | 289 (93.8) | 2.60 (1.51–4.48) | 0.001 | 2.37 (1.36–4.13) | 0.002 |
| Gender | – | – | – | – | – | – |
| Male (n = 312) | 13 (4.2) | 299 (95.8) | Reference | – | Reference | – |
| Female (n = 1140) | 102 (9.0) | 1038 (91.0) | 0.44 (0.24–0.80) | 0.007 | 0.45 (0.24–83) | 0.010 |
| Influenza vaccination in 2020–2021 | – | – | – | – | – | – |
| No (n = 1048) | 90 (8.6) | 958 (91.4) | Reference | – | Reference | – |
| Yes (n = 404) | 25 (6.2) | 379 (93.8) | 1.42 (0.90–2.25) | 0.131 | 1.96 (0.98–3.92) | 0.059 |
| Study site | – | – | – | – | – | – |
| Tirana (n = 914) | 79 (8.6) | 835 (91.4) | Reference | – | Reference | – |
| Fier (n = 291) | 13 (4.5) | 278 (95.5) | 2.02 (1.11–3.69) | 0.022 | 1.83 (0.98–3.40) | 0.057 |
| Durres (n = 247) | 23 (9.3) | 224 (90.7) | 0.92 (0.57–1.50) | 0.742 | 0.57 (0.27–1.21) | 0.145 |
| Patient contact | – | – | – | – | – | – |
| No (n = 66) | 12 (18.2) | 54 (81.8) | Reference | – | Reference | – |
| Yes (n = 1386) | 103 (7.4) | 1283 (92.6) | 2.77 (1.44–5.34) | 0.002 | 3.40 (1.67–6.93) | 0.001 |
| Prior self-reported laboratory confirmed SARS-CoV-2 infection | – | – | – | – | – | – |
| No (n = 939) | 64 (6.8) | 875 (93.2) | Reference | – | Reference | – |
| Yes (n = 513) | 51 (9.9) | 462 (90.1) | 0.66 (0.45–0.97) | 0.036 | 0.67 (0.45–0.99) | 0.048 |
| Pre-existing conditions | – | – | – | – | – | – |
| No (n = 1166) | 100 (8.6) | 1066 91.4) | Reference | – | Reference | – |
| Yes (n = 286) | 15 (5.2) | 271 (94.8) | 1.69 (0.97–2.96) | 0.064 | 1.21 (0.65–2.29) | 0.545 |
| Smoking habits | – | – | – | – | – | – |
| Never smoked (n = 1190) | 105 (8.8) | 1085 (91.2) | Reference | – | Reference | – |
| Current and former smoker (n = 262) | 10 (3.8) | 252 (96.2) | 2.44 (1.26–4.73) | 0.008 | 2.03 (1.01–4.08) | 0.046 |
| Care for older patients (65+) | – | – | – | – | – | – |
| No (n = 649) | 63 (9.7) | 586 (90.3) | Reference | – | Reference | – |
| Yes (n = 803) | 52 (6.5) | 751 (93.5) | 1.55 (1.06–2.28) | 0.024 | 1.11 (0.74–1.70) | 0.607 |
| Body mass indexb | – | – | – | – | – | – |
| ≤24.9 (n = 605) | 61 (10.1) | 544 (89.9) | Reference | – | Reference | – |
| ≥25.0–29.9 (n = 584) | 34 (5.8) | 550 (94.2) | 1.81 (1.17–2.80) | 0.007 | 1.15 (0.71–1.87) | 0.563 |
| ≥30.0 (n = 263) | 20 (7.6) | 243 (92.4) | 1.36 (0.80–2.31) | 0.250 | 0.80 (0.44–1.43) | 0.447 |
| Occupation | – | – | – | – | – | – |
| Physician (n = 297) | 14 (4.7) | 283 (95.3) | Reference | – | Reference | – |
| Nurses, midwifes (n = 696) | 56 (8.1) | 640 (91.9) | 0.57 (0.31–1.03) | 0.063 | 0.80 (0.43–1.49) | 0.477 |
| Support staff (n = 189) | 11 (5.8) | 178 (94.2) | 0.80 (0.36–1.80) | 0.591 | 0.77 (0.33–1.82) | 0.556 |
| Administrative staff (n = 270) | 34 (12.6) | 236 (87.4) | 0.34 (0.18–0.65) | 0.001 | 0.54 (0.27–1.08) | 0.082 |
| Antibodies against SARS-CoV-2 spike protein at enrollment | – | – | – | – | – | – |
| No (n = 409) | 30 (7.3) | 379 (92.7) | Reference | – | – | – |
| Yes (n = 1043) | 85 (8.2) | 958 (91.6) | 0.89 (0.58–1.38) | 0.605 | – | – |
| Household size | – | – | – | – | – | – |
| Live with 1 person (n = 23) | 2 (8.7) | 21 (91.3) | Reference | – | – | – |
| Live with 2 people (n = 161) | 18 (11.2) | 143 (88.8) | 0.76 (0.16–3.50) | 0.721 | – | – |
| Live with 3 people (n = 368) | 26 (7.1) | 342 (92.9) | 1.25 (0.28–5.64) | 0.769 | – | – |
| Live with 4 people (n = 463) | 40 (8.6) | 423 (91.4) | 1.01 (0.23–4.45) | 0.993 | – | – |
| Live with 5+ people (n = 437) | 29 (6.6) | 408 (93.4) | 1.34 (0.30–6.00) | 0.702 | – | – |
| Regular performance aerosol generating procedures | – | – | – | – | – | – |
| No (n = 716) | 57 (8.8) | 659 (92.0) | Reference | – | – | |
| Yes (n = 736) | 58 (7.9) | 678 (92.1) | 1.01 (0.69–1.48) | 0.955 | – | – |
| Patient-facing role | – | – | – | – | – | – |
| No (n = 570) | 47 (8.3) | 523 (91.7) | Reference | – | – | – |
| Yes (n = 882) | 68 (7.7) | 814 (92.3) | 1.08 (0.73–1.59) | 0.712 | – | – |
Excluding 52 participants who only received one COVID-19 vaccine dose.
Body mass index was calculated based on self-reported height and weight and grouped into the following three categories: 1) underweight (<18.5) and healthy weight (18.5–24.9), 2) overweight (25.0–29.9), and 3) obesity (≥30.0).
In the multivariable model (Table 1), factors positively associated with receiving at least the COVID-19 primary vaccine series (P < .05) were being in any age-group ≥35 years (35–44 years: aOR 2.07, 95% CI 1.26–3.41; 45–54 years: aOR 4.62, 95% CI 2.47–8.67; and ≥55 years: aOR 2.37, 95% CI 1.36–4.13), having any patient contact (aOR 3.40, 95% CI 1.67–6.93), and being a current or former smoker (aOR 2.03, 95% CI 1.01–4.08). Female HCWs had lower odds of vaccination (aOR 0.45, 95% CI 0.24–0.83) as did staff with a prior SARS-CoV-2 infection (aOR 0.67, 95% CI 0.45–0.99).
Factors associated with receiving a COVID-19 booster dose
Among participants who completed a primary vaccine series (n = 1337), 255 (19.1%) received a third dose, all but one with Pfizer-BioNTech. In the univariable analysis, booster dose receipt was significantly associated with age-group, gender, presence of medical conditions, smoking status, patient group cared for, BMI, hospital of employment, and household size (Table 2). The Mantel-Haenszel test for homogeneity did not suggest any interaction between gender and age on their effect on receiving a booster dose (χ2 = 1.76, P = .623).
Table 2.
Characteristics associated with uptake of COVID-19 vaccine booster among health care workers receiving at least two doses in Albania, June 2022.
| Characteristics N = 1337 | Vaccination status |
Unadjusted Odds Ratio (OR) |
Adjusted Odds Ratio (aOR) |
|||
|---|---|---|---|---|---|---|
| Vaccinated with two doses N = 1082 (%) | Vaccinated with three doses N = 255 (%) | OR (95% CI) | p-value | aOR (95% CI) | p-value | |
| Age group | – | – | – | – | – | – |
| ≤34 (n = 333) | 303 (91.0) | 30 (9.0) | Reference | – | Reference | – |
| 35–44 (n = 320) | 269 (84.1) | 51 (15.9) | 1.91 (1.18–3.09) | 0.008 | 1.76 (1.05–2.97) | 0.033 |
| 45–54 (n = 395) | 297 (75.2) | 98 (24.8) | 3.33 (2.15–5.17) | <0.001 | 3.11 (1.92 5.05) | <0.001 |
| ≥55 (n = 289) | 213 (73.7) | 76 (26.3) | 3.60 (2.28–5.69) | <0.001 | 3.38 (2.04–5.59) | <0.001 |
| Gender | – | – | – | – | – | – |
| Male (n = 299) | 209 (69.9) | 90 (30.1) | Reference | – | Reference | – |
| Female (n = 1038) | 873 (84.1) | 165 (15.9) | 0.44 (0.33–0.59) | <0.001 | 0.58 (0.41–0.81) | 0.002 |
| Influenza vaccination in 2020–2021 | – | – | – | – | – | – |
| No (n = 958) | 784 (81.8) | 174 (18.2) | Reference | – | Reference | – |
| Yes (n = 379) | 298 (78.6) | 81 (21.4) | 1.22 (0.91–1.65) | 0.179 | 1.78 (1.20–2.64) | 0.004 |
| Study site | – | – | – | – | – | – |
| Tirana (n = 835) | 655 (78.4) | 180 (21.6) | Reference | – | Reference | – |
| Fier (n = 278) | 224 (80.6) | 54 (19.4) | 0.88 (0.62–1.23) | 0.450 | 0.73 (0.50–3.07) | 0.110 |
| Durres (n = 224) | 203 (90.6) | 21 (9.4) | 0.38 (0.23–0.61) | <0.001 | 0.25 (0.14–0.45) | <0.001 |
| Occupation | – | – | – | – | – | – |
| Physician (n = 283) | 165 (58.3) | 118 (41.7) | Reference | – | Reference | – |
| Nurse, midwifes (n = 640) | 544 (85.0) | 96 (15.0) | 0.25 (0.18–0.34) | <0.001 | 0.31 (0.22–0.45) | <0.001 |
| Support staff (n = 178) | 157 (88.2) | 21 (11.8) | 0.19 (0.11–0.31) | <0.001 | 0.19 (0.11–0.32) | <0.001 |
| Administrative staff (n = 236) | 216 (91.3) | 20 (8.5) | 0.13 (0.08–0.22) | <0.001 | 0.15 (0.09–0.26) | <0.001 |
| Prior self-reported laboratory confirmed SARS-CoV-2 infection | – | – | – | – | – | – |
| No (n = 875) | 703 (80.3) | 172 (19.7) | Reference | – | Reference | – |
| Yes (n = 462) | 379 (82.0) | 83 (18.0) | 0.79 (0.58–1.07) | 0.454 | 0.67 (0.48–0.93) | 0.016 |
| Household size | – | – | – | – | – | – |
| Live with 1 person (n = 21) | 11 (52.4) | 10 (47.6) | Reference | – | Reference | – |
| Live with 2 people (n = 143) | 99 (69.2) | 44 (30.8) | 0.49 (0.19–1.24) | 0.130 | 0.54 (0.19–1.49) | 0.235 |
| Live with 3 people (n = 342) | 281 (82.2) | 61 (17.8) | 0.24 (0.10–0.59) | 0.002 | 0.25 (0.09–0.69) | 0.007 |
| Live with 4 people (n = 423) | 351 (83.0) | 72 (17.0) | 0.23 (0.09–0.55) | 0.001 | 0.22 (0.08–0.59) | 0.003 |
| Live with 5+ people (n = 408) | 340 (83.3) | 68 (16.7) | 0.22 (0.09–0.54) | 0.001 | 0.22 (0.08–0.60) | 0.003 |
| Pre-existing conditions | – | – | – | – | – | – |
| No (n = 1066) | 879 (82.5) | 187 (17.5) | Reference | – | Reference | – |
| Yes (n = 271) | 203 (74.9) | 68 (25.1) | 1.57 (1.15–2.16) | 0.005 | 1.06 (0.72–1.55) | 0.784 |
| Smoking habits | – | – | – | – | – | – |
| Never smoked (n = 1085) | 900 (82.9) | 185 (17.1) | Reference | – | Reference | – |
| Current and former smoker (n = 252) | 182 (72.2) | 70 (27.8) | 1.87 (1.36–2.57) | <0.001 | 1.42 (0.96–2.12) | 0.083 |
| Care for older patients (≥65 years) | – | – | – | – | – | – |
| No (n = 586) | 503 (85.8) | 83 (14.3) | Reference | – | Reference | – |
| Yes (n = 751) | 579 (77.1) | 172 (22.9) | 1.80 (1.35–2.40) | <0.001 | 1.27 (0.92–1.75) | 0.152 |
| Patient-facing Role | – | – | – | – | – | – |
| No (n = 523) | 435 (83.2) | 88 (16.8) | Reference | – | Reference | – |
| Yes (n = 814) | 647 (79.5) | 167 (20.5) | 1.28 (0.96–1.70) | 0.094 | 0.93 (0.61–1.44) | 0.761 |
| Body mass indexa | – | – | – | – | – | – |
| ≤24.9 (n = 544) | 468 (86.0) | 76 (14.0) | Reference | – | Reference | – |
| ≥25.0–29.9 (n = 550) | 429 (78.0) | 121 (22.0) | 1.74 (1.27–2.38) | 0.001 | 1.37 (0.95–1.99) | 0.096 |
| ≥30.0 (n = 243) | 185 (76.1) | 58 (23.9) | 1.93 (1.32–2.83) | 0.001 | 1.47 (0.95–2.28) | 0.083 |
| Antibodies against SARS-CoV-2 spike protein at enrollment | – | – | – | – | – | – |
| No (n = 409) | 296 (78.1) | 83 (21.9) | Reference | – | Reference | – |
| Yes (n = 1043) | 786 (82.1) | 172 (17.9) | 0.78 (0.58–1.05) | 0.098 | 0.91 (0.64–1.30) | 0.594 |
| Regular performance of aerosol generating procedures | – | – | – | – | – | – |
| No (n = 659) | 536 (81.3) | 123 (18.7) | Reference | – | – | – |
| Yes (n = 678) | 546 (80.5) | 132 (19.5) | 1.05 (0.80–1.38) | 0.708 | – | – |
| Any patient contact | – | – | – | – | – | – |
| No (n = 54) | 45 (83.3) | 9 (16.7) | Reference | – | – | – |
| Yes (n = 1283) | 1037 (80.8) | 246 (19.2) | 1.19 (0.57–2.45) | 0.646 | – | – |
Body mass index was calculated based on self-reported height and weight and grouped into the following three categories: 1) underweight (<18.5) and healthy weight (18.5–24.9), 2) overweight (25.0–29.9), and 3) obesity (≥30.0).
In the multivariable analysis, HCWs ≥35 years (35–44 years: aOR 1.76, 95% CI 1.05–2.97; 45–54 years: aOR 3.11, 95% CI 1.92–5.05; and ≥55 years: aOR 3.38, 95% CI 2.04–5.59) and HCWs who received the 2020/2021 influenza vaccine (aOR 1.78, 95% CI 1.20–2.64) had statistically significant higher odds of being vaccinated with a booster dose. In contrast, the odds of receiving a booster was lower among female HCWs (aOR 0.58, 95% CI 0.41–0.81), HCWs working in Durres hospital (aOR 0.25, 95% CI 0.14–0.45), non-physicians (e.g. nurses and midwives: aOR 0.31, 95% CI 0.22–0.45), HCWs previously infected with SARS-CoV-2 (aOR 0.67, 95% CI 0.48–0.93), and those living with 3 or more people in the household (e.g. living with 4 people: aOR 0.22, 95% CI 0.08–0.59) (Table 2).
Factors associated with SARS-CoV-2 seropositivity
Eleven participants were vaccinated against COVID-19 > 5 days before their enrollment serology sample was collected and were excluded from the analysis. Of the 1493 study participants included, 1076 (72%) had serological evidence of prior SARS-CoV-2 infection at enrollment. Among these, less than half [492 (45.7%)] reported having had a previous laboratory-confirmed SARS-CoV-2 infection, while 39 (9.4%) of the 417 participants with a negative serological result reported having had a laboratory-confirmed COVID-19 infection prior to enrollment.
In the multivariable logistic regression model, the only factors that remained positively associated with SARS-CoV-2 seropositivity (P < .05) were working as a nurse or midwife (aOR 1.45, 95% CI 1.05–2.02), working as a janitor or food worker (aOR 1.57, 95% CI 1.03–2.41), working at Tirana University Hospital (aOR 1.46, 95% CI 1.09–1.96), and performing AGPs (aOR 1.40, 95% CI 1.01–1.94) (Table 3). Current smokers had statistically significant lower odds of being seropositive compared with non-smokers (aOR 0.55, 95% CI 0.40–0.75).
Table 3.
Characteristics associated with presence of antibodies against severe acute respiratory syndrome coronavirus-2 spike protein among health care workers, February–May 2021a.
| Characteristics N = 1493 | Antibodies against SARS-CoV-2 spike protein |
Unadjusted Odds Ratio (OR) |
Adjusted Odds Ratio (aOR) |
|||
|---|---|---|---|---|---|---|
| Negative N = 417 (%) | Positive N = 1076 (%) | OR (95% CI) | p-value | aOR (95% CI) | p-value | |
| Age group (years) | – | – | – | – | – | – |
| ≤34 (n = 416) | 103 (24.8) | 313 (75.2) | Reference | – | Reference | – |
| 35–59 (n = 972) | 282 (29.0) | 690 (71.0) | 0.81 (0.62–1.05) | 0.105 | 0.88 (0.67–1.16) | 0.362 |
| ≥60 (n = 105) | 32 (30.5) | 73 (69.5) | 0.75 (0.47–1.20) | 0.233 | 0.85 (0.52–1.40) | 0.529 |
| Gender | – | – | – | – | – | – |
| Male (n = 316) | 111 (35.1) | 205 (64.9) | Reference | – | Reference | – |
| Female (n = 1177) | 306 (26.0) | 871 (74.0) | 1.54 (1.18–2.01) | 0.001 | 1.22 (0.90–1.65) | 0.210 |
| Smoking habits | – | – | – | – | – | – |
| Never smoked (n = 1224) | 320 (26.1) | 904 (73.9) | Reference | – | Reference | – |
| Previous smoker (n = 68) | 14 (20.6) | 54 (79.4) | 1.36 (0.74–2.49) | 0.31 | 1.74 (0.94–3.22) | 0.080 |
| Current smoker (n = 201) | 83 (41.3) | 118 (58.7) | 0.50 (0.37–0.69) | <0.001 | 0.55 (0.40–0.75) | <0.001 |
| Study site | – | – | – | – | – | – |
| Fier Hospital (n = 296) | 96 (32.4) | 200 (67.6) | Reference | – | Reference | – |
| Durres Hospital (n = 262) | 85 (32.4) | 177 (67.6) | 1.00 (0.70–1.43) | 0.998 | 1.04 (0.72–1.50) | 0.847 |
| Tirana University Hospital (n = 935) | 236 (25.2) | 699 (74.8) | 1.42 (1.07–1.89 | 0.015 | 1.46 (1.09–1.96) | 0.011 |
| Occupation | – | – | – | – | – | – |
| Physicians (n = 296) | 95 (32.1) | 201 (67.9) | Reference | – | Reference | – |
| Nurse, midwifes (n = 713) | 164 (23.0) | 549 (77.0) | 1.58 (1.17–2.14) | 0.003 | 1.45 (1.05–2.02) | 0.026 |
| Support staff (n = 195) | 55 (28.2) | 140 (71.8) | 1.20 (0.81–1.79) | 0.360 | 1.57 (1.03–2.41) | 0.039 |
| Administrative staff (n = 289) | 103 (35.5) | 186 (64.5) | 0.85 (0.61–1.20) | 0.365 | 1.02 (0.71–1.50) | 0.886 |
| Regular performance of aerosol generating procedures | – | – | – | – | – | – |
| No (n = 741) | 243 (32.8) | 498 (67.2) | Reference | – | Reference | – |
| Yes (n = 752) | 174 (23.1) | 578 (76.9) | 1.62 (1.29–2.04) | <0.001 | 1.40 (1.01-1.94) | 0.044 |
| Patient-facing Role | – | – | – | – | – | – |
| No (n = 594) | 192 (32.3) | 402 (67.7) | Reference | – | Reference | – |
| Yes (n = 899) | 225 (25.0) | 674 (75.0) | 1.43 (1.13–1.80) | 0.002 | 1.01 (0.62–1.63) | 0.976 |
| Influenza vaccination 2020/2021 | – | – | – | – | – | – |
| No (n = 1075) | 287 (26.7) | 788 (73.3) | Reference | – | Reference | – |
| Yes (n = 418) | 130 (31.1) | 288 (68.9) | 0.81 (0.63–1.03) | 0.089 | 0.82 (0 59–1.15) | 0.254 |
| Any patient contact | – | – | – | – | – | – |
| No (n = 70) | 21 (30.0) | 49 (70.0) | Reference | – | – | – |
| Yes (n = 1423) | 396 (27.8) | 1027 (72.2) | 1.11 (0.66–1.88) | 0.693 | – | – |
| Care for older patients (≥65 years) | – | – | – | – | – | – |
| No (n = 682) | 195 (28.6) | 487 (71.4) | Reference | – | – | – |
| Yes (n = 811) | 222 (27.4) | 589 (72.6) | 1.06 (0.85–1.33) | 0.601 | – | – |
| Body mass indexb | – | – | – | – | – | – |
| ≤24.9 (n = 633) | 187 (29.5) | 446 (70.5) | Reference | – | – | – |
| ≥25.0–29.9 (n = 589) | 157 (26.7) | 432 (73.3) | 1.15 (0.90–1.48) | 0.262 | – | – |
| ≥30.0 (n = 271) | 73 (26.9) | 198 (73.1) | 1.13 (0.83–1.56) | 0.428 | – | – |
| Pre-existing conditions | – | – | – | – | – | – |
| No (n = 1203) | 337 (28.0) | 866 (72.0) | Reference | – | – | – |
| Yes (n = 290) | 80 (27.6) | 210 (72.4) | 1.02 (0.77–1.36) | 0.884 | – | – |
| Household size | – | – | – | – | – | – |
| Live with 1 person (n = 23) | 7 (30.4) | 16 (69.6) | Reference | – | – | – |
| Live with 2 people (n = 166) | 41 (24.7) | 125 (75.3) | 1.33 (0.51–3.47) | 0.555 | – | – |
| Live with 3 people (n = 385) | 114 (29.6) | 271 (70.4) | 1.04 (0.42–2.60) | 0.933 | – | – |
| Live with 4 people (n = 475) | 125 (26.3) | 350 (73.7) | 1.23 (0.50–3.05) | 0.663 | – | – |
| Live with ≥5 people (n = 444) | 130 (29.3) | 314 (70.7) | 1.06 (0.42–2.63) | 0.906 | – | – |
Excluding 11 participants who received their first COVID-19 vaccine >5 days before enrollment in the study.
Body mass index was calculated based on self-reported height and weight and grouped into the following three categories: 1) underweight (<18.5) and healthy weight (18.5–24.9), 2) overweight (25.0–29.9), and 3) obesity (≥30.0).
Discussion
In our study evaluating SARS-CoV-2 seroprevalence and COVID-19 vaccine uptake among HCWs in Albania, nearly three-quarters of hospital HCWs had evidence of previous SARS-CoV-2 infection 12 months into the pandemic. In addition, while a high proportion of HCWs (almost 90%) had received two doses of COVID-19 vaccine by June 2022, demand slowed substantially after reaching 60% in the first three months of the programme. Similar initial rapid increase in COVID-19 vaccination uptake among HCWs in the first months of vaccine rollout followed by a slowdown has been observed in a number of other countries.6,21 Moreover, in June 2022, less than one in five HCWs had received a booster dose, despite that national recommendations for a third COVID-19 vaccine dose for HCWs in Albania were in place since 1 November 2021, and numerous studies demonstrating the protective effect of a third vaccine dose, particularly in preventing severe disease.22,23 Similar low uptake of booster doses has been reported among HCWs in other upper MICs in Europe, where on average 7.8% HCWs received a booster, but also in the general population of Albania, where only 11% had received a booster by June 2022.6
In our study, female HCWs were less likely to be vaccinated compared with male colleagues. Vaccination uptake was particularly low among females <35 years (primary series: 78.6%, booster dose: 6.8% - data not shown). Other large post-introduction surveys in Israel and the United States have also documented lower COVID-19 vaccine uptake among female HCWs compared with male HCWs (aOR 0.65, 95% CI 0.58–0.73 and aOR 0.83, 95% CI 0.79–0.88),24,25 respectively. Moreover, several studies on willingness to vaccinate have reported lower intention to receive COVID-19 vaccine among female HCWs of reproductive age.7,9,24,26 This observation has partly been attributed to concerns about adverse effects of COVID-19 vaccines on fertility and pregnancy,26, 27, 28 despite the lack of evidence of an association.29,30 Addressing these concerns, describing the potential harms of SARS-CoV-2 infection in pregnant women and their newborns31,32 and highlighting the potential benefits of COVID-19 vaccine in pregnant women33,34 may help increase vaccine uptake in female HCWs.
Prior and current smoking was associated with a higher uptake of a primary vaccine series compared with never-smokers, although the absolute difference was small (5%). Additionally, the effect was borderline significant (P = .046), and the association may be spurious in the context of multiple testing. A higher proportion of former and current smokers were also vaccinated with a booster dose compared with never-smokers; however, this association did not reach statical significance in the subgroup analysis after multivariable adjustment. It may be that knowledge about increased risk of severe disease and death from COVID-1935 among former and current smokers motivated this group to get vaccinated, but the effect size was small.
Although the difference was small, HCWs with a known SARS-CoV-2 infection prior to enrollment had a lower two- and three-dose vaccine uptake compared to those with no self-reported history of SARS-CoV-2 infection. A large multicenter study in the United States also found that HCWs with prior SARS-CoV-2 infection had lower vaccine uptake (OR 0.55, 95% CI, 0.51–0.58).25 Likewise, a study from Azerbaijan showed that HCWs who were not previously infected with COVID-19 were 7 times more likely to be vaccinated compared with individuals with a previous infection.36 Individuals with a prior infection may decline vaccination due to a belief that naturally acquired immunity reduces the risk of re-infection to the extent that vaccination is no longer beneficial. However, a number of studies have demonstrated the added benefit of vaccination following natural infection (hybrid immunity), which has been shown to increase the durability and breadth of immunity against SARS-CoV-2, and confer very high protection against hospitalization.37, 38, 39, 40 Our study also adds to the growing evidence-base that seasonal influenza vaccination, which has been recommended for all HCWs in Albania since 2014,41 is a predictor for COVID-19 vaccine receipt. A meta-analysis on factors associated with HCWs’ intention to receive COVID-19 vaccination found that HCWs who were previously vaccinated against influenza had a higher likelihood of accepting COVID-19 vaccines (OR 2.97, 95% CI 1.82–4.84).7 Likewise, studies from Azerbaijan and Georgia found that HCWs with prior influenza vaccination were more likely to be vaccinated against COVID-19 (OR 2.30, 95% 1.67–3.2036; and OR 2.98, 95% CI 2.19–4.08),42 respectively). This relationship has been ascribed to an overall positive attitude about vaccination, including a belief that vaccines are safe and effective, a higher perceived threat of respiratory diseases, and hence increased benefit of vaccination.43,44 These findings support the supposition that a seasonal influenza vaccination programme provides a foundation for pandemic preparedness in part by increasing the probability that individuals will receive a future pandemic vaccine,45 even for a pandemic not caused by influenza.
We found that non-physicians were significantly less likely to receive a booster dose compared with physicians, which is particularly concerning in view of the increased risk of SARS-CoV-2 infection observed among nurses and nonclinical support staff in this study. This finding has been described in other studies.9,25
Vaccination uptake varied across study sites; uptake was lowest in Durres hospital for both the primary series and booster dose. Since COVID-19 vaccines were equally accessible at all three hospitals, variation in vaccine uptake may be linked to differences in promotion of vaccination at the hospitals, peer influence, individual motivation, or other factors which could not be investigated within this study.
Additionally, we found that staff living in households with ≥4 members were less likely to receive a third COVID-19 vaccine dose compared with two-person households. This finding may be explained by differences in socio-economic status; other studies have shown that higher income is associated with higher COVID-19 vaccine uptake among HCWs.9 In Albania, households with dependent children have lower reported household income than households without.46 If larger households in our study represent families with children, this could be a proxy for socio-economic status.
While a number of serological studies have been conducted among HCWs in high-income settings in Europe,12 our findings provide a rare insight into potential risk factors for infection among HCWs in an upper middle-income country in Eastern Europe during the first year of the pandemic. The high seroprevalence among HCWs presented here is consistent with high SARS-CoV-2 seropositivity (48.2%) described in a population survey conducted in Albania in December 2020, just 2–4 months prior to our study.47 The considerably higher seroprevalence in our HCW population compared to the general population may reflect both the emergence of the SARS-CoV-2 alpha variant in early 2021 in Albania and occupational exposure. In addition, SARS CoV-2 seroprevalence among HCWs in this study was noticeably higher compared to HCWs in HICs in Europe; 20 studies among HCWs in 9 HICs in Europe during the same period (January–May 2021) found seropositivity rates ranging from 5.2%–53.1%.12 However, our rates were similar to the rate of seropositivity (69.5%) among HCWs in Bosnia and Herzegovina, another middle-income country in the same region, at roughly the same time.48 Variation in risk of nosocomial infections and in intensity of SARS-CoV-2 community transmission between countries may partially explain these differences.
Over half of seropositive participants did not report a prior laboratory-diagnosed SARS-CoV-2 infection. Because COVID-19 testing of symptomatic individuals in Albania was quite common early in the pandemic, this finding likely reflects high numbers of asymptomatic infections. Our findings are consistent with those from a recent meta-analysis, which found that the proportion of asymptomatic infections among SARS-CoV-2 confirmed populations was 41% (95% CI, 34%–48%).49
Study participants who regularly performed AGPs were also significantly more likely to have had a SARS-CoV-2 infection compared with those not performing the procedure, a finding that has been reported in other studies.50 This finding underscores the importance of consistent infection, prevention and control measures for staff at increased occupational risk. Moreover, HCWs working in a nursing or midwifery role, or as support staff (janitorial staff or food workers), were at higher risk of infection compared to physicians. These findings are very similar to those from a large cohort study in the United Kingdom that found that HCWs working in nursing and midwifery roles were more likely to be infected compared with physicians (aOR 1.30, 95% CI 1.11–1.53),51 and to a study in Italy where nurses had a higher odds of infection compared to administrative staff (aOR 1.28, 95% CI, 1.17–1.41), but physicians did not.52 In our study population, nurses, midwifes, and support staff may have been at increased risk of exposure to SARS-CoV-2 due to closer and more prolonged contact with COVID-19 patients or less access to personal protective equipment. Nevertheless, differences in non-occupational risk, including disparities in socio-economic status and household composition (with and without children), which we did not evaluate in this study, may also have contributed to this finding.
Finally, SARS-CoV-2 seroprevalence appeared to be significantly lower among current smokers compared with those who never smoked. Two other studies from England and Italy also found that HCWs who smoked were less likely than non-smokers to have SARS-CoV-2 antibodies; OR 0.63, 95% CI 0.50–0.79,51 and aOR 0.41, 95% CI 0.27–0.61,53 respectively. Lower prevalence of SARS-CoV-2 antibodies among current smokers compared with non-smokers has also been documented in large population-based surveys (aOR 0.64, 95% CI 0.58–0.71).54 Reasons for this observation may include residual confounding. Alternatively, smokers may have avoided infection-prone settings due to awareness of their increased risk of severe disease. However, several studies have suggested that smoking is associated with overall reduced levels of IgA, IgG and IgM titers, and of IgG following SARS-CoV-2 infection or COVID-19 vaccination,55, 56, 57, 58, 59 hence a lower seroprevalence among smokers could be an artefact.
Strengths and limitations
Strengths of our study include a methodologically rigorous protocol with a relatively large sample of HCWs from three hospitals that employ one-third of all hospital-based staff in Albania. In addition, the study was conducted in a region where data on seropositivity and factors associated with COVID-19 vaccine uptake are sparse. The analysis benefitted from the completeness of enrollment data, and the use of serology in addition to laboratory-confirmed outcomes, to determine factors associated with SARS-CoV-2 infection. Finally, the prospective study design allowed us to follow changes in vaccination uptake over time, and the number of participants who withdrew or were lost to follow-up was low.
Our study, however, has a number of limitations. First, because participants were selected by convenience sample, the study may suffer from self-selection bias by potentially attracting HCWs more willing to engage with research and science, more likely to get vaccinated, or more likely to suspect prior infection, which could impact measures of effect and potentially overestimate vaccination uptake and SARS-CoV-2 seroprevalence. Also, although our sample was broadly representative of all hospital staff in terms of demographic and occupational characteristics, females and physicians were slightly overrepresented. Both characteristics were associated with vaccination and seropositivity status; however, the effects were in opposite directions in the two analyses, and we cannot assess how this may have affected the overall vaccine uptake and seroprevalence estimates. Second, we included 68 dropouts in the analysis of predictors for COVID-19 uptake and it is possible that some misclassification regarding vaccination status might have occurred. However, most withdrawals occurred much later in the analysis period, when few HCWs were vaccinated with primary series or booster doses.
Third, while primary series and booster dose vaccine uptake were similar to those reported among all Albanian HCWs for the same period (82.7% and 17% respectively),6 we did not have data on the demographic and occupational distribution of all HCWs in Albania to assess if participants in our study were generally representative of hospital workers in the country. Fourth, we did not collect specific information about potential exposures at the time of SARS-CoV-2 infection, and therefore we could not discern whether infections were occupational or community-acquired. Likewise, information on participant characteristics was only collected at time of enrollment, and therefore we could not control for potential time-varying covariates. However, we believe this limitation is unlikely to have impacted the results significantly: For example, Albanian HCWs rarely change their occupational role, a variable associated with both vaccination uptake and SARS-CoV-2 seropositivity. We also did not have data on SARS-CoV-2 infections occurring between the administration of primary vaccine series and booster dose available for this analysis and other potentially important variables such as socio-economic status, and thus we cannot exclude residual confounding.
Lastly, as the sensitivity of the Wantai test has been shown to have a sensitivity of 87% (95% CI 79–92) and a specificity 100% (95% CI 99–100),60 we may have misclassified a small number (n ≈ 161) of cases as seronegative, slightly underestimating the SARS-CoV-2 seroprevalence in the cohort. Due to the high specificity of the test, false positives were unlikely. Any underestimation would likely be equal among subgroups, and therefore would not change our findings about disparities in seropositivity.
Conclusion
We found very high SARS-CoV-2 seroprevalence among HCWs in Albania one year into the pandemic and identified occupational factors associated with a higher risk of infection. These findings call for further investigation of the reasons for disparate infection rates to inform appropriate preventative measures to reduce work-related SARS-CoV-2 infections. While a large majority of HCWs in our study were vaccinated with two COVID-19 vaccine doses, efforts should be made to better understand the reasons for low booster dose uptake particularly among young, female, non-physician HCWs in order to develop tailored interventions to increase vaccination coverage. This issue should be urgently addressed in order to protect HCWs and preserve essential health services during future waves of SARS-CoV-2.
Contributors
SB, MAK, PJ and RP conceived the cohort study on which this analysis is based; AS, JS, IP, IH, AF, SS, JRS, MK, PJ, AD, RD, MN, MAK and SB planned and implemented the study, including development of study protocols, data quality checks and acquisition of data; PJ, AS, MAK and SB conceived the article; PJ and AS drafted the manuscript and performed the literature search. PJ contributed to the data analysis. PJ, JS and EK directly accessed and verified the raw data and take responsibility for the integrity and accuracy of the analyses.
All authors contributed to the interpretation of the results and critically revised the manuscript. All authors had full access to all the data reported in the study and accept responsibility to submit the paper for publication.
Data sharing statement
Data used to undertake this analysis is not available because the participants of this study did not give written consent for their data to be shared publicly.
Disclaimer
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the World Health Organization or of the U.S. Centers for Disease Control and Prevention.
Declaration of interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Acknowledgements
We are grateful to all of the HCWs for their heroic efforts during the pandemic and to our study participants. We appreciate the continued support from the leadership and field teams at the Tirana, Durres, and Fier Hospitals. We thank Gazmend Bejtja and Gladiola Kashari Kodra (WHO Country Office, Albania), Alina Guseinova and Diogo Simao Lemos (WHO Regional Office for Europe, Denmark), Ledia Agolli (Southeast European Center for Surveillance and Control of Infectious Diseases, Albania), Britni Burkhardsmeier and Dominique Richardson (Task Force for Global Health, United States), and Madelyn Rojas and Marta Valenciano (Epiconcept, France) for their contributions. This study was funded by the Task Force for Global Health (US Centers for Disease Control (CDC) cooperative agreement # NU51IP000873) and the World Health Organization, Regional Office for Europe.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100584.
Appendix A. Supplementary data
References
- 1.Gómez-Ochoa S.A., Franco O.H., Rojas L.Z., et al. COVID-19 in health-care workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol. 2021;190(1):161–175. doi: 10.1093/aje/kwaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2021. The impact of COVID-19 on health and care workers: a closer look at deaths.https://apps.who.int/iris/handle/10665/345300 Available from: [Google Scholar]
- 3.Mutambudzi M., Niedwiedz C., Macdonald E.B., et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med. 2021;78:307–314. doi: 10.1136/oemed-2020-106731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen L.H., Drew D.A., Graham M.S., et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5(9):e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . 2020. WHO sage roadmap for prioritizing uses of COVID-19 vaccines in the context of limited supply: an approach to inform planning and subsequent recommendations based upon epidemiologic setting and vaccine supply scenarios.https://apps.who.int/iris/handle/10665/341445 Version 1. Available from: [Google Scholar]
- 6.World Health Organization . 2022. Regional office for Europe COVID-19 vaccine programme monitor.https://worldhealthorg.shinyapps.io/EURO_COVID-19_vaccine_monitor/ Available from: [Google Scholar]
- 7.Luo C., Yang Y., Liu Y., et al. Intention to COVID-19 vaccination and associated factors among health care workers: a systematic review and meta-analysis of cross-sectional studies. Am J Infect Control. 2021;49(10):1295–1304. doi: 10.1016/j.ajic.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M., Luo Y., Watson R., et al. Healthcare workers' (HCWs) attitudes and related factors towards COVID-19 vaccination: a rapid systematic review. Postgrad Med J. 2021;0:1–7. doi: 10.1136/postgradmedj-2021-140195. [DOI] [PubMed] [Google Scholar]
- 9.Biswas N., Mustapha T., Khubchandani J., Price J.H. The nature and extent of COVID-19 vaccination hesitancy in healthcare workers. J Community Health. 2021;46(6):1244–1251. doi: 10.1007/s10900-021-00984-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou R., Dana T., Buckley D.I., Selph S., Fu R., Totten A.M. Update alert 10: epidemiology of and risk factors for coronavirus infection in health care workers. Ann Intern Med. 2022;175(1):W8–W9. doi: 10.7326/M21-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rostami A., Sepidarkish M., Leeflang M.M.G., et al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(3):331–340. doi: 10.1016/j.cmi.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SeroTracker . 2020. Dashboard and data platform for SARS-CoV-2 serosurveys.https://serotracker.com/en/Explore Available from: [Google Scholar]
- 13.Galanis P., Vraka I., Fragkou D., Bilali A., Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120–134. doi: 10.1016/j.jhin.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.COVID-19 Excess Mortality Collaborators Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21. Lancet. 2022;399(10334):1513–1536. doi: 10.1016/S0140-6736(21)02796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministry of Health Albania Plani kombëtar i vaksinimit Ndaj COVID-19 [National vaccination plan against COVID-19], 2021. https://shendetesia.gov.al/wp-content/uploads/2021/02/4-SHQIP.pdf Available from:
- 16.World Health Organization . 2020. WHO weekly coronavirus (COVID-19) dashboard.https://covid19.who.int/region/euro/country/al Available from: [Google Scholar]
- 17.Sridhar S., Fico A., Preza I., et al. COVID-19 vaccine effectiveness among healthcare workers in Albania (COVE-AL): protocol for a prospective cohort study and cohort baseline data. BMJ Open. 2022;12(3) doi: 10.1136/bmjopen-2021-057741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clinicaltrials.gov . 2021. COVID-19 Vaccine Effectiveness in Albanian Health Workers (COVEAL)https://clinicaltrials.gov/ct2/show/NCT04811391 Available from: [Google Scholar]
- 19.2021. United States, Food and Drug Administration (FDA), WANTAI SARS-CoV-2 Ab Elisa [package insert]https://www.fda.gov/media/140929/download Available from: [Google Scholar]
- 20.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reses H.E., Jones E.S., Richardson D.B., Cate K.M., Walker D.W., Shapiro C.N. COVID-19 vaccination coverage among hospital-based healthcare personnel reported through the department of health and human services unified hospital data surveillance system, United States, January 20, 2021-september 15, 2021. Am J Infect Control. Dec 2021;49(12):1554–1557. doi: 10.1016/j.ajic.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu-Raddad L.J., Chemaitelly H., Ayoub H.H., et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in qatar. N Engl J Med. 2022;386(19):1804–1816. doi: 10.1056/NEJMoa2200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moreira E.D., Kitchin N., Xu X., et al. Safety and efficacy of a third dose of BNT162b2 COVID-19 vaccine. N Engl J Med. 2022;386(20):1910–1921. doi: 10.1056/NEJMoa2200674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilboa M., Tal I., Levin E.G., et al. Coronavirus disease 2019 (COVID-19) vaccination uptake among healthcare workers. Infect Control Hosp Epidemiol. 2021;43:1–6. doi: 10.1017/ice.2021.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farah W., Breeher L., Shah V., et al. Disparities in COVID-19 vaccine uptake among health care workers. Vaccine. 2022;40(19):2749–2754. doi: 10.1016/j.vaccine.2022.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Townsel C., Moniz M.H., Wagner A.L., et al. COVID-19 vaccine hesitancy among reproductive-aged female tier 1A healthcare workers in a United States medical center. J Perinatol. 2021;41(10):2549–2551. doi: 10.1038/s41372-021-01173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shakeel C.S., Mujeeb A.A., Mirza M.S., Chaudhry B., Khan S.J. Global COVID-19 vaccine acceptance: a systematic review of associated social and behavioral factors. Vaccines (Basel) 2022;10:110. doi: 10.3390/vaccines10010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skirrow H., Barnett S., Bell S., et al. Women's views on accepting COVID-19 vaccination during and after pregnancy, and for their babies: a multi-methods study in the UK. BMC Pregnancy Childbirth. 2022;22(1):33. doi: 10.1186/s12884-021-04321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iacobucci G. Covid-19: no evidence that vaccines can affect fertility, says new guidance. BMJ. 2021;372:n509. doi: 10.1136/bmj.n509. [DOI] [PubMed] [Google Scholar]
- 30.Wesselink A.K., Hatch E.E., Rothman K.J., et al. A prospective cohort study of COVID-19 vaccination, SARS-CoV-2 infection, and fertility. Am J Epidemiol. 2022;191(8):1383–1395. doi: 10.1093/aje/kwac011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jafari M., Pormohammad A., Sheikh Neshin S.A., et al. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: a systematic review and meta-analysis. Rev Med Virol. 2021;31(5):1–16. doi: 10.1002/rmv.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei S.Q., Bilodeau-Bertrand M., Liu S., Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193(16):E540–E548. doi: 10.1503/cmaj.202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falsaperla R., Leone G., Familiari M., Ruggieri M. COVID-19 vaccination in pregnant and lactating women: a systematic review. Expert Rev Vaccines. 2021;20(12):1619–1628. doi: 10.1080/14760584.2021.1986390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization . 2022. Questions and Answers: COVID-19 vaccines and pregnancy 15 February 2022.https://apps.who.int/iris/handle/10665/351855?locale-attribute=es& Available from: [Google Scholar]
- 35.Patanavanich R., Siripoon T., Amponnavarat S., Glantz S.A. Active smokers are at higher risk of COVID-19 death: a systematic review and meta-analysis. Nicotine Tob Res. 2022;25:1–11. doi: 10.1093/ntr/ntac085. [DOI] [PubMed] [Google Scholar]
- 36.Doran J., Seyidov N., Mehdiyev S., et al. Factors associated with early uptake of COVID-19 vaccination among healthcare workers in Azerbaijan, 2021. Influenza Other Respir Viruses. 2022;16:626–631. doi: 10.1111/irv.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altarawneh H.N., Chemaitelly H., Ayoub H.H., et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022;387(1):21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plumb I.D., Feldstein L.R., Barkley E., et al. Effectiveness of COVID-19 mRNA vaccination in preventing COVID-19-associated hospitalization among adults with previous SARS-CoV-2 infection–United States, June 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(15):549–555. doi: 10.15585/mmwr.mm7115e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldberg Y., Mandel M., Bar-On Y.M., et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N Engl J Med. 2022;386(23):2201–2212. doi: 10.1056/NEJMoa2118946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nordström P.B.M., Nordström A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: a retrospective, total population cohort study in Sweden. Lancet Infect Dis. 2022;22(6):781–790. doi: 10.1016/S1473-3099(22)00143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preza I., Nelaj E., Bino S. International meeting on emerging diseases and surveillance (IMED) 2018 abstracts; November 9-12, 2018; Vienna, Austria. 2018. Influenza vaccination in Albania. Presented at: [Google Scholar]
- 42.Lucaccioni H., Chakhunashvili G., McKnight C.J., et al. Sociodemographic and occupational factors associated with low early uptake of COVID-19 vaccine in hospital-based healthcare workers, Georgia, March-July 2021. Vaccines (Basel) 2022;10(8):1197. doi: 10.3390/vaccines10081197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J., While A.E., Norman I.J. Knowledge and attitudes regarding influenza vaccination among nurses: a research review. Vaccine. 2010;28(44):7207–7214. doi: 10.1016/j.vaccine.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 44.Terry E., Cartledge S., Damery S., Greenfield S. Factors associated with COVID-19 vaccine intentions during the COVID-19 pandemic; a systematic review and meta-analysis of cross-sectional studies. BMC Public Health. 2022;22(1):1667. doi: 10.1186/s12889-022-14029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bresee J.S., Lafond K.E., McCarron M., et al. The partnership for influenza vaccine introduction (PIVI): supporting influenza vaccine program development in low and middle-income countries through public-private partnerships. Vaccine. 2019;37(35):5089–5095. doi: 10.1016/j.vaccine.2019.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.INSTAT, Institute of Stastictics, Albania . 2020. Income and living conditions in Albania.http://www.instat.gov.al/en/themes/social-condition/income-and-living-conditions-in-albania/publication/2021/income-and-living-conditions-in-albania-2020/ Available from: [Google Scholar]
- 47.Sulcebe G., Ylli A., Cenko F., Kurti-Prifti M. Rapid increase of SARS-CoV-2 seroprevalence during the 2020 pandemic year in the population of the city of Tirana, Albania. medRxiv. 2021 doi: 10.1101/2021.02.18.21251776. [DOI] [Google Scholar]
- 48.Knežević D., Petković M., Božić L., et al. Seroprevalence of SARS-CoV-2 antibodies among primary healthcare workers in the republic of Srpska, Bosnia & Herzegovina: a cross-sectional study. Acta Microbiol Immunol Hung. 2022;69(1):18–26. doi: 10.1556/030.2022.01706. [DOI] [PubMed] [Google Scholar]
- 49.Ma Q., Liu J., Liu Q., et al. Global percentage of asymptomatic SARS-CoV-2 infections among the tested population and individuals with confirmed COVID-19 diagnosis: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(12) doi: 10.1001/jamanetworkopen.2021.37257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kayı İ., Madran B., Keske Ş., et al. The seroprevalence of SARS-CoV-2 antibodies among health care workers before the era of vaccination: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(9):1242–1249. doi: 10.1016/j.cmi.2021.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin C.A., Pan D., Melbourne C., et al. Risk factors associated with SARS-CoV-2 infection in a multiethnic cohort of United Kingdom healthcare workers (UK-REACH): a cross-sectional analysis. PLoS Med. 2022;19(5) doi: 10.1371/journal.pmed.1004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poletti P., Tirani M., Cereda D., et al. Seroprevalence of and risk factors associated with SARS-CoV-2 infection in health care workers during the early COVID-19 pandemic in Italy. JAMA Netw Open. 2021;4(7) doi: 10.1001/jamanetworkopen.2021.15699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lombardi A., Mangioni D., Consonni D., et al. Seroprevalence of anti-SARS-CoV-2 IgG among healthcare workers of a large university hospital in Milan, Lombardy, Italy: a cross-sectional study. BMJ Open. 2021;11(2) doi: 10.1136/bmjopen-2020-047216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ward H., Atchison C., Whitaker M., et al. SARS-CoV-2 antibody prevalence in England following the first peak of the pandemic. Nat Commun. 2021;12(1):905. doi: 10.1038/s41467-021-21237-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomaselli V., Ferrara P., Cantone G.G., et al. The effect of laboratory-verified smoking on SARS-CoV-2 infection: results from the troina sero-epidemiological survey. Intern Emerg Med. 2022;17(6):1617–1630. doi: 10.1007/s11739-022-02975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferrara P., Gianfredi V., Tomaselli V., Polosa R. The effect of smoking on humoral response to COVID-19 vaccines: a systematic review of epidemiological studies. Vaccines (Basel) 2022;10(2):303. doi: 10.3390/vaccines10020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferrara P., Ponticelli D., Agüero F., et al. Does smoking have an impact on the immunological response to COVID-19 vaccines? evidence from the VASCO study and need for further studies. Public Health. 2022;203:97–99. doi: 10.1016/j.puhe.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petersen M.S., Pérez-Alós L., Armenteros J.J.A., et al. Factors influencing the immune response over 15 months after SARS-CoV-2 infection: a longitudinal population-wide study in the Faroe Islands. J Intern Med. 2022;293(1):63–81. doi: 10.1111/joim.13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giuca M.R., Pasini M., Tecco S., Giuca G., Marzo G. Levels of salivary immunoglobulins and periodontal evaluation in smoking patients. BMC Immunol. 2014;15:5. doi: 10.1186/1471-2172-15-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.GeurtsvanKessel C.H., Okba N.M.A., Igloi Z., et al. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat Commun. 2020;11(1):3436. doi: 10.1038/s41467-020-17317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.