Abstract
⮞ The primary means of femoral fixation in North America is cementless, and its use is increasing worldwide, despite registry data and recent studies showing a higher risk of periprosthetic fracture and early revision in elderly patients managed with such fixation than in those who have cemented femoral fixation.
⮞ Cemented femoral stems have excellent long-term outcomes and a continued role, particularly in elderly patients.
⮞ Contrary to historical concerns, recent studies have not shown an increased risk of death with cemented femoral fixation.
⮞ The choice of femoral fixation method should be determined by the patient’s age, comorbidities, and bone quality.
⮞ We recommend considering cemented femoral fixation in patients who are >70 years old (particularly women), in those with Dorr type-C bone or a history of osteoporosis or fragility fractures, or when intraoperative broach stability cannot be obtained.
Cementless femoral fixation, in both total hip arthroplasty (THA) and hemiarthroplasty, has increased dramatically in North America during the past 2 decades and continues to grow worldwide1. Despite the excellent long-term clinical outcomes of cemented femoral fixation, >94% of THAs in the 2020 American Joint Replacement Registry (AJRR) Annual Report were cementless2. This percentage contrasts dramatically with other international registries. This shift to cementless femoral fixation is multifactorial but began in the 1980s to address aseptic loosening inaccurately attributed to cement3. Initially pursued as biologic fixation that was more durable for younger, active patients, who had excellent results, and because of concerns about adverse intraoperative effects of cement, cementless fixation has become the choice of fixation in the U.S. The efficiency of cementless femoral fixation, requiring less operative time and fewer supplies, has added to its popularity4,5. Less exposure to cemented techniques during training and thus decreasing staff comfort with the technique have also likely contributed to the further decline of cemented fixation.
Recent studies and data from multiple international registries have shown higher complication rates with cementless than with cemented femoral fixation, particularly in elderly patients and women6–9. Despite this finding, of the >500,000 primary elective THAs in the AJRR, 86% of patients who were 80 to 89 years old and 67% of patients who were ≥90 years old received cementless stems2. Additionally, most hemiarthroplasties for femoral neck fractures in patients who were ≥90 years old were cementless.
The purposes of this article are to review cemented femoral stem designs and their outcomes, review indications for cemented fixation in hip arthroplasty, and highlight the scenarios in which cemented fixation is more appropriate than cementless. Additionally, we have provided pearls for the cemented technique.
Cemented Stem Designs and Principles of Fixation
It is important to understand the shapes and the principles of fixation for the various cemented femoral stem designs. There are 4 broad categories (Fig. 1)10. We focus on the 2 most utilized stem designs in North America: type I (polished tapered) and type II (composite beam)11. Type-III and type-IV stems demonstrate good long-term survival; however, they are used less frequently12,13.
Fig. 1.
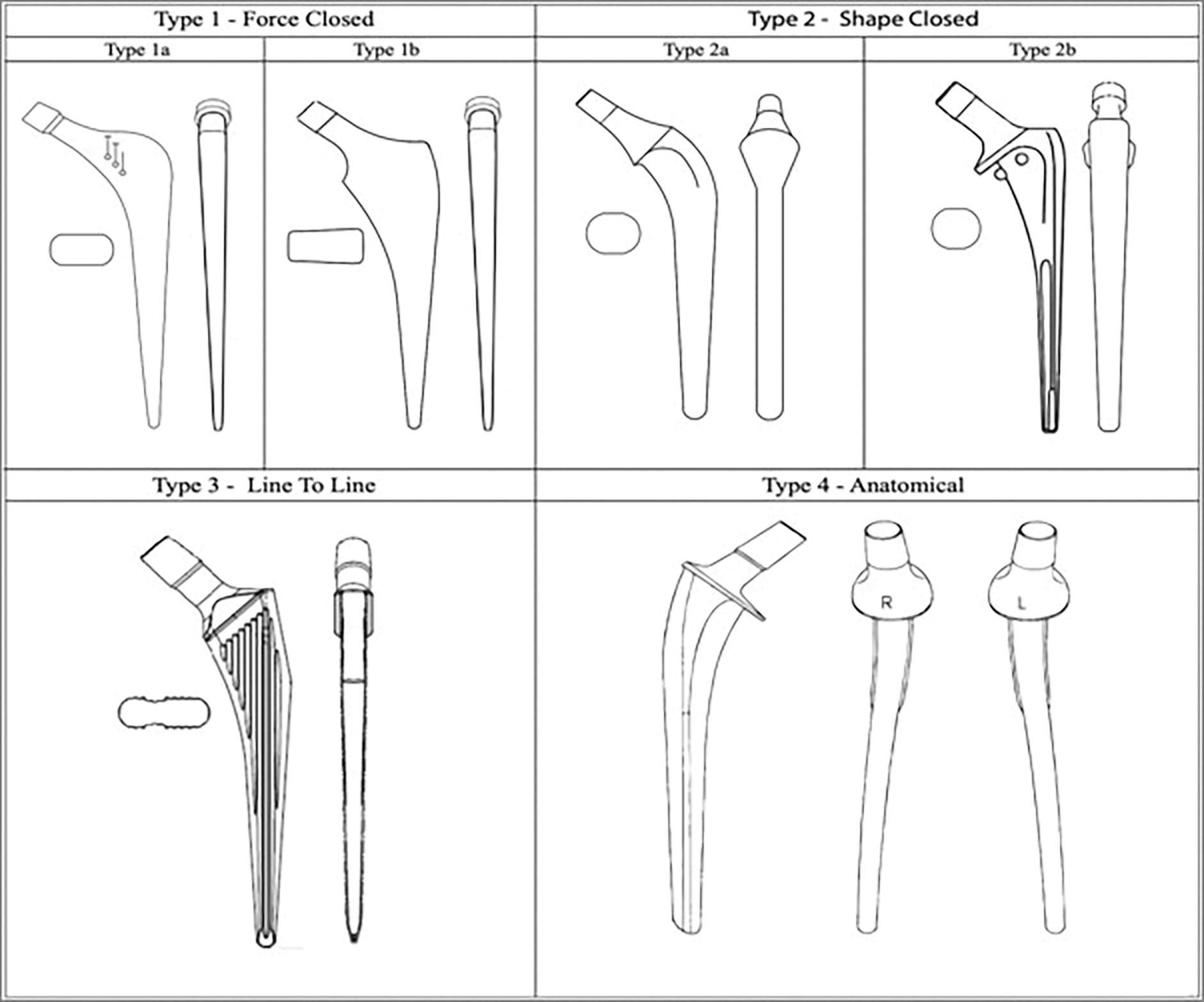
Illustration showing the classification of cemented femoral stem designs. A revision stem for each type can be subclassified into the short (Rs) or long version (Rl,) (e.g., Type 1Rs). (Reproduced from: Cassar-Gheiti AJ, McColgan R, Kelly M, Cassar-Gheiti TM, Kenny P, Murphy CG. Current concepts and outcomes in cemented femoral stem design and cementation techniques: the argument for a new classification system. EFORT Open Rev. 2020;5[4]:241–52. Copyright © 2020 The authors. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7202038/ This is an open access article under the CC BY-NC 4.0 license [http://creativecommons.org/licenses/by-nc-nd/4.0/].)
Type I: Polished Taper
Type-I stems are known by various names including force-closed and polished taper. Fixation is obtained by controlled subsidence into the cement mantle, loading the cement in compression. They are collarless, highly polished, and made of stainless steel or cobalt-chromium, with a dual or triple taper. These characteristics discourage bonding of cement to the prosthesis and allow subsidence of the stem. A flexible distal centralizer that collapses as the stem subsides prevents point loading of the cement by the stem tip. Radiostereometric analysis has consistently demonstrated distal migration of 1 to 2 mm in the first 2 years14. This subsidence causes plastic deformation of the cement mantle, loads the proximal part of the femur, and minimizes shear forces at the bone-cement interface15. Type-I stems have had excellent survival rates in Great Britain’s National Joint Registry (NJR), with survival of 97.9% at 8 years based on >200,000 implants16. In studies with aseptic loosening as the end point, survival rates have consistently been reported near 100%, with long-term (20-year) survival of 98.7%17,18. Similar results for patients who were <40 years old have been reported, with 17-year implant survival rates of 100% with aseptic loosening as the end point19.
Type II: Composite Beam
Type-II stems, also known as shape-closed or composite beam, differ fundamentally from type-I stems as they rely on cement bonding to the prosthesis. These are roughened or precoated with methylmethacrylate and may have grooves to enhance the cement bond. They are typically collared to prevent subsidence and to load the medial proximal aspect of the femur. The Charnley design, the original type-II stem, had a low average roughness (Ra) of 0.1 mm, but subsequent designs have higher Ra values ranging from 0.6 to 0.75 mm20. Multiple studies from the U.S. and Europe have described 25-year survivorship from 85% to 96%21,22. On the basis of the success of the Charnley design, type-II stems enjoy widespread use. In a study of >47,000 modern-design, composite-beam stems in the NJR, the 8-year survival rate was 97.5%16. Long-term data have shown that Charnley stems have a survival rate of 78% at 35 years23.
Comparative Outcomes
Polished tapered and composite-beam stems have excellent clinical results with low revision rates. Few high-level studies have directly compared the 2 types, and most comparative data are from national joint registries. A recent report on 292,987 cemented stems in the NJR found that polished tapered designs had a significantly lower 8-year revision rate for aseptic loosening in primary THA (1.3%) than forced-closed designs (1.7%)16, which is consistent with previous studies24–27. A 2008 randomized controlled trial of 219 hips found no significant difference in revision rates between the 2 types at 5 years28. Failure modes seem to differ, with polished tapered stems failing more often because of fracture and composite-beam stems failing more often because of aseptic loosening16,28,29. Overall, both show excellent clinical outcomes, and implant survival is likely more related to technique than to any modern implant design difference.
Comparison of Outcomes of Cemented and Cementless Femoral Fixation
Total Hip Arthroplasty
Cemented and cementless stems in hip arthroplasty both have excellent long-term outcomes. A recent systematic review of 11 composite-beam stems, 1 polished tapered stem, and 1 type-III cemented stem found a 20-year survivorship of 86% to 98%, with revision for aseptic loosening as the end point30. Similarly, Rajaratnam et al.31 reported a survival rate of 97.4%, with revision for any reason as the end point, in 331 fully coated, cementless stems with a mean follow-up of 17 years. In a cohort of 330 primary cemented composite-beam stems with a minimum follow-up of 35 years, only 10% were revised for aseptic loosening, and overall survivorship was 78% with revision for any reason as an end point23. New Zealand Joint Registry data from 1999 to 2019 on >46,000 patients with an age of ≥65 years showed that early revision rates within 3 months were higher for cementless than for fully cemented THAs32. Using Australian Orthopaedic Association National Joint Replacement Registry data to compare the 3 best-performing cemented stems (2 polished tapered and 1 composite-beam) and 3 best performing cementless stems (1 double-wedge, 1 tapered round, and 1 tapered rectangle) in THA among patients who were >75 years old, Tanzer et al.9 showed that cementless stems were 9 times (95% confidence interval [CI], 5.5 to 15 times) as likely to be revised within the first month, which was mainly attributable to fracture or loosening. No difference was found in the cumulative percentage revision between 3 months and 13 years.
Higher early revision rates for cementless compared with cemented femoral stems are related to a greater risk of periprosthetic femoral fracture (PFF) and early implant loosening seen in elderly patients, most notably women9,11. The risk of early PFF within 3 months was much higher with cementless fixation in an AJRR analysis of >10,000 revisions of THAs and hemiarthroplasties. Cementless femoral fixation accounted for 95% (596) of all 628 early PFFs, whereas cemented accounted for only 5.1% (32 early PFFs). Women were 1.9 times (95% CI, 1.1 to 3.1 times) as likely as men to undergo early revision8. An evaluation of >170,000 THAs, between 1992 and 2007, in the Swedish Hip Arthroplasty Register found that the rate of postoperative PFF leading to stem revision surgery within 2 years was 17% for cementless compared with 6% for fully cemented THA (relative risk [RR], 8; 95% CI, 5 to 14). However, with an end point of revision of any component during the entire study period, the risk of revision related to aseptic loosening was lower for cementless stems than for cemented stems (RR, 0.4; 95% CI, 0.3 to 0.5)7. In nearly 67,000 THAs in the Norwegian Arthroplasty Register, between 2005 and 2017, PFF risk was much higher for cementless THA than for cemented THA (RR, 5.2; 95% CI, 3.2 to 8.5) and for women than for men (RR, 12; 95% CI, 6 to 25)33.
In a large case series of >36,000 THAs performed during a 40-year period, intraoperative fracture occurred in 3% (529) of 17,466 cementless stems and in only 0.23% (35) of 15,178 cemented stems6. Of 564 intraoperative fractures, 94% (529) occurred in patients with cementless stems compared with 6.2% (35) in patients with cemented stems, a 14-fold higher rate6. The odds of having an intraoperative fracture were significantly greater for women than for men (odds ratio [OR], 1.4; 95% CI, 1.2 to 1.7), and patients who were >65 years old had significantly greater odds of fracture than those who were ≤65 years old (OR, 2.5; 95% CI, 2.1 to 3.0)6. A prospective cohort study34 of >8,000 THAs in patients older than 70 years using the Danish National Patient Register and the Danish Hip Arthroplasty Register from 2010 to 2017 found a higher prevalence of PFF after cementless THAs (1.5%; 70 of 4,728 stems) than after fully cemented or hybrid THAs (0.2%; 7 of 3,368 stems)34. These findings are similar to those in other studies6,7,33.
In summary, cemented and cementless femoral fixation have shown excellent long-term outcomes. Primarily on the basis of registry data, cemented femoral fixation may be a better option for patients >70 years old, especially women, to reduce the risk of PPF and early revision. Cementless femoral fixation is more appropriate in younger, highly active patients with adequate bone stock35–37.
Hemiarthroplasty
Similar to the findings after THA, studies of hemiarthroplasty have shown a lower risk of PFF in hips with cement than in those managed without cement. A systematic review and meta-analysis of 7 randomized controlled trials of cemented versus cementless hemiarthroplasty in patients with a mean age of >75 years found lower intraoperative fracture (OR, 0.29; 95% CI, 0.13 of 0.68) and postoperative fracture odds (OR, 0.09; 95% CI, 0.02 of 0.38) with cemented stems34. In a meta-analysis of 6 randomized controlled trials with patients >65 years old who underwent hemiarthroplasty for femoral neck fractures, prosthesis-related complications were less likely for cemented stems (OR, 0.24; 95% CI, 0.14 to 0.41)4. Moreover, in a recent retrospective cohort study38 of >12,000 patients with an age of ≥65 years who underwent hemiarthroplasty for hip fracture, cementless fixation was found to be associated with a greater incidence of aseptic revision at 1 year compared with cemented fixation (cumulative incidence, 3.0% [239 of 6,042] versus 1.3% [136 of 6,449]; hazard ratio [HR], 1.8; 95% CI, 1.4 to 2.2). This was primarily because of a greater rate of PFF in the cementless group (cumulative incidence at 1 year, 1.6% [95% CI, 1.3% to 1.9%]) compared with that in the cemented group (cumulative incidence at 1 year, 0.2% [95% CI, 0.1% to 0.4%]). Compared with cemented fixation, cementless fixation was also associated with higher HRs of aseptic revision in all age groups (HR range, 1.2 to 2.8)38. These results suggest that cemented hemiarthroplasty better protects against PFF and revision, particularly among older patients.
The available data suggest that hemiarthroplasty for displaced femoral neck fractures in the elderly should be performed with cemented femoral fixation. In 2014, the American Academy of Orthopaedic Surgeons issued a moderate-strength recommendation for using cemented femoral stems in patients who were >65 years old and undergoing hemiarthroplasty for femoral neck fractures39. Despite the recommendation, only 37.1% of patients who were 70 to 79 years old, 43.3% of those who were 80 to 89 years old, and 49.2% of those ≥90 years old received cemented stems in hemiarthroplasty for femoral neck fractures2.
Cemented femoral fixation should be used in hemiarthroplasty for displaced femoral neck fractures in patients who are ≥65 years old, as cementless femoral fixation in hemiarthroplasty is associated with increased risk of intraoperative and postoperative PFF, as well as increased risk of aseptic loosening.
Bone Cement Implantation Syndrome and Death
Reports of cardiovascular collapse related to cementation were described in the 1970s and became known as bone cement implantation syndrome (BCIS)40,41. Parvizi et al.42 demonstrated this increased risk with cementing in a review of 38,488 hip arthroplasties performed between 1969 and 1997. There were 23 intraoperative deaths associated with cardiorespiratory disruption during cementation, and none were seen in the 15,411 hips managed with uncemented hip arthroplasty. Microemboli from bone marrow were observed in the lungs of 11 of 13 patients who underwent autopsy, and methylmethacrylate particles were found in the lungs of 3 patients. Modifications of the technique and minimizing intramedullary pressure resulted in a >3.5-fold decrease in intraoperative mortality rate in the later years of the study42.
In a comprehensive review, Donaldson et al.43 proposed that BCIS is characterized by “hypoxia, hypotension or both and/or unexpected loss of consciousness occurring around the time of cementation, prosthesis insertion, reduction of the joint or, occasionally, tourniquet deflation in a patient undergoing cemented bone surgery.” They described 3 grades of increasing severity (Table I)43. The most severe, grade III (cardiovascular collapse), is rare, occurring in 0.4% to 1.7% of patients having hemiarthroplasty for a femoral neck fracture43–45, but it can lead to intraoperative or early postoperative death46. The pathophysiology of BCIS is unknown but likely multifactorial, related to an embolic shower from pressurization and a histamine response to the cement monomer43. Much of our understanding comes from retrospective studies of hemiarthroplasty for femoral neck fractures and oncologic conditions47,48.
TABLE I.
| Grade | Characteristic |
|---|---|
|
| |
| I | Moderate hypoxia (SpO2 of <94%) ora decrease (20%–40%) in systolic blood pressure |
| II | Severe hypoxia (SpO2 of <88%) or a decrease (>40%) in systolic blood pressure |
| III | Cardiovascular collapse requiring cardiopulmonary resuscitation |
SpO2 = oxygen saturation.
Rassir et al.46 retrospectively studied BCIS in 915 patients with a mean age of 85 years who had cemented hemiarthroplasties from 2008 to 2019. They reported that grade-III BCIS occurred in 0.44% (4) of the 915 patients, none of whom survived despite immediate resuscitation attempts. Severe BCIS was associated with a greater likelihood of death within 30 days postoperatively compared with less severe or no BCIS (HR, 3.5; 95% CI, 2.1 to 5.8)46. In another retrospective cohort study of 1,095 patients (mean age, ≥82 years) who underwent hemiarthroplasty for femoral neck fracture from 2008 to 2011, those treated with cemented hemiarthroplasty had higher rates of hypotension and/or hypoxia (28%; 272 of 986 patients) than those treated with cementless hemiarthroplasty (17%; 18 of 109 patients) (p = 0.003)44. They also reported a greater incidence of death within 48 hours after surgery in the cemented group (2%) than in the cementless group (0%) (p = 0.001). Moreover, the use of cement was independently associated with a higher hazard of death at 1 year (HR, 1.9; 95% CI, 1.3 to 2.7) after adjusting for sex, age, and comorbidities44.
More severe BCIS has been associated with increasing patient age, particularly patients who are >75 years old and those with an American Society of Anesthesiologists (ASA) physical status classification of ≥3, renal impairment, chronic obstructive pulmonary disease, cancer and lung metastases, and use of diuretics or warfarin43,46,49. Identifying patients with severe systemic disease prior to surgery is essential and should involve a thorough medical evaluation to address comorbidities to the extent possible43,48,50,51. Severe BCIS, although rare, can be fatal. More high-level research is needed to better understand this topic and associated risks.
Mortality
THA
Recent studies have shown no difference in mortality rates between cemented and cementless femoral fixation in THA. Richardson et al.52 compared the mortality rates after hybrid (cemented femoral stem and cementless acetabular cups) and after cementless hip arthroplasty in nearly 6,000 patients with femoral neck fractures. They found a lower mortality rate during hospital stay in the hybrid group than in the cementless group (OR, 0.70; 95% CI, 0.56 to 0.87). Additionally, they observed lower mortality rates in the hybrid fixation group at 1 month (OR, 0.56; 95% CI, 0.47 to 0.66), 3 months (OR, 0.56; 95% CI, 0.48 to 0.64), and 1 year postoperatively (OR, 0.56; 95% CI, 0.50 to 0.63)52. Likewise, in a large, matched cohort study of nearly 180,000 patients in the Swedish Hip Arthroplasty Register who underwent THA for primary osteoarthritis between 1992 and 2012, a supplementary analysis showed no significant difference in mortality rates up to 14 days after hybrid compared with cementless THA53.
Hemiarthroplasty
Several randomized controlled trials found no differences in mortality rates from 30 days to 5 years after cemented and cementless hemiarthroplasty54–56. A single-center study of 657 patients who were ≥65 years old with ASA physical status classification of ≥3 who underwent hemiarthroplasty for femoral neck fracture between 2010 and 2016 found no differences with respect to all-cause mortality, infection, or reoperation between the patients managed with cemented stems and those managed with cementless stems at 1 year postoperatively57. In a recent study of >30,000 patients from the Norwegian Hip Fracture Register who were ≥70 years old and underwent hemiarthroplasty from 2005 to 2017, no differences in mortality rates at 1 year were found between the cemented and cementless hemiarthroplasty groups58. Moreover, a study38 of >12,000 patients with an age of >65 years who underwent hemiarthroplasty for femoral neck fracture at a large U.S. integrated health-care system between 2009 and 2017 found no differences according to cementation status in in-hospital or overall mortality rates at 1 year postoperatively.
Cemented femoral fixation is not associated with an increased risk of death and can be protective against PFF in elderly patients. Cemented femoral fixation should be used cautiously in patients with severe systemic disease, including cardiopulmonary disease and cancer and lung metastases48.
Technical Principles and Pearls
Goal of Cementation
Technique is critical to safely implanting cemented femoral stems and ensuring longevity of the construct. Although the principles of fixation differ between type-I and type-II cemented stems, the goals of cementation and technique are the same: to obtain a uniform cement mantle of 2 to 4 mm with sufficient interdigitation of the cement with the cancellous bone. These characteristics have been associated with longer implant survivorship59–61. The quality of the cement mantle is graded on orthogonal postoperative radiographs. The grading system of A through D described by Barrack et al.62 is the most widely used (Table II).
TABLE II.
Grading System of the Quality of Femoral Stem Cementation According to Barrack et al.62
| Grade | Cementation |
|---|---|
|
| |
| A | Uniform cement mantle without any stem-bone contact and excellent interdigitation of cement, which results in a “white out” |
| B | Radiolucency at the cement-bone interface, covering <50% of the implant |
| C | 50% to 99% radiolucency at the cement-bone interface |
| D | 100% radiolucency at the cement-bone interface and absence of cement distal to the tip of the stem |
Modern Cementing Techniques, Fourth Generation
Since Charnley first described the use polymethylmethacrylate in hip replacement63, the technique has continuously evolved to optimize the cement mantle and the longevity of the construct (Table III).
TABLE III.
Summary of Prior Cementing Techniques
| Generation | Description |
|---|---|
|
| |
| First21,23,63 | Originated in the 1960s No osseous preparation, such as washing Cement inserted in antegrade fashion using finger packing, which led to inadequate penetration of cement into cancellous bone, inclusion of blood in cement, and poor cement mantle |
| Second62,65 | Canal preparation through cleaning to remove blood and fat Cement restrictor Cement inserted using gun in retrograde fashion Improved penetration into bone resulted in decreased risk of femoral component loosening |
| Third66 | Added the use of vacuum mixing to reduce porosity of cement Also focused on maintaining pressurization before and during insertion of femoral prosthesis |
| Fourth | Femoral canal preparation using a brush, followed by pulsatile lavage Canal packing with gauze soaked in hemostatic solution Vacuum cement mixing Retrograde cement introduction using long-nozzle cement gun, use of distal cement restrictor Maintaining cement pressurization |
The key aspects of the current, or fourth generation, technique include osseous debris removal with a brush, pulsatile irrigation of the osseous bed, use of a distal cement restrictor, vacuum-mixed cement, retrograde introduction of cement, and cement pressurization. These techniques are approach-independent and can be readily adopted by all surgeons. Although not entirely avoidable, BCIS can be limited by patient selection, appropriate technique, close management together with the anesthesia team, and other measures outlined in Table IV.
TABLE IV.
Intraoperative Steps to Avoid Bone Cement Implantation Syndrome
| Notify anesthesia team of the plan to use cement and again at time of cementation |
| Use invasive monitoringjudiciously in at-risk patients |
| Communicate with anesthesia team regarding blood loss, volume status, and blood pressure |
| Ensure adequate volume maintenance before and during cementation |
| Prepare for use of vasopressors |
| Avoid cementation in patients already on vasopressors |
| Thoroughly irrigate the femoral canal before instrumentation |
| Place cement later in curing phase (minimizes monomer exposure) |
| Avoid overpressurizing of the cement in susceptible patients |
The first step to an ideal cement mantle begins with preparation of the femur (Fig. 2). A canal seeker is used to identify a starting point for entry into the femoral canal. Removal of the remaining lateral femoral neck with a rongeur or box osteotome helps to prevent varus positioning. Broaching proceeds sequentially until loose cancellous bone has been removed and the broach is both axially and rotationally stable. With proper broaching, 2 to 4 mm of supportive cancellous bone will be compacted adjacent to the cortical bone. Care during femoral preparation must be taken to avoid removal of this supportive cancellous bone with curets or the suction tip because it is necessary for proper cement interdigitation.
Fig. 2.
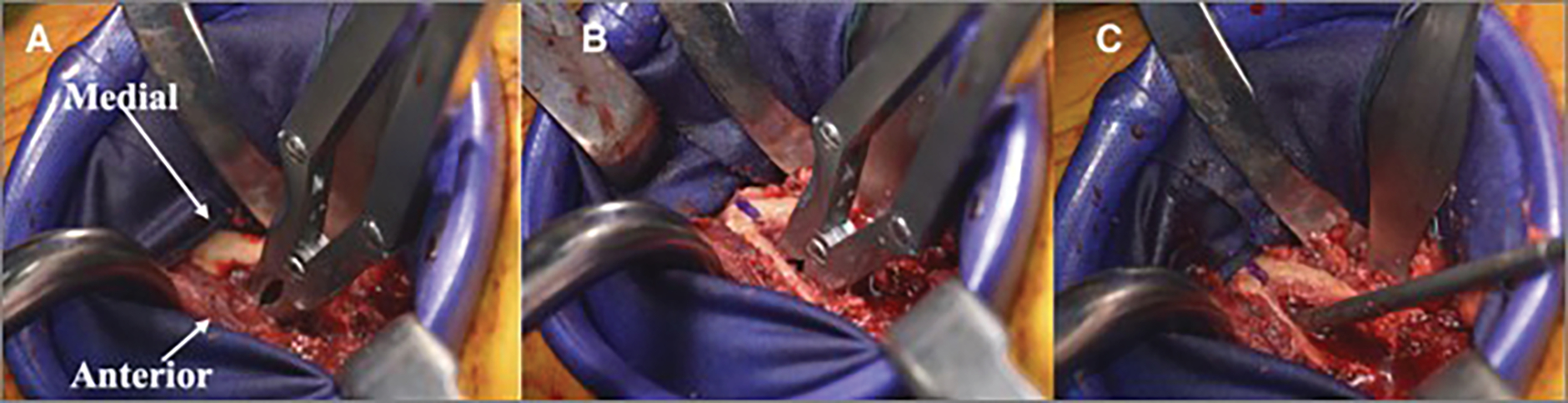
Figs. 2-A, 2-B, and 2-C Intraoperative photographs of a hip arthroplasty showing how to establish appropriate entry into the femoral canal without perforation. Fig. 2-A A rongeur is used to remove the lateral femoral neck. The medial femoral neck is denoted by the arrow. Fig. 2-B A rongeur is used to initially enter the femoral neck. Fig. 2-C A canal seeker is used to enter the femoral canal.
After broaching, a canal brush can be used to remove loose pieces. The prepared bed of bone is irrigated extensively with pulsatile lavage to remove remaining bone marrow and blood. A distal cement restrictor is sized and placed, allowing at least a 1-cm distal cement mantle. The canal is irrigated again with pulsatile lavage. At this point, the cancellous bed should be clean and free of any visible bone marrow contents. A narrow suction catheter is placed in the canal. The canal is then packed tightly with damp gauze that has been soaked in a hemostatic solution of the surgeon’s choice (Fig. 3). Cement is mixed under vacuum at room temperature according to manufacturer recommendations. We prefer a high-viscosity cement for better penetration and pressurization and a longer working time.
Fig. 3.
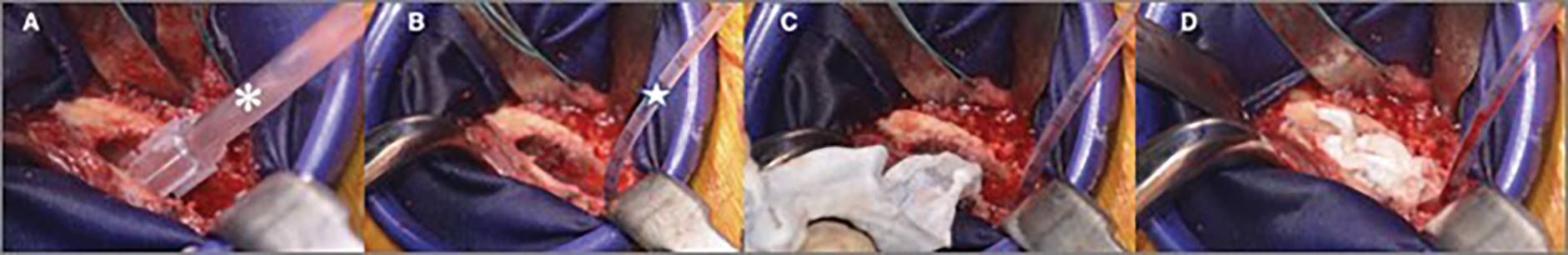
Figs. 3-A through 3-D Intraoperative photographs showing femoral canal cement preparation for a hip arthroplasty. Fig. 3-A A canal brush (asterisk) is used to clean the canal of debris. Fig. 3-B A flexible suction catheter (star) is advanced distally in the canal to remove blood. Fig. 3-C Gauze soaked with halfstrength hydrogen peroxide are packed into the canal distally to proximally. Fig. 3-D Sponges packed within the canal.
The cement is ready for application when it has reached the working phase (i.e., it no longer adheres to the surgeon’s glove). The gauze is removed from the canal while the suction catheter remains in place to remove any blood that may pool on the cement restrictor. The catheter is removed, and the cement is introduced in a retrograde fashion with a cement gun. One of the senior authors prefers to leave the catheter while cementing to vent the canal and avoid further blood pooling and to remove it after cementing while placing pressure over the cement column with a clean thumb or gauze. The cement is then pressured. Care is taken to hold prolonged pressure against the cement to enable interdigitation into the cancellous bone. If properly pressured, bone marrow should be seen extravasating out of the cortical bone of the proximal part of the femur. Any remaining blood on the proximal cement is removed (Fig. 4).
Fig. 4.
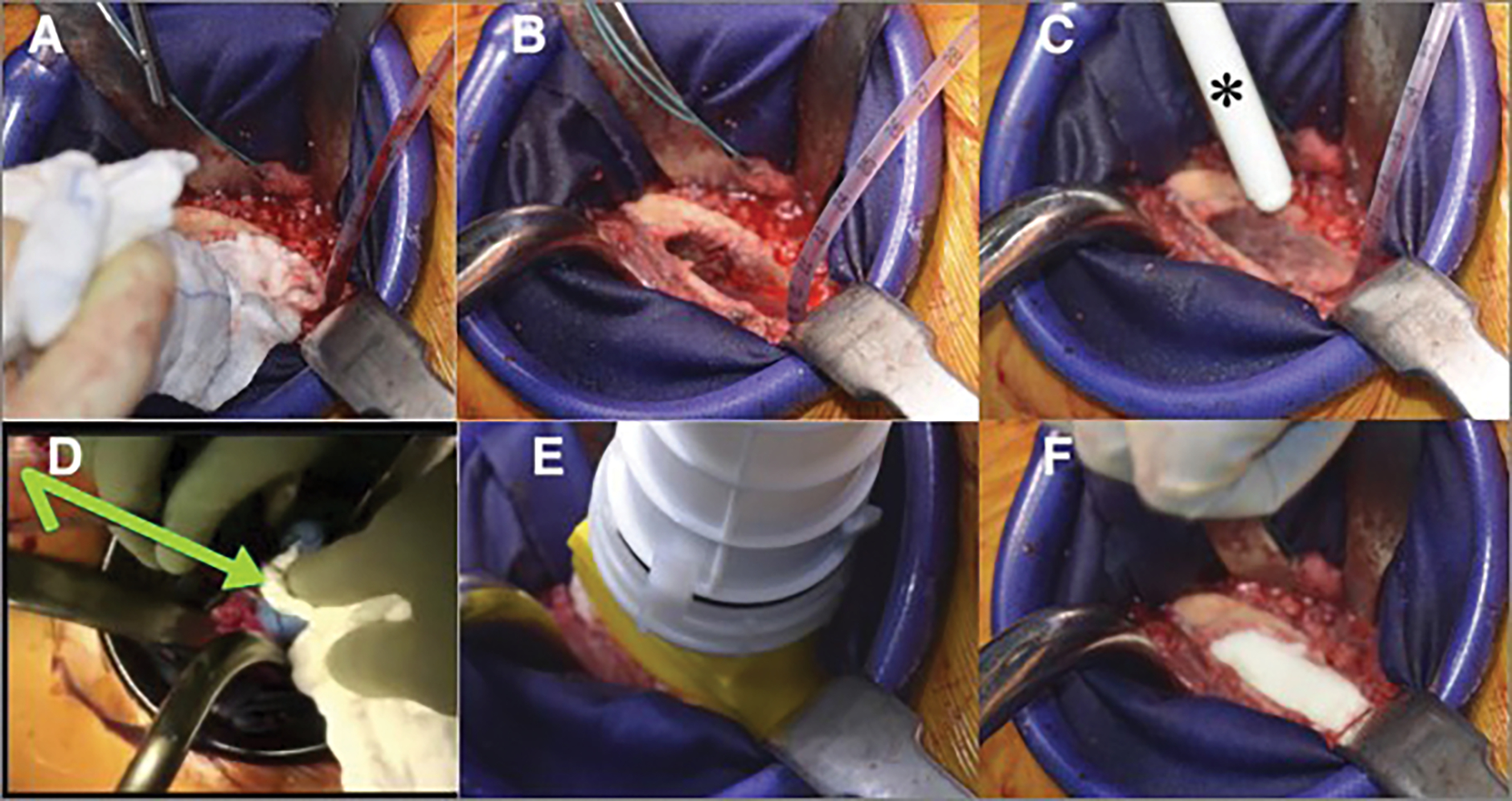
Figs. 4-A through 4-F Intraoperative photographs of femoral canal cementation. Fig. 4-A The packed sponge is removed. Fig. 4-B The canal suction catheter remains in place. Fig. 4-C The cement is introduced in a retrograde fashion with a cement gun (asterisk), allowing the pressure of the cement to push it out the nozzle. Note that the endosteal canal is devoid of blood. Fig. 4-D Pressure is held over the cement column (arrow) while removing the catheter. Fig. 4-E Cement is pressurized with the gun. Fig. 4-F The cement within the canal after pressurization. Again, note the attempts to avoid any blood mixing with cement.
After pressurization, the femoral prosthesis is introduced with care to position it centrally in the coronal and sagittal planes. A thumb is placed over the calcar to help to prevent varus positioning and to further pressurize the cement as the stem is introduced. The final position should match that determined during the trialing process. Care must be taken to avoid any motion of the leg or pressure on the trunnion by retractors so that the stem does not move in the cement as it cures (Fig. 5). A summary of these steps is outlined in Table V.
Fig. 5.
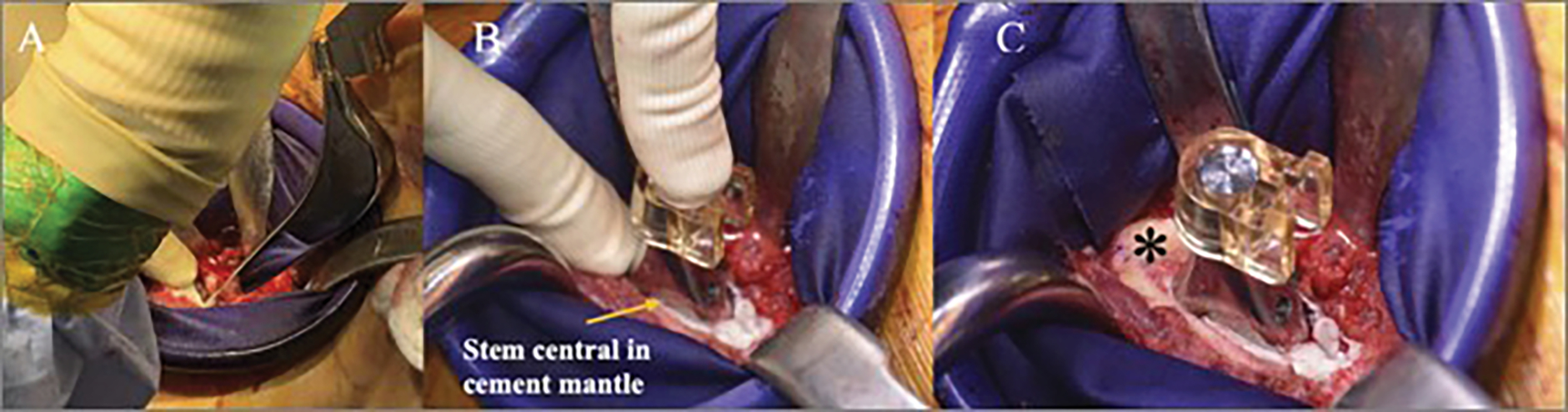
Figs. 5-A, 5-B, and 5-C Intraoperative photographs of femoral stem insertion. Fig. 5-A The femoral prosthesis is introduced centrally in the cement mantle. Fig. 5-B A thumb is placed over the calcar to help to prevent varus positioning and to further pressurize the cement as the stem is introduced. Fig. 5-C The final cemented construct. Note that the version matches that desired, as noted by the asterisk medially.
TABLE V.
Steps for Femoral Preparation and Cemented Stem Insertion*
| Step | Description |
|---|---|
|
| |
| Anesthetic considerations | Notify the anesthesia team approximately 20 minutes before cementing to allow for FiO2 increase and fluid resuscitation and to make vasopressors available |
| Femoral preparation | Large rongeur used to remove medullary contents from lateral femoral neck Curved canal finder rasp used to enter femoral canal Flexible reamer used to sound femoral canal Broach with increasingly sized femoral broaches to templated and/or appropriate size Trial reduction to confirm appropriately sized/positioned implants Irrigate and suction femoral canal Place cement restrictor Place whistle-tip suction catheter in base of femoral canal; pack ribbon gauze into canal Place gauze in acetabulum |
| Cementing and pressurization | Cement is ready to be inserted when it can be easily molded in surgeon’s hand without adhering to the glove Remove ribbon gauze; keep suction catheter in place Fill canal in retrograde fashion with cement gun with long nozzle Use finger to hold pressure over cement and remove suction catheter Remove long cement nozzle and place foam nozzle on cement gun and replace over canal Apply firm pulses of pressure for 30–60 seconds and observe for fat and marrow contents extruding from cortex |
| Stem insertion | Insert stem by hand with long hand attachment, with thumb holding pressure over medial calcar Advance stem to two-thirds of its length into canal and remove excess cement; check position Advance stem to final depth and remove excess cement; do not alter final position while cement cures |
FiO2 = fraction of inspired oxygen.
Overview
Evidence supports the use of cemented femoral fixation in patients who are >70 years old, especially women, and patients with osteoporosis, because of the lower rates of PFF and revision surgery in these patients compared with those who undergo cementless fixation8,33,64. Compared with those undergoing cementless fixation, patients undergoing cemented femoral fixation for femoral neck fracture have a lower risk of PFF, reoperation, and aseptic revision4,38,58.
We recommend that cemented femoral fixation be considered in the following scenarios: patients who are >70 years old (particularly women), those with a history of osteoporosis or fragility fracture, those with Dorr type-C bone (Table VI)33,64, displaced femoral neck fragility fractures, or when intraoperative broach stability cannot be obtained in attempting cementless fixation (Table VII). With careful fourth-generation cementation technique and appropriate perioperative management, cemented femoral stems provide excellent outcomes and minimize complications in these patients.
TABLE VI.
Dorr Classification of Femoral Bone67
| Type | Characteristics |
|---|---|
|
| |
| A | Thick, distinct cortices on anteroposterior and lateral radiographs, “champagne flute” appearance, and cortical thickness index* of <0.5 |
| B | Indicates bone loss from medial and posterior cortices, wider diaphyseal canal, thinning of posterior cortex on lateral radiographs, and cortical thickness index of 0.5 to 0.75 |
| C | Substantial loss of medial and posterior cortices, “stovepipe” appearance, thinning of cortices on both anteroposterior and lateral radiographs, and cortical thickness index of >0.75 |
Cortical thickness index is the ratio of the difference between the diaphyseal diameter and canal diameter, divided by the diaphyseal diameter, 10 cm distal to the midportion of the lesser trochanter.
TABLE VII.
Grades of Recommendation for the Use of Cemented Femoral Stem Fixation in Hip Arthroplasty
| Grade* | Recommendation |
|---|---|
|
| |
| B | Elderly patients who are >70 years old, especially women |
| B | Patients with poor bone stock, thin femoral cortices, and wide medullary canals (Dorr type C) |
| B | History of osteoporosis or fragility fracture |
| B | Displaced femoral neck fracture (except in young patients with fractures related to high-energy mechanisms) |
| I | When intraoperative broach stability cannot be obtained in attempting cementless fixation |
According to Wright68, grade A indicates good evidence (level-I studies with consistent findings) for or against recommending intervention; grade B indicates fair evidence (level-II or III studies with consistent findings) for or against recommending intervention; grade C indicates poor-quality evidence (level-IV or V studies with consistent findings) for or against recommending intervention; and grade I indicates insufficient or conflicting evidence precluding a recommendation for or against intervention.
Source of Funding
No outside funding was received for this study.
Note: The authors thank Jenni Weems, MS, Kerry Kennedy, BA, and Rachel Box, MS, in the Editorial Services group of The Johns Hopkins Department of Orthopaedic Surgery, for their editorial assistance.
Footnotes
Disclosure: The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJS/G974).
References
- 1.Lehil MS, Bozic KJ. Trends in total hip arthroplasty implant utilization in the United States. J Arthroplasty. 2014. Oct;29(10):1915–8. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Orthopaedic Surgeons. The American Joint Replacement Registry Annual Report 2020. Accessed May 20, 2021. https://aaos.org/registries/publications/ajrr-annual-report/
- 3.Jones LC, Hungerford DS. Cement disease. Clin Orthop Relat Res. 1987. Dec;(225):192–206. [PubMed] [Google Scholar]
- 4.Kumar P, Rajnish RK, Neradi D, Kumar V, Agarwal S, Aggarwal S. Hemiarthroplasty for neck of femur fractures: to cement or not? A systematic review of literature and meta-analysis. Eur J Orthop Surg Traumatol. 2019. May;29(4):731–46. [DOI] [PubMed] [Google Scholar]
- 5.Veldman HD, Heyligers IC, Grimm B, Boymans TA. Cemented versus cementless hemiarthroplasty for a displaced fracture of the femoral neck: a systematic review and meta-analysis of current generation hip stems. Bone Joint J. 2017. Apr;99-B(4):421–31. [DOI] [PubMed] [Google Scholar]
- 6.Abdel MP, Watts CD, Houdek MT, Lewallen DG, Berry DJ. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. Bone Joint J. 2016. Apr;98-B(4):461–7. [DOI] [PubMed] [Google Scholar]
- 7.Hailer NP, Garellick G, Kärrholm J. Uncemented and cemented primary total hip arthroplasty in the Swedish Hip Arthroplasty Register. Acta Orthop. 2010. Feb;81(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Springer BD, Etkin CD, Shores PB, Gioe TJ, Lewallen DG, Bozic KJ. Perioperative Periprosthetic Femur Fractures are Strongly Correlated With Fixation Method: an Analysis From the American Joint Replacement Registry. J Arthroplasty. 2019. Jul;34(7S):S352–4. [DOI] [PubMed] [Google Scholar]
- 9.Tanzer M, Graves SE, Peng A, Shimmin AJ. Is Cemented or Cementless Femoral Stem Fixation More Durable in Patients Older Than 75 Years of Age? A Comparison of the Best-performing Stems. Clin Orthop Relat Res. 2018. Jul;476(7):1428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassar-Gheiti AJ, McColgan R, Kelly M, Cassar-Gheiti TM, Kenny P, Murphy CG. Current concepts and outcomes in cemented femoral stem design and cementation techniques: the argument for a new classification system. EFORT Open Rev. 2020. Apr 2;5(4):241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The New Zealand Joint Registry. Sixteen year report January 1999 to December 2014. Accessed May 20, 2021. https://nzoa.org.nz/sites/default/files/Web_DH7657_NZJR2014Report_v4_12Nov15.pdf
- 12.Nikolaou VS, Korres D, Lallos S, Mavrogenis A, Lazarettos I, Sourlas I, Efstathopoulos N. Cemented Müller straight stem total hip replacement: 18 year survival, clinical and radiological outcomes. World J Orthop. 2013. Oct 18;4(4):303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Junnila M, Laaksonen I, Eskelinen A, Pulkkinen P, Ivar Havelin L, Furnes O, Marie Fenstad A, Pedersen AB, Overgaard S, Kärrholm J, Garellick G, Malchau H, Mäkelä KT. Implant survival of the most common cemented total hip devices from the Nordic Arthroplasty Register Association database. Acta Orthop. 2016. Dec;87(6):546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefánsdóttir A, Franzén H, Johnsson R, Ornstein E, Sundberg M. Movement pattern of the Exeter femoral stem; a radiostereometric analysis of 22 primary hip arthroplasties followed for 5 years. Acta Orthop Scand. 2004. Aug;75(4):408–14. [DOI] [PubMed] [Google Scholar]
- 15.Berry DM, Lieberman J. Surgery of the Hip. 1st ed. Elsevier; 2012. [E-Book]. [Google Scholar]
- 16.Kazi HA, Whitehouse SL, Howell JR, Timperley AJ. Not all cemented hips are the same: a register-based (NJR) comparison of taper-slip and composite beam femoral stems. Acta Orthop. 2019. Jun;90(3):214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khatun F, Gill DF, Atrey A, Porteous M. Exeter Universal cemented femoral component. Bone Joint J. 2020. Oct;102-B(10):1319–23. [DOI] [PubMed] [Google Scholar]
- 18.Yates PJ, Burston BJ, Whitley E, Bannister GC. Collarless polished tapered stem: clinical and radiological results at a minimum of ten years’ follow-up. J Bone Joint Surg Br. 2008. Jan;90(1):16–22. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz MW, Bronsema E, de Kam DC, Gardeniers JW, Veth RP, Schreurs BW. Results of the cemented Exeter femoral component in patients under the age of 40 : an update at ten to 20 years’ follow-up. Bone Joint J. 2017. Feb;99-B(2):192–8. [DOI] [PubMed] [Google Scholar]
- 20.Crowninshield RD, Jennings JD, Laurent ML, Maloney WJ. Cemented femoral component surface finish mechanics. Clin Orthop Relat Res. 1998. Oct;(355):90–102. [DOI] [PubMed] [Google Scholar]
- 21.Berry DJ, Harmsen WS, Cabanela ME, Morrey BF. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg Am. 2002. Feb;84(2):171–7. [DOI] [PubMed] [Google Scholar]
- 22.Caton J, Prudhon JL. Over 25 years survival after Charnley’s total hip arthroplasty. Int Orthop. 2011. Feb;35(2):185–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callaghan JJ, Bracha P, Liu SS, Piyaworakhun S, Goetz DD, Johnston RC. Survivorship of a Charnley total hip arthroplasty. A concise follow-up, at a minimum of thirty-five years, of previous reports. J Bone Joint Surg Am. 2009. Nov;91(11):2617–21. [DOI] [PubMed] [Google Scholar]
- 24.Della Valle AG, Zoppi A, Peterson MG, Salvati EA. A rough surface finish adversely affects the survivorship of a cemented femoral stem. Clin Orthop Relat Res. 2005. Jul;(436):158–63. [DOI] [PubMed] [Google Scholar]
- 25.Howie DW, Middleton RG, Costi K. Loosening of matt and polished cemented femoral stems. J Bone Joint Surg Br. 1998. Jul;80(4):573–6. [DOI] [PubMed] [Google Scholar]
- 26.Middleton RG, Howie DW, Costi K, Sharpe P. Effects of design changes on cemented tapered femoral stem fixation. Clin Orthop Relat Res. 1998. Oct;(355):47–56. [DOI] [PubMed] [Google Scholar]
- 27.Ong A, Wong KL, Lai M, Garino JP, Steinberg ME. Early failure of precoated femoral components in primary total hip arthroplasty. J Bone Joint Surg Am. 2002. May;84(5):786–92. [DOI] [PubMed] [Google Scholar]
- 28.Lachiewicz PF, Kelley SS, Soileau ES. Survival of polished compared with precoated roughened cemented femoral components. A prospective, randomized study. J Bone Joint Surg Am. 2008. Jul;90(7):1457–63. [DOI] [PubMed] [Google Scholar]
- 29.Thien TM, Chatziagorou G, Garellick G, Furnes O, Havelin LI, Mäkelä K, Overgaard S, Pedersen A, Eskelinen A, Pulkkinen P, Kärrholm J. Periprosthetic femoral fracture within two years after total hip replacement: analysis of 437,629 operations in the nordic arthroplasty register association database. J Bone Joint Surg Am. 2014. Oct 1;96(19):e167. [DOI] [PubMed] [Google Scholar]
- 30.Bedard NA, Callaghan JJ, Stefl MD, Liu SS. Systematic review of literature of cemented femoral components: what is the durability at minimum 20 years followup? Clin Orthop Relat Res. 2015. Feb;473(2):563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajaratnam SS, Jack C, Tavakkolizadeh A, George MD, Fletcher RJ, Hankins M, Shepperd JAN. Long-term results of a hydroxyapatite-coated femoral component in total hip replacement: a 15- to 21-year follow-up study. J Bone Joint Surg Br. 2008. Jan;90(1):27–30. [DOI] [PubMed] [Google Scholar]
- 32.The New Zealand Joint Registry. Twenty-one year report January 1999 to December 2019. Accessed May 20, 2021. https://nzoa.org.nz/sites/default/files/DH8426_NZJR_2020_Report_v5_30Sep.pdf
- 33.Dale H, Børsheim S, Kristensen TB, Fenstad AM, Gjertsen JE, Hallan G, Lie SA, Furnes O. Fixation, sex, and age: highest risk of revision for uncemented stems in elderly women - data from 66,995 primary total hip arthroplasties in the Norwegian Arthroplasty Register. Acta Orthop. 2020. Feb;91(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin FF, Chen YF, Chen B, Lin CH, Zheng K. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: A meta-analysis of randomized controlled trails. Medicine (Baltimore). 2019. Feb;98(8):e14634. Erratum in: Medicine (Baltimore). 2019 Apr;98(14):e15216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cherian JJ, Jauregui JJ, Banerjee S, Pierce T, Mont MA. What Host Factors Affect Aseptic Loosening After THA and TKA? Clin Orthop Relat Res. 2015. Aug;473(8):2700–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLaughlin JR, Lee KR. Total Hip Arthroplasty With an Uncemented Tapered Femoral Component in Patients Younger Than 50 Years of Age: A Minimum 20-Year Follow-Up Study. J Arthroplasty. 2016. Jun;31(6):1275–8. [DOI] [PubMed] [Google Scholar]
- 37.Mei XY, Gong YJ, Safir O, Gross A, Kuzyk P. Long-term outcomes of total hip arthroplasty in patients younger than 55 years: a systematic review of the contemporary literature. Can J Surg. 2019. Aug 1;62(4):249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okike K, Chan PH, Prentice HA, Paxton EW, Burri RA. Association Between Uncemented vs Cemented Hemiarthroplasty and Revision Surgery Among Patients With Hip Fracture. JAMA. 2020. Mar 17;323(11):1077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American Academy of Orthopaedic Surgeons. Management of hip fractures in the elderly: Evidence-based clinical practice guideline. Accessed May 20, 2021. https://www.aaos.org/globalassets/quality-and-practice-resources/hip-fractures-in-the-elderly/hip-fractures-elderly-clinical-practice-guideline-4-24-19-2.pdf
- 40.Kallos T, Enis JE, Gollan F, Davis JH. Intramedullary pressure and pulmonary embolism of femoral medullary contents in dogs during insertion of bone cement and a prosthesis. J Bone Joint Surg Am. 1974. Oct;56(7):1363–7. [PubMed] [Google Scholar]
- 41.Herndon JH, Bechtol CO, Crickenberger DP. Fat embolism during total hip replacement. A prospective study. J Bone Joint Surg Am. 1974. Oct;56(7):1350–62. [PubMed] [Google Scholar]
- 42.Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res. 1999. Dec;(369):39–48. [DOI] [PubMed] [Google Scholar]
- 43.Donaldson AJ, Thomson HE, Harper NJ, Kenny NW. Bone cement implantation syndrome. Br J Anaesth. 2009. Jan;102(1):12–22. [DOI] [PubMed] [Google Scholar]
- 44.Olsen F, Hå rd Af, Segerstad M, Nellgå rd B, Houltz E, Ricksten SE. The role of bone cement for the development of intraoperative hypotension and hypoxia and its impact on mortality in hemiarthroplasty for femoral neck fractures. Acta Orthop. 2020. Jun;91(3):293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olsen F, Kotyra M, Houltz E, Ricksten SE. Bone cement implantation syndrome in cemented hemiarthroplasty for femoral neck fracture: incidence, risk factors, and effect on outcome. Br J Anaesth. 2014. Nov;113(5):800–6. [DOI] [PubMed] [Google Scholar]
- 46.Rassir R, Schuiling M, Sierevelt IN, van der Hoeven CWP, Nolte PA. What Are the Frequency, Related Mortality, and Factors Associated with Bone Cement Implantation Syndrome in Arthroplasty Surgery? Clin Orthop Relat Res. 2021. Apr 1;479(4):755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larsen CG, Crockatt WK, Fitzgerald M, Matos N, Goodman HJ, Kenan S, Kenan S. Outcomes of press-fit uncemented versus cemented hip arthroplasty in the oncologic patient. J Orthop. 2020. May 4;22:198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwarzkopf E, Sachdev R, Flynn J, Boddapati V, Padilla RE, Prince DE. Occurrence, risk factors, and outcomes of bone cement implantation syndrome after hemi and total hip arthroplasty in cancer patients. J Surg Oncol. 2019. Nov;120(6):1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mudgalkar N, Ramesh KV. Bone cement implantation syndrome: A rare catastrophe. Anesth Essays Res. 2011. Jul-Dec;5(2):240–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emara AK, Ng M, Krebs VE, Bloomfield M, Molloy RM, Piuzzi NS. Femoral Stem Cementation in Hip Arthroplasty: The Know-How of a “Lost” Art. Curr Rev Musculoskelet Med. 2021. Feb;14(1):47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scanelli JA, Reiser GR, Sloboda JF, Moskal JT. Cemented Femoral Component Use in Hip Arthroplasty. J Am Acad Orthop Surg. 2019. Feb 15;27(4):119–27. [DOI] [PubMed] [Google Scholar]
- 52.Richardson CG, Lethbridge LN, Dunbar MJ. Increased Mortality with the Use of Cementless Fixation for Femoral Neck Fractures: Analysis of 5883 Hip Arthroplasty Cases. J Arthroplasty. 2020. Dec;35(12):3627–30. [DOI] [PubMed] [Google Scholar]
- 53.Garland A, Gordon M, Garellick G, Kärrholm J, Sköldenberg O, Hailer NP. Risk of early mortality after cemented compared with cementless total hip arthroplasty: a nationwide matched cohort study. Bone Joint J. 2017. Jan;99-B(1):37–43. [DOI] [PubMed] [Google Scholar]
- 54.Deangelis JP, Ademi A, Staff I, Lewis CG. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: a prospective randomized trial with early follow-up. J Orthop Trauma. 2012. Mar;26(3):135–40. [DOI] [PubMed] [Google Scholar]
- 55.Langslet E, Frihagen F, Opland V, Madsen JE, Nordsletten L, Figved W. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: 5-year followup of a randomized trial. Clin Orthop Relat Res. 2014. Apr;472(4):1291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor F, Wright M, Zhu M. Hemiarthroplasty of the hip with and without cement: a randomized clinical trial. J Bone Joint Surg Am. 2012. Apr 4;94(7):577–83. [DOI] [PubMed] [Google Scholar]
- 57.Song JSA, Dillman D, Wilson D, Dunbar M, Richardson G. Higher periprosthetic fracture rate associated with use of modern uncemented stems compared to cemented stems in femoral neck fractures. Hip Int. 2019. Mar;29(2):177–83. [DOI] [PubMed] [Google Scholar]
- 58.Kristensen TB, Dybvik E, Kristoffersen M, Dale H, Engesæter LB, Furnes O, Gjertsen JE. Cemented or Uncemented Hemiarthroplasty for Femoral Neck Fracture? Data from the Norwegian Hip Fracture Register. Clin Orthop Relat Res. 2020. Jan;478(1):90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ayers D, Mann K. The importance of proximal cement filling of the calcar region: a biomechanical justification. J Arthroplasty. 2003. Oct;18(7)(Suppl 1):103–9. [DOI] [PubMed] [Google Scholar]
- 60.Ebramzadeh E, Sarmiento A, McKellop HA, Llinas A, Gogan W. The cement mantle in total hip arthroplasty. Analysis of long-term radiographic results. J Bone Joint Surg Am. 1994. Jan;76(1):77–87. [DOI] [PubMed] [Google Scholar]
- 61.Ramaniraka NA, Rakotomanana LR, Leyvraz PF. The fixation of the cemented femoral component. Effects of stem stiffness, cement thickness and roughness of the cement-bone surface. J Bone Joint Surg Br. 2000. Mar;82(2):297–303. [PubMed] [Google Scholar]
- 62.Barrack RL, Mulroy RD Jr, Harris WH. Improved cementing techniques and femoral component loosening in young patients with hip arthroplasty. A 12-year radiographic review. J Bone Joint Surg Br. 1992. May;74(3):385–9. [DOI] [PubMed] [Google Scholar]
- 63.Charnley J Anchorage of the femoral head prosthesis to the shaft of the femur. J Bone Joint Surg Br. 1960. Feb;42-B:28–30. [DOI] [PubMed] [Google Scholar]
- 64.Carli AV, Negus JJ, Haddad FS. Periprosthetic femoral fractures and trying to avoid them: what is the contribution of femoral component design to the increased risk of periprosthetic femoral fracture? Bone Joint J. 2017. Jan;99-B(1)(Supple A): 50–9. [DOI] [PubMed] [Google Scholar]
- 65.Mulroy RD Jr, Harris WH. The effect of improved cementing techniques on component loosening in total hip replacement. An 11-year radiographic review. J Bone Joint Surg Br. 1990. Sep;72(5):757–60. [DOI] [PubMed] [Google Scholar]
- 66.Wixson RL, Lautenschlager EP, Novak MA. Vacuum mixing of acrylic bone cement. J Arthroplasty. 1987;2(2):141–9. [DOI] [PubMed] [Google Scholar]
- 67.Wilkerson J, Fernando ND. Classifications in Brief: The Dorr Classification of Femoral Bone. Clin Orthop Relat Res. 2020. Aug;478(8):1939–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wright JG. Revised grades of recommendation for summaries or reviews of orthopaedic surgical studies. J Bone Joint Surg Am. 2006. May;88(5):1161–2. [DOI] [PubMed] [Google Scholar]


