Abstract
With age, the presence of multiple neuropathologies in a single individual becomes increasingly common. Given that traumatic brain injury and the repetitive head impacts (RHIs) that occur in contact sports have been associated with the development of many neurodegenerative diseases, including chronic traumatic encephalopathy (CTE), Alzheimer’s disease, Lewy body disease, and amyotrophic lateral sclerosis, it is becoming critical to understand the relationship and interactions between these pathologies. In fact, comorbid pathology is common in CTE and likely influenced by both age and the severity and type of exposure to RHI as well as underlying genetic predisposition. Here, we review the major comorbid pathologies seen with CTE and in former contact sports athletes and discuss what is known about the associations between RHI, age, and the development of neuropathologies. In addition, we examine the distinction between CTE and age-related pathology including primary age-related tauopathy and age-related tau astrogliopathy.
Keywords: chronic traumatic encephalopathy, traumatic brain injury, neurodegenerative disease, Alzheimer’s disease, comorbidity
The cooccurrence of multiple neuropathologies is the most common driver of cognitive decline and dementia with increasing age.1,2 Occam’s razor, the “law of parsimony,” in essence states that the simplest solutions are more likely correct then complex ones. In diagnostic neuropathology, this approach compels us to seek a single diagnosis, rather than conclude multiple complex processes are occurring simultaneously. While this, in principle, seems reasonable, in practice, it is not. In the aging brain, comorbid or mixed pathologies with similar features are increasingly recognized as the rule, rather than the exception.1 In fact, in almost 80% of autopsies included in a recent study, 2 or more of the 9 most common neuropathologies were identified, and there were > 230 different neuropathological combinations.1 Recent advances in our understanding of the cellular, genetic, and molecular bases of neurodegenerative diseases have allowed us to greatly improve our ability to recognize distinct pathological processes and thus diagnose comorbidities. Overall, dementia is often caused by mixed pathologies, and age is one of the most significant driving factors.
In addition to age, head impacts, either in the form of traumatic brain injury (TBI) or repetitive head impacts (RHIs) such as in contact sports, may be a risk factor for the development of multiple neuropathologies. Although nascent, recent studies suggest that TBI and RHI can have similar, yet distinct heterogeneous pathologies.3-8 Remote moderate to severe TBI has long been viewed as a risk factor for Alzheimer’s disease (AD), yet recent large cohort studies indicate that TBI of all severities (mild, moderate, severe) is a risk factor for dementia, the neuropathology of which is largely unknown.3-10 Following acute TBI, amyloid precursor protein (APP) and β-amyloid (Aβ) increase in brain tissue and cerebrospinal fluid (CSF), and there can be rapid formation of diffuse cortical Aβ plaques.11 Both APP and Aβ increase within damaged axons, and the release of Aβ into the surrounding tissue may be the basis for plaque formation.12 RHIs are associated with chronic traumatic encephalopathy (CTE), a neurodegenerative disease. In addition, recent studies suggest that RHI may lead to, or alter, multiple neuropathologies including cerebral amyloid angiopathy (CAA), Lewy body disease (LBD), amyotrophic lateral sclerosis (ALS), and others.
Approaching CTE in the context of comorbidities is challenging given its complex clinical presentation, with symptoms that are variable and overlap with other brain diseases. Variable degrees of coexistent cognitive, motor, movement, and psychiatric dimensions are all common and blur diagnostic certainty. In contrast to the clinical criteria for CTE, which are still in development, the neuropathological criteria that are applied on the tissue level are highly specific and sensitive especially at later disease stages.13 Advanced neuroimaging and biomarkers in blood and CSF are in development and hold promise for improving diagnosis ante-mortem14,15; until that time, a postmortem examination is paramount, as it allows for evaluation of the molecular and cellular changes occurring in degenerating neurons and glia and localization to specific brain regions. Sorting out these changes is the foundation to making these challenging diagnoses.
The main molecular feature used to evaluate neurodegenerative diseases is intracellular aggregates of abnormal protein inclusions such as tau, α-synuclein, and TDP-43. Extracellular aggregates can also occur, the most common being the Aβ plaque in AD. Identification of these aggregates in postmortem brain is critical, but not sufficient, for making the diagnosis, as they must also be localized to specific brain regions and cells types. In this way, the overlap and blurred distinctions seen in the clinical context are mirrored on the tissue level where advancements in understanding disease-specific cellular vulnerability have greatly increased our ability to resolve differences. Here, we summarize the multiple neuropathologies seen in association with RHI and CTE and describe the similarities and differences in their pathological presentations. In addition, we discuss the current models linking RHI to multiple neuropathologies and discuss the role of aging and age-related pathologies in CTE.
Beta-Amyloid in CTE
Unlike AD, which requires the presence of both tau pathology and Aβ deposition for diagnosis, CTE is defined by the unique spatial distribution of tau pathology only. However, though Aβ is not a defining feature of CTE, it is the most frequent comorbid pathology and may play a role in disease progression. Deposition of Aβ has been reported in approximately 30% of cases of acute trauma and APP abnormalities occur following axonal injury.11,12,16-19 Early reports using Aβ immunohistochemistry demonstrated that the majority of boxers with CTE had widespread diffuse Aβ deposits.20,21 However, as more cases were described, it was found that many cases of CTE exhibit no Aβ pathology; this was particularly true in young individuals22-26 and early stages of CTE.27 Postmortem analysis of 114 contact sport athletes and military veterans with CTE who had a mean age of death of 60 years demonstrated that Aβ deposition was present in just over half (52%) and varied by both age and stage.28 Various types of Aβ plaque can be distinguished by their morphology. Two major types typically used for AD classification include neuritic plaques with argyrophilic dystrophic neurites, with or without dense amyloid cores and diffuse plaques with noncompact amyloid and no apparent dystrophic neurites.29 The type of Aβ plaque was significantly different in CTE: in contrast to AD where neuritic plaques are necessary for the diagnosis, participants with CTE were far more likely to have diffuse Aβ plaques, while only about one-third of participants had neuritic plaques (►Table 1). Furthermore, the deposition of Aβ occurred at an earlier age and at an accelerated rate in CTE compared with a normal aging population.30 Similar to these findings, a recent report of 11 cases of soccer and rugby players found that AD pathology was a common comorbidity with CTE in older athletes.31
Table 1.
Neuropathological comorbidities and reported frequencies in CTE
| Frequencya | Mean age (y) | Reference | |
|---|---|---|---|
| Beta-amyloid plaques | |||
| - Diffuse | 59/55 (52%) | 60 | Stein et al 201528 |
| - Neuritic | 41/73 (36%) | 60 | Stein et al 201528 |
| Cerebral amyloid angiopathy | 72/179 (29%) | 60 | Standring et al 201946 |
| Alzheimer diseaseb | 23/154 (13%) | 67 | Mez et al 201763 |
| Lewy body disease | |||
| - Any | 53/86 (38%) | 57 | Adams et al 201858 |
| - Neocortical | 13/238 (5.2%) | 60 | Standring et al 201946 |
| Amyotrophic lateral sclerosis | 11/166 (6%) | 67 | Mez et al 201763 |
| Arteriolosclerosis (moderate-severe) | 85/95 (47%) | 68 | Alosco et al 201981 |
| Primary age-related tauopathy (PART) | Unknown | – | – |
| Age-related tau astrogliopathy (ARTAG) | Unknown | – | – |
Abbreviation: CTE, chronic traumatic encephalopathy.
Data are presented as with/without (% with).
Based on NIA-Reagan criteria.
The biochemical type and pattern of Aβ deposition was subtly different in CTE. Quantitative enzyme-linked immuno-sorbent assays showed that levels of Aβ1–40, but not Aβ1–42 were greater in the sulci than the gyri in CTE-AD subjects. This correlates with the location of peak mechanical force following RHI and with ptau pathology in CTE and is distinct from the even distribution of Aβ in gyri and sulci in AD.28 Biochemical analysis of an early tau phosphorylation site present in AD (ptau231) showed that the presence of neuritic plaques was associated with increased ptau231 levels in CTE, but that levels were still greatest in AD or in subjects with both CTE and AD. Thus, ptau231 appears to be a phosphorylation site that is largely driven by Aβ pathology in CTE.
The presence of Aβ plaques in CTE was associated with other more severe pathologies. Linear regression analysis adjusting for age showed that CTE subjects with Aβ had worse tau pathology and were more likely to have clinical symptoms, dementia, and parkinsonism.28 These findings suggest that RHI may accelerate Aβ deposition and that Aβ, in turn, accelerates ptau pathology and worsens clinical outcomes in CTE.
Cerebral Amyloid Angiopathy in CTE and Contact Sports
CAA consists of Aβ deposition within the basement membrane of meningeal and superficial cortical arteries and arterioles that leads to the replacement of smooth muscle cells.32 CAA is a frequent comorbidity with AD, but may also occur independently. Moderate to severe CAA is also associated with cerebral hemorrhages, cortical microinfarcts, and dementia independent of AD pathology.33,34 CAA is more common in the posterior cerebral cortex (parietal and occipital lobes) than the frontal lobes in AD and the general aging population.35-39 Aβ deposition in the blood vessel wall can cause progressive weakening and dysfunction of the vessel wall and lead to rupture and subsequent intracerebral hemorrhage.38,40,41 Independent of other pathologies, CAA is associated with age as well as impaired cognitive function and dementia likely due to a combination of hemorrhage, ischemia, and synaptic damage.42,43
Previous studies have documented the presence of CAA in postmortem subjects with a moderate to severe TBI history.44 However, a retrospective study of three large autopsy cohorts did not find an association between TBI and the presence of moderate to severe CAA,5 although alterations in the distribution of CAA were not examined. RHIs may be distinct from TBI and may lead to small vessel pathology such as CAA. For instance, amateur football players were found to have blood–brain barrier dysfunction by dynamic contrast-enhanced magnetic resonance imaging analysis after a single season of play compared with noncontact sport athletes.45 In addition, both TBI and RHI may lead to an altered distribution of CAA.
A recent postmortem study of contact sport participants found that CAA was most severe in the frontal leptomeningeal vessels in 251 participants with CTE.46 This distribution was significantly different from AD where CAA was more likely to involve the intracortical vessels and the parietal and occipital lobes. The frontal predilection of CAA also aligns with where tau pathology first occurs and where injury is likely greatest. Indeed, head collisions predominantly involve the anterior aspect of the skull in American football, and mathematical models of a helmet-to-helmet collision in football predicted that the greatest strain occurs in the frontal convexities and at the depths of sulci.47-49 Examination of three different brain bank groups demonstrated that in subjects with CAA, RHI was associated with increased CAA severity in the frontal leptomeningeal vessels, adjusting for AD, apolipoprotein E (APOE) ε4, and age. Overall, RHI and CTE were associated with increased leptomeningeal CAA, and CAA was independently associated with worse pathological and clinical outcomes, suggesting that altered CAA is another mechanism by which RHI may lead to cognitive decline.46
Lewy Body Disease in CTE and Contact Sports
While the hypothesis that TBI might play a role in the pathogenesis of Parkinson’s disease (PD) dates back to the original description,50 several recent studies have begun to elucidate this link.6,51-54 Analysis of a large veteran cohort showed that a history of TBI, including mild TBI, was associated with increased risk of PD.6 Furthermore, TBI with loss of consciousness greater than 1 hour occurring early in life was associated with increased risk of cortical Lewy bodies in pooled brain banks from three community aging cohorts. This study also found a clinical association between TBI with loss of consciousness and incident PD in one cohort and progression of parkinsonian signs in two others.5
Parkinsonism appears to be relatively common in patients thought to have CTE.55,56 On the other hand, one study did not find significant deficits in motor function in Canadian football players compared with a small group of controls.57 However, few studies have examined associations between RHI, PD symptoms, and pathology. A recent study by our group examined the clinical and pathological relationships between RHI exposure, CTE, and LBD within a group of deceased athletes with RHI exposure, a community-based aging cohort, and a cohort enriched for AD. The number of years of exposure to RHI through contact sports was associated with the development of neocortical LBD in the community-based aging cohort, the contact sport athlete cohort, and in a pooled analysis controlling for age, sex, and APOE ε4 allele status. The distribution of LBD in CTE was distinct from that in AD such that in CTE, LBD was most often limbic, neocortical, or brainstem, while in AD, the distribution of Lewy bodies was more likely to be amygdala-predominant. Clinically, across three autopsy groups, dementia was significantly associated with neocortical LBD, CTE stage, AD, and age at death. Parkinsonism was significantly associated with LBD and age at death, but not CTE stage.58 Therefore, the increased frequency of LBD following RHI exposure may partially explain the extrapyramidal motor symptoms sometimes observed in CTE. However, it remains to be determined whether tau or other pathology in the substantia nigra in CTE might also partially contribute to Parkinsonism in those cases without LBD.
Given that even a single TBI might be associated with LBD and Parkinsonism, the threshold for the number of years of contact sport participation might be low. Analysis of the community-based aging cohort and the contact sport athlete group showed that a threshold of 8 years of contact sport play was the greatest predictor for neocortical LBD, suggesting this may be an important threshold.58 However, this analysis is limited by retrospective and autopsy-based selection biases as well as the fact that the athlete cohort was selected for a high level of contact sport play. Future prospective studies will be needed to determine how the risk of LBD is influenced by the type and amount of contact sport play as well as the development of CTE.
Amyotrophic Lateral Sclerosis and CTE
Motor neuron disease or ALS is a progressive, fatal neurodegenerative disorder involving degeneration of both motor cortex and spinal motor neurons. ALS is more common in males, military veterans, and former American football players.59-62 It is a relatively common comorbid pathology with CTE, occurring in approximately 6% of American football players with CTE in an autopsy group (►Table 1).63 The role of TBI in the development of ALS is unclear. One study found ALS was more likely in those that reported multiple head injuries within the past 10 years prior to diagnosis,64 although other studies have not found a modifying effect of head injury on ALS.65 In a veteran cohort, those with head injuries, especially within 15 years prior to diagnosis, were found to be significantly more likely to develop ALS.66 We found CTE in approximately 6% of a veteran ALS cohort (n = 155).67 Participants with ALS + CTE were more likely to have a TBI history, have served during the first Persian Gulf War, and have more severe ptau pathology in the frontal cortex and spinal cord. One-half of ALS participants with a history of TBI had comorbid CTE. In those with ALS + CTE, the most common RHI exposures were from contact sport exposure (e.g., tackle football) (44%), military service (frequent aircraft carrier landings) (11%), or both (bull riding and grenade blast exposure) (11%). Most of these exposures have been previously related to the development of CTE, including bull riding.68 CTE is sometimes associated with TDP-43 inclusions even in the absence of ALS.27 It may be that trauma potentiates TDP-43 pathology and that this is one mechanism by which RHI and CTE are associated with ALS. In fact, in the veteran cohort there was a significantly increased TDP-43 stage69 in participants with ALS + CTE.67 Clinically, participants with ALS + CTE were more likely to have bulbar onset ALS, behavioral changes, and/or mood changes, which may represent a combination or synergy of the two diseases. Overall, compared with ALS alone, comorbid ALS + CTE is associated with RHI and TBI and has an altered clinical and pathological presentation.
Vascular Disease and White Matter Damage in CTE
Cerebrovascular disease is often comorbid with neurodegenerative disease and contributes to cognitive decline.70-75 Arteriolosclerosis, infarcts, microinfarcts, and white matter (WM) degeneration are common with aging and cardiovascular disease. Cardiovascular disease is frequent in former American football players,76,77 and WM degeneration and cerebrovascular disease are common comorbidities in CTE.27,78-80 Therefore, RHI may be associated with cerebrovascular disease and contribute to cognitive decline in CTE.
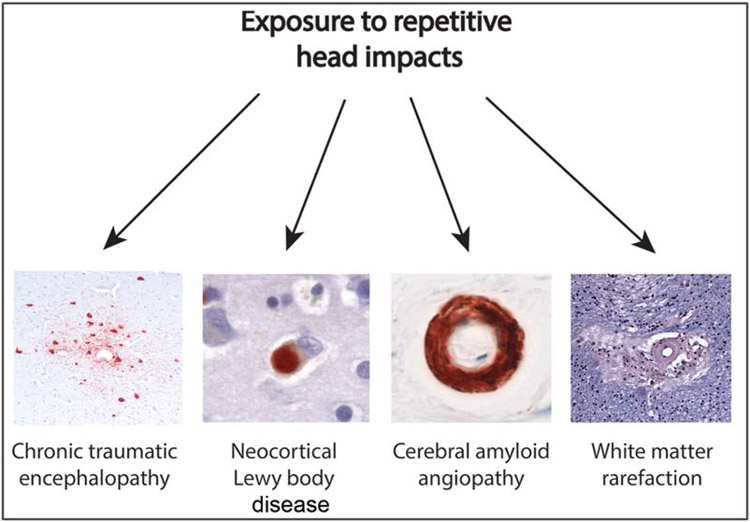
A recent study found that moderate to severe WM rarefaction (46.6%) and arteriolosclerosis (47.2%) are common in 180 deceased football players with autopsy-confirmed CTE (Table 1).81 Simultaneous equations regression model controlling for age and race showed more years of play corresponded to more severe WM rarefaction and greater tau pathology accumulation. Arteriolosclerosis and years of play were not related, but arteriolosclerosis was independently associated with dementia. The odds of dementia were further increased with more severe WM rarefaction and arteriolosclerosis independent of tau pathology. Among older football players with CTE, more years of football participation corresponded to more severe WM rarefaction and greater burden of tau pathology.81 Overall, dementia in CTE may result from multiple neuropathologies linked to RHI, including WM rarefaction and tau pathology, as well as pathologies not related to TBI or RHI, such as arteriolosclerosis and other age-related disease (►Fig. 1).
Fig. 1.
Recent studies have linked total years of exposure to repetitive head impacts from contact sports to a variety of neuropathologies including chronic traumatic encephalopathy (CTE),27,58,122 neocortical Lewy body disease,58 severity of cerebral amyloid angiopathy,46 and white matter rarefaction.81 Examples of each pathology in subjects with CTE are shown.
Primary Tauopathies
CTE is just one of several pathological entities and contexts in the brain associated with the presence of abnormal tau aggregates.82 Among these entities, the frequency varies considerably and would thus be expected to exist comorbidly with CTE to varying degrees. The term tauopathy was originally coined to describe a subset of frontotemporal lobar degeneration (FTLD) patients with germline autosomal dominant MAPT gene mutations, but these patients are very rare.83 Subsequently, the term tauopathy has come to encompass all brain diseases where tangles occur.
Neurofibrillary tangles and gliofibrillary tangles are nearly ubiquitous in usual aging by about the age of 50 years old.84 Remarkably, cross-sectional studies have revealed that the very first evidence of tau abnormalities can occur in the teen years in the brain stem.85 These changes progress though the lifespan, involving midbrain and forebrain structures to varying degrees and leading to a wide spectrum of clinical and neuropathological outcomes. This heterogeneity is thought to depend on several intrinsic and extrinsic factors, including environment and genetics. Thus, depending on age, some degree of tau pathology is expected and differentiating CTE from common age-related changes is critical. Various factors contribute to the heterogeneity in tau pathology, including selective vulnerability of brain regions and cell types, and understanding these mechanisms is key to rendering diagnoses. In the absence of a MAPT mutation, the approach to differentiating tauopathies relies heavily on three factors: (1) regional (i.e., neuroanatomical) vulnerability, (2) cellular vulnerability (mainly neurons and glia), and (3) biochemical differences in tau isoform expression. Other factors, such as tau filament ultrastructure and tau hyperphosphorylation, are not currently useful as differentiating factors.
Primary Age-Related Tauopathy
Primary age-related tauopathy (PART) is a term used to describe AD type neurofibrillary tangle pathology appearing in a limited neuroanatomical distribution, predominantly the medial temporal lobe and other structures, in the absence amyloid plaques.86 PART is commonly observed in cognitively normal individuals after the age of 50, but it is generally not very severe. The frequency and severity of tau pathology increases with age. Interestingly, but not surprisingly, it was recently found in a supercentenarian.87 With increased burden of neurofibrillary pathology and neurodegeneration in PART, the likelihood of cognitive impairment is higher.88 Symptomatic PART is generally manifested as amnestic mild cognitive impairment (MCI) or dementia,89 but other symptoms may occur. PART patients are often clinically diagnosed with AD, given the prominent amnestic component, but they may have some clinical features that diverge. For example, recent studies suggest that cognitive impairment declines more slowly in PART90,91 and that these patients may have relative preservation of certain cognitive domains, such as semantic memory.92 This remains unclear, however, as some studies have suggested that higher stages of neurofibrillary tangle burden in PART might be associated with more rapid decline on tasks involving episodic and semantic memory along with tests of processing speed and attention.93
Postmortem examination in PART has revealed that the tangles are regionally, morphologically, biochemically, and ultrastructurally very similar to those seen in AD.86 They are present in neurons in the entorhinal cortex and hippocampus proper and variably present in other temporal lobe regions. There may be preferential involvement of CA2. Outside the medial temporal lobe, neurofibrillary tangles in PART can be seen in the brainstem, including the locus coeruleus and substantia nigra, as well as inferior frontal cortex and nucleus accumbens. These tangles are present in neurons, structurally similar to the classic flame-shaped tangles in AD that accumulate and take on the shape of the pyramidal neurons in which they commonly develop. Biochemical studies have consistently demonstrated an isoform profile that is similar to AD, with both tau isoforms containing 3 and 4 microtubule binding domain repeats. Ultrastructurally, they contain Alzheimer-type paired helical filaments. Just as in all tauopathies, the tau contains numerous secondary modifications, including hyperphosphorylation, but there are no specific markers for PART at this time.
The genetics of PART is not fully explored, although emerging evidence suggests that individuals with PART have divergent genetic risk from AD.94 There is no increase in the frequency of the APOE ε4 allele which is strongly associated with amyloid deposition and AD. Instead, PART is associated with an increased allele frequency of the protective APOE ε2 allele.95 Theoretically, individuals with PART may harbor other genetic variations that protect from amyloid deposition alongside risk for tau accumulation, such as the MAPT 17q21.31 H1 haplotype.95,96
Differentiating PART from CTE can be accomplished as the regional tau accumulation in the neocortical sulcal and perivascular lesions that define CTE are not a component of PART. However, medial temporal lobe pathology is common in the late stages of CTE, and the regional pattern of the two diseases overlaps and may be difficult to discriminate if the hippocampal formation is examined in isolation. Thus far, the differential involvement of the subfields of the hippocampus between PART and CTE is unknown, though CTE may preferentially affect CA4.27 For this reason, we recommend extensive sampling of neocortical regions when suspicion merits.
Aging-Related Tau Astrogliopathy
Aging-related tau astrogliopathy (ARTAG) is a term coined to describe a morphological spectrum of pathological accumulation of abnormal tau protein in astrocytes in aging.97 ARTAG is mainly observed in subjects over the age of 60 years where it has been observed in up to 38% of brains in one series.98 The ARTAG spectrum includes thorn-shaped astrocytes at the glial limitans and in the WM as well as granular fuzzy astrocytes in the gray matter. It is not clear whether these different glial fibrillary forms represent different or single pathological processes. ARTAG is distinct from the pathology seen in other tauopathies with prominent glial changes, such as progressive supranuclear palsy (PSP) and cortical basal degeneration, which are characterized by tufted astrocytes99 and astrocytic plaques,100 respectively.
ARTAG is most readily observable using immunohistochemistry, especially 4R isoform-specific antisera. Connection 43 and aquaporin 4 expression are also strongly correlated with ARTAG.101 These abnormal astrocytes can be seen in various compartments, including subpial, subependymal, perivascular, WM, and gray matter. ARTAG can be seen diffusely throughout the central nervous system, but the peri-amygdala WM is preferentially vulnerable.102 While ARTAG may spread through the brain in a similar pattern as has been proposed for neurofibrillary tau, the propagation is more complex with only some forms of ARTAG showing clear hierarchical progression.101
Understanding of the clinical implications of ARTAG is minimal. Early studies revealed argyrophilic thorny astrocyte clusters in primary progressive aphasia associated with AD pathology.103 Later, thorn-shaped astrocytes were linked to cognitive symptoms, but this was variable.104,105 Intriguingly, one recent study found evidence that cortical ARTAG is associated with dementia, but limbic and brainstem ARTAG was not.106 Further studies are required to clarify the clinical impact of ARTAG.
Given its prevalence, ARTAG would be expected to be observed comorbidly with numerous other diseases, and reports have documented it in numerous and disparate contexts, including human immunodeficiency virus,107 FTLD,108 and Huntington’s disease.109 The relationship between CTE and ARTAG is worth noting. Astrogliosis in CTE patients can be robust.110 In addition, there are significant morphological overlaps. This includes localization of tau pathology to subpial, perivascular, and gray matter astrocytes, as with ARTAG. The key feature of the CTE-associated lesions is neuronal tau, however, so the presence of pure astrocyte tau, even in a perivascular distribution, should not be considered diagnostic of CTE. Studies of ARTAG progression have invited speculation that ARTAG may be a precursor lesion for CTE, however further investigation is required.101
Argyrophilic Grain Disease
Argyrophilic grain disease (AGD) was first reported as a neurodegenerative disease with late-onset displaying small spindle- or comma-shaped, silver stain positive lesions, termed argyrophilic grains.111 Follow-up studies revealed that these lesions were composed of hyperphosphorylated-tau. These are observed in combination with oligodendroglial coiled bodies and numerous pretangles. While AGD is commonly seen in the context of MCI and aging, it is very commonly comorbid with other neurodegenerative diseases, and identifying a distinctive clinical correlate has been challenging.112,113 One understudied aspect of CTE is the dot-like lesion.114 These lesions are common in CTE and track the other thread and tau pathology in CTE. They have been proposed to be related to AGD, but this requires further investigation.115
Frontotemporal Lobar Degeneration
FTLD is the neuropathological category that is the counterpart to the clinical term frontotemporal dementia, a heterogeneous group of syndromes, including behavioral variant FTD, progressive nonfluent aphasia, semantic dementia, and corticobasal syndrome. These diseases have a range of neuropathological changes, with the most common being accumulation of TDP-43 termed FTLD-TDP43. This is followed by tau pathology, termed FTLD-tau, which can be familial in kindreds with autosomal dominant MAPT mutations, or sporadic. Other forms of FTLD have been reported, but these are very rare.
CTE has been reported to occur comorbidly with FTLD-tau. For example, sporadic PSP was observed in a 75-year-old ex-professional boxer.116 This finding was intriguing because, unlike autosomal dominant genetic forms of FTLD where causality is established, the pathogenesis of sporadic FTLD remains less clear and it raises the possibility that mechanical injury may play a role. As discussed previously, TBI and RHI are likely risk factors for other neurodegenerative diseases beyond CTE, including AD, PD, and ALS, but a link with PSP has not yet been established and further studies are required.117
Other Neurodegenerative and Brain Diseases
In addition to the entities described above, CTE has been reported comorbidly with several neurodegenerative and psychiatric illnesses. For example, CTE has been seen alongside prion disease, with three cases of histopathologically validated CTE with coexisting sporadic Creutzfeldt–Jakob disease.118 Features consistent with stage I CTE were also demonstrated in comorbid in a single case of Huntington’s disease (i.e., rare perivascular aggregates of tau-positive neurons, astrocytes, and processes were identified at sulcal depths).109 This patient did not have a history of contact sport play, but did have documented falls. Similarly, CTE was found in approximately 6% of patients with multiple systems atrophy,119 another disorder associated with frequent falls. Finally, CTE has also been seen in the context of schizophrenia,120 where TBI might be a risk factor.121
Conclusion
Altogether, comorbid pathology is common in CTE and likely influenced by both age and the severity and type of exposure to RHI as well as underlying genetic predisposition. Recent studies suggest that RHI may lead to or alter the presentation of multiple neuropathologies including Aβ deposition, CAA, LBD, and WM degeneration. Age and APOE genotype further contribute to the development and progression of pathology. The prevalence of rare diseases such as ALS comorbid with CTE suggests a possible association, but better powered and prospective studies are necessary. Overall, recognition of risk factors and detection of comorbid pathology will be important for prevention, diagnosis, and treatment.
Acknowledgments
We gratefully acknowledge the use of resources and facilities at the Edith Nourse Rogers Memorial Veterans Hospital (Bedford, MA), the outreach and recruitment contributions of Dr. Christopher Nowinski from the Concussion Legacy Foundation, and all the individuals whose participation and contributions made this work possible.
Funding
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Clinical Sciences Research and Development Merit Award (I01-CX001038); Veterans Affairs Biorepository (BX002466); Department of Defense (W81XWH-14–1-0399); National Institute of Aging (RF1AG054156, R56AG057768, R01AG057768, R01AG054008, R01NS095252, R01AG062348, RF1AG060961); National Institute of Neurological Disorders and Stroke (U01NS086659); National Institute of Aging Boston University AD Center (P30AG13846; supplement 0572063345–5); Concussion Legacy Foundation, the Tau Consortium (Rainwater Charitable Trust), and the Alzheimer’s Association (NIRG-15–363188). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
None declared.
References
- 1.Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol 2018;83(01):74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 2018;141(07):2181–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes DE, Byers AL, Gardner RC, Seal KH, Boscardin WJ, Yaffe K. Association of mild traumatic brain injury with and without loss of consciousness with dementia in US Military Veterans. JAMA Neurol 2018;75(09):1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plassman BL, Havlik RJ, Steffens DC, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 2000;55(08):1158–1166 [DOI] [PubMed] [Google Scholar]
- 5.Crane PK, Gibbons LE, Dams-O’Connor K, et al. Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol 2016;73(09):1062–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner RC, Byers AL, Barnes DE, Li Y, Boscardin J, Yaffe K. Mild TBI and risk of Parkinson disease. Neurology 2018;90(20):e1771–e1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugarman MA, McKee AC, Stein TD, et al. Failure to detect an association between self-reported traumatic brain injury and Alzheimer’s disease neuropathology and dementia. Alzheimers Dement 2019;15(05):686–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dams-O’Connor K, Guetta G, Hahn-Ketter AE, Fedor A. Traumatic brain injury as a risk factor for Alzheimer’s disease: current knowledge and future directions. Neurodegener Dis Manag 2016;6(05):417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plassman BL, Grafman J. Traumatic brain injury and late-life dementia. Handb Clin Neurol 2015;128:711–722 [DOI] [PubMed] [Google Scholar]
- 10.Pischiutta F, Micotti E, Hay JR, et al. Single severe traumatic brain injury produces progressive pathology with ongoing contralateral white matter damage one year after injury. Exp Neurol 2018;300:167–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikonomovic MD, Uryu K, Abrahamson EE, et al. Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol 2004;190(01):192–203 [DOI] [PubMed] [Google Scholar]
- 12.Smith DH, Chen X-H, Iwata A, Graham DI. Amyloid beta accumulation in axons after traumatic brain injury in humans. J Neurosurg 2003;98(05):1072–1077 [DOI] [PubMed] [Google Scholar]
- 13.McKee AC, Cairns NJ, Dickson DW, et al. ; TBI/CTE group. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 2016;131(01):75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zetterberg H, Blennow K. Chronic traumatic encephalopathy: fluid biomarkers. Handb Clin Neurol 2018;158:323–333 [DOI] [PubMed] [Google Scholar]
- 15.Lin A, Charney M, Shenton ME, Koerte IK. Chronic traumatic encephalopathy: neuroimaging biomarkers. Handb Clin Neurol 2018;158:309–322 [DOI] [PubMed] [Google Scholar]
- 16.Roberts GW, Gentleman SM, Lynch A, Murray L, Landon M,Graham DI. Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 1994;57(04):419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts GW, Gentleman SM, Lynch A, Graham DI. beta A4 amyloid protein deposition in brain after head trauma. Lancet 1991;338(8780):1422–1423 [DOI] [PubMed] [Google Scholar]
- 18.Gentleman SM, Greenberg BD, Savage MJ, et al. A beta 42 is the predominant form of amyloid beta-protein in the brains of short-term survivors of head injury. Neuroreport 1997;8(06):1519–1522 [DOI] [PubMed] [Google Scholar]
- 19.Olsson A, Csajbok L, Ost M, et al. Marked increase of beta-amyloid (1-42) and amyloid precursor protein in ventricular cerebrospinal fluid after severe traumatic brain injury. J Neurol 2004;251(07):870–876 [DOI] [PubMed] [Google Scholar]
- 20.Roberts GW, Allsop D, Bruton C. The occult aftermath of boxing. J Neurol Neurosurg Psychiatry 1990;53(05):373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tokuda T, Ikeda S, Yanagisawa N, Ihara Y, Glenner GG. Re-examination of ex-boxers’ brains using immunohistochemistry with antibodies to amyloid beta-protein and tau protein. Acta Neuropathol 1991;82(04):280–285 [DOI] [PubMed] [Google Scholar]
- 22.Hof PR, Bouras C, Buée L, Delacourte A, Perl DP, Morrison JH. Differential distribution of neurofibrillary tangles in the cerebral cortex of dementia pugilistica and Alzheimer’s disease cases. Acta Neuropathol 1992;85(01):23–30 [DOI] [PubMed] [Google Scholar]
- 23.Geddes JF, Vowles GH, Robinson SF, Sutcliffe JC. Neurofibrillary tangles, but not Alzheimer-type pathology, in a young boxer. Neuropathol Appl Neurobiol 1996;22(01):12–16 [PubMed] [Google Scholar]
- 24.Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 2005;57(01):128–134, discussion 128–134 [DOI] [PubMed] [Google Scholar]
- 25.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 2009;68(07):709–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 2012;4(134):134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013;136(Pt 1):43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein TD, Montenigro PH, Alvarez VE, et al. Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol 2015;130(01):21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montine TJ, Phelps CH, Beach TG, et al. ; National Institute on Aging; Alzheimer’s Association. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic as-sessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 2012;123(01):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 2011;70(11):960–969 [DOI] [PubMed] [Google Scholar]
- 31.Lee EB, Kinch K, Johnson VE, Trojanowski JQ, Smith DH, Stewart W. Chronic traumatic encephalopathy is a common co-morbidity, but less frequent primary dementia in former soccer and rugby players. Acta Neuropathol 2019;138(03):389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keable A, Fenna K, Yuen HM, et al. Deposition of amyloid β in the walls of human leptomeningeal arteries in relation to perivascular drainage pathways in cerebral amyloid angiopathy. Biochim Biophys Acta 2016;1862(05):1037–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charidimou A, Boulouis G, Roongpiboonsopit D, et al. Cortical superficial siderosis multifocality in cerebral amyloid angiopathy: a prospective study. Neurology 2017;89(21):2128–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauer A, van Veluw SJ, William CM, et al. Microbleeds on MRI are associated with microinfarcts on autopsy in cerebral amyloid angiopathy. Neurology 2016;87(14):1488–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Attems J, Jellinger KA, Lintner F. Alzheimer’s disease pathology influences severity and topographical distribution of cerebral amyloid angiopathy. Acta Neuropathol 2005;110(03):222–231 [DOI] [PubMed] [Google Scholar]
- 36.Pfeifer LA, White LR, Ross GW, Petrovitch H, Launer LJ. Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study. Neurology 2002;58(11):1629–1634 [DOI] [PubMed] [Google Scholar]
- 37.Tian J, Shi J, Bailey K, Mann DMA. Negative association between amyloid plaques and cerebral amyloid angiopathy in Alzheimer’s disease. Neurosci Lett 2003;352(02):137–140 [DOI] [PubMed] [Google Scholar]
- 38.Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke 1987;18(02):311–324 [DOI] [PubMed] [Google Scholar]
- 39.Tian J, Shi J, Bailey K, Mann DMA. Relationships between arteriosclerosis, cerebral amyloid angiopathy and myelin loss from cerebral cortical white matter in Alzheimer’s disease. Neuropathol Appl Neurobiol 2004;30(01):46–56 [DOI] [PubMed] [Google Scholar]
- 40.Attems J, Jellinger K, Thal DR, Van Nostrand W. Review: sporadic cerebral amyloid angiopathy. Neuropathol Appl Neurobiol 2011;37(01):75–93 [DOI] [PubMed] [Google Scholar]
- 41.Itoh Y, Yamada M, Hayakawa M, Otomo E, Miyatake T. Cerebral amyloid angiopathy: a significant cause of cerebellar as well as lobar cerebral hemorrhage in the elderly. J Neurol Sci 1993;116(02):135–141 [DOI] [PubMed] [Google Scholar]
- 42.Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry 2012;83(02):124–137 [DOI] [PubMed] [Google Scholar]
- 43.Keage HAD, Carare RO, Friedland RP, et al. Population studies of sporadic cerebral amyloid angiopathy and dementia: a systematic review. BMC Neurol 2009;9(01):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leclercq PD, Murray LS, Smith C, Graham DI, Nicoll JA, Gentleman SM. Cerebral amyloid angiopathy in traumatic brain injury: association with apolipoprotein E genotype. J Neurol Neurosurg Psychiatry 2005;76(02):229–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weissberg I, Veksler R, Kamintsky L, et al. Imaging blood-brain barrier dysfunction in football players. JAMA Neurol 2014;71(11):1453–1455 [DOI] [PubMed] [Google Scholar]
- 46.Standring OJ, Friedberg J, Tripodis Y, et al. Contact sport participation and chronic traumatic encephalopathy are associated with altered severity and distribution of cerebral amyloid angiopathy. Acta Neuropathol 2019;138(03):401–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Broglio SP, Sosnoff JJ, Shin S, He X, Alcaraz C, Zimmerman J. Head impacts during high school football: a biomechanical assess-ment. J Athl Train 2009;44(04):342–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crisco JJ, Fiore R, Beckwith JG, et al. Frequency and location of head impact exposures in individual collegiate football players. J Athl Train 2010;45(06):549–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghajari M, Hellyer PJ, Sharp DJ. Computational modelling of traumatic brain injury predicts the location of chronic traumatic encephalopathy pathology. Brain 2017;140(02):333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parkinson J An essay on the shaking palsy. 1817. J Neuropsychi-atry Clin Neurosci 2002;14(02):223–236, discussion 222 [DOI] [PubMed] [Google Scholar]
- 51.Gardner RC, Burke JF, Nettiksimmons J, Goldman S, Tanner CM, Yaffe K. Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol 2015;77(06):987–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perry DC, Sturm VE, Peterson MJ, et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J Neurosurg 2016;124(02):511–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee P-C, Bordelon Y, Bronstein J, Ritz B. Traumatic brain injury, paraquat exposure, and their relationship to Parkinson disease. Neurology 2012;79(20):2061–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jafari S, Etminan M, Aminzadeh F, Samii A. Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Mov Disord 2013;28(09):1222–1229 [DOI] [PubMed] [Google Scholar]
- 55.Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology 2013;81(13):1122–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montenigro PH, Corp DT, Stein TD, Cantu RC, Stern RA. Chronic traumatic encephalopathy: historical origins and current perspective. Annu Rev Clin Psychol 2015;11(January):309–330 [DOI] [PubMed] [Google Scholar]
- 57.Tarazi A, Tator CH, Wennberg R, et al. Motor function in former professional football players with history of multiple concussions. J Neurotrauma 2018;35(08):1003–1007 [DOI] [PubMed] [Google Scholar]
- 58.Adams JW, Alvarez VE, Mez J, et al. Lewy body pathology and chronic traumatic encephalopathy associated with contact sports. J Neuropathol Exp Neurol 2018;77(09):757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weisskopf MG, Cudkowicz ME, Johnson N. Military service and amyotrophic lateral sclerosis in a population-based cohort. Epidemiology 2015;26(06):831–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cragg JJ, Johnson NJ, Weisskopf MG. Military service and amyotrophic lateral sclerosis in a population-based cohort: extended follow-up 1979-2011. Epidemiology 2017;28(02):e15–e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired National Football League players. Neurology 2012;79(19):1970–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brady CB, Trevor KT, Stein TD, et al. The Department of Veterans Affairs Biorepository Brain Bank: a national resource for amyotrophic lateral sclerosis research. Amyotroph Lateral Scler Frontotemporal Degener 2013;14(7-8):591–597 [DOI] [PubMed] [Google Scholar]
- 63.Mez J, Daneshvar DH, Kiernan PT, et al. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 2017;318(04):360–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen H, Richard M, Sandler DP, Umbach DM, Kamel F. Head injury and amyotrophic lateral sclerosis. Am J Epidemiol 2007;166(07):810–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fournier CN, Gearing M, Upadhyayula SR, Klein M, Glass JD. Head injury does not alter disease progression or neuropathologic outcomes in ALS. Neurology 2015;84(17):1788–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmidt S, Kwee LC, Allen KD, Oddone EZ. Association of ALS with head injury, cigarette smoking and APOE genotypes. J Neurol Sci 2010;291(1-2):22–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walt GS, Burris HM, Brady CB, et al. Chronic traumatic encephalopathy within an amyotrophic lateral sclerosis brain bank cohort. J Neuropathol Exp Neurol 2018;77(12):1091–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keene CD, Latimer CS, Steele LM, Mac Donald CL. First confirmed case of chronic traumatic encephalopathy in a professional bull rider. Acta Neuropathol 2018;135(02):303–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brettschneider J, Del Tredici K, Toledo JB, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol 2013;74(01):20–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alosco ML, Duskin J, Besser LM, et al. Modeling the relationships among late-life body mass index, cerebrovascular disease, and Alzheimer’s disease neuropathology in an autopsy sample of 1,421 subjects from the National Alzheimer’s Coordinating Center data set. J Alzheimers Dis 2017;57(03):953–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deramecourt V, Slade JY, Oakley AE, et al. Staging and natural history of cerebrovascular pathology in dementia. Neurology 2012;78(14):1043–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kalaria RN, Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord 1999;13(Suppl 3):S115–S123 [DOI] [PubMed] [Google Scholar]
- 73.Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim Biophys Acta 2016;1862(05):887–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Toledo JB, Arnold SE, Raible K, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 2013;136(Pt 9):2697–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol 2016;15(09):934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baron SL, Hein MJ, Lehman E, Gersic CM. Body mass index, playing position, race, and the cardiovascular mortality of retired professional football players. Am J Cardiol 2012;109(06):889–896 [DOI] [PubMed] [Google Scholar]
- 77.Tucker AM, Vogel RA, Lincoln AE, et al. Prevalence of cardiovascular disease risk factors among National Football League players. JAMA 2009;301(20):2111–2119 [DOI] [PubMed] [Google Scholar]
- 78.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med 1973;3(03):270–303 [DOI] [PubMed] [Google Scholar]
- 79.Buée L, Hof PR, Bouras C, et al. Pathological alterations of the cerebral microvasculature in Alzheimer’s disease and related dementing disorders. Acta Neuropathol 1994;87(05):469–480 [DOI] [PubMed] [Google Scholar]
- 80.Doherty CP, O’Keefe E, Wallace E, et al. Blood-brain barrier dysfunction as a hallmark pathology in chronic traumatic encephalopathy. J Neuropathol Exp Neurol 2016;75(07):656–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alosco ML, Stein TD,Tripodis Y, et al. Association of white matter rarefaction, arteriolosclerosis, and tau with dementia in chronic traumatic encephalopathy. JAMA Neurol 2019(August) Doi: 10.1001/jamaneurol.2019.2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kovacs GG. Invited review: neuropathology of tauopathies: principles and practice. Neuropathol Appl Neurobiol 2015;41(01):3–23 [DOI] [PubMed] [Google Scholar]
- 83.Spillantini MG, Goedert M, Crowther RA, Murrell JR, Farlow MR, Ghetti B. Familial multiple system tauopathy with presenile dementia: a disease with abundant neuronal and glial tau filaments. Proc Natl Acad Sci U S A 1997;94(08):4113–4118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bouras C, Hof PR, Morrison JH. Neurofibrillary tangle densities in the hippocampal formation in a non-demented population define subgroups of patients with differential early pathologic changes. Neurosci Lett 1993;153(02):131–135 [DOI] [PubMed] [Google Scholar]
- 85.Braak H, Del Tredici K. Evolutional aspects of Alzheimer’s disease pathogenesis. J Alzheimers Dis 2013;33(Suppl 1):S155–S161 [DOI] [PubMed] [Google Scholar]
- 86.Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 2014;128(06):755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Takao M, Hirose N, Arai Y, Mihara B, Mimura M. Neuropathology of supercentenarians - four autopsy case studies. Acta Neuropathol Commun 2016;4(01):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Besser LM, Crary JF, Mock C, Kukull WA. Comparison of symptomatic and asymptomatic persons with primary age-related tauopathy. Neurology 2017;89(16):1707–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abner EL, Kryscio RJ, Schmitt FA, et al. Outcomes after diagnosis of mild cognitive impairment in a large autopsy series. Ann Neurol 2017;81(04):549–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cerami C, Dodich A, Iannaccone S, et al. A biomarker study in long-lasting amnestic mild cognitive impairment. Alzheimers Res Ther 2018;10(01):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bell WR, An Y, Kageyama Y, et al. Neuropathologic, genetic, and longitudinal cognitive profiles in primary age-related tauopathy (PART) and Alzheimer’s disease. Alzheimers Dement 2019;15(θ1):8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Besser LM, Mock C, Teylan MA, Hassenstab J, Kukull WA, Crary JF. Differences in cognitive impairment in primary age-related tauopathy versus Alzheimer disease. J Neuropathol Exp Neurol 2019;78(03):219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jefferson-George KS, Wolk DA, Lee EB, McMillan CT. Cognitive decline associated with pathological burden in primary age-related tauopathy. Alzheimers Dement 2017;13(09):1048–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McMillan CT, Lee EB, Jefferson-George K, et al. Alzheimer’s genetic risk is reduced in primary age-related tauopathy: a potential model of resistance? Ann Clin Transl Neurol 2018;5(08):927–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Santa-Maria I, Haggiagi A, Liu X, et al. The MAPT H1 haplotype is associated with tangle-predominant dementia. Acta Neuropathol 2012;124(05):693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Janocko NJ, Brodersen KA, Soto-Ortolaza AI, et al. Neuropathologically defined subtypes of Alzheimer’s disease differ significantly from neurofibrillary tangle-predominant dementia. Acta Neuropathol 2012;124(05):681–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kovacs GG, Ferrer I, Grinberg LT, et al. Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol 2016;131(01):87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Forrest SL, Kril JJ, Wagner S, et al. Chronic traumatic encephalopathy (CTE) is absent from a European community-based aging cohort while cortical aging-related tau astrogliopathy (ARTAG) is highly prevalent. J Neuropathol Exp Neurol 2019;78(05):398–405 [DOI] [PubMed] [Google Scholar]
- 99.Yamada T, McGeer PL, McGeer EG. Appearance of paired nucleated, Tau-positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci Lett 1992;135(01):99–102 [DOI] [PubMed] [Google Scholar]
- 100.Feany MB, Dickson DW. Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 1995;146(06):1388–1396 [PMC free article] [PubMed] [Google Scholar]
- 101.Kovacs GG, Xie SX, Robinson JL, et al. Sequential stages and distribution patterns of aging-related tau astrogliopathy (ARTAG) in the human brain. Acta Neuropathol Commun 2018;6(01):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kovacs GG, Robinson JL, Xie SX, et al. Evaluating the patterns of aging-related tau astrogliopathy unravels novel insights into brain aging and neurodegenerative diseases. J Neuropathol Exp Neurol 2017;76(04):270–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Munoz DG, Ros R, Fatas M, Bermejo F, de Yebenes JG. Progressive nonfluent aphasia associated with a new mutation V363I in tau gene. Am J Alzheimers Dis Other Demen 2007;22(04):294–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mesulam M, Wicklund A, Johnson N, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol 2008;63(06):709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bigio EH, Mishra M, Hatanpaa KJ, et al. TDP-43 pathology in primary progressive aphasia and frontotemporal dementia with pathologic Alzheimer disease. Acta Neuropathol 2010;120(01):43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Robinson JL, Corrada MM, Kovacs GG, et al. Non-Alzheimer’s contributions to dementia and cognitive resilience in The 90+ Study. Acta Neuropathol 2018;136(03):377–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morgello S, Jacobs M, Murray J, et al. Alzheimer’s disease neuropathology may not predict functional impairment in HIV: a report of two individuals. J Neurovirol 2018;24(05):629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gómez-Tortosa E, Baradaran-Heravi Y, González Alvarez V, et al. ; EU EOD Consortium. Presence of tau astrogliopathy in frontotemporal dementia caused by a novel Grn nonsense (Trp2*) mutation. Neurobiol Aging 2019;76:214.e11–214.e15 [DOI] [PubMed] [Google Scholar]
- 109.Baskota SU, Lopez OL, Greenamyre JT, Kofler J. Spectrum of tau pathologies in Huntington’s disease. Lab Invest 2019;99(07):1068–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hsu ET, Gangolli M, Su S, et al. Astrocytic degeneration in chronic traumatic encephalopathy. Acta Neuropathol 2018;136(06):955–972 [DOI] [PubMed] [Google Scholar]
- 111.Braak H, Braak E. Argyrophilic grains: characteristic pathologyof cerebral cortex in cases of adult onset dementia without Alzheimer changes. Neurosci Lett 1987;76(01):124–127 [DOI] [PubMed] [Google Scholar]
- 112.Josephs KA, Whitwell JL, Parisi JE, et al. Argyrophilic grains: a distinct disease or an additive pathology? Neurobiol Aging 2008;29(04):566–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rodriguez RD, Grinberg LT. Argyrophilic grain disease: an underestimated tauopathy. Dement Neuropsychol 2015;9(01):2–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McKee AC, Stein TD, Kiernan PT, Alvarez VE. The neuropathology of chronic traumatic encephalopathy. Brain Pathol 2015;25(03):350–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Armstrong RA, McKee AC, Stein TD, Alvarez VE, Cairns NJ. A quantitative study of tau pathology in 11 cases of chronic traumatic encephalopathy. Neuropathol Appl Neurobiol 2017;43(02):154–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ling H, Kara E, Revesz T, et al. Concomitant progressive supranuclear palsy and chronic traumatic encephalopathy in a boxer. Acta Neuropathol Commun 2014;2(01):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Golbe LI, Rubin RS, Cody RP, et al. Follow-up study of risk factors in progressive supranuclear palsy. Neurology 1996;47(01):148–154 [DOI] [PubMed] [Google Scholar]
- 118.Nemani SK, Notari S, Cali I, et al. Co-occurrence of chronic traumatic encephalopathy and prion disease. Acta Neuropathol Commun 2018;6(01):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Koga S, Dickson DW, Bieniek KF. Chronic traumatic encephalopathy pathology in multiple system atrophy. J Neuropathol Exp Neurol 2016;75(10):963–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Farrell M, Aherne S, O’Riordan S, O’Keeffe E, Greene C, Campbell M. Blood-brain barrier dysfunction in a boxer with chronic traumatic encephalopathy and schizophrenia. Clin Neuropathol 2019;38(02):51–58 [DOI] [PubMed] [Google Scholar]
- 121.Molloy C, Conroy RM, Cotter DR, Cannon M. Is traumatic brain injury a risk factor for schizophrenia? A meta-analysis of case-controlled population-based studies. Schizophr Bull 2011;37(06):1104–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stein TD, Alvarez VE, McKee AC. Concussion in chronic traumatic encephalopathy. Curr Pain Headache Rep 2015;19(10):47. [DOI] [PMC free article] [PubMed] [Google Scholar]