Abstract
Burnout is a form of negative emotional and physical response to job stress. This study aimed to investigate the prevalence of burnout among healthcare workers responding to the coronavirus disease 2019 (COVID-19) outbreak in Korea and to explore correlates of burnout among healthcare workers. A nationwide questionnaire-based survey was conducted from December 1, 2020, to January 29, 2021 on 1425 healthcare workers who worked in one of the 16 healthcare facilities designated for COVID-19 care, in public health centers, or as paramedics in Korea. Burnout was assessed using 16 Korean-adapted items based on the Oldenburg Burnout Inventory (OLBI). Data were collected using a structured questionnaire and analyzed using the R version 4.1.1 software program. OLBI results indicate clinically exhaustion in 84.5% (1204/1425) and clinically disengagement in 91.1% (1298/1425), and 77.3% (1102/1425) met the score criteria for both the exhaustion and disengagement subscales for burnout. Burnout rate was significantly increased in the group with chronic fatigue symptoms (Fatigue Severity Scale ≥ 3.22) after the outbreak of COVID-19 (OR, 3.94; 95% CI 2.80–5.56), in the female group (OR, 2.05; 95% CI 1.46–2.86), in the group with physical symptoms (Patient Health Questionnaire-15 ≥ 10) after the outbreak of COVID-19 (OR, 2.03; 95% CI 1.14–3.60), in the group with a higher Global Assessment of Recent Stress scale (OR, 1.71; 95% CI 1.46–2.01), in the group with post-traumatic stress symptoms (Primary Care Post-Traumatic Stress Disorder-5 ≥ 2) (OR, 1.47; 95% CI 1.08–2.01), and in the younger age group(OR, 1.45; 95% CI 1.22–1.72). The chronic fatigue symptoms were correlated with cumulative days of care (OR, 1.18; 95% CI 1.02–1.37). The physical symptoms were correlated with average contact hours with COVID-19 patients per day (OR, 1.34; 95% CI 1.17–1.54), and cumulative days of care (OR, 1.21; 95% CI 1.06–1.38). Most Korean healthcare workers suffered from burnout related to excessive workload during the COVID-19 pandemic. During a widespread health crisis like COVID-19, it is necessary to regularly check the burnout status in healthcare workers and reduce their excessive workload by supplementing the workforce and providing appropriate working hours sufficient rest hours.
Subject terms: Psychology, Diseases, Health care, Health occupations, Risk factors
Introduction
The World Health Organization (WHO) declared the coronavirus disease 2019 (COVID-19) as a pandemic on March 11, 2020; subsequently, more than 300 million confirmed cases of COVID-19 were reported worldwide by January 1, 20221,2. With the prolonged nature of the COVID-19 pandemic, across the globe, healthcare workers involved in COVID-19 care have hit their physical and mental limits. A healthcare system’s collapse due to a pandemic caused by a novel infectious disease, such as COVID-19, can expose healthcare workers to stress. During the H1N1 pandemic, nurses involved in patient care developed mental health conditions, such as stress, anxiety, depression, and hostility. They avoided being involved in caring for patients with the infectious disease due to the fear of exposing themselves or their families to the virus and the consequent mental stress3. During the severe acute respiratory syndrome (SARS) pandemic, nurses caring for patients with SARS had higher levels of stress due to the use of personal protection equipment (PPE), risk of virus exposure, infectious disease management, infection management protocol, and patients’ demands4. As the COVID-19 pandemic continues, studies on depression, anxiety, and stress of healthcare workers related to COVID-19 have been conducted in several countries5–8. Since these workers often face novel and difficult situations, various symptoms ranging from psychological distress to mental disorders can occur, and policies to prevent these symptoms are urgently needed9,10.
“Burnout” is a form of negative emotional and physical response to job stress that was first described in 1974 by a German American psychologist named Freudenberger11. It can occur when members of an organization do not receive the expected reward or can no longer manage their stress due to excessively committing to interpersonal relationships in the workplace. The Job demands-resources (JD-R) model suggests that work-related burnout progresses through two mechanisms. The first one is related to ‘exhaustion’ from excessive job demands and the second one is related to ‘disengagement’ from lack of job resources12. JD-R model proposes job demands, such as high workload, time pressure, and emotional demands, may initiate the processes of losing energy and impairing health, which in turn lead to chronic exhaustion and burnout12,13. During an outbreak of a novel infectious disease such as COVID-19, healthcare workers may be exposed to exhaustion and burnout due to increased job demands such as staff shortage and complicated protocols14. A high level of burnout among healthcare staff involved in COVID-19 care has already been documented in multiple countries that have experienced a collapse of the healthcare system due to the COVID-19 pandemic, including the United States, India, Italy, the United Kingdom, and Singapore15–19. The burnout of healthcare workers can negatively affect their relationship with patients, reduce the quality of medical services, and negatively affect their personal lives, such as turnover20.
At the beginning of the COVID-19 pandemic, South Korea’s strong national response to COVID-19 provided several important lessons for other countries21,22. The Korean government designated hospitals for the care of COVID-19 patients centered on public hospitals, and quickly created negative-pressure isolation rooms using portable negative-pressure devices so that all patients diagnosed with COVID-19 at the pandemic could be hospitalized21. The government tried to recruit unpaid volunteers, and hospitals recruited new employees, but it was difficult to solve the shortage of healthcare workers. The excessive workload required for the success of containment measures has led to the burnout of healthcare workers23. This study is the first large-scale nationwide study conducted in Korea to determine the prevalence of burnout in healthcare workers during the COVID-19 pandemic and analyze predictive factors for it.
Method
Participants and sample size
The study population comprised physicians, nurses, other healthcare workers, public health center staff, and epidemiologists, who worked in one of the 16 healthcare facilities designated for COVID-19 care and public emergency medical service workers between January 20, 2020, and December 1, 2020. Table 1 is a list of participating medical institutions nationwide.
Table 1.
List of participating medical institutions by region.
| City or Province | Number | Name of Medical Institution |
|---|---|---|
| Seoul Metropolitan City | 6 | Gangbuk Samsung Hospital |
| Hanyang University Seoul Hospital | ||
| Konkuk University Hospital | ||
| SoonChunHyang University Seoul Hospital | ||
| Seoul National University Hospital | ||
| National Medical Center | ||
| Daegu Metropolitan City | 3 | Kyongpook National University Hospital |
| Kyongpook National University Chilgok Hospital | ||
| Daegu Medical Center | ||
| Busan Metropolitan City | 1 | Pusan National University Hospital |
| Daejeon Metropolitan City | 1 | Konyang University Hospital |
| Gwangju Metropolitan City | 1 | Chonnam National University Hospital |
| Gangwon Province | 1 | Kangwon National University Hospital |
| Gyeongsangbuk Province | 1 | Andong Medical Center |
| Gyeongsangnam Province | 1 | Masan Medical Center |
| Jeju Special Self-Governing Province | 1 | Jeju National University Hospital |
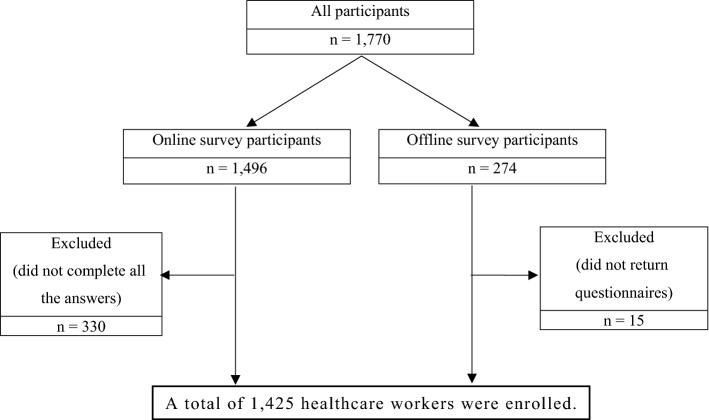
Considering the sampling accuracy and economic cost of increasing the sample size, the optimal population sample size was approximately 1000–1500 people24. The target sample size for each job group was determined to be 150 physicians, 700 nurses, 300 other workers (e.g., clinical pathologists, radiologic technologists, cleaning staff, and administrative staff) in consideration of the number of healthcare workers involved in COVID-19-related work. In the case of emergency medical service workers working outside the healthcare facilities, about 300 people were set as the target sample, and with the help of the National Emergency Management Agency, an online survey website link was sent through a message. Finally, 1166 of 1496 people who participated in the online survey completed all the answers, and 259 of 274 people who received the offline questionnaire completed and returned it, resulting in a total 1425 responses analyzed (Fig. 1).
Figure 1.
Flow chart describes the study enrollment steps.
Study design
A text message with a link to an online survey web site was sent to potential candidates who worked in one of the 16 healthcare facilities designated for COVID-19 care, public health centers, or emergency medical services in Korea from December 1, 2020, to January 29, 2021. During the same period, a paper-and-pencil survey was sent by mail to those candidates who wanted an offline survey. The online questionnaire was accessed via the SurveyMonkey link, and those who read the information page and consented to participate were allowed to proceed with the questionnaire. The offline questionnaire was distributed along with a study consent form, and the completed questionnaires were collected either in person or via mail.
Measures
An supplementary note shows the questionnaire that was developed with items about the physical and mental impact of COVID-19 care during the COVID-19 pandemic [see Supplementary Note file]. Sociodemographic factors included gender, age, occupation, work location, and length of employment. For healthcare workers involved in COVID-19 care, the following were additionally surveyed: information about the number of confirmed patients they had provided care for, duration of COVID-19 care, duration of contact with COVID-19 patients, experiences with especially difficult patients, such as those critically or mentally ill, use of PPE, exposure to infection risk, and training for wearing and removing PPE. Physical and mental health assessment included instruments to assess perceived physical symptoms, chronic fatigue symptoms, post-traumatic stress disorder (PTSD) symptoms, depression symptoms, anxiety disorder, sleep disorder, burnout, perceive stress, positive resources, job satisfaction, and intent to practice nursing or turnover upon another outbreak of a novel infectious disease. The following scales were selected in reference to several previous studies on effective factors that determine the prevalence of burnout in healthcare workers6,10,18–20. In particular, the positive resource scale using the Positive Resources Test (POREST) was a protective factor in a previous study on burnout during the MERS epidemic in Korea25, but there are few studies on the positive resource and burnout. Therefore, we tried to investigate the effect of positive resources on burnout using POREST in our study.
Perceived physical symptoms were assessed using the Patient Health Questionnaire-15 (PHQ-15), with 0 for “none,” 1 for “mild,” and 2 for “very severe.” A total score of 10 or higher indicated moderate physical symptoms26. Cronbach’s α was 0.80 in Kroenke et al.’s study26, and in this study, it was 0.82 for questions before COVID-19 and 0.88 for questions after COVID-19.
Chronic fatigue symptoms were assessed using the nine-item Fatigue Severity Scale (FSS). Each item was rated on a seven-point scale, and an average score of 3.22 or higher indicated fatigue27. Cronbach’s α was 0.93 in Krupp et al.’s study28, and in this study, it was 0.93 for questions before COVID-19 and 0.94 for questions after COVID-19.
PTSD symptoms were assessed using the Primary Care Post-Traumatic Stress Disorder-5 (PC-PTSD-5), with 0 for “no” and 1 for “yes.” A score of 2 was considered “moderate PTSD,” and a score of 3 or higher was considered “severe PTSD”29. In Jung et al.’s study, Cronbach’s α was 0.8729, and it was. 76 in this study.
Depression symptoms were assessed using the Korean version of the Patient Health Questionnaire-9 (PHQ-9), which asks about the frequency of symptoms in the previous two weeks using a scale with 0 for “none,” 1 for “two days or more,” 2 for “one week or longer,” and 3 for “almost every day.” A total score of 10 or higher was defined as “moderate or more severe depression symptoms”30,31. In Spitzer et al.’s study, Cronbach’s α was 0.9432, and in this study, it was 0.87 for the question before COVID-19 and 0.89 for the question after COVID-19.
Anxiety symptoms were assessed using the Generalized Anxiety Disorder Scale (GAD-7), with the same scoring criteria as that for depression (0–3). A score of 10 or higher was defined as “moderate or more severe anxiety symptoms”33,34. In Spitzer et al.’s study, Cronbach’s α was 0.9235, and in this study, it was 0.89 for both pre- and post-COVID-19 questions.
Insomnia was assessed using seven items in the Korean-adapted version (2013) of the Insomnia Severity Index developed by Bastien et al.36. Each item was rated on a 0–4 scale, and a total score of 16 or higher was defined as insomnia36,37. In Cho et al.38 Cronbach’s α was 0.92, and in this study it was 0.92 as well.
Stress was assessed using the Global Assessment of Recent Stress (GARS) scale, which measures perceived stressors in the previous week. Each of the eight items about work, school life, interpersonal relationships, changes in relationship, disease and injury, economic problems, unordinary events, changes in daily life, and overall perceived stress was rated on a scale from 0 (no stress at all) to 9 (extreme stress). A higher score indicated more significant perceived stress39. In Koh et al.’s study, Cronbach’s α was 0.8639, and in this study, it was 0.89.
Positive resources were assessed using the POREST developed by Kim et al. in 2013. The 23-item test comprised seven items for positivity, six for purpose and hope, five for self-control, three for social support, and two for caregiving and service. Each item was rated on a 1–5 scale, and the total score range was 23–115, where a higher score indicated greater positive resources40,41. In Chae et al.’s study, Cronbach’s α was 0.9242, and in this study, it was 0.92 as well.
Burnout was assessed using 16 Korean-adapted items by Na based on the Oldenburg Burnout Inventory (OLBI) developed by Demerouti, which comprises eight items for exhaustion and eight items for disengagement43–45. Each items rated on a 4-point Likert scale with options of “Strongly disagree,” “Disagree,” “Agree,” and “Strong agree,” and the burnout response is the highest with 4 points and the lowest with 1 point. Eight of the items are reverse-scored. The means were calculated for each items for two domains, exhaustion and disengagement. Burnout was determined with a cutoff score of 2.25 for exhaustion and 2.10 or higher for disengagement43. In Peterson’s study, Cronbach’s α was. 8343, and in this study, it was 0.90.
Data analysis
All analyses were conducted using the R version 4.1.1 software. The participants’ demographics and information assessing burnout were summarized using standard descriptive statistics. Descriptive statistics were also used to compare the burnout rate of groups according to demographic characteristics, presence of physical and psychological symptoms, and workload related COVID-19. Univariate logistic analysis of participants’ characteristics and information was performed to evaluate the predictive factors of burnout. The differences in categorical variables other than the stress and positive resources scores were analyzed using Pearson’s Chi-square test and Fisher’s exact test. The differences in the continuous variables (stress score and positive resources score) were analyzed using student t-tests or a Wilcoxon test. All factors with a p-value of less than 0.05 in univariate analysis were placed in a multivariable logistic regression analysis using a stepwise approach to investigate predictors of burnout. We also reported the receiver operating characteristic (ROC) curve of statistically significant variables after multivariable logistic regression analysis and calculate the area under curve (AUC) to evaluate the sensitivity and specificity of this logistic regression model in predicting the burnout. A p-value of less than 0.05 was interpreted as significant. Due to concerns about the validity of the analysis of burnout as categorical variable, correlations between the total score of each of the two domains of burnout and clinical variables were examined using Pearson’s r and Spearman’s rho. Multiple linear regression was used to control confounding variables and identify the predictors of higher burnout scores.
Institutional review board statement
The study was approved by the Public Institutional Review Board Designated by Ministry of Health and Welfare, Seoul, South Korea (IRB number: P-01-202011-23-001). Written informed consent was obtained from each participant who volunteered to participate after reading the information about the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Results
Burnout according to the demographic characteristics
Table 2 depicts the demographic characteristics and burnout rate of the participants. Among the 1,425 participants, 923 were women (64.8%), and more than 80% of the participants were aged 20–29 (n = 581, 40.8%) and 30–39 (n = 565, 39.6%) years. Most participants (n = 712, 50%) were nurses, and the majority worked in a university hospital (n = 741, 52.0%). The most common employment period was one to five years (n = 686, 48.1%), followed by six to ten years (n = 279, 19.6%), and less than one year (n = 175, 12.3%).
Table 2.
Characteristics of the study population and burnout rate according to the characteristics.
| Variable | Total (N = 1425) |
Burnout (N = 1102) |
No Burnout (N = 323) |
P-value |
|---|---|---|---|---|
| Gender | P < 0.001 | |||
| Men | 502 | 309 (61.6%) | 193 (38.4%) | |
| Women | 923 | 793 (85.9%) | 130 (14.1%) | |
| Age group | P < 0.001 | |||
| 20–29 years | 581 | 480 (82.6%) | 101 (17.4%) | |
| 30–39 years | 565 | 440 (77.9%) | 125 (22.1%) | |
| 40–49 years | 173 | 123 (71.1%) | 50 (28.9%) | |
| 50–59 years | 90 | 51 (56.7%) | 39 (43.3%) | |
| > 60 years | 16 | 8 (50.0%) | 8 (50.0%) | |
| Current region of work | P < 0.001 | |||
| Seoul metropolitan area | 704 | 545 (77.4%) | 159 (22.6%) | |
| Gangwon | 47 | 38 (80.9%) | 9 (19.1%) | |
| Chungcheong | 110 | 82 (74.5%) | 28 (25.5%) | |
| Jeolla | 71 | 31 (43.7%) | 40 (56.3%) | |
| Gyeongsang | 485 | 400 (82.5%) | 85 (17.5%) | |
| Jeju | 8 | 6 (75%) | 2 (25%) | |
| Type of facility | P < 0.001 | |||
| National/public medical center | 320 | 254 (79.4%) | 66 (20.6%) | |
| University hospital (Tertiary hospital) | 741 | 614 (82.9%) | 127 (17.1%) | |
| Public emergency medical service | 326 | 203 (62.3%) | 123 (37.7%) | |
| Others | 38 | 31 (81.6%) | 7 (18.4%) | |
| Length of current employment | 0.018 | |||
| < 1 year | 175 | 121 (69.1%) | 54 (30.9%) | |
| 1–5 years | 686 | 544 (79.3%) | 142 (20.7%) | |
| 6–10 years | 279 | 223 (79.9%) | 56 (20.1%) | |
| > 10 years | 285 | 214 (75.1%) | 71 (24.9%) | |
| Occupation/profession | P < 0.001 | |||
| Physician | 167 | 128 (76.6%) | 39 (23.4%) | |
| Nurse | 712 | 633 (88.9%) | 79 (11.1%) | |
| Paramedic | 297 | 184 (62.0%) | 113 (38.0%) | |
| Nurse aid, transport staff, cleaning staff, and radiologic technologist | 135 | 77 (57.0%) | 58 (43.0%) | |
| Hospital administrative staff and others | 114 | 80 (70.2%) | 34 (29.8%) |
A total of 1204 (84.5%) participants had an exhaustion score of 2.25 or higher, and 1,298 (91.1%) had a disengagement score of 2.1 or higher. Moreover, 1102 participants (77.3%) met the score criteria for both subscales for burnout. The burnout rate was higher among women (793/923, 85.9%) than men (309/502, 61.6%), and higher among the 20–29 years age group (480/581, 82.6%) than in the 30–39 years age group (440/565, 77.9%). Regarding employment duration, the burnout rate was high in the one to five years group (544/686, 79.3%) and in the six to ten years group (223/279, 79.9%). Regarding occupation, nurses had the highest burnout rate (633/712, 88.9%), followed by physicians (128/167, 76.6%), hospital administrative staff, epidemiologists, and civic servants (80/114, 70.2%), paramedics (184/297, 62.0%), nurse aids, transport staff, cleaning staff, radiologic technologists, and clinical pathologists (77/135, 57.0%).
An supplementary table about the burnout rate according to the job position and clinical career of physicians shows that among the doctors, the burnout rate was the highest among residents (71/82, 86.6%) [see Table S1 in Supplementary Tables file], and another table about burnout rate according to the job position and clinical career of nurses shows that among the nurses, the burnout rate was the highest among charge nurses (28/29, 96.6%), followed by staff nurses (556/612, 89.5%) [see Table S2 in Supplementary Tables file].
Burnout according to COVID-19-related work and work experience
Table 3 shows the burnout rate of healthcare workers according to COVID-19-related work. Among the total respondents, 1066 (74.8%) had direct contact with COVID-19 patients (e.g., nurse, doctor, transport staff, testing staff, and cleaning staff). Of these 1066 participants, 855 (80.2%) experienced burnout, which is a higher burnout rate than healthcare workers working without direct contact with COVID-19 patients (247/359, 68.8%). According to an average contact time with COVID-19 patients per day, the burnout rate was the highest among workers who had an average two to six hours of exposure to patients (311/349, 89.1%), and according to days of care for COVID-19 patients, the burnout rate was the highest in 90 days or longer group (368/440, 83.6%). According to work location, healthcare workers who worked in the COVID-19 ward (399/471, 84.7%) and worked in the emergency room (167/201, 83.1%) had a higher burnout rate than those who did not. Those who worked in the ambulance (156/228, 68.4%) had a lower burnout rate than those who did not. According to the work experience with difficult COVID-19 patients, the burnout rates of those with experience in caring for critically ill and mentally ill patients were 83.6% (398/476) and 83.7% (484/578), higher than those without experience. In addition, the burnout rates of those with experience wearing level D PPE and powered air-purifying respirators (PAPR) were 82.2% (525/639) and 84.5% (523/619), higher than those without experience.
Table 3.
Burnout rate according to COVID-19-related work experience.
| Variable | Total (N = 1425) |
Burnout (N = 1102) |
No Burnout (N = 323) |
P-value |
|---|---|---|---|---|
| Worked in direct contact with COVID-19 patients | P < 0.001 | |||
| Yes | 1066 | 855 (80.2%) | 211 (19.8%) | |
| No | 359 | 247 (68.8%) | 112 (31.2%) | |
| Currently working in direct contact with COVID-19 patients | 0.016 | |||
| Yes | 556 | 449 (80.8%) | 107 (19.2%) | |
| No | 869 | 653 (75.1%) | 216 (24.9%) | |
| Average contact time with COVID-19 patients per day | P < 0.001 | |||
| Not at all | 369 | 253 (68.6%) | 116 (61.4%) | |
| < 30 min | 269 | 205 (76.2%) | 64 (23.8%) | |
| 30 min–2 h | 349 | 261 (74.8%) | 88 (25.2%) | |
| 2–6 h | 349 | 311 (89.1%) | 38 (10.9%) | |
| > 6 h | 89 | 72 (80.9%) | 17 (19.1%) | |
| Number of COVID-19 patients cared for | P < 0.001 | |||
| 0 | 366 | 252 (68.9%) | 114 (31.1%) | |
| 1–10 | 409 | 324 (79.2%) | 85 (20.8%) | |
| 11–20 | 145 | 119 (82.1%) | 26 (17.9%) | |
| > 21 | 505 | 407 (80.6%) | 98 (19.4%) | |
| Days of care for COVID-19 patients | P < 0.001 | |||
| 0 | 373 | 257 (68.9%) | 116 (31.1%) | |
| 1–29 days | 403 | 309 (76.7%) | 94 (23.3%) | |
| 30–59 days | 115 | 92 (80.0%) | 23 (20.0%) | |
| 60–89 days | 94 | 76 (80.9%) | 18 (19.1%) | |
| ≥ 90 days | 440 | 368 (83.6%) | 72 (16.4%) | |
| Work location | ||||
| COVID-19 ward | P < 0.001 | |||
| Yes | 471 | 399 (84.7%) | 72 (15.3%) | |
| No | 954 | 703 (73.7%) | 251 (26.3%) | |
| Emergency room | 0.036 | |||
| Yes | 201 | 167 (83.1%) | 34 (16.9%) | |
| No | 1224 | 935 (76.4%) | 289 (23.6%) | |
| COVID-19 intensive care unit | 0.238 | |||
| Yes | 312 | 249 (79.8%) | 63 (20.2%) | |
| No | 1113 | 853 (76.6%) | 260 (23.4%) | |
| COVID-19 screening center | 0.544 | |||
| Yes | 163 | 123 (75.5%) | 40 (24.5%) | |
| No | 1262 | 979 (77.6%) | 283 (22.4%) | |
| COVID-19 community care center | 0.398 | |||
| Yes | 35 | 25 (71.4%) | 10 (28.6%) | |
| No | 1390 | 1077 (77.5%) | 313 (22.5%) | |
| Ambulance | P < 0.001 | |||
| Yes | 228 | 156 (68.4%) | 72 (31.6%) | |
| No | 1197 | 946 (79.0%) | 251 (21.0%) | |
| Work experience with difficult COVID-19 patients | ||||
| Critically ill | P < 0.001 | |||
| Yes | 476 | 398 (83.6%) | 78 (16.4%) | |
| No | 949 | 704 (74.2%) | 245 (25.8%) | |
| Dementia, delirium, other mental illness | P < 0.001 | |||
| Yes | 578 | 484 (83.7%) | 94 (16.3%) | |
| No | 847 | 618 (73.0%) | 229 (27.0%) | |
| Experience with wearing PPE | ||||
| Level D PPE* | P < 0.001 | |||
| Yes | 639 | 525 (82.2%) | 114 (17.8%) | |
| No | 786 | 577 (73.4%) | 209 (26.6%) | |
| PAPR** | P < 0.001 | |||
| Yes | 619 | 523 (84.5%) | 96 (15.5%) | |
| No | 806 | 579 (71.8%) | 227 (28.2%) | |
*PPE personal protection equipment.
**PAPR powered air-purifying respirator.
Burnout according to physical and psychological symptoms
Table 4 shows the burnout rate of healthcare workers according to physical, chronic fatigue, depression, and anxiety symptoms; mental disorder diagnosis, PTSD symptoms; GARS score; and positive resources before and after the COVID-19 outbreak. A total of 103 out of 110 participants (93.6%) who had physical symptoms (PHQ-15 ≥ 10) since before the outbreak, experienced burnout, and 407 out of 426 participants (95.5%) with physical symptoms (PHQ-15 ≥ 10) after the outbreak, experienced burnout. At any time before or after the onset of COVID-19, the burnout rate among healthcare workers with physical symptoms (PHQ-15 ≥ 10) was higher than those without physical symptoms (PHQ-15 ≥ 10). A total of 791 of 887 participants (89.2%) who had chronic fatigue symptoms (FSS ≥ 3.22) since before the outbreak experienced burnout, and 964 of 1089 participants (88.5%) with chronic fatigue symptoms (FSS ≥ 3.22) after the outbreak experienced burnout. Chronic fatigue symptoms, like physical symptoms, had a higher rate of burnout in those who had symptoms at any time before or after the onset of COVID-19. The burnout rates among those with depression symptoms (PHQ-9 ≥ 10) since before and after the outbreak were 94.4% (51/54) and 96.4% (268/278), respectively, and the burnout rate was 100% among those who had anxiety symptoms (GAD-7 ≥ 10) since before (n = 28) and after (n = 43) the outbreak. In total, burnout was reported by 39 of 41 (95.2%) and 18 of 20 (90.0%) participants diagnosed with a mental disorder before and after the outbreak, respectively. Regarding PTSD symptoms within the month before the survey period, 158 of 171 (92.4%) participants who experienced moderate PTSD symptoms (PC-PTSD-5 = 2), and 282 of 299 (94.3%) participants who experienced severe PTSD symptoms (PC-PTSD-5 ≥ 3), experienced burnout. The burnout rate among those with insomnia was 94.9% (277/292), while it was 72.8% (825/1,133) in those without insomnia.
Table 4.
Burnout rate according to the presence of physical and mental symptoms before and after the COVID-19 pandemic.
| Variable | Total (N = 1425) |
Burnout (N = 1102) |
No Burnout (N = 323) |
P-value |
|---|---|---|---|---|
| Physical symptoms (PHQ-15 ≥ 10) | ||||
| Before the COVID-19 pandemic | P < 0.001 | |||
| Yes | 110 | 103 (93.6%) | 7 (6.3%) | |
| No | 1315 | 999 (76.0%) | 316 (24.0%) | |
| After the COVID-19 pandemic | P < 0.001 | |||
| Yes | 426 | 407 (95.5%) | 19 (4.5%) | |
| No | 999 | 695 (69.6%) | 304 (30.4%) | |
| Chronic fatigue symptoms (FSS ≥ 3.22) | ||||
| Before the COVID-19 pandemic | P < 0.001 | |||
| Yes | 887 | 791 (89.2%) | 96 (10.8%) | |
| No | 538 | 311 (57.8%) | 227 (42.2%) | |
| After the COVID-19 pandemic | P < 0.001 | |||
| Yes | 1089 | 964 (88.5%) | 125 (11.5%) | |
| No | 336 | 138 (41.1%) | 198 (58.9%) | |
| Depression symptoms (PHQ-9 ≥ 10) | ||||
| Before the COVID-19 pandemic | 0.004 | |||
| Yes | 54 | 51 (94.4%) | 3 (5.6%) | |
| No | 1371 | 1051 (76.7%) | 320 (23.3%) | |
| After the COVID-19 pandemic | P < 0.001 | |||
| Yes | 278 | 268 (96.4%) | 10 (3.6%) | |
| No | 1147 | 834 (72.7%) | 282 (27.3%) | |
| Anxiety symptoms (GAD-7 ≥ 10) | ||||
| Before the COVID-19 pandemic | 0.004 | |||
| Yes | 28 | 28 (100%) | 0 (0.0%) | |
| No | 1397 | 1074 (76.9%) | 323 (23.1%) | |
| After the COVID-19 pandemic | P < 0.001 | |||
| Yes | 43 | 43 (100%) | 0 (0.0%) | |
| No | 1382 | 1059 (76.6%) | 323 (23.4%) | |
| Diagnosis of mental disorders before the COVID-19 pandemic | 0.006 | |||
| Yes | 41 | 39 (95.2%) | 2 (4.8%) | |
| No | 1384 | 1063 (76.8%) | 321 (23.2%) | |
| Diagnosis of mental disorders after the COVID-19 pandemic | 0.2793 | |||
| Yes | 20 | 18 (90.0%) | 2 (10.0%) | |
| No | 1405 | 1084 (77.2%) | 321 (22.8%) | |
| Symptoms of post-traumatic stress symptoms in the past month (PC-PTSD-5 ≥ 2) | P < 0.001 | |||
| Normal | 955 | 662 (69.3%) | 293 (30.7%) | |
| Mild-moderate | 171 | 158 (92.4%) | 13 (7.6%) | |
| Severe | 299 | 282 (94.3%) | 17 (5.7%) | |
| Insomnia | P < 0.001 | |||
| Yes | 292 | 277 (94.9%) | 15 (5.1%) | |
| No | 1133 | 825 (72.8%) | 308 (27.2%) | |
Burnout according to stress and positive resources
Table 5 shows the GARS and POREST scores according to burnout. The mean GARS score was 3.2 among 1102 participants with burnout, 1.7 points higher than that among 323 participants without burnout, indicating that those experiencing burnout had more significant perceived stress. In particular, the stress score for job in the burnout group was 4.7, the highest among all GARS subscale scores. The POREST score was 78.5 in the burnout group and 89.9 in the non-burnout group. The burnout group scored higher than the non-burnout group in all items of the POREST.
Table 5.
The mean (SD) GARS scale and POREST scores according to burnout.
| Variable | Total (N = 1425) Mean (SD) |
Burnout (N = 1102) Mean (SD) |
No Burnout (N = 323) Mean (SD) |
P-value |
|---|---|---|---|---|
| GARS scale | ||||
| Average | 2.9 (1.6) | 3.2 (1.5) | 1.7 (1.0) | P < 0.001 |
| Job, School | 4.2 (2.1) | 4.7 (1.9) | 2.5 (1.6) | P < 0.001 |
| Family interpersonal | 3.1 (2.1) | 3.5 (2.1) | 1.9 (1.5) | P < 0.001 |
| Changes in relationships | 2.7 (2.2) | 3.0 (2.3) | 1.6 (1.6) | P < 0.001 |
| Sickness, injury | 3.1 (2.2) | 3.5 (2.2) | 1.8 (1.7) | P < 0.001 |
| Financial | 2.9 (2.2) | 3.2 (2.2) | 1.9 (1.8) | P < 0.001 |
| Unusual events | 2.3 (2.0) | 2.5 (2.1) | 1.3 (1.4) | P < 0.001 |
| Change routine | 1.6 (1.7) | 1.8 (1.8) | 0.8 (1.0) | P < 0.001 |
| Overall global | 3.0 (2.1) | 3.5 (2.1) | 1.4 (1.3) | P < 0.001 |
| POREST scores | ||||
| Optimism | 24.7 (4.5) | 23.7 (4.2) | 28.1 (3.6) | P < 0.001 |
| Purpose and hope | 20.7 (3.9) | 20.0 (3.9) | 22.7 (3.5) | P < 0.001 |
| Self-control | 16.9 (3.2) | 16.4 (3.1) | 18.9 (2.7) | P < 0.001 |
| Social resource support | 11.5 (2.1) | 11.3 (2.2) | 12.4 (1.8) | P < 0.001 |
| Care | 7.3 (1.5) | 7.1 (1.5) | 7.8 (1.4) | P < 0.001 |
| Total | 81.1 (12.7) | 78.5 (12.1) | 89.9 (10.6) | P < 0.001 |
GARS global assessment of recent stress scale, POREST positive resources test.
Correlates of burnout
Table 6 shows the correlates of burnout using univariate and multivariable logistic regression. In this study, gender, age, physical symptoms and chronic fatigue symptoms after the COVID-19 outbreak, PTSD symptoms, and GARS score were related to burnout. In multiple regression model adjusted for other confounders, the odds for burnout were 2.05 times higher for women (95% confidence interval [CI] = 1.46–2.86; p < 0.001) than men and 1.45 times higher in younger groups than older ones (95% CI = 1.22–1.72; p < 0.001). The odds for burnout were 2.03 times higher (95% CI = 1.14–3.60; p = 0.016) among those who developed physical symptoms (PHQ-15 ≥ 10) after the COVID-19 outbreak and 3.94 times higher (95% CI 2.80–5.56; p < 0.001) among those with chronic fatigue symptoms (FSS ≥ 3.22; p < 0.001). The odds for burnout were 1.47 times higher (95% CI 1.08–2.01; p = 0.014) in the groups that experienced moderate or severe PTSD symptoms (PC-PTSD-5 ≥ 2) than in the non-PTSD group. The odds for burnout increased by 1.71 times (95% CI 1.46–2.01; p < 0.001) with a one-point increase in the GARS score. Regarding the factors of positive resources, optimism and caring were found to significantly reduce the risk for burnout, with a one-point increase in both optimism and caring decreasing the odds for burnout by 0.84 times (95% CI 0.80–0.88; p < 0.001) and 0.86 times (95% CI 0.77–0.99; p = 0.030), respectively. The AUC value of the multivariable logistic regression model was acceptable (0.893).
Table 6.
Logistic regression to identify the correlates of burnout.
| Variable | Univariate logistic regression analysis | Multivariable logistic regression analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Women (compared to men) | 3.81 (2.94, 4.93) | P < 0.001 | 2.05 (1.46, 2.86) | P < 0.001 |
| Age* | 1.48 (1.31, 1.69) | P < 0.001 | 1.45 (1.22, 1.72) | P < 0.001 |
| Physical symptoms** after COVID-19 pandemic | 9.37 (5.80, 15.13) | P < 0.001 | 2.03 (1.14, 3.60) | 0.016 |
| Chronic fatigue symptoms*** after COVID-19 pandemic | 11.07 (8.31, 17.73) | P < 0.001 | 3.94 (2.80, 5.56) | P < 0.001 |
| Post-traumatic stress symptoms**** | 3.09 (2.40, 3.98) | P < 0.001 | 1.47 (1.08, 2.01) | 0.014 |
| GARS Scale (for every 1-point increase) | 2.79 (2.42, 3.21) | P < 0.001 | 1.71 (1.46, 2.01) | P < 0.001 |
| Optimism score of POREST (for every 1-point increase) | 0.75 (0.72, 0.78) | P < 0.001 | 0.84 (0.80, 0.88) | P < 0.001 |
| Caring score of POREST (for every 1-point increase) | 0.74 (0.67, 0.81) | P < 0.001 | 0.87 (0.77, 0.99) | 0.030 |
CI confidence interval, GARS global assessment of recent stress scale, POREST positive resources test.
*60 years and older, 50–59 years, 40–49 years, 30–39 years, 20–29 years, as the age group decreases from the older age group to the lower age group.
**Physical symptoms mean a score of 10 or higher on the Patient Health Questionnaire-15.
***Chronic fatigue symptoms mean a score of 3.22 or higher on the Fatigue Severity Scale.
****Post-traumatic stress symptoms mean a score of 2 or higher on the Primary Care Post-Traumatic Stress Disorder-5 scale.
Physical (PHQ-15 ≥ 10) and chronic fatigue (FSS ≥ 3.22) symptoms after the COVID-19 outbreak according to patient care work
Tables 7 and 8 show the predictors of physical symptoms (PHQ-15 ≥ 10) and chronic fatigue symptoms (FSS ≥ 3.22) after the COVID-19 outbreak according to patient care work using univariate and multivariable logistic regression. The odds for physical symptoms (PHQ-15 ≥ 10) and chronic fatigue symptoms (FSS ≥ 3.22) after the COVID-19 outbreak were 2.04 times (95% CI = 1.52–2.78; p < 0.001) and 2.14 times (95% CI = 1.52–3.02; p < 0.001) higher among those who worked in medical institutions as frontline workers (COVID-19 ward, emergency room and COVID-19 intensive care unit) in multivariable logistic regression analysis.
Table 7.
Univariate logistic analysis of physical (PHQ-15 ≥ 10) and chronic fatigue (FSS ≥ 3.22) symptoms after the COVID-19 outbreak according to patient care work.
| Variable | Univariate logistic analysis | |||
|---|---|---|---|---|
| Physical symptoms | Chronic fatigue symptoms | |||
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Work location | ||||
| Medical institutions (COVID-19 ward, emergency room, COVID-19 intensive care unit) | 2.46 (1.93, 3.14) | P < 0.001 | 2.22 (1.73, 2.85) | P < 0.001 |
| Non-medical institutions (residential treatment center, ambulance, others) | 0.46 (0.34, 0.62) | P < 0.001 | 0.67 (0.51, 0.89) | 0.005 |
| Work experience with difficult COVID-19 patient | ||||
| Critically ill | 1.81 (1.43, 2.30) | P < 0.001 | 1.62 (1.23, 2.13) | P < 0.001 |
| Dementia, delirium, other mental illness | 2.16 (1.72, 2.73) | P < 0.001 | 1.91 (1.47, 2.49) | P < 0.001 |
| Number of COVID-19 patients cared for* | 1.24 (1.13, 1.36) | P < 0.001 | 1.21 (1.09, 1.34) | P < 0.001 |
| Days of care for COVID-19 patients** | 1.26 (1.17, 1.35) | P < 0.001 | 1.22 (1.13, 1.32) | P < 0.001 |
| Currently working in direct contact with COVID-19 patient | 1.81 (1.44, 2.28) | P < 0.001 | 1.61 (1.24, 2.09) | P < 0.001 |
| Average contact time with COVID-19 patients per day*** | 1.37 (1.25, 1.51) | P < 0.001 | 1.29 (1.17, 1.43) | P < 0.001 |
| Experience with wearing Level D PPE | 1.63 (1.29, 2.04) | P < 0.001 | 1.56 (1.21, 2.01) | P < 0.001 |
| Experience with wearing PAPR | 1.77 (1.41, 2.22) | P < 0.001 | 1.90 (1.47, 2.46) | P < 0.001 |
CI confidence interval, PPE personal protection equipment, PAPR powered air-purifying respirator.
*0, 1–10 patients, 11–20 patients, more than 20 patients, as the number of patients to care increases from a small group to a large group.
**0, 1–29 days, 30–59 days, 60–89 days, more than 90 days, as the number of days of care for COVID-19 patients increases from a small group to a large group.
***No, less than 30 min, 30 min–2 h, 2–6 h, more than 6 h, as the contact time with COVID-19 patients per day increases from a small group to a large group.
Table 8.
Multivariable logistic regression analysis of physical (PHQ-15 ≥ 10) and chronic fatigue (FSS ≥ 3.22) symptoms after the COVID-19 outbreak according to patient care work.
| Variable | Multivariable logistic analysis | |||
|---|---|---|---|---|
| Physical symptoms | Chronic fatigue symptoms | |||
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Work location | ||||
| Medical institutions (COVID-19 ward, emergency room, COVID-19 intensive care unit) | 2.04 (1.52, 2.78) | P < 0.001 | 2.14 (1.52, 3.02) | P < 0.001 |
| Number of COVID-19 patients cared for* | 0.81 (0.67, 0.94) | P = 0.026 | 0.82 (0.68, 0.99) | P = 0.034 |
| Days of care for COVID-19 patients** | 1.21 (1.06, 1.38) | P = 0.004 | 1.18 (1.02, 1.37) | P < 0.001 |
| Average contact time with COVID-19 patients per day*** | 1.34 (1.17, 1.54) | P < 0.001 | ||
CI confidence interval.
*0, 1–10 patients, 11–20 patients, more than 20 patients, as the number of patients to care increases from a small group to a large group.
**0, 1–29 days, 30–59 days, 60–89 days, more than 90 days, as the number of days of care for COVID-19 patients increases from a small group to a large group.
***No, less than 30 min, 30 min–2 h, 2–6 h, more than 6 h, as the contact time with COVID-19 patients per day increases from a small group to a large group.
Exhaustion score and disengagement score according to multiple variables
We analyzed correlation between scores for each of the two domains of burnout, exhaustion and disengagement, and clinical variables including demographic characteristics. Tables 9 and 10 show the strength of adjusted associations from linear regression analysis between the covariates and the score of each domains of burnout. The predictors explained 57.6% of exhaustion score and 46.9% of disengagement score. Chronic fatigue symptoms after the outbreak of COVID-19 had the strongest relationship with both exhaustion (standardized β = 1.96; p < 0.001) and disengagement score (standardized β = 1.89; p < 0.001). Consistent with the results in previous paragraphs, in addition to chronic fatigue symptoms after COVID-19, women, younger age, and GARS score were positively correlated with both exhaustion and disengagement score.
Table 9.
Multiple linear regression analyses predicting exhaustion score.
| Variable | Exhaustion multiple regression | |
|---|---|---|
| Standardized β (95% CI) | P-value | |
| Women (compared to men) | 0.70 (0.38, 1.02) | P < 0.001 |
| Age* | 0.45 (0.29, 0.60) | P < 0.001 |
| Physical symptoms** after COVID-19 pandemic | 0.51 (0.14, 0.88) | 0.007 |
| Chronic fatigue symptoms*** after COVID-19 pandemic | 1.96 (1.59, 2.33) | P < 0.001 |
| Post-traumatic stress symptoms**** | 0.28 (0.07, 0.48) | 0.009 |
| Depression symptoms***** before COVID-10 pandemic | − 1.14 (− 1.91, − 0.37) | 0.004 |
| Depression symptoms***** after COVID-10 pandemic | 0.88 (0.44, 1.33) | P < 0.001 |
| Presence of insomnia | 0.54 (0.15, 0.93) | 0.007 |
| GARS Scale (for every 1-point increase) | 0.58 (0.47, 0.69) | P < 0.001 |
| Work at COVID-19 ward (compared to other location) | 0.43 (0.11, 0.75) | 0.009 |
| Optimism score of POREST (for every 1-point increase) | − 0.23 (− 0.28, − 0.18) | P < 0.001 |
| Self-control score of POREST (for every 1-point increase) | − 0.13 (− 0.19, − 0.07) | P < 0.001 |
| Social resource support score of POREST (for every 1-point increase) | 0.11 (0.02, 0.19) | 0.018 |
| Adjusted R square | 0.576 | P < 0.001 |
CI confidence interval, GARS global assessment of recent stress scale, POREST positive resources test.
*60 years and older, 50–59 years, 40–49 years, 30–39 years, 20–29 years, as the age group decreases from the older age group to the lower age group.
**Physical symptoms mean a score of 10 or higher on the Patient Health Questionnaire-15.
***Chronic fatigue symptoms mean a score of 3.22 or higher on the Fatigue Severity Scale.
****Post-traumatic stress symptoms mean a score of 2 or higher on the Primary Care Post-Traumatic Stress Disorder-5 scale.
Table 10.
Multiple linear regression analyses predicting disengagement score.
| Variable | Disengagement multiple regression | |
|---|---|---|
| Standardized β (95% CI) | P-value | |
| Women (compared to men) | 0.64 (0.32, 0.96) | P < 0.001 |
| Age* | 0.49 (0.30, 0.68) | P < 0.001 |
| Length of current employment | ||
| 1–5 years | 0.46 (0.00, 0.92) | 0.052 |
| 6–10 years | 0.65 (0.12, 1.19) | 0.017 |
| > 10 years | 0.81 (0.24, 1.34) | 0.006 |
| Chronic fatigue symptoms** before COVID-19 pandemic | − 0.47 (− 0.89, − 0.05) | 0.027 |
| Chronic fatigue symptoms** after COVID-19 pandemic | 1.89 (1.39, 2.38) | P < 0.001 |
| Depression symptoms*** after COVID-10 pandemic | 0.90 (0.49, 1.32) | P < 0.001 |
| Anxiety symptoms**** before COVID-19 pandemic | − 1.68 (− 2.75, − 0.61) | 0.002 |
| GARS Scale (for every 1-point increase) | 0.41 (0.29, 0.52) | P < 0.001 |
| Presence of mental disorders before the COVID-19 pandemic | 1.10 (0.22, 1.98) | 0.015 |
| Optimism score of POREST (for every 1-point increase) | − 0.19 (− 0.24, − 0.13) | P < 0.001 |
| Purpose and hope score of POREST (for every 1-point increase) | − 0.20 (− 0.26, − 0.15) | P < 0.001 |
| Social resource support score of POREST (for every 1-point increase) | 0.23 (0.14, 0.33) | P < 0.001 |
| Caring score of POREST (for every 1-point increase) | − 0.28 (− 0.40, − 0.17) | P < 0.001 |
| Adjusted R square | 0.469 | P < 0.001 |
CI confidence interval, GARS global assessment of recent stress scale, POREST positive resources test.
*60 years and older, 50–59 years, 40–49 years, 30–39 years, 20–29 years, as the age group decreases from the older age group to the lower age group.
**Physical symptoms mean a score of 10 or higher on the Patient Health Questionnaire-15.
***Depression symptoms mean a score of 10 or higher on the Patient Health Questionnaire-9.
****Anxiety symptoms mean a score of 10 or higher on the General Anxiety Disorder-7.
Discussion
In our study, of 1425 healthcare workers, 1204 reported feeling exhausted (84.5%), and 1298 (91.1%) were disengaged; 1102 (77.3%) met both the criteria and were thus deemed to have burnout. These numbers are higher than the exhaustion (65.5%) and disengagement (79.5%) rates reported by 171 healthcare workers surveyed during the Middle East respiratory syndrome (MERS) epidemic in Korea from 2015 to 201625. In addition, the burnout rate in our study was higher than in previous COVID-19-related studies of healthcare workers conducted in Singapore, India, the United Kingdom, and Poland16,17,46,47. However, the burnout assessments for healthcare workers in those countries were performed in March–June 2020, approximately six months before our study. The duration of the MERS epidemic in Korea was less than three months (May 2015 to July 28, 2015). Taken together, these results suggest that healthcare worker burnout has been exacerbated by the prolonged duration of the COVID-19 pandemic. A recently published systematic review and meta-analysis on the psychological distress of healthcare workers treating COVID-19 patients in Asia also speculated that the high burnout rate among healthcare workers during the COVID-19 pandemic, compared to that during the SARS and MERS outbreaks, is due to the prolonged pandemic, as it has persisted for more than a year now48. In addition, a Canadian study on burnout among hospital healthcare workers, published in October 2021, reported that the burnout rate among healthcare workers surveyed in the spring of 2021 exceeded 60%, an increase from approximately 30–40% in the spring of 2020, highlighting the urgency of assessing organizational interventions and current systems, and discovering solutions to reduce burnout among healthcare workers49.
The burnout rate was higher among women than men in our study. Nurses were at greater risk of exposure to depression, anxiety, and stress because they were primarily involved in the direct care of COVID-19 patients compared to participants in other jobs. This could be attributable to the high-risk of burnout and since most nurses are women, it could explain the high burnout rate among women50,51. In addition, stress-related disorders, such as depression and anxiety disorder, are approximately two-fold higher among women than in men52,53, suggesting that women may be more vulnerable to depression, anxiety, and stress than men even in a similar environment, which may elevate their risk for burnout54.
The burnout rate increased with decreasing age in our study. A Chinese survey on healthcare workers treating COVID-19 patients also reported that the burnout rate was higher in the < 30-years age group than that in the 30–39-years and ≥ 40-years age groups55. A Turkish study of nurses during the COVID-19 pandemic also showed that the burnout rate increased with decreasing age56. The most significant reason for this result may be that younger nurses are less experienced, and the unfamiliarity of their tasks increases their stress and burnout. Another reason may be that younger individuals tend to be more involved in leisure activities and private social gatherings than their older counterparts, and their burnout may naturally be aggravated due to the restrictions imposed on these activities due to the COVID-19 pandemic57. In our study, 581 of 1425 participants (40.8%) were aged 20–29 years, which was the youngest age group of our study population, and 71 of them were physicians or nurses with a clinical career of less than one year, 55 of whom (77.5%) were found to experience burnout. Accordingly, in order to prevent turnover and burnout of new healthcare workers during a pandemic such as COVID-19, it is considered important to provide more education and training to them than trained healthcare workers, and periodically listen to their concerns to improve the work environment.
PTSD manifests as an extreme psychological response to a severe event wherein the individual feels traumatized by continuously reliving the experience. A Norwegian study on PTSD among 1773 healthcare workers during the COVID-19 pandemic reported that PTSD was significantly correlated with burnout58. A COVID-19-related study of 2579 healthcare workers in the United States also reported significant correlations among PTSD, burnout, difficulty with work, and interpersonal relationships59. A systematic review of 24 studies on PTSD among healthcare workers during SARS, MERS, and COVID-19 outbreaks showed that the risk for PTSD was higher among the frontline staff, those who work at wards with increased exposure to high-risk patients, and those with prolonged contact with patients60. The relationship between PTSD symptoms and burnout has been reported in several studies. Several studies have investigated the effect of PTSD on burnout61,62 and several studies have investigated the role of burnout in the development of PTSD63,64. As mentioned earlier, exposure to traumatic and stressful events can lead to the development of both PTSD symptoms and burnout. Therefore, PTSD symptoms and burnout are closely related but it may be difficult to define a causal relationship. Considering these results, it is necessary to develop measures to reduce exposure to infection risk to reduce both PTSD symptoms and burnout among healthcare workers during the COVID-19 pandemic and enforce regulations on the appropriate duration of contact with patients per healthcare worker.
The presence of Physical (PHQ-15 ≥ 10) and chronic fatigue symptoms (FSS ≥ 3.22) after the outbreak of COVID-19 were statistically significant associations of burnout in our study. Although we evaluated healthcare workers’ physical and chronic fatigue symptoms both since before and after the COVID-19 outbreak, but only the presence of physical and chronic fatigue symptoms after the COVID-19 outbreak are significant positive associations of burnout in a multivariable logistic regression analysis. To identify factors related to physical (PHQ-15 ≥ 10) and chronic fatigue symptoms (FSS ≥ 3.22) after the outbreak of COVID-19, we analyzed significant variables according to the COVID-19 patient care work in Tables 7 and 8. The risk for both symptoms was higher among those working in medical institutions such as the emergency department, intensive care unit (ICU), and COVID-19 ward––areas at high-risk of exposure to COVID-19 patients, with an increased number of days of care work, and increased daily average contact time with COVID-19 patients. Multivariable regression analysis showed that the greater the number of COVID-19 patients to be cared for, the lower the risk of physical symptoms (PHQ-15 ≥ 10) and chronic fatigue symptoms (FSS ≥ 3.22), but there is a limit to interpreting the number of COVID-19 patients to be cared for as proportional to the workload since we surveyed the cumulative number of patients to be cared for up to the time of the survey, not the number of patients to be cared for per day. In addition, when caring for critically ill or mentally ill patients, although the number of patients may be small, the workload may be higher because the severity of illness is high. Experiences with especially difficult patients, such as critically or mentally ill patients, and experiences with level D PPE or PAPR can also be risk predictors associated with physical and chronic fatigue symptoms. An Indian study conducted in December 2020 on the physiological effects of the use of N95 masks and PPE reported that among 75 healthcare workers who vigorously worked in a COVID-19 ICU while wearing an N95 mask and PPE for an average of 3.1 h a day, 90.1%, 70.7%, and 60% suffered from headache, fatigue, and dyspnea, respectively65. In addition, a Saudi Arabian survey of 1060 healthcare workers who worked at a hospital during the COVID-19 pandemic reported that the risk of headache increased with the increasing duration of PPE use in people with and without pre-existing headaches. Moreover, the use of PPE also provoked nausea, vomiting, sensitivity to light, sound, and motion, and throat discomfort66. In a study on burnout among healthcare workers who worked in a high-risk region in China during the COVID-19 pandemic, physical symptoms and acute stress were significantly correlated with emotional exhaustion and disengagement (cynicism), indicating burnout67, and a study in 2001 on the relationship between workload and burnout reported that excessive workload leads to emotional exhaustion, which in turn leads to disengagement (cynicism), which can trigger physical symptoms68. These results show that excessive workload can worsen physical symptoms, chronic fatigue symptoms, stress, and burnout, highlighting the urgent need to improve the current working environment. This aligns with the need for improvement of work environment, which is perceived by the participants as most important, as shown in an supplementary table [see Table S3 in Supplementary Tables file]. Thus, measures to lower excessive workload need to be implemented to reduce physical symptoms and chronic fatigue symptoms and ultimately to prevent burnout in healthcare workers treating COVID-19 patients; particularly, standard limits for patient contact and number of days of COVID-19 work need to be established for healthcare workers who work in direct contact with COVID-19 patients.
In this study, the optimism and caring score components of the POREST were identified as significant negative associations with burnout. This means that individuals who were more emotionally positive and had a greater tendency to try to help others had a lower degree of job burnout. The POREST measure was developed in Korea in 2018; although as yet there are few related data, a study on risk factors for burnout conducted in Korea during the MERS epidemic found that the lower the purpose and hope scores of the POREST, the higher the risk of burnout25. In another study conducted among 217 clinical nurses at a public hospital in Korea, the relationship between all positive resources of the POREST and burnout showed a significant negative correlation69. Therefore, to prevent job burnout of healthcare workers, it is also necessary to evaluate positive resources and develop programs that can increase positive resources.
Currently, countries worldwide are actively vaccinating citizens to eradicate the COVID-19 pandemic and are striving to develop effective therapeutics. Despite such endeavors, the COVID-19 pandemic persists, and healthcare workers responding to this threat are experiencing severe burnout. The WHO Regional Office of Europe published a report on the policies supporting healthcare workers treating COVID-19 patients in several countries in Europe in 2020. The report shows that Malta and Poland had already enforced policies to manage mental health, offered a financial reward, parenting support, and vacation compensation for healthcare workers treating COVID-19 patients since the initial days of the COVID-19 outbreak70. Korea had been preparing for the emerging infectious disease pandemic since the outbreak of MERS in 2015, but when the explosive COVID-19 outbreak at the epicenter occurred, there was a shortage of healthcare workers and medical facilities23. This shortage has resulted in work overload and burnout for healthcare workers. We need to accurately assess the state of burnout among healthcare workers involved in COVID-19 care and implement measures to reduce their burnout.
This study has the following limitations. First, the questionnaire was administered from December 1, 2020, to January 29, 2021, a period during the third wave, primarily throughout the Seoul metropolitan area with more than 1000 newly diagnosed cases every day. Despite that, only 556 out of 1425 (39%) healthcare workers were involved in COVID-19 care during the survey time. Healthcare workers actively overloaded with COVID-19-related care may not have participated in the survey, which is a limitation of a large-scale questionnaire survey wherein the respondents cannot be selected. Second, the burnout prevalence among Korean healthcare workers before the COVID-19 pandemic could not be assessed, and some questions from a past point in time (before COVID-19) were based on retrospective recalls of the past, there may be limitations. However, even if there is a memory bias for retrospective recalls, it is necessary to consider the physical and psychological symptoms before the outbreak of COVID-19 to more accurately identify risk factors for burnout due to the COVID-19 outbreak. Third, there is a confounding variable that influences both the independent and dependent variables. We used multivariable logistic regression analysis in this study to attempt to adjust as much as possible for potential confounding variables such as gender (e.g., nursing is a female dominated profession), but there may be limitations in interpreting the meaning of the results. In addition, some overlapping items in the self-questionnaire evaluating burnout, chronic fatigue symptoms, physical symptoms, depression symptoms and stress symptoms can increase their correlations with each other, and some variables with overlapping items for burnout can be overestimated as risk factors for burnout. In this study, it was not possible to ascertain causal relationship between burnout and the analyzed confounding variables (e.g., physical symptoms, chronic fatigue symptoms, depression symptoms and PTSD symptoms), and additional research will be needed. Finally, we did not assess the workload of healthcare workers who did not have direct contact with COVID-19 patients, such as COVID-19 epidemiologists and nursing civil servants at public health centers, since we only assessed workers involved with direct care of COVID-19 patients. Future research is needed to investigate healthcare workers who do not provide direct COVID-19 care but who experience burnout due to work overload.
Conclusion
This study aimed to assess burnout among healthcare workers due to the COVID-19 pandemic and identify burnout risk factors to develop measures to lower its prevalence in this population. The results confirmed that the risk of burnout was higher among women and younger workers. Further, the risk of burnout was higher among those who developed physical (PHQ-15 ≥ 10) and chronic fatigue (FSS ≥ 3.22 symptoms) after the outbreak of COVID-19, those with PTSD symptoms, and those with a higher GARS score while the risk was lower among those with high scores in the optimism and caring component of the POREST. To reduce these factors that aggravate burnout, periodically interviewing healthcare workers to determine the state of burnout and implementing educational and other programs that lower stress and enhance positivity may be helpful. The most crucial aspect, however, is to ease their workload. Healthcare workers in direct contact with COVID-19 patients are physically more strained due to PPE, which further elevates their risk of burnout. Thus, specific limitations on the number of work hours spent in direct contact with COVID-19 patients must be established, and healthcare workers should be guaranteed sufficient break periods. In addition, policies and systems to prevent burnout, such as securing more healthcare personnel and providing compensation, are urgently needed.
Supplementary Information
Acknowledgements
We appreciate the contributions and dedication of all the healthcare workers taking care of patients with COVID-19. We would also like to thank all participants who took part in this study and the researcher who helped with the statistics of this report.
Abbreviations
- COVID-19
Coronavirus disease 2019
- WHO
World Health Organization
- MERS
Middle East respiratory syndrome
- SARS
Severe acute respiratory syndrome
- PTSD
Post-traumatic stress disorder
- FSS
Fatigue severity scale
- PC-PTSD-5
Primary care post-traumatic stress disorder-5
- POREST
Positive resources test
- GARS
Global assessment of recent stress scale
- PPE
Personal protection equipment
- PHQ-15
Patient health questionnaire-15
- PHQ-9
Patient health questionnaire-9
- GAD-7
Generalized anxiety disorder scale
- ICU
Intensive care units
- PAPR
Powered air-purifying respirator
Author contributions
K.T.K., and S.H.L. contributed to the study conception and design. S.W.K., H.H.C., Y.K., S.B., H.S.C., S.Y.P., B.K., S.L., J.P., S.T.H., W.S.O., Y.K., K.H.P., C.K.K., N.O., S.J.L., S.Y., J.W.S., H.W.R., U.S.C., J.Y.L., H.Y.P., S.B., and J.L. were involved in material preparation, data collection, and statistical analysis. J.Y.S., S.H., K.T.K., S.H.L. reviewed statistical analysis and interpreted the data. S.H., K.T.K., and S.H.L. wrote the manuscript and revised it critically for important intellectual content. All co-authors read and approved the final manuscript. All authors agree to publish.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of health and welfare (MOHW), Republic of Korea (Grant Number: HC20C0003).
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ki Tae Kwon, Email: ktkwon@knu.ac.kr.
So Hee Lee, Email: sohee.lee@nmc.or.kr.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-30372-x.
References
- 1.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2023. https://covid19.who.int/of subordiante document. Accessed 3 Apr 2023.
- 3.Wong EL, Wong SY, Kung K, Cheung AW, Gao TT, Griffiths S. Will the community nurse continue to function during H1N1 influenza pandemic: A cross-sectional study of Hong Kong community nurses? BMC Health Serv. Res. 2010;10(1):1–8. doi: 10.1186/1472-6963-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holroyd E, McNaught C. The SARS crisis: Reflections of Hong Kong nurses. Int. Nurs. Rev. 2008;55(1):27–33. doi: 10.1111/j.1466-7657.2007.00586.x. [DOI] [PubMed] [Google Scholar]
- 5.Tengilimoğlu D, Zekioğlu A, Tosun N, Işık O, Tengilimoğlu O. Impacts of COVID-19 pandemic period on depression, anxiety and stress levels of the healthcare employees in Turkey. Leg. Med. (Tokyo) 2021;48:101811. doi: 10.1016/j.legalmed.2020.101811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luceño-Moreno L, Talavera-Velasco B, García-Albuerne Y, Martín-García J. Symptoms of posttraumatic stress, anxiety, depression, levels of resilience and burnout in Spanish health personnel during the COVID-19 pandemic. Int. J. Environ. Res. Public Health. 2020;17(15):5514. doi: 10.3390/ijerph17155514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verma S, Mishra A. Depression, anxiety, and stress and socio-demographic correlates among general Indian public during COVID-19. Int. J. Soc. Psychiatry. 2020;66(8):756–762. doi: 10.1177/0020764020934508. [DOI] [PubMed] [Google Scholar]
- 8.Vagni M, Maiorano T, Giostra V, Pajardi D. Coping with COVID-19: Emergency stress, secondary trauma and self-efficacy in healthcare and emergency workers in Italy. Front. Psychol. 2020;11:2294. doi: 10.3389/fpsyg.2020.566912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmassi C, Cerveri G, Bui E, Gesi C, Dell’Osso L. Defining effective strategies to prevent post-traumatic stress in healthcare emergency workers facing the COVID-19 pandemic in Italy. CNS Spectr. 2020 doi: 10.1017/S1092852920001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.d’Ettorre G, Ceccarelli G, Santinelli L, Vassalini P, Innocenti GP, Alessandri F, et al. Post-traumatic stress symptoms in healthcare workers dealing with the COVID-19 pandemic: A systematic review. Int. J. Environ. Res. Public Health. 2021;18(2):601. doi: 10.3390/ijerph18020601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freudenberger HJ. Staff burn-out. J. Soc. Issues. 1974;30(1):159–165. doi: 10.1111/j.1540-4560.1974.tb00706.x. [DOI] [Google Scholar]
- 12.Demerouti E, Bakker AB, Nachreiner F, Schaufeli WB. The job demands-resources model of burnout. J. Appl. Psychol. 2001;86(3):499–512. doi: 10.1037/0021-9010.86.3.499. [DOI] [PubMed] [Google Scholar]
- 13.Bakker, A. B. & Demerouti, E. Multiple levels in job demands-resources theory: Implications for employee well-being and performance. In Noba Scholar Handbook Series: Handbook of well-being (eds. Diener, E., et al.), http://www.nobascholar.com/books/1 (Salt Lake City, UT: DEF Publishers, 2018).
- 14.Barello S, Caruso R, Palamenghi L, Nania T, Dellafiore F, Bonetti L, et al. Factors associated with emotional exhaustion in healthcare professionals involved in the COVID-19 pandemic: An application of the job demands-resources model. Int. Arch. Occup. Environ. Health. 2021;94(8):1751–1761. doi: 10.1007/s00420-021-01669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Card AJ. Burnout and sources of stress among health care risk managers and patient safety personnel during the COVID-19 pandemic: A pilot study. Disaster Med. Public Health Prep. 2021 doi: 10.1017/dmp.2021.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srikrishna N, Vinnakota A, Srinivas S, Shvetha C, Abhilash GV, Vidya S, et al. Burnout and its impact on mental health of physicians during the COVID-19 pandemic: A cross-sectional study from South India. Telangana J. Psychiatry. 2020;6(2):160. [Google Scholar]
- 17.Barello S, Palamenghi L, Graffigna G. Burnout and somatic symptoms among frontline healthcare professionals at the peak of the Italian COVID-19 pandemic. Psychiatry Res. 2020;290:113129. doi: 10.1016/j.psychres.2020.113129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferry AV, Wereski R, Strachan FE, Mills NL. Predictors of UK healthcare worker burnout during the COVID-19 pandemic. QJM. 2021;114(6):374–380. doi: 10.1093/qjmed/hcab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan BY, Kanneganti A, Lim LJ, Tan M, Chua YX, Tan L, Sia CH, Denning M, Goh ET, Purkayastha S. Burnout and associated factors among health care workers in Singapore during the COVID-19 pandemic. J. Am. Med. Dir. Assoc. 2020;21(12):1751–1758. e1755. doi: 10.1016/j.jamda.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munnangi S, Dupiton L, Boutin A, Angus L. Burnout, perceived stress, and job satisfaction among trauma nurses at a level I safety-net trauma center. J. Trauma Nurs. 2018;25(1):4–13. doi: 10.1097/JTN.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 21.Kim J-H, An JA-R, Min P-k, Bitton A, Gawande AA. How South Korea responded to the COVID-19 outbreak in Daegu. NEJM Catal. Innov. Care Deliv. 2020 doi: 10.1056/CAT.20.0159. [DOI] [Google Scholar]
- 22.Oh J, Lee J-K, Schwarz D, Ratcliffe HL, Markuns JF, Hirschhorn LR. National response to COVID-19 in the Republic of Korea and lessons learned for other countries. Health Syst. Reform. 2020;6(1):e1753464. doi: 10.1080/23288604.2020.1753464. [DOI] [PubMed] [Google Scholar]
- 23.Cheong HS, Kwon KT, Hwang S, Kim S-W, Chang H-H, Park SY, Kim B, Lee S, Park J, Heo ST. Workload of healthcare workers during the COVID-19 outbreak in Korea: A nationwide survey. J. Korean Med. Sci. 2022;37(6):e49. doi: 10.3346/jkms.2022.37.e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheaffer, R. L., Mendenhall III, W., Ott, R. L. & Gerow, K. G. Elementary survey sampling: Cengage Learning (2011).
- 25.Seo YE, Kim HC, Yoo SY, Lee KU, Lee HW, Lee SH. Factors associated with burnout among healthcare workers during an outbreak of MERS. Psychiatry Investig. 2020;17(7):674. doi: 10.30773/pi.2020.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: Validity of a new measure for evaluating the severity of somatic symptoms. Psychosom. Med. 2002;64(2):258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Chung K-I, Song C-H. Clinical usefulness of fatigue severity scale for patients with fatigue, and anxiety or depression. Korean J. Psychosom. Med. 2001;9(2):164–173. [Google Scholar]
- 28.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 29.Prins A, Bovin MJ, Smolenski DJ, Marx BP, Kimerling R, Jenkins-Guarnieri MA, et al. The primary care PTSD screen for DSM-5 (PC-PTSD-5): Development and evaluation within a veteran primary care sample. J. Gen. Intern. Med. 2016;31(10):1206–1211. doi: 10.1007/s11606-016-3703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroenke K, Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. J. Gen. Intern. Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi HS, Choi JH, Park KH, Joo KJ, Ga H, Ko HJ, et al. Standardization of the Korean version of Patient Health Questionnaire-9 as a screening instrument for major depressive disorder. J. Korean Acad. Fam. Med. 2007;28(2):114–119. [Google Scholar]
- 32.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. JAMA Open. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 33.Plummer F, Manea L, Trepel D, McMillan D. Screening for anxiety disorders with the GAD-7 and GAD-2: A systematic review and diagnostic metaanalysis. Gen. Hosp. Psychiatry. 2016;39:24–31. doi: 10.1016/j.genhosppsych.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Kroenke K, Spitzer RL, Williams JB, Monahan PO, Löwe B. Anxiety disorders in primary care: Prevalence, impairment, comorbidity, and detection. Ann. Intern. Med. 2007;146(5):317–325. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]
- 35.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 36.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 37.Cho YW, Lee H, Lee JH, Han SY, Lee MY. Sleep disorders in maintenance dialysis patients with end-stage renal disease. J. Korean Neurol. Assoc. 2003;21(5):492–497. [Google Scholar]
- 38.Cho YW, Song ML, Morin CM. Validation of a Korean version of the insomnia severity index. J. Clin. Neurol. 2014;10(3):210–215. doi: 10.3988/jcn.2014.10.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koh K-B, Park J-K. Validity and reliability of the Korean version of the global assessment of recent stress scale. Korean J. Psychosom. Med. 2000;8(2):201–211. [Google Scholar]
- 40.Kim SY, Min JA, Chae JH, Kim M, Kim JH. Development and validation of the Positive Resources Test (POREST) Proc. Korean Psychol. Assoc. Conf. 2013;2013(2):143–143. [Google Scholar]
- 41.Huh HJ, Kim SY, Min JA, Chae JH. Development of the clinical short-form positive resources test. Korean J. Stress Res. 2018;26(2):77–87. doi: 10.17547/kjsr.2018.26.2.77. [DOI] [Google Scholar]
- 42.Park Y, Chae JH. The effect of mindfulness meditation on positive resources and positive affects in outpatients with depressive disorder and anxiety disorder. Mood Emot. 2017;15(2):67–72. [Google Scholar]
- 43.Peterson U, Demerouti E, Bergström G, Åsberg M, Nygren Å. Work characteristics and sickness absence in burnout and nonburnout groups: A study of Swedish health care workers. Int. J. Stress Manag. 2008;15(2):153. doi: 10.1037/1072-5245.15.2.153. [DOI] [Google Scholar]
- 44.Na, Y. J. The Construct Validity of the Oldenburg Burnout Inventory (OLBI) (The Graduate School of Ajou University, Suwon, 2013).
- 45.Demerouti E, Bakker AB, Vardakou I, Kantas A. The convergent validity of two burnout instruments: A multitrait-multimethod analysis. Eur. J. Psychol. Assess. 2003;19(1):12. doi: 10.1027//1015-5759.19.1.12. [DOI] [Google Scholar]
- 46.McKinley N, McCain RS, Convie L, Clarke M, Dempster M, Campbell WJ, et al. Resilience, burnout and coping mechanisms in UK doctors: A cross-sectional study. BMJ. 2020;10(1):e031765. doi: 10.1136/bmjopen-2019-031765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heinen MM, van Achterberg T, Schwendimann R, Zander B, Matthews A, Kózka M, et al. Nurses’ intention to leave their profession: A cross sectional observational study in 10 European countries. Int. J. Nurs. Stud. 2013;50(2):174–184. doi: 10.1016/j.ijnurstu.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 48.Ching SM, Ng KY, Lee KW, Yee A, Lim PY, Ranita H, et al. Psychological distress among healthcare providers during COVID-19 in Asia: Systematic review and meta-analysis. PLoS ONE. 2021;16(10):e0257983. doi: 10.1371/journal.pone.0257983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maunder R, Heeney N, Strudwick G. Burnout in hospital-based healthcare workers during COVID-19. Sci. Br. Ont. COVID-19 Sci. Adv. Table. 2021;2:46. [Google Scholar]
- 50.Lai J, Ma S, Wang Y, Cai Z, Hu J, Wei N, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw. Open. 2020;3(3):e203976–e203976. doi: 10.1001/jamanetworkopen.2020.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torrente M, Sousa PA, Sánchez-Ramos A, Pimentao J, Royuela A, Franco F, et al. To burn-out or not to burn-out: A cross-sectional study in healthcare professionals in Spain during COVID-19 pandemic. BMJ Open. 2021;11(2):e044945. doi: 10.1136/bmjopen-2020-044945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Audet M-C. Stress-induced disturbances along the gut microbiota-immune-brain axis and implications for mental health: Does sex matter? Front. Neuroendocrinol. 2019;54:100772. doi: 10.1016/j.yfrne.2019.100772. [DOI] [PubMed] [Google Scholar]
- 53.Baxter AJ, Scott KM, Vos T, Whiteford HA. Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychol. Med. 2013;43(5):897–910. doi: 10.1017/S003329171200147X. [DOI] [PubMed] [Google Scholar]
- 54.Zhu Z, Xu S, Wang H, Liu Z, Wu J, Li G, et al. COVID-19 in Wuhan: Sociodemographic characteristics and hospital support measures associated with the immediate psychological impact on healthcare workers. EClinicalMedicine. 2020;24:100443. doi: 10.1016/j.eclinm.2020.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huo L, Zhou Y, Li S, Ning Y, Zeng L, Liu Z, et al. Burnout and its relationship with depressive symptoms in medical staff during the COVID-19 epidemic in China. Front. Psychol. 2021;12:544. doi: 10.3389/fpsyg.2021.616369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sayilan AA, Kulakaç N, Uzun S. Burnout levels and sleep quality of COVID-19 heroes. Perspect. Psychiatr. Care. 2021;57(3):1231–1236. doi: 10.1111/ppc.12678. [DOI] [PubMed] [Google Scholar]
- 57.Alsulimani LK, Farhat AM, Borah RA, AlKhalifah JA, Alyaseen SM, Alghamdi SM, et al. Health care worker burnout during the COVID-19 pandemic: A cross-sectional survey study in Saudi Arabia. Saudi Med. J. 2021;42(3):306–314. doi: 10.15537/smj.2021.42.3.20200812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson SU, Ebrahimi OV, Hoffart A. PTSD symptoms among health workers and public service providers during the COVID-19 outbreak. PLoS ONE. 2020;15(10):e0241032. doi: 10.1371/journal.pone.0241032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Norman SB, Feingold JH, Kaye-Kauderer H, Kaplan CA, Hurtado A, Kachadourian L, et al. Moral distress in frontline healthcare workers in the initial epicenter of the COVID-19 pandemic in the United States: relationship to PTSD symptoms, burnout, and psychosocial functioning. Depress. Anxiety. 2021;38(10):1007–1017. doi: 10.1002/da.23205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carmassi C, Foghi C, Dell'Oste V, Cordone A, Bertelloni CA, Bui E, et al. PTSD symptoms in healthcare workers facing the three coronavirus outbreaks: What can we expect after the COVID-19 pandemic. Psychiatry Res. 2020;292:113312. doi: 10.1016/j.psychres.2020.113312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crabbe J, Bowley D, Boffard K, Alexander DA, Klein S. Are health professionals getting caught in the crossfire? The personal implications of caring for trauma victims. Emerg. Med. J. 2004;21(5):568–572. doi: 10.1136/emj.2003.008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oh J-H, Lim N-Y. Analysis of factors influencing secondary traumatic stress, burnout, and physical symptoms in firefighters. J. Korean Fund. Nurs. 2006;13(1):96–106. [Google Scholar]
- 63.Jo I, Lee S, Sung G, Kim M, Lee S, Park J, et al. Relationship between burnout and PTSD symptoms in firefighters: The moderating effects of a sense of calling to firefighting. Int. Arch. Occup. Environ. Health. 2018;91(1):117–123. doi: 10.1007/s00420-017-1263-6. [DOI] [PubMed] [Google Scholar]
- 64.Hilton NZ, Addison S, Ham E, Rodrigues NC, Seto MC. Workplace violence and risk factors for PTSD among psychiatric nurses: Systematic review and directions for future research and practice. J. Psychiatr. Ment. Health Nurs. 2022;29(2):186–203. doi: 10.1111/jpm.12781. [DOI] [PubMed] [Google Scholar]
- 65.Choudhury A, Singh M, Khurana DK, Mustafi SM, Ganapathy U, Kumar A, et al. Physiological effects of N95 FFP and PPE in healthcare workers in COVID intensive care unit: A prospective cohort study. Indian J. Crit. Care Med. 2020;24(12):1169–1173. doi: 10.5005/jp-journals-10071-23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alreshidi NM, Alghamdi S, Shibily F, Mahsoon A, Alasmee N, Sharif L, et al. The association between using personal protective equipment and headache among healthcare workers in Saudi Arabia hospitals during the COVID-19 pandemic. Nurs. Rep. 2021;11(3):568–583. doi: 10.3390/nursrep11030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li D, Wang Y, Yu H, Duan Z, Peng K, Wang N, et al. Occupational burnout among frontline health professionals in a high-risk area during the COVID-19 outbreak: A structural equation model. Front. Psychiatry. 2021;12:575005. doi: 10.3389/fpsyt.2021.575005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greenglass ER, Burke RJ, Fiksenbaum L. Workload and burnout in nurses. J. Community Appl. Soc. Psychol. 2001;11(3):211–215. doi: 10.1002/casp.614. [DOI] [Google Scholar]
- 69.Kim SO, Wang MS. A study on emotional labor, positive resources and job burnout in clinical Nurses. J. Korea Acad. Ind. Coop. Soc. 2015;16(2):1273–1283. [Google Scholar]
- 70.Williams GA, Scarpetti G, Bezzina A, Vincenti K, Grech K, Kowalska-Bobko I, et al. How are countries supporting their health workers during COVID-19? Eurohealth. 2020;26(2):58–62. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.