Abstract
Advancements in nanomedicine helped scientists design a new class of nanoparticles known as hybrid nanoparticles (core/shell) for diagnostic and therapeutic purposes. An essential requirement for the successful use of nanoparticles in biomedical applications is their low toxicity. Therefore, toxicological profiling is necessary to understand the mechanism of nanoparticles. The current study aimed to assess the toxicological potential of CuO/ZnO core/shell nanoparticles with a size of 32 nm in Albino female rats. In vivo toxicity was evaluated by oral administration of 0, 5, 10, 20, and 40 (mg/L) of CuO/ZnO core/shell nanoparticles to a female rate for 30 consecutive days. During the time of treatment, no deaths were observed. The toxicological evaluation revealed significant (p < 0.01) alteration in white blood cells (WBC) at a 5 (mg/L) dose. Also, increase in red blood cells (RBC) at 5, 10 (mg/L) doses, while hemoglobin (Hb) levels and hematocrit (HCT) increased at all doses. This maybe indicates that the CuO/ZnO core/shell nanoparticles stimulated the rate of blood corpuscle generation. The anaemia diagnostic indices (mean corpuscular volume MCV and mean corpuscular haemoglobin MCH) remained unchanged throughout the experiment for all the doses tested 5, 10, 20, and 40 (mg/L). According to the results of this study, exposure to CuO/ZnO core/shell NPs deteriorates the Triiodothyronine hormone (T3) and a Thyroxine hormone (T4) activated by Thyroid‐Stimulating Hormone (TSH), which is generated and secreted from the pituitary gland. There is possibly related to an increase in free radicals and a decrease in antioxidant activity. Significant (p < 0.01) growth retardation in all groups treated due to rats’ infection by Hyperthyroidism induced by thyroxine (T4) level increase. Hyperthyroidism is a catabolic state related to increased energy consumption, protein turnover, and lipolysis. Usually, these metabolic effects result in weight reduction and a decrease in fat storage and lean body mass. The histological examination indicates that the low concentrations of CuO/ZnO core/shell nanoparticles are safe for desired biomedical applications.
Keywords: Argon plasma jets; CuO/ZnO core/shell nanoparticles; Oral exposure; In vivo toxicity; Histological, haematological, and thyroid hormones; Growth retardation
Introduction
In the last few years, the field of non-thermal plasma has expanded significantly. The topic of chemical interactions between non-thermal plasma and liquids attracted the attention of researchers due to its potential medical and economic benefits (Rumbach et al. 2013; Mohammed et al. 2022a, b). Recently, nanomaterial (NM) synthesis through plasma–liquid interactions (PLIs) is a new branch of plasma research that has grown rapidly. This is mostly a result of multiple recently discovered plasma sources that operate at low and atmospheric pressures (Chen et al. 2015). Nanotechnology is a field that combines the physical, chemical, and biological sciences. It also involves the processing and using materials ≤ 100 nm in one or more dimensions. Thus, with the nanotechnology field’s development, there has been a global increase in nanomaterials (Chatterjee et al. 2014; Gopinath et al. 2016; Singh et al. 2019). Nano-enabled theranostics depend on the improved properties of nanosystems. Nano-enabled biomedical applications help generate effective treatment and therapy with minimal dosage and fewer negative effects than bulk materials (Kumar 2020). Metal-based pharmaceuticals, mainly inorganic nanomaterials, have been researched as next-generation nanomedicine. These nanoparticles exhibit their unique medicinal features and novel pharmacological functions. Metal-based nanotherapeutics with controllable characteristics such as particle size and porosity are helpful for biomedical applications of drug delivery and therapeutic activity (Jin and Jin 2019). Different types of metal oxide nanoparticles (MONPs) have been well reported in the literature for decades due to their countless biomedical applications. Among these medications are copper oxide and zinc oxide nanoparticles (Grigore et al. 2016; Sruthi et al. 2018; Patrinoiu et al. 2019; Akintelu et al. 2020). Moreover, their physicochemical and physiological behavior determines in vivo interaction of biomolecules, which further determines their extent of toxicological behavior compared with their bulk counterpart. Because after reaching inside the organism, it takes some time to get dissolved and integrate into the bioligands, which confirms their prolonged existence in the system and reach their target. Therefore, these unique properties are considered an important factor in toxicology (Singh et al. 2019). One of the most common mechanisms leading to toxicity is generating reactive oxygen species (ROS). ROS are active molecules produced during cell metabolism, and in biology, ROS indicates superoxide anion radicals , hydroxyl radicals (OH−), and hydrogen peroxide (H2O2). Excessive production of ROS can break the redox balance, resulting in oxidative stress, inflammation, and consequent damage to proteins, cell membranes, and DNA. Several factors influence the level of ROS generated induced by nanoparticles, including the size, shape, surface, composition, solubility, aggregation/agglomeration, particle uptake, the presence of mutagens, and transition metals affiliated with the particles (Yao et al. 2019; Sengul et al. 2020). Several studies were carried out to in vivo assess the toxicity of liver, kidney, and spleen induced by CuO and ZnO nanoparticles. Bugata et al. (2019) reported that oral exposure to CuO nanoparticles in female albino rats with (30, 300, and 1000 mg/kg) doses for 28 days; high doses caused significantly high abnormalities in the liver, kidney, and brain histoarchitecture. Tang et al. (2018) examed the acute toxic effects correlated with particle size (30, 50, 80 nm, and 1 μm) induced by Cu nanoparticles on the brain, heart, lung, testes, liver, kidney, and spleen of rats for 7 days by oral exposure. The liver, kidney, and spleen were the major organs most affected by Cu NPs. Physiological results indicated that 80 nm of Cu NPs were the most toxic to rats when animals were repeatedly treated for short periods. The toxicity effects of rats generated by Cu NPs were investigated by Zhou et al. (2019) for 28 days, with a size of 75 nm; the results were apparent spleen damage and oxidative inflammatory-immune alterations. The study by Moatamed et al. (2019) demonstrated the exposure of male rats to three different doses (25, 50, and 100 mg/kg) of ZnO NPs (39 nm) for 10 days caused the dysfunction of hepatic impairments, including changes in liver enzymes and histopathological structure, and redox state. Also, Yousef et al. (2019a, b) reported the oral exposure of male rats to a high dose (100 mg/kg) of ZnO NPs treatment for 75 days caused pronounced hepatorenal toxicities and systemic inflammation. Core/shell nanoparticles have recently gained increasing attention due to their low cytotoxicity, chemical liable surface, and potential in fields like tumor therapy, treatment with biomedical imaging, and drug delivery (Rajabi and Sohrabnezhad 2018; Jin and Jin 2019).
In vitro cultures seem unable to include helpful information about the physiological system response under investigation. Thus, the main aim of the current study is to examine the in vivo toxicity of CuO/ZnO core/shell nanoparticles synthesized by environmentally friendly plasma jets technique. The toxicity of the CuO and ZnO NPs has been previously reported. To the best of our knowledge and according to a literature survey, no report is available on the acute lethal effect and toxicity of biologic CuO/ZnO core/shell NPs. Thus, the present study’s biomedical application includes investigating in vivo toxicity via haematology, biochemistry, and histopathology analysis.
Materials and methods
Manufactured CuO/ZnO core/shell nanoparticles
Our previous study (Mohammed et al. 2022a, b) reported the synthesis of CuO/ZnO core/shell nanoparticles with argon plasma jets.
Animals model
Five-week-old albino female rats that were healthy and weighed 65–93 g were purchased from the University of Al-Nahrain/Biotechnology Research Center/Animal House. Air-conditioned rooms were employed to house the rats in plastic cages, which were kept at a light/dark cycle of 12 h. The room’s temperature ranged from 22 to 24 °C ± 2 °C. The rats had free access to food and water. Twenty-five rats were randomly divided into five groups (five rats in each group), with one group assigned as the control group and the other four as test groups. They administered various doses of CuO/ZnO core/shell NPs. After one week of acclimation, the animals were 6 weeks old when the experiment began. The rats in the control group were not treated. Various doses of CuO/ZnO core/shell NPs solution were orally administered to test groups to assess the toxicity. Each rat in groups 2–5 received 1 mL of a CuO/ZnO core/shell NPs solution containing doses of 5, 10, 20, or 40 (mg/L), respectively. Each rat was observed twice daily for any clinical signs of toxicity. The body weight was measured each week throughout the test period. The rats were sacrificed after 30 days to examine the toxicity through hematological, thyroid hormones, and histological analyses. All animal-handling procedures were performed according to the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines associated with the Care and Use of Laboratory Animals Institute for Laboratory Animal Research Division https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf
Haematological analysis
Female rats were anesthetized with isoflurane (manufactured by Piramal Enterprises Limited: Telangana, India). Blood samples were obtained through an intra-cardiac puncture and collected in coated tubes immediately for a hematological examination following anaesthetization. Hematological parameters include Red blood cells (RBCs), White blood cells (WBCs), Hematocrit (HCT), Hemoglobin (Hb) levels, Mean corpuscular hemoglobin (MCH), Mean corpuscular volume (MCV), Mean corpuscular hemoglobin concentration (MCHC).
Thyroid hormones analysis
An automated analyzer (Hitachi 912 Chemistry Analyzer) centrifuged the serum for 10 min at 2000 (rpm) to collect it. To measure thyroid gland function, were examine the Triiodothyronine (T3) and thyroxine (T4) hormones released by the thyroid gland, also as the thyroid-stimulating hormone (TSH), which is released by the anterior pituitary gland.
Histopathology analysis
To perform a histological examination, the rats’ kidney, liver, and spleen were examined to see if the CuO/ZnO core/shell NPs affected their morphology. Deionized water was used to remove the blood from the organs specimens before they were fixed in 10% formalin, embedded in paraffin blocks, and cut into 5 m thick sections. Hematoxylin and Eosin (H and E) staining was conducted to evaluate the fixed sections of tissue. The fixed tissue was viewed under a binocular microscope at magnifications of 40, 100, and 400, and photomicrographs were taken.
Statistical analysis
ANOVA followed by GraphPad Prism version 8 (GraphPad Software Inc., La Jolla, CA) was used to examine the differences between the mean values, and P value ≤ 0.05 was regarded as statistically significant. The results of each group are expressed in mean ± standard deviation (n = 5).
Results and discussion
Animals observation
Each animal was looked at twice daily for any signs of toxicity or death during the test period. During the time of treatment, no deaths were observed. Except for hair loss in some groups, growth retardation, and pupil changes, the animals in all the groups did not show severe clinical poisoning symptoms, like decreased mobility, skin color change, and loss of appetite. Throughout the experiment, the rats were weighed every week. There was a statistically significant difference in the mean body weights of the groups treated with the CuO/ZnO core/shell NPs at various doses (5, 10, 20, and 40 mg/L) compared with the control group (p < 0.01), as shown in Fig. 1. This study’s findings show that female rats have growth retardation. The growth retardation of the female rats can be related to being infected with Hyperthyroidism, as mentioned in the section on thyroid hormone analyses. Growth retardation is the main characteristic of NP-intoxicated animals. Toxicological effects of NPs, according to a report from the American National Research Council, include growth inhibition. Growth hormone, insulin-like growth factor-binding proteins, and insulin-like growth factor-I production are all decreased by NPs, resulting in growth retardation (Shakibaie et al. 2013).
Fig. 1.
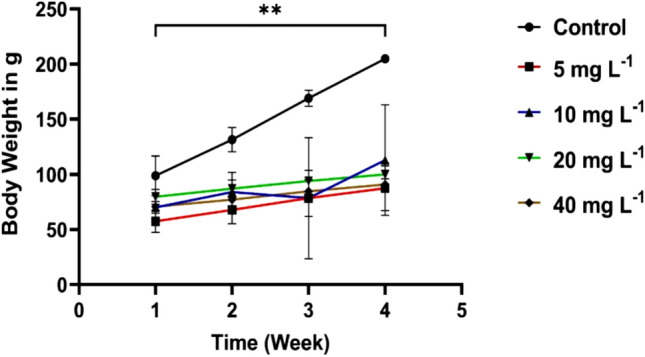
Mean body weight of female rats exposed to CuO/ZnO core/shell NPs at 5, 10, 20, and 40 mg/L doses for 30 consecutive days. Values are means ± SD (n = 5), (**p < 0.01, compared with the control)
Hematology analysis
The measurement of blood parameters is an important step in establishing the toxicity of NPs. After 30 days of oral exposure to different doses of CuO/ZnO core/shell NPs, the hematological profiles of the animals were evaluated. Hematological parameters could be utilized to estimate the severity of a foreign compound's toxicity to an animal’s blood components. Since hematological system changes effectively predict toxicity when translating data from animal studies to human toxicity, such testing is pertinent to risk assessment (Ashafa et al. 2012).
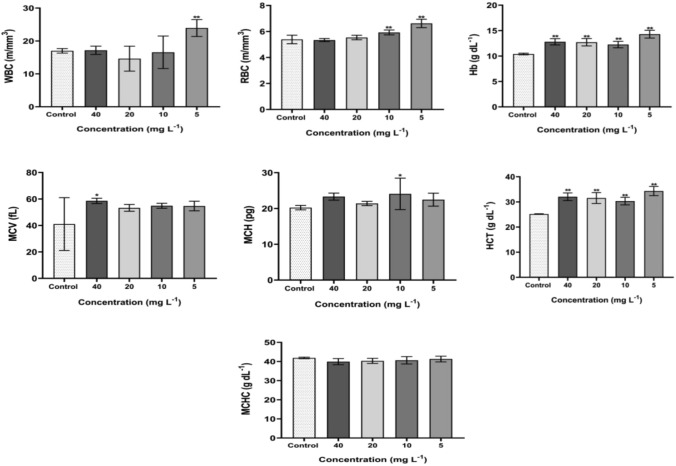
Table 1 shows the concentration-dependent hematology results of CuO/ZnO core/shell NPs. The result reported in Table 1 showed that the CuO/ZnO core/shell NPs at all the doses tested 5, 10, 20, and 40 (mg/L) investigated did not significantly alter the mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), and mean corpuscular haemoglobin concentration (MCHC). MCV and MCH, which are anaemia diagnostic indices, remained unchanged throughout the experiment. This implies that repeated oral administration of CuO/ZnO core/shell NPs does not affect RBC average size or the weight of haemoglobin per RBC (Ashafa et al. 2012; Suryavanshi et al. 2017). Observed only at 40 (mg/L) dose, the mean corpuscular volume (MCV) (P < 0.02) elevated; this implies increased platelet destruction, and compensation for bone marrow function caused MCV levels to be raised (Wang et al. 2017).
Table 1.
Hematological parameters in female rats exposed to various doses of CuO/ZnO core/shell NPs
| Hematological parameters | Control | 5 (mg/L) | 10 (mg/L) | 20 (mg/L) | 40 (mg/L) |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| WBC | 17.00 ± 0.685 | 23.94 ± 2.563** | 16.56 ± 4.943 | 14.62 ± 3.790 | 17.16 ± 1.260 |
| RBC | 5.386 ± 0.326 | 6.618 ± 0.322** | 5.926 ± 0.187** | 5.534 ± 0.173 | 5.340 ± 0.117 |
| Hb | 10.40 ± 0.158 | 14.30 ± 0.764** | 12.26 ± 0.630** | 12.72 ± 0.725** | 12.80 ± 0.620** |
| MCV | 41.06 ± 19.94 | 54.68 ± 3.586 | 54.86 ± 1.884 | 53.26 ± 1.962 | 58.56 ± 1.962* |
| MCH | 20.24 ± 0.623 | 22.46 ± 1.811 | 24.08 ± 4.387 | 21.40 ± 0.595 | 23.32 ± 0.985 |
| MCHC | 41.86 ± 0.397 | 41.30 ± 1.483 | 40.64 ± 1.923 | 40.32 ± 1.337 | 39.94 ± 1.590 |
| HCT | 25.16 ± 0.114 | 34.32 ± 1.819** | 30.32 ± 1.501** | 31.54 ± 2.164** | 32.04 ± 1.532** |
Data of each group presented as mean ± SD (n = 5) and *Refer P < 0.05 and **Refer P < 0.01 vs control
A significant alteration was observed in white blood cells (WBC) at a 5 (mg/L) dose. An increase in WBC shows the immune system’s reaction to nanoparticles as a foreign factor (Kavianiet al. 2019), also may indicate haematological tissue dysfunction (kidney and spleen) or certain infectious diseases (Khabbazi et al. 2015). In contrast, we observed a significant increase in red blood cells (RBC) at 5, 10 (mg/L) doses, while haemoglobin (Hb) levels and hematocrit (HCT) increased at all doses. Because all of these parameters are related to one another, the significant effect of the CuO/ZnO core/shell NPs on RBC, Hb, and HCT maybe indicate that the CuO/ZnO core/shell nanoparticles stimulated the rate of blood corpuscle generation. It may also suggest that CuO/ZnO core/shell NPs could stimulate the kidney's erythropoietin secretion, a humoral regulator of red cells (Sheta et al. 2019). Due to disturbing normal cells, physiology shows abnormal oxygenation from the lungs, indicating the absence of oxygen in blood with elevated RBC, Hb, and HCT values (Peterson et al. 2016; Amereh et al. 2019). These values are considered normal parameters, and further studies should focus on them. Figure 2 shows the mean value of hematologic parameters ± SD of groups treated with CuO/ZnO core/shell NPs at various doses compared to the control group. Changes in blood cells occur when blood component anomalies interfere with normal functions (Khabbazi et al. 2015). Although these values are considered normal parameters, further studies should focus on them. Figure 2 shows the mean value of hematologic parameters ± SD of groups treated with CuO/ZnO core/shell NPs at vari-ous doses compared to the control group.
Fig. 2.
The hematological results for rats exposed to CuO/ZnO core/shell NPs following 30 days at different doses (5, 10, 20, and 40 mg/L). Values are means ± SD (n = 5), (*Refer P < 0.05 and **Refer P < 0.01, compared with the control)
Thyroid hormones function
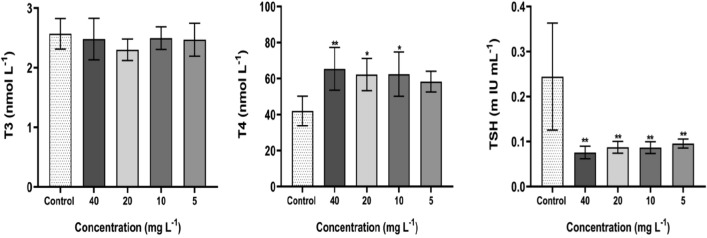
After 30 days, the blood serum of albino female rats in control and treated groups was examined for the harmful effect of CuO/ZnO core/shell NPs on thyroid gland hormone function (T3 and T4, and TSH). Thyroid hormones include the Triiodothyronine hormone (T3) and a Thyroxine hormone (T4) activated by Thyroid‐Stimulating Hormone (TSH), which is generated and secreted from the pituitary gland. The decreased concentrations of T3 and T4 levels stimulate the pituitary gland to release TSH. T3 and T4 hormones are mostly bound to the plasma protein [thyroxine binding globulin (TBG)] and circulate in the bloodstream (Sheta et al. 2019). Although the T4 hormone concentration is significantly greater than T3 (T4 is generated about 80% of the thyroid hormones, while just 20% is generated as T3), T3 affects physiological processes 4 times greater than T4. T3 is critical in various physiological processes including growth, metabolism, skeletal and organ systems development, heart rate, and body temperature (Amereh et al. 2019; Sheta et al. 2019). The effect of CuO/ZnO core/shell NPs on thyroid gland hormones is listed in Table 2, indicating a significant elevation in T4 levels in treated rats compared with the control group. Also, a significant reduction has been observed in the TSH concentrations at the tested doses. The pituitary gland prevents TSH production when increasing T3 and T4 levels in the blood (Sheta et al. 2019). The increase in T4 level indicates that the rats were infected by Hyperthyroidism, which explains their weight reduction. Hyperthyroidism is a catabolic state related to increased energy consumption, protein turnover, and lipolysis. Usually, these metabolic effects result in weight reduction and a decrease in fat storage and lean body mass (Peterson et al. 2016). However, the levels of T3 remained unaltered throughout the experimental period. The statistically significant differences in T3, T4, and TSH in blood serum of female rats are due to oral administration of CuO/ZnO core/shell NPs, as shown in Fig. 3. Exposure to CuO/ZnO core/shell NPs deteriorates thyroid hormones (T4, T3, and TSH), according to the results of this study. There is possibly related to an increase in free radicals and a decrease in antioxidant activity (Yousef et al. 2019a, b).
Table 2.
Results of effect thyroid hormones in female rats exposed to various doses of CuO/ZnO core/shell NPs
| Hormones | Control | 5 (mg/L) | 10 (mg/L) | 20 (mg/L) | 40 (mg/L) |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| T3 | 2.568 ± 0.256 | 2.470 ± 0.276 | 2.496 ± 0.189 | 2.300 ± 0.180 | 2.480 ± 0.348 |
| T4 | 41.96 ± 8.200 | 58.27 ± 5.754 | 62.42 ± 12.32* | 62.21 ± 8.950* | 65.36 ± 11.86** |
| TSH | 0.2442 ± 0.1189 | 0.096 ± 0.011** | 0.086 ± 0.013** | 0.087 ± 0.013** | 0.075 ± 0.014** |
Data of each group presented as mean ± SD (n = 5) and *Refer P < 0.05 and **Refer P < 0.01 vs control
Fig. 3.
Thyroid hormones (T3, T4, TSH) for rats exposed to CuO/ZnO core/shell NPs following 30 days at different doses (5, 10, 20, and 40 mg/L).Values are means ± SD (n = 5) (*Refer P < 0.05 and **Refer P < 0.01, compared with the control)
Histopathological analysis
A histological study of female rats' liver, kidney, and spleen after 30 days of consecutive oral exposure was performed to determine the toxicity of CuO/ZnO core/shell NPs. The results of a histological study of these three organs are shown below.
Liver toxicity
In the human body, the liver is the major organ for detoxification. It has the ability to deoxidize, bile formation, protein synthesis, store glycogen, and general homeostasis. By isolating and removing various foreign exogenous compounds via phagocytosis, the liver functions as a biological barrier (Yao et al. 2019; Boey and Boey 2020). The liver is a complex network of interconnected cells. The basic functioning unit of the liver is the hepatocyte; the hepatic lobule constitutes about 60% of the hepatocytes (solid cells) and 30–35% of the Kupffer cells, hepatic stellate cells, liver sinusoidal, and endothelial cells (non-solid cells). The liver's normal physiological and biochemical functions can be affected by nanoparticles deposited in liver tissue, affecting liver cells and important physiological processes (Yao et al. 2019).
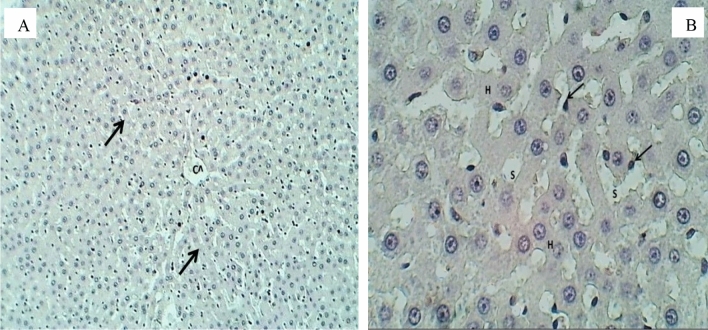
The histological photographs of the liver of albino female rats in the control group show that the central vein and hepatic cords were normally arranged, as in Fig. 4A. Also, Fig. 4B shows that the hepatic lobules, the hepatocytes, the sinusoids, and the Kupffer cells were also normal. Figures 5, 6, 7 and 8 show pathological changes in the liver of female rats due to accumulated CuO/ZnO core/shell NPs in the liver at various doses compared with the control group Fig. 4A.
Fig. 4.
Histopathological evaluation of the liver for the control group after 30 days of oral exposure to CuO/ZnO core/shell NPs (H&E stained), sections observed at, A 100× magnification, Central vein (Cv), and hepatic cords (Arrows) appear normal. B 400× magnification, no abnormal histological changes were observed for hepatocytes (H), sinusoids (S), and Kupffer cells (Arrow)
Fig. 5.
Sections of the liver (H&E stained) for the group exposure to 5 (mg/L) doses, A 100× magnification, central vein (Cv) and hepatic cords (Arrows) normal appear. B 400× magnification, show mild micro vaculation of hepatocyte (Black arrows) and little stellate cells (Red arrows)
Fig. 6.
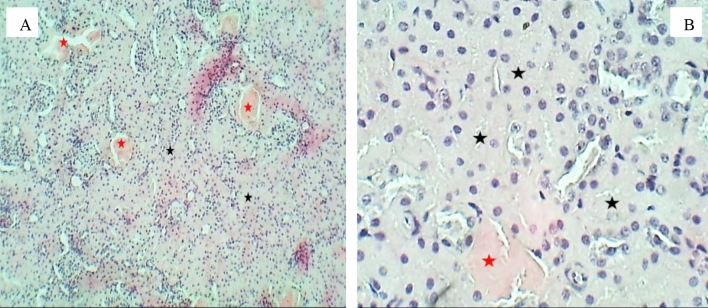
Sections of the liver (H&E stained) for the group exposure to 10 (mg/L) doses, A 100× magnification, a section of the liver shows disarrangement of hepatic cords with vacular degeneration (Arrows). B 400× magnification, a section of the liver lobule shows mild cellular swelling with granular degeneration (Asterisks) and necrosis (N) of hepatocytes
Fig. 7.
Sections of the liver (H&E stained) for the group exposure to 20 (mg/L) doses, A 40× magnification, a liver section shows disarrangement of hepatic cords with cellular necrosis (Arrows). B 400 × magnification, the section of the liver lobule shows moderate cellular swelling associated with nuclear pyknosis (Red arrow) and, necrosis (N) of hepatocytes & sinusoidal congestion
Fig. 8.
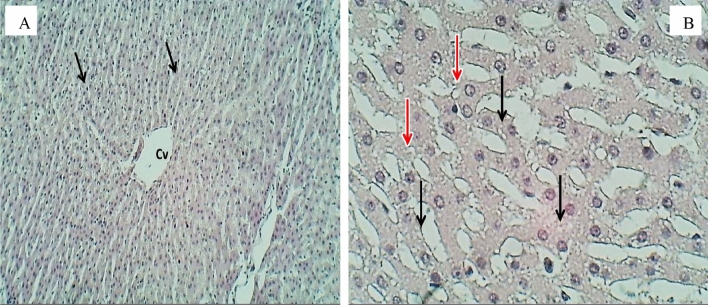
Sections of the liver (H&E stained) for the group exposure to 40 (mg/L) doses, A 100× magnification, section of the liver shows normal arranged of hepatic cords with the normal central vein (Cv). B 400× magnification, a section of the liver lobule normal of hepatocytes (H), sinusoid (S), and Kupffer cells
The tissue of rats treated with a 5 (mg/L) dose of CuO/ZnO core/shell NPs appear in Fig. 5A. The central vein and hepatic cords are regularly arranged. Figure 5B shows the magnified sections of hepatic lobules and hepatic cords and revealed mild micro vacultion within cytoplasmic hepatocytes and little formation of stellate cells. The group treated with a 10 (mg/L) dose showed disarrangement of hepatic cords with vacular degeneration, as shown in Fig. 6A. The magnified sections Fig. 6B revealed mild cellular swelling with granularity cytoplasmic degeneration of hepatocytes within the peripheral zone of hepatic lobules and necrosis. Figure 7A related to the sections of hepatic lobules of rats treated with 20 (mg/L) showed disarranged hepatic cords with cellular necrosis. Figure 7B, the magnified sections showed notable figures of nuclear associated with severe granular cellular degeneration, necrosis hepatocytes, and sinusoidal dilation and congestion. In contrast, the sections of hepatic lobules of rats treated with 40 (mg/L) were similar to those in the control group, as shown in Fig. 8A and B.
Our findings revealed that a low CuO/ZnO core/shell NPs dose caused more severe histopathological alterations than a higher dose. According to several workers, lower doses of NPs are more toxic than higher doses (Khorsandi et al. 2018). Metal nanoparticles (MNPs) cause cell damage and death via hepatocyte necrosis by generating reactive oxygen species (ROS). The necrosis of parenchymal cells in organs is promoted by inflammation. Thus, one of the prominent features of acute liver injury is necrosis. Plasma membrane rupture, mitochondrial swelling, and intracellular contents release are all characteristics of necrosis (Yao et al. 2019). The histological analysis results indicate that hepatocyte necrosis was induced at a 10, 20 (mg/L) dose of CuO/ZnO core/shell NPs.
Kidney toxicity
The kidney has different functions. It excretes metabolic end-products and regulates the production and release of hormones such as erythropoietin and renin. Because of its high blood flow and ability to concentrate waste, the kidney is one of the most sensitive organs in the body to toxic substances. Renal clearance is the second most important route to remove nanoparticles and greatly filters nanoparticles out of the blood and reduces their potential toxicity. The kidney comprises three distinct regions: the renal cortex, the medulla, and the pelvis. Nephrons are the kidney’s fundamental structural and functional units. They are found in the cortex and medulla and consist of the renal corpuscle, distal tubule, collecting ducts, proximal tubule, Henle loop, and peritubular capillaries surrounding the tubules in the tubulointerstitium (Du et al. 2018; Makhdoumi et al. 2020).
Kidney histology analysis indicated that the exposure to CuO/ZnO core/shell NPs at various doses caused pathological alterations in the kidney compared with the control group. The renal sections of the rats in the control group were normal, as shown in Fig. 9A and B; the renal cortex, glomeruli, collecting tubule, proximal, and distal convoluted tubules were normal. Figure 10A related to rats treated with 5 (mg/L) of CuO/ZnO core/shell NPs, shows the sections of renal cortex and medulla showed mildvacular degeneration of epithelial cells of renal tubules. In contrast, the renal glomeruli appeared normal, as shown in Fig. 10B. Also, the kidney of the female rats treated with 10 (mg/L) displayed that the renal glomeruli seemed normal Fig. 11A. In contrast, the renal cortex and medulla sections showed mild vacular degeneration of renal tubules, as shown in Fig. 11B. The morphological change in the kidney with different histopathological alterations induced by the 20 (mg/L) dose appears in Fig. 12A and B. Sections of the renal cortex and medulla showed marked multiple hemorrhagic foci and massive tubular cloudy swelling. The change of renal toxicity induced by 40 (mg/L) appears in Fig. 13A and B; the sections of the renal cortex showed mild vacular degeneration of renal tubules. Meanwhile, the renal medulla showed mild tubular cloudy swelling of collecting tubules.
Fig. 9.
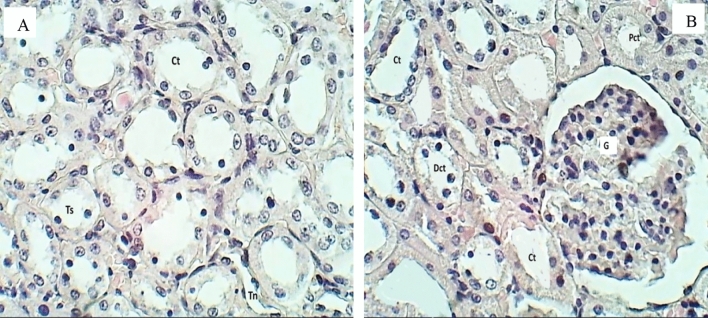
Histopathological evaluation of the kidney for the control group after 30 days of oral exposure to CuO/ZnO core/shell NPs (H&E stained), sections observed at 400× magnification, A Section of the renal cortex shows glomeruli (G), collecting tubule (Ct), proximal (Pct), and distal convoluted tubules (Dct) were normal. B Section of renal medulla shows: collecting tubules (Ct), thick segment (Ts) & thin segment (Tn) of a loop of Henle
Fig. 10.
Sections of the kidney (H&E stained) for the group exposure to 5 (mg/L) doses, A 100× magnification, section of the renal cortex shows mild vacular degeneration of renal tubules (Arrows). B 400× magnification, a section of renal cortex shows mild vacular degeneration of epithelial cells of renal tubules (Arrows)
Fig. 11.
Sections of the kidney (H&E stained) for the group exposure to 10 (mg/L) doses, A 100× magnification, section of the renal cortex shows normal appearance of the renal cortex, renal tubules, and glomeruli. B 400× magnification, a section of renal cortex shows mild granular degeneration of renal tubules (Arrows)
Fig. 12.
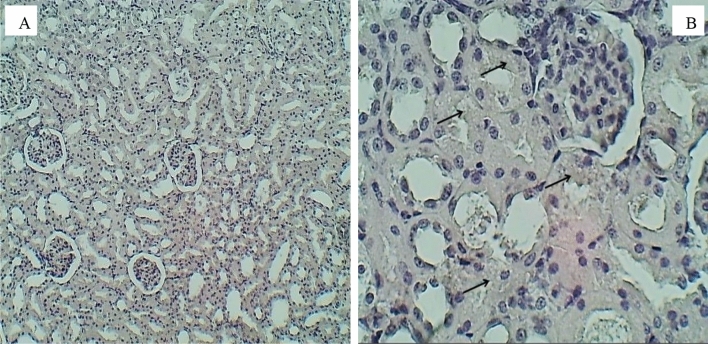
Sections of the kidney (H&E stained) for the group exposure to 20 (mg/L) doses, A 100× magnification, section of the renal cortex shows multiple hemorrhagic foci (Red asterisks) and massive tubular cloudy swelling (Black asterisks). B 400× magnification, a section of renal cortex shows hemorrhage (Red asterisks) and tubular cloudy swelling (Black asterisks)
Fig. 13.
Sections of the kidney (H&E stained) for the group exposure to 40 (mg/L) doses, A 100× magnification, section of the renal cortex shows mild vacular degeneration (asterisks), B 400× magnification, a section of renal cortex shows tubular cloudy swelling (Asterisks)
Spleen toxicity
When nanoparticles are deposited systemically, the main targets for deposition are immune system cells and organs (Zhou et al. 2019). Previous studies on humans and animals have indicated that the immune system includes potential target organs, and the damage to this system can result in morbidity and even mortality. Immune cells are present in the tissues and bloodstream, and the spleen comes to mind at talking about immune organs as a secondary lymphoid organ. Many researchers have discovered that nanoparticles can escape the immune system and induce damage to spleen tissue. The spleen is the largest immunological organ, including many lymphocytes, and is indispensable in keeping a body in a favorable environment. The inside components of the spleen are constructed of red and white pulp, and the red pulp contains lymphatic nodules surrounding the white pulp (Sang et al. 2014). Toxicants have a significant effect on the immune system. The primary role of the spleen is to eliminate damaged, aging, and necrotic cells while also contributing to the body’s immune response. Toxicants at low doses are toxic to the mice's immune systems but are relatively safe for other organs (Zhou et al. 2019).
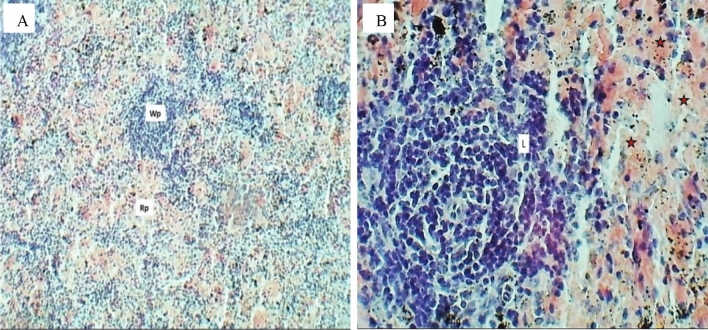
The splenic sections of the rats in the control group were normal, as shown in Fig. 14A and B appear to have a normal spleen architecture, and the white pulps and red pulps appear normal. Figure 15A and B show the spleen tissue of female rats treated with 5 (mg/L) of CuO/ZnO core/shell nanoparticles, indicating that this group did not exhibit any abnormal pathological alterations in the spleen compared with the control group. Exposure to a 10 (mg/L) dose caused some histological changes in the spleen of rats, as appears in Fig. 16A. Sections of splenic white pulp showed marked atrophied lymphatic follicles of white pulp, while the cytoarchitecture of red pulp showed a normal appearance, as shown in Fig. 16B.
Fig. 14.
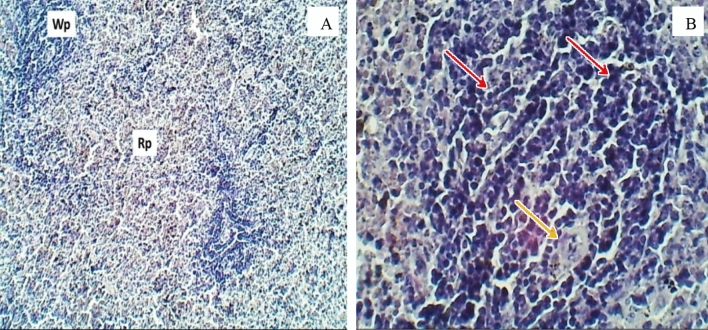
Histopathological evaluation of the spleen for the control group after 30 days of oral exposure to CuO/ZnO core/shell NPs (H&E stained), sections observed at A 40× magnification, a section of the spleen shows normal appearance of splenic white pulp (Wp) & Red pulp (Rp). B 400× magnification shows central arteriole (A) lymphocytes (Black arrow), macrophage (Red arrows), and splenic cord (Sc)
Fig. 15.
Sections of the spleen (H&E stained) for the group exposure to 5 (mg/L) doses, A 100× magnification, a section of the spleen shows normal appearance of the splenic red pulp (Rp) and white pulp (Wp). B 400× magnification, normal appearance of a lymphocytic follicle (Red arrows) and central arteriole (Wp) (color figure online)
Fig. 16.
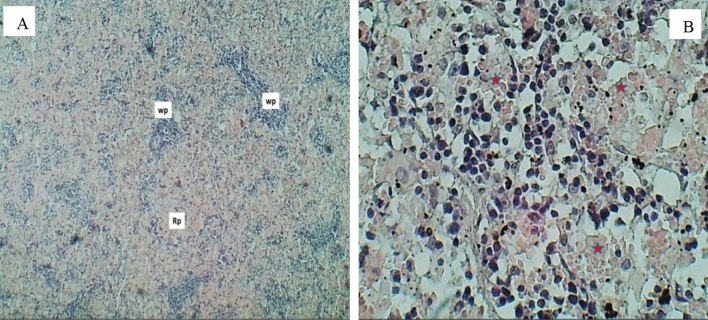
Sections of the spleen (H&E stained) for the group exposure to 10 (mg/L) doses, sections were observed at 400 × magnification. A A section of the spleen shows it marked atrophied lymphatic follicle with white pulp (Wp) and increased area of the red pulp. B The normal appearance of a red splenic sinusoid with red pulp (Asterisks) (color figure online)
The histological changes in the spleen of rats treated with a 20 (mg/L) dose are shown in Fig. 17, suggesting that this group had abnormal histological changes in the spleen. Sections of splenic white pulp showed marked hyperplasia with lymphatic follicles of white pulp, as shown in Fig. 17A. In contrast, the cytoarchitecture of red pulp showed mild sinusoidal congestion and an increased population of macrophages that engulf hemosiderin pigment, as shown in Fig. 17B. Figure 18 shows the histological changes in the rat’s spleen induced by 40 (mg/L) doses. Sections of splenic white pulp showed marked atrophy of lymphatic follicles of white pulp, as in Fig. 18A. In contrast, the cytoarchitecture of red pulp showed moderate sinusoidal congestion and an increase in the population of macrophages, which engulf hemosiderin pigment, as in Fig. 18B.
Fig. 17.
Sections of the spleen (H&E stained) for the group exposure to 20 (mg/L) doses sections were observed at 400 × magnification (A); section of the spleen shows hyperplasia of a lymphatic follicle within white pulp (Asterisks) with normal red pulp. B show lymphatic follicle within white pulp (lp) with mild congestion of sinusoid (Asterisks) and increased macrophages engulfing hemosiderin pigments (arrows) (color figure online)
Fig. 18.
Sections of the spleen (H&E stained) for the group exposure to 40 (mg/L) doses sections were at A 40× magnification, a section of the spleen shows atrophied lymphatic follicle within white pulp (Wp) sinusoidal congestion of red pulp (Rp). B 400× magnification, show atrophied lymphatic follicle within white pulp (L) with sinusoidal congestion of red pulp (Asterisks) (color figure online)
In terms of toxicity, the results indicate that the toxicity of CuO/ZnO core/shell NPs due to copper has higher dissolution in the stomach (De et al. 2019). This implies that the CuO/ZnO NPs with a size of 30 nm determined their relative order of toxicity and their effect on target organs. The practical implication of our histopathology study showed that CuO/ZnO core/shell nanoparticles could induce morphological and histological changes in the liver, kidneys, and spleen. The damage of this change is much lower and milder than that reported in the literature related to the toxicity of CuO and ZnO. This discrepancy might be due to various factors, like the size of particles and the doses. This also suggests that covering CuO nanoparticles with ZnO nanoparticles improves their toxicity properties.
Limitations of the study
The results of this study have to be seen in the light of several potential limitations:
The first limitation concerns the conditions of the study conducted in December/2020 and the difficulty in obtaining laboratory animals due to the COVID-19 Pandemic, which caused the closure of most research centers.
The second limitation is time constraints. A PhD students have to meet deadlines for submitting a thesis. Therefore, the time available for the toxicity estimation of CuO/ZnO core/shell NPs on other organs (such as the heart, brain, stomach, bone marrow, etc.) was insufficient. So we recommend the need for a future study (e.g., a longitudinal toxicity study) to determine the helpful medical applications
Lack of previous research studies on the topic. Citing and referencing prior research studies constitutes the basis of the literature review for the study. To the best of our knowledge and according to a literature survey, no report is available on the acute lethal effect and toxicity of biologic CuO/ZnO CS NPs.
Conclusion
All the animals did not show severe clinical signs of poisoning that would have led to mortality. Growth retardation in rats after exposure to CuO/ZnO core/shell nanoparticles was attributed to the infection with Hyperthyroidism. Due to the immune system's reaction to nanoparticles as a foreign factor, a 5 (mg/L) dose of CuO/ZnO core/shell NPs caused a significant alteration in white blood cell count. The considerable increase in RBC, Hb, and HCT at the dose of 5 (mg/L) suggests that these nanoparticles enhanced the rate of blood corpuscle production. These doses of NPs may assist in treating anemia. The elevated T4 level shows the rat’s infection by Hyperthyroidism. These results suggest that a dose of 5 mg/L CuO/ZnO NPs is less harmful to the liver and kidneys, which may help regulate thyroid hormones and treat hypothyroidism. Our findings indicate that CuO/ZnO core/shell NPs may have increased ROS generation, which may explain the toxicity observed in the liver, kidney, and spleen of treated female rats. Dosage quantity (1 mL) and long-term exposure, during which rats acclimate to CuO/ZnO core/shell nanoparticles exposure, may also explain the toxicity. This mechanism limits the absorption and excretion of CuO/ZnO core/shell nanoparticles, reducing their toxicity to animals. As demonstrated by hematological and histological investigations, low concentrations of CuO/ZnO core/shell nanoparticles are safe for the intended biomedical uses.
Acknowledgements
The authors would like to thank the support of the University of Al-Nahrain/Biotechnology Research Center/Animal House.
Funding
The authors declare that no funds, grants, or other support were received for conducting this study.
Data availability
All data related to generated or analyzed CuO/ZnO core/shell nanoparticles in this study are available in the [Synthesis of CuO/ZnO and MgO/ZnO Core/Shell Nanoparticles with Plasma Jets and Study of their Structural and Optical Properties, https://web.archive.org/web/20220508022312id_/https://kijoms.uokerbala.edu.iq/cgi/viewcontent.cgi?article=3225&context=home]. Other data related in medical application for CuO/ZnO core/shell nanoparticles during this study are included in this article.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akintelu SA, Folorunso AS, Folorunso FA, et al. Green synthesis of copper oxide nanoparticles for biomedical application and environmental remediation. Heliyon. 2020;6(7):04508. doi: 10.1016/j.heliyon.2020.e04508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amereh F, Eslami A, Fazelipour S, et al. Thyroid endocrine status and biochemical stress responses in adult male Wistar rats chronically exposed to pristine polystyrene nanoplastics. Toxicol Res. 2019;8(6):953–963. doi: 10.1039/c9tx00147f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashafa AOT, Orekoya LO, Yakubu MT. Toxicity profile of ethanolic extract of Azadirachta indica stem bark in male Wistar rats. Asian Pac J Trop Biomed. 2012;2(10):811–817. doi: 10.1016/S2221-1691(12)60234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boey A, Ho HK. All roads lead to the liver: metal nanoparticles and their implications for liver health. Small. 2020;16(21):2000153. doi: 10.1002/smll.202000153. [DOI] [PubMed] [Google Scholar]
- Bugata LSP, Venkata PP, Gundu AR, et al. Acute and subacute oral toxicity of copper oxide nanoparticles in female albino Wistar rats. J Appl Toxicol. 2019;39(5):702–716. doi: 10.1002/jat.3760. [DOI] [PubMed] [Google Scholar]
- Chatterjee K, Sarkar S, Rao KJ, et al. Core/shell nanoparticles in biomedical applications. Adv Colloid Interface Sci. 2014;209:8–39. doi: 10.1016/j.cis.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Chen Q, Li J, Li Y. A review of plasma–liquid interactions for nanomaterial synthesis. J Phys D: Appl Phys. 2015;48:424005. doi: 10.1088/0022-3727/48/42/424005. [DOI] [Google Scholar]
- De Jong WH, De Rijk E, Bonetto A, et al. Toxicity of copper oxide and basic copper carbonate nanoparticles after short-term oral exposure in rats. Nanotoxicology. 2019;13(1):50–72. doi: 10.1080/17435390.2018.1530390. [DOI] [PubMed] [Google Scholar]
- Du B, Yu M, Zheng J. Transport and interactions of nanoparticles in the kidneys. Nat Rev Mater. 2018;3(10):358–374. doi: 10.1038/s41578-018-0038-3. [DOI] [Google Scholar]
- Gopinath V, Priyadarshini S, Al-Maleki AR, et al. In vitro toxicity, apoptosis and antimicrobial effects of phyto-mediated copper oxide nanoparticles. RSC Adv. 2016;6:110986. doi: 10.1039/c6ra13871c. [DOI] [Google Scholar]
- Grigore ME, Biscu ER, Holban AM, et al. Methods of synthesis, properties and biomedical applications of CuO nanoparticles. Pharmaceuticals. 2016;9:75. doi: 10.3390/ph9040075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SE, Jin HE. Synthesis, characterization, and three-dimensional structure generation of zinc oxide-based nanomedicine for biomedical applications. Pharmaceutics. 2019;11(11):575. doi: 10.3390/pharmaceutics11110575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaviani E, Naeemi A, Salehzadeh A. Influence of copper oxide nanoparticle on hematology and plasma biochemistry of Caspian trout (Salmo trutta caspius), following acute and chronic exposure. Pollution. 2019;5(1):225–234. doi: 10.22059/POLL.2018.251034.383. [DOI] [Google Scholar]
- Khabbazi M, Harsij M, Hedayati SAA, et al. Effect of CuO nanoparticles on some hematological indices of rainbow trout Oncorhynchus mykiss and their potential toxicity. Nanomed J. 2015;2(1):67–73. [Google Scholar]
- Khorsandi L, Heidari Moghadam A, Jozi Z. Nephrotoxic effects of low-dose zinc oxide nanoparticles in rats. J Nephropathol. 2018;7(3):158–165. doi: 10.15171/jnp.2018.35. [DOI] [Google Scholar]
- Kumar R, Mondal K, Panda PK, et al. Core-shell nanostructures: perspectives towards drug delivery applications. J Mater Chem B. 2020;8(2020):8992–9027. doi: 10.1039/D0TB01559H. [DOI] [PubMed] [Google Scholar]
- Makhdoumi P, Karimi H, Khazaei M. Review on metal-based nanoparticles: role of reactive oxygen species in renal toxicity. Chem Res Toxicol. 2020;33(10):2503–2514. doi: 10.1021/acs.chemrestox.9b00438. [DOI] [PubMed] [Google Scholar]
- Moatamed ER, Hussein AA, El-desoky MM, et al. Comparative study of zinc oxide nanoparticles and its bulk form on liver function of Wistar rat. Toxicol Ind Health. 2019;35(10):1–11. doi: 10.1177/0748233719878970. [DOI] [PubMed] [Google Scholar]
- Mohammed RS, Aadim KA, Ahmed KhA. Estimation of in vivo toxicity of MgO/ZnO core/shell nanoparticles synthesized by eco-friendly non-thermal plasma technology. Appl Nanosci. 2022 doi: 10.1007/s13204-022-02608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed RS, Aadim KA, Ahmed KhA. Synthesis of CuO/ZnO and MgO/ZnO core/shell nanoparticles with plasma jets and study of their structural and optical properties. Karbala Int J Mod Sci. 2022;8(2):213–222. doi: 10.33640/2405-609X.3225. [DOI] [Google Scholar]
- Patrinoiua G, Hussienb MD, Calderón-Morenoa JM, et al. Eco-friendly synthesized spherical ZnO materials: effect of the core-shell to solid morphology transition on antimicrobial activity. Mater Sci Eng C. 2019;97:438–450. doi: 10.1016/j.msec.2018.12.063. [DOI] [PubMed] [Google Scholar]
- Peterson ME, Castellano CA, Rishniw M. Evaluation of body weight, body condition, and muscle condition in cats with hyperthyroidism. J Vet Intern Med. 2016;30(6):1780–1789. doi: 10.1111/jvim.14591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabi S, Sohrabnezhad S. Fabrication and characteristic of Fe3O4@ MOR@ CuO core-shell for investigation antibacterial properties. J Fluor Chem. 2018;206:36–42. doi: 10.1016/j.jfluchem.2017.12.010. [DOI] [Google Scholar]
- Rumbach P, Witzke M, Sankaran RM, et al. Decoupling interfacial reactions between plasmas and liquids: charge transfer vs plasma neutral reactions. J Am Chem Soc. 2013;135:16264–16267. doi: 10.1155/2014/426028. [DOI] [PubMed] [Google Scholar]
- Sang X, Fei M, Sheng L, et al. Immunomodulatory effects in the spleen-injured mice following exposure to titanium dioxide nanoparticles. J Biomed Mater Res A. 2014;102(10):3562–3572. doi: 10.1002/jbm.a.35034. [DOI] [PubMed] [Google Scholar]
- Sengul AB, Asmatulu E. Toxicity of metal and metal oxide nanoparticles: a review. Environ Chem Lett. 2020;18:1659–1683. doi: 10.1007/s10311-020-01033-6. [DOI] [Google Scholar]
- Shakibaie M, Shahverdi AR, Faramarzi MA, et al. Acute and subacute toxicity of novel biogenic selenium nanoparticles in mice. Pharm Biol. 2013;51(1):58–63. doi: 10.3109/13880209.2012.710241. [DOI] [PubMed] [Google Scholar]
- Sheta SM, El-Sheikh SM, Abd-Elzaher MM. Promising photoluminescence optical approach for triiodothyronine hormone determination based on smart copper metal-organic framework nanoparticles. Appl Organomet Chem. 2019;33(9):5069. doi: 10.1002/aoc.5069. [DOI] [Google Scholar]
- Singh N, Das MK, Gautam R, et al. Assessment of intermittent exposure of zinc oxide nanoparticle (ZNP)–mediated toxicity and biochemical alterations in the splenocytes of male Wistar rat. Environ Sci Pollut Res. 2019;26:33642–33653. doi: 10.1007/s11356-019-06225-4. [DOI] [PubMed] [Google Scholar]
- Sruthi S, Ashtami J, Mohanan PV. Biomedical application and hidden toxicity of zinc oxide nanoparticles. Mater Today Chem. 2018;10:175–186. doi: 10.1016/j.mtchem.2018.09.008. [DOI] [Google Scholar]
- Suryavanshi A, Khanna K, Sindhu K, et al. Magnesium oxide nanoparticle-loaded polycaprolactone composite electrospun fiber scaffolds for bone–soft tissue engineering applications: in-vitro and in-vivo evaluation. Biomed Mater. 2017;12(5):055011. doi: 10.1088/1748-605X/aa792b. [DOI] [PubMed] [Google Scholar]
- Tang H, Xu M, Zhou X, et al. Acute toxicity and biodistribution of different sized copper nanoparticles in rats after oral administration. Mater Sci Eng C. 2018;93:649–663. doi: 10.1016/j.msec.2018.08.032. [DOI] [PubMed] [Google Scholar]
- Wang D, Li H, Liu Z, et al. Acute toxicological effects of zinc oxide nanoparticles in mice after intratracheal instillation. Int J Occup Environ Health. 2017;23(1):11–19. doi: 10.1080/10773525.2016.1278510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Zang Y, Qu J, et al. The toxicity of metallic nanoparticles on liver: the subcellular damages, mechanisms, and outcomes. Int J Nanomed. 2019;14(2019):8787–8804. doi: 10.2147/IJN.S212907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef M, Al-Hamadani M, Kamel M. Reproductive toxicity of aluminum oxide nanoparticles and zinc oxide nanoparticles in male rats. Nanoparticle. 2019 doi: 10.35702/nano.10003. [DOI] [Google Scholar]
- Yousef MI, Mutara TF, El-Nabi Kamel MA. Hepato-renal toxicity of oral sub-chronic exposure to aluminum oxide and/or zinc oxide nanoparticles in rats. Toxicol Rep. 2019;6:336–346. doi: 10.1016/j.toxrep.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Zhao L, Luo J, et al. The toxic effects and mechanisms of Nano-Cu on the spleen of rats. Int J Mol Sci. 2019;20(6):1469. doi: 10.3390/ijms20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data related to generated or analyzed CuO/ZnO core/shell nanoparticles in this study are available in the [Synthesis of CuO/ZnO and MgO/ZnO Core/Shell Nanoparticles with Plasma Jets and Study of their Structural and Optical Properties, https://web.archive.org/web/20220508022312id_/https://kijoms.uokerbala.edu.iq/cgi/viewcontent.cgi?article=3225&context=home]. Other data related in medical application for CuO/ZnO core/shell nanoparticles during this study are included in this article.