Abstract

The excitatory amino acid transporter 2 (EAAT2) plays a key role in the clearance and recycling of glutamate - the major excitatory neurotransmitter in the mammalian brain. EAAT2 loss/dysfunction triggers a cascade of neurodegenerative events, comprising glutamatergic excitotoxicity and neuronal death. Nevertheless, our current knowledge regarding EAAT2 in neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS) and Alzheimer’s disease (AD), is restricted to post-mortem analysis of brain tissue and experimental models. Thus, detecting EAAT2 in the living human brain might be crucial to improve diagnosis/therapy for ALS and AD. This perspective article describes the role of EAAT2 in physio/pathological processes and provides a structure–activity relationship of EAAT2-binders, bringing two perspectives: therapy (activators) and diagnosis (molecular imaging tools).
1. Introduction
Glutamate, the primary excitatory neurotransmitter in the mammalian brain, is a poorly blood-brain barrier (BBB) penetrant nonessential amino acid, requiring synthesis within the central nervous system (CNS).1−3 In the CNS, glutamate is mostly synthesized via two classical pathways. The first is a de novo synthetic route using glucose and the anaplerotic enzyme pyruvate carboxylase, yielding glutamate by further transamination of α-ketoglutarate.4,5 The second is the glutamine-glutamate cycle which involves the exchange of these two amino acids between neurons and astrocytes (Figure 1).6 Astrocytes cease glutamatergic neurotransmission by removing glutamate from the synaptic cleft via the excitatory amino acid transporter 2 (EAAT2, human isoform) or the glutamate transporter 1 (GLT-1, rodent isoform that shares 96% identity with the human EAAT2).7−9
Figure 1.
Glutamate-glutamine cycle in the healthy brain. (A) Astrocytes are known to provide the glutamine required by neurons to synthesize GABA and glutamate. The glutamine efflux from the astrocytes is mediated via the system N transporter (SN1). Once in the extracellular space, neurons capture glutamine through the system A transporters (SA1 and SA2). In the neuronal intracellular space, glutamine is metabolized into glutamate by the mitochondrial enzyme glutaminase. Glutamate is then packed within synaptic glutamatergic vesicles (VGluT) via a Mg2+/ATP-dependent process and released to the extracellular compartment from the vesicles by a Ca2+-dependent mechanism. Of note, the glutamate concentration in the intracellular and extracellular space is 10 mM and 10 μM, respectively. Once glutamate is released in the synaptic cleft, it produces an excitatory postsynaptic potential, which is tightly controlled by a wide range of neuronal receptors, including ionotropic and metabotropic glutamate receptors (iGluRs and mGluRs). A balance between glutamate release and clearance is essential. Glutamate in the extracellular space is captured by astrocytes via EAAT2. (B) Topology diagram of EAAT2. The transport domain consists TM3, TM6, TM7, TM8, HP1, HP2 and the connecting HP1 and HP2 loops.
The reduced capacity of removing extracellular glutamate by EAAT2—due to transporter mislocalization10 or degradation11—is associated with a pathological phenomenon termed glutamatergic excitotoxicity, a common feature in neurodegenerative diseases such as Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD), and Huntington’s disease (HD).12−15 Experimental models and post-mortem analysis of human brain tissue from patients with AD, ALS, PD, and HD suggest that EAAT2 loss/dysfunction could be an early trigger evolving into chronic reactive astrogliosis, oxidative stress, and neuronal death.16−18 In this context, identifying molecules able to increase EAAT2 levels (increasing EAAT2 expression through translational activation or preventing EAAT2 degradation) or enhance EAAT2 function (positive allosteric modulator, PAM, promoting glutamate binding) could lead the way for the development of therapeutic agents, while EAAT2 binders (inhibitors and PAM) could inspire the design of novel imaging diagnostic tools.
Minimal invasive neuroimaging techniques, such as positron emission tomography (PET), are key to evaluate neurotransmitter trafficking and transporter density. Although the use of PET radiolabeled glutamate (e.g., [11C]glutamate) may work to investigate neurotransmitter trafficking and availability of its transporters/receptors, it would lack of selectivity and potentially be converted to glutamine, confounding imaging results.19,20 Note that there are no brain-penetrant EAAT2-selective PET radiotracers available for clinical research (an early phase 1 EAAT2 targeted positron emitting agent, [18F]fluorenylasparaginate methyl ester - [18F]RP-115 - started to recruit on May 2022 for which no published data are available yet, clinical trials: NCT05374378).
The initial step for developing a PET radiotracer targeting EAAT2 should be the selection of a molecule with high affinity and selectivity toward EAAT2. Therefore, in this perspective article, we conduct a structure–activity relationship of the library of EAAT2 inhibitors and PAMs defining appropriate chemical features that might help with the development of EAAT2 selective PET radiotracers. In addition, we discuss important findings regarding EAAT2 structure/isoforms and examine experimental evidence of EAAT2 dysfunction/loss in neurodegenerative diseases to emphasize the importance of detecting EAAT2 in vivo.
2. A Snapshot of the Glutamatergic Neurotransmission and Transporters
The brain is formed by a complex and intertwined network of cells that communicate in a subsecond frequency. Excitatory neurotransmission is largely—more than 90%—accounted by the glutamatergic system.2,21,22 Glutamate, once released by neurons, binds to a group of high-affinity ionotropic receptors (NMDAr , KAr and AMPAr) and eight isoform metabotropic glutamatergic receptors (e.g., mGluR1–8),23−25 until eventually most of the extracellular glutamate is captured by EAAT’s.26,27 There are five known EAATs in the mammalian body: EAAT1 (GLAST for rodents),28 EAAT2 (GLT-1 for rodents),29 EAAT3 (EAAC1 for rodents),29 EAAT4,30 and EAAT5.31 Immunohistochemical analyses suggested that EAAT1 and EAAT2 are mainly localized in the membrane of astrocytes in the CNS.32,33 EAAT3 is predominantly found in the soma and dendrites of both excitatory and inhibitory neurons as well as EAAT4 is largely expressed in Purkinje cells and in the membrane of postsynaptic neurons.34,35 EAAT5 is exclusively located in the retinal ganglion cells.36
2.1. Excitatory Amino Acid Transporter 2
The EAAT2, a membrane-bound protein composed of 574 amino acids, is responsible for more than 95% of total glutamate uptake in the forebrain region of the adult mammalian brain37 (steady state affinity (Km) of 10–20 μM and binding affinity (Kd) of 140 μM).38 Importantly, more than 80% of total EAAT2 expression in the mammalian brain is detected in the membrane of astrocytes.39 Glutamate transport via EAAT2 is accompanied by the entry of three sodium ions and one proton, combined with the release of one potassium ion (Figure 1).28 EAAT2 has eight α-helical transmembrane regions (TMs 1–8) and two helical hairpin loops (HP1 and HP2).40 The EAAT2 usually forms a homotrimer to enable glutamate uptake, in which TMs 2–5 are responsible for trimerization of EAAT2.41 The transport domain consists of four TMs (TM3, TM6, TM7, and TM8), HP1, HP2 and the connecting HP1 and HP2 loops, respectively.
2.2. EAAT2 Splicing and Localization
The EAAT2 belongs to the solute carrier 1 (SLC1) family encoded by the SLC1A2 gene. A single functional nucleotide polymorphism, rs4354668 (181 T/G), located in the gene promoter region seems to modulate the expression of EAAT2.28 Indeed, EAAT2 has a complex pattern of alternative splicing mostly at the C-terminal position. The main splice variant of EAAT2 in the human brain is termed EAAT2b (99.5% identity to the full-length EAAT2), which uniquely differs in the C-terminal amino acid sequence.42 The full-length EAAT2 represents more than 90% of total EAAT2 content in the forebrain region (25-fold higher than EAAT2b in the whole brain).43,44 EAAT2 is mainly expressed in the caudate nucleus, nucleus basalis of Meynert, spinal ventral horn, cerebral cortex, and hippocampus and found in lower levels in other CNS regions.45 In addition, expression of EAAT2 (GLT-1) in rat hippocampus and cerebellum presents 12,000 and 2800 molecules per μm3 tissue, respectively.46
2.3. Binding Sites
A crystal structure analysis of the glutamate transporter of Pyrococcus horikoshii (GltPh)—a bacterial homologue that shares 37% identity with human EAAT2—helped build our knowledge about EAAT2 binding pockets.40 To date, one binding site (BS1) for substrates (glutamate/aspartate) and two binding sites (BS2 and BS3) for modulators have been identified (Figure 2). BS1 is formed in the C-terminal portion of each EAAT2 monomer, which includes HP1, HP2, TM7, and TM8 (Figures 1 and 2). In fact, the D475 and R478 amino acid residues in TM8 are suggested to be the main players in the formation of this binding pocket.47−49 Two independent research groups have releveled the cryo-electron microscopy (cryo-EM) structural information on human EAAT2 in complex at BS1 with glutamate50 (Figure 2) and a selective inhibitor WAY-213613.50,51 BS2 is formed by the H71 amino acid residue in the TM2, L295 and K299 in TM5, and W472 in TM8 (Figure 2).52 More recently, BS3 defined by M477 on TM8, and F345, F348, F352, W355 on HP1 was identified as a binding pocket for negative allosteric modulators (Figure 2).51,53
Figure 2.

Three-dimensional structure of EAAT2 in complex with glutamate (PDB code 7XR4). Close-ups on the glutamate binding site (BS1). Two peripherical binding sites (BS2 and BS3) are also shown.
3. EAAT2 in Brain Disorders
Over the last decades, EAAT2 loss/dysfunction in neurodegenerative diseases became a potential target for pharmacological intervention in the brain. Here, we briefly discuss experimental findings and immunohistochemical reports of post-mortem human brain tissue which suggest that EAAT2 impairment and glutamatergic excitotoxicity precede neuronal loss and clinical manifestations in ALS and AD and other neurodegenerative diseases.
3.1. Amyotrophic Lateral Sclerosis
ALS is a progressive adult-onset neurodegenerative disease affecting neurons associated with voluntary muscle movement.54 ALS is the most common motor neuron disease in adults, and only 5–10% of cases have a genetic link—familial ALS (fALS).55 In fALS mutated superoxide dismutase 1 (SOD1)—a critical enzyme for maintaining cellular redox homeostasis—adopts an aberrant conformation causing protein aggregation.56 The common ALS clinical symptoms are fasciculations, tight muscles, difficulty chewing, and muscle cramps.57 Such clinical manifestations are primarily due to selective degeneration of upper and lower motor neurons, which are located in the motor cortex and in the brainstem and spinal cord, characterizing the main CNS regions affected in this disease.58 Even though ALS etiology remains unknown, glutamate excitotoxicity and EAAT2 impairment could be potential triggers in ALS development.59 Interestingly, sporadic ALS patients have considerable loss in EAAT2 protein levels in the motor cortex (71 ± 12%) and spinal cord (57 ± 12%) compared to healthy subjects.14 In addition, it seems that EAAT2 loss could originate from aberrant mRNA processing.60 Specifically, brain regions most affected by ALS pathology presented reduced levels of EAAT2 mRNA variants that retained intron-7 or skipped exon-9 (EAAT2-e9).60,61 Further work could not confirm that the reduction of this EAAT2 translational variant was specific of ALS, thus whether or not the intron-7 and the exon-9 variants are differentially expressed in ALS patients compared to healthy subjects remains under debate.62,63
A transgenic mouse model bearing the Cu2+/Zn2+ SOD1 mutation was developed to explore ALS pathophysiology.64,65 Interestingly, in SOD1 mice the EAAT2 loss of function and expression preceded neuronal loss and disease onset.66 Of note, disturbances on glutamatergic neurotransmission are associated with disease progression and seem to be region-specific, mostly affecting the spinal cord.67In vitro studies corroborate these results, showing hypertrophy of glial fibrillary acidic protein (GFAP)-positive astrocytes (also an index of reactive astrogliosis) and alterations in EAAT2 levels prior to motor neuron loss.68 Reactive astrocytes in ALS are known to mediate the release of inflammatory cytokines such as tumor necrosis factor-α (TNF-α).69 TNF-α and downstream NFκB signaling have been previously shown to suppress EAAT2 expression.70 Interestingly, it seems that deletion of membralin, an endoplasmic reticulum protein, in SOD-1 mice is a key figure in this process. More specifically, membralin deletion exacerbated the activation of TNF-α receptor and NFκB pathway and reduced EAAT2 transcription.71
Gibb and colleagues investigated if EAAT2 splicing could be involved in the EAAT2 dysfunction in ALS.72 Using the SOD-1 mouse model, the authors observed that EAAT2 impairment could be linked to caspase-3 activation.72 Caspase-3 cleaved EAAT2 at the cytosolic C-terminal domain producing two fragments: (1) truncated EAAT2 and (2) carboxy terminus of EAAT2 (CTE). Then, CTE is SUMOylated (CTE-SUMO-1) and accumulates in the spinal cord of SOD-1 mice.72 CTE-SUMO-1 accumulation was mostly seen in the presymptomatic stage and specific mouse brain regions related to ALS.72 In agreement with these findings, a mutation on the EAAT2 site for caspase-3 cleavage extended mice life span and delayed the progression of motor changes.73
3.2. Alzheimer’s Disease
Over the last decades, EAAT2 loss/dysfunction in AD, the most common cause of dementia, became a potential target for pharmacological intervention. Here, we briefly discuss immunohistochemical reports from post-mortem human brains and experimental models showing that EAAT2 dysfunction/loss and glutamatergic excitotoxicity may precede neurodegeneration and clinical manifestations in the AD continuum.
AD is characterized by a slow decline in memory, thinking, and reasoning abilities, mainly occurring in its sporadic form. Genetic-linked AD represents less than 1% of the cases (mostly related to mutations in the genes of amyloid precursor protein, presenilin 1 and 2).74 The clinical manifestation of AD includes memory loss, space/time disorientation, and behavioral symptoms such as depression and personality changes.75 AD pathogenesis, according to the National Institute of Aging – Alzheimer’s Association research framework, is defined by a biological construct (based on fluid and imaging biomarkers) comprising Aβ deposition (A), pathologic tau (T) and neurodegeneration (N) – the AT(N) system.76 Remarkably, biological changes in AD patients are suggested to start 20–30 years before the symptomatic stages; therefore, the need for identifying new biomarkers to detect these early alterations is fundamental to improving disease diagnosis and therapy.77 In this context, glial cells (mostly astrocytes and microglia) are currently suggested to be early responders to pathological changes in AD.78−80 Astrocytes undergo functional, morphological, and molecular changes in response to AD pathology—a phenomenon termed reactive astrogliosis. Early evidence showing that astrocytes become reactive in AD was mostly associated with upregulated mRNA and protein levels of the GFAP.81,82 Nevertheless, the current view on reactive astrogliosis indicates that a single biomarker, such as GFAP upregulation, is unlikely to represent the whole picture of reactive astrocytes in the human brain.83 For instance, decreased expression level of EAAT2 characterizes an additional biomarker to identify reactive astrocytes in AD.83 The several lines of research on EAAT2 in AD are discussed below and subdivided in human brain findings and animal/cellular models of AD.
3.3. Immunohistochemical Reports of Post-Mortem Human AD Brain Tissue
Immunohistochemical reports of post-mortem AD brain tissue indicated that EAAT2 density loss correlates with upregulated levels of GFAP in the temporal cortex of AD individuals, a region of high Aβ pathology.84 In a recent meta-analysis of post-mortem immunohistochemical reports, EAAT2 was downregulated in AD versus control brains.85 Although lower expression of EAAT2 in AD brains is not a consensus,86,87 the presence of EAAT2 mRNA variants could also account for these inconsistencies observed by different groups.88 Specifically, higher expression of two exon-skipping mRNA variants (EAAT2-e7 and EAAT2-e7e9) was observed in brain regions commonly affected by AD pathology, while wild-type EAAT2 mRNA levels were decreased in post-mortem brain tissue of AD patients.88 Remarkably, the increase of mRNA variants of EAAT2-e7 and EAAT2-e7e9 seems to correlate with pathology severity in these brains.88 Furthermore, reactive astrocytes are very prone to oxidative stress, and, EAAT2, in particular, stands out as a specific target of reactive oxygen species in astrocytes.89 Woltjer et al. demonstrated that detergent insoluble EAAT2—possibly derived from oxidation processes—accumulates in the hippocampus and frontal cortex of AD patients and correlates with disease progression.90 In keeping with this, post-translational modifications by either defective mRNA splicing or oxidative damage may affect the availability of EAAT2 in the cell surface, reducing the glutamate uptake capacity and ultimately causing neuronal death.
3.4. Animal Models of Aβ Pathology: Immunohistochemistry Findings
Animal and cellular models are useful tools to further explore potential mechanisms that may explain the interplay between Aβ pathology, reactive astrogliosis, and EAAT2 loss.91 Different animal models seek to reproduce the main pathological hallmarks of AD. However, it is important to emphasize that rodent models such as APP/PS1 (mice containing human transgenes for APP Swedish mutation and PSEN1 containing an L166P mutation)92 or 5xFAD (mice containing 5 human AD-linked mutations) do not reflect the complexity of AD pathogenesis, but rather mimic amyloidosis in AD. Below, we discuss the main findings regarding EAAT2 loss/dysfunction in different models of Aβ pathology.
One of the first reports to evaluate EAAT’s levels in animal models of Aβ pathology, using transgenic mouse overexpressing human APP bearing the London mutation, demonstrated that decreased EAAT2 expression precedes the formation of insoluble Aβ aggregates in the neocortex, while EAAT2 mRNA levels remain stable.93 Similarly, Schallier and colleagues, using AβPP23 mice, showed that lower expression levels of EAAT2 in the pre-Aβ plaque stage occurs independently of GFAP upregulation in the frontal cortex and hippocampus.94 Furthermore, in APP/PS1 mice, partial loss of EAAT2 (genetically modified mice lacking one EAAT2 allele) accelerated memory impairment onset95 and associated with insoluble Aβ deposits.96 In a similar extent, lower levels of hippocampal EAAT2 and accelerated cognitive deficits were also observed in adeno-associated virus-based APP/PS1 mice.97 Nonetheless, it is important to mention that in the medial prefrontal cortex of 3xTg-AD mouse, EAAT2 levels were comparable to the wild-type animals.98
In summary, we speculate that functional changes (i.e., EAAT2 loss and decreased glutamate uptake capacity) may precede astrocytic morphological alterations (e.g., GFAP increased levels) and deposition of insoluble Aβ aggregates in the early stages of AD. In addition, alterations in EAAT2 levels, which were not observed in all brain regions, suggest that EAAT2 loss is not a universal marker for reactive astrogliosis (i.e., some astrocytic populations may maintain EAAT2 levels unaltered), thus, corroborating with the concept of astrocyte heterogeneity in AD.
3.5. In Vitro Insights on Aβ-EAAT2 Interaction and Glutamatergic Excitotoxicity
Studies in vitro (cell culture and brain slices) offer the possibility of exploring mechanistic insights into the association between preplaque Aβ species (e.g., soluble Aβ oligomers, AβOs) and EAAT2 loss in astrocytes. In primary culture of astrocytes, soluble AβOs internalize EAAT2 in the astrocytic membrane disturbing glutamate uptake.99 Studies on brain hippocampal slices showed that blocking glutamate uptake by EAATs, reduced synaptic response via AMPAr, induced activation of extrasynaptic NMDA receptors and depolarization of astrocytic membrane.100 Other mechanisms have been proposed to explain EAAT2 loss in the membrane of astrocytes in the context of Aβ pathology, such as EAAT2 interaction with presenilin 1 (PS1)—the catalytic component of the amyloid precursor protein-processing enzyme, γ-secretase—and cholesterol metabolism.101,102 Specifically, increased cholesterol 24S-hydroxylase activity caused the reduction of cholesterol levels in the astrocytic membrane and led to the dissociation of EAAT2 from lipid rafts and, consequently, EAAT2 loss.102
Altogether, these findings highlight the strong association between initial Aβ pathology and EAAT2 dysfunction in AD, either in a direct manner (AβOs-induced decrease on EAAT2 levels by physical interaction and internalization of EAAT2 in the membrane of astrocytes or via downstream signaling pathways, e.g., CN/NFAT pathway103) or by alternative mechanisms associated with Aβ aggregation, such as cholesterol dyshomeostasis (a catalyzer of Aβ aggregation process104) and PS1 expression (associated with APP cleavage and Aβ formation).
3.6. Tau and EAAT2 Dysfunction
Disturbances in EAAT expression/function are tightly associated with early AD changes, including reactive astrogliosis and Aβ pathology, extensively discussed above. However, a link between pathological tau (usually viewed as a later pathological event in the AD continuum) and EAAT2 was demonstrated in temporal cortex homogenates from AD brains.105 Specifically, EAAT2 was shown to interact with hyperphosphorylated tau rather than nonpathological tau.105 In a transgenic mouse model of astrocytic tau pathology (GFAP/tau Tg mice), decreased EAAT2 levels were evident throughout the spinal cord and reflected on motor impairment in these animals. To a lesser extent, EAAT2 loss was observed in the occipital and frontal cortices.106 Complimentarily, in a very recent work it was shown that tau oligomers, following internalization into the astrocytic intracellular space, inhibited EAAT2 expression and decreased the capacity of astrocytes to uptake glutamate.107 These findings suggest that, perhaps, EAAT2 dysfunction and reactive astrogliosis are not only associated with Aβ pathology in AD but also with pathological tau in the later stages of the AD continuum.
3.7. Other Neurodegenerative Diseases
The implication of EAAT2 dysfunction/loss in other common neurodegenerative disease, such as HD and PD, has also been investigated, although not as much as compared to studies in AD and ALS brains and experimental models.
HD is a neurodegenerative disease characterized by cognitive, motor, and psychiatric dysfunction. The pathophysiology of HD is typically associated with an inherited gene—huntingtin—which disrupts multiple cellular processes leading to neuronal loss in basal ganglia and cortical regions of the human brain.108 Consistent evidence, based on immunohistochemical analysis of post-mortem HD brain tissue, indicated prominent loss of EAAT2 expression and mRNA levels in subcortical regions of the human brain such as striatum, neostriatum, and putamen.109,110 Interestingly, Arzberger and colleagues found that global EAAT2 mRNA levels decreased in neostriatum of HD, in correlation to disease severity, but identified increased number of astrocytes expressing EAAT2, putting forward a compensatory astrocytic mechanism to prevent glutamate excitotoxicity and neuronal death.110
PD is the second most prevalent neurodegenerative disease in which clinical manifestation, in the context of motor function impairment, overlaps with HD comprising bradykinesia, tremor and postural instability. PD pathophysiology is associated with misfolding of α-synuclein—a protein whose aggregation leads to the formation of inclusions termed Lewy bodies—and the progressive loss of dopaminergic neurons in the substantia nigra (a component of the basal ganglia). The involvement of EAAT2 was mostly demonstrated in experimental models of PD, highlighting the strong association between reactive astrocytes and EAAT2 loss in the striatum.111−114 Interestingly, Wnt1 promoted increased EAAT2 expression in astrocytes which induced protective effects on dopaminergic neurons.115 Another study reported that exercise intervention in 6-hydroxydopamine-induced PD rats significantly improved the motor dysfunction of PD model rats, increased the ability of striatal glutamate reuptake significantly, and upregulated the expression levels of EAAT2 protein.116 In human brains, a recent work showed that EAAT2 trafficking in the astrocytic membrane is affected by the leucine-rich repeat kinase 2 (LRRK2) pathogenic variant G2019S, which is considered a contributor of late onset familial PD.117 Specifically, EAAT2 downregulation in the striatum correlated with increased GFAP positive astrocytes in caudate and putamen of PD patients as compared to healthy controls.117 Research in the context of reactive astrogliosis in PD pathogenesis is prominent.118,119 Therefore, detecting EAAT2 in vivo could be an additional tool to increase our knowledge on the role of reactive astrocytes in the early stages of PD.
4. EAAT2 Activators
The involvement of EAAT2 loss/dysfunction in neurodegenerative diseases suggests that restoring its expression/function could be an alternative to halt the downstream cascade of neurodegenerative processes or, at least, to attenuate the excitotoxic process. In this context, transcriptional (increase gene transcription) and translational (increase the protein expression) activators and/or reducing protein turnover can increase EAAT2 expression, while PAMs restore the EAAT2 function.18 Therapeutic targeting EAAT2 seems promising, since higher EAAT2 expression was associated with improved cognitive function and brain energetic metabolism.120,121 In the paragraphs below, we discuss the whole library of EAAT2 activators and PAMs.
4.1. EAAT2 Transcriptional Activators
The beta-lactam antibiotic ceftriaxone increases EAAT2 expression at the transcriptional level through NF-κB-mediated EAAT2 promoter activation and restored EAAT2 function in animal models of ALS, AD, and PD by delaying loss of neurons and muscle strength, improving cognitive performance, and increasing survival.96,122−125 The clinical efficacy of ceftriaxone was tested so far in ALS,126,127 and PD (on going, NCT03413384). However, Fumagalli et al. showed that ceftriaxone was unable to prevent loss of EAAT2 levels and activity following growth factor withdrawal in primary mouse striatal astrocyte.128 Dexamethasone has been previously shown to be an efficient inducer of EAAT2 in cortical astrocytes and increase in activity in cortical and striatal astrocytes.129,130 Tamoxifen and Raloxifene increased EAAT2 protein expression by the activation of multiple signaling pathways including ERK, EGFR, and CREB mediated by estrogen receptors (ERs) ER-α, ER-β, and GPR30 as well as increased EAAT2 mRNA in astrocytes.131−133 Nevertheless, since mRNA levels of EAAT2 might not be affected in AD brains,87,134 transcriptional activators may not be the strategy to pursue for therapeutic intervention.
4.2. EAAT2 Translational Activators
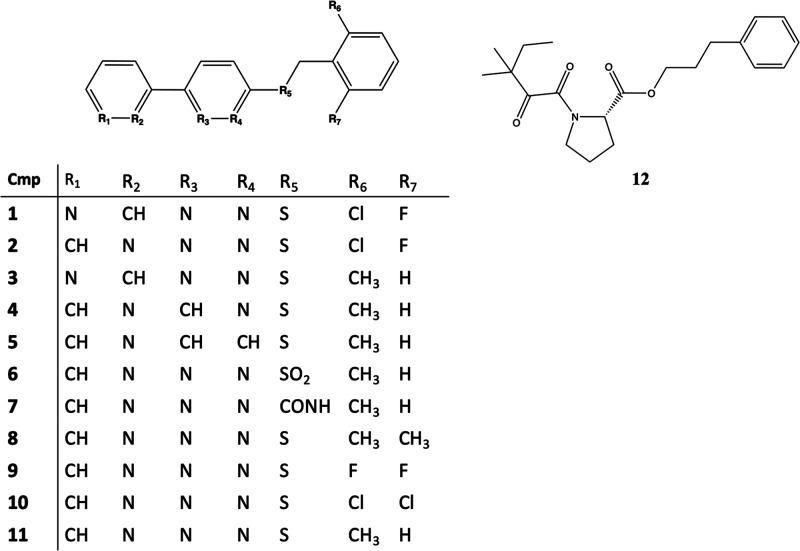
An initial high-throughput screen of 140,000 compounds identified translational activators of EAAT2 such as compound 1 (Figure 3), which increases EAAT2 protein levels 2-fold.135 Further exploitation of pyridazine-based compounds may help in determining the specific molecular components required for enhancing EAAT2 protein levels. The increase in EAAT2 protein levels compared to vehicle (dimethyl sulfoxide only) were adopted to estimate the potency of each compound. At first, it was demonstrated that replacing the 2-pyridyl in 1 for a 3-pyridyl (2) had a similar effect in increasing EAAT2 levels. Importantly, modifications in the pyridazine ring had a negative impact.136 Instead replacing the 2-Cl-6-F-Bn (1) with a 2-Me-Bn (3) or an unsubstituted Bn group, the EAAT2 level increased >3-fold. By removing one (4) or two nitrogen atoms (5) of the pyridazine ring, the increase on EAAT2 protein levels is completely lost for 4 or reduced by half compared to compound 1, respectively.136 Furthermore, the sulfur link of 1 seems crucial for increasing EAAT2 levels, as oxidation into a Sulphone (6) or replacement to an amide group (7) decreased activity from 2-fold to 1.1- and 1.4-fold, respectively.136 The addition of a further methyl group on the aromatic ring of the benzyl group resulted in stronger activity as observed by comparing compounds bearing a 2,6-Di-Me-Bn (6.5-fold increase, 8) to compound 1 (3.5-fold increase).136 Interestingly, the substitution of the two methyl groups in 8 with two fluorine atoms (9) or two chlorine atoms (10) decreases the effect from 6.5-fold to 2.2-fold or 3.9-fold, respectively. To further explore the efficacy of these pyridazine-based compounds, in a mixed culture of astrocytes and neurons, compound 11 (LDN/OSU-0212320) prevented glutamate excitotoxicity by selective increase on EAAT2 expression.137 In keeping with this, translational activators of EAAT2 may have a potential therapeutic application in neurodegenerative diseases in which EAAT2 damage occurs mostly at the posttranslational level, such as AD.88,138 Loss of EAAT2 protein in APPSw,Ind mice is caused by disturbances at the post-transcriptional level because EAAT2 mRNA is not decreased. The treatment with 11 of APPSw, animal model of AD, reversed memory and learning deficits after a short period of treatment, sustained beneficial effects on cognitive functions even after 1 month of treatment cessation, indicating a potential for disease modification, restored synaptic integrity, and increased EAAT2 expression via the translational rather than the transcriptional activation mechanism, resolving the central problem of reduced EAAT2 expression.139 Other translational activators are sulbactam, amitriptyline, riluzole, GPI-1046, and MS-153. Sulbactam upregulated EAAT2 expression in rats and prevented or reversed the EAAT2 downregulation normally induced in the ischemic rat brain.140,141 Chronic amitriptyline administration to rats produced upregulation of EAAT1 and EAAT2 in spinal cord.142 Riluzole, an FDA-approved benzothiazole drug, may exert its neuroprotective effects by changing the relative affinity for glutamate to EAAT2128 and increasing EAAT2 levels.129 Moreover, Liu et al. showed that riluzole increased EAAT2 protein expression in vitro, a phenomenon associated with the increased expression of HSP70 and HSP90.143 The non-immunosuppressant neuroimmunophilin GPI-1046 (12) exerted both neuroprotective and neuroregenerative effects in cell culture and in animal models of ALS. 12 induced both expression and activity of EAAT2 in rat spinal cord cultures and in rat brain homogenates without appreciable effects on gene expression. Chronic oral administration of 12 prolonged survival in transgenic mouse model of ALS and produced increased levels of spinal cord EAAT2 protein.144 MS-153 enhanced the function of EAAT2 in vitro, and it has shown neuroprotective effects in multiple experimental models associated with glutamate excitotoxicity (including traumatic brain injury, ischemia, addiction, and anxiety).145−149 Nevertheless, it seems that MS-153 activity is mostly linked to modulation of calcium channel currents via interaction with protein kinase C, and not through EAAT2 activation.150
Figure 3.
EAAT2 translational activators. Compound 11 is also known as LDN/OSU-0212320.
4.3. EAAT2 PAM
Activators/enhancers of EAAT2 activity could restore glutamate uptake and prevent glutamatergic excitotoxicity.151 Multiple attempts have been made over the last decades to design selective EAAT2 activators/enhancers.152 An example is Parawixin1, a compound isolated from the Parawixia bistriata spider, which enhanced EAAT2-mediated glutamate uptake by interacting with the trimerization region, located between TM2 and TM5 of EAAT2.153,154
Kortagere and colleagues155 identified three selective PAMs of EAAT2, in which compound 13 (GT951, Figure 4) was considered the best mediator of glutamate excitotoxicity by interacting with the TM2, TM5, and TM8 (BS2, Figure 2) of EAAT2.151,155 In primary culture of neurons and astrocytes, compound 13 was neuroprotective improving the uptake of glutamate via EAAT2 in a noncompetitive mechanism.151 Glia incubated for 24 h with or without a compound similar to 13, where the (trifluoromethyl) phenyl) piperazin motif is substituted with a cyclohexylpiperazin (GT949), revealed that EAAT2 expression is unaffected.155 Despite the high efficacy of 13, it has very poor drug-like properties including high lipophilicity and poor aqueous solubility which limit its bioavailability in vivo. Next, an optimization campaign led to the design of 14 (GTS511, EC50 3.8 ± 2.2 nM) with more favorable drug-like properties. Docking studies showed that 13 and 14 bind to BS2 with strong interactions forming hydrogen-bond, cation-π, and hydrophobic interactions, suggesting direct interactions with M86, L295, K299 (TM5), S465, and W472.155,156
Figure 4.

Structures of PAMs.
The PAM 15 ((R)-AS-1, Figure 4) revealed favorable anticonvulsant and safety profiles, displaying a good permeability in the parallel artificial membrane permeability assay (PAMPA), an excellent metabolic stability in human liver microsomes (HLMs), no influence on CYP3A4/CYP2D6 activity, as well as no hepatotoxic properties in HepG2 cells. 15 protected mice significantly against seizures in acute animal models of seizures. 15 showed a potency of 25 ± 21 nM and efficacy of 174 ± 13% for glutamate uptake augmentation in cultured glia. Studies in cultured glial cells revealed that the addition of a fluorine atom in 16 (Figure 4) had a more potent effect (with an EC50 of 0.1 ± 0.3 nM) than what was observed for 15 (∼20 nM) with a similar efficacy of glutamate transport augmentation. Similarities between 13 and 15–16 included bioisosteric replacement of the 5,6-dihydropyridin-2(1H)-one ring with pyrrolidine-2,5-dione; the tetrazole moiety with an amide fragment, as well as exchange of phenylethyl substituent to benzyl. Molecular docking simulations on EAAT2 revealed that binding site of 15–16 coincides with the binding site of 13. Molecular interactions stabilizing the bound form include a cation-π interaction between K299/R476 and the benzene ring of the compounds, strong hydrophobic interactions involving, e.g., M86, and hydrogen bonds between the pyrrolidine-2,5-dione ring and K90 and D238.1570
Because positive allosteric modulation is a fast direct process, it does not require synthesis and trafficking of new protein and will consequently have immediate acute effects and not rely on prophylactic pretreatments. PAMs of EAAT2 have a promising clinical potential by enhancing the removal of excessive glutamate in the synaptic cleft and preventing glutamate-mediated excitotoxicity. Future preclinical studies on PAMs in rodent models of neurodegeneration are needed to evaluate their efficacy.
5. EAAT2 Inhibitors
The ever-growing interest in understanding glutamate uptake and EAAT2 function led to the development of multiple EAAT2 inhibitors. Specifically, these compounds are of great interest to the development of PET radiotracers that could help to assess EAAT2 density in the living brain. In the following subsections, we describe the plethora of restricted glutamate analogues and aspartate derivatives that bind to EAAT2, constructing a structure–activity relationship to indicate which compounds have higher affinity and selectivity.
5.1. EAAT2 Inhibitors: Aspartate Analogues
l-glutamate and d- or l-aspartate bind to EAAT2.157−159 Back in the 1970s, Balcar and Johnston synthesized the aspartate analogue threo-3-hydroxy-l-aspartate (17, Figure 5), which has been described, for many years, as the most potent inhibitor of the EAAT’s (IC50 = 31 ± 5.8, Table 1).16017 contains an amino and carboxylic acid (highlighted in blue in Figure 5), a hydrogen-bond donor/acceptor carboxylic acid (black), and an alcohol functional group (Figure 5). To understand the preferable conformation and isomeric forms to inhibit the glutamate uptake via EAATs, different stereoisomers of 17 were synthesized. A comparison between l-, d-, and dl-mixture demonstrated similar affinity to EAATs (IC50 = 3.2, 5.6, and 4.0 μM, respectively). Further studies established that 17 behaves as a EAAT’s substrate (i.e., uptake by the transporter)36 and lacks selectivity to inhibit glutamate uptake,30 binding also to NMDA receptors.161 These works have encouraged the development of selective aspartate analogues binding EAAT2. The hydroxyl group at the C3-position of 17 allows for exploring the structure–activity relationship of novel aspartate analogues and has provided strong inhibitors of the EAAT2 over the last decades.162
Figure 5.
EAAT2 binders as aspartic acid analogues 17–31.
Table 1. Aspartate Analogues Inhibitors of EAAT2.
| IC50 (μM) |
Ratio |
|||||
|---|---|---|---|---|---|---|
| Compounds | EAAT1 | EAAT2 | EAAT3 | Ratio EAAT1 vs EAAT2 | Ratio EAAT3 vs EAAT2 | Ref |
| 17 | 96 ± 13 | 31 ± 5.8 | – | 3.1 | – | (163) |
| 18 | 67 ± 7.5 | 5.5 ± 1 | – | 12.1 | – | (163) |
| 19 | 0.116 | 0.059 | 2.4 | 2.0 | 40.6 | (170) |
| 20 | – | 1.2 | 4.1 | – | 3.4 | (170) |
| 21 | 0.022 | 0.017 | 0.3 | 1.3 | 17.6 | (170, 171) |
| 22 | – | 0.021 | 0.034 | – | 1.6 | (170) |
| 23 | 2 | 0.1 | 10 | 20 | 100 | (173) |
| 24a | 0.3 | 0.2 | 0.65 | 1.5 | 3.3 | (173) |
| 24b | 0.15 | 0.1 | 0.2 | 1.5 | 2 | (173) |
| 24c | 0.1 | 0.08 | 0.05 | 1.3 | 0.6 | (173) |
| 24d | 3.8 | 0.1 | 2.4 | 38 | 24 | (173) |
| 25 | 2.9 | 0.13 | 14.5 | 22 | 111.5 | (173) |
| 26 | 5 | 0.08 | 3.8 | 62.5 | 47.5 | (173) |
| 27 | 0.8 | 2.4 | 1.2 | 0.3 | 0.5 | (168) |
| 28 | 18 | 28 | 13 | 0.6 | 0.5 | (168) |
| 29 | 1.6 | 4.7 | 2.0 | 0.3 | 0.4 | (168) |
| 30 | 100 | 2.8 | 150 | 35.7 | 53.5 | (168) |
| 31 | 2.3 | 0.5 | 20 | 4.6 | 40 | (168) |
The dl-threo-β-benzyloxyaspartate (18, Figure 5) was synthesized by the addition of a aromatic substituent (highlighted in green in Figure 5) at the alcohol of 17,163 with an attempt to avoid the substrate-like behavior of 17.36 Indeed, 18 had a nonsubstrate inhibitor profile and selectivity for EAAT2 over GluRs (>100-fold).163 Currently, the 18 is commercially available and is widely applied in studies involving both physiological and pathophysiological roles of EAAT2.164−166 Yet, 18 lacked selectivity toward EAAT3, EAAT4, and EAAT5.167 The substitution of OCH2 group with amide group displayed a weak inhibitory activity at EAAT2 (IC50 ∼ 70 μM) while being inactive at EAAT1 and EAAT3 at concentrations up to 300 μM.168 Maintaining the ether group of 18, the modification at the benzyloxy ring has been explored in the search of additional interactions in BP1 in order to improve EAAT2 selectivity.169 Indeed, slight variations in residues Leu467 and Val468 located around the tip of the aromatic group (Figure 6) are substituted with different sets of residues in EAAT1 and EAAT3, making the area of BP1 smaller, e.g., Leu467 (Figure 1) in EAAT2 is substituted with isoleucine in EAAT1 and EAAT3. Kato et al. using point mutations showed that these residues forming the cavities are closely related to the sensitivities of the EAAT subtypes to inhibitors.51 Indeed, the addition of an aromatic group (red in Figure 5) via amide bond (19, IC50 = 59 nM) increased the activity by ∼100-fold vs EAAT2. 19 was slightly more selective to EAAT2 than EAAT1(∼2-fold) and EAAT3 (and 40-fold).170 The substitution of the para-metoxyphenyl group with an aliphatic chain had a negative effect on activity (IC50 of 20 = 1200 nM, Figure 5, Table 1), suggesting that the molecule aromatic group favor the interaction with the EAAT2.169 The introduction of electron-withdrawing CF3 (21, also known as TFB-TBOA) instead of a methoxy group (19) increased IC50 by 3.5 times (IC50= 17 nM) and in a further comparison using COS-1 cells, showed a 17-fold selectivity vs EAAT3, but not over EAAT1.170,171 Leuenberger et al. have shown that the relative stereochemistry of 21 is crucial for high inhibitory activity, with the 2,3-syn (threo) isomers being clearly more active than the 2,3-anti (erythro) isomers.172 The substitution of the CF3 group with an aryl ring (22, Figure 4, Table 1) decreased the affinity to EAAT2 by 2 times as the bulky ring might not fit in the BS1. Of note, analogues 17–22 are unlikely to cross the BBB,170 limiting their application in vivo.
Figure 6.

Binding mode of 26 (WAY213613, green) to EAAT2 (blue cartoon) (PDB ID code: 7XR6).
To improve physicochemical properties and aiming for a molecule that may penetrate the BBB, a series of aspartamide and 2,3-diaminopropionic-acid were developed.173 Aspartamide molecules are composed of only one free carboxylic acid, which decreases the number of hydrogen bond acceptors and charge favoring BBB penetration. It was first observed that the biphenyl in compound 23 (Figure 5) is essential for inhibitory activity.173 In addition, increasing the rigidity of 23 into a fluorene (Figure 5) and adding an halogen atom resulted in similar affinity (IC50 = 0.2 μM (24a, Br) > 0.1 μM (24b, Cl) = 0.1 μM (24d, CF3) > 0.07 μM (24c, F)), with slight improvement with the use of electron-withdrawing fluorine but losing selectivity versus EAAT3. The increased affinity obtained with perturbations in the biaryl bond of 23 led to the development of ether derivatives. The ether derivative 25 (Figure 5) bearing two fluorine atoms maintained the same affinity as 24c but owns higher EAAT2 selectivity.
Introducing a bromide atom in 26 (Figure 5) resulted in the best-in class compound with IC50 of 0.08 μM toward EAAT2 and 59- and 45-fold higher selectivity versus EAAT1 and EAAT3, respectively (Table 1). Noteworthy, 26, which is commercially available as WAY213613, remains the most selective and potent EAAT2 inhibitor to date.173,174 Cryo-EM analysis has recently shown that WAY213613 inhibits glutamate uptake via EAAT2 by either interaction at the glutamate-binding site and sterically preventing HP2 loop movement (Figure 6).51 The l-asparagine (Figure 6, blue and black squares) and 4-(2-bromo-4,5-difluorophenoxy) phenyl (Figure 6, green and red squares) moieties of 26 played distinct roles in the EAAT2 inhibition, by competing with the glutamate binding and sterically preventing the HP2 loop gating suspending the transport cycle of glutamate, respectively.
Hansen et al. synthesized thirty-two β-aspartate analogues (Figure 5).168 The authors proposed a key role for the β-position of aspartate in the glutamate uptake inhibition. Interestingly, the β-sulfonamide scaffold (27) has inhibitory activity in the low micromolar range (IC50 = 2.4 μM) but lacks selectivity to EAAT2. Of note, the l-threo conformation of 27 has an IC50 of 2.4 μM, while the l-erythro is inactive (IC50 = 200 μM). Thus, l-threo27 became the main scaffold to develop further EAAT2 selective inhibitors. Methylation of the sulfonamido group or rotational restriction of the phenyl ring by introduction of two ortho-chloro atoms decreased the inhibitory EAAT potency significantly. Heteroaromatic analogues 28 (Figure 5) demonstrated considerable loss of selectivity. The 4-fluoro analogue 29 demonstrated similar inhibitory potency at EAAT2 (IC50 4.7 μM) as 27; however, increasing the number of fluorine atoms decreased the inhibitory potency of the analogues toward EAAT2. The meta-CF3 substituted analogue 30 is a potent inhibitor of EAAT2 and possesses high selectivity toward EAAT1 and EAAT3.168 As anticipated, the EAAT2 selectivity might be also reached by the addition of an aromatic ring, indeed, 31 bearing a phenyl ring in para position displayed high inhibitory potency at EAAT1 and EAAT2 (IC50 values of 2.3 and 0.50 μM, respectively) and 10–40-fold lower potency at EAAT3.
5.2. EAAT2 Inhibitors: Glutamate Analogues
To date, many conformationally restricted glutamate analogues have been synthesized to help shed light on the characterization of glutamate receptors and EAAT’s (Figure 7). Concomitantly to the development of aspartate derivatives in the beginning of the 1970s, the glutamate analogue 32 (also known as kainic acid, Figure 7) was shown to inhibit EAAT2 (Ki = 59 ± 18 μM, Table 2).157,175 Specific binding in the rat brain using [3H]32 was observed in the striatum, hippocampus, cerebral cortex, hypothalamus, and cerebellum.176 The analogue 33 (also known as DHK, Figure 6) is twice as potent as 32(175) and highly selective to EAAT2 vs glutamate receptors.157 Further analyses of 32 and 33 mechanisms of inhibition demonstrated that both compounds are competitive antagonists of EAAT2-mediated glutamate uptake.157 Interestingly, 34 (also known as PDC) has a inhibitory activity in the low micromolar range (Ki = 8 μM) but with lower selectivity toward EAAT2 (EAAT1 = 10-fold, EATT3 = 8-fold).157 In mice primary cortical cocultures of neurons and astrocytes, treatment with 34 triggered an elevation of extracellular glutamate concentration, induced neuronal calcium influx, and NMDA receptor (NMDAR) mediated-neuronal death without having any direct agonist activity on NMDARs.177 Although compound 35 exhibited a high degree of overlap with 34, it was proved to be an excellent substrate of EAAT2.178
Figure 7.
EAAT2 inhibitors as glutamate analogues 32–39.
Table 2. Glutamate Analogues Inhibitors of the Excitatory Amino Acid Transporter 2.
Vandenberg et al. investigated a series of methyl derivatives at 3- or 4-position of glutamate on EAAT2 by modeling the conformations of the methylglutamate derivatives to the restricted conformation of 32, which inhibited the transport of glutamate by blocking the transporter, and found that 4-methylglutamate derivative ((2S,4R)-4-methylglutamate) 36 were transported by EAAT1 but potently blocked glutamate transport via EAAT2.179 Interestingly, the epimer (2S,4S) of 36 was inactive either as a blocker or as a substrate for both EAAT1 and EAAT2.179 The threo-3-methylglutamate derivative 37 (Figure 6) is a competitive blocker of EAAT2, while the erythro-isomer did not inhibit the transport glutamate. This highlights the importance of stereoisomerism at positions 3 and 4 in defining a nonsubstrate inhibitor profile.179
Conformationally constrained azetidine 38 was identified as an inhibitor and showed selectivity for the EAAT2 subtype (versus EAAT1) and potency on EAAT2 equiv to 33.179 The selectivity toward the EAAT2 subtype was lost by elimination of a the 4-alkyl substituent.179 The similarities on 32–38 suggest that all three molecules might bind to the same or closely related sites on EAAT2 near the extracellular surface of the protein. Pharmacophores have also assisted in the identification of the chemical properties and geometry that favor a nonsubstrate EAAT2 inhibitor.178 Based on this, Dunlop et al. developed compound 39 (WAY-855) which contains a glutamate backbone in a six-member heptane dicarboxylate ring.18039 has 45- and 11-fold selectivity over EAAT1 and EAAT3, respectively, and behaves as a nonsubstrate inhibitor of EAAT2 (IC50 = 2.2 μM). Thus, conformational restriction and bulkiness is a key characteristic to obtain a selective nonsubstrate inhibitor of EAAT2.180 Application of 39 to EAAT2-injected oocytes blocked the inward current generated by glutamate. Results from the cell line uptake studies indicate selectivity for EAAT2 inhibition over EAAT3 and EAAT1 of 11- and 45-fold, respectively
Glutamate analogues with different functionalities in C4-position showed similar findings.181 The bulky substituent on the amide nitrogen of compound 40 resulted in intermediate affinity (Ki = 95 μM, Table 2) to EAAT2 and ∼31-fold selectivity over EAAT1 and EAAT3.181
6. Developing a PET Radiotracer Targeting EAAT2
PET radiotracers are unique tools for imaging receptors and transporters in the living brain.182 Nevertheless, the design of novel PET radiotracers comprises a few parameters that must be carefully considered, such as the selection of a molecule with high affinity and selectivity to the target. In section 5 we discussed the library of known EAAT2 inhibitors, in which the compound 24c (commercially available as WAY213613) represents the most promising molecule for designing a PET radiotracer with high affinity and selectivity to EAAT2.
Importantly, the affinity required for a radiotracer is highly dependent on Bmax , the concentration of the target in the region of interest (ROI). (For a more detailed discussion on the designing and testing of radiotracers, see ref (183).) EAAT2 Bmax in the hippocampus of an adult rat has a value of ∼199 nM.46 In keeping with this, the desired inhibitory affinity should be approximately 20 nM or less to detect EAAT2 in the hippocampus. For imaging brain receptors, the minimum ratio of the Bmax/Kd should be at least 10, in order to achieve an acceptable signal in the in vivo PET imaging analysis.184 Nevertheless, an intermediate affinity (e.g., 20 nM < Kd > 100 nM) may be tolerated if selectivity is high.184 The compound 24c is 59- and 45-fold higher selective vs EAAT1 and EAAT3 with affinity toward EAAT2 of 80 nM. Molar activity of the PET radiotracer at the time of injection should also be considered (for a more detailed discussion, see ref (185)).
Of note, in addition to the compound affinity and selectivity, accessibility to the ROI must be considered. PET radiotracers are commonly administered intravenously, thus, it seems reasonable to estimate whether the selected molecule is likely to “access the brain”, i.e., cross the BBB, and bind to the EAAT2. Wager and colleagues proposed the CNS multiparameter optimization desirability tool, in which a few physicochemical parameters must be evaluated to increase the chances of a molecule to cross the BBB.186 In this context, the free carboxylic acid functionality of 24c may impact the molecule permeability through the BBB due to its high polarity and negative charge at physiological pH.187 To overcome this possible limitation and increase BBB permeability, different approaches could be applied, such as replacing the carboxylic acid for tetrazole isostere.188 In addition, the radiosynthesis of the first EAAT2-selective radiotracer highlighted that replacing the free carboxylic acid for a carboxylic ester increased the BBB permeability without compromising EAAT2 affinity and selectivity (a clinical trial is ongoing: NCT05374278). This strategy in which the radiotracer mimics a pro-drug could be identified as the “pro-radiotracer approach”. Indeed, dynamic PET imaging of 6-[18F]bromo-7-(2-fluoroethyl) purine ([18F]41, Figure 8), a PET “pro-radiotracer”, demonstrated good brain uptake and fast metabolic conversion, suggesting that this could be, indeed, a promising strategy to deliver PET radiotracers selective for targets in the brain.189 Another possibility to develop a PET radiotracer could be to radiolabel with carbon-11 at the methoxy group 13–14 PAMs or with fluorine-18 compounds 13 and 16 as these compounds have pharmacokinetic profiles suitable to image EAAT2 in the brain.
Figure 8.
Pro-radiotracer approach to increase the BBB permeability for EAAT2 inhibitors. (A) The EAAT2 specific inhibitor developed 24c by Greenfield et al. possesses a free carboxylic acid–a disadvantage for brain penetration.173 A pro-radiotracer approach may be applied to increase BBB permeability. The 18F-pro-radiotracer [18F]41 was developed following the addition of a methyl ester in the C-terminal of the EAAT2 inhibitor. After in vivo administration, the [18F]41 is hydrolyzed in the brain, producing the EAAT2-specific 18F-radiotracer [18F]24c inside the brain. (B) Schematic mechanism of a brain pro-radiotracer.
7. Concluding Remarks
EAAT2 plays a key role in maintaining glutamatergic homeostasis in the mammalian brain. Human and rodent data support the involvement of EAAT2 loss/dysfunction in neurodegenerative diseases. These findings led to the development of innovative PAMs and translational activators as potential therapeutic agents to prevent glutamate excitotoxicity via EAAT2. Nevertheless, the appropriate time point for therapeutic intervention, in which EAAT2-targeting drugs might be effective to prevent neurodegeneration, is still unknown. Therefore, it is crucial to find noninvasive ways to improve our knowledge on EAAT2 function/expression in the living brain. In this context, neuroimaging techniques such as PET hold promise, but no PET radiotracer with high selectivity and affinity to EAAT2 has been available for clinical research so far. We believe that the availability of the cryo-EM structures of EAAT2 will offer the opportunity to initiate a structural-based drug design campaign to facilitate the development and screening of high-affinity PAMs able to modulate EAAT2 activity while using them as imaging tools to detect change in EAAT2 density in neurodegenerative diseases.
Acknowledgments
This work was supported by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z].
Glossary
Abbreviations Used
- AβOs
amyloid-β oligomers
- AT(N)
amyloid, tau and neurodegeneration
- BP
binding pocket
- CN
calcineurin
- CREB
cAMP-response element binding protein
- cryo-EM
cryogenic electron microscopy
- CYP46
cholesterol 24S-hydroxylase
- EAAC1
excitatory amino acid carrier 1
- EAAT
excitatory amino acid transporter
- EAAT2-e9
excitatory amino acid transporter 2 skipped exon-9
- fALS
familial amyotrophic lateral sclerosis
- GFAP
glial fibrillary acidic protein
- GLT-1
glutamate transporter 1
- HD
Huntington’s disease
- HP
hairpin
- LRRK2
leucine-rich repeat kinase 2
- NFAT
nuclear factor activated T-cells
- ROI
region of interest
- SLC1
solute carrier 1
- SN
system N transporter
- SOD1
superoxide dismutase 1
- TM
transmembrane
- vGluT
vesicular glutamate transporter
Biographies
Igor C. Fontana has a bachelor’s degree in Industrial Chemistry (2015), a master’s degree in Pharmaceutical Sciences (2017), and a Ph.D. in Biochemistry (2021) at the Universidade Federal do Rio Grande do Sul (UFGRS). During his Ph.D., Igor was awarded an international scholarship to conduct half of his Ph.D. research in the School of Biomedical Engineering and Imaging Sciences at King’s College London. Currently, Igor is working on his second year as a postdoctoral fellow at Karolinska Institutet in Stockholm, Sweden, with Professor Agneta Nordberg. His research is focused on positron emission tomography radiotracers specific for astrocytes, with the goal of understanding how these brain cells are linked to Alzheimer’s disease and other neurodegenerative diseases.
Débora G. Souza has a bachelor’s degree in Pharmacy (2009) and a Ph.D. in Biochemistry (2016). Currently, Dr. Souza is a Biochemistry lecturer (2016), an associated advisor (2020), and an Alzheimer’s Association research fellow (2022) at the Universidade Federal do Rio Grande do Sul and an associated researcher (2022) at the Brain Institute of Rio Grande do Sul. Her area of research is neurochemistry, with a focus on brain energetics and astrocytes. In a preclinical setting, she uses brain imaging, high-resolution respirometry, and omics technologies to better understand the astrocyte biochemistry. In a clinical setting, she is assessing biomarkers of neurodegeneration, blood-brain barrier injury, and astrocyte reactivity in patients that had experienced severe diseases.
Diogo O. Souza is Professor of Biochemistry at the Universidade Federal do Rio Grande do Sul (UFRGS) as a Faculty of Medicine. He obtained his bachelor’s degree in Medicine at the Universidade Católica de Pelotas and a Master’s degree and Ph.D. in Biochemistry at the Universidade Federal do Rio de Janeiro (UFRJ). Since then, Diogo worked as a postdoctoral fellow and visiting professor in the University of London and Universidad Autónoma de Madrid, respectively. His research has always been focused on neuroscience with a subspeciality towards understanding glutamatergic signaling, with both basic science and clinical approaches, in brain diseases.
Antony Gee is Professor of PET and Radiochemistry in the Division of Imaging Sciences at King’s College London. He obtained a BSc (Hons) in Chemistry at the University of Sussex (1985) and his Ph.D. in Radiopharmaceutical Organic Chemistry at Uppsala University, Sweden (1991). Since then, he has worked as the Director of PET Chemistry at the Guy’s and St Thomas’ Hospitals Clinical PET Centre, UMDS, London, the Aarhus University Hospital PET Centre in Aarhus, Denmark, before spending 10 years at GlaxoSmithKline to spearhead the use of PET imaging in drug discovery and development.
Eduardo R. Zimmer is an Assistant Professor of Pharmacology at the Universidade Federal do Rio Grande do Sul (UFRGS) and an Adjunct Professor at McGill University (Canada). He obtained a bachelor’s degree in Pharmacy (2009), Master’s degree (2011) and Ph.D. (2015) in Biological Sciences/Biochemistry at UFRGS, the latter with a exchange period at McGill University, Canada. His work involves understanding the communication between neurons and glial cells in Alzheimer’s disease. The main lines of research seek to decipher neurodegenerative mechanisms, develop innovative therapeutic strategies, and identify Alzheimer’s disease early, before the symptoms. His laboratory is comprised of a multidisciplinary team, conducting translational research with an emphasis on neuroimaging, pharmacology, and neurochemistry.
Salvatore Bongarzone attained his Ph.D. in Physics and Chemistry of Biological Systems at the International School for Advanced Studies (SISSA, Italy). Subsequent postdoctoral positions were held at the Institute for Research in Biomedicine (IRB, Spain) cofounded by Marie Curie Action fellowship and later at the School of Biomedical Engineering and Imaging Sciences at King’s College London (KCL, UK) supported by the Medical Research Council capacity building fellowship. In April 2018, he was appointed Translational Radiochemist at the Wellcome/EPSRC Centre for Medical Engineering (KCL). At KCL, Dr. Bongarzone conceived novel radiochemical reactions for developing PET imaging probes ([11C]niacin, [11C]biotin, [18F]FAMTO, and [11C]FPSZM1) and their preclinical characterization. Since 2021, he works as Senior Scientist in radiochemistry at Novartis. In February 2022, Dr. Bongarzone was qualified as Associate Professor in Organic Chemistry as well as Associate Professor in Medicinal, Toxicological, and Nutritional Chemistry and Applied Technologies from the Italian Minister of University and Research.
Author Present Address
† Technical Research and Development, Novartis, via Ribes 5, Colleretto Giacosa 10010, Italy
The authors declare the following competing financial interest(s): E.R.Z. serves on the scientific advisory board of Next Innovative Therapeutics (Nintx).
After this paper was published ASAP February 14, 2023, a new reference was added (ref 157). The corrected version was reposted online February 23, 2023.
Special Issue
Published as part of the Journal of Medicinal Chemistry virtual special issue “Diagnostic and Therapeutic Radiopharmaceuticals”.
References
- Deco G.; et al. How local excitation-inhibition ratio impacts the whole brain dynamics. J. Neurosci. 2014, 34, 7886–7898. 10.1523/JNEUROSCI.5068-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J. Neurochem. 1984, 42, 1–11. 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A. The blood-brain barrier and glutamate. Am. J. Clin. Nutr. 2009, 90, 867S–874S. 10.3945/ajcn.2009.27462BB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira G. C.; et al. Metabolism of [1,6-(13) C]glucose in the cerebellum of 18-day-old rats: Comparison with cerebral metabolism. J. Neurochem. 2021, 157, 1946–1962. 10.1111/jnc.15326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman D. L.; et al. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 2011, 24, 943–957. 10.1002/nbm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M.; Silver I. A. Metabolism and role of glutamate in mammalian brain. Prog. Neurobiol. 1990, 35, 245–296. 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G.; Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol. Pharmacol. 1997, 52, 6–15. 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- Bjornsen L. P.; et al. The GLT-1 (EAAT2; slc1a2) glutamate transporter is essential for glutamate homeostasis in the neocortex of the mouse. J. Neurochem. 2014, 128, 641–649. 10.1111/jnc.12509. [DOI] [PubMed] [Google Scholar]
- Rothstein J. D.; et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 1996, 16, 675–686. 10.1016/S0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Abdul H. M.; et al. Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J. Neurosci. 2009, 29, 12957–12969. 10.1523/JNEUROSCI.1064-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoni C.; et al. Increased internalisation and degradation of GLT-1 glial glutamate transporter in a cell model for familial amyotrophic lateral sclerosis (ALS). J. Cell. Sci. 2004, 117, 5417–5426. 10.1242/jcs.01411. [DOI] [PubMed] [Google Scholar]
- O’Donovan S. M.; et al. The role of glutamate transporters in the pathophysiology of neuropsychiatric disorders. NPJ. Schizophr. 2017, 3, 32. 10.1038/s41537-017-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A.; et al. Divergent roles of astrocytic versus neuronal EAAT2 deficiency on cognition and overlap with aging and Alzheimer’s molecular signatures. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 21800–21811. 10.1073/pnas.1903566116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein J. D.; et al. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann. Neurol. 1995, 38, 73–84. 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- Masliah E.; et al. Deficient glutamate transport is associated with neurodegeneration in Alzheimer’s disease. Ann. Neurol. 1996, 40, 759–766. 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- Kim K.; et al. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J. Cell. Physiol. 2011, 226, 2484–2493. 10.1002/jcp.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin G. M.; et al. Glutamate transporters, EAAT1 and EAAT2, are potentially important in the pathophysiology and treatment of schizophrenia and affective disorders. World J. Psychiatry 2018, 8, 51–63. 10.5498/wjp.v8.i2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A. C. Current approaches to enhance glutamate transporter function and expression. J. Neurochem. 2015, 134, 982–1007. 10.1111/jnc.13200. [DOI] [PubMed] [Google Scholar]
- Zielke H. R.; et al. Compartmentation of [14C]glutamate and [14C]glutamine oxidative metabolism in the rat hippocampus as determined by microdialysis. J. Neurochem. 1998, 71, 1315–1320. 10.1046/j.1471-4159.1998.71031315.x. [DOI] [PubMed] [Google Scholar]
- Shegani A.; et al. Radiosynthesis, Preclinical, and Clinical Positron Emission Tomography Studies of Carbon-11 Labeled Endogenous and Natural Exogenous Compounds. Chem. Rev. 2023, 123, 105. 10.1021/acs.chemrev.2c00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum B. S. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J. Nutr. 2000, 130, 1007S–1015S. 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- Petroff O. A. GABA and glutamate in the human brain. Neuroscientist 2002, 8, 562–573. 10.1177/1073858402238515. [DOI] [PubMed] [Google Scholar]
- Reiner A.; Levitz J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. 10.1016/j.neuron.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M.; et al. Beyond the role of glutamate as a neurotransmitter. Nat. Rev. Neurosci. 2002, 3, 748–755. 10.1038/nrn916. [DOI] [PubMed] [Google Scholar]
- Nakanishi S.; et al. Glutamate receptors: brain function and signal transduction. Brain Res. Brain Res. Rev. 1998, 26, 230–235. 10.1016/S0165-0173(97)00033-7. [DOI] [PubMed] [Google Scholar]
- Amara S. G.; Fontana A. C. Excitatory amino acid transporters: keeping up with glutamate. Neurochem. Int. 2002, 41, 313–318. 10.1016/S0197-0186(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Chen W.; et al. The glutamate transporter GLT1a is expressed in excitatory axon terminals of mature hippocampal neurons. J. Neurosci. 2004, 24, 1136–1148. 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storck T.; et al. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc. Natl. Acad. Sci. U. S. A. 1992, 89, 10955–10959. 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y.; Hediger M. A. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature 1992, 360, 467–471. 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- Fairman W. A.; et al. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature 1995, 375, 599–603. 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- Wersinger E.; et al. The glutamate transporter EAAT5 works as a presynaptic receptor in mouse rod bipolar cells. J. Physiol. 2006, 577, 221–234. 10.1113/jphysiol.2006.118281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre K. P.; et al. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J. Neurosci. 1995, 15, 1835–1853. 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu B. Cryo-EM structures of excitatory amino acid transporter 3 visualize coupled substrate, sodium, and proton binding and transport. Sci. Adv. 2021, 7, eabf5814. 10.1126/sciadv.abf5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao S.; et al. EAAT4, a glutamate transporter with properties of a chloride channel, is predominantly localized in Purkinje cell dendrites, and forms parasagittal compartments in rat cerebellum. Neuroscience 1997, 78, 929–933. 10.1016/S0306-4522(97)00021-3. [DOI] [PubMed] [Google Scholar]
- Helms H. C. C.; et al. Glutamate Transporters in the Blood-Brain Barrier. Adv. Neurobiol. 2017, 16, 297–314. 10.1007/978-3-319-55769-4_15. [DOI] [PubMed] [Google Scholar]
- Arriza J. L.; et al. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc. Natl. Acad. Sci. U. S. A. 1997, 94, 4155–4160. 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K.; et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 1997, 276, 1699–1702. 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Bergles D. E.; et al. Comparison of coupled and uncoupled currents during glutamate uptake by GLT-1 transporters. J. Neurosci. 2002, 22, 10153–10162. 10.1523/JNEUROSCI.22-23-10153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmele T. S.; Rosenberg P. A. GLT-1: The elusive presynaptic glutamate transporter. Neurochem. Int. 2016, 98, 19–28. 10.1016/j.neuint.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yernool D.; et al. Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature 2004, 431, 811–818. 10.1038/nature03018. [DOI] [PubMed] [Google Scholar]
- Reyes N.; et al. Transport mechanism of a bacterial homologue of glutamate transporters. Nature 2009, 462, 880–885. 10.1038/nature08616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill S. M.; et al. Differential regulation of two isoforms of the glial glutamate transporter EAAT2 by DLG1 and CaMKII. J. Neurosci. 2015, 35, 5260–5270. 10.1523/JNEUROSCI.4365-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmseth S.; et al. The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience 2009, 162, 1055–1071. 10.1016/j.neuroscience.2009.03.048. [DOI] [PubMed] [Google Scholar]
- Lauriat T. L.; et al. Quantitative analysis of glutamate transporter mRNA expression in prefrontal and primary visual cortex in normal and schizophrenic brain. Neuroscience 2006, 137, 843–851. 10.1016/j.neuroscience.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Milton I. D.; et al. Expression of the glial glutamate transporter EAAT2 in the human CNS: an immunohistochemical study. Brain Res. Mol. Brain Res. 1997, 52, 17–31. 10.1016/S0169-328X(97)00233-7. [DOI] [PubMed] [Google Scholar]
- Lehre K. P.; Danbolt N. C. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J. Neurosci. 1998, 18, 8751–8757. 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J.; Amara S. G. New views of glutamate transporter structure and function: advances and challenges. Neuropharmacology 2011, 60, 172–181. 10.1016/j.neuropharm.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichman S.; Kanner B. I. Aspartate-444 is essential for productive substrate interactions in a neuronal glutamate transporter. J. Gen. Physiol. 2007, 129, 527–539. 10.1085/jgp.200609707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudker O.; et al. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature 2007, 445, 387–393. 10.1038/nature05455. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; et al. Structural basis of ligand binding modes of human EAAT2. Nat. Commun. 2022, 13, 3329. 10.1038/s41467-022-31031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T.; et al. Structural insights into inhibitory mechanism of human excitatory amino acid transporter EAAT2. Nat. Commun. 2022, 13, 4714. 10.1038/s41467-022-32442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen O. V.; et al. Molecular determinants of transport stimulation of EAAT2 are located at interface between the trimerization and substrate transport domains. J. Neurochem. 2015, 133, 199–210. 10.1111/jnc.13047. [DOI] [PubMed] [Google Scholar]
- Damm-Ganamet K. L.; et al. A computational approach yields selective inhibitors of human excitatory amino acid transporter 2 (EAAT2). J. Biol. Chem. 2020, 295, 4359–4366. 10.1074/jbc.AC119.011190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafede R.; Mariotti R. ALS Pathogenesis and Therapeutic Approaches: The Role of Mesenchymal Stem Cells and Extracellular Vesicles. Front Cell Neurosci. 2017, 11, 80. 10.3389/fncel.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldink J. H. ALS genetic epidemiology ’How simplex is the genetic epidemiology of ALS?’. J. Neurol. Neurosurg. Psychiatry 2017, 88, 537. 10.1136/jnnp-2016-315469. [DOI] [PubMed] [Google Scholar]
- Prudencio M.; et al. Variation in aggregation propensities among ALS-associated variants of SOD1: correlation to human disease. Hum. Mol. Genet. 2009, 18, 3217–3226. 10.1093/hmg/ddp260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrika S.; et al. Cognitive, Emotional and Psychological Manifestations in Amyotrophic Lateral Sclerosis at Baseline and Overtime: A Review. Front Neurosci. 2019, 13, 951. 10.3389/fnins.2019.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles T.; Swash M. Amyotrophic lateral sclerosis: current understanding. J. Neurosci. Nurs. 2001, 33, 245–253. 10.1097/01376517-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Rothstein J. D.; et al. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N. Engl. J. Med. 1992, 326, 1464–1468. 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- Lin C. L.; et al. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron 1998, 20, 589–602. 10.1016/S0896-6273(00)80997-6. [DOI] [PubMed] [Google Scholar]
- Flomen R.; Makoff A. Increased RNA editing in EAAT2 pre-mRNA from amyotrophic lateral sclerosis patients: involvement of a cryptic polyadenylation site. Neurosci. Lett. 2011, 497, 139–143. 10.1016/j.neulet.2011.04.047. [DOI] [PubMed] [Google Scholar]
- Jackson M.; et al. Polymorphisms in the glutamate transporter gene EAAT2 in European ALS patients. J. Neurol. 1999, 246, 1140–1144. 10.1007/s004150050532. [DOI] [PubMed] [Google Scholar]
- Flowers J. M.; et al. Intron 7 retention and exon 9 skipping EAAT2 mRNA variants are not associated with amyotrophic lateral sclerosis. Ann. Neurol. 2001, 49, 643–649. 10.1002/ana.1029. [DOI] [PubMed] [Google Scholar]
- van Es M. A.; et al. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. 10.1016/S0140-6736(17)31287-4. [DOI] [PubMed] [Google Scholar]
- Gurney M. E.; et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 1994, 264, 1772–1775. 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Howland D. S.; et al. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS). Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 1604–1609. 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y.; et al. Extensive dysregulations of oligodendrocytic and astrocytic connexins are associated with disease progression in an amyotrophic lateral sclerosis mouse model. J. Neurochem. 2014, 11, 42. 10.1186/1742-2094-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis N.; et al. Combined excitotoxic-oxidative stress and the concept of non-cell autonomous pathology of ALS: insights into motoneuron axonopathy and astrogliosis. Neurochem. Int. 2012, 61, 523–530. 10.1016/j.neuint.2012.02.026. [DOI] [PubMed] [Google Scholar]
- Vargas M. R.; Johnson J. A. Astrogliosis in amyotrophic lateral sclerosis: role and therapeutic potential of astrocytes. Neurotherapeutics 2010, 7, 471–481. 10.1016/j.nurt.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine S. M.; et al. Tumor necrosis factor alpha inhibits glutamate uptake by primary human astrocytes. Implications for pathogenesis of HIV-1 dementia. J. Biol. Chem. 1996, 271, 15303–15306. 10.1074/jbc.271.26.15303. [DOI] [PubMed] [Google Scholar]
- Jiang L. L.; et al. Membralin deficiency dysregulates astrocytic glutamate homeostasis leading to ALS-like impairment. J. Clin. Invest. 2019, 129, 3103–3120. 10.1172/JCI127695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb S. L.; et al. A caspase-3-cleaved fragment of the glial glutamate transporter EAAT2 is sumoylated and targeted to promyelocytic leukemia nuclear bodies in mutant SOD1-linked amyotrophic lateral sclerosis. J. Biol. Chem. 2007, 282, 32480–32490. 10.1074/jbc.M704314200. [DOI] [PubMed] [Google Scholar]
- Rosenblum L. T.; et al. Mutation of the caspase-3 cleavage site in the astroglial glutamate transporter EAAT2 delays disease progression and extends lifespan in the SOD1-G93A mouse model of ALS. Exp. Neurol. 2017, 292, 145–153. 10.1016/j.expneurol.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane C. A.; et al. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- Santacruz Escudero J. M.; et al. Neuropsychiatric Symptoms as Predictors of Clinical Course in Neurodegeneration. A Longitudinal Study. Front Aging Neurosci. 2019, 11, 176. 10.3389/fnagi.2019.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C. R. Jr; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R.; et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA 2015, 313, 1939–1949. 10.1001/jama.2015.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boza-Serrano A.; et al. Innate immune alterations are elicited in microglial cells before plaque deposition in the Alzheimer’s disease mouse model 5xFAD. Sci. Rep. 2018, 8, 1550. 10.1038/s41598-018-19699-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl W. C. Discerning the relationship between microglial activation and Alzheimer’s disease. Brain 2017, 140, 1825–1828. 10.1093/brain/awx151. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes R. E.; et al. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front Cell Neurosci. 2017, 10, 427. 10.3389/fnmol.2017.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A.; et al. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am. J. Pathol. 2011, 179, 1373–1384. 10.1016/j.ajpath.2011.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A.; et al. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. 10.1038/s41593-020-00783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Esparcia P.; et al. Glutamate Transporter GLT1 Expression in Alzheimer Disease and Dementia With Lewy Bodies. Front Aging Neurosci. 2018, 10, 122. 10.3389/fnagi.2018.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viejo L.; et al. Systematic review of human post-mortem immunohistochemical studies and bioinformatics analyses unveil the complexity of astrocyte reaction in Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2022, 48, e12753 10.1111/nan.12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstrom H.; et al. Interindividual differences in the levels of the glutamate transporters GLAST and GLT, but no clear correlation with Alzheimer’s disease. J. Neurosci. Res. 1999, 55, 218–229. . [DOI] [PubMed] [Google Scholar]
- Wood O. W. G.; et al. EAAT2 as a therapeutic research target in Alzheimer’s disease: A systematic review. Front Neurosci. 2022, 16, 952096. 10.3389/fnins.2022.952096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H. A.; et al. Glutamate transporter variants reduce glutamate uptake in Alzheimer’s disease. Neurobiol. Aging 2011, 32, 553. 10.1016/j.neurobiolaging.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Trotti D.; et al. Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration?. Trends Pharmacol. Sci. 1998, 19, 328–334. 10.1016/S0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- Woltjer R. L.; et al. Aberrant detergent-insoluble excitatory amino acid transporter 2 accumulates in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2010, 69, 667–676. 10.1097/NEN.0b013e3181e24adb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana I. C.; et al. Amyloid-beta oligomers in cellular models of Alzheimer’s disease. J. Neurochem. 2020, 155, 348. 10.1111/jnc.15030. [DOI] [PubMed] [Google Scholar]
- Radde R.; et al. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006, 7, 940–946. 10.1038/sj.embor.7400784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E.; et al. Abnormal glutamate transport function in mutant amyloid precursor protein transgenic mice. Exp. Neurol. 2000, 163, 381–387. 10.1006/exnr.2000.7386. [DOI] [PubMed] [Google Scholar]
- Schallier A.; et al. Region- and age-specific changes in glutamate transport in the AbetaPP23 mouse model for Alzheimer’s disease. J. Alzheimers Dis. 2011, 24, 287–300. 10.3233/JAD-2011-101005. [DOI] [PubMed] [Google Scholar]
- Mookherjee P.; et al. GLT-1 loss accelerates cognitive deficit onset in an Alzheimer’s disease animal model. J. Alzheimers Dis. 2011, 26, 447–455. 10.3233/JAD-2011-110503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefendehl J. K.; et al. Mapping synaptic glutamate transporter dysfunction in vivo to regions surrounding Abeta plaques by iGluSnFR two-photon imaging. Nat. Commun. 2016, 7, 13441. 10.1038/ncomms13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain M.; et al. Alzheimer’s disease-like APP processing in wild-type mice identifies synaptic defects as initial steps of disease progression. Mol. Neurodegener. 2016, 11, 5. 10.1186/s13024-016-0070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulijewicz-Nawrot M.; et al. Astrocytes and glutamate homoeostasis in Alzheimer’s disease: a decrease in glutamine synthetase, but not in glutamate transporter-1, in the prefrontal cortex. Ann. Neurol. 2013, 5, 273–282. 10.1042/AN20130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H.; et al. Amyloid-beta peptide decreases expression and function of glutamate transporters in nervous system cells. Int. J. Biochem. Cell Biol. 2017, 85, 75–84. 10.1016/j.biocel.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Srivastava I.; et al. Reducing Glutamate Uptake in Rat Hippocampal Slices Enhances Astrocytic Membrane Depolarization While Down-Regulating CA3-CA1 Synaptic Response. Front Synaptic Neurosci. 2020, 12, 37. 10.3389/fnsyn.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoltowska K. M.; et al. Novel interaction between Alzheimer’s disease-related protein presenilin 1 and glutamate transporter 1. Sci. Rep. 2018, 8, 8718. 10.1038/s41598-018-26888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G.; et al. Increased expression of cholesterol 24S-hydroxylase results in disruption of glial glutamate transporter EAAT2 association with lipid rafts: a potential role in Alzheimer’s disease. J. Neurochem. 2010, 113, 978–989. 10.1111/j.1471-4159.2010.06661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompol P.; et al. Calcineurin/NFAT Signaling in Activated Astrocytes Drives Network Hyperexcitability in Abeta-Bearing Mice. J. Neurosci. 2017, 37, 6132–6148. 10.1523/JNEUROSCI.0877-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habchi J.; et al. Cholesterol catalyses Abeta42 aggregation through a heterogeneous nucleation pathway in the presence of lipid membranes. Nat. Chem. 2018, 10, 673–683. 10.1038/s41557-018-0031-x. [DOI] [PubMed] [Google Scholar]
- Sasaki K.; et al. Excitatory amino acid transporter 2 associates with phosphorylated tau and is localized in neurofibrillary tangles of tauopathic brains. FEBS Lett. 2009, 583, 2194–2200. 10.1016/j.febslet.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Dabir D. V.; et al. Impaired glutamate transport in a mouse model of tau pathology in astrocytes. J. Neurosci. 2006, 26, 644–654. 10.1523/JNEUROSCI.3861-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Puma D. D.; et al. Extracellular tau oligomers affect extracellular glutamate handling by astrocytes through downregulation of GLT-1 expression and impairment of NKA1A2 function. Neuropathol. Appl. Neurobiol. 2022, 48, e12811 10.1111/nan.12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C.; et al. The corticostriatal pathway in Huntington’s disease. Prog. Neurobiol. 2007, 81, 253–271. 10.1016/j.pneurobio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faideau M.; et al. In vivo expression of polyglutamine-expanded huntingtin by mouse striatal astrocytes impairs glutamate transport: a correlation with Huntington’s disease subjects. Hum. Mol. Genet. 2010, 19, 3053–3067. 10.1093/hmg/ddq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzberger T.; et al. Changes of NMDA receptor subunit (NR1, NR2B) and glutamate transporter (GLT1) mRNA expression in Huntington’s disease-an in situ hybridization study. J. Neuropathol. Exp. Neurol. 1997, 56, 440–454. 10.1097/00005072-199704000-00013. [DOI] [PubMed] [Google Scholar]
- Pinheiro Campos A. Effect of Subthalamic Stimulation and Electrode Implantation in the Striatal Microenvironment in a Parkinson’s Disease Rat Model. Int. J. Mol.Sci. 2022, 23, 12116. 10.3390/ijms232012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng L.; et al. Erythrocytic alpha-synuclein contained in microvesicles regulates astrocytic glutamate homeostasis: a new perspective on Parkinson’s disease pathogenesis. Acta Neuropathol. Commun. 2020, 8, 102. 10.1186/s40478-020-00983-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; et al. Generation of a Novel Mouse Model of Parkinson’s Disease via Targeted Knockdown of Glutamate Transporter GLT-1 in the Substantia Nigra. ACS Chem. Neurosci. 2020, 11, 406–417. 10.1021/acschemneuro.9b00609. [DOI] [PubMed] [Google Scholar]
- Ren C.; et al. Induction of Parkinsonian-Like Changes via Targeted Downregulation of Astrocytic Glutamate Transporter GLT-1 in the Striatum. J. Parkinsons Dis. 2022, 12, 295–314. 10.3233/JPD-212640. [DOI] [PubMed] [Google Scholar]
- Wei L.; et al. Wnt1 Promotes EAAT2 Expression and Mediates the Protective Effects of Astrocytes on Dopaminergic Cells in Parkinson’s Disease. Neural Plast. 2019, 2019, 1247276. 10.1155/2019/1247276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y.; et al. Exercise increases striatal Glu reuptake and improves motor dysfunction in 6-OHDA-induced Parkinson’s disease rats. Exp. Brain Res. 2021, 239, 3277–3287. 10.1007/s00221-021-06186-6. [DOI] [PubMed] [Google Scholar]
- Iovino L.; et al. Trafficking of the glutamate transporter is impaired in LRRK2-related Parkinson’s disease. Acta Neuropathol. 2022, 144, 81–106. 10.1007/s00401-022-02437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M. A.; et al. Astrogliosis in aging and Parkinson’s disease dementia: a new clinical study with (11) C-BU99008 PET. Brain Commun. 2022, 4, fcac199. 10.1093/braincomms/fcac199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H.; et al. Imidazoline 2 binding sites reflecting astroglia pathology in Parkinson’s disease: an in vivo11C-BU99008 PET study. Brain 2019, 142, 3116–3128. 10.1093/brain/awz260. [DOI] [PubMed] [Google Scholar]
- Kobayashi E.; et al. Activated forms of astrocytes with higher GLT-1 expression are associated with cognitive normal subjects with Alzheimer pathology in human brain. Sci. Rep. 2018, 8, 1712. 10.1038/s41598-018-19442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. C.; et al. Riluzole, a glutamate modulator, slows cerebral glucose metabolism decline in patients with Alzheimer’s disease. Brain 2021, 144, 3742–3755. 10.1093/brain/awab222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S.; et al. Ceftriaxone Improves Cognitive Function and Upregulates GLT-1-Related Glutamate-Glutamine Cycle in APP/PS1Mice. J.Alzheimers Dis. 2018, 66, 1731–1743. 10.3233/JAD-180708. [DOI] [PubMed] [Google Scholar]
- Hamidi N.; et al. Effect of ceftriaxone on paired-pulse response and long-term potentiation of hippocampal dentate gyrus neurons in rats with Alzheimer-like disease. Life Sci. 2019, 238, 116969. 10.1016/j.lfs.2019.116969. [DOI] [PubMed] [Google Scholar]
- Fan S.; et al. Ceftriaxone regulates glutamate production and vesicular assembly in presynaptic terminals through GLT-1 in APP/PS1 mice. Neurobiol. Learn. Mem. 2021, 183, 107480. 10.1016/j.nlm.2021.107480. [DOI] [PubMed] [Google Scholar]
- Chotibut T.; et al. Ceftriaxone reduces L-dopa-induced dyskinesia severity in 6-hydroxydopamine parkinson’s disease model. Mov. Disord. 2017, 32, 1547–1556. 10.1002/mds.27077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry J. D.; et al. Design and initial results of a multi-phase randomized trial of ceftriaxone in amyotrophic lateral sclerosis. PLoS One 2013, 8, e61177 10.1371/journal.pone.0061177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz M. E.; et al. Safety and efficacy of ceftriaxone for amyotrophic lateral sclerosis: a multi-stage, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2014, 13, 1083–1091. 10.1016/S1474-4422(14)70222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli E.; et al. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur. J. Pharmacol. 2008, 578, 171–176. 10.1016/j.ejphar.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Carbone M.; et al. Riluzole elevates GLT-1 activity and levels in striatal astrocytes. Neurochem. Int. 2012, 60, 31–38. 10.1016/j.neuint.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschocke J.; et al. Differential promotion of glutamate transporter expression and function by glucocorticoids in astrocytes from various brain regions. J. Biol. Chem. 2005, 280, 34924–34932. 10.1074/jbc.M502581200. [DOI] [PubMed] [Google Scholar]
- Lee E.; et al. Transforming growth factor-alpha mediates estrogen-induced upregulation of glutamate transporter GLT-1 in rat primary astrocytes. Glia 2012, 60, 1024–1036. 10.1002/glia.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P.; et al. Mechanism of raloxifene-induced upregulation of glutamate transporters in rat primary astrocytes. Glia 2014, 62, 1270–1283. 10.1002/glia.22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P.; et al. cAMP response element-binding protein (CREB) and nuclear factor kappaB mediate the tamoxifen-induced up-regulation of glutamate transporter 1 (GLT-1) in rat astrocytes. J. Biol. Chem. 2013, 288, 28975–28986. 10.1074/jbc.M113.483826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung J. H. Y.; et al. EAAT2 Expression in the Hippocampus, Subiculum, Entorhinal Cortex and Superior Temporal Gyrus in Alzheimer’s Disease. Front Cell Neurosci. 2021, 15, 702824. 10.3389/fncel.2021.702824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton C. K.; et al. Identification of translational activators of glial glutamate transporter EAAT2 through cell-based high-throughput screening: an approach to prevent excitotoxicity. J. Biomol. Screen. 2010, 15, 653–662. 10.1177/1087057110370998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing X.; et al. Structure-activity relationship study of pyridazine derivatives as glutamate transporter EAAT2 activators. Bioorg. Med. Chem. Lett. 2011, 21, 5774–5777. 10.1016/j.bmcl.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q.; et al. Small-molecule activator of glutamate transporter EAAT2 translation provides neuroprotection. J. Clin. Invest. 2014, 124, 1255–1267. 10.1172/JCI66163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T.; et al. Alternative splicing of the glutamate transporter EAAT2 (GLT-1). Neurosci. Lett. 1998, 241, 68–70. 10.1016/S0304-3940(97)00973-7. [DOI] [PubMed] [Google Scholar]
- Takahashi K.; et al. Restored glial glutamate transporter EAAT2 function as a potential therapeutic approach for Alzheimer’s disease. J. Exp. Med. 2015, 212, 319–332. 10.1084/jem.20140413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X.; et al. Sulbactam Plays Neuronal Protective Effect Against Brain Ischemia via Upregulating GLT1 in Rats. Mol. Neurobiol. 2015, 51, 1322–1333. 10.1007/s12035-014-8809-3. [DOI] [PubMed] [Google Scholar]
- Qi J.; et al. Sulbactam Protects Hippocampal Neurons Against Oxygen-Glucose Deprivation by Up-Regulating Astrocytic GLT-1 via p38 MAPK Signal Pathway. Front Cell Neurosci. 2018, 11, 281. 10.3389/fnmol.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Q. X.; Yang T. D. Amitriptyline upregulates EAAT1 and EAAT2 in neuropathic pain rats. Brain Res. Bull. 2010, 81, 424–427. 10.1016/j.brainresbull.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Liu A. Y.; et al. Neuroprotective drug riluzole amplifies the heat shock factor 1 (HSF1)- and glutamate transporter 1 (GLT1)-dependent cytoprotective mechanisms for neuronal survival. J. Biol. Chem. 2011, 286, 2785–2794. 10.1074/jbc.M110.158220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganel R.; et al. Selective up-regulation of the glial Na+-dependent glutamate transporter GLT1 by a neuroimmunophilin ligand results in neuroprotection. Neurobiol. Dis. 2006, 21, 556–567. 10.1016/j.nbd.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Li X.; et al. Effect of MS-153 on the acquisition and expression of conditioned fear in rats. Eur. J. Pharmacol. 2004, 505, 145–149. 10.1016/j.ejphar.2004.10.041. [DOI] [PubMed] [Google Scholar]
- Nakagawa T.; et al. Effect of MS-153, a glutamate transporter activator, on the conditioned rewarding effects of morphine, methamphetamine and cocaine in mice. Behav. Brain Res. 2005, 156, 233–239. 10.1016/j.bbr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Aal-Aaboda M.; et al. Effects of (R)-(−)-5-methyl-1-nicotinoyl-2-pyrazoline on glutamate transporter 1 and cysteine/glutamate exchanger as well as ethanol drinking behavior in male, alcohol-preferring rats. J. Neurosci.Res. 2015, 93, 930–937. 10.1002/jnr.23554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A. C.; et al. Neuroprotective Effects of the Glutamate Transporter Activator (R)-(−)-5-methyl-1-nicotinoyl-2-pyrazoline (MS-153) following Traumatic Brain Injury in the Adult Rat. J. Neurotrauma 2016, 33, 1073–1083. 10.1089/neu.2015.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada F.; et al. The neuroprotective agent MS-153 stimulates glutamate uptake. Eur. J. Pharmacol. 1999, 386, 263–270. 10.1016/S0014-2999(99)00735-9. [DOI] [PubMed] [Google Scholar]
- Uenishi H.; et al. Ion channel modulation as the basis for neuroprotective action of MS-153. Ann. N.Y. Acad. Sci. 1999, 890, 385–399. 10.1111/j.1749-6632.1999.tb08018.x. [DOI] [PubMed] [Google Scholar]
- Falcucci R. M.; et al. Novel Positive Allosteric Modulators of Glutamate Transport Have Neuroprotective Properties in an in Vitro Excitotoxic Model. ACS Chem. Neurosci. 2019, 10, 3437–3453. 10.1021/acschemneuro.9b00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile A.; et al. Laquinimod ameliorates excitotoxic damage by regulating glutamate re-uptake. J. Neurochem. 2018, 15, 5. 10.1186/s12974-017-1048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A. C.; et al. Enhancing glutamate transport: mechanism of action of Parawixin1, a neuroprotective compound from Parawixia bistriata spider venom. Mol. Pharmacol. 2007, 72, 1228–1237. 10.1124/mol.107.037127. [DOI] [PubMed] [Google Scholar]
- Forster Y. M.; et al. Elucidation of the Structure and Synthesis of Neuroprotective Low Molecular Mass Components of the Parawixia bistriata Spider Venom. ACS Chem. Neurosci. 2020, 11, 1573–1596. 10.1021/acschemneuro.0c00007. [DOI] [PubMed] [Google Scholar]
- Kortagere S.; et al. Identification of Novel Allosteric Modulators of Glutamate Transporter EAAT2. ACS Chem. Neurosci. 2018, 9, 522–534. 10.1021/acschemneuro.7b00308. [DOI] [PubMed] [Google Scholar]
- Das S.; et al. Design and Characterization of Novel Small Molecule Activators of Excitatory Amino Acid Transporter 2. ACS Med. Chem. Lett. 2022, 13, 1628–1633. 10.1021/acsmedchemlett.2c00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abram M.; et al. Discovery of (R)-N-Benzyl-2-(2,5-dioxopyrrolidin-1-yl)propanamide [(R)-AS-1], a Novel Orally Bioavailable EAAT2 Modulator with Drug-like Properties and Potent Antiseizure Activity In Vivo. J. Med. Chem. 2022, 65, 11703–11725. 10.1021/acs.jmedchem.2c00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriza J. L.; et al. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J. Neurosci. 1994, 14, 5559–5569. 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegelashvili G.; Schousboe A. Cellular distribution and kinetic properties of high-affinity glutamate transporters. Brain Res. Bull. 1998, 45, 233–238. 10.1016/S0361-9230(97)00417-6. [DOI] [PubMed] [Google Scholar]
- Besson M. T.; et al. Selective high-affinity transport of aspartate by a Drosophila homologue of the excitatory amino-acid transporters. Curr. Biol. 2000, 10, 207–210. 10.1016/S0960-9822(00)00339-0. [DOI] [PubMed] [Google Scholar]
- Balcar V. J.; et al. Stereospecificity of the inhibition of L-glutamate and L-aspartate high affinity uptake in rat brain slices by threo-3-hydroxyaspartate. J. Neurochem. 1977, 28, 1145–1146. 10.1111/j.1471-4159.1977.tb10682.x. [DOI] [PubMed] [Google Scholar]
- Marini A.; Novelli A. DL-threo-3-hydroxyaspartate reduces NMDA receptor activation by glutamate in cultured neurons. Eur. J. Pharmacol. 1991, 194, 131–132. 10.1016/0014-2999(91)90136-E. [DOI] [PubMed] [Google Scholar]
- Fu H.; et al. Chemoenzymatic Synthesis and Pharmacological Characterization of Functionalized Aspartate Analogues As Novel Excitatory Amino Acid Transporter Inhibitors. J. Med. Chem. 2018, 61, 7741–7753. 10.1021/acs.jmedchem.8b00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto K.; et al. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol. Pharmacol. 1998, 53, 195–201. 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- Cechova S.; Zuo Z. Inhibition of glutamate transporters increases the minimum alveolar concentration for isoflurane in rats. Br. J. Anaesth. 2006, 97, 192–195. 10.1093/bja/ael152. [DOI] [PubMed] [Google Scholar]
- Montiel T.; et al. Differential effects of the substrate inhibitor l-trans-pyrrolidine-2,4-dicarboxylate (PDC) and the non-substrate inhibitor DL-threo-beta-benzyloxyaspartate (DL-TBOA) of glutamate transporters on neuronal damage and extracellular amino acid levels in rat brain in vivo. Neuroscience 2005, 133, 667–678. 10.1016/j.neuroscience.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Bonde C.; et al. Neurotoxic and neuroprotective effects of the glutamate transporter inhibitor DL-threo-beta-benzyloxyaspartate (DL-TBOA) during physiological and ischemia-like conditions. Neurochem. Int. 2003, 43, 371–380. 10.1016/S0197-0186(03)00024-X. [DOI] [PubMed] [Google Scholar]
- Shigeri Y.; et al. Effects of threo-beta-hydroxyaspartate derivatives on excitatory amino acid transporters (EAAT4 and EAAT5). J. Neurochem. 2001, 79, 297–302. 10.1046/j.1471-4159.2001.00588.x. [DOI] [PubMed] [Google Scholar]
- Hansen J. C.; et al. beta-Sulfonamido Functionalized Aspartate Analogues as Excitatory Amino Acid Transporter Inhibitors: Distinct Subtype Selectivity Profiles Arising from Subtle Structural Differences. J. Med. Chem. 2016, 59, 8771–8786. 10.1021/acs.jmedchem.6b01066. [DOI] [PubMed] [Google Scholar]
- Leighton B. H.; et al. A hydrophobic domain in glutamate transporters forms an extracellular helix associated with the permeation pathway for substrates. J. Biol. Chem. 2002, 277, 29847–29855. 10.1074/jbc.M202508200. [DOI] [PubMed] [Google Scholar]
- Shimamoto K.; et al. Characterization of novel L-threo-beta-benzyloxyaspartate derivatives, potent blockers of the glutamate transporters. Mol. Pharmacol. 2004, 65, 1008–1015. 10.1124/mol.65.4.1008. [DOI] [PubMed] [Google Scholar]
- Bozzo L.; Chatton J. Y. Inhibitory effects of (2S, 3S)-3-[3-[4-(trifluoromethyl) benzoylamino]benzyloxy]aspartate (TFB-TBOA) on the astrocytic sodium responses to glutamate. Brain Res. 2010, 1316, 27–34. 10.1016/j.brainres.2009.12.028. [DOI] [PubMed] [Google Scholar]
- Leuenberger M.; et al. Concise Asymmetric Synthesis and Pharmacological Characterization of All Stereoisomers of Glutamate Transporter Inhibitor TFB-TBOA and Synthesis of EAAT Photoaffinity Probes. ACS Chem. Neurosci. 2016, 7, 534–539. 10.1021/acschemneuro.5b00311. [DOI] [PubMed] [Google Scholar]
- Greenfield A.; et al. Synthesis and biological activities of aryl-ether-, biaryl-, and fluorene-aspartic acid and diaminopropionic acid analogs as potent inhibitors of the high-affinity glutamate transporter EAAT-2. Bioorg. Med. Chem. Lett. 2005, 15, 4985–4988. 10.1016/j.bmcl.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Dunlop J.; et al. Characterization of novel aryl-ether, biaryl, and fluorene aspartic acid and diaminopropionic acid analogs as potent inhibitors of the high-affinity glutamate transporter EAAT2. Mol. Pharmacol. 2005, 68, 974–982. 10.1124/mol.105.012005. [DOI] [PubMed] [Google Scholar]
- Johnston G. A.; et al. Action of the neurotoxin kainic acid on high affinity uptake of L-glutamic acid in rat brain slices. J. Neurochem. 1979, 32, 121–127. 10.1111/j.1471-4159.1979.tb04518.x. [DOI] [PubMed] [Google Scholar]
- Simon J. R.; et al. Binding of [3H] kainic acid, and analogue of Lglutamate, to brain membranes. J. Neurochem. 1976, 26, 141–147. [PubMed] [Google Scholar]
- Gouix E.; et al. Reverse glial glutamate uptake triggers neuronal cell death through extrasynaptic NMDA receptor activation. Mol. Cell. Neurosci. 2009, 40, 463–473. 10.1016/j.mcn.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Koch H. P.; et al. Differentiation of substrate and nonsubstrate inhibitors of the high-affinity, sodium-dependent glutamate transporters. Mol. Pharmacol. 1999, 56, 1095–1104. 10.1124/mol.56.6.1095. [DOI] [PubMed] [Google Scholar]
- Vandenberg R. J.; et al. Contrasting modes of action of methylglutamate derivatives on the excitatory amino acid transporters, EAAT1 and EAAT2. Mol. Pharmacol. 1997, 51, 809–815. 10.1124/mol.51.5.809. [DOI] [PubMed] [Google Scholar]
- Dunlop J.; et al. WAY-855 (3-amino-tricyclo[2.2.1.02.6]heptane-1,3-dicarboxylic acid): a novel, EAAT2-preferring, nonsubstrate inhibitor of high-affinity glutamate uptake. Br. J. Pharmacol. 2003, 140, 839–846. 10.1038/sj.bjp.0705509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot E.; et al. Chemo-enzymatic synthesis of (2S,4R)-2-amino-4-(3-(2,2-diphenylethylamino)-3-oxopropyl) pentanedioic acid: a novel selective inhibitor of human excitatory amino acid transporter subtype 2. J. Med. Chem. 2008, 51, 4085–4092. 10.1021/jm800091e. [DOI] [PubMed] [Google Scholar]
- Sander C. Y.; Hesse S. News and views on in-vivo imaging of neurotransmission using PET and MRI. Q. J. Nucl. Med. Mol. Imaging. 2017, 61, 414–428. 10.23736/S1824-4785.17.03019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee A. D.et al. Small Molecules as Radiopharmaceutical Vectors. In Radiopharmaceutical Chemistry, Lewis J. S.; Windhorst A. D.; Zeglis B. M., Eds.; Springer International Publishing: Cham, 2019; pp 119–136. [Google Scholar]
- Eckelman W. C.; et al. Receptor-binding radiotracers: a class of potential radiopharmaceuticals. J. Nucl. Med. 1979, 20, 350–357. [PubMed] [Google Scholar]
- Luurtsema G.; et al. EANM guideline for harmonisation on molar activity or specific activity of radiopharmaceuticals: impact on safety and imaging quality. EJNMMI Radiopharm. Chem. 2021, 6, 34. 10.1186/s41181-021-00149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T. T.; et al. Central Nervous System Multiparameter Optimization Desirability: Application in Drug Discovery. ACS Chem. Neurosci. 2016, 7, 767–775. 10.1021/acschemneuro.6b00029. [DOI] [PubMed] [Google Scholar]
- Clark D. E. In silico prediction of blood-brain barrier permeation. Drug Discovery Today 2003, 8, 927–933. 10.1016/S1359-6446(03)02827-7. [DOI] [PubMed] [Google Scholar]
- Yuan H.; Silverman R. B. Structural modifications of (1S,3S)-3-amino-4-difluoromethylenecyclopentanecarboxylic acid, a potent irreversible inhibitor of GABA aminotransferase. Bioorg. Med. Chem. Lett. 2007, 17, 1651–1654. 10.1016/j.bmcl.2006.12.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galante E.; et al. Development of purine-derived 18F-labeled pro-drug tracers for imaging of MRP1 activity with PET. J. Med. Chem. 2014, 57, 1023–1032. 10.1021/jm401764a. [DOI] [PMC free article] [PubMed] [Google Scholar]