Abstract
Immunoglobulin therapy has a crucial role in the treatment of primary and secondary immunodeficiencies as well as in a multitude of neurologic, hematologic, infectious, and autoimmune conditions. In the current study, a preliminary pilot scale needs assessment survey was conducted to examine the need for IVIG among patients in Addis Ababa, Ethiopia, and in so doing justify local manufacturing of IVIG products. The survey was performed by administering a structured questionnaire to private and government hospitals, a national blood bank, a regulatory body, and healthcare researchers working in academia and pharmaceutical companies. The questionnaire encompassed demographics and specific IVIG-related questions designed for each institution. Responses supplied in the study provide qualitative data. Our findings indicated that IVIG has been registered by the regulatory body for use in Ethiopia and there is a demand for the product in the country. The study also highlights that patients go as far as to clandestine markets to procure IVIG products at a cheaper price. To impede such illegal routes and make the product readily accessible, a small-scale and low-cost approach such as a mini-pool plasma fractionation technique could be implemented to locally purify and prepare IVIG using plasma collected through the national blood donation program.
Keywords: Convalescent sera, immunoglobulin, immunotherapy, IVIG, plasma fractionation
Introduction
Immunoglobulins (IGs) or antibodies are glycoproteins that are produced by B cells in response to specific antigenic stimuli such as bacteria, viruses, fungi, parasites, cellular antigens, chemicals, and synthetic substances.1 Based on differences in the amino acid sequence, five major antibody isotypes have been identified: IgA, IgD, IgE, IgG, and IgM.2 In serum, the IgG isotype comprises 75% to 80% of the antibodies2 and it is the only immunoglobulin that crosses the placentae and thus the most abundant isotype in neonates.3
Disorders of IGs can result from intrinsic genetic defects, secondary to malnutrition and chronic diseases such as cancer, autoimmune, dermatologic, and infectious diseases. The most common types of antibody disorders, worldwide, are X-linked agammaglobulinemia, transient hypogammaglobulinemia of new-borns, selective immunoglobulin immunodeficiencies, super IgM syndrome, and common variable immunodeficiency disorder.4 In such humoral immune disorders, intravenous immunoglobulin G (IVIG) replacement therapy is the treatment of choice.5 The composition of IVIG preparations closely relates to the IGs found in normal human plasma and it contains concentrated IgG immunoglobulins pooled from the plasma of thousands of healthy blood donors.
The therapeutic applications of IVIG go beyond antibody replacement therapy in patients with antibody deficiency. Ever since its inception, the number of inflammatory and autoimmune diseases for which IVIG is used has been expanding enormously. These diverse disorders range from transplant rejection to blistering skin diseases and neurologic diseases. IVIG is now widely accepted for use in patients with multiple other diseases including, but not limited to, Guillain-Barré syndrome, multiple myeloma, myasthenia gravis, acquired factor VIII inhibitor syndrome, chronic inflammatory demyelinating polyradiculoneuropathy, autoimmune neutropenia, post-transfusion purpura, and polymyositis or dermatomyositis.6,7 Unlike subcutaneous IGs, treatment with IVIG is carried out every 3 to 4 weeks with rare local infusion site reactions. Adverse events associated with IVIG treatment are commonly systemic and can be either mild or moderate and rarely patients may develop severe reactions.8 These adverse events are commonly seen in treatment naïve patients, especially during their first infusion, and the reactions usually subside along with treatment. These symptoms include mild to moderate headache, nausea, fever, fatigue, and “flu-like” symptoms.9
Historical precedents also showed the safety and efficacy of IVIG therapy as an anti-infective agent in a growing array of diseases including the West African Ebola epidemic,10 novel influenza A virus pandemic,11 severe acute respiratory syndrome of coronavirus 1 (SARS-CoV-1)12 and Spanish Flu pandemic.13 In the current SARS-CoV-2 pandemic, infusion of human convalescent sera preparations obtained from individuals who have recovered from the infection has been shown as a reliable treatment approach in critically ill patients.14 As such, the clinical importance of IGs as a source of neutralizing antibodies for specific pathogens can never be overemphasized.
In addition, biotherapeutics such as monoclonal antibodies and recombinant proteins offer some degree of clinical advantage in terms of safety, efficacy, and convenience over the standard of care and are pivotal components of modern medicine. However, these treatment options are generally not readily available in low- and middle-income countries (LMICs) including Ethiopia, partly due to their higher costs compared to available conventional alternatives. Another reason could be attributed to health system challenges that create a lack of information in identifying patients who could otherwise benefit from biotherapeutics. For example, some promising and lifesaving biotherapeutics that have proven to considerably increase the overall survival of patients in high-burden conditions such as human epidermal growth factor receptor 2 positive breast cancer take a long time to be integrated into the public health systems.15-17 This is one reason why a higher proportion of premature deaths from cancer is more prevalent in LMICs than in developed countries.18 Therefore, as therapies are now shifting from pharmaceuticals to monoclonal antibodies and RNA-based therapies, it is high time for LMICs to start venturing into these potentially lifesaving treatment options.
Fundamentally, plasma fractionation and IVIG preparation are done in state-of-the-art facilities that follow current principles of good manufacturing practice (cGMP) to ensure the production of high-quality plasma products with lot-to-lot consistency. However, construction of such facilities invariably requires a sizable investment capital pool of about 60 to 100 million US dollars.19 This ultimately limits the availability of IVIG products in resource-constrained developing countries including Ethiopia and patients who need IVIG care normally have to import the product from abroad at high and unaffordable costs. Additionally, the vast majority of patients who require the therapy do not have the financial resources to cover the high treatment costs and associated expenses. As such, it is imperative to look for alternative production techniques that still adhere to cGMP working standards and are successful in maintaining the integrity and quality of plasma products. A mini-pool plasma fractionation system had previously been successfully designed as a prospective alternative production approach for producing IG products in a closed sterile bag system.19-22 Such a technique will be adapted and tailored to the demands and requirements of patients in Ethiopia. This inevitably has the potential to culminate in expensive treatment costs that patients have to deal with while obtaining treatment from abroad. In addition, according to the ministry of Health of Ethiopia, the total units of blood collected per annum had increased by 57% in 2019/2020 due to a growth in the trend of blood donors.23 This eventually makes it possible to support the local preparation and purification of IVIG products. However, before investing in local IVIG preparation, it is necessary to determine the country’s IVIG requirements. As such, the purpose of this preliminary pilot scale need assessment survey is to gather information on the IVIG requirements by consulting with physicians working in private and government-owned hospitals in Addis Ababa, Ethiopia, a regulatory body, a national blood bank and researchers working in academia and pharmaceutical companies.
Methodology
A preliminary pilot scale need assessment survey on IVIG requirements was conducted using a structured questionnaire administered to Myungsung Christian General Hospital (a private general teaching hospital) and Black Lion Specialized Hospital (a tertiary government teaching hospital), Ethiopian National Blood Bank Service (ENBBS), Ethiopian Food and Drug Authority (EFDA) and healthcare researchers from academia and pharmaceutical companies (Ethiopian Pharmaceuticals Manufacturing Shared company [EPHARM] and East African Pharmaceuticals [EAP]). These hospitals were chosen because they often serve high loads of patients with a variety of diagnoses or illnesses in the country. The questionnaires encompassed demographics and specific IVIG-related questions designed for each institution. Responses supplied in the study provide qualitative data. In total, there were 14 physicians from the 2 hospitals, 1 from ENBBS, EFDA, pharmaceutical companies, and academia who participated in the survey. Physician participants were selected from departments that are, through literature review, known to have higher utilization of IVIG. As a result, physicians with extensive clinical experience and an academic role in haemato-oncology, adult and pediatric neurology, internal medicine, critical care medicine, and pediatrics were included. The responses obtained from each institution were thoroughly examined and reported in the study. The questionnaire designed for each institution is provided in the Supplemental Material.
Result and Discussion of Findings
Hospitals
In total, 14 physicians who participated in the study are hematologists, pediatricians and neurologists working in the 2 major hospitals in Addis Ababa, Ethiopia; Myungsung Christian General Hospital and Black Lion Specialized Hospital. According to the physician’s response, the hospitals have the necessary equipment and staff to provide IG care and monitor patients. Some of the clinical assessment and evaluation procedures followed to diagnose patients in those hospitals were: patient history, physical examinations such as vital signs, imaging, electrophysiological studies, and laboratory investigations. However, there are no locally drafted guidelines available to prescribe IVIG preparations in Ethiopia because IVIG is not listed in the country’s essential medicines list according to the Food, Medicine and Healthcare Administration and Control Authority of Ethiopia. As such, the physicians follow internationally recognized standard prescription guidelines such as the American Academy of Allergy, Asthma & Immunology, IV immunoglobulin prescription chart-Leeds formulary, and American academy of neurology to administer IVIG preparations.
According to the physicians, the most frequently diagnosed disorders in their practice for which IG care is essential are Guillain–Barré syndrome (100%), followed by myasthenia gravis (38%), chronic inflammatory demyelinating polyneuropathy (31%), acute disseminated encephalomyelitis (23%), Dermatomyositis (23%), and Acute transverse myelitis (23%) (Figure 1).
Figure 1.
Percentage of frequently diagnosed disorders for which IVIG therapy is required. The denominator used to calculate the percentage was n = 14.
Most of the side effects experienced by patients while receiving IVIG treatment are mild and include headache, chills, rigor, and myalgia. Furthermore, the physicians underscored that while approximately 415 patients between 2016 and 2021 required IVIG therapy, only around 18% received treatment while the remaining 81.44% of patients were without treatment (Figure 2) due to the unavailability of locally produced IVIG products and lack of financial means to cover the high treatment costs; which is around 5720 USD over a 5 days treatment course. It was also noted that among the 18% of those who underwent treatment, their IVIG products were obtained through a clandestine market. This invariably puts the quality, efficacy, safety and integrity of the IVIG products in question and predisposes patients to unnecessary medical adversities. To impede such illegal routes, reduce the high treatment costs, and enhance on-time patient care, all the physicians recommended that local IVIG production is imperative for the country and therefore the authors recommend that it should receive special attention from the ministry of health and related organizations.
Figure 2.
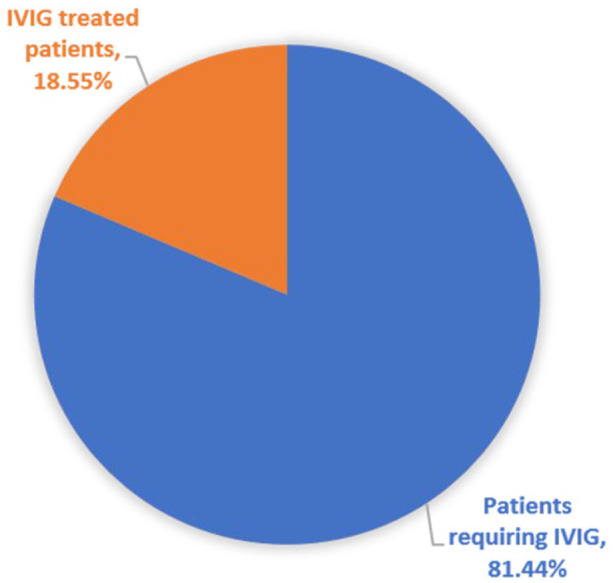
Percentage of patients who received IVIG treatment and those who required IVIG between 2016 and 2021.
Ethiopian national blood bank service
The Ethiopian National Blood Bank Service (ENBBS) is a non-profit governmental organization whose core functions, among others, include community mobilization and education on voluntary blood donation, blood collection, laboratory processing, testing, and blood component production. As an informant, the director of ENBBS underscored that the total national whole blood collected by ENBBS in the first 11 months of 2021 was 256 473 units. Some of the blood components produced by ENBBS include plasma, platelets and cryoprecipitate. The ENBBS tests the obtained whole blood for HIV, HBC, HCV, and syphilis as the principal pathogenic illnesses. To ensure the quality and integrity of the testing procedure, ENBBS has in place a quality management system, and the pathogen test kits are thoroughly examined by the quality laboratory before usage. However, the organization does not use pathogen-inactivation methods to treat and use donated blood.
Apheresis services such as plasmapheresis and plateletpheresis as sources of plasma are not provided by ENBBS, although they are included in the organization’s 5-year transformation strategy. In addition, the ENBBS’s long-term goal includes the manufacturing of immunoglobulins and other products derived from human plasma. The organization also believes that local immunoglobulin manufacturing is crucial for IVIG-dependent patients, and it is working under commitment with the ministry of health to build a platform to support the initiation and strengthen the capacity of immunoglobulin therapy services in Ethiopia.
Ethiopian food and drug authority
The Ethiopian Food and Drug Authority (EFDA) is a regulatory body in Ethiopia that oversees the safety, efficacy, and quality of health and health-related products and services. According to the medicine assessor response, IVIG has been registered by EFDA for use in Ethiopia and a generic pharmaceutical manufacturing establishment guideline is used as the regulatory requirement for the local production of IVIG products. However, the organization does not have a guideline for the production of IVIG products through blood collection, although there are initiatives put in place. The respondent also highlighted that for registration of IVIG products in Ethiopia, a guideline for registration of bio-therapeutic products can be used. However, depending on the method of manufacturing, other medicine registration guidelines may be used. If a guideline for a particular product is not available at EFDA, the organization uses European Medicines Agency guidelines for evaluation purposes. The medicine assessor also noted that, previously, there were applications for importing and distributing IVIG products from abroad, for which, some of these products have been registered and others are in the assessment process. Lastly, the respondent concurred that there is a need to locally produce IVIG preparations in Ethiopia.
Healthcare researchers
The survey included 2 research and development managers from Ethiopian pharmaceuticals manufacturing shared company and East African Pharmaceuticals, as well as 3 professors from academia: (1) a professor in Pharmacology and Clinical Pharmacy from the School of Pharmacy, College of Health Sciences, Addis Ababa University, (2) a professor of pediatrics and child health, and (3) a professor and head of the neurology department (who is also the current president of the Ethiopian Neurology Society) at Black Lion specialized hospital, under the college of health science of Addis Ababa University. So far in their careers, the researchers have not made investigational research on blood components but have shown intention to perform research on the preparation and purification of blood components. All the researchers indicated that local IVIG manufacturing is imperative for the advancement of the healthcare industry within the country and for assisting IVIG-dependent patients in overcoming the challenges they face while receiving therapy.
Conclusion
The preliminary findings obtained from the different stakeholders involved in this pilot scale need assessment survey strongly justify local purification and preparation of IVIG products. In addition, our need assessment survey establishes a solid ground as to why local manufacturing of IVIG products in resource-constrained countries such as Ethiopia is critical to saving the lives of patients who would otherwise be at risk. The survey also highlighted the most prevalent IVIG-required illnesses as well as the number of patients receiving IVIG therapy and the lack thereof in Addis Ababa, Ethiopia. To support the need for local manufacturing of immunoglobulin products, the Ethiopian National Blood Bank Service has taken initiatives to work under commitment with the ministry of health to build a platform and strengthen the capacity of immunoglobulin services in Ethiopia. As treatments are now shifting from conventional pharmaceuticals to the use of biologics, it is high time for Ethiopia and other developing countries to venture into this field of technology and thus local manufacturing of IVIG products is an initial step that paves the way. Fundamentally, IVIG production is done in state-of-the-art facilities that are expensive to be implemented in resource-constrained countries including Ethiopia. However, a mini-pool plasma fractionation system is an alternative approach that is relatively less expensive, cGMP compliant and efficient for producing high-quality plasma products with lot-to-lot consistency. This exploratory pilot scale study, in general, paves the ground for a quantitative study to be conducted to obtain more comprehensive IVIG need assessment data by incorporating multiple healthcare stakeholders in commercial and government-owned institutions across Ethiopia.
Supplemental Material
Supplemental material, sj-docx-1-his-10.1177_11786329231157467 for Intravenous Immunoglobulin G (IVIG) Need Assessment Survey Toward Local Manufacturing of IVIG Using a Mini-Pool Plasma Fractionation Technique by Bisrat Bekele, Zekarias Masresha, Mekdelawit Alemayehu, Berhanu Seyoum, Liya Wassie and Markos Abebe in Health Services Insights
Acknowledgments
The authors would like to thank all the physicians from Myungsung Christian General Hospital and Black Lion Specialized Hospital, the director general of ENBBS, the medicine assessor at EFDA, research and development managers from Ethiopian Pharmaceuticals Manufacturing Shared company and East African Pharmaceuticals, and Addis Ababa University Professor of Pharmacology and Clinical Pharmacy for participating in the need assessment survey.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ Contribution: Conceptualization, M.A; Investigation, B.B, M.A, Z.M, B.S, Mek. A, L.W; Writing – preparation of original manuscript and questionnaire, B.B; Writing – review and editing of manuscript and questionnaire, M.A, B.S, L.W; Writing – review and editing of the manuscript, Mek., Z.M.
Ethics Approval Statement: The survey did not require ethics approval because it did not involve patients or patient record charts; rather, participants responded based on their years of professional experience in their area of duty.
Statement of Informed Consent: The survey was voluntary, and participants were given a brief overview of the survey’s purpose. Before delivering the questionnaire, informed consent from the respondents was acquired.
ORCID iDs: Bisrat Bekele  https://orcid.org/0000-0001-8773-7467
https://orcid.org/0000-0001-8773-7467
Zekarias Masresha  https://orcid.org/0000-0002-5836-9594
https://orcid.org/0000-0002-5836-9594
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Burton DR. Antibody: the flexible adaptor molecule. Trends Biochem Sci. 1990;15:64-69. [DOI] [PubMed] [Google Scholar]
- 2. de Taeye SW, Rispens T, Vidarsson G. The ligands for human IgG and their effector functions. Antibodies. 2019;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21:3365-3369. [DOI] [PubMed] [Google Scholar]
- 4. Gernez Y, Baker MG, Maglione PJ. Humoral immunodeficiencies: conferred risk of infections and benefits of immunoglobulin replacement therapy. Transfusion. 2018;58:3056-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arumugham VB, Rayi A. Intravenous Immunoglobulin (IVIG). StatPearls; 2021. [PubMed] [Google Scholar]
- 6. Jolles S, Sewell WA, Misbah SA. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol. 2005;142:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kivity S, Katz U, Daniel N, Nussinovitch U, Papageorgiou N, Shoenfeld Y. Evidence for the use of intravenous immunoglobulins—a review of the literature. Clin Rev Allergy Immunol. 2010;38:201-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Espanol T, Olding L, Prevot J, Drabwell J, Sondhi S. Improving current immunoglobulin therapy for patients with primary immunodeficiency: quality of life and views on treatment. Patient Prefer Adherence. 2014;8:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonilla FA. Intravenous immunoglobulin: adverse reactions and management. J Allergy Clin Immunol. 2008;122:1238-1239. [DOI] [PubMed] [Google Scholar]
- 10. van Griensven J, De Weiggheleire A, Delamou A, et al. The use of ebola convalescent plasma to treat ebola virus disease in resource-constrained settings: a perspective from the field. Clin Infect Dis. 2016;62(1):69-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rockman S, Lowther S, Camuglia S, et al. Intravenous immunoglobulin protects against severe pandemic Influenza infection. EBioMedicine. 2017;19:119-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kubota-Koketsu R, Terada Y, Yunoki M, et al. Neutralizing and binding activities against SARS-CoV-1/2, MERS-CoV, and human coronaviruses 229E and OC43 by normal human intravenous immunoglobulin derived from healthy donors in Japan. Transfusion. 2021;61:356-360. [DOI] [PubMed] [Google Scholar]
- 13. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145:599-609. [DOI] [PubMed] [Google Scholar]
- 14. Liu STH, Lin H-M, Baine I, et al. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat Med. 2020;26:1708-1713. [DOI] [PubMed] [Google Scholar]
- 15. Martei YM, Binagwaho A, Shulman LN. Affordability of cancer drugs in Sub-Saharan Africa: effects of pricing on needless loss of life. JAMA Oncol. 2017;3:1301-1302. [DOI] [PubMed] [Google Scholar]
- 16. Barrios CH, Reinert T, Werutsky G. Access to high-cost drugs for advanced breast cancer in Latin America, particularly trastuzumab. Ecancermedicalscience. 2019;13:898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trapani D, Lengyel CG, Habeeb BS, et al. The global landscape of availability, accessibility and affordability of essential diagnostics and therapeutics for the management of HER2-positive breast cancer: the ONCOLLEGE-001 survey. J Cancer Policy. 2021;28:100285. [DOI] [PubMed] [Google Scholar]
- 18. Morin S, Segafredo G, Piccolis M, et al. Expanding access to biotherapeutics in low-income and middle-income countries through public health non-exclusive voluntary intellectual property licensing: considerations, requirements, and opportunities. Lancet Glob Health. 2023;11:e145-e154. [DOI] [PubMed] [Google Scholar]
- 19. El-Ekiaby M, Radosevich M, Goubran H, El Sayed M, Burnouf T. New methods of plasma fractionation - a presentation of the ‘mini-pool’ fractionation procedure developed in Egypt. ISBT Sci Ser. 2009;4:99-106. [Google Scholar]
- 20. Burnouf T, Caron C, Radosevich M, Goubran HA, Goudemand J, El-Ekiaby M. Properties of a concentrated minipool solvent-detergent treated cryoprecipitate processed in single-use bag systems. Haemophilia. 2008;14:956-962. [DOI] [PubMed] [Google Scholar]
- 21. El-Ekiaby M, Sayed MA, Caron C, et al. Solvent-detergent filtered (S/D-F) fresh frozen plasma and cryoprecipitate minipools prepared in a newly designed integral disposable processing bag system. Transfus Med. 2010;20:48-61. [DOI] [PubMed] [Google Scholar]
- 22. El-Ekiaby M, Vargas M, Sayed M, et al. Minipool caprylic acid fractionation of plasma using disposable equipment: a practical method to enhance immunoglobulin supply in developing countries. PLoS Negl Trop Dis. 2015;9:e0003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ministry of Health-Ethiopia. Health sector transformation plan II. Ministry of Health-Ethiopia; 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-his-10.1177_11786329231157467 for Intravenous Immunoglobulin G (IVIG) Need Assessment Survey Toward Local Manufacturing of IVIG Using a Mini-Pool Plasma Fractionation Technique by Bisrat Bekele, Zekarias Masresha, Mekdelawit Alemayehu, Berhanu Seyoum, Liya Wassie and Markos Abebe in Health Services Insights



