Abstract
Purpose
There is still no consensus on the therapeutic strategies for patients with International Federation of Gynecology and Obstetrics (FIGO) stage IV ovarian cancer (OC). We aim to outline the clinical characteristics and optimal therapeutic strategies of patients with FIGO stage IV OC.
Methods
This single center retrospective study analyzed the clinical features and survival of patients with FIGO stage IV OC that underwent cytoreduction or received at least one course of chemotherapy between January 2014 and December 2020.
Results
One hundred and twenty patients were included. Surgery, especially optimal cytoreduction without residual mass improved the overall survival of patients in surgery group (P = .047, HR .432, 95% CI .181-.987). Secondly, the completion of chemotherapy improved median overall survival of patients either with (53.0 months vs 25.0 months, P < .001, HR 7.015, 95% CI 1.372-35.881) or without cytoreduction (43.0 months vs 6.0 months, P = .006, HR 5.969, 95% CI 1.115-31.952). In patients with FIGO stage IVB, those with only extra-abdominal lymph node metastases had better survival.
Conclusions
In patients with FIGO stage IV, complete resection of intra-abdominal tumor foci and completion of chemotherapy provided considerable survival benefits to patients with FIGO stage IV OC. Among patients with FIGO stage IVB, those with only extra-abdominal lymph node metastases had a better prognosis.
Keywords: ovarian cancer, international federation of gynecology and obstetrics stage IV, cytoreduction, chemotherapy, prognosis
Introduction
Ovarian cancer remains the most leading cause of death from gynecological cancer in women worldwide. Due to the lack of typical clinical symptoms and effective screening methods, more than 60% of patients are diagnosed with advanced OC, and about 30% of patients are diagnosed with International Federation of Gynecology and Obstetrics (FIGO) stage IV for distant metastases. Despite advances in treatment, patients diagnosed with FIGO stage IV OC have poor prognosis with a 5-year overall survival (OS) rate of less than 20%.1,2
FIGO staging of OC was updated in 2014, defining patients with malignant pleural effusion but without other extra-abdominal or parenchymal metastases as stage IVA and the rest of patients with distant metastases as FIGO stage IVB. FIGO staging is an important predictive factor of prognosis,1,3 several studies have studied on the prognostic value of new staging method but no significantly positive result was observed.4–6
The residual disease after cytoreduction is one of the independent risk factors of prognosis.7 Patients with FIGO stage IV OC often have extensive foci in pelvic and abdominal cavity combined with distant metastases or parenchymal organ involvement, as a result, the cytoreduction is of high complexity. Recently, gynecologic oncologists tend to seek multi-disciplinary teamwork to perform thorough cytoreduction.8,9 However, whether intraoperative and postoperative complications associated with complex procedure compromise the survival benefits of the procedure itself remains controversial.10 There is still no consensus on the optimal therapeutic strategies for patients with FIGO stage IV OC.
The aim of this study was to summarize the clinical features and to testify optimal therapeutic strategies of patients with FIGO stage IV OC.
Materials and Methods
This retrospective was conducted at a tertiary cancer center. The study was approved by the board of the Institutional Ethics Committee of The First Affiliated Hospital of Chongqing Medical University on 17 February 2022 (approval number, 2022-K32). Due to the retrospective nature of the study, the requirement for informed consent in this study was waived. All patient details have been de-identified. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.11
Study Population
The enrolled patients met the following criteria: (a) newly diagnosed with FIGO stage IV epithelial ovarian cancer (EOC), primary fallopian tube cancer (PFTC) and primary peritoneal cancer (PC), admitted for primary debulking surgery (PDS) or interval debulking surgery (IDS) incorporated with neoadjuvant chemotherapy (NACT) or at least one course of chemotherapy without cytoreduction in The First Affiliated Hospital of Chongqing Medical University between January 2014 and December 2020; (b) no other malignant tumors; and (c) data on regular follow-up after initial treatment was available. The exclusion criteria were as follows: (a) age <18 years old, (b) borderline tumor, recurrence, or metastatic tumor.
We excluded patients who refused or could not tolerate cytoreduction or standard chemotherapy. Patients were divided into cytoreduction group (CR) and chemotherapy alone group (CT) based on whether they underwent cytoreduction or not. Patients who underwent only diagnostic laparoscopic or biopsy were not included in the CR group. Preoperative workup included at least a chest computed tomography scan (CT-scan) combined with abdominal-pelvic CT-scan or magnetic resonance imaging (MRI), or positron emission tomography/computed tomography (PET/CT). An experienced multidisciplinary oncological team then conducted a comprehensive evaluation of the patients. If intra-abdominal foci were considered resectable and the procedure was tolerated, the surgery was recommended. All surgeries were performed by professional gynecologic oncologist with the goal of achieving optimal intra-abdominal cytoreduction. The procedures of cytoreduction included hysterectomy and bilateral salpingo-oophorectomy, infragastric omentectomy, pelvic and paraaortic lymphadenectomy and the procedures that might be needed for optimal debulking surgery. NACT followed by IDS would be delivered when optimal PDS was infeasible. If unresectable foci remained after six or more cycles of NACT, the patient would be considered ineligible for cytoreduction and enrolled in the CT group.
All diagnoses were confirmed by the Department of Pathology of the hospital, all staging were based on FIGO staging principle updated in 2014. Patients with FIGO stage IVA had histologically evidence of malignant pleural effusion.
The diagnosis of distant lymph node metastasis was based on RECIST (Response Evaluation Criteria in Solid Tumors, version (1.1) criteria, when the short axis of a lymph node was >10 mm, the lymph node was regarded as enlarged and metastasis was considered. Patients who receive more than four course of chemotherapy regimen (recommended by gynecologic oncologist) were regarded as chemotherapy completion. Cytoreduction with no intra-abdominal residual mass was recorded as R0 resection. Cytoreduction with the maximum diameter of intra-abdominal residual tumors less or no less than 1 cm was recorded as R1 resection (optimal cytoreduction) and R2 resection, respectively.
Patients in stage IVB were divided into three subgroups according to the patterns of distant metastases: (i) lymph node group: only with distant lymph node metastases, without malignant pleural effusion, no restriction on the site(s) and number of positive lymph node(s); (ii) parenchymal group: only with parenchymal organ involvement, without malignant pleural effusion, regardless of the site(s) and number of organ(s) involved; (iii) multi-metastases group: all stage IVB patients except the above two groups.
Data Collection
Age, initial treatment, histological type, surgical data, chemotherapy regimen, and other data were retrospectively collected from the record system. The initial treatment date was defined as the date of cytoreduction for the PDS group and the date of the first course of NACT or chemotherapy for the IDS group or CT group. Overall survival (OS) was calculated from the date of initial treatment to death. Progression-free survival (PFS) was calculated from the initial treatment date to any progression or death, whichever occurred first. If no endpoints were observed, the last follow-up was considered.
Postoperative Management
Patients underwent outpatient assessment every 3 months in the first 2 years, every 6 months in the following 3 years, and annual follow-up thereafter or following the individualized follow-up plans developed by gynecologic oncologist if necessary. The indicators of follow-up included serologic tumor markers combined with pelvic and abdominal imaging examinations. Recurrence was defined as a continuous increase in serum CA-125 levels >2 times the upper limit or positive imaging findings based on the RECIST criteria or had any clinical symptoms associated with disease progression such as ascites.
Statistical Analysis
The Kaplan-Meier method was used for survival curves. Multivariate analysis was performed using the Cox proportional hazards model. Hazard ratios (HRs) were presented with 95% confidence intervals. The χ2 test or Fisher’s exact test was used for categorical variables. Statistical analysis was performed using SPSS version 21 (IBM Corporation, Armonk, NY, USA).
Results
Patient Population
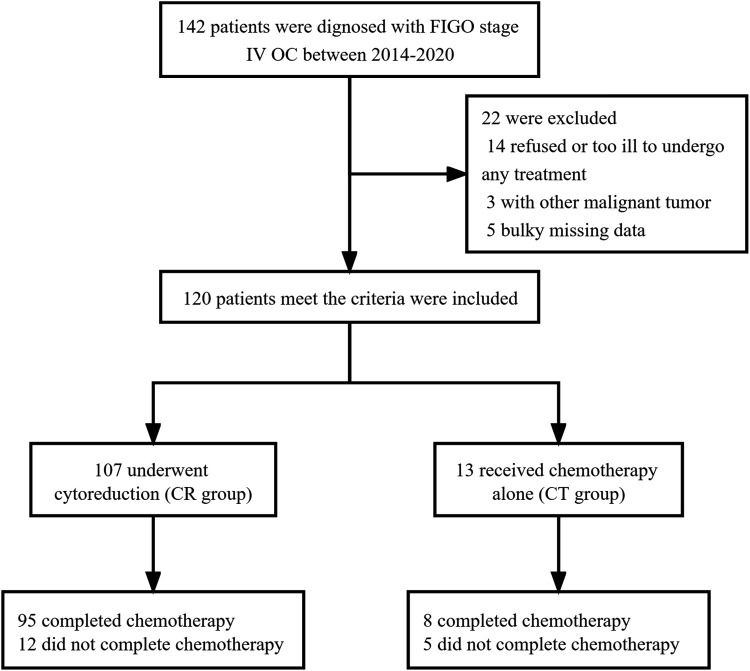
One hundred and forty-two patients were newly diagnosed with FIGO stage IV OC at our center between January 2014 and November 2020. As shown in Figure 1, one hundred and twenty patients met the inclusion criteria, One hundred and seventeen patients were included in the survival analysis for 3 were lost to follow-up. The last follow-up was in November 2021 and the median follow-up time was 25 months (range 3-89). The characteristics of the patients are shown in Table 1.
Figure 1.
Flowchart of patients for analysis.
Table 1.
Clinical Characteristics of Patients with FIGO Stage IV.
| Characteristics | NO. (%), (median, range) | P value | |
|---|---|---|---|
| Surgery (n = 107) | No-surgery (n = 13) | ||
| Age | 51 (31-73) | 58 (35-85) | .138 |
| Histological type | 1.000 | ||
| Serous | 92 (86.0) | 11 (84.6) | |
| Non-serousa | 15 (14.0) | 2 (15.4) | |
| Grade | 1.000 | ||
| High | 102 (95.3) | 11 (84.6) | |
| Low | 3 (2.8) | - | |
| Unknow | 2 (1.9) | 2 (15.4) | |
| FIGO stage | .929 | ||
| IVA | 19 (17.8) | 3 (23.1) | |
| IVB | 88 (82.2) | 10 (76.9) | |
| Lymph node metastasis | 12 (11.2) | 2 (15.4) | |
| Parenchymal metastasis | 30 (28.3) | 4 (30.8) | |
| Multi-metastasis | 46 (43.0) | 4 (30.8) | |
| Pleural effusion | .894 | ||
| Yes | 55 (51.4) | 7 (53.8) | |
| No | 51 (47.7) | 6 (46.2) | |
| Unknow | 1 (.9) | - | |
| Diaphragmatic angle lymph node metastasis | .085 | ||
| Yes | 16 (15.0) | 5 (38.5) | |
| No | 91 (85.0) | 8 (61.5) | |
| Neoadjuvant chemotherapy | |||
| Yes | 77 (82.0) | - | |
| No | 30 (28.0) | - | |
| Operative type | |||
| laparoscopic | 54 (50.5) | - | |
| laparotomy | 53 (49.5) | - | |
| Residual mass | |||
| <1 cm | 77 (78.0) | - | |
| ≥1 cm | 30 (28.0) | - | |
| Chemotherapy | .018 | ||
| Complete | 95 (88.8) | 8 (61.5) | |
| Incomplete | 11 (10.3) | 5 (38.5) | |
| Unknow | 1 (.9) | - | |
a6 clear cell carcinoma, 3 endometrioid carcinoma, 2 Mucous carcinoma, 4 unknow.
Survival
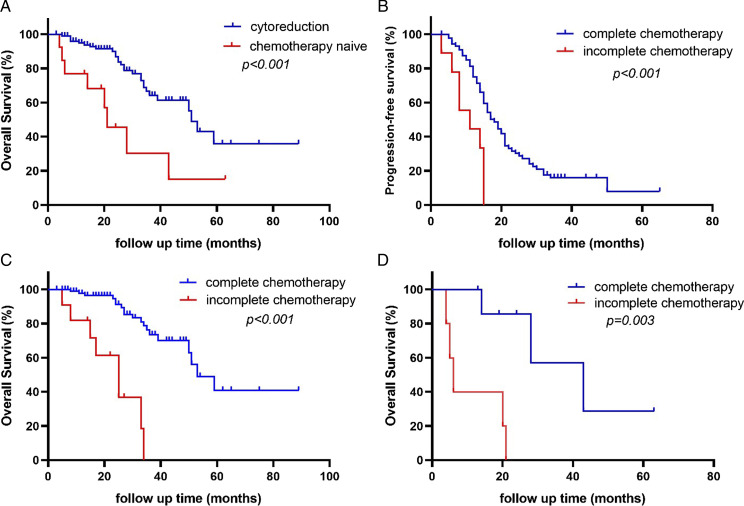
The median PFS of CR group was 16.0 months (95% CI 14.1-17.9), the three-year and five-year progression-free survival rate was 14.0% and a 7.0%, respectively. The median OS of CR group was 51.0 months (95% CI 46.5-55.5), the three-year and five-year overall survival rate was 65.0% and 36.2%, respectively. The median OS of CT group was 21.0 months (95% CI 11.7-30.3), the three-year and five-year overall survival rate was 30.4% and 15.2%, respectively. Patients in CR group had a better prognosis (51.0 months vs 21.0 months, P < .001, HR 2.43, 95% CI 1.11-5.33; Figure 2A).
Figure 2.
A, Overall survival of patients with or without cytoreduction; B, Progression-free survival of patients underwent cytoreduction with or without complete chemotherapy; C, Overall survival of patients underwent cytoreduction with or without complete chemotherapy; D, Overall survival of patients did not underwent cytoreduction with or without complete chemotherapy.
Among CR group, 93 (86.9%) patients with survival data completed chemotherapy, these who completed chemotherapy had longer median PFS (18.0 months vs 11.0 months, P < .001, HR 3.928, 95% CI 1.935-7.973) and median OS (53.0 months vs 25.0 months, P < .001, HR 7.015, 95% CI 1.372-35.881) than that of patients who did not (Figure 2B, Figure 2C).
Of patients who completed first line chemotherapy, 71 (66.4%) underwent IDS and 22 (20.6%) underwent PDS, the median overall chemotherapy course of IDS group and PDS group were 8 (range 4-13) and 6 (range 4-8), respectively. There was no difference in median PFS (P = .227) and median OS (P = .854) between the two groups.
Similarly, among CT group, eight (61.5%) patients completed chemotherapy and 5 (38.5%) patients did not. The median OS was longer in patients who completed chemotherapy (43.0 months vs 6.0 months, P = .006, HR 5.969, 95% CI 1.115-31.952; Figure 2D).
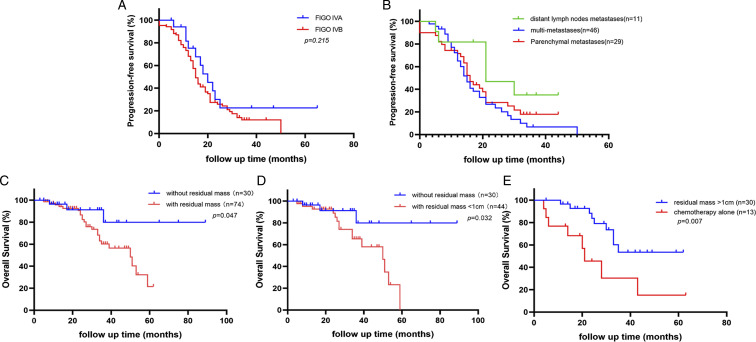
Stage-based stratified analysis showed no significant difference in median PFS (P = .215; Figure 3A) and median OS (P = .103) between patients with FIGO stage IVA and IVB. Besides, the results suggested that median PFS was longer in lymph node group when compared with the parenchymal group (21.0 months vs 15.0 months, P = .041, HR 2.458, 95% CI .982-6.151) and the multi-metastasis group (21.0 months vs 15.0 months, P = .039, HR 2.396.95% CI .995-5.773; Figure 3B). However, the median PFS was not statistically significant different between the parenchymal group and multi-metastasis group, and the median OS was not significantly different in any of the three groups.
Figure 3.
A, Progression-free survival of patients with FIGO IVA and FIGO IVB stage; B, Progression-free survival of patients with FIGO IVB stage. C, Overall survival of patients without and with residual intra-abdominal mass. D, Overall survival of patients without and with residual intra-abdominal mass less than 1 cm.E, Overall survival of patients with residual mass no less than 1 cm and with chemotherapy alone.
The median PFS was longer in laparoscopic group than that in laparotomy group (18.0 months vs 15.0 months, P = .030, HR 1.633 95% CI 1.031-2.589; Figure 3C), however, the difference in the median OS between two groups was not statistically significant. Further analysis indicated that laparoscopic group had higher optimal cytoreduction rate (81.5% vs 62.3%, P = .027).
Metastatic and Recurrence Sites
As shown in Table 2, the most common distant metastatic sites at initial diagnosis in the CR group were pleural effusion (n = 55, 51.4%), liver (n = 48, 44.8%), and distant lymph nodes (n = 36, 33.6%), and the diaphragmatic angle lymph node (n = 17, 15.9%) was the most common distant lymph node metastasis. Seventy-two (67.3%) patients in CR group had recurrence during follow-up, the recurrence sites were detailed in Table 3. The most common recurrence site was pelvic-abdominal recurrence (n = 43, 59.7%), and the most common parenchymal organ recurrence was the liver (n = 7, 9.7%).
Table 2.
Distant Metastatic Site at Initial Diagnosis. (n = 120).
| Site | NO. (%) | P value | |
|---|---|---|---|
| Surgery (n = 107) | No-surgery (n = 13) | ||
| Pleural effusion | 55 (51.4) | 7 (53.8) | .894 |
| Distant lymph node | 36 (33.6) | 6 (46.2) | .042 |
| Diaphragmatic angle | 17 (615.9) | 5 (38.5) | |
| Inguinal | 10 (9.3) | 3 (23.1) | |
| Supraclavicular | 8 (7.5) | 0 | |
| Axillary | 6 (5.6) | 0 | |
| Mediastinal | 5 (4.7) | 0 | |
| Othersa | 5 (4.7) | 0 | |
| Liver | 48 (44.8) | 6 (46.2) | .929 |
| Spleen | 18 (16.8) | 0 | .233 |
| Lung | 11 (10.3) | 5 (38.5) | .017 |
| Bone | 4 (3.7) | 1 (7.7) | .442 |
| Othersb | 2 (1.9) | 0 | 1.000 |
a1 subpleural lymph node, 2 cervical lymph node, 2 hilar lymph nodes.
b1 breast, 1 adrenal gland.
Table 3.
Recurrence Site of Patients Underwent Surgery (n = 72).
| Site | NO. (%) |
|---|---|
| Abdominopelvic cavity | 43 (59.7) |
| Distant lymph node | 5 (6.9) |
| Elevated CA125 | 16 (22.2) |
| Parenchymal organ | 11 (15.2) |
| Liver | 7 (9.7) |
| Spleen | 2 (2.8) |
| Othersa | 4 (5.6) |
a1 breast, 1 lung, 1 brain, 1bone.
Among CR group, 5 had bowel resection and 2 of them had fistula, 3 had hepatectomy, 1 had splenectomy, 1 had cholecystectomy. No patient underwent extra-abdominal surgery except 2 had inguinal lymph node dissected. No patient died within 30 days after surgery.
Univariate and Multivariate Analysis in CR Group
Univariate analysis on Table 4 showed that surgery type (P = .037), completed postoperative chemotherapy (P < .001) were significant prognostic factors for PFS. We attributed variables that differed significantly in univariate analysis to multivariate analysis. The results revealed that completed postoperative chemotherapy (P < .001) was an independent risk prognostic factor for survival.
Table 4.
Univariate and Multivariate Analyses of Patients Underwent Surgery on Progression-free Survival. (n = 104).
| Characteristics | n (%) | Univariate analyses | Multivariate analyses | ||
|---|---|---|---|---|---|
| P value | HR (95% CI) | P value | HR (95% CI) | ||
| FIGO stage | .230 | ||||
| IVA | 18 | 1 | |||
| IVB | 86 | 1.482 (.780-2.816) | |||
| Metastasis pattern | |||||
| Lymph node metastasis | 11 | 1 | .357 | .656 (.267-1.610) | |
| Parenchymal metastasis | 29 | .041 | 2.458 (.982-6.151) | ||
| Multi-metastasis | 46 | .039 | 2.396 (.995-5.773) | ||
| Multi-metastasis | .082 | .162 | 1.418 (.870-2.312) | ||
| No | 57 | 1 | |||
| Yes | 46 | 1.510 (.949-2.402) | |||
| Pleural effusion | .938 | ||||
| No | 49 | 1 | |||
| Yes | 54 | 1.018 (.654-1.538) | |||
| Neoadjuvant chemotherapy | .241 | ||||
| Yes | 77 | 1 | |||
| No | 27 | .713 (.405-1.256) | |||
| Operative type | .037 | .112 | 1.467 (.915-2.350) | ||
| Laparoscopic | 53 | 1 | |||
| Laparotomy | 51 | 1.633 (1.031-2.589) | |||
| Histological type | .971 | ||||
| Serous | 91 | 1 | |||
| Non-Serous | 13 | 1.013 (.499-2.059) | |||
| Residual massa | .226 | ||||
| ≤1 cm | 74 | 1 | |||
| >1 cm | 30 | 1.350 (.831-2.193) | |||
| Chemotherapy | <.001 | <.001 | 3.927 (1.875-8.226) | ||
| Complete | 93 | 1 | |||
| Incomplete | 11 | 3.928 (1.935-7.973) | |||
| Lung metastasis | .556 | ||||
| No | 92 | 1 | |||
| Yes | 11 | 1.248 (.597-2.607) | |||
| Liver metastasis | .231 | ||||
| No | 56 | 1 | |||
| Yes | 47 | 1.327 (.835-2.108) | |||
| Diaphragmatic angle lymph node metastasis | .497 | ||||
| No | 88 | 1 | |||
| Yes | 16 | 1.240 (.666-2.311) | |||
aResidual intra-abdominal foci.
As shown in Table 4, among CR group. The residual intra-abdominal foci was not an independent risk factor of the median PFS of patients. However, further analysis showed that the median OS of patients without residual intra-abdominal mass was longer than those with residual mass (P = .047, HR .432, 95% CI .181-.987; Figure 3C). Even among patients who had optimal cytoreduction (the maximum diameter of residual mass less than 1 cm), only those with no residuals had significantly longer OS (P = .032, HR .350, 95% CI .134-.913; Figure 3D). Compared with the median OS of patients with residual mass (50.0 months, with 95% CI of 30.4 - 64.7 months), the median OS of those without residual foci was not available for the incidence of endpoint did not reach 50%. Besides, the median OS of patients with R2 resection was longer than CT group (P = .007, HR .194, 95% CI .059-.636; Figure 3E).
Discussion
FIGO staging is an independent risk factor of prognosis of patients with OC.2 Some studies have pointed out that patients with FIGO stage IVA had similar survival outcomes to that with stage IVB, but those with only extra-abdominal lymph node metastases had better prognosis.6,12,13 In the study by Timmermans et al,12 the prognosis of patients in stage IVB that only with extra-abdominal lymph node metastases was statistically better and the conclusion held true for both clinically suspicious lymph node metastases and those confirmed by postoperative pathological examination. Similarly, Suh et al14 Found that patients had supraclavicular lymph node as the only extra-abdominal metastases foci were related to better prognosis and were less likely to have multiple distant metastasis. Our results were consistent with the studies, no significant difference in prognosis between patients with stage IVA and stage IVB was observed, and among FIGO stage IVB, patients in lymph node group had better prognosis.
Residual disease is closely related to the survival of patients with advanced OC,15 and achieving optimal cytoreduction with maximum effort is the prime goal of surgery. Dabi et al16 suggested that cytoreduction provide survival benefits to patients with FIGO stage IV regardless of PDS or IDS. Our results found that achieving optimal cytoreduction without any intra-abdominal residuals improved the OS of patients in CR group, implying the pivotal impact of R0 resection of intra-abdominal tumor load, as well as the necessity of preoperative evaluation. Since in case of recurrence, the decision to perform regional treatment with more aggressive efforts depends on the tumor burden and distribution. Intra-abdominal foci combined with distant metastases tumor are a common reason for palliative care and might be associated with a worse prognosis. Superior survival benefit of surgery to chemotherapy alone could still be observed even when we compared the OS between those with R2 resection vs CT group, which supports the potential significance of reducing tumor load in surgery for improving the efficacy of chemotherapy.
Optimal resection of pelvic and abdominal foci is not a thorough cytoreduction for patients with distant metastasis. The presence of extra-abdominal tumor still carries the risk of disease progression.17 Of the patients included in our study, none underwent extra-abdominal tumor resection except for two who underwent inguinal lymph node dissection. For untreated distant metastatic tumors, chemotherapy might be the only non-surgical option proven to be effective.18,19 Our results indicated that completion of chemotherapy prescribed by gynecologic oncologists significantly prolonged median OS of patients regardless of whether the cytoreduction was performed, emphasizing the critical importance of chemotherapy, even for these who cannot tolerate surgery, chemotherapy is still strongly recommended.
The severity of FIGO stage IV OC means that patients probably have to undergo extensive and challenging surgical procedures, increasing the incidence of intraoperative and postoperative complications and even delaying the initiation of postoperative adjuvant therapy, thus compromise the survival benefit of the procedure itself.9 Aletti et al1 suggested that patients with a high American Society of Anesthesiologists (ASA) risk classification (≥ grade 3) and multiple hepatic parenchymal metastases cannot benefit from cytoreduction. Besides, chi et al20 concluded that performing upper abdominal surgery increased the rate of optimal cytoreduction and thus improved survival of patients. Bogani et al21 reported a significant improvement in the prognosis of patients with stage IV FIGO by performing diaphragmatic resection, with a mild but manageable increase in the incidence of postoperative complications such as hemopneumothorax. Debates on how to balance the benefits of cytoreduction with the compromise of complications related to surgery, and whether chemotherapy is the preferred option for specific patients, and if so, how to identify such patients still need to be further explored.
We found that the most common sites of distant metastases in FIGO stage IV OC patients were malignant pleural fluid (51.4%), liver (44.8%), and extra-abdominal lymph nodes (33.6%). Diaphragmatic angle lymph node metastasis (15.9%) was the most common extra-abdominal lymph node metastasis, which is consistent with previous studies.3,12 Diaphragmatic angle lymph nodes are located above the diaphragm, some studies have revealed that the enlargement of diaphragmatic lymph nodes might be negatively associated with the prognosis.22,23 Although evidence confirmed the safety of diaphragmatic angle lymph node dissection, the correlation between this procedure and prognosis remains unclear. In our results, no correlation between diaphragmatic angle lymph nodes metastasis and prognosis was observed.
Isolated extra-abdominal recurrence without concomitant abdominal recurrence is rarely seen when relapsed. In our study, only 8 (11.1%) patients were diagnosed with recurrence due to the isolated extra-abdominal progression. Highlight the prominence of cytoreduction and adjuvant therapy of abdominal disease in patients with FIGO stage IV OC and the necessity of long-term management as well.
Strengths and Limitations
We included only patients with newly diagnosed FIGO stage IV OC after the implementation of the new version (FIGO 2014) of the staging scheme which could avoid errors due to incorrect staging or re-staging. The short time interval of include criteria and the identical surgical team of this study to some extent guarantee the consistency of the treatment protocol.
Limitations remained in this study. First, there is potential selection biases limited by the single center study and retrospective design and the power calculation for estimation of the required sample size was not conducted. Second, we cannot neglect the impact of some other important factors on prognosis that we did not perform valid analysis, such as preoperative CA-125 levels, the cycles of neoadjuvant chemotherapy, the Peritoneal Cancer Index (PCI) and BRCA mutation or HRD status of patients. Besides, although we adjusted for variables with regression, propensity score matching among subgroups might have better adjust for confounding factors and yield more robust results if larger sample size were available. In addition, the worse survival of patients in CT group might be associated with heavier tumor burden and worse performance status which affect the therapeutic efficacy. Finally, performing laparoscopic cytoreduction in patients with advanced OC has not reached a consensus yet and the above results should be carefully interpreted before the publishing of more convincing results.
Conclusions
In patients with FIGO stage IV, complete resection of intra-abdominal tumor foci and completion of chemotherapy provided considerable survival benefits to patients with FIGO stage IV OC. Among patients with FIGO stage IVB, those with only extra-abdominal lymph node metastases had a better prognosis. Larger trials are required to be conducted to confirm these findings.
Appendix.
Abbreviations
- 1. OC
Ovarian cancer
- 2. FIGO
International federation of gynecology and obstetrics
- 3. OS
Overall survival
- 4. EOC
Epithelial ovarian cancer
- 5. PC
Primary peritoneal cancer
- 6. PFTC
Primary fallopian tube cancer
- 7. PDS
Primary debulking surgery
- 8. IDS
Interval debulking surgery
- 9. NACT
Neoadjuvant chemotherapy
- 10. CR
Cytoreduction group;
- 11. CT
Chemotherapy alone group;
- 12. RECIST
Response evaluation criteria in solid tumors
- 13. PFS
Progression-free survival
- 14. HRs
Hazard ratios
- 15. ASA:
American society of anesthesiologists
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: This retrospective study was approved by the board of the Institutional Ethics Committee of The First Affiliated Hospital of Chongqing Medical University (No.1 Youyi Road, Yuzhong District, Chongqing, China) on 17 February 2022 (approval number, 2022-K32). Due to the retrospective nature of the study, the requirement for informed consent in this study was waived.
Data Availability: The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Consent to Participate: The need for written informed consent was waived due to the retrospective nature of the study.
Consent to Publish: All authors have consented to publication of the results presented in this manuscript.
ORCID iD
References
- 1.Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Analysis of factors impacting operability in stage IV ovarian cancer: Rationale use of a triage system. Gynecol Oncol. 2007;105(1):84-89. doi: 10.1016/j.ygyno.2006.10.055 [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284-296. doi: 10.3322/caac.21456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ataseven B, Chiva LM, Harter P, Gonzalez-Martin A, du Bois A. FIGO stage IV epithelial ovarian, fallopian tube and peritoneal cancer revisited. Gynecol Oncol. 2016;142(3):597-607. doi: 10.1016/j.ygyno.2016.06.013 [DOI] [PubMed] [Google Scholar]
- 4.Tajik P, van de Vrie R, Zafarmand MH, et al. The FIGO stage IVA versus IVB of ovarian cancer: prognostic value and predictive value for neoadjuvant chemotherapy. Int J Gynecol Cancer. 2018;28(3):453-458. doi: 10.1097/igc.0000000000001186 [DOI] [PubMed] [Google Scholar]
- 5.Jamieson A, Sykes P, Eva L, Bergzoll C, Simcock B. Subtypes of stage IV ovarian cancer; response to treatment and patterns of disease recurrence. Gynecol Oncol. 2017. Aug;146(2):273-278. doi: 10.1016/j.ygyno.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 6.Nasioudis D, Chapman-Davis E, Frey MK, Caputo TA, Witkin SS, Holcomb K. Should epithelial ovarian carcinoma metastatic to the inguinal lymph nodes be assigned stage IVB? Gynecol Oncol. 2017. Oct;147(1):81-84. doi: 10.1016/j.ygyno.2017.07.124 [DOI] [PubMed] [Google Scholar]
- 7.Timmermans M, van der Hel O, Sonke GS, Van de Vijver KK, van der Aa MA, Kruitwagen RF. The prognostic value of residual disease after neoadjuvant chemotherapy in advanced ovarian cancer; A systematic review. Gynecol Oncol. 2019. May;153(2):445-451. doi: 10.1016/j.ygyno.2019.02.019 [DOI] [PubMed] [Google Scholar]
- 8.Kehoe SM, Eisenhauer EL, Chi DS. Upper abdominal surgical procedures: liver mobilization and diaphragm peritonectomy/resection, splenectomy, and distal pancreatectomy. Gynecol Oncol. 2008;111(2 suppl l):S51-S55. doi: 10.1016/j.ygyno.2008.07.053 [DOI] [PubMed] [Google Scholar]
- 9.Hoffman MS, Tebes SJ, Sayer RA, Lockhart J. Extended cytoreduction of intraabdominal metastatic ovarian cancer in the left upper quadrant utilizing en bloc resection. Am J Obstet Gynecol. 2007. Aug;197(2):209.e1-209.e5. discussion 209.e4-5. doi: 10.1016/j.ajog.2007.04.049 [DOI] [PubMed] [Google Scholar]
- 10.Chi DS, Zivanovic O, Levinson KL, et al. The incidence of major complications after the performance of extensive upper abdominal surgical procedures during primary cytoreduction of advanced ovarian, tubal, and peritoneal carcinomas. Gynecol Oncol. 2010. Oct;119(1):38-42. doi: 10.1016/j.ygyno.2010.05.031 [DOI] [PubMed] [Google Scholar]
- 11.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med. 2007. Oct 16;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 12.Timmermans M, Sonke GS, Van de Vijver KK, et al. Localization of distant metastases defines prognosis and treatment efficacy in patients with FIGO stage IV ovarian cancer. Int J Gynecol Cancer. 2019;29(2):392-397. doi: 10.1136/ijgc-2018-000100 [DOI] [PubMed] [Google Scholar]
- 13.Hjerpe E, Staf C, Dahm-Kähler P, et al. Lymph node metastases as only qualifier for stage IV serous ovarian cancer confers longer survival than other sites of distant disease - a Swedish Gynecologic Cancer Group (SweGCG) study. Acta Oncol. 2018;57(3):331-337. doi: 10.1080/0284186x.2017.1400691 [DOI] [PubMed] [Google Scholar]
- 14.Suh DH, Kim TH, Kim JW, et al. Improvements to the FIGO staging for ovarian cancer: reconsideration of lymphatic spread and intraoperative tumor rupture. J Gynecol Oncol. 2013. Oct;24(4):352-358. doi: 10.3802/jgo.2013.24.4.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naik R, Nordin A, Cross PA, Hemming D, de Barros Lopes A, Monaghan JM. Optimal cytoreductive surgery is an independent prognostic indicator in stage IV epithelial ovarian cancer with hepatic metastases. Gynecol Oncol. 2000. Aug;78(2):171-175. doi: 10.1006/gyno.2000.5841 [DOI] [PubMed] [Google Scholar]
- 16.Dabi Y, Huchon C, Ouldamer L, et al. Patients with stage IV epithelial ovarian cancer: Understanding the determinants of survival. J Transl Med. 2020. Mar 23;18(1):134. doi: 10.1186/s12967-020-02295-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winter WE,, 3rd, Maxwell GL, Tian C, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: A gynecologic oncology group study. J Clin Oncol. 2008. Jan 1;26(1):83-89. doi: 10.1200/jco.2007.13.1953 [DOI] [PubMed] [Google Scholar]
- 18.Mahner S, Eulenburg C, Staehle A, et al. Prognostic impact of the time interval between surgery and chemotherapy in advanced ovarian cancer: Analysis of prospective randomised phase III trials. Eur J Cancer( Oxford, England: 1990). 2013. Jan;49(1):142-149. 10.1016/j.ejca.2012.07.023 [DOI] [PubMed] [Google Scholar]
- 19.Rauh-Hain JA, Rodriguez N, Growdon WB, et al. Primary debulking surgery versus neoadjuvant chemotherapy in stage IV ovarian cancer. Ann Surg Oncol. 2012;19(3):959-965. doi: 10.1245/s10434-011-2100-x [DOI] [PubMed] [Google Scholar]
- 20.Chi DS, Franklin CC, Levine DA, et al. Improved optimal cytoreduction rates for stages IIIC and IV epithelial ovarian, fallopian tube, and primary peritoneal cancer: A change in surgical approach. Gynecol Oncol. 2004;94(3):650-654. doi: 10.1016/j.ygyno.2004.01.029 [DOI] [PubMed] [Google Scholar]
- 21.Bogani G, Ditto A, Martinelli F, et al. Surgical techniques for diaphragmatic resection during cytoreduction in advanced or recurrent ovarian carcinoma: A systematic review and meta-analysis. Int J Gynecol Cancer. 2016. Feb;26(2):371-380. doi: 10.1097/igc.0000000000000597 [DOI] [PubMed] [Google Scholar]
- 22.Wang B, Wang S, Ren W. Development and validation of a nomogram to predict survival outcome among epithelial ovarian cancer patients with site-distant metastases: A population-based study. BMC Cancer. 2021. May 25;21(1):609. doi: 10.1186/s12885-021-07977-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raban O, Peled Y, Krissi H, et al. The significance of paracardiac lymph-node enlargement in patients with newly diagnosed stage IIIC ovarian cancer. Gynecol Oncol. 2015. Aug;138(2):259-262. doi: 10.1016/j.ygyno.2015.05.007 [DOI] [PubMed] [Google Scholar]