Abstract
Subjective tinnitus is the perception of sound in the absence of external stimulation. Neuromodulation is a novel method with promising properties for application in tinnitus management. This study sought to review the types of non-invasive electrical stimulation in tinnitus to provide the foothold for further research. PubMed, EMBASE, and Cochrane databases were searched for studies on the modulation of tinnitus by non-invasive electrical stimulation. Among the four forms of non-invasive electrical modulation, transcranial direct current stimulation, transcranial random noise stimulation, and transauricular vagus nerve stimulation yielded promising results, whereas the effect of transcranial alternating current stimulation in the treatment of tinnitus has not been confirmed. Non-invasive electrical stimulation can effectively suppress tinnitus perception in some patients. However, the heterogeneity in parameter settings leads to scattered and poorly replicated findings. Further high-quality studies are needed to identify optimal parameters to develop more acceptable protocols for tinnitus modulation.
Keywords: electrical stimulation, non-invasive, tACS, taVNS, tDCS, tinnitus, tRNS
Introduction
Subjective tinnitus is the perception of sound in the absence of external stimulation, with a reported prevalence of about 10–20% in adults.1 Severe tinnitus can bring about a range of psychological comorbidities such as depression, anxiety, and insomnia and can seriously affect the quality of life.2–4 According to a cross-sectional study in the United States, 26.1% and 25.6% of patients with tinnitus had anxiety and depression, respectively, with shorter sleep duration at night.5 Many approaches have been developed over the years to treat tinnitus encompassing pharmacotherapy, acoustic therapy, cognitive behavioral therapy (CBT), and surgical intervention. However, no consensus has been reached on the optimal therapeutic approach. Ginkgo biloba extract, steroids, and glutamate antagonists are the most applied agents to treat tinnitus. Interestingly, a review that included four randomized controlled trials (RCTs) found no benefit of ginkgo biloba extract in patients with tinnitus.6 Negative results were also found with steroids and glutamate antagonists.7,8 Acoustic therapy includes customized and non-customized sound, such as masking therapy, Heidelberg neuromusic therapy, and tailor-made notched music training (TMNMT).9 Although some patients experienced tinnitus suppression, strong evidence is still lacking.10,11 CBT has shown promising results in many studies, but due to the heterogeneity between studies, no definitive recommendation can be made at this time.12–14 Cochlear implants have also shown positive results on tinnitus suppression but limited efficacy for patients with severe hearing loss.15–17
Tinnitus is often related to hearing loss. Peripheral hearing loss causes decreased input from the cochlea to the auditory cortex (AC), resulting in increased firing rate and neuron synchronization. Moreover, this leads to changes in the state of excitement and inhibition, which is called thalamic cortical rhythm disorder. Studies in tinnitus patients without any hearing abnormalities suggest that the non-auditory brain network also plays a specific role in the occurrence and development of tinnitus. These networks include attention, salience, distress, and memory function. The activation of these non-auditory networks also leads to comorbidities, such as sleep disorder, anxiety, and depression. In general, tinnitus occurs due to changes in the neuroplasticity of the auditory and non-auditory systems. Given that functional changes in neuronal activity cause tinnitus, it could theoretically be suppressed through the corresponding neuromodulation mode.
Invasive and non-invasive neuromodulation techniques have been studied for tinnitus relief.18,19 Invasive techniques such as deep brain stimulation and epidural stimulation are limited,20–22 while non-invasive techniques are portable, cost-effective, easy-to-use, and highly acceptable. Transcranial magnetic stimulation (TMS) uses the magnetic field generated by a coil in contact with the scalp to affect the activity of neurons through the skull and resulting in depolarization.23,24 In recent years, many studies have reviewed the development and application of TMS in tinnitus.25,26 The remaining non-invasive technologies harness electric current to stimulate the cerebral cortex inducing changes in cortical excitability.27 Considering the rapid pace at which the research in this field is moving forward, it is important to summarize the state of the art. Several excellent related narrative or systematic reviews have recently been published.28–33 However, these reviews inevitably lose some information of this theme due to exclusion of unqualified studies or the limited scope. Therefore, this review sought to bring together the continuous work from the literature and summarize the existing types of non-invasive electrical stimulation (NIES) and their application value in tinnitus treatment without excluding studies based on their quality.
Methods
A review was undertaken in September 2021. All studies that fulfill inclusion criteria were included as long as they addressed the topic. The eligibility criteria were as follows: (1) participants: adult patients with subjective chronic tinnitus (>3 months); (2) interventions: NIES; (3) comparison: sham or no treatment or other treatment methods; and (4) outcome: tinnitus distress and loudness measured with validated questionnaires or visual analog scales (VASs). The exclusion criteria included animal studies, non-English articles, case reports or series, and conference and opinion papers.
Our search strategy consisted of two steps. In the first step, we identified relevant studies in PubMed, Embase, and Cochrane (CENTRAL). The retrieval strategy included medical subject headings, keywords, and free words. The search strategy used the following words: ‘transcutaneous electric nerve stimulation’ OR ‘TENS’ OR ‘non-invasive brain stimulation’ OR ‘transcranial direct current stimulation’ OR ‘tDCS’ OR ‘transcranial alternating current stimulation’ OR ‘tACS’ OR ‘transcranial random noise stimulation’ OR ‘tRNS’ OR ‘transcutaneous vagal nerve stimulation’ OR ‘tVNS’ AND ‘tinnitus’. In the second step, we manually searched the list of references for articles to identify more eligible literature. Two investigators (S.C. and M.D.) independently reviewed the abstracts and the full text of all articles retrieved. They made decisions about including or excluding them. Discrepancies in eligibility were discussed until agreement was achieved. The literature search and flow diagram of study selection are depicted in Supplementary Figure 1.
Results
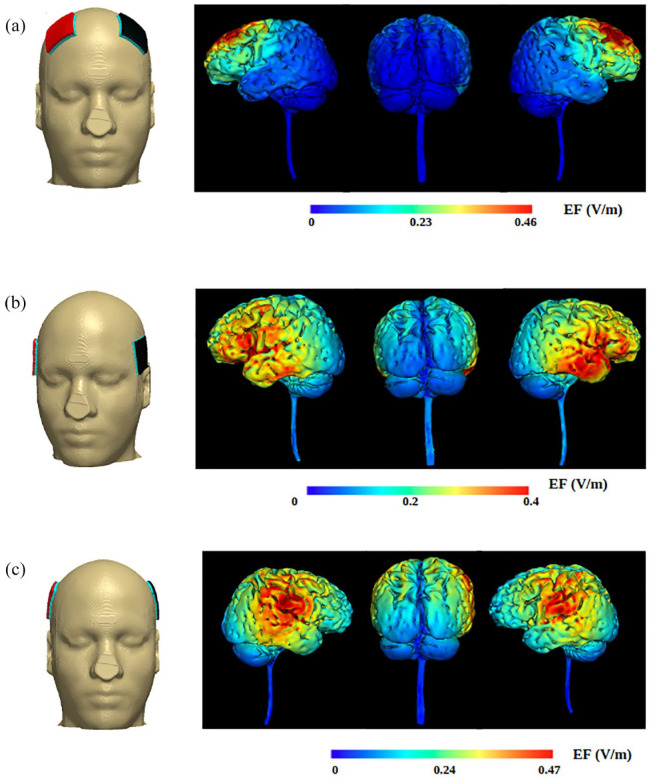
NIES can be sorted into transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), transcranial random noise stimulation (tRNS), and transcutaneous auricular vagus nerve stimulation (taVNS). tDCS, tACS, and tRNS involve placing electrodes on the surface of the skull to deliver current for cortical modulation (Figure 1). taVNS stimulates the auricular branch of the vagus nerve (ABVN) for central modulation (Figure 2).28
Figure 1.
Simulation of electrode positioning and current flow of tDCS with Soterix HD-Explor (Soterix Medical): (a) dorsolateral prefrontal cortex (DLPFC), (b) auditory cortex (AC), and (c) left temporoparietal area (LTA). Each montage used 2 mA current and 5×5 cm electrode pads. Copyright © Soterix, Inc. used with permission.
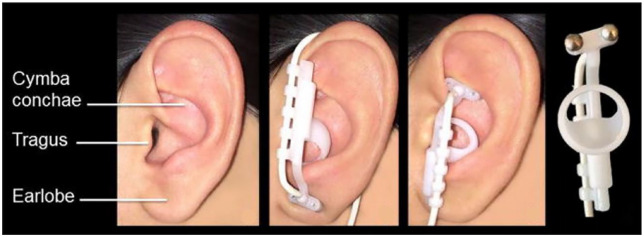
Figure 2.
Electrode position of transcutaneous auricular vagal nerve stimulation (taVNS). (Reprinted from Frangos E, Ellrich J, Komisaruk BR. Non-invasive Access to the Vagus Nerve Central Projections via Electrical Stimulation of the External Ear: fMRI Evidence in Humans. Brain Stimul. 2015;8(3):624–636. Copyright © 2015 Elsevier, Inc. used with permission.)
Transcranial direct current stimulation
The tDCS has long been used as a neuromodulation method and is now widely used to treat cognitive, psychiatric, psychological, and other neurological disorders.34–36 The tDCS involves two electrode pads, one located in the brain area of interest and the other reference electrode at any position on the body surface. A relatively weak and constant current is applied and passed through the cerebral cortex under the electrodes. The classical view is that the anode induces neural depolarization, which produces an excitation effect on the cerebral cortex, while the cathodal stimulation results in inhibitory effects by hyperpolarization.37 However, this cannot be regarded as a general rule because the conclusion was drawn from motor cortex stimulation. Subsequent studies have confirmed that in addition to the stimulation polarity, intensity, geometry and electric properties of electrode pads, the distance to the electrode also affects the spatial distribution of the electric field.38,39 The effect of the electric field on the excitability of local neurons depends on the direction of the axon or somatic dendritic axis with respect to the electric field.40 Finally, in terms of clinical effect, the physiological performance of stimulation also depends on whether the affected network of related neural disorders is inhibitory or excitatory. Demographic, clinical, and parameters details extracted from the studies are reported in Table 1.
Table 1.
General data from studies of tDCS included in the review.
| References | Study type | Intervention | Sample size | Stimulation area | Intensity (mA) | Stimulation (min) | Anode location | Cathode location | Scheme | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Fregni et al.41 | RCT | tDCS | 7 | LTA | 1 | 3 | LTA/RSO | LTA/RSO | 1 session | Positive: anodal stimulation |
| (with sham control) | ||||||||||
| Garin et al.42 | RCT | tDCS | 21 | LTA | 1 | 20 | LTA/between T4 and F8 | LTA/between T4 and F8 | 3 sessions in 2 weeks | Positive: anodal stimulation |
| (with sham control) | ||||||||||
| Shekhawat et al.43 | RCT | tDCS | 25 | LTA | 1.5, 2.0 | 10, 15, 20 | LTA | Between T4 and F8 | 6 sessions | Positive |
| Shekhawat et al.44 | RCT | tDCS + HA | 40 | LTA | 2 | 20 | LTA | Between T4 and F8 | 1 session/day, 5 sessions | Negative |
| (with sham control) | ||||||||||
| Shekhawat et al.45 | RCT | tDCS + RI | 10 | LTA | 2 | 20 | LTA | Between T4 and F8 | 1 session/day, 4 sessions | Negative |
| (with sham control) | ||||||||||
| Forogh et al.46 | RCT | tDCS | 22 | LTA | 2 | 20 | LTA | RSO | 1 session/day, 5 sessions | Negative |
| (with sham control) | ||||||||||
| Souza et al.47 | RCT | tDCS | 24 | LTA | 2 | 20 | LTA | Right DLPFC | 1 session/day, 5 sessions | Positive |
| (with sham control) | ||||||||||
| Hyvärinen et al.48 | RCT | tDCS | 43 | LTA | 2 | 20 | LTA | Right frontal area | 1 session/day, 10 sessions | Negative |
| (with sham control) | DLPFC | Left frontal area | Right frontal area | |||||||
| Teismann et al.49 | RCT | tDCS + TMNMT | 34 | AC | 2 | 30 | Left AC/RSO | Left AC/RSO | 1 session/day, 5 sessions | Negative |
| (with sham control) | ||||||||||
| Joos et al.50 | Cohort | tDCS | 175 | AC | 1.5, 2.0 | 20 | Left/Right AC | Left/Right AC | 1 session | Positive: 2.0 mA |
| Pal et al.51 | RCT | tDCS | 42 | AC | 2 | 20 | PFC | Bilateral AC | 1 session/day, 5 sessions | Negative |
| (with sham control) | ||||||||||
| Minami et al.52 | Cohort | tDCS | 9 | AC | 1 | 10 | Right AC | Left AC | 1 session | Negative |
| Henin et al.53 | RCT | HD-tDCS | 14 | AC | 2 | 20 | Bilateral AC | Bilateral PFC | 2 sessions | Negative |
| (with sham control) | HD-tDCS + CAS | |||||||||
| Abtahi et al.54 | RCT | tDCS | 51 | AC | 2 | 20 | Left/Right AC | Left/Right AC | 1 session | Positive: anode |
| (with sham control) | ||||||||||
| Vanneste et al.55 | RCT | tACS | 111 | AC | 1.5 | 20 | Left/Right AC | Left/Right AC | 1 session | Negative |
| tDCS | ||||||||||
| tRNS | ||||||||||
| Vanneste et al.56 | Cohort | tDCS | 543 | DLPFC | 1.5 | 20 | Left/Right DLPFC | Left/Right DLPFC | 1 session | Positive: right anode |
| Negative: left anode | ||||||||||
| Frank et al.57 | Cohort | tDCS | 32 | DLPFC | 1,5 | 30 | Right DLPFC | Left DLPFC | 2 sessions/week, 6 sessions | Positive: VAS |
| Faber et al.58 | RCT | tDCS | 15 | DLPFC | 1.5 | 20 | Left/Right DLPFC | Left/Right DLPFC | 6 sessions in 2 weeks | Positive |
| (with sham control) | ||||||||||
| De Ridder and Vanneste59 | Cohort | tDCS | 675 | DLPFC | NA | NA | Right DLPFC | Left DLPFC | NA | Positive: DLPFC |
| EEG-driven location | Highest theta band FC | Highest gamma band FC | Negative: EEG-driven location | |||||||
| Vanneste et al.60 | RCT | tDCS, tACS | 50 | DLPFC | 2 | 20 | Right DLPFC | Left DLPFC | 1 session | Positive |
| (with sham control) | ||||||||||
| Shekhawat et al.61 | RCT | HD-tDCS | 27 | DLPFC | 1, 2 | 10, 20 | Right DLPFC | F2, FC4, F6, AF4 | 2 sessions | Positive |
| LTA | LTA | C5, TP7, CP3, P5 | ||||||||
| Lee et al.62 | Cohort | tDCS + TMNMT | 14 | DLPFC | 1.5 | 20 | Right DLPFC | Left DLPFC | 4 sessions in 2 weeks | Positive |
| Rabau et al.63 | RCT | tDCS | 65 | DLPFC | 2 | 20 | Right DLPFC | Left DLPFC/Shoulder | 2 sessions/week, 8 sessions | Negative |
| To et al.64 | RCT | tDCS | 40 | DLPFC (tDCS) | tDCS: 1.5 | tDCS: 20 | Right DLPFC | Left DLPFC | 2 sessions/week, 8 sessions | Positive: TQ, VAS |
| tDCS + tRNS | AC (tRNS) | tRNS: 2 | tRNS: 20 | Negative: THI | ||||||
| Shekhawat and Vanneste65 | RCT | tDCS | 111 | DLPFC | 1.5, 2.0 | 20, 30 | Right DLPFC | Left DLPFC | 2, 4, 6, 8, 10 sessions, respectively | Positive |
| Shekhawat and Vanneste66 | RCT | HD-tDCS | 13 | DLPFC | 2 | 20 | Right DLPFC | F2, FC4, F6, AF4 | 1 session | Positive |
| (with sham control) | ||||||||||
| Jacquemin et al.67 | Cohort | tDCS | 117 | DLPFC/LTA | 2 | 20 | Right DLPFC/RSO | Left DLPFC/LTA | 2 sessions/week, 8 sessions | Positive: TFI |
| HD-tDCS | Right DLPFC | F2, FC4, F6, AF4 | ||||||||
| Lee68 | Cohort | tDCS + Conventional | 70 | DLPFC | 1.5 | 20 | Right DLPFC | Left DLPFC | 1–6 sessions | Positive |
| Jacquemin et al.69 | Cohort | HD-tDCS | 22 | DLPFC | 2 | 20 | Right DLPFC | F2, FC4, F6, AF4 | 2 sessions/week, 8 sessions | Positive |
| Bae et al.70 | RCT | tDCS | 80 | DLPFC | 1.5 | 20 | Left DLPFC | Right DLPFC | 1 session | Positive |
| tDCS + TMS | ||||||||||
| Jacquemin et al.71 | Cohort | HD-tDCS | 117 | DLPFC | 2 | 20 | Right DLPFC | F2, FC4, F6, AF4 | 2 sessions/week, 6 sessions | Positive: TFI, TQ |
| Negative: HQ, HADS, VAS | ||||||||||
| Bae et al.72 | Cohort | tDCS | 70 | DLPFC | 1 | 10 | Right DLPFC | Left DLPFC | 1 session/day, 5 sessions | Positive: VAS |
| Negative: THI |
AC, auditory cortex; CAS, compensatory auditory stimulation; DLPFC, dorsolateral prefrontal cortex; EEG, electroencephalogram; HA, hearing aid; HADS, hospital anxiety and depression scale; HD-tDCS, high-definition transcranial direct current stimulation; HQ, hyperacusis questionnaire; LTA, left temporoparietal area; PFC, prefrontal cortex; RCT, randomized controlled trial; RI, residual inhibition; RSO, right supraorbital area; tACS, transcranial alternating current stimulation; tDCS, transcranial direct current stimulation; TFI, tinnitus functional index; THI, tinnitus handicap inventory; TMNMT, tailor-made notched music training; TMS, transcranial magnetic stimulation; TQ, tinnitus questionnaire; tRNS, transcranial random noise stimulation; VAS, visual analog scale.
Electrode location according to the 10–20 system of EEG.
Left temporoparietal area
The first application of tDCS in the treatment of tinnitus was documented in 2006 by Fregni et al.41 They compared the inhibitory effect of anodal tDCS, cathodal tDCS, 10 Hz rTMS and sham control on tinnitus. Interestingly, 42% of patients experienced a significant reduction in tinnitus distress after anodal tDCS of left temporoparietal area (LTA), but the effect lasted only for a few minutes. Garin et al.42 investigated the differences between anodal and cathodal stimulation on tinnitus suppression in setting up a sham control. Consistent with the above results, anodal tDCS yielded a significant suppressive effect on tinnitus intensity immediately after stimulation. Shekhawat et al.43 conducted a dose-response trial for the parameters of the stimulus. Currents of 1 and 2 mA for 10, 15, and 20 min were used, and all subjects received the six resultant settings of a two-by-two sum of the above current intensity and stimulation time parameters, with a 10 min washout time between each stimulus. The final results showed that a current of 2 mA for 20 min was the most effective stimulus parameter for anodal tDCS of LTA. It is important to note that interpretation of these results requires caution because it is uncertain that the wash-time is enough to eliminate the accumulation effects of multiple stimulations. Contradicting results were reported by Forogh et al.,46 Hyvärinen et al.,48 and Souza et al.,47 who used effective stimulus parameter settings, anodal LTA stimulation, 2 mA current intensity, and 20 min stimulation time. Shekhawat et al.45 combined tDCS and acoustic therapy to explore the joint effects of tinnitus suppression and found that tDCS with residual inhibition yielded no significant effect on tinnitus minimum masking level. The tDCS combined with hearing aids showed a significant reduction in the overall tinnitus functional index score with time, but no significant difference was observed between sham tDCS and tDCS groups.44 Therefore, this trial could only prove the inhibitory effect of hearing aids on tinnitus, and the effects were independent of tDCS.
Auditory cortex
A total of seven studies discussed the effect of AC stimulation on tinnitus suppression using anode stimulation (n = 1),53 cathode stimulation (n = 2),51,52 and both (n = 4).49,50,54,55 Joos et al.50 and Abtahi et al.54 reported positive results, while other experiments did not observe the positive effect of AC stimulation on tinnitus regulation, irrespective of anode or cathode regulation. Although positive results were documented by Joos et al.,50 there was no sham-control group in the study. The positive results were limited to a current of 2.0 mA, while a current of 1.5 mA was not effective for tinnitus treatment. Although a sham-control group was set up in the study of Abtahi et al.,54 from the description in the original text, it remains unclear whether the study followed the general paradigm of sham-tDCS setting. Interestingly, in these two positive studies, the researchers placed the reference electrode on the opposite upper limb of the subject, unlike in other studies where it was placed on the head. Rabau et al.63 found that placing the reference electrode on the shoulder did not affect the stimulation effect of dorsolateral prefrontal cortex (DLPFC). We hypothesize that the location of the reference electrode outside the skull may not affect the stimulation electrode on the AC. However, the relationship between the tinnitus suppressive effect and the location of the reference electrode remains largely understudied, and its impact warrants further exploration by rigorous experiments.
Teismann et al.49 and Pal et al.51 conducted an RCT study with a sham control and adopted the design scheme of 2 mA current intensity and five sessions. The former only reported the positive effect of TMNMT and did not observe any inhibition effect by AC stimulation on tinnitus. Pul et al.51 used cathodic stimulation but did not observe any positive effect.
Dorsolateral prefrontal cortex
A total of 18 studies involving DLPFC were found.48,56–72 Vanneste et al.60 showed that single bilateral DLPFC stimulation significantly inhibited tinnitus loudness and annoyance. Faber et al.58 reported that multiple sessions of tDCS of DLPFC only yielded benefits in improving tinnitus distress, while Shekhawat and Vanneste66 found that DLPFC was effective for improving tinnitus loudness. Although the degree of distress of subjects after stimulation improved, the difference was not statistically significant.
Vanneste et al.56 conducted a tDCS trial focusing on the bilateral DLPFC with a large sample of 543 participants. A significant reduction in tinnitus intensity and distress was observed in 29.9% of patients with anodal right/cathodal left tDCS, while reversed location stimulation showed no effect. In a large retrospective study including 675 participants, De Ridder and Vanneste59 compared the effects of bilateral DLPFC stimulation with electroencephalogram (EEG)-driven tDCS. The rationale is that, based on the pathophysiology of tinnitus and the polarity-dependent effects of tDCS, the cathode in the area of tinnitus-related gamma energy band activity or the area of gamma energy band functional connectivity may be more effective for tinnitus suppression since functional connectivity determines the tinnitus network. It was found that both bifrontal tDCS and EEG-driven tDCS could significantly suppress tinnitus loudness, and distress, but no advantage was observed with the latter.
Shekhawat and Vanneste65 conducted a dose-response trial to explore the optimal stimulation parameters for DLPFC. This study discussed the current intensities of 1.5 and 2.0 mA, the stimulation time of 20 and 30 min, and the effects of different stimulation sessions on tinnitus perception. Tinnitus loudness decreased significantly after tDCS with DLPFC. There was no significant difference between stimulus intensity and duration. The tinnitus loudness reached a nadir after six treatment sessions. Finally, the author recommended six sessions with a current of 1.5 mA for 20 min over 3 weeks with a washout period of 3–4 days to apply tDCS stimulation by DLPFC.
In the study by Rabau et al.,63 a cathode was placed on the left DLPFC and the left shoulder, respectively, and the anode on the right DLPFC. The former stimulated the DLPFC and hippocampus, while the latter stimulated the cingulate cortex, right hippocampus, and temporal lobe. However, there was no significant difference in VAS and TFI scores between the two groups. Other studies on tDCS stimulation of DLPFC validated its inhibitory effect on tinnitus perception
High-definition tDCS
The spatial resolution of tDCS is limited because of the low current conductivity of the skull. It has been found that the rectangular pad configuration peak–induced electric field magnitude was not directly under the pads but at an intermediate lobe, which brings difficulties to the anatomical interpretation of the tinnitus inhibitory effect.73 HD-tDCS, including the 4×1 ring configuration, results in enhanced spatial focus, with peak induced electric field magnitude directly underneath the active electrode.74,75 At present, six studies have explored the effect of HD-tDCS in chronic tinnitus relief,53,61,66,67,69,71 Three studies of DLPFC stimulation by Jacquemin et al.67,69,71 found that tinnitus improved in 47%, 36%, and 31% of patients. The RCT study by Shekhawat et al.61 found that 77.8% of subjects responded to HD-tDCS, with no difference between DLPFC and LTA stimulation effects. The most effective inhibition was achieved after 15 min stimulation with a current of 2 mA. Henin et al.53 did not clarify the effect of tDCS due to the small sample size.
Taken together, the above findings suggest that HD-tDCS stimulation at the right DLPFC with a 2 mA current intensity for 20 min yielded promising results.
Transcranial alternating current stimulation
The tACS uses alternating currents with specified frequency to affect cortical oscillation to regulate the neuronal activity of the target brain area. Therefore, tACS is suitable for regulating the functions closely related to brain oscillation at a specific frequency.76,77 Recent studies have substantiated that tACS at alpha frequency leads to an elevation of alpha power, and the coupling in alpha- and gamma-phase synchronization between frontal, parietal, and cingulate brain areas is reportedly related to tinnitus distress.78–81 Only three clinical studies have assessed tACS in tinnitus treatment using alpha-modulated tACS (6–13 Hz), but no significant results were found irrespective of whether AC or DLPFC was stimulated.55,60,82 Demographic, clinical, and parameters details extracted from the studies are reported in Table 2.
Table 2.
General data from studies of tACS included in the review.
| References | Study type | Intervention | Sample size | Stimulation area | Intensity (mA) | Stimulation (min) | Frequency (Hz) | Scheme | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Claes et al.82 | Cohort | tACS, tRNS | 228 | AC | 2 | 20 | tACS (6–13) | 8 sessions | Negative |
| tRNS (0.1–100) | |||||||||
| Vanneste et al.55 | RCT | tACS | 111 | AC | 1.5 | 20 | tACS (6–13) tRNS (0.1–100) |
1 session | Negative |
| tDCS | |||||||||
| tRNS | |||||||||
| Vanneste et al.60 | RCT | tDCS, tACS | 50 | DLPFC | 2 | 20 | tACS (6–13) | 1 session | Negative |
| (with sham control) |
AC, auditory cortex; DLPFC, dorsolateral prefrontal cortex; RCT, randomized controlled trial; tACS, transcranial alternating current stimulation; tDCS, transcranial direct current stimulation; tRNS, transcranial random noise stimulation.
Transcranial random noise stimulation
The tRNS is a non-invasive brain stimulation method using alternating currents with random frequencies to interfere with oscillatory neural activity.83 This technology can be applied in the low frequency (0.1–100 Hz) or the high-frequency range (101–640 Hz). One proposed mechanism is that tRNS may induce repeated opening of Na(+) channels, which shortens the hyperpolarization phase and causes an inward sodium current, resulting in a depolarization of the neural membrane.84 Direct evidence of tRNS interfering with the auditory-evoked activity of the AC was provided by an EEG study.85 Claes et al.82 and Vanneste et al.55 found that tRNS induced the most significant suppressive effect on tinnitus loudness compared with tDCS and tACS. However, another prospective study on tRNS yielded the opposite results. Joos et al.86 reported that low-frequency tRNS and high-frequency tRNS could significantly reduce tinnitus loudness and distress, whereas whole-frequency tRNS yielded no effect on tinnitus in a single-session scheme. Mohsen et al.87 investigated the effect of multiple-sessions treatment regimens on tinnitus suppression and showed significant suppression of tinnitus loudness and annoyance compared with the single-session scheme. However, the study by Kreuzer et al.88 reported negative results for tRNS in the treatment of tinnitus. Demographic, clinical, and parameters details extracted from the studies are reported in Table 3.
Table 3.
General data from studies of tRNS included in the review.
| References | Study type | Intervention | Sample size | Stimulation area | Intensity (mA) | Stimulation (min) | Frequency (Hz) | Scheme | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Joos et al.86 | Cohort | tRNS | 154 | AC | 2 | 20 | 0.1–100 | 1 session | Positive: 0.1–100, 100–640 Hz |
| 100–640 | Negative: 0.1–640 Hz | ||||||||
| 0.1–640 | |||||||||
| Kreuzer et al.88 | Cohort | tRNS | 30 | AC | 2 | 20 | 100–640 | 10 sessions | Negative |
| Mohsen et al.87 | RCT | tRNS | 29 | AC + DLPFC | 2 | 20 | 0.1–100 | 1 session/8 sessions | Positive |
| 100–640 | |||||||||
| To et al.64 | RCT | tDCS | 40 | DLPFC (tDCS) | tDCS: 1.5 | tDCS: 20 | 0.1–100 | 8 sessions | Positive |
| tDCS + tRNS | AC (tRNS) | tRNS: 2 | tRNS: 20 | ||||||
| Claes et al.82 | Cohort | tACS, tRNS | 228 | AC | 2 | 20 | tACS (6–13) | 8 sessions | Positive |
| tRNS (0.1–100) | |||||||||
| Vanneste et al.55 | RCT | tACS | 111 | AC | 1.5 | 20 | tACS (6–13) tRNS (0.1–100) |
1 session | Positive |
| tDCS | |||||||||
| tRNS |
AC, auditory cortex; DLPFC, dorsolateral prefrontal cortex; RCT, randomized controlled trial; tACS, transcranial alternating current stimulation; tDCS, transcranial direct current stimulation; tRNS, transcranial random noise stimulation.
Transcutaneous auricular vagal nerve stimulation
The vagus nerve is the tenth pair of cranial nerves consisting of approximately 80% sensory afferent fibers and 20% motor afferent fibers.89 Stimulation of the vagus nerve modulates the release of norepinephrine and acetylcholine.90,91 These neuromodulators enhance neuroplasticity by modulating the cortex, hippocampus, and amygdala. It has also been demonstrated that norepinephrine and acetylcholine can affect the selective plasticity of auditory cortical neurons.92–94 Engineer et al.95 used VNS paired with tones that exclude the tinnitus frequency to successfully eliminate the behavioral and physiologic manifestations of tinnitus in a rat model. The follow-up pilot clinical trials achieved significant results in about half of tinnitus patients.96 Despite the high acceptability of patients with implanted VNS,97 invasive intervention is inevitably accompanied by potential risks. Acute side effects include infection, vocal cord paralysis, lower facial weakness, and so on. Long-term risks include voice changes, hoarseness, and sore throat.98 Anatomy and imaging studies have confirmed that stimulation of ABVN can also activate the central nerve pathway, similar to implanted VNS.99–101 Therefore, taVNS has huge prospects and exploration value in treating tinnitus.
Four clinical studies reported meaningful results for applying taVNS combined with sound therapy (ST) in tinnitus relief.102–105 The taVNS + TRT alleviated tinnitus stress in 76% of patients at 1-year follow-up.105 Half of the patients in the study by Shim et al.102 reported symptom relief after taVNS plus notched music therapy. Lehtimӓki et al.103 demonstrated the neuromodulatory effect of taVNS and tailored ST on evoked auditory cortical response. There was significant heterogeneity in parameter settings, follow-up time, and the types of ST used in these studies. Three studies used taVNS only as an intervention to clarify the effect of taVNS on tinnitus inhibition.106–108 In the cohort study by Kreuzer et al.,106 phase I was terminated prematurely due to cardiac side effects, with a slight significant decrease in TQ score reported. Although there were no adverse events in the second phase, the difference observed before and after treatment was not significant. Suk et al.107 reported a positive outcome after 2 weeks of taVNS treatment. Importantly, the results of this study were collected 1 month after the end of stimulation, suggesting the possible long-term after-effects of taVNS. A recent RCT study found that taVNS had a significant inhibitory effect on unilateral and bilateral tinnitus.108 Although the control group also showed a significant decrease in tinnitus handicap inventory (THI) score, further analysis showed that the decreased THI score of taVNS was significantly higher than the control group.
The pre-clinical research obtained promising results using implanted VNS combined with acoustic stimulation. The effect of taVNS on AC was observed in functional magnetic resonance imaging (fMRI) studies. However, significant heterogeneity is found in current clinical reports, and the quality of trials is not high. The results of three studies using taVNS alone are inconsistent, making it difficult to evaluate the clinical effect of taVNS. Demographic, clinical, and parameters details extracted from the studies are reported in Table 4.
Table 4.
General data from studies of taVNS included in the review.
| References | Study type | Intervention | Sample size | Stimulation area | Intensity (mA) | Stimulation (min) | Frequency (Hz) | Scheme | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Lehtimäki et al.103 | Cohort | taVNS + tailored ST | 10 | Left tragus | Suprathreshold | 45–60 | 25 | 7 sessions in 10 days | Positive |
| Mei et al.104 | RCT | taVNS + sound masking | 63 | Concha | 1 | 20 | 20 | 2 sessions/day, 8 weeks | Positive |
| Ylikoski et al.105 | Cohort | taVNS + TRT | 78 | Left tragus | Suprathreshold | 60–90 | 25 | 1 session/day, 5 days/week, 1 year | Positive |
| Shim et al.102 | Cohort | taVNS + notched music | 30 | Left concha | Suprathreshold | 30 | 25 | 10 sessions | Positive |
| Kreuzer et al.106 | Cohort | taVNS | 50 | Cymba conchae | Suprathreshold | phase 1: 6 h/day | 25 | 24 weeks | Negative |
| phase 2: 4 h/day | |||||||||
| Suk et al.107 | Cohort | taVNS | 24 | Cavum, cymba, outer surface of the tragus | Suprathreshold | 4 at each site | 30 | 4 sessions | Positive |
| Tutar et al.108 | RCT | taVNS | 60 | Cymba conchae | Suprathreshold | 30 | 200 | 10 sessions | Positive |
RCT, randomized controlled trial; ST, sound therapy; taVNS, transcutaneous vagal nerve stimulation; TRT, tinnitus-retraining therapy.
Discussion
Tinnitus is a disease with significant heterogeneity in terms of etiology, perception, and degree of severity, ranging from mild annoyance to disastrous impact on daily life.109 Our review of the literature identified four non-invasive electrical therapy themes. Most studies that focused on tDCS provided significant results. tACS and tRNS have also been applied as novel modulation technology, but the clinical research in tinnitus is still in its early stage. taVNS has demonstrated promising effects in tinnitus management, but there is a lack of high-quality research to exclude the placebo effect.
Although there is a rich literature on tDCS available, significant heterogeneity in scheme design and results prevail. In clinical studies that applied tDCS for tinnitus treatment, the stimulation sites included AC, LTA, and DLPFC. The selection of these areas depends on neuroimaging and electrophysiological evidence of tinnitus.110–113 AC is a part of the classical auditory pathway, and the functional changes of DLPFC are considered to be a deficiency of the top–down inhibitory system in tinnitus patients.114 Plewnia et al.115 documented that LTA is the most effective scalp position for repeated TMS to inhibit tinnitus, validated in a subsequent tDCS study by Fregni et al.41 Many studies selected DLPFC as the stimulation area and reported significant improvement in tinnitus irrespective of the number of sessions. Moreover, there is no lack of high-quality RCT studies with sham control and some studies even observed the after-effects 1 month after treatment. However, in the study with AC or LTA as the stimulation target area, the number of positive results is close to negative results. It is well-established that AC and LTA are very close stimulation areas, corresponding to the halfway point between C3 and T5 and T3 (EEG 10/20 system), respectively. Applying 35 cm2 electrodes in these studies may activate additional cortical regions around the target area, which inevitably lead to partial overlap. This finding accounts for the comparable stimulation effects of AC and LTA on tinnitus. These findings suggest that DLPFC stimulation with tDCS seems to be more promising for tinnitus suppression.
The difference between tACS and tRNS lies in the difference in current frequency. tACS often adopts 6–13 Hz, while the tRNS frequency range is 0.1–640 Hz. In this review, no tACS studies reported significant effects on tinnitus perception. tRNS was found to significantly inhibit the loudness and distress of tinnitus irrespective of the number of sessions, with superior effects compared with tDCS. Interestingly, the study by To et al.64 found an added value of tRNS to the DLPFC tDCS protocol for tinnitus relief. This finding brings hope for the joint application of two or even more neuromodulation modes in the later stage.
In addition to the stimulation area, the stimulation parameters are also worthy of further exploration. Stimulation with a current of 2 mA for 20 min was adopted by most studies on tDCS. Simulation of electric field distribution has proven its safety in the human body.116,117 LTA stimulation with a current intensity of 2 mA and 20 min duration was most effective for transient tinnitus suppression. However, the study did not attempt to optimize the number of sessions. The main adverse events of tDCS were itching, tingling, headache, discomfort, and burning sensation and were generally mild and disappeared soon after stimulation.118,119 No serious adverse events have been reported in existing studies about tDCS in tinnitus. The current tDCS parameters have been demonstrated to be relatively safe in tinnitus research, and further high-quality RCTs with sham control are needed to explore different parameter settings on tinnitus suppression. At present, tACS and tRNS are rarely used in tinnitus, and almost all of the studies adopted 2.0 mA intensity and 20 min duration. tACS has no inhibitory effect on tinnitus, but tRNS showed significant inhibition on tinnitus loudness and annoyance. The current tRNS studies suggest the effect of high and low frequencies on tinnitus inhibition, and the effect of multiple sessions seems to yield a more significant effect, but the number of studies on tRNS is significantly less than that on tDCS, with no RCT study to control placebo effect. Accordingly, double-blinded sham-controlled trials with different parameter settings are needed in future studies.
Over the years, taVNS has been widely used in the psychological fields of depression, epilepsy, migraine, pain, and tinnitus.120 Most taVNS studies reported a positive effect on tinnitus suppression, with taVNS intervention alone or in combination with ST. However, the lack of sham-control design leads to an inevitable placebo effect. The body surface distribution map of ABVN depends on anatomical evidence, which has been limited due to the difficulty of nerve anatomy.99 According to anatomical evidence, ABVN is distributed in the external auditory meatus (EAM), especially in the posterior wall of the EAM. However, fMRI studies found that stimulation of the cymba concha, inner tragus, and anterior wall of the EAM could activate the vagal afferent pathway.121 The stimulation sites used in the clinical study of taVNS include the inner tragus, cymba concha, and cavum concha. The significant clinical effects can be mutually verified with the results of fMRI. Moreover, the research by Yakunina et al.122 further pointed out that the cymba concha may be a more suitable site because stimulating the cymba concha can result in the most robust activation of the vagal afferent pathway in the brainstem. Almost all taVNS studies adopted suprathreshold current intensity settings in this review. Current flow patterns and intensity, and resulting regions of interest influence, were highly specific to taVNS electric montage.123 Considering the substantial influence of electrode and tissue impedance, the amount or amplitude of energy delivered to the tissue remains largely unknown, suggesting that using suprathreshold is an alternative choice.124 In this review, only the study by Kreuzer et al.106 reported two cardiac events that were not related to the taVNS. Evidence substantiates that taVNS is a safe and well-tolerated technology.125 The most common side effects are local skin irritation caused by electrode placement, headache, and nasopharyngitis. No special therapies were required. However, the results of existing studies of pure taVNS application were inconsistent. Whether pairing of the vagus stimulation with non-tinnitus or tinnitus-matched sounds is essential is still to be determined.126 In conclusion, taVNS is a feasible and safe technique, but much heterogeneity surrounds the parameter settings making it challenging to summarize and reproduce. Further research should follow the recommendations for reporting standards in research on taVNS to achieve transparency, completeness, and reproducibility.127
Conclusion
NIES broadens the therapeutic landscape for the treatment of subjective tinnitus. This approach is safe and with only a few relatively minor side effects. The variety of options (tDCS, tACS, tRNS, taVNS) and stimulation parameters opens up unlimited neuromodulation possibilities in tinnitus management. More high-quality studies are warranted to substantiate the robustness of our results.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223221148061 for State of the art: non-invasive electrical stimulation for the treatment of chronic tinnitus by Shanwen Chen, Maoshan Du, Yang Wang, Yifan Li, Busheng Tong, Jianxin Qiu, Feihu Wu and Yehai Liu in Therapeutic Advances in Chronic Disease
Acknowledgments
We would like to give our sincere appreciation to the reviewers for their helpful comments on this article.
Footnotes
ORCID iDs: Shanwen Chen  https://orcid.org/0000-0002-4204-2968
https://orcid.org/0000-0002-4204-2968
Yehai Liu  https://orcid.org/0000-0003-3977-2481
https://orcid.org/0000-0003-3977-2481
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Shanwen Chen, Department of Otorhinolaryngology–Head and Neck Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, P.R. China.
Maoshan Du, Department of Otorhinolaryngology–Head and Neck Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, P.R. China.
Yang Wang, Department of Otorhinolaryngology–Head and Neck Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, P.R. China.
Yifan Li, Department of Otorhinolaryngology–Head and Neck Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, P.R. China.
Busheng Tong, Department of Otorhinolaryngology–Head and Neck Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, P.R. China.
Jianxin Qiu, Department of Otorhinolaryngology–Head and Neck Surgery, The First Affiliated Hospital of Anhui Medical University, Hefei, P.R. China.
Feihu Wu, Department of Otorhinolaryngology–Head and Neck Surgery, The First Affiliated Hospital of Anhui University of Chinese Medicine, 117 Meishan Road, Hefei 230031, Anhui, P.R. China.
Yehai Liu, Department of Otorhinolaryngology–Head and Neck Surgery, The First Affiliated Hospital of Anhui Medical University, 218 Jixi Road, Hefei 230022, Anhui, P.R. China.
Declarations
Ethics approval and consent to participate: Ethical approval is not required as the current study was based on published data.
Consent for publication: Not applicable.
Author contributions: Shanwen Chen: Conceptualization; Data curation; Formal analysis; Writing – original draft; Writing – review & editing.
Maoshan Du: Data curation; Formal analysis; Methodology; Writing – review & editing.
Yang Wang: Conceptualization; Data curation; Formal analysis; Writing – review & editing.
Yifan Li: Conceptualization; Writing – review & editing.
Busheng Tong: Conceptualization; Writing – review & editing.
Jianxin Qiu: Conceptualization; Writing – review & editing.
Feihu Wu: Conceptualization; Project administration; Writing – review & editing.
Yehai Liu: Conceptualization; Project administration; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Project of Science and Technology of National Health Commission (No. 20003), Anhui Province collaborative research project of Chinese and Western medicine for major and difficult diseases (Grant No. 2021-70), and Project of Health Commission of Anhui Province (Grant No. AHWJ2021b160).
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: All data are included in the article.
References
- 1. Baguley D, McFerran D, Hall D. Tinnitus. Lancet 2013; 382: 1600–1607. [DOI] [PubMed] [Google Scholar]
- 2. Weidt S, Delsignore A, Meyer M, et al. Which tinnitus-related characteristics affect current health-related quality of life and depression? A cross-sectional cohort study. Psychiatry Res 2016; 237: 114–121. [DOI] [PubMed] [Google Scholar]
- 3. Zeman F, Koller M, Langguth B, et al. Which tinnitus-related aspects are relevant for quality of life and depression: results from a large international multicentre sample. Health Qual Life Outcomes 2014; 12: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bartels H, Middel BL, van der Laan BF, et al. The additive effect of co-occurring anxiety and depression on health status, quality of life and coping strategies in help-seeking tinnitus sufferers. Ear Hear 2008; 29: 947–956. [DOI] [PubMed] [Google Scholar]
- 5. Bhatt JM, Bhattacharyya N, Lin HW. Relationships between tinnitus and the prevalence of anxiety and depression. Laryngoscope 2017; 127: 466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zenner HP, Delb W, Kröner-Herwig B, et al. A multidisciplinary systematic review of the treatment for chronic idiopathic tinnitus. Eur Arch Otorhinolaryngol 2017; 274: 2079–2091. [DOI] [PubMed] [Google Scholar]
- 7. Choi SJ, Lee JB, Lim HJ, et al. Intratympanic dexamethasone injection for refractory tinnitus: prospective placebo-controlled study. Laryngoscope 2013; 123: 2817–2822. [DOI] [PubMed] [Google Scholar]
- 8. Figueiredo RR, Langguth B, Mello de, Oliveira P, et al. Tinnitus treatment with memantine. Otolaryngol Head Neck Surg 2008; 138: 492–496. [DOI] [PubMed] [Google Scholar]
- 9. Wang H, Tang D, Wu Y, et al. The state of the art of sound therapy for subjective tinnitus in adults. Ther Adv Chronic Dis 2020; 11: 2040622320956426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sereda M, Xia J, El Refaie A, et al. Sound therapy (using amplification devices and/or sound generators) for tinnitus. Cochrane Database Syst Rev 2018; 12: CD013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hobson J, Chisholm E, El Refaie A. Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database Syst Rev 2012; 11: CD006371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fuller T, Cima R, Langguth B, et al. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev 2020; 1: CD012614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beukes EW, Baguley DM, Allen PM, et al. Audiologist-guided internet-based cognitive behavior therapy for adults with tinnitus in the United Kingdom: a randomized controlled trial. Ear Hear 2018; 39: 423–433. [DOI] [PubMed] [Google Scholar]
- 14. Beukes EW, Allen PM, Baguley DM, et al. Long-term efficacy of audiologist-guided internet-based cognitive behavior therapy for tinnitus. Am J Audiol 2018; 27: 431447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poncet-Wallet C, Mamelle E, Godey B, et al. Prospective multicentric follow-up study of cochlear implantation in adults with single-sided deafness: tinnitus and audiological outcomes. Otol Neurotol 2020; 41: 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Távora-Vieira D, Marino R, Krishnaswamy J, et al. Cochlear implantation for unilateral deafness with and without tinnitus: a case series. Laryngoscope 2013; 123: 1251–1255. [DOI] [PubMed] [Google Scholar]
- 17. Levy DA, Lee JA, Nguyen SA, et al. Cochlear implantation for treatment of tinnitus in single-sided deafness: a systematic review and meta-analysis. Otol Neurotol 2020; 41: e1004–e1012. [DOI] [PubMed] [Google Scholar]
- 18. Deklerck AN, Marechal C, Pérez Fernández AM, et al. Invasive neuromodulation as a treatment for tinnitus: a systematic review. Neuromodulation 2020; 23: 451–462. [DOI] [PubMed] [Google Scholar]
- 19. Lefebvre-Demers M, Doyon N, Fecteau S. Non-invasive neuromodulation for tinnitus: a meta-analysis and modeling studies. Brain Stimul 2021; 14: 113–128. [DOI] [PubMed] [Google Scholar]
- 20. Shi Y, Burchiel KJ, Anderson VC, et al. Deep brain stimulation effects in patients with tinnitus. Otolaryngol Head Neck Surg 2009; 141: 285–287. [DOI] [PubMed] [Google Scholar]
- 21. Dijkstra E, Figee M, Schuurman PR, et al. Effective deep brain stimulation of intractable tinnitus: a case study. Brain Stimul 2018; 11: 1205–1207. [DOI] [PubMed] [Google Scholar]
- 22. Cheung SW, Racine CA, Henderson-Sabes J, et al. Phase I trial of caudate deep brain stimulation for treatment-resistant tinnitus. J Neurosurg. Epub ahead of print 24 September 2019. DOI: 10.3171/2019.4.JNS19347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lehner A, Schecklmann M, Greenlee MW, et al. Triple-site rTMS for the treatment of chronic tinnitus: a randomized controlled trial. Sci Rep 2016; 6: 22302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noh TS, Kyong JS, Park MK, et al. Dual-site rTMS is more effective than single-site rTMS in tinnitus patients: a blinded randomized controlled trial. Brain Topogr 2020; 33: 767–775. [DOI] [PubMed] [Google Scholar]
- 25. Theodoroff SM, Folmer RL. Repetitive transcranial magnetic stimulation as a treatment for chronic tinnitus: a critical review. Otol Neurotol 2013; 34: 199–208. [DOI] [PubMed] [Google Scholar]
- 26. Meng Z, Liu S, Zheng Y, et al. Repetitive transcranial magnetic stimulation for tinnitus. Cochrane Database Syst Rev 2011; 2011: CD007946. [DOI] [PubMed] [Google Scholar]
- 27. Merrill DR, Bikson M, Jefferys JGR. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods 2005; 141: 171–198. [DOI] [PubMed] [Google Scholar]
- 28. Peter N, Kleinjung T. Neuromodulation for tinnitus treatment: an overview of invasive and non-invasive techniques. J Zhejiang Univ Sci B 2019; 20: 116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Langguth B. Non-invasive neuromodulation for tinnitus. J Audiol Otol 2020; 24: 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang T, Zhang J, Wang B, et al. Electrical stimulation to treat tinnitus: a meta-analysis and systemic review of randomized controlled trials. Ther Adv Chronic Dis. Epub ahead of print 13 September 2021. DOI: 10.1177/20406223211041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martins ML, Souza DDS, Cavalcante MEOB, et al. Effect of transcranial direct current stimulation for tinnitus treatment: a systematic review and meta-analysis. Neurophysiol Clin 2022; 52: 1–16. [DOI] [PubMed] [Google Scholar]
- 32. Labree B, Hoare DJ, Gascoyne LE, et al. Determining the effects of transcranial direct current stimulation on tinnitus, depression, and anxiety: a systematic review. Brain Sci 2022; 12: 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stegeman I, Velde HM, Robe P, et al. Tinnitus treatment by vagus nerve stimulation: a systematic review. PLoS ONE 2021; 16: e0247221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palm U, Hasan A, Strube W, et al. tDCS for the treatment of depression: a comprehensive review. Eur Arch Psychiatry Clin Neurosci 2016; 266: 681–694. [DOI] [PubMed] [Google Scholar]
- 35. Knotkova H, Hamani C, Sivanesan E, et al. Neuromodulation for chronic pain. Lancet 2021; 397: 2111–2124. [DOI] [PubMed] [Google Scholar]
- 36. Elsner B, Kugler J, Pohl M, et al. Transcranial direct current stimulation (tDCS) for idiopathic Parkinson’s disease. Cochrane Database Syst Rev 2016; 7: CD010916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000; 527: 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Opitz A, Paulus W, Will S, et al. Determinants of the electric field during transcranial direct current stimulation. Neuroimage 2015; 109: 140–150. [DOI] [PubMed] [Google Scholar]
- 39. Saturnino GB, Antunes A, Thielscher A. On the importance of electrode parameters for shaping electric field patterns generated by tDCS. NeuroImage 2015; 120: 25–35. [DOI] [PubMed] [Google Scholar]
- 40. Bikson M, Inoue M, Akiyama H, et al. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol 2004; 557: 175–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fregni F, Marcondes R, Boggio PS, et al. Transient tinnitus suppression induced by repetitive transcranial magnetic stimulation and transcranial direct current stimulation. Eur J Neurol 2006; 13: 996–1001. [DOI] [PubMed] [Google Scholar]
- 42. Garin P, Gilain C, Van Damme JP, et al. Short- and long-lasting tinnitus relief induced by transcranial direct current stimulation. J Neurol 2011; 258: 1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shekhawat GS, Stinear CM, Searchfield GD. Transcranial direct current stimulation intensity and duration effects on tinnitus suppression. Neurorehabil Neural Repair 2013; 27: 164–172. [DOI] [PubMed] [Google Scholar]
- 44. Shekhawat GS, Searchfield GD, Stinear CM. Randomized trial of transcranial direct current stimulation and hearing aids for tinnitus management. Neurorehabil Neural Repair 2014; 28: 410–419. [DOI] [PubMed] [Google Scholar]
- 45. Shekhawat GS, Kobayashi K, Searchfield GD. Methodology for studying the transient effects of transcranial direct current stimulation combined with auditory residual inhibition on tinnitus. J Neurosci Methods 2015; 239: 28–33. [DOI] [PubMed] [Google Scholar]
- 46. Forogh B, Mirshaki Z, Raissi GR, et al. Repeated sessions of transcranial direct current stimulation for treatment of chronic subjective tinnitus: a pilot randomized controlled trial. Neurol Sci 2016; 37: 253–259. [DOI] [PubMed] [Google Scholar]
- 47. Souza DDS, Almeida AA, Andrade SMDS, et al. Transcranial direct current stimulation improves tinnitus perception and modulates cortical electrical activity in patients with tinnitus: a randomized clinical trial. Neurophysiol Clin 2020; 50: 289–300. [DOI] [PubMed] [Google Scholar]
- 48. Hyvärinen P, Mäkitie A, Aarnisalo AA. Self-administered domiciliary tDCS treatment for tinnitus: a double-blind sham-controlled study. PLoS ONE 2016; 11: e0154286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Teismann H, Wollbrink A, Okamoto H, et al. Combining transcranial direct current stimulation and tailor-made notched music training to decrease tinnitus-related distress – a pilot study. PLoS ONE 2014; 9: e89904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Joos K, De Ridder D, Van de Heyning P, et al. Polarity specific suppression effects of transcranial direct current stimulation for tinnitus. Neural Plast 2014; 2014: 930860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pal N, Maire R, Stephan MA, et al. Transcranial direct current stimulation for the treatment of chronic tinnitus: a randomized controlled study. Brain Stimul 2015; 8: 1101–1107. [DOI] [PubMed] [Google Scholar]
- 52. Minami SB, Oishi N, Watabe T, et al. Auditory resting-state functional connectivity in tinnitus and modulation with transcranial direct current stimulation. Acta Otolaryngol 2015; 135: 1286–1292. [DOI] [PubMed] [Google Scholar]
- 53. Henin S, Fein D, Smouha E, et al. The effects of compensatory auditory stimulation and high-definition transcranial direct current stimulation (HD-tDCS) on tinnitus perception – a randomized pilot study. PLoS ONE 2016; 11: e0166208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Abtahi H, Okhovvat A, Heidari S, et al. Effect of transcranial direct current stimulation on short-term and long-term treatment of chronic tinnitus. Am J Otolaryngol 2018; 39: 94–96. [DOI] [PubMed] [Google Scholar]
- 55. Vanneste S, Fregni F, De Ridder D. Head-to-head comparison of transcranial random noise stimulation, transcranial AC stimulation, and transcranial DC stimulation for tinnitus. Front Psychiatry 2013; 4: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vanneste S, Plazier M, Ost J, et al. Bilateral dorsolateral prefrontal cortex modulation for tinnitus by transcranial direct current stimulation: a preliminary clinical study. Exp Brain Res 2010; 202: 779–785. [DOI] [PubMed] [Google Scholar]
- 57. Frank E, Schecklmann M, Landgrebe M, et al. Treatment of chronic tinnitus with repeated sessions of prefrontal transcranial direct current stimulation: outcomes from an open-label pilot study. J Neurol 2012; 259: 327–333. [DOI] [PubMed] [Google Scholar]
- 58. Faber M, Vanneste S, Fregni F, et al. Top down prefrontal affective modulation of tinnitus with multiple sessions of tDCS of dorsolateral prefrontal cortex. Brain Stimul 2012; 5: 492–498. [DOI] [PubMed] [Google Scholar]
- 59. De Ridder D, Vanneste S. EEG driven tDCS versus bifrontal tDCS for tinnitus. Front Psychiatry 2012; 3: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vanneste S, Walsh V, Van De Heyning P, et al. Comparing immediate transient tinnitus suppression using tACS and tDCS: a placebo-controlled study. Exp Brain Res 2013; 226: 25–31. [DOI] [PubMed] [Google Scholar]
- 61. Shekhawat GS, Sundram F, Bikson M, et al. Intensity, duration, and location of high-definition transcranial direct current stimulation for tinnitus relief. Neurorehabil Neural Repair 2016; 30: 349–359. [DOI] [PubMed] [Google Scholar]
- 62. Lee HY, Choi MS, Chang DS, et al. Combined bifrontal transcranial direct current stimulation and tailor-made notched music training in chronic tinnitus. J Audiol Otol 2017; 21: 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rabau S, Shekhawat GS, Aboseria M, et al. Comparison of the long-term effect of positioning the cathode in tDCS in tinnitus patients. Front Aging Neurosci 2017; 9: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. To WT, Ost J, Hart J, Jr, et al. The added value of auditory cortex transcranial random noise stimulation (tRNS) after bifrontal transcranial direct current stimulation (tDCS) for tinnitus. J Neural Transm (Vienna) 2017; 124: 79–88. [DOI] [PubMed] [Google Scholar]
- 65. Shekhawat GS, Vanneste S. Optimization of transcranial direct current stimulation of dorsolateral prefrontal cortex for tinnitus: a non-linear dose-response effect. Sci Rep 2018; 8: 8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shekhawat GS, Vanneste S. High-definition transcranial direct current stimulation of the dorsolateral prefrontal cortex for tinnitus modulation: a preliminary trial. J Neural Transm (Vienna) 2018; 125: 163–171. [DOI] [PubMed] [Google Scholar]
- 67. Jacquemin L, Shekhawat GS, Van de Heyning P, et al. Effects of electrical stimulation in tinnitus patients: conventional versus high-definition tDCS. Neurorehabil Neural Repair 2018; 32: 714–723. [DOI] [PubMed] [Google Scholar]
- 68. Lee HY. Adjunctive role of bifrontal transcranial direct current stimulation in distressed patients with severe tinnitus. J Korean Med Sci 2019; 34: e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jacquemin L, Mertens G, Van de Heyning P, et al. An exploratory study on the use of event-related potentials as an objective measure of auditory processing and therapy effect in patients with tinnitus: a transcranial direct current stimulation study. Otol Neurotol 2019; 40: e868–e875. [DOI] [PubMed] [Google Scholar]
- 70. Bae EB, Lee JH, Song JJ. Single-session of combined tDCS-TMS may increase therapeutic effects in subjects with tinnitus. Front Neurol 2020; 11: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jacquemin L, Mertens G, Shekhawat GS, et al. High definition transcranial direct current stimulation (HD-tDCS) for chronic tinnitus: outcomes from a prospective longitudinal large cohort study. Prog Brain Res 2021; 263: 137–152. [DOI] [PubMed] [Google Scholar]
- 72. Bae SH, Moon SJ, Lee JG, et al. Comparison of treatment outcome between repetitive transcranial magnetic stimulation (rTMS) and transcutaneous direct current stimulation (tDCS) in intractable tinnitus. J Clin Med 2021; 10: 635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nitsche MA, Doemkes S, Karaköse T, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol 2007; 97: 3109–3117. [DOI] [PubMed] [Google Scholar]
- 74. Datta A, Elwassif M, Battaglia F, et al. Transcranial current stimulation focality using disc and ring electrode configurations: FEM analysis. J Neural Eng 2008; 5: 163–174. [DOI] [PubMed] [Google Scholar]
- 75. Datta A, Bansal V, Diaz J, et al. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul 2009; 2: 201–207, 207.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science 2004; 304: 1926–1929. [DOI] [PubMed] [Google Scholar]
- 77. Herrmann CS, Rach S, Neuling T, et al. Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Front Hum Neurosci 2013; 7: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zaehle T, Rach S, Herrmann CS. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS ONE 2010; 5: e13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vossen A, Gross J, Thut G. Alpha power increase after transcranial alternating current stimulation at alpha frequency (α-tACS) reflects plastic changes rather than entrainment. Brain Stimul 2015; 8: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cabral-Calderin Y, Williams KA, Opitz A, et al. Transcranial alternating current stimulation modulates spontaneous low frequency fluctuations as measured with fMRI. NeuroImage 2016; 141: 88–107. [DOI] [PubMed] [Google Scholar]
- 81. Vanneste S, Plazier M, der Loo E, et al. The neural correlates of tinnitus-related distress. NeuroImage 2010; 52: 470–480. [DOI] [PubMed] [Google Scholar]
- 82. Claes L, Stamberger H, Van de Heyning P, et al. Auditory cortex tACS and tRNS for tinnitus: single versus multiple sessions. Neural Plast 2014; 2014: 436713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Terney D, Chaieb L, Moliadze V, et al. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J Neurosci 2008; 28: 14147–14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Schoen I, Fromherz P. Extracellular stimulation of mammalian neurons through repetitive activation of Na+ channels by weak capacitive currents on a silicon chip. J Neurophysiol 2008; 100: 346–357. [DOI] [PubMed] [Google Scholar]
- 85. Van Doren J, Langguth B, Schecklmann M. Electroencephalographic effects of transcranial random noise stimulation in the auditory cortex. Brain Stimul 2014; 7: 807–812. [DOI] [PubMed] [Google Scholar]
- 86. Joos K, De Ridder D, Vanneste S. The differential effect of low- versus high-frequency random noise stimulation in the treatment of tinnitus. Exp Brain Res 2015; 233: 1433–1440. [DOI] [PubMed] [Google Scholar]
- 87. Mohsen S, Pourbakht A, Farhadi M, et al. The efficacy and safety of multiple sessions of multisite transcranial random noise stimulation in treating chronic tinnitus. Braz J Otorhinolaryngol 2019; 85: 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kreuzer PM, Poeppl TB, Rupprecht R, et al. Daily high-frequency transcranial random noise stimulation of bilateral temporal cortex in chronic tinnitus – a pilot study. Sci Rep 2019; 9: 12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yu ZJ, Weller RA, Sandidge K, et al. Vagus nerve stimulation: can it be used in adolescents or children with treatment-resistant depression? Curr Psychiatry Rep 2008; 10: 116–122. [DOI] [PubMed] [Google Scholar]
- 90. Bonaz B, Picq C, Sinniger V, et al. Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol Motil 2013; 25: 208–221. [DOI] [PubMed] [Google Scholar]
- 91. Manta S, Dong J, Debonnel G, et al. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J Psychiatry Neurosci 2009; 34: 272–280. [PMC free article] [PubMed] [Google Scholar]
- 92. Seol GH, Ziburkus J, Huang S, et al. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron 2007; 55: 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Manunta Y, Edeline JM. Noradrenergic induction of selective plasticity in the frequency tuning of auditory cortex neurons. J Neurophysiol 2004; 92: 1445–1463. [DOI] [PubMed] [Google Scholar]
- 94. Edeline JM, Manunta Y, Hennevin E. Induction of selective plasticity in the frequency tuning of auditory cortex and auditory thalamus neurons by locus coeruleus stimulation. Hear Res 2011; 274: 75–84. [DOI] [PubMed] [Google Scholar]
- 95. Engineer ND, Riley JR, Seale JD, et al. Reversing pathological neural activity using targeted plasticity. Nature 2011; 470: 101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tyler R, Cacace A, Stocking C, et al. Vagus nerve stimulation paired with tones for the treatment of tinnitus: a prospective randomized double-blind controlled pilot study in humans. Sci Rep 2017; 7: 11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Engineer ND, Rosellini WM, Tyler RS. Willingness to accept and pay for implantable tinnitus treatments: a survey. Neuromodulation 2013; 16: 154–162. [DOI] [PubMed] [Google Scholar]
- 98. Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol 2002; 1: 477–482. [DOI] [PubMed] [Google Scholar]
- 99. Butt MF, Albusoda A, Farmer AD, et al. The anatomical basis for transcutaneous auricular vagus nerve stimulation. J Anat 2020; 236: 588–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kraus T, Hösl K, Kiess O, et al. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm (Vienna) 2007; 114: 1485–1493. [DOI] [PubMed] [Google Scholar]
- 101. Kraus T, Kiess O, Hösl K, et al. CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal – a pilot study. Brain Stimul 2013; 6: 798–804. [DOI] [PubMed] [Google Scholar]
- 102. Shim HJ, Kwak MY, An YH, et al. Feasibility and safety of transcutaneous vagus nerve stimulation paired with notched music therapy for the treatment of chronic tinnitus. J Audiol Otol 2015; 19: 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lehtimäki J, Hyvärinen P, Ylikoski M, et al. Transcutaneous vagus nerve stimulation in tinnitus: a pilot study. Acta Otolaryngol 2013; 133: 378–382. [DOI] [PubMed] [Google Scholar]
- 104. Mei ZG, Yang SB, Cai SJ, et al. Treatment of tinnitus with electrical stimulation on acupoint in the distribution area of ear vagus nerve combining with sound masking: randomized controlled trial. World J Acup – Moxibust 2014; 24: 30–35. [Google Scholar]
- 105. Ylikoski J, Markkanen M, Pirvola U, et al. Stress and tinnitus; transcutaneous auricular vagal nerve stimulation attenuates tinnitus-triggered stress reaction. Front Psychol 2020; 11: 570196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kreuzer PM, Landgrebe M, Resch M, et al. Feasibility, safety and efficacy of transcutaneous vagus nerve stimulation in chronic tinnitus: an open pilot study. Brain Stimul 2014; 7: 740–747. [DOI] [PubMed] [Google Scholar]
- 107. Suk WC, Kim SJ, Chang DS, et al. Characteristics of stimulus intensity in transcutaneous vagus nerve stimulation for chronic tinnitus. J Int Adv Otol 2018; 14: 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tutar B, Atar S, Berkiten G, et al. The effect of transcutaneous electrical nerve stimulation (TENS) on chronic subjective tinnitus. Am J Otolaryngol 2020; 41: 102326. [DOI] [PubMed] [Google Scholar]
- 109. Stouffer JL, Tyler RS. Characterization of tinnitus by tinnitus patients. J Speech Hear Disord 1990; 55: 439–453. [DOI] [PubMed] [Google Scholar]
- 110. Giraud AL, Chéry-Croze S, Fischer G, et al. A selective imaging of tinnitus. Neuroreport 1999; 10: 1–5. [DOI] [PubMed] [Google Scholar]
- 111. Mirz F, Pedersen B, Ishizu K, et al. Positron emission tomography of cortical centers of tinnitus. Hear Res 1999; 134: 133–144. [DOI] [PubMed] [Google Scholar]
- 112. Seydell-Greenwald A, Leaver AM, Turesky TK, et al. Functional MRI evidence for a role of ventral prefrontal cortex in tinnitus. Brain Res 2012; 1485: 22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Alain C, Woods DL, Knight RT. A distributed cortical network for auditory sensory memory in humans. Brain Res 1998; 812: 23–37. [DOI] [PubMed] [Google Scholar]
- 114. Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron 2010; 66: 819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Plewnia C, Bartels M, Gerloff C. Transient suppression of tinnitus by transcranial magnetic stimulation. Ann Neurol 2003; 53: 263–266. [DOI] [PubMed] [Google Scholar]
- 116. Bikson M, Grossman P, Thomas C, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul 2016; 9: 641–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Im C-H, Park J-H, Shim M, et al. Evaluation of local electric fields generated by transcranial direct current stimulation with an extracephalic reference electrode based on realistic 3D body modeling. Phys Med Biol 2012; 57: 2137–2150. [DOI] [PubMed] [Google Scholar]
- 118. Matsumoto H, Ugawa Y. Adverse events of tDCS and tACS: a review. Clin Neurophysiol Pract 2017; 2: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Brunoni AR, Amadera J, Berbel B, et al. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol 2011; 14: 1133–1145. [DOI] [PubMed] [Google Scholar]
- 120. Wang Y, Li SY, Wang D, et al. Transcutaneous auricular vagus nerve stimulation: from concept to application. Neurosci Bull 2021; 37: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Badran BW, Dowdle LT, Mithoefer OJ, et al. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: a concurrent taVNS/fMRI study and review. Brain Stimul 2018; 11: 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yakunina N, Kim SS, Nam EC. Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation 2017; 20: 290–300. [DOI] [PubMed] [Google Scholar]
- 123. Kreisberg E, Esmaeilpour Z, Adair D, et al. High-resolution computational modeling of the current flow in the outer ear during transcutaneous auricular Vagus Nerve Stimulation (taVNS). Brain Stimul 2021; 14: 1419–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Yap JYY, Keatch C, Lambert E, et al. Critical review of transcutaneous vagus nerve stimulation: challenges for translation to clinical practice. Front Neurosci 2020; 14: 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Redgrave J, Day D, Leung H, et al. Safety and tolerability of transcutaneous vagus nerve stimulation in humans; a systematic review. Brain Stimul 2018; 11: 1225–1238. [DOI] [PubMed] [Google Scholar]
- 126. De Ridder D, Langguth B, Vanneste S. Vagus nerve stimulation for tinnitus: a review and perspective. Prog Brain Res 2021; 262: 451–467. [DOI] [PubMed] [Google Scholar]
- 127. Farmer AD, Strzelczyk A, Finisguerra A, et al. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (Version 2020). Front Hum Neurosci 2020; 14: 568051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223221148061 for State of the art: non-invasive electrical stimulation for the treatment of chronic tinnitus by Shanwen Chen, Maoshan Du, Yang Wang, Yifan Li, Busheng Tong, Jianxin Qiu, Feihu Wu and Yehai Liu in Therapeutic Advances in Chronic Disease