Abstract
Among the three primary gynecological malignancies, ovarian cancer has the lowest incidence but the worst prognosis. Because of the poor prognosis of ovarian cancer patients treated with existing treatments, immunotherapy is emerging as a potentially ideal alternative to surgery, chemotherapy, and targeted therapy. Among immunotherapies, immune checkpoint inhibitors have been the most thoroughly studied, and many drugs have been successfully used in the clinic. CD47, a novel immune checkpoint, provides insights into ovarian cancer immunotherapy. This review highlights the mechanisms of tumor immune evasion via CD47-mediated inhibition of phagocytosis and provides a comprehensive insight into the progress of the relevant targeted agents in ovarian cancer.
Keywords: ovarian cancer, immunotherapy, CD47, immune checkpoint, immune checkpoint inhibitors
Introduction
Ovarian cancer (OC) is one of the deadliest female malignancies, with the lowest incidence but the worst prognosis among all gynecological malignancies. According to clinical guidelines and expert consensus, cytoreductive surgery followed by platinum-based chemotherapy is the standard treatment for most patients with OC.1 Related clinical symptoms are relieved under this standard treatment; however, OC is prone to recurrence and drug resistance, resulting in a five-year survival rate of less than 50%.2 Despite significant progress in surgical techniques and drug therapies, the survival of patients with advanced OC has not improved. Therefore, there is an urgent need to get OC out of therapeutic dilemmas.3
Growing evidence has demonstrated that malignancy is a heterogeneous disease with immunogenicity, and its occurrence, development, and metastasis rely on immune suppression.4 Immunotherapy targeting immunogenicity is a hotspot of anti-tumor therapies and has been successfully used in clinics as a novel anti-tumor option after traditional treatments (e.g., surgery, radiotherapy, chemotherapy, and endocrine therapy).5 Along with the discovery of immune checkpoints (ICs), immune checkpoint inhibitors (ICIs) have become the focus of research in tumor immunotherapy.6–8 ICIs restore self-clearing and monitor the function of the immune system by blocking inhibitory signals. Various ICIs have shown anti-tumor activity in preclinical models, and some have been successfully used in clinics.6–11 Programmed cell death protein-1 (PD-1) and its ligand (PD-L1) inhibitors have been the most successful and widely used ICIs.12 Nevertheless, new ICIs are continuously being identified and developed.
The known ICIs primarily act on the adaptive immune system. However, only 10%-30% of patients with OC show long-term and durable responses, followed by acquired resistance, which remains a substantial dilemma.7,12–14 CD47 has been identified as the first innate IC, restoring macrophages phagocytosis by blocking the “don’t eat me” signal.15,16 CD47 is a promising therapeutic target to provide insights into new treatment options for patients with OC.
The Development of ICIs
Adaptive ICs
T cells mediated adaptive immune dominates in anti-tumor reaction.5,14 Both the primary signals produced by the interaction between the major histocompatibility complex and T cells receptor (TCR) and the secondary signals offered by the co-stimulatory molecules are necessary to elicit intact T cell responses.14 Functioning as the assistant of T cell activation and proliferation, co-stimulatory molecules are also essential regulators of humoral immunity and cytokine production.17 However, increasing evidence has shown that some members, such as cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), PD-L1, PD-L2, PD-1 homolog, and B7-H3, also provide critical inhibitory secondary signals.18 These inhibitory signals act as a “brake” to protect normal cells from the excessive T cells’ attack by attenuating T cell response.18,19 Conversely, multiple tumors suppress anti-tumor immune responses and evade immune attacks by overexpressing these molecules.7
These brake-like molecules and related inhibitors have been dubbed as ICs and ICIs in cancer immunotherapy because of the satisfactory anti-tumor effects of targeting CTLA-4. 14,20,21 Mechanically, the increased anti-tumor effects appear to be the result of the simultaneous enhancement of effector T cell function and concurrent inhibition of Treg activity. In 2011, the FDA (the Food and Drug Administration) authorized the first ICI, Ipilimumab, a CTLA-4 monoclonal antibody (mAb), for the treatment of advanced melanoma. Notably, subsequent approvals of Ipilimumab for the treatment of other malignancies have involved its use with the PD-1/PD-L1 ICIs.22–24 PD-1/PD-L1 inhibitors are the most successful ICIs, which primarily act in the adaptive immune system to trigger tumor-specific T cells responses.25 PD-L1 overexpresses on various human tumors and induces the phosphorylation ordephosphorylation process of intracellular signaling pathways when it binds to PD-1.26–29 These signaling cascades suppress T cell proliferation, activation, and cytokine production, inhibiting the anti-tumor immune responses and promoting tumor growth.9,13 Various anti-PD-1/PD-L1 mAbs have been shown to have effective anti-tumor activities in, for example, melanoma, lung cancer, and Hodgkin’s lymphoma (HL).29–32 Multifarious PD-1/PD-L1 inhibitors, for example, Pembrolizumab, Atezolizumab, and Nivolumab, have been approved worldwide for various malignancies.33,34
Despite their proven efficacy, only 10%-30% of patients with solid tumors receiving PD-1/PD-L1 or CTLA-4 ICIs show long-term, durable responses, and the remainder mostly do not respond. This result is true in OC.35–37 How to overcome acquired resistance in the development of ICIs is critical, prompting us to explore new targets in tumor immunotherapy to solve this predicament.
Innate ICs
The immune system consists of the innate and adaptive immune systems, both of which must be activated simultaneously to obtain sufficient anti-tumor effects.38 CTLA-4 and PD-L1 has garnered considerable attention, which mainly acts on adaptive immunity, but the impact of innate immunity is ignored. In innate immune responses, antigen-presenting cells (APCs) present antigens after phagocytes take up tumor-specific antigens; NK cells directly kill tumor cells, or APCs trigger an adaptive immune response to participate in anti-tumor responses by presenting antigens to T cells.39 Brake-like molecules are also found during the innate immune response.
CD47 is the first identified innate IC,40 and Oldenborg described its anti-phagocytic effect by observing the rapid clearance of erythrocytes from CD47−/− mice injected into wild-type mice, but macrophage depletion removed this effect.41 Van Buerger found that macrophages rapidly cleared senescent erythrocytes with reduced CD47 expression.42 Notably, CD47 has become a research hotspot since the revelation of its innate IC identity.
Biological Characteristics and Function of CD47
CD47 is a 50-kDa highly glycosylated-transmembrane immunoglobulin43 possessing an extracellular IgV-like domain at the N-terminal, five highly hydrophobic membrane-spanning regions, and a short intracellular tail at the C-terminal, ubiquitously expressing on various normal cells.44,45 The known ligands of CD47 include integrin,40 thrombospondin-1 (TSP-1), signal regulatory protein α (SIRPα), SIRPγ.16,45 Integrin or TSP-1 binding to CD47 is involved in cell adhesion, migration, aging, angiogenesis, platelet activation, and other biological functions.45–51 Additionally, the connection of TSP-1 and CD47 inhibits anti-tumor immunity by regulating the immune system.49,52 On the one hand, TSP-1/CD47-dependent signaling inhibits T cell function via limiting TCR-mediated first signal transduction and blocking H2S-mediated T cell activation.50,53 On the other hand, TSP-1 reduces macrophage activation by limiting lipopolysaccharide-induced IL-1β expression and total protein production in macrophages.51 Notably, mice myeloid macrophages without TSP1 or CD47 showed the reduced induction of mature IL-1β of lipopolysaccharide.49,54 Simultaneously, decreased macrophage infiltration was observed in the absence of TSP-1.55 Moreover, TSP-1 was initially identified as an angiogenesis inhibitor to suppress tumor growth by limiting angiogenesis and perfusion in the tumor vasculature.56,57 Perhaps the bi-phasic effects of TSP-1 in cancers that therapies targeting TSP-1/CD47 have not progressed from preclinical studies into human trials.
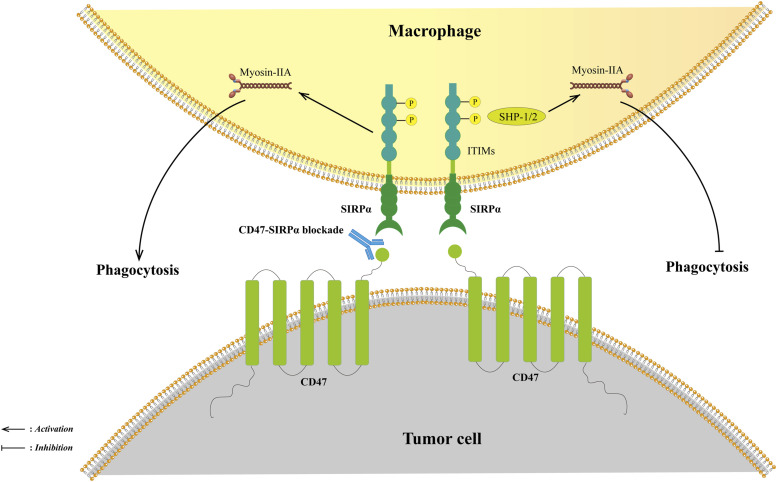
SIRPα is the most researched ligand of CD47, which is mainly restricted to its expression on myeloid cells.46,47,58,59 SIRPα consists of three immunoglobulin-like extracellular domains, a transmembrane region, and a cytoplasmic domain containing four immunoreceptor tyrosine inhibitory motifs (ITIMs).59 The interactions between CD47 and SIRPα on macrophages recruit Src homology-2 (SH2)-containing tyrosine phosphatase 1 (SHP-1) and SHP-2, leading to the phosphorylation of ITIMs. These upstream signals prevent myosin-IIA accumulation to form phagocytic synapses, leading to phagocytosis inhibitions.45,47,59–61 (Figure 1) This phenomenon could further induce APCs cross-presentation and initiate adaptive immunity. In a sense, the CD47-SIRPα axis bridges innate and adaptive immunity.11,62,63 Overall, CD47 sends a “don’t eat me” suppressant signal to maintain immune tolerance and avoid excessive phagocytosis.
Figure 1.
The CD47-SIRPα signal axis. The interaction between CD47 and SIRPα recruits SHP-1/2, leading to the phosphorylation of two tyrosine residues of ITIMs in the intracellular structural domain of SIRPα. Phosphorylation of SHP-1/2 prevents the accumulation of myosin-IIA, which forms phagocytic synapses, resulting in sending a “don’t eat me” signal. Blocking the CD47-SIRPa axis could restore phagocytosis. Abbreviations: SHP-1, Src homology 2 containing tyrosine phosphatase 1; SHP-2, Src homology 2 containing tyrosine phosphatase 2; ITIMs, immunoreceptor tyrosine inhibitory motifs.
CD47 Expression in OC and Its Possible Regulatory Mechanism
CD47 is overexpressed on OC cells and was identified as an OC tumor-specific marker in 1986.63 Subsequently, diverse insights into its expression and regulation in OC have been proposed.
Regulatory factors, including Myc, NF-κB, and HIF-1, induce CD47 expression at the transcriptional level.64–66 Myc oncogene has been confirmed to regulate CD47 and PD-L1 expression through direct joining to their promoters. CD47 and PD-L1 expression was decreased in a mouse xenograft tumor model with Myc suppression, and the anti-tumor immune response was enhanced. Conversely, the opposite phenomenon was observed with Myc activation.64 HIF-1 binding to the promoter initiates transcription and increases CD47 expression, protecting breast cancer cells from phagocytosis.65 Moreover, NF-κB directly connects with a super-enhancer near the CD47 gene to promote its expression upon stimulation by TNF-α in breast cancer.67 Notably, the aforementioned transcriptional regulatory pathways must be further verified in OC models.
At the cellular level, the CD47 expression levels of exosomes in OC cells were observed to correlate with macrophage phagocytosis. Inhibition of exosome release and uptake can downregulate CD47 expression, increasing macrophage phagocytosis.68 In general, the multifaceted regulatory mechanisms of CD47 expression imply diverse perspectives on CD47-targeted therapies.
Clinical Significance of Abnormal CD47 Expression in Patients With OC
Similar to various malignancies, CD47 high expression is associated with disease development and adverse clinical outcomes in OC.38,46,69
In 2012, Weissman et al first observed that the expression level of CD47 mRNA is associated with prognosis in OC and other solid tumors. They revealed that CD47 expression in tumor tissues was more than three times that in the surrounding normal tissues. Further analysis demonstrated that high CD47 mRNA expression correlated with poorer progression-free survival and overall survival (OS) in OC.46 Another team found that CD47 overexpression enhanced growth and motility in TOV OC cell lines.70 Wang et al not only observed high CD47 expression in 86 ovarian clear cell carcinomas by IHC but also confirmed that CD47 expression was correlated with surgical stage, drug resistance, and prognosis. Furthermore, it was an independent risk factor for prognosis.71 Subsequently, the same researchers proposed that CD47 comprised Lewis-y antigen, implicated in tumor cells adhesion, metastasis, and resistance. CD47 and Lewis-y were confirmed to be significantly highly expressed in OC, and their expressions were linearly correlated. High expression of CD47 and Lewis-y was statistically correlated with the FIGO stage, lymph node metastasis, and differentiation degree.72 The effective component of CD47 was clarified in this study.
Opinions on the association between CD47 expression and prognosis differ. A retrospective exploratory analysis of 316 serous patients with OC in the Cancer Genome Atlas database showed no difference in disease-free survival and OS between patients with different CD47 expression levels. IHC was used on another 265 patient tumor samples to validate this conclusion. Upregulation of CD47 expression was observed in 48.7% of the patients, and the low-expression group showed better treatment responses. However, this result did not improve their prognosis, which might partially be explained by the presence of multiple immune evasion mechanisms in the tumor immune microenvironment (TME) of OC.73
A recent bioinformatics study offers several references to this confusion. The OC TME with CD47 overexpression in the Tumor Immune Estimation Resource database contained more M2 and Treg cell infiltration, promoting tumor cell immune escape. Additionally, the positive correlation between CD47 overexpression and T cell exhaustion further confirmed that CD47 might influence the infiltration abundance of immune cells in the TME to regulate OC biological behavior.74
Mechanism of CD47-SIRPα Axis Targeted Therapy in OC
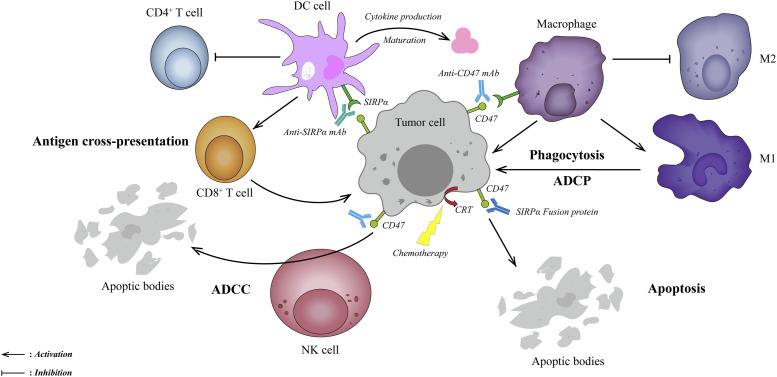
In 2009, Weissman et al observed the disappearance of AML cells in vitro and in xenografted mice with B6H12.2 (anti-human CD47 mAb). Surprisingly, the depletion of macrophages eliminated this phenomenon. It suggested that targeting the CD47-SIRPα axis eliminates tumors by restoring macrophage phagocytosis.75 Anti-CD47/SIRPα mAbs and recombinant SIRPα-fusion proteins are thus considered promising immunotherapies based on these inspiring theories. These attractive immunotherapies may facilitate tumor clearance through the following pathways: (Figure 2).
Figure 2.
Mechanisms of blocking CD47-SIRPα axis therapies in OC. First, blocking the CD47-SIRPα axis could clear tumor cells by restoring macrophage phagocytosis and promoting tumor-associated macrophages to differentiate toward the M1 subtype (upper right). Second, anti-CD47/SIRPα-blocking mAbs or SIRPα-fusion proteins could clear tumor cells through classical Fc-dependent mechanisms, including NK cell-mediated ADCC (lower left) and macrophage-mediated ADCP (upper right). Third, blocking the CD47-SIRPα axis might indirectly stimulate adaptive immunity by promoting DCs uptake by tumor cells and cross-presentation to CD8+ T cells (upper left). Fourth, blocking the CD47-SIRPα axis could directly clear tumor cells by caspase-independent apoptosis (lower right). Abbreviations: ADCC, antibody-dependent cytotoxicity; ADCP, antibody-dependent phagocytosis; DC, dendritic cells; M1, M1-like macrophages; M2, M2-like macrophages; NK cells, natural killer cells; OC, ovarian cancer.
First, anti-CD47/SIRPα-blocking mAbs restore macrophage-mediated phagocytosis by blocking the “don’t eat me” signal and non-blocking antibodies without such function. In xenotransplantation models, the exhaustion of clodronate-mediated phagocytes eliminated such mAbs-dependent anti-tumor effects, confirming that phagocytes as the primary effectors.15,46,76 In this pathway, tumor-associated macrophages differentiate toward the M1-like phenotype, shifting immune-tolerant TME to attacking tumor cells.46,77
Second, the balance between pro-phagocytic and anti-phagocytic signals determines phagocytosis. In other words, blocking the “don’t eat me” signal alone may be insufficient to gain effective anti-tumor effects.62,78 Calreticulin (CRT), a dominant pro-phagocytic signal, highly expresses on various cancer cells’ surfaces, whereas barely on normal cells.62,79 The Fc receptor is another pro-phagocytic molecule, which eliminates tumor cells through NK cells’ mediated antibody-dependent cytotoxicity (ADCC) and macrophage-mediated antibody-dependent phagocytosis (ADCP). Importantly, most anti-CD47/SIRPα mAbs or SIRPα fusion proteins contain an IgG (Fcγ) skeleton. The SIRPα-Fc fusion protein constructed by Huang et al. significantly enhanced ADCC and highlighted a potent anti-tumor effect in SK-OV3 and HO8910 OC cells.80 Moreover, further research is necessary to clarify the roles of other pro-phagocytic molecules, including SLAMF and PtdSer, during the CD47-SIRPα axis blockade.62,79
Third, inhibition of the CD47-SIRPα axis bridges innate and adaptive immunity. Weissman et al first observed that anti-CD47 antibody-mediated phagocytosis of tumor cells promoted CD8+ T cell activation but inhibited CD4+ T cell initiation in vitro and in vivo.81 Furthermore, the interactions of CD47 with SIRPα suppress the biological behaviors of dendritic cells (DCs), such as maturation and cytokine production, resulting in the inhibition of T cell antigen presentation and adaptive immunity initiation.82 Blocking CD47-SIRPα can remove these inhibitory effects, enabling the cross-presentation of tumor antigens and activating adaptive immunity.83,84
Fourth, direct induction of apoptosis is another mechanism, which has been observed in breast cancer, chronic myeloid leukemia (CML), and multiple myeloma (MM).15,76,85,86 Notably, anti-CD47 antibodies mediated apoptosis via a caspase-independent pathway.86
Clinical Drug Development Targeting CD47-SIRPα Axis in OC
Although no drugs targeting the CD47-SIRPα axis have been officially approved, related clinical trials have set off booms worldwide. As of August 29, 2022, 74 and 25 related clinical trials were registered in the United States National Clinical Trials Registry (NCT) system and China Drug Trials Registry (CDT) system, respectively. (Table 1) Additionally, we summarized and updated the latest clinical research involving OC. (Table 2)
Table 1.
Registered Clinical Trials of Drugs Blocking CD47-SIRPα Axis in the NCT and CDT System.
| Agent | CD47 Isotype | Mechanism | Condition or Disease | Phase | Enrollment | Start Date | Status | Preliminary clinical data reported (Ref.) |
|---|---|---|---|---|---|---|---|---|
| B6H12.2 | IgG4 | Humanized anti-CD47 mAb | Advanced Malignancies (include ovarian cancer) | Cell lines animal mode | NA | NA | NA | 43,46,75,87–89 |
| Hu5F9-G4 (Magrolimab) | IgG4 | Humanized anti-CD47 mAb | Solid Tumor (includes fallopian tube and ovarian cancers) | 1 | 88 | 2014-08 | Completed | 89, NCT0221640990 |
| AML/MDS | 1 | 20 | 2015-12 | Completed | NCT0267833891 | |||
| Solid Tumor | 1 | 78 | 2016-12-01 | Completed | NCT02953782 | |||
| NHL | 1 | 178 | 2016-12-21 | Active, not recruiting | NCT02953509 | |||
| Hematological Malignancies | 1 | 258 | 2017-09-08 | Active, not recruiting | NCT02678338 | |||
| Hematological Malignancies | 1 | 258 | 2017-09-08 | Active, not recruiting | NCT03248479 | |||
| OC | 1 | 34 | 2018-05-23 | Completed | NCT03558139 | |||
| NHL/DLBCL | 1 | 30 | 2018-06-19 | Completed | NCT03527147 | |||
| Urothelial Carcinoma | 1b/2 | 645 | 2019-06-01 | Recruiting | NCT03869190 | |||
| AML | 1 | 13 | 2019-10-08 | Terminated | NCT03922477 | |||
| Solid Tumor | 1 | 7 | 2020-07-28 | Completed | NCT04403308 | |||
| AML | 1/2 | 98 | 2020-07-28 | Recruiting | NCT04435691 | |||
| MDS | 3 | 520 | 2020-09-09 | Recruiting | NCT04313881 | |||
| T-cell Lymphoma | 1b/2 | 18 | 2020-12-24 | Suspended | NCT04541017 | |||
| Neuroblastoma/Osteosarcoma | 1 | 82 | 2021-04-16 | Recruiting | NCT04751383 | |||
| AML | 3 | 346 | 2021-06-01 | Recruiting | NCT04778397 | |||
| MM | 2 | 128 | 2021-06-28 | Recruiting | NCT04778410 | |||
| Head and Neck Squamous Cell Carcinoma | 2 | 230 | 2021-09-07 | Recruiting | NCT04854499 | |||
| Solid Tumor | 2 | 116 | 2021-10-01 | Recruiting | NCT04827576 | |||
| MM | 2 | 153 | 2021-12-09 | Recruiting | NCT04892446 | |||
| Triple-Negative Breast Cancer | 2 | 144 | 2021-12-14 | Recruiting | NCT04958785 | |||
| B-cell Malignancies | 1 | 76 | 2021-12-16 | Recruiting | NCT04599634 | |||
| HL | 2 | 24 | 2022-06-21 | Recruiting | NCT04788043 | |||
| AML | 3 | 432 | 2022-07-07 | Recruiting | NCT05079230 | |||
| CC-90002 | IgG4 | Humanized anti-CD47 mAb | Hematologic Neoplasms | 1 | 60 | 2015-03-12 | Completed | 92, NCT02367196 |
| Hematologic Neoplasms | 1 | 28 | 2016-03-01 | Terminated | NCT02641002 | |||
| TTI-621 | IgG1 | SIRPα-IgG1 Fc Fusion Protein | Hematologic Malignancies/Solid Tumor | 1 | 250 | 2016-01-31 | Active, not recruiting | 93,94, NCT0266351895,96 |
| Solid Tumor/Mycosis Fungoides | 1 | 56 | 2016-09 | Terminated | 97, NCT0289036898 | |||
| Leiomyosarcoma | 2 | 80 | 2021-06-22 | Recruiting | NCT04996004 | |||
| MM | 1 | 32 | 2021-10-28 | Recruiting | NCT05139225 | |||
| TTI-622 | IgG4 | SIRPα-IgG4 Fc Fusion Protein | Hematological Malignancies | 1 | 386 | 2018-07-07 | Recruiting | 99, NCT03530683 |
| MM | 1 | 32 | 2021-10-28 | Recruiting | NCT05139225 | |||
| OC | 1/2 | 50 | 2022-02-14 | Recruiting | NCT05261490 | |||
| ALX148 (Evorpacept) | IgG4 | Humanized anti-CD47 mAb | Solid Tumor/NHL | 1 | 174 | 2017-02-03 | Active, not recruiting | 100, NCT03013218101 |
| Higher Risk MDS | 1/2 | 173 | 2020-10-02 | Recruiting | NCT04417517 | |||
| Head and Neck Cancer | 2 | 183 | 2021-04-02 | Recruiting | NCT04675294 | |||
| AML | 1/2 | 97 | 2021-05-05 | Active, not recruiting | NCT04755244 | |||
| Head and Neck Cancer | 2 | 168 | 2021-05-10 | Recruiting | NCT04675333 | |||
| HER2+ Cancers | 1/2 | 93 | 2021-09-15 | Recruiting | NCT05027139 | |||
| B-cell NHL | 1/2 | 52 | 2021-10-13 | Recruiting | NCT05025800 | |||
| Gastric Cancer | 2/3 | 450 | 2022-01-15 | Recruiting | NCT05002127 | |||
| Ti-061 | IgG4 | Humanized anti-CD47 mAb | Advanced Malignancies | 1 | 160 | 2017-04-05 | Terminated | EudraCT number, 2016-004372-22102 |
| SRF231 | IgG4 | Humanized anti-CD47 mAb | Advanced Solid/Hematologic Cancers | 1 | 148 | 2018-03-13 | Completed | 103, NCT03512340104 |
| SHR-1603 | IgG4 | Humanized anti-CD47 mAb | Advanced Malignancies | 1 | 128 | 2018-12-13 | Suspended | 105, NCT03722186 |
| IBI188 | IgG4 | Humanized anti-CD47 mAb | Advanced Malignancies | 1 | 49 | 2019-01-10 | Completed | NCT03717103 |
| Advanced Malignancies | 1 | 20 | 2019-01-19 | Completed | NCT03763149 | |||
| MDS | 1 | 32 | 2020-08-24 | Suspended | NCT04511975 | |||
| AML | 1/2 | 126 | 2020-09-25 | Unknown | 106, NCT04485052 | |||
| MDS | 1 | 12 | 2020-09-30 | Recruiting | NCT04485065 | |||
| Solid Tumor | 1 | 120 | 2021-05-25 | Recruiting | NCT04861948 | |||
| IBI322 | IgG4 | Humanized anti-CD47/PD-L1 BsAb | Advanced Malignancies | 1 | 218 | 2020-07-31 | Recruiting | 107, NCT04328831 |
| Advanced Lymphomas | 1 | 51 | 2021-01-14 | Recruiting | NCT04338659 | |||
| Hematologic Malignancy | 1 | 230 | 2021-05-07 | Recruiting | NCT04795128 | |||
| Advanced Solid Tumor | 1 | 36 | 2021-06-20 | Recruiting | NCT04912466 | |||
| Myeloid Tumor | 1 | 124 | 2021-12-31 | Not yet recruiting | NCT05148442 | |||
| NSCLC | 2 | 80 | 2022-07-20 | Not yet recruiting | NCT05296278 | |||
| SCLC | 2 | 40 | 2022-07-22 | Not yet recruiting | NCT05296603 | |||
| AO-176 | IgG2 | Humanized anti-CD47 mAb | Solid Tumor (includes epithelial ovarian carcinoma) | 1/2 | 183 | 2019-02-04 | Active, not recruiting | 108,109, NCT03834948110 |
| MM | 1/2 | 157 | 2020-12-30 | Active, not recruiting | NCT04445701 | |||
| TG-1801 | IgG4 | Humanized anti- CD47/CD19 BsAb | B-Cell Lymphoma | 1 | 16 | 2019-03-05 | Recruiting | NCT03804996 |
| B-Cell Lymphoma/CLL | 1 | 60 | 2021-04-23 | Recruiting | NCT04806035 | |||
| TJ4 (TJ011133) | IgG4 | Humanized anti-CD47 mAb | Solid Tumor/Lymphoma | 1 | 98 | 2019-04-16 | Active, not recruiting | 111, NCT03934814112 |
| AML/MDS | 1/2 | 100 | 2020-03-25 | Recruiting | NCT04202003113 | |||
| AML/MDS | 1 | 38 | 2021-06-25 | Active, not recruiting | NCT04912063 | |||
| Advanced Solid Tumor | 1/2 | 96 | 2021-12-30 | Recruiting | NCT05148533 | |||
| MM | 1 | 8 | 2022-01-17 | Terminated | NCT04895410 | |||
| BI 765063 (OSE-172) | IgG4 | Humanized against SIRPα mAb | Solid Tumor | 1 | 116 | 2019-4-16 | Recruiting | NCT03990233 |
| Solid Tumor | 1 | 18 | 2020-12-15 | Completed | NCT04653142 | |||
| HX009 | IgG4 | Humanized anti- CD47/PD-1 BsAb | Advanced Solid Tumor | 1 | 21 | 2019-06-12 | Active, not recruiting | NCT04097769 |
| Advanced Solid Tumor | 2 | 210 | 2021-05-12 | Active, not recruiting | NCT04886271 | |||
| Relapsed/Refractory Lymphoma | 1/2 | 99 | 2021-12-31 | Recruiting | NCT05189093 | |||
| IMM01 | IgG1 | SIRPα-IgG1 Fc Fusion Protein | Relapsed/Refractory Lymphoma | 1 | 30 | 2019-09-09 | Recruiting | CTR20191531114 |
| AML/MDS | 1/2 | 76 | 2022-01-01 | Not yet recruiting | NCT05140811 | |||
| AK117 | IgG4 | Humanized anti-CD47 mAb | Neoplasms Malignant | 1 | 159 | 2020-04-25 | Active, not recruiting | NCT04349969115 |
| Neoplasms Malignant | 1 | 162 | 2021-01-27 | Recruiting | NCT04728334 | |||
| MDS | 1/2 | 190 | 2021-06-18 | Recruiting | NCT04900350 | |||
| AML | 1/2 | 160 | 2021-07 | Recruiting | NCT04980885 | |||
| Triple-negative Breast Cancer | 2 | 80 | 2022-03-23 | Recruiting | NCT05227664 | |||
| Advanced Malignant Tumors | 1/2 | 114 | 2022-05-04 | Recruiting | NCT05229497 | |||
| Advanced Malignant Tumors | 1/2 | 130 | 2022-07-12 | Recruiting | NCT05235542 | |||
| Metastatic Colorectal Cancer | 2 | 114 | 2022-07-27 | Recruiting | NCT05382442 | |||
| ZL-1201 | IgG4 | Humanized anti-CD47 mAb | Advanced Cancer | 1 | 37 | 2020-05-11 | Active, not recruiting | NCT04257617 |
| IMC-002 | IgG4 | Humanized anti-CD47 mAb | Solid Tumor/Lymphoma | 1 | 24 | 2020-06-05 | Recruiting | NCT04306224 |
| Advanced Cancer | 1 | 24 | 2022-05-10 | Recruiting | NCT05276310 | |||
| SL-172154 | IgG4 | Bispecific SIRPα-Fc-CD40L Fusion Protein | OC | 1 | 40 | 2020-07-29 | Recruiting | NCT04406623116 |
| DSP107 | IgG4 | Humanized anti- CD47/4-1BB BsAb | Advanced Solid Tumor | 1/2 | 100 | 2020-10-07 | Recruiting | 117, NCT04440735 |
| Hematological Malignancies | 1 | 36 | 2022-01-31 | Recruiting | NCT04937166 | |||
| SHR2150 | IgG4 | Selective TLR7 Agonist | Solid Tumor | 1/2 | 50 | 2020-10-10 | Recruiting | 118, NCT04588324 |
| IMM0306 | IgG1 | Humanized anti- CD47/CD20 BsAb | B-cell NHL | 1 | 90 | 2021-01-15 | Recruiting | 119, NCT04746131 |
| PF-07257876 | IgG4 | Humanized anti-CD47/PD-L1 BsAb | Selected Advanced Tumors (include OC) | 1 | 90 | 2021-08-15 | Recruiting | 120, NCT04881045 |
| STI-6643 | IgG4 | Humanized anti-CD47 mAb | Advanced Solid Tumor | 1 | 24 | 2021-12-21 | Recruiting | NCT04900519 |
Abbreviations: AML, acute myeloid leukemia; CLL, chronic lymphatic leukemia; DLBCL, diffuse large B cell lymphoma; HL, Hodgkin's lymphoma; MDS, myelodysplastic syndromes; MM, multiple myeloma; NA, not available; NHL, non-Hodgkin’s lymphoma; NSCLC, non-small cell lung carcinoma; SCLC, small cell lung carcinoma; TLR7, Toll-like receptor 7.
The related references cited in Table 1 can be found in Anonymous Main Document. Some important ICIs in the table are written in bold.
Table 2.
Blocking CD47-SIRPα axis Applied in OC.
| Treatment | Agent | Target | Model | Reference |
|---|---|---|---|---|
| Monotherapy | B6H12 | CD47 | SK-OV-3 OC Cell Lines | 46 |
| Anti-47 mAb | CD47 | TOV-112D and TOV-21G EAOC Cell Lines | 87 | |
| Anti-47 mAb | CD47 | Ovarian CSCs | 87 | |
| Hu5F9-G4 | CD47 | Ovarian/Fallopian Tube Cancer | 89,90 | |
| BI 765063 | SIRPα | OC | 121 | |
| AO-176 | CD47 | Epithelial Ovarian Carcinoma | 108–110 | |
| Combined with Tumor-targeting Antibodies/Adaptive ICIs | Hu5F9-G4 + Avelumab (PD-L1) | CD47+PD-L1 | Platinum-resistant/Refractory OC | 122 |
| Bispecific Antibodies | PF-07257876 | CD47+PD-L1 | OC | NCT04881045 |
| SL-172154 | SIRPα+CD40 L | NCT04406623116 | ||
| Other Combination Therapies | Anti-CD47 mAbs + Chemotherapy + Photodynamic Therapy | CD47 | Ovarian carcinoma OVCAR-3 cell Lines | 123 |
| Dual CAR-T Cells | CD47+TAG-72 | Ovarian carcinoma OVCAR-3 cell Lines | 124 | |
| OV-αCD47 | CD47 | OC Cell Line A2780 | 125 | |
| TTI-662+ PLD | CD47 | OC | NCT05261490 |
Abbreviations: CAR-T, chimeric antigen receptor T; OV, oncolytic virus; PLD, PEGylated liposomaldoxorubicin; TAG-72, tumor-associated glycoprotein 72.
The related references cited in Table 2 can be found in Anonymous Main Document.
Blocking CD47-SIRPα Axis Monotherapy
The research onanti-CD47 mAbs in OC started later than the research on AML, ALL, NHL, breast cancer, and bladder cancer, which may be related to its complex histological types and heterogeneities. In 2012, Weissman et al found that B6H12.2 and Bric126 effectively promoted human and mouse macrophage phagocytosis of SK-OV-3 OC cells in vitro, respectively. Furthermore, tumor growth suppression and significant survival improvement were demonstrated in xenotransplantation mice.46 In 2017, Liu et al further observed increased macrophage infiltration abundance with B6H12.2 in xenograft OCs. In this study, OC stem cells (CSCs) were also found to highly express CD47, and robust phagocytosis of CSCs was induced with anti-CD47 mAbs. CSCs are known for their proliferation, recurrence, and resistance, suggesting that anti-CD47 mAbs may prevent and treat metastatic and recurrent OC.87,126 Significant inhibition of tumor cell growth, migration, and invasion in TOV OC cell lines has been observed by applying CD47 shRNA and anti-CD47 mAbs.70
BI 765063, a selective humanized-IgG4 mAb, blocks the CD47-SIRPα axis by antagonizing SIRPα. During a phase I study including nine advanced patients with OC, BI 765063 was well tolerated without reported dose-limited toxicities or hemotoxic adverse drug reactions and showed well-pharmacokinetics and efficacy (NCT04653142).121 Details are available on clinicaltrials.gov.
Blocking CD47-SIRPα Axis Therapy Combined With Tumor-Targeting Antibody
Blocking the CD47-SIRPα axis monotherapy has shown promising utilization, but the combination strategies of CD47-SIRPα inhibition may offer more therapeutic potential than that treatment.
The pro-phagocytic effects of CD47-SIRPα axis mAbs combined with the Fc-dependent effects (ADCC and ADCP) of tumor-targeting antibodies exert synergistic roles for maximum efficacy.63,83 In 2010, Cao et al combined B6H12.2 with anti-CD20 mAb (rituximab) to eliminate lymphoma in mice engrafted with primary human NHL.88 Hu5F9-G4 (Magrolimab), a humanized-IgG4 antibody, is the first CD47-targeting mAb with good clinical data to be tested in humans.90 In a subsequent phase Ib trial, Magrolimab combined with rituximab showed promising activity and well-tolerated safety events in aggressive and indolent patients with NHL (NCT02953509).127 Similarly, trastuzumab-mediated ADCP can significantly suppress ADCC-tolerant HER2+ breast cancer growth with the combination of anti-CD47 mAb.128
In the absence of specific targets for OC, the combination of targeting PD1/PD-L1 therapies is the mainstream research in this field. Blocking the CD47-SIRPα axis has been shown to simultaneously restore macrophage phagocytosis and activate T cells, indicating that combinations of innate and adaptive ICIs may yield promising results.64,81,129 In 2016, Weissman combined anti-CD47 mAb with anti-PD-L1 mAb to synergistically promote tumor cell phagocytosis in vitro, and tumor growth was suppressed in vivo, demonstrating the therapeutic synergy between innate and adaptive ICIs.130 Subsequently, a complete phase 1b trial of Hu5F9-G4 combined with avelumab (PD-L1) showed a 56% stable disease rate in 18 platinum-resistant or refractory patients with OC, which facilitated further phase II trial evaluation (NCT03558139).122 AO-176 (NCT03834948),108 TJ011133 (NCT03934814),113 and TTI-621 (NCT02663518)96 in combination with various PD-1/PD-L1 ICIs are now in different trial stages.
Blocking CD47-SIRPα Axis With Bispecific Antibody
Bispecific antibodies (BsAbs) have been developed based on combination therapy with targeting-tumor antibodies, which are expected to be more effective and precise. The BsAbs skeleton contains two binding arms, one arm blocks the CD47-SIRPα axis, and the other arm binds tumor-specific antigens, which ensures that BsAbs specifically kill cancer cells.
Majeti et al constructed a BsAb targeting CD47 and CD20 arms with less affinity for CD47 and more affinity for CD20 than the parental antibodies. Decreased tumor burden and prolonged survival in human NHL-engrafted mice were observed with BsAb; more importantly, less blood cell destruction occurred in the BsAb group than in the combination therapy group.119 IMM0306, the related agent, is in the recruitment stage (NCT04746131).
Similar to combination therapy, BsAbs were designed to co-target CD47/SIRPα and PD-1/PD-L1 in OC. PF-07257876, a dual checkpoint inhibiting BsAb, simultaneously blocks CD47 and PD-L1 to maximize anti-tumor immunity. The preferential targeting of tumor cells in the TME is attributed to its high affinity for PD-L1 and weak affinity for CD47. It can additionally activate DCs and macrophages and increase CD8+ T cells in the TME.119 In a phase I dose escalation and expansion study of PF-07257876, patients with OC tolerated the priming and subsequent dose (NCT04881045).
Other Combination Therapy
The strategy of anti-CD47/SIRPα monotherapy combined with chemotherapy relies on a pro-phagocytosis effect rather than an Fc-dependent effect. Almost all chemotherapies can cause inflammatory reactions and further increase macrophage infiltration in the TME.131 As aforementioned, CRT is an essential pro-phagocytic signal, and some chemotherapy drugs, such as anthracyclines, can induce additional CRT translocation to the cell surface, which might enhance the activity of anti-CD47 mAbs. Notably, normal cells also highly express CRT with these chemotherapies, suggesting that this strategy may increase toxicity.60,78 Hongrapipat et al. found that the combination of chemotherapy and photodynamic therapy drugs with the Fab’ segment of anti-CD47 antibody might improve the reactivity of OC OVCAR-3 cells to drugs.123
CAR-T cells have revolutionized anti-tumor therapy for hematological malignancies but have shown disappointing activity in most solid tumors.132 Shu et al generated dual CAR-T cells co-targeting CD47 and TAG-72. TAG-72 is an aberrantly glycosylated glycoprotein overexpressed in adenocarcinomas, including ovarian, colorectal, and gastric adenocarcinomas. These dual CAR-T cells effectively eliminated OVCAR-3 cells in vitro and suppressed tumor growth in OC xenografted mice. Furthermore, the damage to normal tissues was reduced owing to the specific targeting of TAG-72.124 The ability of oncolytic virus (OV) to infiltrate drugs into the TME makes it an attractive alternative for anti-tumor therapy.133 Researchers constructed OV-αCD47, which delivered anti-CD47 mAb. OV-αCD47, especially those with the IgG1 skeleton, prolonged survival in OC-engrafted mice by enhancing innate immunity (NK cell cytotoxicity and macrophage phagocytosis) and performing an oncolytic function.125
Most of these studies are in the preclinical stage, and additional efforts are warranted to promote the early application of research results to clinics.
Limitations and Future Perspectives
Although preclinical data on CD47-SIRPα axis blockade has highlighted the promising therapeutic potential of blocking the “don’t eat me” signal to recover phagocytosis, the development of related drugs is not all plain sailing. The most common AE of anti-CD47 mAbs is the off-target effect caused by widespread expression of CD47 in normal cells, among which hematotoxicity is the most intractable.
As early as 2017, the phase 1 clinical trial of Ti-061(CD47 mAb) was prematurely terminated owing to unexpected case deaths.134 Subsequently, CD47 mAb CC-90002 was declared to have failed the phase I clinical trial (NCT02641002) owing to severe hemagglutination.92 The repetitive failures have resulted in the uncertain future of such drug development. The superior phase 1b results of Magrolimab in combination with azacitidine in patients with MDS and AML promoted the enthusiasm for CD47-targeted drug development to a high priority until 2019. A low priming dose (1 mg/kg) was used to remove the aged erythrocytes and induce compensatory hematopoiesis to overcome anemia. Moreover, newborn erythrocytes can tolerate the maintenance (30 mg/kg) dose.127,135 Unfortunately, the FDA suspended some clinical trials (i.e., NCT04313881) of the Magrolimab in combination with azacitidine owing to apparent imbalances of suspected unexpected serious adverse reactions across study arms in January 2022. However, most experts have posited that these SUSARs may be more related to the hematotoxicity of Magrolimab or the additive toxicity of azacitidine than to affecting the overall CD47 mAbs. As expected, the FDA quickly lifted the restrictions after reviewing the combined safety data from each trial in April. Pharmaceutical companies have different attitudes toward CD47-targeted drugs. The recent discontinuation of several CD47-targeting programs by AbbVie and Zai Lab has been attributed to strategic reasons rather than safety concerns. By contrast, Innovent and Akesobio are actively advancing CD47-related projects.
In summary, within controlled AEs, CD47-targeted drugs remain the focus of tumor immunotherapy. Notably, strategies have been developed to overcome these limitations, such as anti-SIRPα mAbs, SIRPα-fusion proteins, BsAbs, dual ICIs BsAbs, dual CAR-T cells, and OC-CD47. Alternatively, researchers have engineered TAX2, the first-ever antagonist of the TSP-1/CD47 axis. TAX2 selectively targets tumor-overexpressed TSP-1 and inhibits tumor angiogenesis while activating the anti-tumor immune system without destroying blood cells.136
Although blocking the CD47-SIRPα axis has shown promising activities in OC preclinical models, based on the experience with PD-1/PD-L1,137 this novel therapy might have use as an adjunctive therapy or in combination with other therapies to maximize efficacy. In the future, with the disclosure of the complete upstream and downstream pathways of the CD47-SIRPα axis, more potent blocking CD47-SIRPα axis therapies with fewer side effects will be certain to the clinical application of OC treatment.
Conclusion
In conclusion,CD47-SIRPα axis plays a notable role in inhibiting macrophage phagocytosis, promoting tumors to evade immune surveillance through sending “don’t eat me” anti-phagocytic signals. Therapeutic approaches that inhibit the CD47-SIRPα axis are novel, promising treatments for OC. However, the limitations of these therapies cannot be ignored, and further studies and clinical trials are necessary for continuous improvement.
Appendix.
Abbreviation
- ADCC
antibody-dependent cytotoxicity
- ADCP
antibody-dependent phagocytosis
- ALL
acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- APCs
antigen-presenting cells
- BsAbs
bispecific antibodies
- CAR-T
chimeric antigen receptor T
- CD47
cluster of differentiation 47
- CDT
China drug trials
- CLL
chronic lymphatic leukemia
- CML
chronic myeloid leukemia
- CRT
calreticulin
- CSC
cell stem cell
- CTLA-4
cytotoxic T-lymphocyte-associated protein-4
- DCs
dendritic cells
- DLBCL
diffuse large B cell lymphoma
- Fc
fragment crystallizable
- FDA
Food and Drug Administration
- FIGO
Federation International of Gynecology and Obstetrics
- HER2
human epidermal growth factor receptor 2
- HIF-1
hypoxia-inducible factor-1
- HL
Hodgkin’s lymphoma
- ICIs
immune checkpoint inhibitors
- ICs
immune checkpoints
- IgG
immunoglobulin G
- IHC
immunohistochemistry
- IL-1β
interleukin-1β
- ITIM
immunoreceptor tyrosine inhibitory motif
- M1
M1-like macrophage
- M2
M2-like macrophage
- mAbs
monoclonal antibodies
- MDS
myelodysplastic syndromes
- MM
multiple myeloma
- NA
not available
- NCT
national clinical trials
- NF-κB
nuclear factor kappa-B
- NHL
non-Hodgkin’s lymphoma
- NK
cells natural killer cells
- NSCLC
non-small cell lung carcinoma
- OC
ovarian cancer
- OS
overall survival
- OV
oncolytic virus
- PD-1
programmed cell death protein-1
- PD-L1
programmed cell death ligand-1
- PtdSer
phosphatidylserine
- PLD
pegylatedliposomaldoxorubicin
- SCLC
small cell lung carcinoma
- SHP-1
Src homology 2 containing tyrosine phosphatase 1
- SHP-2
Src homology 2 containing tyrosine phosphatase 2
- SIRPα
signal regulatory protein α
- SIRPγ
signal regulatory protein γ
- SLAMF
signaling lymphocyte activation molecule family
- TAG-72
tumor-associated glycoprotein 72
- TAMs
tumor-associated macrophages
- TCR
T cells receptor
- TME
tumor immune microenvironment
- TNF-α
tumor necrosis factor α
- TSP-1
thrombospondin-1
- TLR7
Toll-like receptor 7
- Treg cells
regulatory T cells
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Obstetrics and Gynecology Hospital of Fudan University.
Ethical Approval: All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ORCID iD
Xukai Luo https://orcid.org/0000-0001-7754-9710
References
- 1.Wang YY, Lee KT, Lim MC, Choi JH. TRPV1 antagonist DWP05195 induces ER stress-dependent apoptosis through the ROS-p38-CHOP pathway in human ovarian cancer cells. Cancers. 2020;12(6):1702. doi: 10.3390/cancers12061702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin LA, Huang B, Miller RW, et al. Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol. 2012;120(3):612-618. doi: 10.1097/AOG.0b013e318264f794 [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics. CA Cancer J Clin. 2012;65(2):87-108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 4.Krishnan V, Schaar B, Tallapragada S, Dorigo O. Tumor associated macrophages in gynecologic cancers. Gynecol Oncol. 2018;149(1):205-213. doi: 10.1016/j.ygyno.2018.01.014 [DOI] [PubMed] [Google Scholar]
- 5.Yang Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J Clin Invest. 2015;125(9):3335-3337. doi: 10.1172/JCI83871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galluzzi L, Humeau J, Buque A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17(12):725-741. doi: 10.1038/s41571-020-0413-z [DOI] [PubMed] [Google Scholar]
- 7.Sharma P, Siddiqui BA, Anandhan S, et al. The next decade of immune checkpoint therapy. Cancer Discov. 2021;11(4):838-857. doi: 10.1158/2159-8290.CD-20-1680 [DOI] [PubMed] [Google Scholar]
- 8.Tan AC, Bagley SJ, Wen PY, et al. Systematic review of combinations of targeted or immunotherapy in advanced solid tumors. J Immunother Cancer. 2021;9(7):e002459. doi: 10.1136/jitc-2021-002459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: A novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27(3):409-416. [DOI] [PubMed] [Google Scholar]
- 10.Burugu S, Dancsok AR, Nielsen TO. Emerging targets in cancer immunotherapy. Semin Cancer Biol. 2018;52(Pt 2):39-52. doi: 10.1016/j.semcancer.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Liu L, Ren Z, et al. Dual targeting of innate and adaptive checkpoints on tumor cells limits immune evasion. Cell Rep. 2018;24(8):2101-2111. doi: 10.1016/j.celrep.2018.07.062 [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: Past, present, and future. J Clin Invest. 2015;125(9):3384-3391. doi: 10.1172/JCI80011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064-5074. doi: 10.1158/1078-0432.CCR-13-3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nandi D, Pathak S, Verma T, et al. T cell costimulation, checkpoint inhibitors and anti-tumor therapy. J Biosci. 2020;45(1):50. doi: 10.1007/s12038-020-0020-2 [DOI] [PubMed] [Google Scholar]
- 15.Chao MP, Weissman IL, Majeti R. The CD47-SIRPα pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24(2):225-232. doi: 10.1016/j.coi.2012.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao XW, Matlung HL, Kuijpers TW, van den Berg TK. On the mechanism of CD47 targeting in cancer. Proc Natl Acad Sci U S A. 2012;109(42):E2844-E2845. doi: 10.1073/pnas.1209265109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515-548. doi: 10.1146/annurev.immunol.23.021704.115611 [DOI] [PubMed] [Google Scholar]
- 18.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467-477. doi: 10.1038/nri2326 [DOI] [PubMed] [Google Scholar]
- 19.Seliger B, Quandt D. The expression, function, and clinical relevance of B7 family members in cancer. Cancer Immunol Immunother. 2012;61(8):1327-1341. doi: 10.1007/s00262-012-1293-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182(2):459-465. doi: 10.1084/jem.182.2.459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734-1736. doi: 10.1126/science.271.5256.1734 [DOI] [PubMed] [Google Scholar]
- 22.Graziani G, Lisi L, Tentori L, Navarra P. Monoclonal antibodies to CTLA-4 with focus on ipilimumab. Exp Suppl. 2022;113:295-350. doi: 10.1007/978-3-030-91311-3_10 [DOI] [PubMed] [Google Scholar]
- 23.Davies MA, Flaherty KT. Melanoma in 2017: Moving treatments earlier to move further forwards. Nat Rev Clin Oncol. 2018;15(2):75-76. doi: 10.1038/nrclinonc.2017.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206(8):1717-1725. doi: 10.1084/jem.20082492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, costimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365-1369. [DOI] [PubMed] [Google Scholar]
- 26.Hino R, Kabashima K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116(7):1757-1766. doi: 10.1002/cncr.24899 [DOI] [PubMed] [Google Scholar]
- 27.Shimoji M, Shimizu S, Sato K, et al. Clinical and pathologic features of lung cancer expressing programmed cell death ligand 1 (PD-L1). Lung Cancer. 2016;98:69-75. doi: 10.1016/j.lungcan.2016.04.021 [DOI] [PubMed] [Google Scholar]
- 28.de Guillebon E, Roussille P, Frouin E, Tougeron D. Anti program death-1/anti program death-ligand 1 in digestive cancers. World J Gastrointest Oncol. 2015;7(8):95-101. doi: 10.4251/wjgo.v7.i8.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015-4022. doi: 10.1200/JCO.2015.62.3397 [DOI] [PubMed] [Google Scholar]
- 30.Abdel-Rahman O. PD-L1 expression and outcome of advanced melanoma patients treated with anti-PD-1/PD-L1 agents: A meta-analysis. Immunotherapy. 2016;8(9):1081-1089. [DOI] [PubMed] [Google Scholar]
- 31.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Normann MC, Turzer M, Diep LM, et al. Early experiences with PD-1 inhibitor treatment of platinum resistant epithelial ovarian cancer. J Gynecol Oncol. 2019;30(4):e56. doi: 10.3802/jgo.2019.30.e56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varga A, Piha-Paul S, Ott PA, et al. Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: Analysis of KEYNOTE-028. Gynecol Oncol. 2019;152(2):243-250. doi: 10.1016/j.ygyno.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 35.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 Inhibition. N Engl J Med. 2017;377(25):2500-2501. doi: 10.1056/NEJMc1713444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: Skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19(3):345-361. doi: 10.1007/s40257-017-0336-3 [DOI] [PubMed] [Google Scholar]
- 37.Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20(1):25-39. doi: 10.1038/s41577-019-0218-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lv B, Wang Y, Ma D, et al. Immunotherapy: Reshape the tumor immune microenvironment. Front Immunol. 2022;13:844142. doi: 10.3389/fimmu.2022.844142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9(1):57-63. [DOI] [PubMed] [Google Scholar]
- 40.Brown E, Hooper L, Ho T, Gresham H. Integrin-associated protein: A 50-kD plasma membrane antigen physically and functionally associated with integrins. J Cell Biol. 1990;111(6 Pt 1):2785-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051-2054. doi: 10.1126/science.288.5473.2051 [DOI] [PubMed] [Google Scholar]
- 42.Burger P, Hilarius-Stokman P, de Korte D, van den Berg TK, van Bruggen R. CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood. 2012;119(23):5512-5521. doi: 10.1182/blood-2011-10-386805 [DOI] [PubMed] [Google Scholar]
- 43.Lindberg FP, Gresham HD, Schwarz E, Brown EJ. Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in alpha v beta 3-dependent ligand binding. J Cell Biol. 1993;123(2):485-496. doi: 10.1083/jcb.123.2.485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mawby WJ, Holmes CH, Anstee DJ, Spring FA, Tanner MJ. Isolation and characterization of CD47 glycoprotein: a multispanning membrane protein which is the same as integrin-associated protein (IAP) and the ovarian tumour marker OA3. Biochem J. 1994;304 ( Pt 2)(2):525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown EJ, Frazier WA. Integrin-associated protein (cd47) and its ligands. Trends Cell Biol. 2001;11(3):130-135. doi: 10.1016/s0962-8924(00)01906-1 [DOI] [PubMed] [Google Scholar]
- 46.Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109(17):6662-6667. doi: 10.1073/pnas.1121623109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol. 2009;19(2):72-80. doi: 10.1016/j.tcb.2008.12.001 [DOI] [PubMed] [Google Scholar]
- 48.Kale A, Rogers NM, Ghimire K. Thrombospondin-1 CD47 Signalling: From mechanisms to medicine. Int J Mol Sci. 2021;22(8):4062. doi: 10.3390/ijms22084062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaur S, Bronson SM, Pal-Nath D, Miller TW, Soto-Pantoja DR, Roberts DD. Functions of thrombospondin-1 in the tumor microenvironment. Int J Mol Sci. 2021;22(9):4570. doi: 10.3390/ijms22094570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller TW, Kaur S, Ivins-O'Keefe K, Roberts DD. Thrombospondin-1 is a CD47-dependent endogenous inhibitor of hydrogen sulfide signaling in T cell activation. Matrix Biol. 2013;32(6):316-324. doi: 10.1016/j.matbio.2013.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stein EV, Miller TW, Ivins-O'Keefe K, Kaur S, Roberts DD. Secreted thrombospondin-1 regulates macrophage interleukin-1β production and activation through CD47. Sci Rep. 2016;6:19684. doi: 10.1038/srep19684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaur S, Roberts DD. Divergent modulation of normal and neoplastic stem cells by thrombospondin-1 and CD47 signaling. Int J Biochem Cell Biol. 2016;81(Pt A):184-194. doi: 10.1016/j.biocel.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaur S, Schwartz AL, Miller TW, Roberts DD. CD47-dependent regulation of H-S biosynthesis and signaling in T cells. Methods Enzymol. 2015;555:145-168. doi: 10.1016/bs.mie.2014.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopez-Dee Z, Pidcock K, Gutierrez LS. Thrombospondin-1: multiple paths to inflammation. Mediators Inflamm. 2011;2011:296069. doi: 10.1155/2011/296069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161(3):831-839. doi: 10.1016/s0002-9440(10)64243-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J Biol Chem. 2010;285(50):38923-38932. doi: 10.1074/jbc.M110.172304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kazerounian S, Yee KO, Lawler J. Thrombospondins in cancer. Cell Mol Life Sci. 2008;65(5):700-712. doi: 10.1007/s00018-007-7486-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X, Kwon H, Li Z, Fu YX. Is CD47 an innate immune checkpoint for tumor evasion? J Hematol Oncol. 2017;10(1):12. doi: 10.1186/s13045-016-0381-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6(6):457-464. doi: 10.1038/nri1859 [DOI] [PubMed] [Google Scholar]
- 60.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54-61. doi: 10.1038/nm1523 [DOI] [PubMed] [Google Scholar]
- 61.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386(6621):181-186. [DOI] [PubMed] [Google Scholar]
- 62.Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, Weissman IL. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer. 2019;19(10):568-586. doi: 10.1038/s41568-019-0183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Logtenberg MEW, Scheeren FA, Schumacher TN. The CD47-SIRPα immune checkpoint. Immunity. 2020;52(5):742-752. doi: 10.1016/j.immuni.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Casey SC, Tong L, Li Y, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352(6282):227-231. doi: 10.1126/science.aac9935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H, Lu H, Xiang L, et al. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci U S A. 2015;112(45):E6215-E6223. doi: 10.1073/pnas.1520032112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jia X, Yan B, Tian X, et al. CD47/SIRPα pathway mediates cancer immune escape and immunotherapy. Int J Biol Sci. 2021;17(13):3281-3287. doi: 10.7150/ijbs.60782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Betancur PA, Abraham BJ, Yiu YY, et al. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat Commun. 2017;8:14802. doi: 10.1038/ncomms14802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shimizu A, Sawada K, Kobayashi M, et al. Exosomal CD47 plays an essential role in immune evasion in ovarian cancer. Mol Cancer Res. 2021;19(9):1583-1595. doi: 10.1158/1541-7786.MCR-20-0956 [DOI] [PubMed] [Google Scholar]
- 69.Jiang Z, Sun H, Yu J, Tian W, Song Y. Targeting CD47 for cancer immunotherapy. J Hematol Oncol. 2021;14(1):180. doi: 10.1186/s13045-021-01197-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang CL, Lin MJ, Hsu CY, et al. CD47 promotes cell growth and motility in epithelial ovarian cancer. Biomed Pharmacother. 2019;119:109105. doi: 10.1016/j.biopha.2019.109105 [DOI] [PubMed] [Google Scholar]
- 71.Wang H, Tan M, Zhang S, et al. Expression and significance of CD44, CD47 and c-met in ovarian clear cell carcinoma. Int J Mol Sci. 2015;16(2):3391-3404. doi: 10.3390/ijms16023391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tan M, Zhuang H, Zhu L, et al. Lewis Y antigen modified CD47 is an independent risk factor for poor prognosis and promotes early ovarian cancer metastasis. Am J Cancer Res. 2015;5(9):2777-2787. [PMC free article] [PubMed] [Google Scholar]
- 73.Brightwell RM, Grzankowski KS, Lele S, et al. The CD47 “don't eat me signal” is highly expressed in human ovarian cancer. Gynecol Oncol. 2016;143(2):393-397. doi: 10.1016/j.ygyno.2016.08.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen C, Wang R, Chen X, Hou Y, Jiang J. Targeting CD47 as a Novel Immunotherapy for Breast Cancer. Front Oncol. 2022;12:924740. doi: 10.3389/fonc.2022.924740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138(2):286-299. doi: 10.1016/j.cell.2009.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feng R, Zhao H, Xu J, Shen C. CD47: The next checkpoint target for cancer immunotherapy. Crit Rev Oncol Hematol. 2020;152:103014. doi: 10.1016/j.critrevonc.2020.103014 [DOI] [PubMed] [Google Scholar]
- 77.Zhang M, Hutter G, Kahn SA, et al. Anti-CD47 treatment stimulates phagocytosis of glioblastoma by M1 and M2 polarized macrophages and promotes M1 polarized macrophages in Vivo. PLoS One. 2016;11(4):e0153550. doi: 10.1371/journal.pone.0153550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chao MP, Jaiswal S, Weissman-Tsukamoto R, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2(63):63ra94. doi: 10.1126/scitranslmed.3001375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lecoultre M, Dutoit V, Walker PR. Phagocytic function of tumor-associated macrophages as a key determinant of tumor progression control: A review. J Immunother Cancer. 2020;8(2):e001408. doi: 10.1136/jitc-2020-001408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang Y, Lv SQ, Liu PY, et al. A SIRPα-Fc fusion protein enhances the antitumor effect of oncolytic adenovirus against ovarian cancer. Mol Oncol. 2020;14(3):657-668. doi: 10.1002/1878-0261.12628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tseng D, Volkmer JP, Willingham SB, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A. 2013;110(27):11103-11108. doi: 10.1073/pnas.1305569110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Demeure CE, Tanaka H, Mateo V, Rubio M, Delespesse G, Sarfati M. CD47 engagement inhibits cytokine production and maturation of human dendritic cells. J Immunol. 2000;164(4):2193-2199. doi: 10.4049/jimmunol.164.4.2193 [DOI] [PubMed] [Google Scholar]
- 83.Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPα) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014;32:25-50. doi: 10.1146/annurev-immunol-032713-120142 [DOI] [PubMed] [Google Scholar]
- 84.Liu X, Pu Y, Cron K, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21(10):1209-1215. doi: 10.1038/nm.3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Manna PP, Frazier WA. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res. 2004;64(3):1026-1036. [DOI] [PubMed] [Google Scholar]
- 86.Mateo V, Lagneaux L, Bron D, et al. CD47 ligation induces caspase-independent cell death in chronic lymphocytic leukemia. Nat Med. 1999;5(11):1277-1284. [DOI] [PubMed] [Google Scholar]
- 87.Liu R, Wei H, Gao P, et al. CD47 promotes ovarian cancer progression by inhibiting macrophage phagocytosis. Oncotarget. 2017;8(24):39021-39032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chao MP, Alizadeh AA, Tang C, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142(5):699-713. doi: 10.1016/j.cell.2010.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu J, Wang L, Zhao F, et al. Pre-clinical development of a humanized Anti-CD47 antibody with Anti-Cancer therapeutic potential. PLoS One. 2015;10(9):e0137345. doi: 10.1371/journal.pone.0137345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sikic BI, Lakhani N, Patnaik A, et al. First-in-human, first-in-class phase I trial of the Anti-CD47 antibody HU5F9-G4 in patients with advanced cancers. J Clin Oncol. 2019;37(12):946-953. doi: 10.1200/JCO.18.02018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brierley CK, Staves J, Roberts C, et al. The effects of monoclonal anti-CD47 on RBCs, compatibility testing, and transfusion requirements in refractory acute myeloid leukemia. Transfusion. 2019;59(7):2248-2254. doi: 10.1111/trf.15397 [DOI] [PubMed] [Google Scholar]
- 92.Narla RK, Modi H, Bauer D, et al. Modulation of CD47-SIRPα innate immune checkpoint axis with Fc-function detuned anti-CD47 therapeutic antibody. Cancer Immunol Immunother. 2022;71(2):473-489. doi: 10.1007/s00262-021-03010-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Petrova PS, Viller NN, Wong M, et al. TTI-621 (SIRPαFc) a CD47-blocking innate immune checkpoint inhibitor with broad antitumor activity and minimal erythrocyte binding. Clin Cancer Res. 2017;23(4):1068-1079. doi: 10.1158/1078-0432.CCR-16-1700 [DOI] [PubMed] [Google Scholar]
- 94.Lin GHY, Chai V, Lee V, et al. TTI-621 (SIRPαFc), a CD47-blocking cancer immunotherapeutic, triggers phagocytosis of lymphoma cells by multiple polarized macrophage subsets. PLoS One. 2017;12(10):e0187262. doi: 10.1371/journal.pone.0187262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johnson LDS, Banerjee S, Kruglov O, et al. Targeting CD47 in Sézary syndrome with SIRPαFc. Blood Adv. 2019;3(7):1145-1153. doi: 10.1182/bloodadvances.2018030577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ansell SM, Maris MB, Lesokhin AM, et al. Phase I study of the CD47 blocker TTI-621 in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2021;27(8):2190-2199. doi: 10.1158/1078-0432.CCR-20-3706 [DOI] [PubMed] [Google Scholar]
- 97.Kruglov O, Johnson LDS, Minic A, et al. The pivotal role of cytotoxic NK cells in mediating the therapeutic effect of anti-CD47 therapy in mycosis fungoides. Cancer Immunol Immunother. 2022;71(4):919-932. doi: 10.1007/s00262-021-03051-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Querfeld C, Thompson JA, Taylor MH, et al. Intralesional TTI-621, a novel biologic targeting the innate immune checkpoint CD47, in patients with relapsed or refractory mycosis fungoides or Sézary syndrome: A multicentre, phase 1 study. Lancet Haematol. 2021;8(11):e808-e817. doi: 10.1016/s2352-3026(21)00271-4 [DOI] [PubMed] [Google Scholar]
- 99.Lin GHY, Viller NN, Chabonneau M, et al. Abstract 2709: TTI-622 (SIRPα-IgG4 Fc), a CD47-blocking innate immune checkpoint inhibitor, suppresses tumor growth and demonstrates enhanced efficacy in combination with antitumor antibodies in both hematologic and solid tumor models. Cancer Res. 2018;78(13_suppl):2709. [Google Scholar]
- 100.Kauder SE, Kuo TC, Harrabi O, et al. ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLoS One. 2018;13(8):e0201832. doi: 10.1371/journal.pone.0201832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lakhani NJ, Chow LQM, Gainor JF, et al. Evorpacept alone and in combination with pembrolizumab or trastuzumab in patients with advanced solid tumours (ASPEN-01): A first-in-human, open-label, multicentre, phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2021;22(12):1740-1751. doi: 10.1016/s1470-2045(21)00584-2 [DOI] [PubMed] [Google Scholar]
- 102.Evans TJ, Italiano A, Eskens F, et al. Phase 1-2 study of TI-061 alone and in combination with other anti-cancer agents in patients with advanced malignancies. J Clin Oncol. 2017;35(15_suppl l):TPS3109-TPS3109. doi: 10.1200/JCO.2017.35.15_suppl.TPS3109 [DOI] [Google Scholar]
- 103.Peluso MO, Adam A, Armet CM, et al. The Fully human anti-CD47 antibody SRF231 exerts dual-mechanism antitumor activity via engagement of the activating receptor CD32a. J Immunother Cancer. 2020;8(1):e000413. doi: 10.1136/jitc-2019-000413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patnaik A, Spreafico A, Paterson AM, et al. Results of a first-in-human phase I study of SRF231, a fully human, high-affinity anti-CD47 antibody. J Clin Oncol. 2020;38(15_suppl l):3064-3064. doi: 10.1200/JCO.2020.38.15_suppl.3064 [DOI] [Google Scholar]
- 105.Gao Y, Zhang D, Yang C, Duan X, Li X, Zhong D. Two validated liquid chromatography-mass spectrometry methods with different pretreatments for the quantification of an anti-CD47 monoclonal antibody in rat and cynomolgus monkey serum compared with an electrochemiluminescence method. J Pharm Biomed Anal. 2019;175:112792. doi: 10.1016/j.jpba.2019.112792 [DOI] [PubMed] [Google Scholar]
- 106.Ni H, Cao L, Wu Z, et al. Combined strategies for effective cancer immunotherapy with a novel anti-CD47 monoclonal antibody. Cancer Immunol Immunother. 2022;71(2):353-363. doi: 10.1007/s00262-021-02989-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Y, Ni H, Zhou S, et al. Tumor-selective blockade of CD47 signaling with a CD47/PD-L1 bispecific antibody for enhanced anti-tumor activity and limited toxicity. Cancer Immunol Immunother. 2021;70(2):365-376. doi: 10.1007/s00262-020-02679-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Andrejeva G, Capoccia BJ, Hiebsch RR, et al. Novel SIRPα antibodies that induce single-agent phagocytosis of tumor cells while preserving T Cells. J Immunol. 2021;206(4):712-721. doi: 10.4049/jimmunol.2001019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Puro RJ, Bouchlaka MN, Hiebsch RR, et al. Development of AO-176, a next-generation humanized anti-CD47 antibody with novel anticancer properties and negligible red blood cell binding. Mol Cancer Ther. 2020;19(3):835-846. doi: 10.1158/1535-7163.MCT-19-1079 [DOI] [PubMed] [Google Scholar]
- 110.Burris III HA, Spira AI, Taylor MH, et al. A first-in-human study of AO-176, a highly differentiated anti-CD47 antibody, in patients with advanced solid tumors. J Clin Oncol. 2021;39(15_suppl l):2516-2516. doi: 10.1200/JCO.2021.39.15_suppl.251634101483 [DOI] [Google Scholar]
- 111.Meng Z, Wang Z, Guo B, Cao W, Shen H. TJC4, a differentiated anti-CD47 antibody with novel epitope and RBC sparing properties. Blood. 2019;134(suppl ment_1):4063-4063. doi: 10.1182/blood-2019-122793 [DOI] [Google Scholar]
- 112.Mehta A, Harb W, Xu C, et al. Lemzoparlimab, a differentiated anti-CD47 antibody in combination with rituximab in relapsed and refractory non-hodgkin’s lymphoma: Initial clinical results. Blood. 2021;138(suppl 1):3542-3542. doi: 10.1182/blood-2021-150606 [DOI] [Google Scholar]
- 113.Qi J, Li J, Jiang B, et al. A phase i/iia study of Lemzoparlimab, a monoclonal antibody targeting CD47, in patients with relapsed and/or refractory acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS): Initial phase I results. Blood. 2020;136(suppl 1):30-31. [Google Scholar]
- 114.Sun M, Qi J, Zheng W, et al. Preliminary results of a first-in-human phase I dtudy of IMM01, SIRPα Fc protein in patients with relapsed or refractory lymphoma. J Clin Oncol. 2021;39(15_suppl l):2550-2550. doi: 10.1200/JCO.2021.39.15_suppl.2550 [DOI] [Google Scholar]
- 115.Gan HK, Coward J, Mislang ARA, et al. Safety of AK117, an anti-CD47 monoclonal antibody, in patients with advanced or metastatic solid tumors in a phase I study. J Clin Oncol. 2021;39(15_suppl l):2630-2630. doi: 10.1200/JCO.2021.39.15_suppl.2630 [DOI] [Google Scholar]
- 116.Lakhani N, Richardson D, Kristedja T, et al. 429 Phase 1 dose escalation study of the agonist redirected checkpoint, SL-172154 (SIRPα-Fc-CD40L) in subjects with platinum-resistant ovarian cancer. J Immunother Cancer. 2021;9(suppl 2):A459-A459. doi: 10.1136/jitc-2021-SITC2021.429 [DOI] [Google Scholar]
- 117.Cendrowicz E, Jacob L, Greenwald S, et al. DSP107 combines inhibition of CD47/SIRPα axis with activation of 4-1BB to trigger anticancer immunity. J Exp Clin Cancer Res. 2022;41(1):4197. doi: 10.1186/s13046-022-02256-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ni K, Luo T, Culbert A, Kaufmann M, Jiang X, Lin W. Correction to nanoscale metal-organic framework co-delivers TLR-7 agonists and anti-CD47 antibodies to modulate macrophages and orchestrate cancer immunotherapy. J Am Chem Soc. 2022;144(34):15907. doi: 10.1021/jacs.2c07990 [DOI] [PubMed] [Google Scholar]
- 119.Piccione EC, Juarez S, Liu J, et al. A bispecific antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells. MAbs. 2015;7(5):946-956. doi: 10.1080/19420862.2015.1062192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen SH, Dominik PK, Stanfield J, et al. Dual checkpoint blockade of CD47 and PD-L1 using an affinity-tuned bispecific antibody maximizes antitumor immunity. J Immunother Cancer. 2021;9(10):e003464. doi: 10.1136/jitc-2021-003464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Champiat S, Cassier PA, Kotecki N, et al. Safety, pharmacokinetics, efficacy, and preliminary biomarker data of first-in-class BI 765063, a selective SIRPα inhibitor: Results of monotherapy dose escalation in phase 1 study in patients with advanced solid tumors. J Clin Oncol. 2021;39(15_suppl l):2623-2623. doi: 10.1200/JCO.2021.39.15_suppl.2623 [DOI] [Google Scholar]
- 122.Lakhani NJ, Patnaik A, Liao JB, et al. A phase Ib study of the anti-CD47 antibody magrolimab with the PD-L1 inhibitor avelumab (A) in solid tumor (ST) and ovarian cancer (OC) patients. J Clin Oncol. 2020;38(5_suppl l):18-18. doi: 10.1200/JCO.2020.38.5_suppl.18 [DOI] [Google Scholar]
- 123.Hongrapipat J, Kopecˇkova P, Liu J, Prakongpan S, Kopecek J. Combination chemotherapy and photodynamic therapy with Fab’ fragment targeted hpma copolymer conjugates in human ovarian carcinoma cells. Mol Pharm. 2008;5(5):696-709. doi: 10.1021/mp800006e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shu R, Evtimov VJ, Hammett MV, et al. Engineered CAR-T cells targeting TAG-72 and CD47 in ovarian cancer. Mol Ther Oncolytics. 2021;20:325-341. doi: 10.1016/j.omto.2021.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tian L, Xu B, Teng KY, et al. Targeting Fc receptor-mediated effects and the “don’t eat me” signal with an oncolytic virus expressing an anti-CD47 antibody to treat metastatic ovarian cancer. Clin Cancer Res. 2022;28(1):201-214. doi: 10.1158/1078-0432.CCR-21-1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang Y, Ju B, Tian J, et al. Ovarian cancer stem cell-specific gene expression profiling and targeted drug prescreening. Oncol Rep. 2014;31(3):1235-1248. doi: 10.3892/or.2014.2976 [DOI] [PubMed] [Google Scholar]
- 127.Advani R, Flinn I, Popplewell L, et al. CD47 Blockade by Hu5F9-G4 and rituximab in non-hodgkin’s lymphoma. N Engl J Med. 2018;379(18):1711-1721. doi: 10.1056/NEJMoa1807315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Upton R, Banuelos A, Feng D, et al. Combining CD47 blockade with trastuzumab eliminates HER2-positive breast cancer cells and overcomes trastuzumab tolerance. Proc Natl Acad Sci U S A. 2021;118(29):e2026849118. doi: 10.1073/pnas.2026849118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495-499. doi: 10.1038/nature22396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sockolosky JT, Dougan M, Ingram JR, et al. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci U S A. 2016;113(19):E2646-E2654. doi: 10.1073/pnas.1604268113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Le T, Williams K, Senterman M, Hopkins L, Faught W, Fung-Kee-Fung M. Histopathologic assessment of chemotherapy effects in epithelial ovarian cancer patients treated with neoadjuvant chemotherapy and delayed primary surgical debulking. Gynecol Oncol. 2007;106(1):160-163. doi: 10.1016/j.ygyno.2007.03.029 [DOI] [PubMed] [Google Scholar]
- 132.Sadelain M, Riviere I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545(7655):423-431. doi: 10.1038/nature22395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xu B, Ma R, Russell L, et al. An oncolytic herpesvirus expressing E-cadherin improves survival in mouse models of glioblastoma. Nat Biotechnol. 2018;37:45-54. doi: 10.1038/nbt.4302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Evans TJ, Italiano A, Eskens F, et al. Phase 1-2 study of TI-061 alone and in combination with other anti-cancer agents in patients with advanced malignancies. J Clin Oncol. 2017;35(15):TPS3109. doi: 10.1200/JCO.2017.35.15_supp [DOI] [Google Scholar]
- 135.Sallman DA, Donnellan WB, Asch AS, et al. The first-in-class anti-CD47 antibody Hu5F9-G4 is active and well tolerated alone or with azacitidine in AML and MDS patients: Initial phase 1b results. J Clin Oncol. 2019;37(15_suppl l):7009-7009. doi: 10.1200/JCO.2019.37.15_suppl.7009 [DOI] [Google Scholar]
- 136.Jeanne A, Sarazin T, Charle M, et al. Targeting ovarian carcinoma with TSP-1:CD47 antagonist TAX2 activates Anti-Tumor immunity. Cancers. 2021;13(19):5019. doi: 10.3390/cancers13195019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375(18):1767-1778. doi: 10.1056/NEJMra1514296 [DOI] [PMC free article] [PubMed] [Google Scholar]