Abstract
Background:
A cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) + endocrine therapy is recommended as first-line treatment for hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2−) advanced breast cancer (ABC). Quality of life (QoL) is an important endpoint that affects treatment decisions. Understanding the relevance of CDK4/6i treatment on QoL is gaining importance given use in earlier treatment lines for ABC and an emerging role in treating early breast cancer in which QoL may be more impactful. In the absence of head-to-head trial data, a matching-adjusted indirect comparison (MAIC) permits comparative efficacy between trials.
Objective:
In this analysis, patient-reported QoL for MONALEESA-2 [ribociclib + aromatase inhibitor (AI)] and MONARCH 3 (abemaciclib + AI) was compared using MAIC with a focus on individual domains.
Design:
An anchored MAIC of QoL comparing ribociclib + AI versus abemaciclib + AI was performed using data from the European Organization for Research and Treatment of Cancer quality of life questionnaire (QLQ)-C30 and BR-23 questionnaires.
Methods:
Individual patient data from MONALEESA-2 and published aggregated data from MONARCH 3 were included in this analysis. Time to sustained deterioration (TTSD) was calculated as the time from randomization to a ⩾10-point deterioration with no later improvement above this threshold.
Results:
Patients from the ribociclib (n = 205) and placebo (n = 149) arms of MONALEESA-2 were matched with patients from the abemaciclib (n = 328) and placebo (n = 165) arms of MONARCH 3. After weighting, baseline patient characteristics were well balanced. TTSD significantly favored ribociclib versus abemaciclib in appetite loss [hazard ratio (HR), 0.46; 95% confidence interval (CI), 0.27–0.81], diarrhea (HR, 0.42; 95% CI, 0.23–0.79), fatigue (HR, 0.63; 95% CI, 0.41–0.96), and arm symptoms (HR, 0.49; 95% CI, 0.30–0.79). TTSD did not significantly favor abemaciclib compared with ribociclib in any functional or symptom scale of the QLQ-C30 or BR-23 questionnaires.
Conclusions:
This MAIC indicates that ribociclib + AI is associated with better symptom-related QoL than abemaciclib + AI for postmenopausal patients with HR+/HER2− ABC treated in the first-line setting.
Trial registration:
NCT01958021 (MONALEESA-2) and NCT02246621 (MONARCH 3)
Keywords: abemaciclib, CDK4/6 inhibitor, MONALEESA-2, MONARCH 3, quality of life, ribociclib
Introduction
All three approved cyclin-dependent kinase 4/6 inhibitors (CDK4/6is; ribociclib, abemaciclib, and palbociclib) reported statistically significant progression-free survival (PFS) benefits in the intent-to-treat (ITT) populations of their phase III trials of patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2−) advanced breast cancer (ABC).1–7 Ribociclib plus endocrine therapy (ET) has demonstrated consistent and statistically significant overall survival (OS) benefits over ET alone in patients with HR+/HER2− ABC throughout its entire phase III program to date, including MONALEESA-2 [hazard ratio (HR), 0.76; 95% confidence interval (CI), 0.63–0.93; p = 0.008], MONALEESA-3 (HR, 0.72; 95% CI, 0.57–0.92; p = 0.00455), and MONALEESA-7 (HR, 0.71; 95% CI, 0.54–0.95; p = 0.00973).8–10 A statistically significant OS benefit was reported for abemaciclib plus ET in the MONARCH 2 trial (HR, 0.757; 95% CI, 0.61–0.95; p = 0.01), while the second interim analysis of the MONARCH 3 trial demonstrated no significant OS benefit (HR, 0.75; 95% CI, 0.58–0.97; p = 0.0301) and final OS results are pending.11,12 No significant OS benefit was observed in either of the phase III trials of palbociclib plus ET, PALOMA-2 (HR, 0.956; 95% CI, 0.777–1.177; p = 0.3378) and PALOMA-3 (HR, 0.81; 95% CI, 0.64–1.03; p = 0.09).12,13 A CDK4/6i + ET is recommended for first-line treatment of HR+/HER2− ABC.14 To date, ribociclib, in combination with ET, is the only CDK4/6i to demonstrate statistically significant OS benefit over ET alone in the first-line setting.8,10,12
The CDK4/6is are known to have different safety profiles attributed to differences in target inhibition.15 Treatment-related side effects, even when mild, can impact quality of life (QoL). In support of this, a multi-country, cross-sectional survey of oncologists, nurses, advocates, and patients identified diarrhea, fatigue, and appetite loss as key adverse events that had a moderate to severe impact on QoL among patients treated with CDK4/6is.16 Many of the trials of CDK4/6is in ABC have reported data on QoL; therefore, understanding the impact of these treatments on QoL is of great importance in clinical decision-making.17–23
No head-to-head study comparing CDK4/6is exists to directly observe the differential effects of these agents on patient QoL. A matching-adjusted indirect comparison (MAIC) is strongly advocated and employed for indirect comparisons in the absence of a direct head-to-head study because it adjusts for differences in the study populations, as opposed to an unadjusted indirect comparison.24 MAICs have been widely used and accepted by several national health technology assessment bodies across diverse therapeutic areas to estimate relative treatment effects to inform reimbursement decisions.24
In this analysis, MAIC was performed using patient-reported outcomes from MONALEESA-2 and MONARCH 3, with a focus on individual domains, to compare QoL in patients treated with first-line ribociclib + aromatase inhibitor (AI) versus abemaciclib + AI. The PALOMA-2 trial of first-line palbociclib + AI in a similar patient population used different QoL scales than these studies and hence could not be considered for this analysis.22
Methods
Overview
An anchored MAIC of QoL with ribociclib + AI versus abemaciclib + AI was performed using patient-reported outcome data from the European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaire (QLQ-C30) and the breast cancer-specific quality of life questionnaire (QLQ-BR-23) used in the MONALEESA-2 (NCT01958021) and MONARCH 3 (NCT02246621) studies. The EORTC QLQ-30 consists of 30 questions assessing functional scales (physical, social, role, cognitive, and emotional), symptom-related scales (fatigue, nausea/vomiting, pain, dyspnea, sleep disturbances, appetite loss, constipation, and diarrhea), financial impact, and overall QoL or global health status (GHS). GHS is not an aggregate score of the different functional or symptomatic scales, and thus the GHS and specific domains are not directly linked. The EORTC QLQ-BR-23 is a breast cancer-specific module consisting of 23 questions assessing functional scales (body image, sexual functioning, sexual enjoyment, and future perspective) and symptom-related scales [systemic therapy side effects, breast symptoms, arm symptoms (including pain in arm or shoulder, swollen arm or hand, and difficulty in raising arm), and upset by hair loss]. All available QoL data from the studies were used in this analysis.
Individual patient data from MONALEESA-2 (patients were randomized from 24 January 2014 to 24 March 2015; data cutoff, 10 June 2021) and published data from MONARCH 3 (patients were randomized from 18 November 2014 and 11 November 2015; data cutoff, 3 November 2017) were used in this analysis.21 Inclusion and exclusion criteria were generally similar between the studies (Table 1). The median follow-up for MONALEESA-2 was 79.7 months, and the median duration of follow-up at which QoL data were reported for MONARCH 3 was 26.7 months. Patients in MONALEESA-2 completed questionnaires at the start of each visit: at screening; every 8 weeks for the first 18 months; then every 12 weeks until disease progression, death, loss to follow-up, or withdrawal of consent; and at treatment discontinuation.17 For MONARCH 3, questionnaires were completed at baseline, every two cycles (cycles 2–19) followed by every three cycles, and approximately 30 days after discontinuation.21
Table 1.
Comparison of key inclusion/exclusion criteria.
| Parameter | MONALEESA-2 | MONARCH 3 |
|---|---|---|
| Disease | HR+/HER2− locally advanced/metastatic BC | HR+/HER2− locally advanced/metastatic BC |
| Menopausal status | Postmenopausal only | Postmenopausal only |
| Gender | Only female | Only female |
| Age | 18 years and older | 18 years and older |
| ECOG performance status | 0 or 1 | 0 or 1 |
| Patients included | Received no systemic treatment for ABC Have measurable disease as defined by RECIST 1.1 or ⩾1 predominantly lytic bone lesion • TFI > 12 months after prior (neo)adjuvant NSAIa |
Received no systemic treatment for ABC Have measurable disease or non-measurable bone-only disease (blastic, lytic, or mixed) as defined by RECIST 1.1 Have had adequate organ function • TFI > 12 months after any prior (neo)adjuvant ET |
| Exclusions | Prior treatment with CDK4/6 inhibitors Presence of active cardiac disease or history of cardiac dysfunction, including QTcF >450 msec • Presence of inflammatory breast cancer |
Prior treatment with everolimus or a CDK4/6
inhibitor Presence of visceral crisis, lymphangitic spread, or leptomeningeal carcinomatosis Presence of inflammatory breast cancer • Evidence or history of CNS metastases |
ML-2 allowed TFI ⩽ 12 months if the (neo)adjuvant therapy was tamoxifen.
ABC, advanced breast cancer; BC, breast cancer; CDK4/6; cyclin-dependent kinases 4 and 6; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; ET, endocrine therapy; HR+/HER2-, hormone receptor-positive/human epidermal growth factor receptor 2-negative; NSAI, nonsteroidal aromatase inhibitor; QTcF, corrected QT interval by Fredericia’s formula; RECIST, Response Evaluation Criteria in Solid Tumors; TFI, treatment-free interval.
MAIC methods
Both studies enrolled postmenopausal patients with HR+/HER2− ABC to be treated with a CDK4/6i + AI or placebo + AI. MONALEESA-2 and MONARCH 3 have a common comparator arm (placebo + AI); thus, an anchored MAIC was used in this analysis with a focus on the comparison between ribociclib and abemaciclib. Patients in both arms of MONALEESA-2 were weighted by the inverse of their propensity score to balance the covariate distribution with that of the aggregated data of MONARCH 3 (Figure 1). Distributions of inverse probability of treatment weights for patients in MONALEESA-2 were plotted as histograms (Figure 2), and effective sample sizes were calculated.
Figure 1.
MONALEESA-2 patient selection. ESS reflects sample size after balancing.
ESS, effective sample size; MAIC, matching-adjusted indirect comparison; ML-2, MONALEESA-2; MON-3, MONARCH 3.
Figure 2.
Distribution of MAIC weights for patients in MONALEESA-2 meeting the inclusion criteria for MONARCH 3 in the placebo + AI and ribociclib + AI arms.
aRescaled weights ranged from (A) 0.06 to 7.33 (median, 0.6) for placebo + AI and (B) 0.16 to 5.16 (median, 0.7) for ribociclib + AI.
AI, aromatase inhibitor; MAIC, matching-adjusted indirect comparison.
Statistical analysis
A Cox proportional hazards model was used to generate HRs; anchored HRs were calculated using the Bucher method. A QoL deterioration of ⩾10 points in the EORTC QoL scales has historically been considered clinically meaningful. In this analysis, time to sustained deterioration (TTSD) was calculated as the time from randomization to a ⩾10-point deterioration in scale scores relative to baseline with no later improvement above this threshold observed during the treatment period or death due to any cause. If a patient had ⩾10-point deterioration followed by improvement, they would be censored and not counted for TTSD. However, if a patient improved after ⩾10-point deterioration, but afterwards had ⩾10-point deterioration again with no further improvement, this second deterioration would be considered for TTSD.
Results
In total, 205 patients treated with ribociclib + AI and 149 patients treated with placebo + AI in MONALEESA-2 were matched with 328 patients treated with abemaciclib + AI and 165 patients treated with placebo + AI in MONARCH 3 (Figure 1 and Table 2). Treatment and placebo arms were matched separately. After weighting, patient characteristics were well balanced (Figure 2 and Table 2). Rescaled weights for patients in MONALEESA-2 who matched the inclusion criteria for MONARCH 3 ranged from 0.06 to 7.33 for the placebo arm and 0.16 to 5.16 for the ribociclib arm, with a median of 0.7 for ribociclib + AI and 0.6 for placebo + AI (Figure 2). The effective sample size was 205 for the ribociclib arm (sample size reduction of 39% from the ITT) and 149 for the placebo arm (reduction of 55%). None of the baseline characteristics reported for MONARCH 3 were removed from the analysis.
Table 2.
Characteristics of patients in MONALEESA-2 and MONARCH 3 meeting the inclusion criteria of MONARCH 3.
| Characteristics | Value | Before weighting | After weighting | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ML-2 | MON-3 | ML-2 | MON-3 | ||||||
| RIB + AI | PBO + AI | ABE + AI | PBO + AI | RIB + AI | PBO + AI | ABE + AI | PBO + AI | ||
| Patients, n | 334 | 334 | 328 | 165 | 205 | 149 | 328 | 165 | |
| Median age, years | 62 | 63 | 63 | 63 | 63 | 63 | 63 | 63 | |
| Race, % | Caucasian | 80.5 | 83.8 | 56.7 | 61.8 | 56.7 | 61.8 | 56.7 | 61.8 |
| Others | 19.5 | 16.2 | 43.3 | 38.2 | 43.3 | 38.2 | 43.3 | 38.2 | |
| ECOG PS, % | 1 | 38.9 | 39.5 | 41.5 | 37.0 | 41.5 | 37.0 | 41.5 | 37.0 |
| 0 | 61.1 | 60.5 | 58.5 | 63.0 | 58.5 | 63.0 | 58.5 | 63.0 | |
| De novo metastatic disease, % | Yes | 34.1 | 33.8 | 41.2 | 37.0 | 41.2 | 37.0 | 41.2 | 37.0 |
| No | 65.9 | 66.2 | 58.8 | 63.0 | 58.8 | 63.0 | 58.8 | 63.0 | |
| PR status, % | Negative | 16.5 | 14.7 | 21.3 | 21.8 | 21.3 | 21.8 | 21.3 | 21.8 |
| Positive | 81.1 | 83.2 | 77.7 | 77.0 | 77.7 | 77.0 | 77.7 | 77.0 | |
| Metastatic site, % | Visceral | 56.6 | 57.8 | 52.7 | 53.9 | 52.7 | 53.94 | 52.7 | 53.9 |
| Bone only | 20.7 | 23.4 | 21.0 | 24.2 | 21.0 | 24.24 | 21.0 | 24.2 | |
| Other | 22.8 | 18.9 | 26.2 | 21.8 | 26.2 | 21.82 | 26.2 | 21.8 | |
| Prior (neo)adjuvant chemotherapy, % | Yes | 43.7 | 43.4 | 38.1 | 40.0 | 38.1 | 40.0 | 38.1 | 40.0 |
| No | 56.3 | 56.6 | 61.9 | 60.0 | 61.9 | 60.0 | 61.9 | 60.0 | |
| ET, % | Prior AI | 34.1 | 32.9 | 25.9 | 30.3 | 25.9 | 30.3 | 25.9 | 30.3 |
| Other prior ET | 24.9 | 24.6 | 20.1 | 18.2 | 20.1 | 18.2 | 20.1 | 18.2 | |
| No prior ET | 41.0 | 42.5 | 54.0 | 51.5 | 54.0 | 51.5 | 54.0 | 51.5 | |
| Measurable disease, % | Yes | 77.5 | 73.4 | 81.4 | 80.0 | 81.4 | 80.0 | 81.4 | 80.0 |
| No | 22.5 | 26.6 | 18.6 | 20.0 | 18.6 | 20.0 | 18.6 | 20.0 | |
| No. of organs at baseline, % | 3+ | 34.1 | 33.5 | 46.3 | 47.3 | 46.3 | 47.3 | 46.3 | 47.3 |
| 2 | 35.3 | 31.1 | 23.5 | 24.8 | 23.5 | 24.8 | 23.5 | 24.8 | |
| 1 | 29.9 | 35.0 | 29.9 | 27.3 | 29.9 | 27.3 | 29.9 | 27.3 | |
ABE, abemaciclib; AI, aromatase inhibitor; ECOG PS, Eastern Cooperative Oncology Group performance status; ET, endocrine therapy; ML-2, MONALEESA-2; MON-3, MONARCH 3; PBO, placebo; PR, progesterone receptor; RIB, ribociclib.
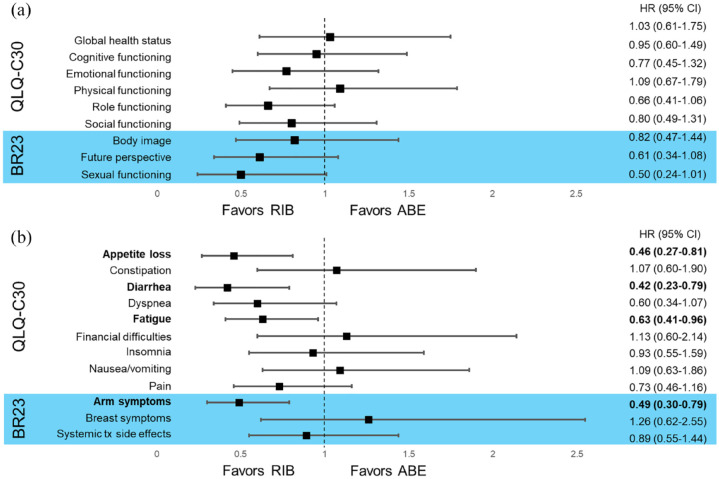
With respect to symptom scales, TTSD analysis significantly favored ribociclib + AI over abemaciclib + AI in four symptom scales (Figure 3): appetite loss (HR, 0.46; 95% CI, 0.27–0.81), diarrhea (HR, 0.42; 95% CI, 0.23–0.79), fatigue (HR, 0.63; 95% CI, 0.41–0.96), and arm symptoms, including pain in arm or shoulder, swollen arm or hand, and difficulty raising arm (HR, 0.49; 95% CI, 0.30–0.79). TTSD analysis numerically favored ribociclib + AI over abemaciclib + AI in three symptom scales (Figure 3): dyspnea (HR, 0.60; 95% CI, 0.34–1.07), pain (HR, 0.73; 95% CI, 0.46–1.16), and systemic treatment side effects (HR, 0.89; 95% CI, 0.55–1.44). No significant differences were observed in any of the EORTC QLQ-C30 or BR-23 functional domains (Figure 3), including GHS (HR, 1.03; 0.61–1.75). However, the TTSD analysis numerically favored ribociclib + AI over abemaciclib + AI in emotional (HR, 0.77; 95% CI, 0.45–1.32), role (HR, 0.66; 95% CI, 0.41–1.06), and social (HR, 0.80; 95% CI, 0.49–1.31) functioning, as well as body image (HR, 0.82; 95% CI, 0.47–1.44), future perspective (HR, 0.61; 95% CI, 0.34–1.08), and sexual functioning (HR, 0.50; 95% CI, 0.24–1.01) (Figure 3). Notably, the TTSD analysis did not significantly favor abemaciclib + AI over ribociclib + AI in any functional or symptom scale of the QLQ-C30 or BR-23 but did numerically trend in favor of abemaciclib + AI in financial difficulties (HR, 1.13; 95% CI, 0.60–2.14) and breast symptoms (HR, 1.26; 95% CI, 0.62–2.55).
Figure 3.
TTSD in functional (a) and symptom (b) scales for ribociclib + AI versus abemaciclib + AI.
ABE, abemaciclib; BR23, breast cancer-specific quality of life questionnaire; CI, confidence interval; HR, hazard ratio; QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; RIB, ribociclib; TTSD, time to sustained deterioration; tx, treatment.
Discussion
This MAIC used matched and weighted individual patient data from MONALEESA-2 and published data from MONARCH 3 to compare QoL with first-line use of ribociclib + AI versus abemaciclib + AI. Results showed that the TTSD analysis significantly favored ribociclib + AI over abemaciclib + AI in diarrhea, fatigue, appetite loss, and arm symptoms. Although not statistically significant, the TTSD analysis favored ribociclib + AI over abemaciclib + AI in emotional, role, and social functioning, as well as body image, future perspective, and sexual functioning. The TTSD analysis did not significantly favor abemaciclib over ribociclib in any of the functional or symptom scales, but TTSD for constipation, physical functioning, financial difficulties, nausea/vomiting, and breast symptoms trended numerically in favor of abemaciclib. Similar to the current MAIC, a previously published MAIC evaluated QoL with second-line use of palbociclib + fulvestrant versus abemaciclib + fulvestrant using data from PALOMA-3 and MONARCH 2.25 That MAIC found that abemaciclib was associated with significantly greater impact on several symptom scores, including diarrhea and appetite loss, compared with palbociclib. While previously published QoL data from MONARCH 3 did not demonstrate a meaningful difference between the abemaciclib and placebo arms in most symptoms with the exception of diarrhea,21 data from these MAICs shed light on the impact of AEs associated with abemaciclib on patient QoL relative to other CDK4/6is.
Both MONALEESA-2 and MONARCH 3 reported significant improvements in PFS in their ITT populations, and MONALEESA-2 demonstrated a significant >12-month improvement in OS with ribociclib + ET versus placebo + ET, with a 24% relative reduction in the risk of death.1,7,8 No significant OS benefit with abemaciclib + ET was observed in the second interim analysis of MONARCH 3, and final OS data are still pending at the time this report was written.12 The most common any-grade adverse event reported in the CDK4/6i arm of MONALEESA-2 was neutropenia (74%), while the most common in MONARCH 3 was diarrhea (81%).1,7 Understanding the impact of CDK4/6i-related adverse events on QoL is important for treatment decision-making. It is highly relevant to view these results in the context of findings from a recent survey that highlighted specific CDK4/6i-related adverse events impacting patient QoL.16 In that multi-country survey, patients treated with CDK4/6is reported that diarrhea, fatigue, and appetite loss had a moderate/severe impact on their QoL. The side effect reported by patients treated with a CDK4/6i as having the greatest impact on QoL was diarrhea, which was reported as having a moderate to severe impact on QoL by 75% of patients. Furthermore, several published patient preference surveys, which utilized discrete choice experiments, reported that risk of diarrhea was one of the most important attributes to both patients and physicians when making treatment decisions for ABC and early breast cancer, including whether to use a CDK4/6i.26–28
Analyses like this and the prior MAIC of PALOMA-3 and MONARCH-2 allow for greater understanding of the impact of CDK4/6i-related adverse events and support consideration of QoL, in addition to efficacy and safety, when making treatment decisions.25 The ESMO Magnitude of Clinical Benefit Scale was created to provide a tool to guide clinical decision-making and considers efficacy, safety, and QoL to assign ratings to cancer therapies.29 Scores of 4 and 5 indicate substantial clinical benefit.30 Based on these parameters, ribociclib combined with ET received the highest score (5/5) of the CDK4/6i for the first-line treatment of premenopausal women as per MONALEESA-7 data and the highest score of the CDK4/6is (4/5) for the first-line treatment of postmenopausal women in combination with AI (MONALEESA-2 data), while first-line abemaciclib in combination with AI received a score of 3/5 and is pending the MONARCH 3 OS readout.29,30
MAIC is a well-established and accepted methodology that utilizes individual patient data from one trial and summary data from another. In doing so, this method statistically controls for cross-trial differences in patient populations and provides clinically important comparative results in the absence of head-to-head studies. However, a few limitations should be noted. While MAICs balance treatment-effect-modifying patient characteristics measured in the trials, there may be unmeasured differences between trials that could not be matched. Although the majority of key baseline patient and disease characteristics were matched, the results may be confounded by any unreported factors because only published characteristics for MONARCH 3 were controlled for in this analysis. Interpretation of these results is limited to the subset of patients in MONALEESA-2 who were matched with patients in MONARCH 3. Additionally, the extreme weights required for some patients during matching adjustment may have led to low statistical power for detecting differences between treatments.
In view of the growing role of CDK4/6is in treatment of early breast cancer, it is of increasing importance to give more consideration to QoL in addition to other treatment outcomes. The results from this MAIC indicate that ribociclib + AI was associated with better symptom-related QoL compared with abemaciclib + AI in the first-line treatment of postmenopausal women with HR+/ HER2− ABC. These findings support the overall clinical benefit of ribociclib treatment and the value of understanding that the CDK4/6is are different with respect to all the outcomes, including efficacy, safety, and QoL. It is important to consider these results in light of the most common adverse events identified by patients to have a large impact on day-to-day QoL during treatment with CDK4/6i, including diarrhea, fatigue, and appetite loss.16 Patients treated with abemaciclib experience QoL that is more heavily impacted by these important adverse events compared with those treated with ribociclib.
Acknowledgments
We thank the patients who participated in this trial, their families, and their caregivers; members of the data monitoring committee; members of the study steering committee; staff members who helped with the trial at each site; and Daniele Cary, PhD, of MediTech Media for medical editorial assistance with this manuscript. Ribociclib was discovered by Novartis Institutes for BioMedical Research in collaboration with Astex Pharmaceuticals.
Footnotes
ORCID iD: Aditya Bardia  https://orcid.org/0000-0003-4885-1157
https://orcid.org/0000-0003-4885-1157
Contributor Information
Hope S. Rugo, University of California San Francisco Helen Diller Family Comprehensive Cancer Center, 1825 4th St, 3rd Floor BCC, San Francisco, CA 94158, USA.
Victoria Harmer, Imperial College Healthcare NHS Trust, London, UK.
Joyce O’Shaughnessy, Texas Oncology-Baylor University Medical Center and The US Oncology Research Network, Dallas, TX, USA.
Komal Jhaveri, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Sara M. Tolaney, Dana-Farber Cancer Institute, Boston, MA, USA
Fatima Cardoso, Breast Unit, Champalimaud Clinical Center, Champalimaud Foundation, Lisbon, Portugal.
Aditya Bardia, Massachusetts General Hospital Cancer Center, Harvard Medical School, Boston, MA, USA.
Vikalp Kumar Maheshwari, NBS CONEXTS at Novartis Pharmaceuticals, Hyderabad, India.
Sandeep Tripathi, Novartis Pharmaceuticals, Hyderabad, India.
Sina Haftchenary, Novartis Pharmaceuticals Canada, Montreal, QC, Canada.
Purnima Pathak, Novartis Services Inc, East Hanover, NJ, USA.
Peter A. Fasching, University Hospital Erlangen, Comprehensive Cancer Center Erlangen–European Metropolitan Region of Nuremberg, and Department of Gynecology and Obstetrics, Friedrich-Alexander University Erlangen-Nuremberg, Erlangen, Germany
Declarations
Ethics approval and consent to participate: This trial was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines and was funded by Novartis. The trial protocol and all amendments were approved by the institutional review board at each site or an independent ethics committee. The conduct of the trial was overseen by a trial steering committee composed of participating international investigators as well as representatives of the sponsor. An independent data monitoring committee assessed safety data. Prior to enrollment, all patients provided written informed consent. Representatives of the sponsor were responsible for trial design, data compilation, and confirming the accuracy of analyses. All authors had access to the data and vouch for the completeness and accuracy of the data as well as the fidelity of the trial to the protocol. All authors participated in the writing and review of all manuscript drafts and contributed to the interpretation of the data. Medical writing and editorial support were funded by Novartis.
Consent for publication: Not applicable.
Author contribution(s): Hope S. Rugo: Conceptualization; Writing – review & editing.
Victoria Harmer: Conceptualization; Writing – review & editing.
Joyce O’Shaughnessy: Conceptualization; Writing – review & editing.
Komal Jhaveri: Conceptualization; Investigation; Writing – review & editing.
Sara M. Tolaney: Conceptualization; Investigation; Writing – review & editing.
Fatima Cardoso: Conceptualization; Writing – review & editing.
Aditya Bardia: Conceptualization; Investigation; Writing – review & editing.
Vikalp Kumar Maheshwari: Conceptualization; Formal analysis; Methodology; Writing – review & editing.
Sandeep Tripathi: Conceptualization; Formal analysis; Methodology; Writing – review & editing.
Sina Haftchenary: Conceptualization; Methodology; Writing – review & editing.
Purnima Pathak: Conceptualization; Methodology; Writing – review & editing.
Peter A. Fasching: Conceptualization; Investigation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Sponsored by Novartis.
H. Rugo reports institution grants from Plexxikon, MacroGenics, OBI Pharma, Eisai, Pfizer, Novartis, Eli Lilly, GlaxoSmithKline, Genentech, Celsion, Merck, personal fees for travel, accommodations, and expenses from Novartis, Roche/Genentech, OBI Pharma, Bayer, Pfizer, speaker’s bureau for Genomic Health; V. Harmer reports honorarium from Eli Lily, Gilead, and Novartis; J. O’Shaughnessy reports personal fees for consultant/advisory board from Clovis Oncology, Daiichi Sankyo, Eisai, G1 Therapeutics, Genentech, Gilead Sciences, GRAIL, Halozyme Therapeutics, Heron Therapeutics, Immunomedics, Ipsen Biopharmaceuticals, Lilly, Merck, Myriad, Nektar Therapeutics, Novartis, Pfizer, Pharmacyclics, Pierre Fabre Pharmaceuticals, Puma Biotechnology, Prime Oncology, Roche, Samsung Bioepis, Sanofi, Seagen, Syndax Pharmaceuticals, Taiho Oncology, Takeda, Synthon; K. Jhaveri has nothing to report; S. Tolaney reports grants and personal fees, all funding to institute, from Eli Lilly, Novartis, AstraZeneca, Merck, Nektar, Pfizer, Genentech/Roche, Exelixis, BMS, Eisai, NanoString, Sanofi, Odonate, Gilead, grant from Cyclacel, personal fees from Puma, Seattle Genetics, G1 Therapeutics, Athenex, OncoPep, Kyowa Kirin Pharmaceuticals, Daiichi Sankyo, CytomX, Samsung Bioepis Inc., Certara, Mersana Therapeutics, Oncosec, Chugai Pharma, Ellipses Pharma, 4D Pharma, Beyond Spring Pharma, OncXerna, Infinity Therapeutics, Zentalis, Zymeworks; F. Cardoso reports statistical analysis, medical writer for the work under consideration for Novartis, personal fees outside the submitted work from Amgen, Astellas/Medivation, AstraZeneca, Celgene, Daiichi Sankyo, Eisai, GE Oncology, Genentech, GSK, MacroGenics, Medscape, Merck-Sharp, Merus, Mylan, Mundipharma, Novartis, Pfizer, Pierre Fabre, Prime Oncology, Roche, Sanofi, Samsung Bioepis, Teva, Seagen, Debiopharm, Gilead, Iqvia, touch IME; A. Bardia reports research grants to his institution from Genentech, Novartis, Pfizer, Merck, Sanofi, Radius Health, Immunomedics, Mersana, Innocrin, personal fees for advisory board from Biotheranostics Inc., personal fees for advisory board, steering committee, travel support from Pfizer, Novartis, Genentech, Merck, Radius Health, Immunomedics, Spectrum Pharma, Taiho, Sanofi, personal fees for advisory board from Daiichi Pharma, Puma; V.K. Maheshwari and S. Tripathi have nothing to report; S. Haftchenary and P. Pathak report employment, stock ownership from Novartis; P. Fasching reports personal fees for advisory boards from Novartis, Pfizer, Daiichi Sankyo, AstraZeneca, Eisai, Merck Sharp & Dohme, Lilly, Pierre Fabre, Seagen, Roche, Hexal, Agendia, Sanofi Aventis, Gilead, institutional funding grant from BioNTech, Cepheid, research grant from Pfizer.
Availability of data and materials: Novartis made the study protocols available for MONALEESASIA at the time of primary publications. Individual participant data will not be made available.
References
- 1. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375: 1738–1748. [DOI] [PubMed] [Google Scholar]
- 2. Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 2018; 36: 2465–2472. [DOI] [PubMed] [Google Scholar]
- 3. Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 2018; 19: 904–915. [DOI] [PubMed] [Google Scholar]
- 4. Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res 2016; 18: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turner NC, Ro J, Andre F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 2015; 373: 209–219. [DOI] [PubMed] [Google Scholar]
- 6. Sledge GW, Jr, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017; 35: 2875–2884. [DOI] [PubMed] [Google Scholar]
- 7. Goetz MP, Toi M, Campone M, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017; 35: 3638–3646. [DOI] [PubMed] [Google Scholar]
- 8. Hortobagyi GN, Stemmer SM, Burris HA, et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med 2022; 386: 942–950. [DOI] [PubMed] [Google Scholar]
- 9. Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med 2020; 382(6): 514–524. [DOI] [PubMed] [Google Scholar]
- 10. Im SA, Lu YS, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 2019; 381(4): 307–316. [DOI] [PubMed] [Google Scholar]
- 11. Sledge GW, Jr., Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol 2019; 6: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finn RS, Rugo HS, Dieras V, et al. Overall suvival with first-line palbociclib plus letrozole versus placebo plus letrozole in women with estrogen-receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: analysis from PALOMA-2. Am Soc Clin Oncol 2022; 40: LBA1003. [Google Scholar]
- 13. Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 2018; 379: 1926–1936. [DOI] [PubMed] [Google Scholar]
- 14. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Breast Cancer. version 4.2022 (2022, accessed 21 June 2022). [Google Scholar]
- 15. Chen P, Lee NV, Hu W, et al. Spectrum and degree of CDK drug interactions predicts clinical performance. Mol Cancer Ther 2016; 15: 2273–2281. [DOI] [PubMed] [Google Scholar]
- 16. Cardoso F, Rihani J, Aubel D, et al. Assessment of side effects impacting quality of life in patients undergoing treatment for advanced breast cancer (ABC) in clinical practice: a real-world multi-country survey. Eur Soc Med Oncol Breast Cancer 2022; 33: S208–S209. [Google Scholar]
- 17. Verma S, O’Shaughnessy J, Burris HA, et al. Health-related quality of life of postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer treated with ribociclib + letrozole: results from MONALEESA-2. Breast Cancer Res Treat 2018; 170(3): 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fasching PA, Beck JT, Chan A, et al. Ribociclib plus fulvestrant for advanced breast cancer: health-related quality-of-life analyses from the MONALEESA-3 study. Breast 2020; 54: 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harbeck N, Franke F, Villanueva-Vazquez R, et al. Health-related quality of life in premenopausal women with hormone-receptor-positive, HER2-negative advanced breast cancer treated with ribociclib plus endocrine therapy: results from a phase III randomized clinical trial (MONALEESA-7). Ther Adv Med Oncol 2020; 12: 1758835920943065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaufman PA, Toi M, Neven P, et al. Health-related quality of life in MONARCH 2: abemaciclib plus fulvestrant in hormone receptor-positive, HER2-negative advanced breast cancer after endocrine therapy. Oncologist 2020; 25: e243–e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goetz MP, Martin M, Tokunaga E, et al. Health-related quality of life in MONARCH 3: abemaciclib plus an aromatase inhibitor as initial therapy in HR+, HER2- advanced breast cancer. Oncologist 2020; 25: e1346–e1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rugo HS, Dieras V, Gelmon KA, et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: results from the PALOMA-2 trial. Ann Oncol 2018; 29: 888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harbeck N, Iyer S, Turner N, et al. Quality of life with palbociclib plus fulvestrant in previously treated hormone receptor-positive, HER2-negative metastatic breast cancer: patient-reported outcomes from the PALOMA-3 trial. Ann Oncol 2016; 27: 1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thom H, Jugl S, Palaka E, et al. Matching adjusted indirect comparisons to assess comparative effectiveness of therapies: usage in scientific literature and health technology appraisals. Value Health 2016; 19: A100–A101. [Google Scholar]
- 25. Law E, Gavanji R, Walsh S, et al. Palbociclib versus abemaciclib in HR+/HER2- advanced breast cancer: an indirect comparison of patient-reported end points. J Comp Eff Res 2022; 11: 109–120. [DOI] [PubMed] [Google Scholar]
- 26. Maculaitis MC, Liu X, Will O, et al. Oncologist and patient preferences for attributes of CDK4/6 inhibitor regimens for the treatment of advanced/metastatic HR positive/HER2 negative breast cancer: discrete choice experiment and best-worst scaling. Patient Prefer Adherence 2020; 14: 2201–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Omori Y, Enatsu S, Cai Z, et al. Patients’ preferences for postmenopausal hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer treatments in Japan. Breast Cancer 2019; 26: 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beusterien K, Maculaitis MC, Hallissey B, et al. Patient, oncologist, and payer preferences for adjuvant endocrine therapy and CDK4/6 inhibitor regimens in early-stage breast cancer: a discrete choice experiment. Patient Prefer Adherence 2021; 15: 611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020; 31: 1623–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. ESMO-MCBS scorecards. https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-scorecards (2022, accessed 20 September 2022).