Abstract
Alzheimer's disease (AD) is the most common neurodegenerative disease. Increasing studies suggest that mitochondrial dysfunction is closely related to the pathogenesis of AD. Thioredoxin-1 (Trx-1), one of the major redox proteins in mammalian cells, plays neuroprotection in AD. However, whether Trx-1 could regulate the mitochondrial biogenesis in AD is largely unknown. In the present study, we found that Aβ25−35 treatment not only markedly induced excessive production of reactive oxygen species and apoptosis, but also significantly decreased the number of mitochondria with biological activity and the adenosine triphosphate content in mitochondria, suggesting mitochondrial biogenesis was impaired in AD cells. These changes were reversed by Lentivirus-mediated stable overexpression of Trx-1 or exogenous administration of recombinant human Trx-1. What's more, adeno-associated virus-mediated specific overexpression of Trx-1 in the hippocampus of β-amyloid precursor protein/presenilin 1 (APP/PS1) mice ameliorated the learning and memory and attenuated hippocampal Aβ deposition. Importantly, overexpression of Trx-1 in APP/PS1 mice restored the decrease in mitochondrial biogenesis-associated proteins, including adenosine monophosphate -activated protein kinase (AMPK), silent information regulator factor 2-related enzyme 1 (Sirt1) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α). In addition, Lentivirus-mediated overexpression of Trx-1 in rat adrenal pheochromocytoma (PC12) cells also restored the decrease of AMPK, Sirt1, and PGC1α by Aβ25−35 treatment. Pharmacological inhibition of AMPK activity significantly abolished the effect of Trx-1 on mitochondrial biogenesis. Taken together, our data provide evidence that Trx-1 promoted mitochondrial biogenesis via restoring AMPK/Sirt1/PGC1α pathway in AD.
Keywords: Alzheimer's disease, thioredoxin-1, mitochondrial biogenesis, AMPK/Sirt1/PGC1α, neuroprotection
Introduction
Alzheimer's disease (AD) is the most common form of neurodegenerative diseases (Hodson, 2018). According to the “World Alzheimer's Disease Report 2022,” the number of people suffering from dementia is estimated to be 55 million in 2019, and is expected to increase to 139 million in 2050. According to the “Alzheimer's Disease Report in China 2021,” both prevalence and mortality of AD in China are obviously much higher than the global average levels and are still rising, suggesting that AD has become a major disease and social problem seriously endangering the health of Chinese people. AD has a complex etiopathogenesis that is likely to involve multiple susceptible genes and environmental factors (Migliore & Coppede, 2022). It is generally believed that the progressive neurodegeneration of AD is attributed to the deposition of β-amyloid (Aβ) and the formation of neurofibrillary tau, which could lead to the loss of neurons associated to learning and memory (McAlpine et al., 2021).
Increasing evidence showed that mitochondrial function is central in AD and mitochondrial dysfunction is implicated in the pathogenesis (Hedskog et al., 2012). The impairment of mitochondrial function was found in the brain tissue of both alive and post-mortem AD patients (Kerr et al., 2017). The mutual enhancement between Aβ and reactive oxygen species (ROS) impaired mitochondrial function, which would promote the development of AD (Jia et al., 2021). It was reported that Aβ induced the phosphorylation of dynamin-related protein 1 (Drp1) and promoted excessive mitochondrial fission, which led to neuronal apoptosis (Kim et al., 2016). Thus, maintaining mitochondrial balance may be a promising therapeutic strategy of AD.
Thioredoxin-1 (Trx-1), a multifunctional redox protein, can directly eliminate ROS and maintain the redox balance in mammalian cells (Zeng et al., 2018). Various stress conditions could upregulate the expression of Trx-1 (Jia et al., 2014, 2016; Wang et al., 2022b; Zeng et al., 2020). Accumulating researches have demonstrated that Trx-1 has neuroprotective effects in common neurodegenerative diseases, including AD (Jia et al., 2022a). In a rat model of AD, the increased expression of Trx-1 inhibited Aβ25−35-induced oxidative stress and cognitive dysfunction (Zhuo et al., 2016). Overexpression of Trx-1 in the hippocampus of mice inhibited the neurotoxicity of apolipoprotein E4, a genetic risk factor of AD (Persson et al., 2017). Enhancement of Trx-1 activity also reduced apoptosis of brain neurons in transgenic β-amyloid precursor protein/presenilin 1 (APP/PS1) mice (Wang et al., 2019). Our latest study found that Trx-1 exhibited a potential inhibition on the canonical NOD-like receptor pyrin domain containing 1 (NLRP1)/caspase-1/gasdermin D (GSDMD) pyroptotic pathway (Jia et al., 2022b). However, current research on Trx-1 in AD is limited to inhibition of Aβ toxicity, reduction of apoptosis, etc. It is largely unclear whether Trx-1 could regulate the mitochondrial biogenesis in AD.
In this study, Aβ25−35 was used to mimic the neurotoxic role of Aβ1−42 in experimental studies because it is the neurotoxic fragment of Aβ1−42 (Kaminsky et al., 2010). The dose of Aβ25−35 and time of application were determined in our previous study (Jia et al., 2022b). Here we reported the effect of Trx-1 on mitochondrial biogenesis in AD. Furthermore, we also clarified that Trx-1 promoted mitochondrial biogenesis via reversing adenosine monophosphate-activated protein kinase (AMPK)/silent information regulator factor 2-related enzyme 1 (Sirt1)/peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) pathway.
Materials and Methods
Chemicals
Aβ25−35 was synthetized by the ChinaPeptides Co., Ltd. (Shanghai, China). Dorsomorphin (Compound C, B3252) and G418 (A2513) were purchased from APExBIO (Houston, USA). AMPK mouse monoclonal antibody (bsm-33236 M) and Sirt1 rabbit ployclonal antibody (bs-0921R) were purchased from Biosynthesis Biotechnology Co., Ltd. (Beijing, China). PGC1α rabbit antibody (ab54481) was obtained from Abcam (Shanghai, China). Caspase-3 rabbit monoclonal antibody (D3R6Y) was obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). Aβ mouse monoclonal antibody (TA500973S) was obtained from Origene (Rockville, MD, USA). Active Oxygen Detection Kit (S0033S), Annexin V-mCherry Apoptosis Detection Kit (C1070 M), Mito-Tracker Red CMXRos (C1035), and pCMV-Mito-AT1.03 (D2606) were obtained from Beyotime (Shanghai, China). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) was purchased from Solarbio (Beijing, China).
Cell Culture
Rat adrenal pheochromocytoma (PC12) cells and mouse N2a cells were obtained from National Collection of Authenticated Cell Cultures (Chinese Academy of Sciences, Shanghai, China). PC12 cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen, Grand Island, NY, USA) supplemented with fetal bovine serum (10%) and antibiotics (100 IU/ml penicillin and 100 mg/ml streptomycin). N2a cells were cultured in Dulbecco's modified eagle medium (DMEM) supplemented with fetal bovine serum (10%) and antibiotics (100 IU/ml penicillin and 100 mg/ml streptomycin). All cultures were maintained in a 37°C humid incubator containing 5% CO2.
Stable Overexpression of Trx-1 in PC12 Cells
Lentivirus (LenV) transfection was employed to stably overexpress human Trx-1 (hTrx-1) in PC12 cells. PC12 cells were infected with LenV-Trx-1 (Hanbio, Shanghai, China). After infection for 48 h, puromycin (2.0 μg/ml) was added to kill cells that were not effectively infected. A strain with stable expression of Trx-1 was finally obtained under the maintenance of puromycin.
Preparation of Aβ25−35
Aβ25−35 was dissolved in deionized distilled water at 2 mM, and subsequently aged for 7 days in 37°C water bath before being used.
Cell Viability
Wild type (WT) or Trx-1 overexpressing PC12 cells were seeded in a 96-well plate overnight for adhesion (2 × 104/well). The cells were administrated with Aβ25−35 (40 μM) for 48 h. Cellular viability was tested by MTT assay according to the manufacturer's instructions by using a microplate reader (Tecan SPARK, Austria). Results are presented as percentages of the control group which was defined as 100%.
ROS Detection
ROS were detected by using Active Oxygen Detection Kit. N2a cells were seeded in a 6-well plate (2 × 105/well) overnight for adhesion. After administration with recombinant human Trx-1 (rhTrx-1, 50 μg/ml) for 6 h, the cells were treated with Aβ25−35 for 48 h, followed by incubation with 2',7'-dichlorofluorescein diacetate (DCFH-DA) (10 μM) for 20 min. The ROS levels were in green color and were recorded with a fluorescence microscope.
Cellular Apoptosis Detection
Cellular apoptosis was detected by using Annexin V-mCherry Apoptosis Detection Kit. PC12 cells were seeded in a 6-well plate (2 × 105/well) overnight for adhesion. The cells were treated with Aβ25−35 for 48 h, followed by incubation with Annexin V-mCherry in dark at room temperature for 20 min. The apoptosis was in red color and were recorded with a fluorescence microscope.
Detection of Mitochondrial Number
Number of mitochondria in cells was detected by using Mito-Tracker Red CMXRos, which can specifically label mitochondria with biological activity in cells. PC12 cells were seeded in a 6-well plate (2 × 105/well) overnight for adhesion. The cells were treated with Aβ25−35 for 48 h at the presence or absence of Dorsomorphin (10 μM), followed by incubation with CMXRos (100 nM) for 20 min in a cell incubator. The mitochondrial number (red color) was observed with a fluorescence microscope.
Detection of ATP Content
PC12 cells were seeded in a 6-well plate (1 × 105/well) overnight for adhesion and then transfected with pCMV-Mito-AT1.03 (mitochondrial adenosine triphosphate [ATP] fluorescence probe, green) for 48 h. G418 (500 μg/ml) were added to screen cell lines stably expressing AT1.03 in mitochondria, which target ATP. PC12 cells stably expressing AT1.03 were seeded in a 6-well plate (2 × 105/well) overnight for adherence. After administration with rhTrx-1 (50 μg/ml) for 6 h, the cells were treated with Aβ25−35 for 48 h. The ATP content was observed with a fluorescence microscope.
Animal Experiments
Male FAD4T APP/PS1 mice and WT littermates, 2 months old, were purchased from GemPharmtech (Nanjing, China) and used in this experiment. Mice were housed in cages and maintained on a 12 h light–dark cycle and had free access to water and food. Mice were divided randomly into four groups: WT + AAV-Vector (n = 6), WT + AAV-Trx-1 (n = 6), APP/PS1 + AAV-Vector (n = 5), and APP/PS1+ AAV-Trx-1 groups (n = 5). The adeno-associated virus (AAV-Vector or AAV-Trx-1, Hanbio, Shanghai, China) were stereotaxically injected into the hippocampal cornu ammonis 1 (CA1) of mice. After recovery from the surgery, the animals were kept for virus expression for about 4 weeks before they were used in the experiments. Mice were subjected to Morris Water Maze (MWM) test when they were 5 months old. The experimental procedures were carried out according to guidelines for the use of Experimental Animal Ethics Committee of Jiaxing University Medical College.
Stereotaxical Injection of AAV in Hippocampus
Mice were anesthetized by injecting intraperitoneally with pentobarbital sodium (50 mg/kg), positioned in a stereotaxic frame (RWD Life Science Co., Ltd, Shenzhen, China), and injected stereotaxically with 0.5 μl AAV-Vector or AAV-Trx-1 into dexter SNc of mice (from bregma: anteroposterior = −1.5 mm, lateral = −2.4 mm, depth ventral = −1.5 mm). The injection lasted for 5 min. The needle was left in the position for 5 min after injection and then withdrawn slowly to avoid reflux. After suturing and applying moderate lidocaine cream and triple antibiotic to the wound, the mice were put on an animal heating blanket until they revived.
Morris Water Maze Test
The MWM test was performed to assess spatial learning and memory of mice. This device consists of a circular pool (40 cm in depth with a diameter of 120 cm, filled with water with milk added, 26°C), a hidden platform (1.0 cm below the water surface), and a video recording system. The tank was divided into four quadrants. The entire test consisted of the training to seek for the hidden platform for 5 days (Days 1–5) and the probe test without the platform. In each training, a mouse would be steered to the platform to stay for 30 s if it failed to mount the platform by itself in 1 min. On Day 6, record the escape latency of every mouse in the training period. The probe test was performed after removing the platform. The animals were placed in the opposite quadrant for 2 min. Record the number of platform crossings to assess spatial memory.
Immunohistochemistry
Mice were executed and heart perfusion was implemented with saline. The brains were quickly isolated, fixed in paraformaldehyde solution (4%) for 24 h, and then dehydrated in 25% sucrose solution until the brain tissues sank to the bottom. Brain tissues were embedded in optimal cutting temperature compound and coronally cut into sections with a thickness of 8 μM in a Leica frozen microtome. After incubation with 0.25% Triton X-100 for 10 min, the sections were blocked in blocking solution (5% bovine serum albumin in phosphate balanced saline [PBS]) for 1 h at 37°C and then incubated with primary anti-Aβ antibody overnight at 4°C. The sections were incubated with an appropriate biotinylated secondary antibody for 1 h at 37°C after the primary antibodies were rinsed, and then were blocked with 3,3-diaminobenzidine (DAB). Finally, the staining was imaged using a Leica microscope and was quantified with ImageJ.
Western Blotting
Protein lysates were prepared using the radio immunoprecipitation assay lysate (CWBIO, Taizhou, China) supplemented with protease and phosphatase inhibitors. Protein concentration was determined using a BCA Protein Assay Kit (CWBIO, Taizhou, China). An equal quantity of proteins was separated by sodium dodecyl sulfate-polyacrylamidegel electrophoresis and transferred to a polyvinylidene fluoride membrane (Millipore Corporation, Billerica, MA, USA). The membrane was blocked in 10% skim milk (in PBS containing 0.1% Tween-20) or 5% albumin bovine V (in Tris buffered saline containing 0.1% Tween-20) for 2 h at room temperature, and then incubated with primary antibodies (1:1000) overnight at 4°C followed by incubation with Horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG (1: 10,000) (CWBIO, Taizhou, China). The epitope was visualized by an eECL Western blot Kit (CWBIO, Taizhou, China). ImageJ software was employed to perform densitometry analysis.
Data Analysis
Data were presented as means ± standard error of mean (SEM) values. Statistical Package for the Social Sciences (SPSS) software was employed to perform statistical analysis. The one-way analysis of variance (ANOVA) followed by a multiple comparison test was used to compare control and treated groups. It was considered statistically significant when p values were <.05. All experiments were performed at least three times.
Results
Stable Overexpression of Trx-1 Resisted the Neurotoxicity of Aβ25−35
In order to research the neuroprotection of Trx-1 in AD, PC12 cells were transfected with LenV-Trx-1 followed by puromycin screening and we obtained the PC12 cells with stably overexpressing Trx-1 (Figure 1(A)). Control PC12 cells and Trx-1 overexpressing PC12 cells were stimulated with Aβ25−35 (40 μM) for 48 h. In the bright field, Aβ25−35 significantly altered the morphology of PC12 cells and this change was reversed by Trx-1 overexpression (Figure 1(B)). MTT assay was further employed to assess the effect of Trx-1 on the neurotoxicity of Aβ25−35. As shown in Figure 1(C), Aβ25−35 treatment markedly decreased the viability of PC12 cells and the neurotoxicity were blocked by Trx-1 overexpression.
Figure 1.
Stable overexpression of Trx-1 resisted the neurotoxicity of Aβ25−35. (A) PC12 cells were transfected with LenV-Trx-1 and screened with puromycin. PC12 cells stably overexpressing Trx-1 were obtained. (B) The cell morphology of WT or Trx-1 overexpressed PC12 cells treated with Aβ (40 μM) for 48 h. (C) MTT assay of cell viability. Trx-1 overexpression inhibited the neurotoxicity of Aβ25−35. Statistics were calculated by one-way ANOVA with Tukey's multiple comparisons test and statistical data were expressed as means ± SEM from 5 independent experiments (n = 5). F (3, 16) = 114.1, ***p < .001. ANOVA=analysis of variance; LenV= Lentivirus; Trx-1=thioredoxin-1.
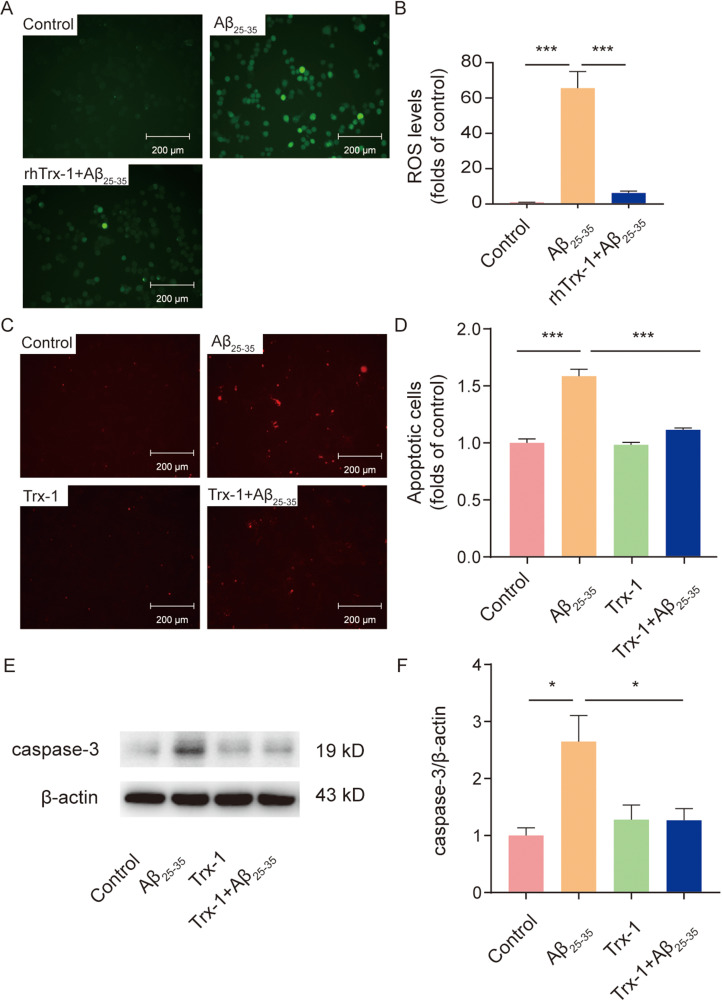
Trx-1 Inhibited Aβ25−35-Induced ROS Production and Apoptosis
We further detected the effects of Trx-1 on Aβ25−35-induced ROS production and cellular apoptosis. As shown in Figure 2(A) and (B), Aβ25−35 treatment significantly induced the production of ROS, which was effectively blocked by rhTrx-1 pre-incubation in N2a cells. Apoptosis analysis was performed by using Annexin V-mCherry Apoptosis Detection Kit. Annexin V could selectively bind phosphosylserine on the outer surface of the membrane of apoptotic cells. The degree of apoptosis was reflected by the fluorescence intensity of Annexin V-coupled mCherry. Aβ25−35 treatment significantly increased the apoptosis of PC12 cells and Trx-1 overexpression inhibited the Aβ25−35-induced cell apoptosis (Figure 2(C) and (D)). Further, we also found that caspase-3 was activated in Aβ25−35-treated PC12 cells, which was significantly inhibited by Trx-1 overexpression (Figure 2(E) and (F)). These data suggest that Trx-1 could inhibit oxidative stress and apoptosis in AD.
Figure 2.
Trx-1 inhibited Aβ25−35-induced ROS production and apoptosis. (A) Pretreatment with rhTrx-1 (50 μg/ml) inhibited Aβ25−35-induced ROS production (green fluorescence) in N2a cells. Nonfluorescent DCFH was oxidized by cellular ROS to DCF with green fluorescence. (B) Statistical analysis of Aβ25−35-induced ROS production in N2a cells. The green fluorescence intensity was quantified with ImageJ. (C) Trx-1 overexpression inhibited Aβ25−35-induced apoptosis (red fluorescence) in PC12 cells. Annexin V-mCherry could couple with the phosphatidylserine on the outer membrane of apoptotic cells and mCherry marked with red fluorescent protein could indicate apoptotic cells. (D) Statistical analysis of Aβ25−35-induced apoptosis in PC12 cells. The red fluorescence intensity was quantified with ImageJ. (E) Trx-1 overexpression inhibited Aβ25−35-induced caspase-3 activation in PC12 cells. (F) Gray scale analysis of caspase-3 expression in Aβ25−35-treated PC12 cells with or without Trx-1 overexpression. Statistical analysis of Aβ25−35-induced apoptosis in PC12 cells. Statistics were calculated by one-way ANOVA with Tukey's multiple comparisons test (B and D) and statistical data were expressed as means ± SEM from three independent experiments (n = 3). F (2, 6) = 43.44 (B), F(3, 8) = 59.96 (D), F(3, 8) = 6.598 (F), *p < .05, ***p < .001. One representative experiment was shown. ANOVA=analysis of variance; ROS= reactive oxygen species; Trx-1=thioredoxin-1.
Trx-1 Promoted Mitochondrial Biogenesis in Aβ25−35-Treated Cells
In cells, mitochondria are the primary organelle to produce ROS, which directly impair the organelle, leading to mitochondrial pathway-mediated apoptosis. Thus, it is highly necessary to detect the effect of Trx-1 on mitochondrial balance in AD cells. Mito-Tracker Red CMXRos, an oxidized red fluorescent dye containing mildly thiol-reactive chloromethyl which could specifically label mitochondria with bioactivity after the dye was passively transported through the cell membrane, was employed to detect the number of mitochondria. Thus, the intensity of the red dye measured with ImageJ could inflect the number of mitochondria with biological activity in cells. As shown in Figure 3(A) and (B), Aβ25−35 treatment significantly decreased the mitochondrial number; while Trx-1 overexpression restored the mitochondrial number. We also detected the effect of Trx-1 on the ATP content in mitochondria. We established the PC12 cells overexpressing Mito-AT1.03, a mitochondrial ATP fluorescence probe (green) with mitochondrial localization signal. Mito-AT1.03 could be used to monitor the real time change of ATP content in mitochondria. The intensity of green fluorescence reflects the ATP content. As shown in Figure 3(C) and (D), Aβ25−35 treatment significantly decreased the ATP levels and rhTrx-1 pretreatment effectively reversed the alteration. These data suggest that Trx-1 promotes mitochondrial biogenesis in AD cells.
Figure 3.
Trx-1 promoted mitochondrial biogenesis in Aβ25−35-treated cells. (A) Mito-Tracker Red CMXRos was used to monitor the number of mitochondria with biological activity in the experiment. Thus, the intensity of the red dye measured with ImageJ could inflect the number of mitochondria with biological activity in cells. Aβ25−35 treatment decreased the number of mitochondria in PC12 cells, which were reversed by Trx-1 overexpression. (B) Statistical analysis of the number of mitochondria. (C) Pretreatment with rhTrx-1 (50 μg/ml) inhibited Aβ25−35-induced decrease in ATP content (green fluorescence) in PC12 cells. (D) Statistical analysis of ATP content. Statistics were calculated by one-way ANOVA with Tukey's multiple comparisons test (B and D) and statistical data were expressed as means ± SEM from three independent experiments (n = 3). F (3, 12) = 42.10 (B), F(2, 6) = 56.61 (D), **p < .01, ***p < .001. ANOVA=analysis of variance; ATP= adenosine triphosphate; Trx-1=thioredoxin-1.
Trx-1 Overexpression Ameliorated the Learning and Memory and Attenuated Hippocampal Aβ Deposition in APP/PS1 Mice
We further investigated the neuroprotective roles of selective Trx-1 overexpression in the hippocampus of APP/PS1 mice by employing brain stereotaxical injection of AAV when mice were 2 months old. The experiment schedule was shown in Figure 4(A). When the mice were 5 months old, they were subjected to MWM training (Days 1–5) and test (Day 6) and then were cervically dislocated to dissociate brain tissues. From the frozen sections, both of the AAV-Vector and AAV-Trx-1 were highly expressed in the hippocampus of mice (Figure 4(B)). After the MWM training for 5 days, the escape latency of the APP/PS1 mice injected with AAV-Vector was significantly extended compared to that of WT mice injected with AAV-Vector, which was significantly shortened in APP/PS1 mice injected with AAV-Trx-1 (Figure 4(C) and (D)). On the contrary, the times crossing the original platform area of the APP/PS1 mice injected with AAV-Vector in 2 min test were significantly reduced compared to that of WT mice injected with AAV-Vector after removing the platform, which were also reversed by Trx-1 overexpression in APP/PS1 mice (Figure 4(E) and (F)). These data suggest that Trx-1 overexpression substantially ameliorated the learning and memory deficit of APP/PS1 mice. Immunohistochemistry (IHC) was employed to further research the neuroprotection of Trx-1 overexpression in AD mice. As shown in Figure 4(G) and (H), hippocampal Aβ deposition of APP/PS1 mice injected with AAV-Vector was markedly increased compared to that of WT mice injected with AAV-Vector, which was effectively inhibited by Trx-1 overexpression in APP/PS1 mice, suggesting that Trx-1 overexpression attenuated hippocampal Aβ deposition in AD mice.
Figure 4.
Trx-1 overexpression ameliorated the learning and memory and attenuated hippocampal Aβ deposition in APP/PS1 mice. (A) The experiment schedule. (B) Brain stereotaxical injection of AAV and their expression. (C) The swimming track of mice before getting on the hidden platform. (D) The escape latency of mice to get on the hidden platform. (E) The swimming track of mice crossing the original platform area. (F) The passing times of mice to cross the original platform area. (G) Hippocampal Aβ deposition in APP/PS1 mice was alleviated by Trx-1 overexpression. (H) The Aβ deposition was quantified with ImageJ. Statistics were calculated by one-way ANOVA with Tukey's multiple comparisons test (D and F) and statistical data were expressed as means ± SEM (n = 6 in WT + AAV-Vector and WT + AAV-Trx-1 groups, n = 3 in APP/PS1 + AAV-Vector and APP/PS1 + AAV-Trx-1 groups). F (3, 18) = 7.261 (D), F(3, 18) = 5.565 (F), F(3, 8) = 112.6 (H), *p < .05, **p < .01, ***p < .001. AAV= adeno-associated virus; ANOVA=analysis of variance; MWM= Morris Water Maze; Trx-1=thioredoxin-1.
Trx-1 Reversed AMPK/Sirt1/PGC1α Pathway in PC12 Cells and the Hippocampus
AMPK/Sirt1/PGC1α pathway has been demonstrated to promote mitochondrial biogenesis in various diseases, such as ischemia/reperfusion, chronic kidney disease, and colitis (El-Ghannam et al., 2022; Li et al., 2022a; Wang, 2022). However, it is still largely unclear whether AMPK/Sirt1/PGC1α pathway plays a role in the neuroprotection of Trx-1 in AD. In this study, western blot (WB) was used to detect the expression of AMPK, Sirt1, and PGC1α. In PC12 cells, Aβ25−35 treatment significantly reduced the expression of AMPK, Sirt1, and PGC1α, which were restored by Trx-1 overexpression (Figure 5(A)–(D)). In the APP/PS1 mice injected with AAV-Vector, the expression of AMPK, Sirt1, and PGC1α in the hippocampus was significantly decreased when compared to WT mice injected with AAV-Vector; while the reduction was also reversed by Trx-1 overexpression in APP/PS1 mice (Figure 5(E)–(H)).
Figure 5.
Trx-1 reversed AMPK/Sirt1/PGC1α pathway. The expression of AMPK, Sirt1, and PGC1α were determined by western blots. (A) The expression of AMPK, Sirt1, and PGC1α in PC12 cells treated with Aβ25–35 was significantly decreased when compared to control group. The reduction was reversed by Trx-1 overexpression in PC12 cells. (B) Gray scale analysis of AMPK in PC12 cells. (C) Gray scale analysis of Sirt1 in PC12 cells. (D) Gray scale analysis of PGC1α in PC12 cells. (E) The expression of AMPK, Sirt1, and PGC1α in the hippocampus of the APP/PS1 mice injected with AAV-Vector was significantly decreased when compared to WT mice injected with AAV-Vector. The reduction was reversed by Trx-1 overexpression in APP/PS1 mice. AMPK and Sirt1 were applied on one gel, PGC1α was applied on another gel. (F) Gray scale analysis of AMPK in mice. (G) Gray scale analysis of Sirt1 in mice. (H) Gray scale analysis of PGC1α in mice. Statistics were calculated by one-way ANOVA with Tukey's multiple comparisons test (B–D and F–H) and statistical data were expressed as means ± SEM from three independent experiments (n = 3). F (3, 8) = 16.28 (B), F (3, 8) = 9.968 (C), F (3, 8) = 47.99 (D), F (3, 8) = 8.352 (F), F (3, 8) = 10.55 (G), F (3, 8) = 14.78 (H), *p < .05, **p < .01, ***p < .001. AAV= adeno-associated virus; AMPK=adenosine monophosphate-activated protein kinase; ANOVA=analysis of variance; PGC1α= peroxisome proliferator-activated receptor gamma coactivator 1-alpha; Sirt1=silent information regulator factor 2-related enzyme 1; Trx-1=thioredoxin-1.
Inhibition of AMPK Abolished the Effect of Trx-1 on Mitochondrial Biogenesis
In order to clarify the role of AMPK/Sirt1/PGC-1α pathway in the promotion of Trx-1 on mitochondrial biogenesis in AD mice, the activity of AMPK was inhibited with a selective inhibitor, Dorsomorphin (Dor). WT or Trx-1 overexpressing PC12 cells were pretreated with or without Dor (10 μM) for 30 min, followed by incubation with Aβ25−35 (40 μM) for 48 h. Mito-Tracker Red CMXRos was used to monitor the number of mitochondria in each group. As shown in Figure 6, the promotion of Trx-1 on mitochondrial biogenesis was abolished after AMPK inhibition. These findings suggest that Trx-1 promotes mitochondrial biogenesis via restoring AMPK/Sirt1/PGC-1α pathway in AD.
Figure 6.
Inhibition of AMPK abolished the effect of Trx-1 on mitochondrial biogenesis. Mito-Tracker Red CMXRos was used to monitor the number of mitochondria with biological activity in the experiment. (A) Trx-1 overexpression reversed Aβ25−35 treatment-induced decrease in the number of mitochondria in PC12 cells, which were abolished by pre-incubation with AMPK inhibitor, Dor (10 μM). (B) Statistical analysis of the number of mitochondria. Statistics were calculated by one-way ANOVA with Tukey's multiple comparisons test and statistical data were expressed as means ± SEM from three independent experiments (n = 3). F (5, 16) = 39.40, ***p < .001; AMPK= adenosine monophosphate-activated protein kinase; ANOVA=analysis of variance; n.s.= no statistical significance; Trx-1=thioredoxin-1.
Discussion
In the present study, we provide evidence that Trx-1 overexpression promoted mitochondrial biogenesis via restoring AMPK/Sirt1/PGC-1α pathway in AD.
AD, the most common neurodegenerative disease, affects about 10 million Chinese people according to the “Alzheimer's Disease Report in China 2021,” which is increasing the social and economic burden. Unfortunately, AD cannot be cured clinically at present because of its complex etiopathogenesis. Thus, it is urgent to find new effective prevention and cure strategies. Accumulating studies suggest that mitochondrial dysfunction occurs in both familial and sporadic AD (Lin & Beal, 2006; McManus et al., 2011). The brain tissues of both living and dead patients with AD showed impaired mitochondrial function, such as decreased glucose uptake and decreased activities of enzymes related to mitochondrial tricarboxylic acid cycle and oxidative phosphorylation (Kerr et al., 2017). Mitochondrial dysfunction produced excessive ROS, which induced apoptosis (Walia et al., 2022). Our study also found that Aβ25−35 treatment yielded excessive ROS and induced cellular apoptosis (Figures 1 and 2). What's more, the number of mitochondria with biological activity and the ATP content in mitochondria were significantly decreased after Aβ25−35 treatment (Figure 3). These data suggest that AD cells suffer a remarkable mitochondrial dysfunction.
Trx-1, one of the major redox proteins in mammalian cells, plays an important neuroprotection in neurodegenerative diseases, including AD (Jia et al., 2022a). The levels of Trx-1 in peripheral tissues from patients with AD, such as cerebrospinal fluid and plasma, are significantly increased (Arodin et al., 2014; Cornelius et al., 2013), which may provide a defense mechanism against oxidative stress. However, Trx-1 is notably reduced in the brain of AD model animals and patients (Akterin et al., 2006; Di Domenico et al., 2010), which leads to the loss of neurons and final neurodegeneration. In the present study, administration of exogenous rhTrx-1 inhibited the production of ROS induced by Aβ25−35 incubation and overexpression of Trx-1 in PC12 cells suppressed Aβ25−35-induced apoptosis (Figure 2). These findings are consistent with the previous studies involving the neuroprotective roles of Trx-1 in AD (Jia et al., 2021). On the other hand, AAV-mediated Trx-1 overexpression in the hippocampus shortened the escape latency of APP/PS1 mice and restored the crossing times (Figure 4(C)–(F)), suggesting that specific Trx-1 overexpression ameliorated the learning and memory deficit of APP/PS1 mice. Our finding are consistent with the study by Yang et al., in which the animals with higher messenger RNA and protein levels of Trx-1 in the hippocampus showed shorter latency and increased numbers to cross the previous target platform in the MWM test (Yang et al., 2012). Our study also found that Trx-1 overexpression attenuated Aβ deposition in the hippocampus of APP/PS1 mice (Figure 4(G) and (H)). This is the first time to directly report the effect of Trx-1 on Aβ deposition in AD models.
Accumulating evidence have demonstrated that amelioration of mitochondrial homeostasis to restore the normal function of mitochondria is beneficial for the prevention or treatment of AD (Li et al. 2022c; Li et al., 2022b). Thus, whether Trx-1 regulates mitochondrial homeostasis in AD was further investigated. We found that Trx-1 overexpression significantly restored the decrease in the number of mitochondria and the ATP content in mitochondria induced by Aβ25−35 incubation (Figure 3), suggesting that Trx-1 overexpression promotes the mitochondrial biogenesis in AD. The deficiency of AMPK/Sirt1/PGC1α signaling pathway is closely associated with AD pathogenesis (Shah et al., 2017; Sun et al., 2019; Zhang et al., 2017). AMPK could activate Sirt1 by elevating the nicotinamide adenine dinucleotide (NAD+) levels in cells, resulting in the activation of PGC1α (Canto et al., 2009; Fulco & Sartorelli, 2008). AAV-mediated overexpression of PGC1α in the lateral parietal association reversed the damage and swelling of mitochondria in cortical neurons through improving abnormal mitochondrial dynamics (Wang et al., 2022a). Dong et al. have defined that Aβ25−35 inhibited the mitochondrial biogenesis in cultured primary hippocampal neurons via attenuating the AMPK/Sirt1/PGC1α signaling pathway (Dong et al., 2016). Thus, the expression of AMPK, Sirt1, and PGC1α in the hippocampus of mice was detected. The western blot results showed that the levels of these proteins were downregulated in Aβ25−35-treated PC12 cells and in the hippocampus of APP/PS1 mice compared to the WT mice (Figure 5). Importantly, Trx-1 overexpression restored their changes (Figure 5) suggesting Trx-1 recovered the AMPK/Sirt1/PGC1α signaling pathway. Pharmacological inhibition of AMPK with a selective inhibitor Dorsomorphin abolished the effect of Trx-1 on promoting the mitochondrial biogenesis (Figure 6). This data is consistent with a recent study in which Dorsomorphin pre-administration partially inhibited liraglutide-induced stimulation of Sirt1 protein in acute demyelination model (Ammar et al., 2022). A previous study also demonstrated that AMPK could enhance Sirt1 activity by increasing cellular NAD+ levels, resulting in the deacetylation and modulation of the activity of downstream Sirt1 targets that include the PGC1α (Canto et al., 2009), suggesting that AMPK might act upstream to promote mitochondrial biogenesis. These data demonstrate that Trx-1 promotes the mitochondrial biogenesis in AD via activating AMPK/Sirt1/PGC1α signaling pathway.
In the present study, Mito-Tracker Red CMXRos was mainly used to evaluate mitochondrial biogenesis. Mito-Tracker dyes not only provided a measure of mitochondrial number or size, but their accumulation is also dependent upon mitochondrial membrane potential. Thus, increased signals of the dyes could reflect changes in membrane potential and not mitochondrial biogenesis. Though the expression of PGC1α, a direct marker of mitochondrial biogenesis, was determined by westerns blot in this study, more indicators, such as mitochondrial ATP production rate, number of mitochondria, mitochondrial DNA copy number, should be detected in the future study to comprehensively reflect the effect of Trx-1 on mitochondrial biogenesis in AD.
Though AMPK could upregulate the expression of Trx-1 under different conditions (Gao et al., 2014; Li et al., 2009), Trx-1 could act as an essential cofactor for AMPK activation because Trx-1 cleaved the disulfides in AMPK proteins and prevents its oxidation (Shao et al., 2014). How Trx-1 overexpression regulates the expression of AMPK need to be further studied. Poly(ADP-ribose) polymerase 1 (PARP-1) plays an important role in the activation of Sirt1 because PARP-1 is a major modulator of NAD+ metabolism (Sajish & Schimmel, 2015). Trx-mimetic peptides inhibited caspase-3 cleavage and prevented the dissociation of PARP-1 (Cohen-Kutner et al., 2013), suggesting that the reduced form of Trx-1 has a potential capacity to regulate PARP-1. The molecular mechanisms underlying that Trx-1 upregulates the AMPK/Sirt1/PGC1α signaling pathway in AD needs to be studied in the future.
Author Contributions: Jinjing Jia was involved in conceptualization, experimental operation, writing-original draft, data analysis, and funding acquisition. Jiayi Yin performed experimental operation and data analysis. Yu Zhang was involved in experimental operation; Guangtao Xu in data analysis; Min Wang in experimental operation; Haiying Jiang in writing-review and editing; Li Li in review and editing; Xiansi Zeng in conceptualization, writing-review and editing; and Dongsheng Zhu in writing-review and editing. All authors have read the paper and approved the submission.
ORCID iD: Xiansi Zeng https://orcid.org/0000-0002-1495-701X
Data Availability Statement: Data will be made available on request.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This research was carried out with the approval of Animal Ethics Committee of Jiaxing University Medical College (No.: JUMC2021-144).
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Zhejiang Provincial Natural Science Foundation of China (grant nos.: LQ22H090003 and LTGY23C090001), Natural Science Foundation of Henan Province (202300410332), Sci-Tech Planning Project of Jiaxing (2022AY30020), and Research Foundation for Advanced Talents of Jiaxing University (CD70520018).
References
- Akterin S., Cowburn R. F., Miranda-Vizuete A., Jimenez A., Bogdanovic N., Winblad B., Cedazo-Minguez A. (2006). Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer's disease. Cell Death & Differentiation, 13(9), 1454–1465. https://doi.org/10.1038/sj.cdd.4401818 [DOI] [PubMed] [Google Scholar]
- Ammar R. A., Mohamed A. F., Kamal M. M., Safar M. M., Abdelkader N. F. (2022). Neuroprotective effect of liraglutide in an experimental mouse model of multiple sclerosis: Role of AMPK/SIRT1 signaling and NLRP3 inflammasome. Inflammopharmacology, 30(3), 919–934. 10.1007/s10787-022-00956-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arodin L., Lamparter H., Karlsson H., Nennesmo I., Bjornstedt M., Schroder J., Fernandes A. P. (2014). Alteration of thioredoxin and glutaredoxin in the progression of Alzheimer's disease. Journal of Alzheimer's Disease: JAD, 39(4), 787–797. 10.3233/JAD-131814 [DOI] [PubMed] [Google Scholar]
- Canto C., Gerhart-Hines Z., Feige J. N., Lagouge M., Noriega L., Milne J. C., Elliott P. J., Puigserver P., Auwerx J. (2009). AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature, 458(7241), 1056–1060. 10.1038/nature07813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Kutner M., Khomsky L., Trus M., Aisner Y., Niv M. Y., Benhar M., Atlas D. (2013). Thioredoxin-mimetic peptides (TXM) reverse auranofin induced apoptosis and restore insulin secretion in insulinoma cells. Biochemical Pharmacology, 85(7), 977–990. 10.1016/j.bcp.2013.01.003 [DOI] [PubMed] [Google Scholar]
- Cornelius C., Trovato Salinaro A., Scuto M., Fronte V., Cambria M. T., Pennisi M., Bella R., Milone P., Graziano A., Crupi R., Cuzzocrea S., Pennisi G., Calabrese V. (2013). Cellular stress response, sirtuins and UCP proteins in Alzheimer disease: Role of vitagenes. Immunity & Ageing, 10(1), 41. https://doi.org/10.1186/1742-4933-10-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico F., Sultana R., Tiu G. F., Scheff N. N., Perluigi M., Cini C., Butterfield D. A. (2010). Protein levels of heat shock proteins 27, 32, 60, 70, 90 and thioredoxin-1 in amnestic mild cognitive impairment: An investigation on the role of cellular stress response in the progression of Alzheimer disease. Brain Research, 1333, 72–81. 10.1016/j.brainres.2010.03.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Wang F., Guo W., Zheng X., Chen Y., Zhang W., Shi H. (2016). Abeta25-35 suppresses mitochondrial biogenesis in primary hippocampal neurons. Cellular and Molecular Neurobiology, 36(1), 83–91. 10.1007/s10571-015-0222-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghannam M. S., Saad M. A., Nassar N. N., El-Yamany M. F., El-Bahy A. A. Z. (2022). Linagliptin ameliorates acetic acid-induced colitis via modulating AMPK/SIRT1/PGC-1alpha and JAK2/STAT3 signaling pathway in rats. Toxicology and Applied Pharmacology, 438, 115906. 10.1016/j.taap.2022.115906 [DOI] [PubMed] [Google Scholar]
- Fulco M., Sartorelli V. (2008). Comparing and contrasting the roles of AMPK and SIRT1 in metabolic tissues. Cell Cycle, 7(23), 3669–3679. 10.4161/cc.7.23.7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K., Chi Y., Sun W., Takeda M., Yao J. (2014). 5'-AMP-activated protein kinase attenuates adriamycin-induced oxidative podocyte injury through thioredoxin-mediated suppression of the apoptosis signal-regulating kinase 1-P38 signaling pathway. Molecular Pharmacology, 85(3), 460–471. 10.1124/mol.113.089458 [DOI] [PubMed] [Google Scholar]
- Hedskog L., Zhang S., Ankarcrona M. (2012). Strategic role for mitochondria in Alzheimer's disease and cancer. Antioxidants & Redox Signaling, 16(12), 1476–1491. https://doi.org/10.1089/ars.2011.4259 [DOI] [PubMed] [Google Scholar]
- Hodson R. (2018). Alzheimer's disease. Nature, 559(7715), S1. 10.1038/d41586-018-05717-6 [DOI] [PubMed] [Google Scholar]
- Jia J., Xu G., Zhu D., Liu H., Zeng X., Li L. (2022a). Advances in the functions of thioredoxin system in central nervous system diseases. Antioxidants & Redox Signaling. https://doi.org/10.1089/ars.2022.0079 [DOI] [PubMed] [Google Scholar]
- Jia J., Zeng X., Xu G., Wang Z. (2021). The potential roles of redox enzymes in Alzheimer's disease: Focus on thioredoxin. ASN Neuro, 13, 1759091421994351. https://doi.org/10.1177/1759091421994351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J., Zhang X., Xu G., Zeng X., Li L. (2022b). Thioredoxin-1 inhibits amyloid-beta25-35-induced activation of NLRP1/caspase-1/GSDMD pyroptotic pathway in PC12 cells. Molecular Biology Reports, 49(5), 3445–3452. 10.1007/s11033-022-07177-8 [DOI] [PubMed] [Google Scholar]
- Jia J. J., Zeng X. S., Li K., Ma L. F., Chen L., Song X. Q. (2016). The expression of thioredoxin-1 in acute epinephrine stressed mice. Cell Stress & Chaperones, 21(5), 935–941. https://doi.org/10.1007/s12192-016-0722-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J. J., Zeng X. S., Zhou X. S., Li Y., Bai J. (2014). The induction of thioredoxin-1 by epinephrine withdraws stress via interaction with beta-arrestin-1. Cell Cycle, 13(19), 3121–3131. 10.4161/15384101.2014.949214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky Y. G., Marlatt M. W., Smith M. A., Kosenko E. A. (2010). Subcellular and metabolic examination of amyloid-beta peptides in Alzheimer disease pathogenesis: Evidence for Abeta(25-35). Experimental Neurology, 221(1), 26–37. 10.1016/j.expneurol.2009.09.005 [DOI] [PubMed] [Google Scholar]
- Kerr J. S., Adriaanse B. A., Greig N. H., Mattson M. P., Cader M. Z., Bohr V. A., Fang E. F. (2017). Mitophagy and Alzheimer's disease: Cellular and molecular mechanisms. Trends in Neurosciences, 40(3), 151–166. 10.1016/j.tins.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. I., Lee K. H., Gabr A. A., Choi G. E., Kim J. S., Ko S. H., Han H. J. (2016). Abeta-induced Drp1 phosphorylation through Akt activation promotes excessive mitochondrial fission leading to neuronal apoptosis. Biochimica et Biophysica Acta, 1863(11), 2820–2834. 10.1016/j.bbamcr.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Li Q., Wu J., Huang J., Hu R., You H., Liu L., Wang D., Wei L. (2022a). Paeoniflorin ameliorates skeletal muscle atrophy in chronic kidney disease via AMPK/SIRT1/PGC-1alpha-mediated oxidative stress and mitochondrial dysfunction. Frontiers in Pharmacology, 13, 859723. 10.3389/fphar.2022.859723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R. L., Wang L. Y., Duan H. X., Zhang Q., Guo X., Wu C., Peng W. (2022b). Regulation of mitochondrial dysfunction induced cell apoptosis is a potential therapeutic strategy for herbal medicine to treat neurodegenerative diseases. Frontiers in Pharmacology, 13, 937289. 10.3389/fphar.2022.937289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Shi Q., Xu H., Xiong Y., Wang C., Le L., Lian J., Wu G., Peng F., Liu Q., Du X. (2022c). Ebselen interferes with Alzheimer's disease by regulating mitochondrial function. Antioxidants (Basel), 11(7),1350. https://doi.org/10.3390/antiox11071350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. N., Song J., Zhang L., LeMaire S. A., Hou X., Zhang C., Coselli J. S., Chen L., Wang X. L., Zhang Y., Shen Y. H. (2009). Activation of the AMPK-FOXO3 pathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes, 58(10), 2246–2257. 10.2337/db08-1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M. T., Beal M. F. (2006). Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature, 443(7113), 787–795. 10.1038/nature05292 [DOI] [PubMed] [Google Scholar]
- McAlpine C. S. Park J. Griciuc A. Kim E. Choi S. H. Iwamoto Y. Kiss M. G. Christie K. A. Vinegoni C. Poller W. C. Mindur J. E. Chan C. T. He S. Janssen H. Wong L. P. Downey J. Singh S. Anzai A. Kahles F.… Swirski F. K. (2021). Astrocytic interleukin-3 programs microglia and limits Alzheimer's disease. Nature, 595(7869), 701–706. 10.1038/s41586-021-03734-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus M. J., Murphy M. P., Franklin J. L. (2011). The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. Journal of Neuroscience, 31(44), 15703–15715. 10.1523/JNEUROSCI.0552-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore L., Coppede F. (2022). Gene-environment interactions in Alzheimer disease: The emerging role of epigenetics. Nature Reviews. Neurology. 18(11) 643-660. https://doi.org/10.1038/s41582-022-00714-w. [DOI] [PubMed] [Google Scholar]
- Persson T., Lattanzio F., Calvo-Garrido J., Rimondini R., Rubio-Rodrigo M., Sundstrom E., Maioli S., Sandebring-Matton A., Cedazo-Minguez A. (2017). Apolipoprotein E4 elicits lysosomal cathepsin D release, decreased thioredoxin-1 levels, and apoptosis. Journal of Alzheimer's Disease, 56(2), 601–617. 10.3233/JAD-150738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajish M., Schimmel P. (2015). A human tRNA synthetase is a potent PARP1-activating effector target for resveratrol. Nature, 519(7543), 370–373. 10.1038/nature14028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. A., Yoon G. H., Chung S. S., Abid M. N., Kim T. H., Lee H. Y., Kim M. O. (2017). Novel osmotin inhibits SREBP2 via the AdipoR1/AMPK/SIRT1 pathway to improve Alzheimer's disease neuropathological deficits. Molecular Psychiatry, 22(3), 407–416. 10.1038/mp.2016.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao D., Oka S., Liu T., Zhai P., Ago T., Sciarretta S., Li H., Sadoshima J. (2014). A redox-dependent mechanism for regulation of AMPK activation by thioredoxin1 during energy starvation. Cell Metabolism, 19(2), 232–245. 10.1016/j.cmet.2013.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Wei L. L., Zhang M., Li T. X., Yang C., Deng S. P., Zeng Q. C. (2019). Rapamycin inhibits activation of AMPK-mTOR signaling pathway-induced Alzheimer's disease lesion in hippocampus of rats with type 2 diabetes mellitus. The International Journal of Neuroscience, 129(2), 179–188. https://doi.org/10.1080/00207454.2018.1491571 [DOI] [PubMed] [Google Scholar]
- Walia V., Kaushik D., Mittal V., Kumar K., Verma R., Parashar J., Akter R., Rahman M. H., Bhatia S., Al-Harrasi A., Karthika C., Bhattacharya T., Chopra H., Ashraf G. M. (2022). Delineation of neuroprotective effects and possible benefits of antioxidants therapy for the treatment of Alzheimer's diseases by targeting mitochondrial-derived reactive oxygen species: Bench to bedside. Molecular Neurobiology, 59(1), 657–680. 10.1007/s12035-021-02617-1 [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Xu Y., Wang X., Guo C., Wang T., Wang Z. Y. (2019). Dl-3-n-butylphthalide inhibits NLRP3 inflammasome and mitigates Alzheimer's-like pathology via Nrf2-TXNIP-TrX axis. Antioxidants & Redox Signaling, 30(11), 1411–1431. https://doi.org/10.1089/ars.2017.7440 [DOI] [PubMed] [Google Scholar]
- Wang G. (2022). Aerobic exercise ameliorates myocardial ischemia/reperfusion injury and thrombosis of diabetic rats via activation of AMPK/Sirt1/PGC-1alpha pathway. General Physiology and Biophysics, 41(4), 319–328. 10.4149/gpb_2022010 [DOI] [PubMed] [Google Scholar]
- Wang J., Liu W. J., Shi H. Z., Zhai H. R., Qian J. J., Zhang W. N. (2022a). A role for PGC-1a in the control of abnormal itochondrial dynamics in Alzheimer's disease. Cells, 11(18), 2849. https://doi.org/10.3390/cells11182849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhou J., Wang C., Fukunaga A., Li S., Yodoi J., Tian H. (2022b). Thioredoxin-1: A promising target for the treatment of allergic diseases. Frontiers in Immunology, 13, 883116. 10.3389/fimmu.2022.883116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. H., Liu H. G., Liu X., Chen J. N. (2012). Thioredoxin and impaired spatial learning and memory in the rats exposed to intermittent hypoxia. Chinese Medical Journal, 125(17), 3074–3080. [PubMed] [Google Scholar]
- Zeng X. S., Geng W. S., Chen L., Jia J. J. (2018). Thioredoxin as a therapeutic target in cerebral ischemia. Current Pharmaceutical Design, 24(25), 2986–2992. 10.2174/1381612824666180820143853 [DOI] [PubMed] [Google Scholar]
- Zeng X. S., Geng W. S., Wang Z. Q., Jia J. J. (2020). Morphine addiction and oxidative stress: The potential effects of thioredoxin-1. Frontiers in Pharmacology, 11, 82. 10.3389/fphar.2020.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. L., Feng H., Li L., Wang J. Y., Wu D., Hao Y. T., Wang Z., Zhang Y., Wu L. L. (2017). Globular CTRP3 promotes mitochondrial biogenesis in cardiomyocytes through AMPK/PGC-1alpha pathway. Biochimica et Biophysica Acta General Subjects, 1861(1 Pt A), 3085–3094. 10.1016/j.bbagen.2016.10.022 [DOI] [PubMed] [Google Scholar]
- Zhuo Y., Guo H., Cheng Y., Wang C., Wang C., Wu J., Zou Z., Gan D., Li Y., Xu J. (2016). Inhibition of phosphodiesterase-4 reverses the cognitive dysfunction and oxidative stress induced by Abeta25-35 in rats. Metabolic Brain Disease, 31(4), 779–791. 10.1007/s11011-016-9814-1 [DOI] [PubMed] [Google Scholar]