Abstract
Sex/gender effects have been demonstrated for multiple aspects of addiction, with one of the most commonly cited examples being the “telescoping effect” where women meet criteria and/or seek treatment of substance use disorder (SUD) after fewer years of drug use as compared with men. This phenomenon has been reported for multiple drug classes including opioids, psychostimulants, alcohol, and cannabis, as well as nonpharmacological addictions, such as gambling. However, there are some inconsistent reports that show either no difference between men and women or opposite effects and a faster course to addiction in men than women. Thus, the goals of this review are to evaluate evidence for and against the telescoping effect in women and to determine the conditions/populations for which the telescoping effect is most relevant. We also discuss evidence from preclinical studies, which strongly support the validity of the telescoping effect and show that female animals develop addiction-like features (e.g., compulsive drug use, an enhanced motivation for the drug, and enhanced drug-craving/vulnerability to relapse) more readily than male animals. We also discuss biologic factors that may contribute to the telescoping effect, such as ovarian hormones, and its neurobiological basis focusing on the mesolimbic dopamine reward pathway and the corticomesolimbic glutamatergic pathway considering the critical roles these pathways play in the rewarding/reinforcing effects of addictive drugs and SUD. We conclude with future research directions, including intervention strategies to prevent the development of SUD in women.
Significance Statement
One of the most widely cited gender/sex differences in substance use disorder (SUD) is the "telescoping effect,” which reflects an accelerated course in women versus men for the development and/or seeking treatment for SUD. This review evaluates evidence for and against a telescoping effect drawing upon data from both clinical and preclinical studies. We also discuss the contribution of biological factors and underlying neurobiological mechanisms and highlight potential targets to prevent the development of SUD in women.
I. Introduction
Despite higher rates of drug use and substance use disorder (SUD) in men, women are more vulnerable than men in many aspects of the disease. One striking example is the "telescoping effect,” which reflects an accelerated course in women versus men for the transition from initiation of substance use to meeting criteria for SUD and/or seeking treatment of SUD. This phenomenon was originally described for alcohol more than 30 years ago (Ashley et al., 1977; Hesselbrock et al., 1985; Piazza et al., 1989). and the observation has been replicated in multiple subsequent studies with alcohol (Hesselbrock et al., 1985; Piazza et al., 1989; Mann et al., 1992, 2005; Randall et al., 1999; McCance-Katz et al., 1999; Hernandez-Avila et al., 2004; Johnson et al., 2005; Diehl et al., 2007; Lewis and Nixon, 2014) as well as with other drug classes, including stimulants (e.g., cocaine, nicotine/tobacco, methamphetamine; Griffin et al., 1989; White et al., 1996; McCance-Katz et al., 1999; Sofuoglu et al., 1999; Haas and Peters, 2000; Brecht et al., 2004; O’Brien and Anthony, 2005; Thorner et al., 2007), opioids (Anglin et al., 1987; Hser et al., 1987; DiFranza et al., 2002; Hernandez-Avila et al., 2004; Back et al., 2011; Lewis et al., 2014; Adelson et al., 2018; Peltier et al., 2021), and cannabis (Haas and Peters, 2000; Hernandez-Avila et al., 2004; Ehlers et al., 2010; Khan et al., 2013; Lewis et al., 2014). It has also been reported for nonpharmacological addictions, such as gambling (Ladd and Petry, 2002; Ibanez et al., 2003; Tavares et al., 2003; Grant et al., 2012).
The telescoping effect in women has been widely noted in studies of SUD, yet there are some inconsistent reports that show either no difference between men and women in the time course for the development of SUD (DiFranza et al., 2007; Alvanzo et al., 2011; Stoltman et al., 2015) or the reverse, a faster course in men than women (Keyes et al., 2010; Slutske et al., 2015). Changes in sociocultural factors, such as a progressive destigmatization of drug use in women over time, have been proposed to account for differences observed between women and men in the original telescoping studies versus more recent ones (Nicolaides, 1996). Recent studies, using population-based surveys, may be further confusing the literature since sex/gender differences in the time course for the development of SUD are confounded by differences in the likelihood of developing SUD and seeking treatment of SUD, both of which are greater in men than women (Greenfield, 2007; Wagner and Anthony, 2007). Some notable exceptions are for psychotherapeutics (i.e., nonmedical use of pain relievers, sedatives, stimulants, and tranquilizers) and tobacco; in these cases, women are more likely than men to develop a SUD (Cotto et al., 2010; Lopez-Quintero et al., 2011). The telescoping effect has also been replicated in several studies conducted during the past). The validity of the telescoping effect is also strongly supported by results from preclinical studies, which show that, like the human situation, female animals develop addiction-like features more readily than male animals (Lynch and Taylor, 2004; Kerstetter et al., 2012; Perry et al., 2013, 2015; Kawa and Robinson, 2019; Towers et al., 2021).
Thus, the purpose of this review is to evaluate evidence for and against the telescoping effect in women and determine the conditions/populations for which the telescoping effect is most relevant. We also discuss preclinical findings of sex differences to establish a biologic basis for the telescoping effect. This evidence is divided into findings from animal models of substance use (see Table 1 for a glossary of terms), which generally use short-access drug self-administration (1–2 h/d) and focus on differences in the acquisition of drug self-administration or maintenance levels of intake or motivation for the drug, versus animal models of SUD, which typically use extended-access drug self-administration (≥6 h/d) and focus on differences in the development and/or expression of addiction-like features like those observed in humans with a SUD (e.g., escalation of drug use, compulsive drug use despite punishment, an enhanced motivation for the drug, enhanced drug-craving/vulnerability to relapse). Mechanisms underlying the telescoping effect are also explored, including the potential for ovarian hormones to drive an enhanced vulnerability in women and female laboratory animals during both initial substance use and with the development of SUD. We also discuss neurobiological mechanisms of substance use and SUD in women and men and male and female laboratory animals focusing on the mesolimbic dopamine reward pathway and corticomesolimbic glutamatergic pathways considering the critical roles these pathways play in the rewarding/reinforcing effects of addictive drugs and SUD. The potential role of sex chromosomes and other signaling pathways, including the potential for stress and the hypothalamic–pituitary–adrenal axis to enhance vulnerability in females, are also briefly discussed. We conclude with implications for sex-specific interventions for SUD and future research directions.
TABLE 1.
Glossary of the key terms used in this review
| Term | Definition |
|---|---|
| Addition-like feature | The expression of a behavior in an animal that resembles a criterion, or symptom, of SUD in humans as defined by the DSM-5 (American Psychiatric Association, 2013). Some of the more commonly modeled features include escalation of drug intake over time, binge/abstinent patterns of drug intake, physical dependence, an enhanced motivation to obtain the drug, compulsive drug use despite adverse consequences, preference for the drug over a nondrug rewards, and enhanced drug-craving/vulnerability to relapse (Lynch 2018). |
| Addition-like phenotype | The expression in an animal of ≥1 characteristics (or addiction-like features) that resemble features of SUD in humans as defined by the DSM-5. For example, the development of an enhanced motivation for the drug has been used to define the development of an addiction-like phenotype since, as in humans, once this feature emerges, it appears to represent a relatively permanent shift to a higher motivational state (Lynch 2018). |
| Acquisition procedure | A procedure that uses a set of performance criteria to define the time-point when an animal has learned a new behavior, such as lever pressing to obtain infusions of a drug. Acquisition procedures can be a strong tool for investigating individual differences in sensitivity to the reinforcing effects of a drug. These effects are ideally studied under low-dose conditions and the question asked is, which animals can detect the reinforcing effects of this low dose of the drug? A faster speed of acquisition and/or greater percent group acquisition is then used to define an enhanced vulnerability to substance use (Lynch et al., 2010). |
| Animal model of substance use | A model used to assess initial vulnerability to use addictive drugs. Short-access drug self-administration procedures (1–2 h/d access) are commonly used and focus on rates and/or percent group acquisition of drug self-administration, maintenance levels of drug use, or motivation to obtain the drug, as assessed using a progressive-ratio schedule or a within-session threshold procedure, following acquisition. |
| Animal model of substance use disorder | A model that has been validated to induce an addiction-like phenotype in animals like that observed in humans with SUD. Extended-access drug self-administration procedures (≤6 h/d access) are the gold-standard for inducing addiction-like features in animals (Lynch 2018). |
| Binge/abstinent pattern | A binge-abstinent of pattern of drug self-administration is characterized by cycles of heavy/prolonged periods of drug use (binge intake) separated by periods of self-imposed abstinence. |
| Choice procedure | A procedure used to determine percent choice, or preference, for one reinforcer over another (or for different magnitudes of a reinforcer). Choice procedures can be a powerful approach for determining individual differences in vulnerability to developing a preference for the drug over other nondrug rewards, such as a highly palatable food reward, and for determining potential interventions that reverse a drug preference back to a nondrug one. |
| Compulsive drug use | A core feature of addiction in humans that is modeled in animals using punishment or choice procedures. The development of this addiction-like feature has been defined as continued drug use despite adverse consequences (e.g., coincident shock) or an exclusive choice (>90%) of the drug over an alternative nondrug reward (Lynch 2018). This addiction-like feature emerges following abstinence l (≥7 days) from extended-access self-administration and the magnitude of its expression increases with longer periods of abstinence (Towers et al., 2021). |
| Enhanced motivation to obtain the drug | A core feature of addiction in humans that is modeled in animals using either a progressive ratio schedule or the threshold procedure. This feature has been defined as ≥15% increase in motivation for the drug relative to short-access controls or baseline prior to extended-access self-administration and abstinence (Lynch 2018). This addiction-like feature emerges following abstinence (≥7 days) from extended-access self-administration and the magnitude of its expression increases with longer periods of abstinence (Towers et al., 2021). |
| Enhanced drug-craving/vulnerability to relapse | A core feature of addiction in humans that is modeled in animals using an extinction/reinstatement procedure or a cue-induced drug-seeking procedure. This addiction-like feature is typically assessed following extended-access self-administration and a period of protracted abstinence (>14 days) since these conditions induce high levels of drug-seeking relative to short-access controls and earlier abstinence time points. The expression of this addiction-like feature progressively increases, or incubates, over abstinence (Lynch 2018). |
| Escalation of drug intake | Escalation of drug intake occurs in animals given extended-access, but not short-access, to the drug and is characterized by a gradual increase in drug intake over time. It is ideally studied following acquisition of drug self-administration, to ensure that increases in intake are reflective of escalation rather than acquisition, and is thought to resemble the loss of control over drug intake feature observed in humans with SUD (Koob 2021). |
| Fixed-ratio schedule | A schedule of reinforcement in which a set number of responses (e.g., 1, 2, or 10) produce a reinforcer delivery, such as a drug infusion. |
| Gender | The characterization of women or men that is socially constructed and varies over time and between cultures (Committee on Understanding the Biology of Sex and Gender Differences 2001). |
| Incubation effect | The incubation effect refers to a progressive increase in drug-seeking from early to later periods of abstinence following extended-access self-administration. A similar phenomenon has also been reported in humans with SUD (Li et al., 2015) and is thought to reflect the development of an enhanced vulnerability to relapse. A similar incubation effect has also been observed for the development of other addiction-like features, including compulsive drug use and an enhanced motivation to obtain the drug (Gancarz-Kausch et al., 2014; Towers et al., 2021). |
| Intermittent-access procedure | A drug self-administration procedure wherein access to the drug is intermittently available, such as in 5-min trials with unrestricted, fixed-ratio 1 access, or in discrete trials, With the most commonly used procedures, animals either have unrestricted, fixed-ratio 1 access to the drug infusions in 5-minute trials that initiate every 30 minutes for ≥6 h/d or to single infusions of the drug in discrete trials that initiate every 15 minutes 12–24 h/d (Fitch and Roberts 1993; Zimmer et al., 2012). Intermittent-access self-administration results in a binge-abstinent pattern of drug intake and spiking brain drug levels (Zimmer et al., 2012). |
| Long-access procedure | A drug self-administration procedure that allows continuous, fixed-ratio 1 access to the drug for ≥6 h/d. This results in high levels of drug intake and an escalating pattern of drug use (Ahmed and Koob 1998). |
| Physical dependence | A core feature of addiction in humans that is assessed in animal models following chronic drug self-administration and defined by withdrawal-induced weight loss and somatic signs of withdrawal (e.g., abdominal constriction, salivation, ptosis, paw tremors; Lynch et al., 2010). |
| Preference for the drug over a nondrug reward | A core feature of addiction in humans that is modeled in animals using a choice procedure. The development of this addiction-like feature is defined as an exclusive choice (>90%) for the drug vs. a nondrug reward (Lynch 2018). |
| Progressive-ratio schedule | A schedule of reinforcement that requires the animal to emit an increasing amount of work (or lever pressing) to obtain each subsequent delivery of the drug within a session. The breakpoint, or the point that the animal stops responding, is used as a measure of motivation to obtain the drug. |
| Punishment procedure | Punishment procedures decrease the probability of responding for the reinforcer. For example, when an aversive stimulus, such as electric shock, is paired with the delivery of the drug, drug-taking decreases. Punishment procedures have also been used to demonstrate compulsive use, a core feature of addiction in humans, wherein animals show a reduced sensitivity to punishment and continue to self-administer high levels of the drug. |
| Reinstatement procedure | A model of relapse/drug-craving whereby the animal is tested on responding on a lever that was formerly associated with the drug under non-reinforced conditions (extinction), and once responding has reached a certain level of nonresponsiveness, the reinstatement of drug-seeking (responding on this same lever) is examined in response to presentations of drug-associated cues, a small “priming” dose of drug, or stress. |
| Sex | The characterization of an individual as female or male according to their reproductive organs and functions derived from their chromosomal complement (generally XX for female and XY for male; Committee on Understanding the Biology of Sex and Gender Differences, 2001). |
| Short-access procedure | A drug self-administration procedure wherein animals have access to the drug for 1–2 h/d. Such access results in relatively stable and low levels of drug intake from day to day. |
| Telescoping effect | A phenomenon that describes a faster progression in females compared with males from initial drug use to meeting the criteria and/or seeking treatment of a SUD (Piazza et al., 1989). |
| Threshold procedure | A procedure used to examine motivation to obtain a reinforcer. For example, the demand for a drug is measured by varying the price (response requirement) and the value (dose) of the drug within a session (Zimmer et al., 2012). |
Human studies were selected based on PubMed and Google Scholar searches using the key words telescoping, time-course, trajectory, alcohol, cocaine, methamphetamine, opioids, fentanyl, heroin, morphine, oxycodone, cannabis, smoking, nicotine, tobacco, illicit drug use, initiation of use, regular use, problem use, addiction, and SUD. Preclinical studies were identified using these terms: acquisition, reinforcing effects, self-administration, addiction phenotype, relapse, enhanced motivation, compulsive use, escalation, binge intake, and extended-access self-administration. Human and animal studies of biologic factors and neurobiological mechanisms focused on these terms: ovarian hormones, estrous cycle, menstrual cycle, luteal, follicular, estradiol, progesterone, dopamine, glutamate, excitability, nucleus accumbens (NAc), ventral tegmental area (VTA), and medial prefrontal cortex (mPFC). Throughout this review, the term sex refers to biologic differences between women and men and male and female laboratory animals related to sex hormones, chromosomes, gene expression, anatomy, or physiology (Committee on Understanding the Biology of Sex and Gender Differences, 2001). The term gender refers to socially determined differences between women and men roles that vary over time and between cultures (Committee on Understanding the Biology of Sex and Gender Differences, 2001).
II. Sex Differences in the Progression to Addiction
A. Evidence for and Against a Telescoping Effect in Women
The original reports of a telescoping effect were based on self-reports and structured interviews from men and women with an alcohol use disorder (AUD; i.e., abuse or dependence based on DSM-III/IV) detailing the timeline of onset of major alcohol-related life events. These events include first drink, first intoxication, continuous consumption, onset of dependence, and first inpatient treatment which have been shown to occur in a chronological sequence with a high level of predictability in both women and men (Schuckit et al., 1995). Using this framework, these studies consistently show that women progress more rapidly from regular alcohol use to developing problematic alcohol use or an AUD (Hesselbrock et al., 1985; Randall et al., 1999; Johnson et al., 2005; Diehl et al., 2007) (Tables 2 and 3). Women also have a shorter course from the onset of problematic use/AUD to seeking treatment of the disorder than men (Ashley et al., 1977; Piazza et al., 1989; Mann et al., 1992, 2005; Randall et al., 1999; McCance-Katz et al., 1999; Hernandez-Avila et al., 2004; Diehl et al., 2007; Lewis and Nixon, 2014). This faster progression to treatment seeking may be attributable to an earlier onset of severe SUD (five or more DSM-V symptoms) considering that at treatment entry, women have more severe clinical profiles than men (e.g., more medical, psychologic, behavioral, and social problems; Greenfield et al., 2010). This conclusion is further supported by studies showing that women have an accelerated course and/or an enhanced sensitivity to alcohol-related health consequences as compared with men. Some of the differences in health decline include a faster course in women than men for the development of alcohol-associated cirrhosis (Loft et al., 1987) and brain atrophy (Mann et al., 1992; Hommer et al., 1996; Hommer et al., 2001; Mann et al., 2005), as well as greater alcohol-associated effects on cardiac and skeletal muscle in women than men (Urbano-Márquez et al., 1995; Fernández-Solà et al., 1997).
TABLE 2.
Summary of human studies on the telescoping effect within treatment-seeking individuals
| Source | Drug | Subjects | Telescoping findings: time (in years unless stated otherwise) between events |
|---|---|---|---|
| Diehl et al. (2007) | Alcohol | 106W/106M | W<M:regular use to dependence (10.0 vs. 11.6) W< dependence to treatment (4.5 vs. 7.9) |
| Johnson et al. (2005) | Alcohol | 785W/1252M | W<M: regular use to problematic use in the older (30+; 7.6 vs. 10.9), but not younger age group (< 29; 4.9 vs. 5.2) |
| Randall et al. (1999) | Alcohol | 419W/1307M | W<M:regular use to problematic use (0.9 vs. 2.3) W<M:loss of control over use to severe alcohol-related problems (5.5 vs. 7.8) W<M:regular use to seeking treatment (11.6 vs. 15.8) |
| Lewis and Nixon (2014) | Alcohol | 257W/274M | W<M:milestones (first use, first intoxication, regular use, problematic use) to treatment (18.1 vs. 23.0, 15.5 vs. 20.7, 13.0 vs. 18.2, 10.3 vs. 14.5) W=M: milestones (first use, first intoxication, regular use) to problematic use/dependence (8.9 vs. 9.7, 6.3 vs. 7.4, 3.2 vs. 4.5) |
| Ashley et al. (1977) | Alcohol | 135W/736M | W<M:problematic use to treatment (14.1 vs. 20.2) |
| Hesselbrock et al. (1985) | Alcohol | 90W/231M | W<M:initial use to problematic use/dependence (7.4 vs. 15.0) |
| Piazza et al. (1989) | Alcohol | 33W/105M | W<M:problematic use to treatment (10.4 vs. 14.7) W=M: initial use to first intoxication (2.9 vs. 1.7) W=M: first intoxication to problematic use (14.0 vs. 14.7) |
| Mann et al. (1992) | Alcohol | 14W/51M | W<M:initial use to treatment (3.8 vs. 9.2) |
| Mann et al. (2005) | Alcohol | 42W/34M | W<M:problematic use/dependence to treatment (5.6 vs. 10.4) |
| McCance-Katz et al. (1999) | Alcohol, Cocaine |
92W/206M | W<M:initial alcohol use to treatment (8.8 vs. 11.4) W<M:initial cocaine use to treatment (5.2 vs. 5.8) |
| Hernandez-Avila et al. (2004) | Alcohol, Cannabis, Opioids |
156W/115M | W<M:regular alcohol use to treatment (14.5 vs. 19.0) W<M:regular cannabis use to treatment (13.0 vs. 18.0) W<M:regular opioid use to treatment (8.0 vs. 12.0) |
| Griffin et al. (1989) | Cocaine | 34W/95M | W<M:initial use to treatment (9.0 vs. 10.2) |
| White et al. (1996) | Cocaine | 27W/60M | W<M:initial use to problematic use (1.6 vs. 3.3) W<M:initial use to treatment (5.1 vs. 10.4) |
| Haas and Peters (2000)a | Alcohol, Cocaine, Cannabis |
42W/118M | W< M: initial cocaine use to problematic use (4.3 vs. 9.8) W=M: initial alcohol or cannabis use to problematic use (2.2 vs. 1.9) |
| Lewis et al. (2014) | Cocaine, Cannabis, Opioids |
288W/255M | W=M: regular cocaine use to problematic use (1.1 vs. 1.8) W<M:regular opioid use to problematic use (0.5 vs. 2.7) W<M:regular cannabis use to problematic use (0.7 vs. 2.0b) |
| Tavares et al. (2003) | Gambling | 70W/70M | W<M:milestones (social, intense, and problematic gambling) to treatment (5.0 vs. 7.9, 0.8 vs. 4.3, 1.9 vs. 6.7) |
| Ladd and Petry (2002) | Gambling | 45W/70M | W<M:problematic gambling to treatment (4.4 vs. 14.6) |
| Ibanez et al. (2003) | Gambling | 22W/47M | W<M:initial gambling to problematic gambling (4.2 vs. 11.0) |
| Grant et al. (2012) | Gambling | 34W/37M | W<M:initial gambling to problematic gambling (8.3 vs. 12.0) |
| Brecht et al. (2004) | Meth | 154W/196M | W<M:initial use to regular use (1.6 and 2.6 yearsb) |
| Peltier et al. (2021) | Opioids | 2794W/45614M | W<M:age diagnosed with OUD (44.9 vs. 51.0) |
| Anglin et al. (1987) | Opioids | 264W/282M | W<M:months from initial use to daily use (14 vs. 21) |
| Adelson et al. (2018) | Opioids | 494W/762M | W<M:initial heroin use to treatment (12.9 vs. 14.8) |
| Hser et al. (1987) | Opioids | 264W/282M | W<M:months from daily use to treatment (82.5 vs. 98.0) |
M, men; Meth, methamphetamine; n.s., nonsignificant; W, women.
aThis treatment population underwent forced treatment due to a drug court program.
bTrend for significant difference (P < 0.1).
TABLE 3.
Summary of human studies on the telescoping effect within nontreatment-seeking individuals
| Source | Drug | Subjects | Telescoping findings: time (in years unless stated otherwise) between events |
|---|---|---|---|
| Alvanzo et al. (2011) | Alcohol | 11862W/9244M | W=M: initial use to dependence (4.9 vs. 5.4) |
| Keyes et al. (2010) | Alcohol | 30125W/23113M | W=M: initial use to dependence in overall sample (5.6 and 5.8) W<M:initial use to dependence in cohort 2 only (3.7 vs. 4.2) W<M:dependence to treatment in overall sample (6.1 vs. 7.0) and in one of 5 cohorts (cohort 5, 19.4 vs. 23.5) |
| Huggett et al. (2018) | Alcohol, Tobacco |
1477W/1297M | W≤M: initial alcohol use to dependence (3.3 vs. 3.8a) W=M: initial tobacco use to dependence (4.5 vs. 4.5) |
| Khan et al. (2013) | Cannabis | 1217W/2080M | W<M:initial use to dependence (2.2 vs. 2.6) |
| Ehlers et al. (2010) | Cannabis | 177W/172M | W<M:initial use to dependence (44.7 vs. 49.3) |
| Sofuoglu et al. (1999) | Cocaine | 21W/23M Study 1 12W/11M Study 2 |
W<M:initial use to dependence in two human laboratory studies (Study 1, 9.2 vs. 11.3; Study 2, 7.4 vs. 13.0) |
| O'Brien and Anthony (2005) | Cocaine | 59488W/54753M | W<M:initial use to dependence (defined by risk within 24 months of first use, W 3–4 times more likely than M) |
| Slutske et al. (2015) | Gambling | 2662W/2001M | W>M: initial gambling to weekly/problematic gambling, disordered gambling symptoms, and diagnosis of disordered gambling (8.6 vs. 8.1, 10.9 vs. 8.3, 12.9 vs. 10.9) |
| DiFranza et al. (2002) | Tobacco | 679W/Mb | W<M:days from monthly smoking to dependence symptoms (21 days vs. 183 days) |
| Scragg et al. (2008) | Tobacco | 14925W/10070Mb | W<M:initial use to dependence (W had less use than M prior to symptoms onset) |
| DiFranza et al. (2007) | Tobacco | 647W/599Mb | W=M: days from initial use to nicotine/tobacco dependence, symptoms, and autonomy loss (no sex effect, data not stated) |
| Sylvestre et al. (2018) | Tobacco | 471W/368Mb | W<M:initial use to dependence symptoms (21 days vs. 183 days) |
| Thorner et al. (2007) | Tobacco | 378W/261Mb | W<M:initial use to daily use (0.9 vs. 1.3) |
| Stoltman et al. (2015) | Opioids | 165W/389M | W=M: initial heroin use to problematic use (2.1 vs. 2.5) |
| Back et al. (2011) | Opioids | 12W/12M | W<M:initial use to regular use (5.0 vs. 8.1) |
M, men; n.s., nonsignificant; W, women.
aTrend for significant difference (P < 0.1).
bConducted in children/adolescents.
Similar methods have been used to establish sex/gender differences in transitions from initial use to regular use, problematic use, and SUD and/or treatment of SUD with other addictive drugs, including opioids, psychostimulants, cannabis, and tobacco (Tables 2 and 3). These studies show that compared with men, women have a shorter duration of opioid (Hser et al., 1987; Hernandez-Avila et al., 2004; Adelson et al., 2018; Peltier et al., 2021), psychostimulants (cocaine and methamphetamine; Griffin et al., 1989; White et al., 1996; McCance-Katz et al., 1999; Sofuoglu et al., 1999; Haas and Peters, 2000; Brecht et al., 2004; O’Brien and Anthony, 2005), and cannabis use (Hernandez-Avila et al., 2004) prior to entering treatment and a faster progression from initial use of opioids (Anglin et al., 1987; Back et al., 2011; Lewis et al., 2014), cocaine (White et al., 1996; Sofuoglu et al., 1999; O’Brien and Anthony, 2005), tobacco (DiFranza et al., 2002; Thorner et al., 2007; Scragg et al., 2008; Sylvestre et al., 2018), and cannabis (Ehlers et al., 2010; Khan et al., 2013; Lewis et al., 2014) to regular or problem use. The same pattern has also been reported for gambling wherein women show a faster progression from the initiation of gambling to developing a problem with gambling or to meeting criteria for pathologic gambling compared to men (Ladd and Petry, 2002; Ibáñez et al., 2003; Tavares et al., 2003; Grant et al., 2012). As with findings with alcohol, women with SUD have more severe clinical profiles than men with SUD at treatment entry (Arfken et al., 2001; Fernandez-Montalvo et al., 2014), and show an accelerated course and/or enhanced vulnerability to drug-related medical consequences including a greater risk of infectious diseases with opioid use [i.e., hepatitis C (Iversen et al., 2010) and AIDS (Des Jarlais et al., 2012)], an earlier age for onset of psychotic disorders with cannabis use (Large et al., 2011), overall greater risk for cocaine-induced death (de la Fuente et al., 2014), shorter time interval between onset of cocaine use and its fatal outcome (Origer et al., 2014; for a review, see Agabio et al., 2016), and increased susceptibility to smoking-associated lung cancer (Kiyohara and Ohno, 2010; Hansen et al., 2018).
However, not all studies have observed a telescoping effect in women (DiFranza et al., 2007; Alvanzo et al., 2011), and findings from nontreatment seeking populations, particularly population-based studies, have been mixed (e.g., Ehlers et al., 2010; Keyes et al., 2010; Back et al., 2011; Khan et al., 2013; Slutske et al., 2015; Stoltman et al., 2015) (Table 2). Probably the most controversial findings are from the Keyes et al. (2010) study, which was a large-scale study of alcohol use trajectories based on population-level data from two US national surveys (conducted in 1991–1992 and 2001–2002) of five birth cohorts (1934–1943, 1944–1953, 1954–1963, 1964–1973, and 1974–1983). They analyzed survival probabilities over time for the transition from initial alcohol use to developing an AUD and from the onset of an AUD to seeking treatment of the disorder. In contrast to predicted effects, men transitioned faster than women from initial alcohol use to AUD and from developing AUD to seeking treatment of the disorder. However, another interpretation is that the data reflect a greater risk in men for developing an AUD and a lower likelihood in women of seeking treatment of AUD. Indeed, the analysis of alcohol use trajectories in the individuals that actually developed an AUD are consistent with previous reports of a telescoping effect. In addition, the mean number of years between initial alcohol use and the development of AUD was shorter in women than men (i.e., alcohol dependence as defined by the DSM-IV; 3.7 years vs. 4.2 years). Similarly, when the analysis was limited to individuals that sought treatment of an AUD, women had fewer years between the onset of an AUD to seeking treatment of the disorder (6.1 vs. 7 years). These differences were modest, however, particularly for the time-course for developing an AUD, and the effect was limited to one of the five birth cohorts (cohort 2). The effect for treatment was statistically significant when data were collapsed across all the cohorts, but analysis within each cohort only yielded significance for cohort 5 (19.4 vs. 23.5).
These data, together with mixed reports of a telescoping effect in nontreatment-seeking populations (Back et al., 2011; Stoltman et al., 2015), indicate that the telescoping effect may be most relevant within treatment-seeking populations, which presumably include only individuals that develop a severe SUD requiring treatment. This idea is also consistent with findings from a population-level study showing that adolescent and young adult females are less likely than their male counterparts to have a mild to moderate illicit drug use disorder (other than cannabis) but equally likely, if not more likely, to have a severe illicit drug use disorder (i.e., classified as dependence according to DSM-IV; Cotto et al., 2010). It is also supported by population-level data (Wave I and II of the National Epidemiologic Survey on Alcohol and Related Conditions) showing that women with a history childhood maltreatment, which is known to be associated with greater addiction severity (for a review, see Puetz and McCrory, 2015), had a faster progression from the onset of drinking to developing an AUD than women without childhood maltreatment and men with and without this history (Schuckher et al., 2018). Importantly, this vulnerable in-treatment population is the population that needs to be studied for insights into prevention and treatment.
While the mechanisms underlying the telescoping effect are not yet known, it is likely that both sociocultural gender differences and biologic sex differences contribute. For example, gender differences in the use of the healthcare system have been suggested as a potential explanation for the telescoping effect since women seek care sooner after initiating substance use or developing a SUD disorder than men. Social stigma against substance use and SUD in women may also cause women to seek treatment earlier after initiating substance use and/or developing a SUD than men. This does not appear to be the case, however, since in contrast to the gender difference for seeking medical care overall, women are not more likely than men to seek treatment of a SUD (Greenfield et al., 2007; Center for Behavioral Health Statistics and Quality, 2015). Women are also more likely than men to be primary caregivers, and fear of losing custody of children is commonly reported as a barrier to seeking care for SUD (Poole and Isaac, 2001; Mackay et al., 2020). Greater socio-relational impairment in women than men has also been reported to serve as a barrier to seeking treatment of a SUD in women. These gender differences may explain the disparity in SUD treatment between men and women and further support the conclusion that women who enter SUD treatment represent a vulnerable population that develops a severe SUD. This explanation also fits the data indicating that at the start of treatment of SUD, women have more severe SUDs and have more psychiatric and medical comorbidities than men. Biologic factors also likely contribute to this vulnerability in women and the telescoping effect considering that similar behavior has been reported in female versus male laboratory animals (as detailed in the following discussion).
B. Sex Differences in Animal Models of Initial Vulnerability to Substance Use
Preclinical studies of sex differences in addiction have focused predominately on vulnerability during early phases of the addiction process, such as acquisition of drug self-administration under short-access conditions. These differences are ideally studied under low drug doses that maximize individual differences; low doses are also less likely than high doses to induce negative side effects that may counter the reinforcing effects of the drug or impact the animal’s ability to respond (Lynch et al., 2010). Results from studies comparing male and female rats have consistently revealed faster rates of acquisition and greater percent group acquisition in females than males under low-dose conditions (e.g., Carroll et al., 2002; Roth and Carroll, 2004; Lynch, 2008). While most of this work has focused on cocaine, similar findings have been reported for other classes of drugs including opioids, alcohol, and cannabis and for other psychostimulants such as nicotine and methamphetamine (for reviews, see Carroll et al., 2004; Becker and Hu, 2008; Lynch, 2006). Females also typically self-administer more drug under short-access conditions (fixed-ratio 1, 1–2-h/d) than males (e.g., Roberts et al., 1989; Smith et al., 2021), but this measure is less sensitive to individual differences, and sex differences are not always observed (e.g., Roth and Carroll, 2004; Towers et al., 2019). The direction of effects can also be difficult to interpret from maintenance levels of intake since lower intake may reflect less sensitivity to the reinforcing effects of the drug (e.g., the dose may function as a reinforcer in only a subset of the animals) or greater sensitivity (e.g., less drug is needed to maintain a preferred level of effect). Motivation to obtain the drug, as assessed under progressive ratio schedules or the threshold procedure, is sensitive to individual differences and is a linear measure of reinforcing effects (i.e., larger doses maintain higher levels of responding). Numerous studies have shown that females are more motivated to obtain infusions of addictive drugs, and this effect has been observed at both low and high drug doses and for multiple addictive drugs (e.g., Roth and Carroll, 2004; Mello et al., 2007; for reviews, see Lynch 2006, 2018). These findings indicate that females have an enhanced sensitivity to reinforcing effects of addictive drugs (Fig. 1).
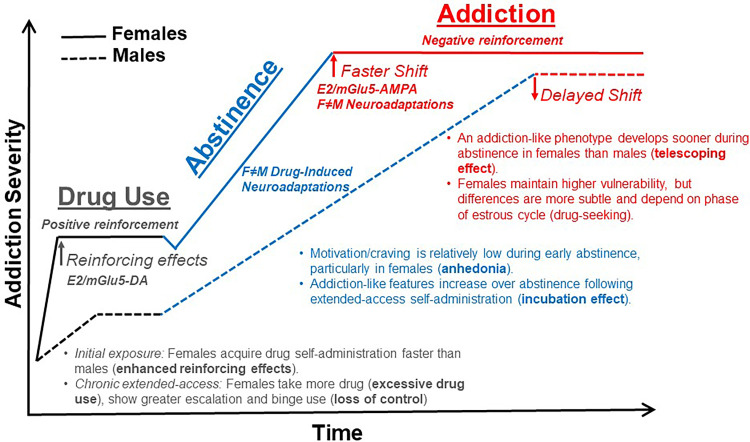
Fig. 1.
Biologic basis for the faster course from drug use to addiction/SUD in females. Females are more sensitive to the positive reinforcing effects of drugs and acquire drug self-administration faster than males. This is mediated through interactions of estradiol and mGlu5, both of which increase drug-evoked dopamine signaling in the mesolimbic reward pathway of females. Craving and motivation to use addictive drugs is typically low during early abstinence, particularly in females, but both features become progressively enhanced over a period of protracted abstinence. Molecular adaptations in response to chronic drug use and abstinence differ between males and females and may drive sex differences in anhedonia, craving, and relapse vulnerability during both early and late abstinence. Addiction-like features, including an enhanced motivation for the drug, compulsive drug use, and vulnerability to relapse, emerge sooner during abstinence and/or after less drug intake in females than males, indicating that the telescoping effect is biologic based. This effect is likely driven by interactions of estradiol and mGlu5, which cause an earlier recruitment of the glutamate system (i.e., AMPA receptors). Once addiction has developed, behavioral differences between males and females become subtle and often depend on estrous cycle phase (e.g., drug craving). The neuroadaptations that underlie addiction also differ between males and females (e.g., NMDA receptor signaling in the dorsomedial prefrontal cortex), even in the absence of behavioral differences. E2 = estradiol. DA = dopamine.
C. Sex Differences in Animal Models of SUD
Much less is known regarding sex differences in vulnerability during later stages of the addiction process and, more specifically, following the development of an addiction-like phenotype. The use of extended-access drug self-administration appears to be critical to inducing this phenotype, which has been defined by the development of one of more key addiction-like features, such as escalation of drug intake over time, binge/abstinence patterns of drug use, compulsive drug use despite negative consequences, the development of physical dependence, an increased preference for the drug over a nondrug reward, an enhanced motivation to obtain the drug, and enhanced drug-craving/vulnerability to relapse (Lynch 2018). While no one procedure captures all 11 diagnostic criteria listed in the DSM-5 (American Psychiatric Association, 2013), there are multiple extended-access procedures that induce two or more of these clinical features, the threshold for a diagnosis of SUD in humans. For example, with the most commonly used extended-access procedure, the long-access procedure (Ahmed and Koob, 1998), animals have unrestricted, fixed-ratio 1 access to infusions of a drug, such as cocaine, heroin, fentanyl, nicotine, methamphetamine, for 6 to 12 h/d. Under these conditions, animals self-administer high levels of the drug and show an escalating pattern of use over time, which is believed to mimic the excessive drug use and loss of control features of SUD in humans. This loss of control feature is also observed in rats given extended, intermittent access to a drug using either a discrete trial (Fitch and Roberts, 1993) or a fixed-ratio 1 procedure (Zimmer et al., 2012), which results in a binge–abstinent of pattern of drug self-administration characterized by cycles of heavy/prolonged periods of drug use (binge intake) separated by periods of self-imposed abstinence. For example, rats given 24-h/d intermittent access to cocaine, heroin, or speedball using a discrete trial procedure (four 10-minute trials/h), self-administer high levels of the drug in binge–abstinent patterns that are dysregulated from the normal diurnal cycle (i.e., responding occurs at high levels throughout the light–dark phase). Similar binge-abstinent patterns have been observed for cocaine and fentanyl under extended, intermittent-access conditions (two 5-minute trials/h) using a fixed-ratio 1 schedule.
Notably, extended-access drug self-administration using the long-access procedure or an intermittent-access procedure leads to the development of other core characteristics of SUD including compulsive drug use, as assessed by continued drug use despite punishment (e.g., foot shock); an enhanced motivation to use the drug, as assessed using a progressive-ratio schedule or a threshold procedure; and enhanced drug-craving/vulnerability to relapse, as assessed using an extinction/reinstatement procedure (Balster and Woolverton, 1982; Fitch and Roberts, 1993; Ahmed and Koob, 1998; Lynch and Carroll, 2001; Allain et al., 2015; Lynch, 2018). Expression of each of these features emerges over abstinence following extended-access self-administration and increases, rather than decreases, in magnitude over time. This “incubation” effect is robust and has been described for cue-induced drug-craving in humans for nicotine (Bedi et al., 2011), methamphetamine (Wang et al., 2013), cocaine (Wang et al., 2013), and alcohol (Li et al., 2014; Bach et al., 2019) and in animals for these drugs along with opioids (for reviews, see Pickens et al., 2011; Li et al., 2015). A similar incubation effect has also been reported for the expression of enhanced motivation with cocaine (Towers et al., 2021) and for compulsive use with cocaine and heroin (Gancarz-Kausch et al., 2014; Towers et al., 2021). Notably, as with humans, the development of some of these addiction-like features (e.g., an enhanced motivation for the drug) are expressed long term and appear to reflect a relatively permanent shift to a higher motivational state (see Lynch et al., 2021). While it is possible to induce these addiction-like features using short-access drug self-administration procedures, it occurs in only a small minority of the rats (approximately 30%; Belin and Deroche-Gamonet, 2012). The phenotype is also more robust following extended- versus short-access self-administration (e.g., Pacchioni et al., 2011; Fischer et al., 2013). Evidence also shows that molecular changes differ following extended- versus short-access self-administration.
Sex differences have been reported for both extended-access self-administration and the induction of an addiction-like phenotype following extended-access self-administration and abstinence (see Fig. 1). Studies have shown that during extended-access self-administration, female rodents self-administer higher levels of drugs including alcohol; opioids, such as heroin, fentanyl, oxycodone, and morphine; and psychostimulants, such as cocaine, methamphetamine, and nicotine, compared with male rodents (Lynch and Taylor, 2004, 2005; Roth and Carroll, 2004; Carroll et al., 2005; Smith et al., 2011; Reichel et al., 2012; Sanchez et al., 2014; Moore and Lynch, 2015; Becker and Koob, 2016; Kawa and Robinson, 2019; Towers et al., 2019, 2022; Nicolas et al., 2019; George et al., 2021; Towers et al., 2022). Female nonhuman primates also self-administer more phencyclidine than male nonhuman primates under long-access conditions (Carroll et al., 2005). Sex differences in intake are most apparent under low-dose conditions and in procedures that do not limit total hourly or daily intake as such procedures increase the likelihood of individual differences. There are also sex differences in patterns of extended-access drug self-administration under both high- and low-dose conditions with female rats and mice showing greater escalation of alcohol, opioids, and psychostimulant intake over time as compared with male rats and mice (Roth and Carroll, 2004; Carroll et al., 2005; Reichel et al., 2012; Melon et al., 2013; George et al., 2021). Female rats and mice also self-administer more heroin during the first hour of a long, continuous-access session (fixed-ratio 1, 6-hour session; Towers et al., 2019) and more fentanyl within active trials under the intermittent access procedure (Towers et al., 2022), have longer initial periods of “binge” cocaine intake (defined as continuous drug use with no breaks from drug self-administration greater than 1 hour) and greater dysregulation in diurnal patterns of cocaine intake under 24-h/d discrete trial procedure (Lynch and Taylor, 2004), and have greater binge-like alcohol drinking under the “drinking-in-the-dark” procedure as compared with males (defined as the amount of ethanol consumed during the first 3 hours of the dark phase; e.g., Sneddon, 2019). These findings indicate that females are more vulnerable than males to excessive drug use and developing a loss of control over drug use. This sex difference also appears to be robust as it has been observed in several species and for multiple drugs.
Importantly, the sex differences observed for the development of an addiction-like phenotype mirror findings of a telescoping effect in women and indicate that this phenotype develops more readily in female as compared with male animals (Lynch and Taylor 2004; Perry et al., 2013; Ramôa et al., 2013, 2014; Lynch 2018) (Table 4). This work has focused on effects with cocaine, with results from the initial study of sex differences showing that females, but not males, developed an enhanced motivation for cocaine under conditions predicted to be the threshold for inducing this phenotype (Fig. 2): 7 days of extended-access cocaine self-administration and 10 days of abstinence (Lynch and Taylor, 2004). We subsequently confirmed that this phenotype is absent in both females and males when assessed under subthreshold self-administration and abstinence conditions (e.g., extended-access self-administration with no intervening period abstinence or following short-access self-administration with or without abstinence; Lynch and Taylor, 2005) and present in both sexes when the conditions are optimized for its development by lengthening the period of extended-access self-administration (i.e., 10 days) and/or the abstinence period (i.e., 14 days; Roberts et al., 2007; Ramôa et al., 2013; Kawa and Robinson, 2019).
TABLE 4.
Summary of preclinical studies on the telescoping effect
| Source | Drug (dose/inf) | Rats | SA conditions | Addiction feature measured (procedure) | Vulnerability to developing addiction-like features |
|---|---|---|---|---|---|
| Kerstetter et al. (2012) | Cocaine (0.4, 1.0 mg/kg) |
39M/29F | ShA (FR1, up to 20 inf or food pellets, 5 days each) | Preference for drug over other rewards (choice procedure): cocaine (0.4 or 1.0 mg/kg) vs. food (45 mg pellet). Sessions began after acquisition and were run for 5 days. | F>M. Females were more likely than males to choose cocaine (low and high dose) over food (low dose, 59% vs. 33%; high dose, 76% vs. 68%) |
| Perry et al. (2013) | Cocaine (0.4 mg/kg) | 12M/12F | ShA (30-min each: pellet only, cocaine only, cocaine vs. pellet choice; FR1, first 3 days then FR5 for 21 days) | Preference for drug over other rewards (choice procedure): Cocaine vs. banana-flavored food pellet (45 mg pellet). Choice testing occurred daily after the pellet and cocaine only sessions. | F>M. Females were likelihood than males to develop a preference for cocaine over food (50% vs. 17%) |
| Perry et al. (2015) | Cocaine (0.4 mg/kg) | 50M/50F | ShA (30-min each: pellet only, cocaine only, cocaine vs. pellet choice; FR1, first 3 days then FR5 for 21 days) | Preference for drug over other rewards (choice procedure): cocaine vs. banana-flavored food pellet (45 mg pellet). Choice testing occurred daily after the pellet and cocaine only sessions. | F>M. Females were more likely than males to develop a preference for cocaine over food (42% vs. 26%) |
| Kawa and Robinson (2019) | Cocaine (0.4 mg/kg) | 28M/24F | ShA (intermittent-access: 2, 5-min trials/h, 5-h/d, 5 days/week, 30 days) | Enhanced motivation for the drug (threshold procedure): threshold tests (FR1, progressively decreasing doses of cocaine 1.28 to 0.004 mg/kg) were run following the 10th and 30th day of SA and again after 14 days of abstinence. | F>M: Females developed an enhanced motivation for cocaine after less abstinence than males (i.e., following ten days of SA vs. following 30 days of SA and 14 days of abstinence) |
| Lynch and Taylor (2004) | Cocaine (1.5 mg/kg) | 18M/20F | ExA (four 10-min trials/h, 24-h/d, 7 days) | Enhanced motivation for the drug (PR schedule): PR testing with cocaine (0.5 mg/kg) was conducted prior to ExA SA and then again after ExA SA and 7 days of abstinence (3 sessions each) | F>M: Females, but not males, developed an enhanced motivation for cocaine under these threshold conditions. |
| Towers et al. (2021) | Cocaine (1.5 mg/kg) | 39M/38F | ExA (four 10-min trials/h, 24-h/d, 10 days) | Enhanced motivation for the drug (PR schedule): PR testing with cocaine (0.5 mg/kg) was conducted prior to ExA SA and then again after ExA SA and 7, 14, or 60 days of abstinence (3 sessions each). Compulsive use (histamine-punishment): Following the third PR session, histamine (0.4 mg/kg) was added to the cocaine solutions and three additional PR sessions were run. |
F>M: Females develop an enhanced motivation for cocaine sooner during abstinence than males (7 vs. 14 days) F>M: Females tested following 7 days of abstinence displayed greater compulsive use than males; males required more abstinence to reach the-female level of compulsivity (14 days). |
| Townsend et al. (2021) | Fentanyl (0.32, 1.0 3.2, 10.0 ug/kg) | 18M/17F | ExA (FR5, 12-h/d, 5-day/wk, 3 weeks) | Preference for drug over other rewards (choice procedure). Fentanyl vs. Ensure. Tested at the end of each week of ExA SA 8 h after last ExA session | F>M: Males, but not females, showed withdrawal-induced increases in preference for fentanyl (at low doses), and methadone attenuated this effect. |
ExA, extended-access; F, female; FR, fixed-ratio; M, male; PR, progressive-ratio; SA, self-administration; ShA, short-access.
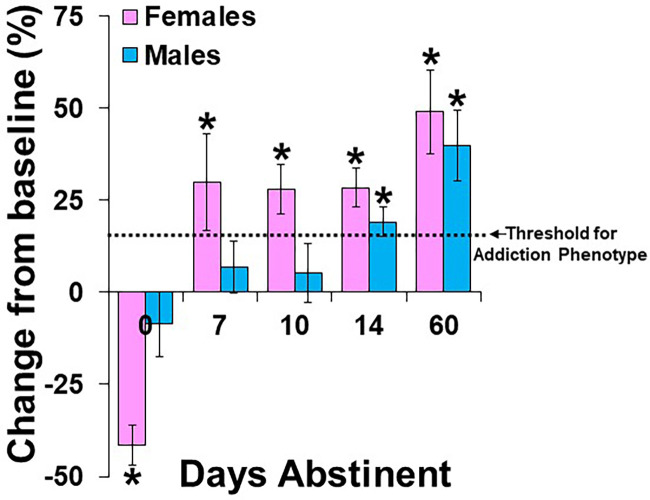
Fig. 2.
Rat model of the telescoping effect with cocaine. Data are plotted as mean percent change (±S.E.M.) from the average number of infusions obtained during three baseline progressive-ratio sessions prior to extended-access cocaine self-administration (24-h/d, 4 discrete trials/h, 1.5 mg/kg/infusion, 7–10 days; refs) versus those obtained at retest following extended-access cocaine self-administration and 0, 7, 10, 14, or 60 days of abstinence. Motivation for cocaine increased progressively over abstinence following extended-access cocaine self-administration. Neither males nor females showed an increase in motivation for cocaine when responding was assessed immediately following extended-access self-administration (0 days abstinent; Lynch and Taylor 2005); in fact, motivation was significantly decreased from baseline in females in the 0-day group. Females, but not males, showed an increase in motivation for cocaine when responding was assessed following 7 (Towers et al., 2021) or 10 days of abstinence (Lynch and Taylor 2004). Both males and females showed an increase in motivation for cocaine when responding was assessed following 14 days of abstinence (Towers et al., 2021), and motivation was highest in both males and females when responding was assessed following 60 days of abstinence (Towers et al., 2021). The threshold for the development of an addiction-like phenotype, as defined by ≥15% increase from baseline and as represented by a dotted line, developed sooner during abstinence in females than males (following 7 vs. 14 days of abstinence). Significant difference from baseline/no change (*). Data were redrawn, with permission, from the previously cited references.
We firmly established a telescoping effect with cocaine in our more recent studies by demonstrating that three key features of SUD—an enhanced motivation for the drug, compulsive drug use, and enhanced drug-craving/vulnerability to relapse—develop sooner during abstinence following extended-access self-administration in females than in males (Towers et al., 2021; Towers et al. revised submission). Specifically, an enhanced motivation for cocaine was evident in females after 7 days of abstinence, whereas, in males it is not evident until after 14 days. Females tested after 7 days of abstinence also displayed greater compulsive cocaine use than males, and while males reached the same level of resistance to punishment as females, this did not occur until after 14 days of abstinence. We also found that in females, cocaine-craving, as defined by total drug-seeking during extinction and cue-induced reinstatement testing, was expressed at high levels during both early and late abstinence, whereas, in males, drug-craving progressively increased from early to later abstinence time-points (following 2 vs. 14 days; E.B. Towers et al., manuscript in preparation). Notably, once these addiction-like features develop, sex differences are subtle, and some studies show greater effects in females than males (e.g., Towers et al., 2021) while others show no differences (e.g., Ramôa et al., 2013). Estrous cycle effects still appear to be relevant, though, considering that numerous studies have shown that drug-craving following abstinence from extended-access self-administration is higher in females tested during estrus versus nonestrus phases (Corbett et al., 2021). Together, these findings show that an addiction-like phenotype with cocaine develops at an accelerated rate in female rats compared with male rats and indicate that the parallel effect in women is biologically based.
It is important to determine whether similar sex differences occur for other drug classes. The initial findings with fentanyl suggest that both the time course for the development of an addiction-like phenotype and the occurrence of sex difference may be different for opioids. Specifically, Townsend et al. (2021) found that males prefer low doses of fentanyl over a nondrug reinforcer (i.e., Ensure) while females required a higher dose of fentanyl to shift their preference from Ensure. In males, preference for fentanyl increased progressively following repeated cycles of extended-access self-administration and withdrawal (8 hours) whereas in females, preference for highest dose of fentanyl decreased during acute withdrawal. While these findings were interpreted to reflect a greater sensitivity in males than females to developing a preference for fentanyl over a nondrug reward, it is notable that even in males the preference observed for fentanyl following the third 1-week cycle of extended-access self-administration and withdrawal was not significantly greater than that observed for the nondrug reward (approximately 50%), and in females, the nondrug reward was strongly preferred (approximately 75%). Considering that both males and females showed a strong preference for high doses of fentanyl prior to extended-access self-administration, this phenotypic difference may be indicative of a sex difference of acute fentanyl withdrawal rather than a sex difference in the development of an addiction-like phenotype. In fact, a similar sex difference was observed with cocaine. Female rats tested following extended-access cocaine self-administration, without an intervening period of abstinence, showed a marked decrease in motivation for cocaine whereas male rats did not show a change from baseline (i.e., prior to extended-access self-administration; Lynch and Taylor, 2005). One caveat to this interpretation, however, is that in males the behavioral phenotype was validated by showing that withdrawal-associated increases in heroin intake were blocked using methadone, Food and Drug Administration–approved treatment of opioid use disorder (OUD). Further research comparing phenotypic changes in females versus males over a period of protracted abstinence following extended-access opioid is necessary to determine whether there are sex differences in the time course for the development of addiction-like features with opioids.
Sex differences have also been observed for the expression of addiction-like features following short-access self-administration, particularly when behavior is examined following a prolonged period of self-administration (1–2 h/d access for ≥30 days). For example, several studies have shown that the development of a preference for the drug (cocaine) over another competing reinforcer (food), another key characteristic of SUD in humans, occurs more readily in females than males tested over a prolonged period of short-access cocaine self-administration (roughly 3–5 weeks; Kerstetter et al., 2012, Perry et al., 2013, 2015); this preference also developed in a greater percentage of females than males (approximately 50% vs. 17%; Perry et al., 2015). The development of a preference for cocaine over food was also associated with the development of two other key addiction-like features—an enhanced motivation for the drug and heightened drug-craving—indicating that females are more vulnerable than males to developing an addiction-like phenotype.
D. Summary and Integration of Preclinical and Clinical Findings
Together, these findings indicate that female laboratory animals display a greater vulnerability than male laboratory animals during the transition from initial drug use to the development of an addiction-like phenotype. Female animals take more psychostimulants, opioids, and alcohol and show greater escalation/binge intake under extended-access conditions than male animals. Female animals also develop an enhanced motivation for cocaine and a preference for cocaine over other reinforcers after less drug exposure and/or shorter periods of abstinence than male animals. It is important to emphasize, however, that the preclinical evidence demonstrating a faster time course for the development of addiction-like features in females than males is based exclusively on findings with cocaine. To our knowledge, no studies have examined sex differences in the time course for the development of addiction-like features following protracted abstinence from extended-access self-administration with other addictive drugs. While the preclinical findings with cocaine provide strong support for its biologic basis, future research studies are necessary to determine whether females also show an accelerated course for the development of addiction-like features in animal models of alcohol, opioid, and other psychostimulant use disorders. These studies are especially important considering that a telescoping effect has consistently been reported in women for cocaine use disorder (CoUD), in both treatment and nontreatment populations, which is in contrast to the findings for AUD and OUD. Future research is also necessary to address molecular mechanisms underlying the telescoping which, as discussed in the following text, are currently unknown.
II. Biologic Factors
A. Ovarian Hormones
Most of the work on potential mechanisms for sex differences in SUD has focused on the role of ovarian hormones. In clinical research, menstrual phase is often used as a proxy for ovarian hormones; several caveats to these studies need to be mentioned. First, it is essential that cycle stage is confirmed by hormone measurements. Without this confirmation, it is likely that nonovulatory cycles and/or cycles with insufficient luteal phase will be included (Younis et al., 2020). In addition, self-reported cycle lengths are often not accurate (Small et al., 2007). Women with polycystic ovarian disease and/or metabolic syndrome need to be excluded as do women on oral contraceptives since their cycles are anovulatory.
1. Human Studies: Ovarian Hormones and Substance Use
There is a large body of literature documenting fluctuations in the subjective and physiologic effects of addictive drugs and patterns and motivation for drug use across the menstrual cycle phase (for reviews, see Lynch et al., 2002; Becker and Koob, 2016). Studies with psychostimulants have focused predominantly on the subjective and physiologic effects of cocaine (in individuals with a cocaine use disorder) and amphetamine (in recreational users or “healthy controls”). These results indicate that the subjective/reinforcing effects of stimulants are higher in women during the late follicular phase, when levels of estradiol are high and progesterone levels are low, versus the mid-luteal phase, when levels of estradiol are moderate and progesterone levels are high (Lukas et al., 1996; Sofuoglu et al., 1999; Justice and de Wit, 2000; Evans et al., 2002; White et al., 2002). Similar conclusions of a faciliatory effect of estradiol and inhibitory effect of progesterone have been reached from clinical studies following exogenous hormone manipulation (Justice and deWit 2000; Lile et al., 2007; DeVito et al., 2014). For example, Lile et al. (2007) conducted a pilot study in 10 women without a SUD to determine the effects of exogenously administered estradiol on subjective ratings of d-amphetamine. They found that estradiol modestly increased the positive subjective effects (e.g., Like Drug) and discriminative stimulus effects of a low dose of d-amphetamine (also see Justice and deWit 2000). Conversely, administration of exogenous progesterone has been shown to decrease the positive subjective effects of psychostimulants in both normal controls and women with SUD (Sofuoglu et al., 2002, 2004; Evans and Foltin, 2006; Peltier and Sofuoglu 2018). Similar findings of enhanced positive subjective effects during the follicular versus luteal phase have been observed for nicotine in smokers (DeVito et al., 2014). While effects with opioids have focused on analgesic effects, these findings similarly show greater morphine analgesia in women during the follicular versus luteal phase (Ribeiro-Dasilva et al., 2011). These findings indicate that estradiol enhances, while progesterone reduces, the positive subjective effects of addictive drugs, particularly psychostimulants although future studies using larger samples are needed to verify the effects of estradiol. Additional studies are also needed to determine whether these effects also translate to other addictive drugs, such as opioids and cannabis.
There is also a large literature on alcohol documenting menstrual cycle effects in social drinkers and individuals with an AUD, but in contrast to literature on psychostimulants, most of these studies have focused on levels of use and craving (Terner and de Wit, 2006) rather than subjective effects (but see Evans and Levin 2011). The results have been less consistent than findings with stimulants. Some studies find greater intake and/or craving premenstrually (late luteal) and during menstruation (early follicular) whereas others show greater consumption/craving during the late follicular/ovulatory phase (for a review, see Joyce et al., 2021). Affective state also fluctuates across the menstrual cycle and may overlap with changes in alcohol consumption and craving. For example, negative affect, including anxiety and depressive affect, peaks in the late luteal/premenstrual phase and early follicular/menstrual phase in response to progesterone withdrawal (Gallo and Smith, 1993; Herzog 1995; Moran et al., 1998; Smith et al., 1998), and positive affect, including feelings of well-being and reward-processing, peak in the late follicular/ovulatory phase when levels of estradiol have are at their apex and progesterone levels are low (Collins et al., 1985; Aganoff and Boyle, 1994). Motivation for drinking similarly varies across the menstrual cycle with women reporting increases in drinking to combat negative affect during the late luteal/menstrual phase and increases in drinking for social motives during late follicular/ovulatory phase (Joyce et al., 2018).
2. Human Studies: Ovarian Hormones and SUD
Motivation to use alcohol and other addictive drugs also likely differ between recreational users and individuals with a SUD given that once addiction has developed, the positive subjective/reinforcing effects of drugs diminish, and the negative reinforcing effects become the principal motivator for drug use (Koob 2021). This idea is also in line with findings showing that in healthy college women (without an AUD), social drinking and craving for alcohol are increased in the follicular phase (vs. luteal phase) and associated with increased levels of estradiol (Martin et al., 1999; Warren et al., 2021) whereas in women with an AUD and/or premenstrual dysphoric disorder, alcohol craving is highest during the late luteal/early follicular phases, when negative affect is highest and progesterone levels are low (Mello et al., 1990; Svikis et al., 2006; Evans and Levin, 2011; Kiesner, 2012). Higher levels of progesterone are also predictive of lower levels of alcohol craving in postmenopausal women with AUD (Weinland et al., 2021). It is also notable that findings with psychostimulants similarly show that, in contrast to positive subjective responses, craving is predicted by progesterone levels. Craving is low when progesterone levels are high (vs. when low or moderate; Sinha et al., 2007; Goletiani et al., 2015; Ethier et al., 2021) and can be offset by treatment with progesterone or its metabolite, allopregnolone (Fox et al., 2013; Peltier and Sofuoglu, 2018). It is also consistent with findings in smokers showing that nicotine withdrawal and depressive symptoms are increased during the late luteal phase, particularly in women who have premenstrual syndrome or premenstrual dysphoric disorder (Mello et al., 1990; Perkins et al., 2000; Svikis et al., 2006; Evans and Levin, 2011; Kiesner, 2012). Findings in daily cannabis users similarly show that cannabis use is higher in the late luteal phase (premenstrually) as compared with the follicular and ovulatory phases (Hanzal et la., 2019; Joyce et al., 2021), and preliminary evidence indicates that progesterone attenuates cannabis craving (Sherman et al., 2019). To our knowledge, no studies have examined the impact of ovarian hormones or menstrual cycle on craving or use of opioids highlighting an area for future research.
Together, these findings indicate that in women the role of ovarian hormones may vary in recreational users versus individuals with a SUD. In initial stages, or under conditions wherein the positive reinforcing actions of the drug are predominantly motivating drug use, estradiol enhances the subjective effects of drugs and likely enhances vulnerability to drug use. At these times, progesterone reduces the subjective effects of addictive drugs and likely reduces vulnerability to drug use. In contrast, progesterone appears to be more critical than estradiol in motivating drug use and craving for addictive drugs in individuals with a SUD and those using addictive drugs for their negative reinforcing effects. Evidence indicates that withdrawal from progesterone enhances drug craving and/or drug use to combat negative affect/craving whereas high levels of progesterone either during the luteal phase or after exogenous administration reduce drug craving and/or use. These results further indicate that the telescoping effect in women may be driven by reward-enhancing actions of estradiol as experienced during initial drug use. In turn, this increases the probability of additional recreational use and the subsequent development of a SUD. Additional research is necessary to determine the effects of ovarian hormones on the subjective effects, levels of use, and craving for opioids.
It is important to note that the relationship between ovarian hormones and drug use/SUD is reciprocal in that ovarian hormones both affect and are affected by drug use and SUD. For example, during cocaine withdrawal, progesterone levels are elevated across the menstrual cycle resulting in significantly lower ratios of estradiol/progesterone as compared with healthy controls (Fox et al., 2008). This occurs in response to elevated cortisol levels and may indicate subfertile cycles (Dobson and Smith 1998). This response is also anxiolytic at first but may lead to the later blunting of the stress response and increased anxiety, reduced tolerance to stress, and depression, which are all stress-related behaviors associated with relapse susceptibility in women with CUD (Fiad et al., 1996; Kampman et al., 2004; Kaplan and Manuck 2004; Sinha et al., 2006). Additionally, hypogonadism is common with chronic opioid use or opioid replacement therapy and is the result of suppression of the pulsatile release of gonadotropin-releasing hormone leading to deficiencies of luteinizing hormone (LH), follicle stimulating hormone (FSH), estradiol, and progesterone (Antony et al., 2020). Chronic alcohol use also causes hypothalamo-pituitary dysfunction and is associated with menstrual irregularities, such as anovulation, luteal-phase defects, recurrent amenorrhea, and early menopause (Hugues et al., 1980). As another example, nicotine reduces the aromatization of testosterone to estradiol, and as such, female smokers have higher testosterone levels and are more likely to experience estradiol deficiency and early menopause than nonsmoker females (Jandikova et al., 2017). Similar reciprocal effects of ovarian hormones and addictive drugs have also been observed in preclinical studies, but given that hormones can be more precisely manipulated in animals (e.g., using hormone replacement in ovariectomized, OVX, animals), these studies have been critical for establishing a causal role of ovarian hormones in substance use and SUD.
3. Animal Studies: Ovarian Hormones and Substance Use
Most of the support for a role of ovarian hormones on vulnerability to addiction has come from preclinical studies. These studies have shown that the reinforcing effects of addictive drugs vary in intact female rodents across the estrous cycle and in OVX females with and without hormone replacement. Studies in intact females have shown that following acquisition of drug self-administration, progressive-ratio responding for cocaine is markedly higher during estrus compared with other phases of the estrous cycle (Roberts et al., 1989; Hecht et al., 1999; Feltenstein and See, 2007; Lynch et al., 2008; Lacy et al., 2016). Findings in nonhuman primates similarly show that progressive-ratio responding for cocaine varies across the menstrual cycle with the highest levels observed during the follicular phase (vs. the late luteal phase); this effect is modest and only apparent at a low dose (Mello et al., 2007). Motivation for nicotine is also higher in female rats during estrus, but this effect is modest and has been observed in some studies (e.g., Lynch 2009), but not others (Donny et al., 2000).
Studies with alcohol have focused on consumption and have shown that consumption is lower in female rats during estrus and proestrus (vs. metestrus and diestrus). However, these effects are modest and are only apparent when estrous cycles are synchronized such that each female is tested in the identical portion of each phase (Roberts et al., 1998; also see Forger and Morin, 1982). In contrast, no estrous cycle effects are observed for maintenance levels of alcohol consumption in free-cycling female rats indicating that the variability in hormone levels within different phases of the estrous cycle is enough to obscure the effects of estrous phase on drug intake. In female nonhuman primates, alcohol intake tends to be highest during mid- to late follicular and the late luteal phase, which is similar to findings in humans (vs. menses; Mello et al., 1984). Studies with opioids have also focused on maintenance levels of intake and have shown that intake is markedly lower during proestrus as compared with other phases of the estrous cycle (Lacy et al., 2016; Schmidt et al., 2021; Smith et al., 2021). This effect appears to be driven by estradiol given that it can be blocked using raloxifene, a selective estrogen receptor modulator/antagonist, and mimicked by administering supplementary estradiol treatments (Sharp et al., 2021; Smith et al., 2021).
Studies in OVX rats have consistently found a significant role for ovarian hormones in mediating the reinforcing effects of addictive drugs. For example, numerous studies have shown that OVX robustly attenuates the acquisition of cocaine self-administration (Lynch and Carroll, 2001; Hu and Becker, 2003; Jackson et al., 2006; Hu and Becker, 2008; Zhao and Becker, 2010; Perry et al., 2013). It also decreases nicotine self-administration (Maher et al., 2022), the acquisition of methamphetamine and cannabinoid (WIN55,212-2, CB1 receptor agonist) self-administration (Fattore et al., 2009; Kucerova et al., 2009), and alcohol consumption and preference during acquisition and maintenance (Forger and Morin, 1982; Cailhol and Mormede, 2002). Studies with cocaine and methamphetamine further show that estradiol replacement in OVX females restores acquisition rates to those observed in ovary-intact females (Lynch and Carroll, 2001; Hu and Becker, 2003; Jackson et al., 2006; Hu and Becker, 2008; Kucerova et al., 2009; Zhao and Becker, 2010; Perry et al., 2013). Notably, concurrent administration of progesterone with estradiol inhibits the effect of estradiol on acquisition of cocaine self-administration (Jackson et al., 2006). Progesterone has also been shown to attenuate cocaine-induced conditioned place preference (Russo et al., 2008) and to decrease impulsive choice for cocaine in ovary-intact females (Smethells et al., 2016). Similar findings for the effects of OVX and hormone replacement have been observed for the rewarding effects of cocaine, alcohol, nicotine, methamphetamine, and amphetamine as assessed under the conditioned place preference paradigm (Chen et al., 2003; Frye and Rhodes, 2006; Silverman and Koenig, 2007; Mirbaha et al., 2009; Torres et al., 2009; Hilderbrand and Lasek, 2018). These findings indicate that estradiol enhances the reinforcing effects of psychostimulants and other drugs while progesterone reduces it, similar to reports in humans.
There are also intriguing new data that suggest that in females, hormonal status at the time of initial drug exposure/conditioning impacts later vulnerability to drug use. Specifically, Johnson et al. (2019) showed that female rats that had undergone Pavlovian conditioning with cocaine (paired with a cue light) during estrus prior to cocaine self-administration were more motivated to obtain infusions of cocaine paired with the light cue as compared with males or to females that had been conditioned during diestrus. Levels of estradiol during the time of initial exposure/conditioning appear to drive this effect considering that in OVX females estradiol supplementation that occurs prior to acquisition effectively restores drug intake to levels observed in intact females whereas supplemental after acquisition does not impact intake (Maher et al., 2022). It is not yet known whether hormonal status during initial drug exposure would also impact vulnerability to developing addiction-like features. Future studies using animal models of SUD are necessary to determine this possibility and to determine whether effects extended to other addictive drugs.
Finally, it is important to highlight a need for additional studies to examine the impact of ovarian hormones on the reinforcing effects of opioids considering that effects of OVX and estradiol on acquisition have been mixed with one study showing facilitation (Roth et al., 2002) and another finding no effect of estradiol replacement (Stewart et al., 1996). In contrast, Smith and colleagues have shown in a series of studies that ovarian hormones markedly impact maintenance levels of opioid self-administration. Specifically, they showed that heroin intake is markedly lower in females during proestrus (Lacy et al., 2016; Schmidt et al., 2021; Smith et al., 2021) and that this the effect could be mimicked by estradiol (in OVX and in ovary-intact females) and blocked by an estrogen receptor antagonist (in ovary-intact females; Sharp et al., 2021; Smith et al., 2021). They also showed that progesterone treatment increased heroin self-administration compared with estradiol treatment in OVX females (Smith et al., 2021; but see Smith et al., 2022). These findings suggest that effects of ovarian hormones may be more robust for maintenance opioid use than initial opioid use, but additional studies are necessary to examine this possibility. Future studies are also necessary to determine the direction of effects of estradiol and progesterone on the reinforcing efficacy of opioids (e.g., using progressive-ratio schedules, the threshold procedure, or choice procedures).
4. Animal Studies: Ovarian Hormones and SUD
Ovarian hormones also appear to underlie the enhanced vulnerability in females to developing addiction-like features, and these effects are apparent during both the induction/extended-access drug self-administration phase, where high levels of drug intake lead to the subsequent development of an addiction-like phenotype and, again, with the development of an addiction-like phenotype. As with effects on maintenance levels of drug use, effects of ovarian hormones on extended-access intake have been subtle in intact females. For example, studies on extended-access alcohol self-administration in rats have failed to demonstrate an effect of estrous cycle on alcohol intake using an intermittent access paradigm (Priddy et al., 2017) or continuous access paradigm (Ford et al., 2002) although patterns of alcohol intake do differ by estrous cycle phase (e.g., greater bout frequency and size during diestrus vs. proestrus; Ford et al., 2002). Similarly, there is no effect of estrous cycle phase on levels of cocaine intake under extended-access conditions (6 h/d; Corbett et al., 2021), but during estrus, females show a greater disruption in temporal patterns of cocaine self-administration (e.g., intake is more erratic/less tightly regulated) as compared with during nonestrus phases (Lynch et al., 2000). Cocaine intake also does not differ across the menstrual cycle in female nonhuman primates with an extensive history of cocaine self-administration (Cooper et al., 2013). These findings are in contrast to those reported by Mello et al. (2007), who observed menstrual cycle effects for cocaine self-administration in female nonhuman primates that were drug-naïve prior to acquisition and progressive-ratio testing. They suggested that the role of ovarian hormones may decrease from initial use to the development of an addiction-like phenotype. This conclusion is further supported by findings showing that in female rats that develop a preference for cocaine over food pellets, the estrous cycle continues to modulate motivation for food pellets but not cocaine.
Alternatively, it is possible that effects of ovarian hormones are obscured following chronic drug self-administration due to the reciprocal effects of addictive drugs and ovarian hormones (i.e., the impact of chronic drug use on ovarian hormone levels). This conclusion is supported by multiple studies with cocaine showing that depletion of ovarian hormones by OVX robustly decreases extended-access cocaine self-administration whereas estradiol replacement robustly increases levels of self-administration (Larson et al., 2007; Ramoa et al., 2013; 2014; Martinez et al., 2016). Similar findings have been observed for alcohol consumption under a 24-h/d, two-bottle choice, continuous-access paradigm (Forger and Morin, 1982; Becker et al., 1985; Ford et al., 2004; Rajasingh et al., 2007; but see Hilderbrand and Lasek, 2018) and nicotine intake under extended-access conditions (Flores et al., 2016). These findings also appear to extend to opioids given our recent findings showing that OVX females with estradiol self-administer markedly higher levels of fentanyl under extended- (24-h/d) intermittent-access conditions (two 5-minute trials/h, 10 days) than vehicle-treated OVX rats. OVX rats with estradiol replacement, but not vehicle treated OVX rats, also escalated their intake of fentanyl between the first and last extended-access sessions (Towers et al., 2022). Additionally, similar to the acquisition and conditioned place placement studies, progesterone attenuates the escalation of cocaine intake under extended-access conditions (Larson et al., 2007) and decreases alcohol consumption under a 24-h/d, two-bottle choice, continuous-access paradigm (Ford et al., 2002). Progesterone treatment has also been shown to decrease cocaine self-administration in intact and OVX female nonhuman primates with or without an extensive history of cocaine self-administration (Mello et al., 2011). Thus, estradiol appears to enhance, while progesterone protects against, the transition from regular to escalated/dysregulated drug use. While additional studies are needed to determine whether the protective effects of progesterone on escalation/dysregulation of cocaine and alcohol self-administration extend to other addictive drugs, such as opioids, there is strong evidence that estradiol similarly enhance vulnerability during extended-access self-administration for a number of addictive drugs, including cocaine, nicotine, opioids, and alcohol.
Findings with cocaine and opioids indicate that ovarian hormones also modulate the expression of addiction-like features following abstinence from extended-access self-administration. Most of this evidence is for drug craving and shows that levels of cocaine, fentanyl, and heroin craving are highest in females tested during estrus (vs. nonestrus phases; Nicolas et al., 2019; Bakhti-Suroosh et al., 2021; Corbett et al., 2021; Towers et al., 2022); estrus also prolongs the time course for incubation of cocaine craving in females (Kerstetter et al., 2008). While surprisingly few studies have examined the role of estradiol in incubated drug craving/relapse vulnerability following extended-access self-administration, our recent findings with fentanyl indicates that it is critically involved. Specifically, we found that OVX females with (vs. without) estradiol replacement had a greater a sensitivity to the reinstating effects of fentanyl-associated cues following extended, intermittent-access fentanyl self-administration and 14 days of abstinence (Towers et al., 2022). However, both the vehicle- and estradiol-treated groups showed an increase in responding following exposure to the cues, indicating that while estradiol modulates the expression of this phenotype, it is not necessary for its development. Similar effects of OVX have been reported for cannabinoid-craving following short-access self-administration where OVX rats showed an attenuated response to drug cues and drug primes (CB1 receptor agonist; Fattore et al., 2010). Progesterone may also be involved given that exogenous treatment has been shown to reduce cocaine-craving in intact females following short-access self-administration (Anker et al., 2007; Feltenstein et al., 2009).
In contrast to effects on drug craving, estradiol appears to be necessary for development of an enhanced motivation for the drug and an enhanced preference for the drug over other reward alternatives. Most of this work has focused on cocaine and has shown that depletion of ovarian hormones either surgically (OVX) or pharmacologically (tamoxifen treatment in ovary-intact females) prevents the development of an enhanced motivation for cocaine even under conditions optimized for its development (following extended-access self-administration and 14 days of abstinence; Ramôa et al., 2013, 2014; Bhakti-Suroosh et al., 2019). In contrast, this phenotype is evident in both vehicle-treated intact females and in OVX females treated with estradiol (Ramôa et al., 2013, 2014; Bhakti-Suroosh et al., 2019). Similar effects of OVX and estradiol have also been observed for the development of a preference for cocaine over food (Kerstetter et al., 2012). We also recently observed similar effects of OVX and estradiol for the development of an enhanced motivation for fentanyl (Towers et al., 2022), indicating that the role of estradiol on the development of this feature of SUD may be similar for both psychostimulants and opioids. However, further research is necessary to confirm its role with other psychostimulants (e.g., methamphetamine, nicotine) and other opioids (e.g., heroin, oxycodone). Additional studies are also needed to confirm effects with fentanyl considering that the parameters for the development of an enhanced motivation for fentanyl are not yet known (i.e., when the phenotype emerges, how much prior drug access is needed, and how long phenotype persists).
Interestingly, pharmacological blockade of estrogen receptors with tamoxifen has been shown to similarly prevent the development of an enhanced motivation for cocaine in ovary-intact females, but unlike the findings in the OVX model, tamoxifen did not decrease cocaine self-administration under extended-access conditions or relapse vulnerability (Bakhti-Suroosh et al., 2019). These findings indicate that differences in level of intake during the induction/extended-access phase, which did not differ between tamoxifen- and control-treated females, is not critical for the development of motivational features of addiction (Bakhti-Suroosh et al., 2019). They also suggest other hormones, such as progesterone, may modulate the expression of certain addiction-like features, such as a loss of control over drug use and relapse vulnerability, but is perhaps not critical for others, such as an enhanced motivation for the drug. For example, where estrogen receptors are antagonized, such as with chronic tamoxifen administration in ovary-intact females, there may be compensatory decreases in progesterone signaling, which leads to increased extended-access drug intake and relapse vulnerability. However, it should be noted that as a selective estrogen receptor modulator, tamoxifen can have both agonist and antagonist effects (Dutertre and Smith 2000); thus, it is possible that its antagonist effects at estrogen receptors were sufficient for preventing the development of an enhanced motivation for cocaine but not for reducing extended-access intake or for attenuating relapse vulnerability. Future studies are needed to resolve these questions.
In summary, estradiol appears to enhance the expression of multiple features of SUD (loss of control over use, relapse vulnerability, preference for drug over other rewards, motivation for the drug) and to be necessary for the development an enhanced motivation for the drug and an enhanced preference for the drug over other rewards. Progesterone may attenuate the expression of addiction-like features and vulnerability maybe heightened when progesterone levels are low, but additional studies are studies are necessary to confirm these possibilities.
B. Sex Chromosomes
One biologic factor that few consider when they assess sex differences is the basic inequality in sex chromosome genes. In the mammals commonly used for preclinical studies and in humans, males have two different sex chromosomes, X and Y, whereas females have two copies of the X-chromosome. The X-chromosome is substantially larger (3× the physical size) and contains about 1000 more coding genes than does the Y-chromosome (Balaton et al., 2018). When the phenomenon of X-inactivation was discovered, we assumed it equalized this discrepancy. If, in fact, the entire second X-chromosome in each cell was inactivated in females, the sexes would still have differences in gene expression by virtue of unique genes on the male-only Y-chromosome. However, it is now clear that many (20% in humans) X-chromosome genes escape inactivation (Disteche and Berletch, 2015; Patrat et al., 2020).
To examine the actions of sex chromosome genes both independently of hormones and interactive effects, the Four Core Genotype (FCG) mouse is frequently used (De Vries et al., 2002). The dam for this cross is a normal XX female, but the sire carries a null mutation of the sex determining gene (Sry) on his Y-chromosome and a transgene for the Sry that has randomly incorporated into chromosome 3 (Lovell-Badge and Robertson 1990; Mahadevaiah et al., 1998; Itoh et al., 2015). The Y-chromosome and the Sry transgene segregate independently producing four genetic offspring from the cross: females with XX or XY chromosome and males with XX or XY sex chromosomes. This model provides a way to disassociate the effects of hormones from the effects of sex chromosome complement. The FCG has been exploited for over 20 years for disease models, studies of neurobiology and behavior (Gatewood et al., 2006; Quinn et al., 2007; Smith-Bouvier et al., 2008; Cisternas et al., 2018; Arnold 2020).
1. Animal Studies: Sex Chromosomes and Substance Use
One study has used the FCG mouse model to determine how sex chromosomes influence vulnerability to drug use (Martini et al., 2020). This study found that females (XX and XY) acquired cocaine self-administration faster than males XY males; XXM also acquired faster than XY males and did not differ from XX or XY females. However, contrary to findings in rats, motivation for cocaine (as assessed under progressive-ratio schedule following acquisition) was highest in XY males as compared with all other groups. Together, these results suggest sex chromosomes may interact with gonadal hormones to impact initial vulnerability to drug use.
2. Animal Studies: Sex Chromosomes and SUD
Two studies have used the FCG mouse model to examine habit formation, which is believed to occur during the transition from recreational drug use to compulsive drug use and addiction. Barker et al. (2010) showed that chromosomal females are slower to develop habitual responding for alcohol reinforcement compared with chromosomal males, but gonadal females consumed more alcohol than gonadal males. Interestingly, the second study showed that chromosomal females are faster to form habitual responding for sucrose compared with chromosomal males, regardless of gonadal phenotype (Quinn et al., 2007). These findings suggest that sex chromosomes may differentially affect the formation of habitual drug versus nondrug reinforcers use. They also indicate that gonadal hormones, but not sex chromones, drive the enhanced vulnerability in females.
C. Summary and Integration of Preclinical and Clinical Findings
Together, these clinical and preclinical studies support an important role for estradiol in vulnerability during early phases of SUD, such as drug use initiation and the transition from use to SUD, thereby making estradiol a potential driver of the telescoping effect in women. These studies also highlight ovarian hormones as a potential target for intervention during initial periods of drug use and prior to the development of SUD. However, the role of ovarian hormones may be different with opioids, particularly during drug use initiation, and future studies are necessary to investigate this possibility. Finally, estradiol and progesterone have broad actions on many neural and nonneural tissues including breast, ovary, and uterus. Any steroid treatments would have to take cancer risk into account.
It is important to consider that nearly all women use contraception in their lifetime (Daniels and Mosher, 2013), and hormonal contraceptives composed of either a combination of estradiol and progesterone or progesterone alone are very popular (Daniels and Abma, 2020; Cooper et al., 2022). Little is known about the influence of hormonal contraceptives on vulnerability to SUD in women or female laboratory animals, and the clinical studies report mixed results with some showing that pill users have lower positive subjective ratings of nicotine (Hinderaker et al., 2015) and nicotine craving (Dickmann et al., 2009) than nonpill takers. However, current smokers are more likely that nonsmokers to use hormonal contraceptives (Lee et al., 2013), and these women have increased ethanol intake, especially if contraceptive use begins at an early age (<20 years; Lund and Jacobsen, 1990). These conflicting reports could be the result of the wide range of hormonal contraceptives available to patients, which include high versus low doses of estradiol or just progesterone. Other characteristics of smokers versus nonsmokers may also influence these results. Further studies need to determine the impact of these commonly used hormonal contraceptives as physicians could tailor their birth control recommendation if substance misuse is a concern, or they could be repurposed as an adjunctive therapy for at risk adolescents since they have been proven to be safe in this patient population.
A final important consideration is pregnancy, which appears to protect females. During pregnancy, progesterone and estradiol levels markedly increase, and coincidentally, rates of drug use, including tobacco, alcohol, cannabis, and any drug not prescribed by a doctor to the individual, also markedly decline (Harrison et al., 2009; Kendler et al., 2017: Volkow et al., 2019). While there are obvious sociocultural-based factors that may also explain these decreased rates of drug use, studies in rats also show marked decreases in drug self-administration during pregnancy, indicating that pregnancy decreases biologic vulnerability to drug use in females. For example, Hecht et al. (1999) showed that in rats, motivation to obtain cocaine under short-access conditions progressively declined from prepregnant levels over the course of pregnancy. One caution to note here, however, is that dose was not adjusted for weight changes during pregnancy, and thus motivation to obtain cocaine may be reduced by relatively low cocaine dose. However, because similar findings have also been observed for nicotine self-administration under extend-access conditions (23 h/d; LeSage et al., 2007), where dose was adjusted for changes in body weight and oral alcohol intake under continuous-access conditions (Gene Forger and Morin, 1982), the results are likely to reflect a reduction in the reinforcing effects of addictive drugs as a result of pregnancy. These effects may differ for opioids, however, considering findings showing that under short-access conditions (1 h/d), pregnant female rats self-administer similar levels of oxycodone as nonpregnant female rats (Vassoler et al., 2018). While these findings in rats appear to contrast with epidemiologic data showing that the prevalence of past-month heroin use is markedly lower in pregnant than in nonpregnant women (0.05% vs. 0.19%, respectively; 15–44 years old; Vanderziel et al., 2020), data obtained during labor and delivery show that the prevalence of opioid use and OUD in pregnant women has quadrupled over the last decade and is present in approximately 3% of pregnancies in the United States (Chang 2020). Thus, the rising levels of progesterone during pregnancy appears to be protective against drug use and possibly the transition to SUD, but this may be different for opioids.
IV. Neurobiological Mechanisms
A. Mesolimbic Dopamine Signaling
Dopamine signaling in the mesolimbic pathway has been nearly the exclusive focus of studies on molecular mechanisms mediating sex differences in SUD. This pathway, which includes dopaminergic projection neurons from the VTA to the NAc, is well established, based mainly on studies conducted in males, to be a core component of the reward circuitry and critical for mediating the positive reinforcing effects of addictive drugs (for reviews, see Pierce and Kumaresan, 2006; Koob and Volkow, 2010). Addictive drugs increase dopamine concentrations in the NAc and antagonizing dopamine receptors, particularly dopamine D1-receptors (D1 and D5, referred to as D1 hereafter), prevent the acquisition of drug self-administration and decrease short-access drug self-administration (for a review, see Volkow and Morales, 2015).
Not surprisingly, the mesolimbic reward pathway is also implicated in the disease state of addiction. Again, these data are based predominantly on effects in men and male laboratory animals and show that neuroadaptations caused by chronic drug use leads to mesolimbic hypofunction, which in turn promotes drug use to combat negative affect/anhedonia induced by dopamine deficits during abstinence (Koob and Volkow, 2016). For example, it is well established based on positron emission tomography imaging studies in humans that individuals with a SUD have marked decreases in dopamine D2 receptor binding (D2, D3, and D4, referred to as D2 hereafter) in the striatum. This molecular switch was first documented by Volkow et al. (1990, 1993, 1996) who showed that individuals with cocaine use disorder had lower D2 receptor availability that corresponded to increased ratings of dysphoria which persisted months after abstinence (relative to healthy individuals). These individuals also showed diminished dopamine release in the striatum and reported lower ratings of positive subjective effects (reduced liking, euphoria) and higher ratings of negative subjective effects (want more, craving) following psychostimulant administration as compared with healthy individuals (Volkow et al., 1996, 1997). This phenomenon has been replicated in many subsequent studies and for multiple SUDs including methamphetamine, nicotine, heroin, and alcohol (Volkow et al., 2001, 2014; Martinez et al., 2004, 2005, 2011, 2012; Martinez et al., 2005; Fehr et al., 2008; van de Giessen et al., 2017; Worhunsky et al., 2017; but see Casey et al., 2014; for a review, see Volkow et al., 2007). This is thought to reflect a shift from positive reinforcing effects, a primary mechanism driving drug use during initial phases of SUD, to negative reinforcement, which drives drug use once addiction has developed to alleviate withdrawal, craving, or negative affect. A similar blunting of the dopaminergic response to psychostimulant drug administration is observed in cannabis use disorder (CaUD), and while this effect is also associated with increased relapse vulnerability, it is not accompanied by a downregulation of D2 receptors (Volkow et al., 2014). A few studies have included both men and women (Volkow et al., 2001; Martinez et al., 2012), and some sex differences have been noted (as discussed in the following text; Brown et al., 2012; Okita et al., 2016; Zakiniaeiz et al., 2019). Results from preclinical studies have revealed similar changes in D2 receptor signaling with evidence to further indicate that mesolimbic D2 receptor signaling contributes to both vulnerability to drug use and the development of key addiction-like features, such as a loss of control/escalation of drug use (for reviews, see Everitt et al., 2008; Trifilieff et al., 2017). There is also compelling evidence indicating the role of dopamine is minimized once SUD is established and that other signaling pathways, particularly those involved in mediating the negative reinforcing effects due to craving, are recruited and drive the enhanced motivation for the drug (e.g., glutamatergic signaling; see later section on glutamate).
While most of the evidence for sex differences in dopaminergic signaling is focused on initial vulnerability, preliminary findings indicate that the mechanisms underlying SUD are different in males versus females and that molecular shifts that contribute to its development occur faster in females than males (as discussed in the following text).
1. Human Studies: Dopamine and Substance Use
Clinical studies using healthy controls report that men and women have similar D2 receptor availability and densities in the striatum, but women have greater dopamine synthesis capacity and dopamine transporter availability in the striatum than men (for a review, see Woodcock et al., 2020). The net effect is that dopamine secretion and transport are more active in women than in men. Findings for evoked dopamine release in the striatum, however, have been mixed and tend to suggest greater effects in healthy men than women in response to psychostimulants and alcohol (Munro et al., 2006; Urban et al., 2010; Oswald et al., 2015; Smith et al., 2019; but see Riccardi et al., 2006). In contrast, Manza et al. (2022) reported more striatal dopamine release in women than men (as measured by displacement of [11Craclopride]) in response to both oral and intravenous administration of the psychostimulant methylphenidate. Women also reported higher ratings of “drug effects” than men (Manza et al., 2022), which is in contrast to the other studies reporting greater psychostimulant-evoked dopamine release and positive subjective effects in men than in women (Munro et al., 2006; Smith et al., 2019). Given that the positive subjective effects of addictive drugs are believed to be driven by mesolimbic dopamine signaling, this difference provides a plausible explanation for the differences between these results. Moreover, as with positive subjective ratings of addictive drugs, sex differences in evoked dopamine release are influenced by hormonal changes over the menstrual cycle. Cycle day and/or hormone data have not been included in some of the previous studies (Munro et al., 2006; Urban et al., 2010; Oswald et al., 2015) or testing was completed in women with low ovarian hormones (Munro et al., 2006; Oswald et al., 2015; Smith et al., 2019). As a specific example, in the Smith et al. (2019) study, the women included were in one of three low estradiol states—either postmenopausal, on hormonal contraceptives, or in the early follicular phase of their menstrual cycle. This is in contrast to the most recent study where hormone data were collected, and at least some of the women included were tested during the mid- to late follicular phase (Manza et al., 2022), when levels of estradiol are high and relatively unopposed by progesterone. However, even in this recent study, details are lacking regarding menstrual cycle status and estradiol levels are available for only 7 of the 11 female subjects. Future studies that measure, or manipulate, levels of estradiol and progesterone are necessary to resolve this issue.
2. Human Studies: Dopamine and SUD
Most of the studies on sex differences in dopamine signaling and SUD have been conducted among tobacco smokers. These studies have shown that as with findings in individuals with CoUD, AUD, and OUD, individuals with a TUD have a blunted dopamine response to psychostimulant administration (Busto et al., 2009; Wiers et al., 2017; Zakiniaeiz et al., 2019; Calakos et al., 2022), with particularly robust effects in women (Cosgrove et al., 2014). There is also a sex difference in the mechanism underlying this effect. In male smokers, the mechanism appears to be similar to that observed for CoUD, OUD, and AUD, decreased striatal D2 receptor binding (Brody et al., 2004; Fehr et al., 2008; Stokes et al., 2012; Brown et al., 2012; Albrecht et al., 2013a). This is not the case in female smokers, however, since striatal D2 receptor binding does not differ between smokers and nonsmokers (Brown et al., 2012; Zakiniaeiz et al., 2019). This is intriguing considering that in male smokers this molecular shift is thought to reflect greater addiction severity and poorer treatment outcomes (Volkow et al., 1999), yet this does not occur in women who show greater addiction severity and worse treatment outcome than males. It is similarly thought to reflect enhanced vulnerability in individuals with a CoUD, AUD, or OUD, but given that these studies have been conducted predominantly in men, it is possible that this molecular shift occurs in males but not females.
Sex differences and effects of smoking status have also been between reported for D2 receptor availability in the midbrain, which includes the VTA (Okita et al., 2016). Female smokers have higher midbrain D2 receptor availability than both female nonsmokers and male smokers; however, no differences are seen between male smokers and nonsmokers (Okita et al., 2016). This difference is thought to underlie the greater suppression of mesolimbic dopamine in female versus male smokers given that D2 receptors are predominantly inhibitory. These differences also parallel sex differences in positive subjective ratings of nicotine and smoking, which have consistently been lower in women than in men with a TUD (for a review, see Perkins, 1999) whereas among nonsmokers, women are more sensitive than men to low doses of nicotine (MacLean et al., 2021). Taken together, these findings indicate that there are sex differences in the molecular mechanisms underlying tobacco use/smoking with the development of TUD. The different mechanisms likely lead to sex difference in motivation to use tobacco/nicotine and a greater shift in women than in men to negative reinforcement and to a diminished role of dopamine. This explanation is also consistent with data showing that smoking in women with a TUD does not produce ventral striatal activation but does so in men with a TUD (Verplaetse et al., 2018). Nicotine replacement is also a less effective treatment strategy for TUD in women than men (Perkins et al., 2008). While future studies are necessary to determine whether similar sex differences exist for other SUDs, it is notable that sex differences in positive subjective drug effects are observed across multiple drug classes and parallel these effects with TUD and typically show greater effects in women than men among recreational drug users, particularly at low doses (Matheson et al., 2020; Liechti et al., 2001; Miller et al., 2009; Vansickel et al., 2010; Fogel et al., 2017; Mayo et al., 2019; Wright et al., 2021), but no difference, or a diminished response, in women versus men among individuals with a SUD (e.g., Lynch et al., 2008; McCane-Katz et al., 2005; Mendelson et al., 1999). Additionally, de Wit et al. (2012) showed that dopamine depletion using a dietary intervention biases women, but not men, toward habitual responding rather than goal-directed behavior, indicating that women are prone to transition from recreational drug use, which is goal-directed, to compulsive use, which is habitual.
Individuals with a CaUD also have blunted dopaminergic responses to psychostimulant administration (Volkow et al., 2014; van de Giessen et al., 2017), but unlike effects in CoUD, AUD, and OUD, this response is not associated with lower striatal D2 receptor availability (Sevy et al., 2008; Stokes et al., 2012; Urban et al., 2012; Albrecht et al., 2013b). The mechanisms underlying the blunted responses also differ between men and women. Specifically, Wiers et al. (2016) examined regional brain glucose metabolism in response to psychostimulant administration in men and women with CaUD versus healthy controls. They found decreased stimulant-induced metabolism in the midbrain and striatum as well as decreased glucose metabolism in the putamen, and these correlated with addiction severity; however, all the effects were driven by changes in women. In men, no metabolic differences were observed between healthy controls and individuals with a CaUD. Women with a CaUD also had higher subjective ratings of craving in response to methylphenidate than healthy controls of either sex whereas no difference was observed between healthy men and men with a CaUD (Wiers et al., 2016). These results indicate that the neuroadaptations underlying SUD differ between men and women.
Sex differences in the time course for these molecular shifts in dopamine signaling/receptor populations that are concurrent with the development of SUD have not been examined. However, data from young adult men and women at risk for an AUD indicate they are possible. Specifically, Urban et al. (2010) showed that in young adults at high risk for an AUD based on levels of drinking (>10–15 drinks/wk, 15 drinks/wk for males, and 18 drinks/wk for females), men had greater and more widespread increases in striatal dopamine release than women in response to alcohol administration. Subjective ratings of “activation” in response to alcohol were also positively correlated with dopamine release in the ventral striatum in men whereas subjective ratings of alcohol were not correlated with dopamine release in women. These findings suggest that the shift toward a diminished role of dopamine signaling, believed to reflect greater addiction severity, may occur sooner in females than males. However, future studies are necessary to determine whether this effect is reliable and consistent across SUDs.
3. Animal Studies: Dopamine and Substance Use
Results from preclinical studies also suggest that there are sex differences in the dopamine signaling pathway. Although there are divergent data on whether the density of D1 receptors in the NAc differs between males and females (Andersen and Teicher 2000; Festa et al., 2006), markers of D1–cAMP–PKA cell signaling, which is associated with greater vulnerability to drug use, are enhanced in drug naïve females compared with drug naïve males (Lynch et al., 2007). However, it is important to note that markers of vulnerability, which have been generated based predominantly on findings in males, may differ between males and females. For example, Morgan et al. (2002) showed that dominant male cynomolgus monkeys have higher D2 receptor availability and are less vulnerable to the reinforcing effects of cocaine as compared with subordinate male monkeys. While dominant female cynomolgus monkeys also had higher D2 receptor availability than subordinate female cynomolgus monkeys, dominant females were more vulnerable to the reinforcing effects of cocaine as compared with subordinate females (Nader et al., 2012), indicating that the relationship between D2 receptor availability and vulnerability to cocaine is opposite in females versus males.
Preclinical findings also demonstrate that ovarian hormones modulate dopaminergic signaling in the reward pathway in females. Neuron firing rates in the VTA reach peak levels in females during estrus (vs. diestrus; Calipari et al., 2017), and drug-induced dopamine release is greater during proestrus/estrus (vs. metestrus/diestrus; Becker and Cha, 1989; Calipari et al., 2017). Results from nonhuman primates show that striatal D2 dopamine receptor availability is lower during the follicular phase than the luteal phase (Czoty et al., 2009). OVX has also been shown to increase striatal D2 receptors, reduces VTA firing rates, and drug-induced dopamine release in the NAc, while estradiol replacement restores each of these effects in female rats (Becker, 1990; Castner et al., 1993; Zhang et al., 2008; Cummings et al., 2014; Shams et al., 2016; Shams et al., 2018). Estradiol also increases tyrosine hydroxylase, the rate-limiting enzyme for dopamine synthesis and production, decreases sensitivity of D2 auto receptors, enhances D1 receptor activation (Festa et al., 2006), and reduces the reuptake of dopamine, all of which enhance mesolimbic dopamine signaling (Calipari et al., 2017). Estradiol-induced changes in dopamine signaling have been linked to an increased sensitivity to the rewarding effects of cocaine, assessed using conditioned place-preference (Calipari et al., 2017), and the reinforcing effects of alcohol (Vandegrift et al., 2017).
Interestingly, progesterone can both potentiate and inhibit estradiol’s effects on dopamine release with enhancement observed shortly after estradiol and progesterone administration in OVX rats and inhibition observed 24 hours after administration (Glaser et al., 1983). These differences likely explain estrus-induced enhancements of dopamine release given that both estradiol and progesterone peak prior to the beginning of estrus (Becker and Ramirez, 1981; Becker et al., 1984; Dluzen and Ramirez, 1984; Becker and Rudick, 1999; for a review, see Yoest et al., 2018). Thus, estradiol enhancement of mesolimbic dopamine signaling appears to increase the rewarding and reinforcing properties of addictive drugs and likely drives the enhanced sensitivity in females during initiation/acquisition of drug use. While similar effects have been observed for cocaine, amphetamine, and alcohol, further research is necessary to determine whether findings extend to other drug classes, including opioids and cannabis.
4. Animal Studies: Dopamine and SUD
While dopamine-estrogen interactions likely contribute to the enhanced sensitivity in females during initiation/acquisition of drug use (for a review, see Kokane and Perrotti 2020), it is not yet clear whether similar mechanisms underlie vulnerability during later phases of SUD or the faster time course to addiction in females versus males. Such effects are possible given findings from multiple studies showing that estradiol increases cocaine, alcohol, nicotine, and fentanyl intake under extended-access conditions (Ford et al., 2004; Larson et al., 2007; Ramoa et al., 2013, 2014; Flores et al., 2016; Martinez et al., 2016; Towers et al., 2022). Findings with alcohol also show that estradiol potentiates alcohol-induced excitation of dopamine neurons in VTA and that targeted knockdown of estrogen receptors in the VTA reduces binge alcohol drinking in female, but not male mice (Vandegrift et al., 2017, 2020). Thus, as with effects during drug use initiation, estradiol may enhance vulnerability to the development of addiction-like features by enhancing drug-induced dopamine signaling in the reward pathway.
Estradiol may also be necessary in females for the molecular switch to a diminished role of mesolimbic dopamine that accompanies the development of an addiction-like phenotype. Specifically, we showed that OVX prevents both the development of an enhanced motivation for cocaine and the corresponding molecular shift to a diminished role of NAc dopamine and that both effects can be restored by estradiol replacement (Ramôa et al., 2013; 2014). In our work, NAc D1 receptors remained the critical mechanism motivating cocaine use in vehicle-treated OVX rats that did not develop an addiction-like phenotype (Doyle et al., 2014; Ramôa et al., 2014). Similarly, Perry et al. (2015) showed that female rats that developed a preference for cocaine over a nondrug reward (i.e., palatable food pellets) also displayed attenuated cocaine-induced dopamine release in the NAc. In addition, the rats that developed a cocaine preference, the estrous cycle continued to modulate motivation for the palatable food pellets, but not cocaine (Perry et al., 2015) indicating that ovarian hormones may not be necessary for the expression of this feature of SUD. Thus, estradiol accelerates, and is necessary for, drug-induced changes in dopamine signaling that underlie the development of addiction, but it may not be necessary for the expression of the addiction-like behaviors once they have been established (although estradiol still modulates their expression as discussed in the subsequent section on cocaine craving).
B. Corticomesolimbic Glutamate
Studies in animals have established that estradiol enhances mesolimbic dopaminergic signaling via interactions with metabotropic glutamate receptors (mGlu); this likely contributes to the enhanced sensitivity in females versus males to the reinforcing effects of addictive drugs (as detailed in the following discussion). Glutamatergic signaling in corticomesolimbic regions, including projection neurons from the mPFC to the NAc, is also a strong candidate mechanism underlying the faster course to addiction in females. This pathway is critical for the development of multiple features of addiction including escalation of drug use, compulsive drug use, enhanced motivation for the drug, and enhanced craving/vulnerability to relapse (Koob and Volkow, 2016). Preclinical findings indicate that estradiol interacts with mGlu to enhance mesolimbic dopaminergic signaling, which may contribute to the enhanced sensitivity in females versus males to the reinforcing effects of addictive drugs. Glutamatergic signaling in corticomesolimbic regions, including projection neurons from the mPFC to the NAc, is also a strong candidate mechanism underlying the faster course to addiction in females. This pathway is critical for the development of multiple features of addiction including escalation of drug use, compulsive drug use, enhanced motivation for the drug, and enhanced craving/vulnerability to relapse. Glutamatergic projections from the mPFC to the NAc modulate the behavioral consequences of extended-access drug self-administration (Schmidt and Pierce 2010; Kalivas and Volkow, 2005, 2011; Quintero, 2013), and several studies have shown that extended-, but not short-access self-administration produces long-lasting adaptations in glutamate NMDA and AMPA receptors in the mPFC and NAc in humans, nonhuman primates, and rats (Backes and Hemby 2003; Tang et al., 2004; Hemby et al., 2005). This signaling pathway is known as the “final common pathway to relapse” since it is activated in response to relapse triggered by drug-associated cues, priming doses of the drug, and stress and for multiple drug classes, including psychostimulants, nicotine, opioids, and alcohol (Kalivas and McFarland 2003; Peters et al., 2008; Knackstedt and Kalivas, 2009).
Corticomesolimbic glutamate pathways also underlie the progressive increase, or incubation, of drug-craving over abstinence. Glutamatergic signaling in this pathway changes dramatically during abstinence, from hypoglutamatergic during early abstinence, when levels of drug-craving are low (first 1–3 days), to hyperglutamatergic during protracted abstinence, when craving has incubated to high levels (after 7 days; Ben-Shahar et al., 2009; Chen et al., 2013; Sun et al., 2014; Funk et al., 2016; Koob and Volkow, 2016; Barry and McGinty, 2017; Hearing et al., 2018; Szumlinski and Shin, 2018; Siemsen et al., 2019; Caffino et al., 2020; Roura‐Martínez et al., 2020). NMDA receptors are critically involved in both the early-withdrawal molecular cascade that triggers the incubation of craving (Barry and McGinty, 2017), as well as the enhanced cue-induced craving following protracted abstinence (Chen et al., 2013; Barry and McGinty, 2017; Szumlinski and Shin, 2018). These preclinical data are also consistent with pathophysiology of SUD in humans (Enoch et al., 2014; Hafenbreidel et al., 2017). AMPAR transmission is also critically involved, and this effect appears to be driven by Ca2+-permeable AMPAR, which accumulate in the synapses of neurons in the NAc core over a period of protracted abstinence following extended-, but not short-, access drug self-administration (Conrad et al., 2008; Purgianto et al., 2013; Caffino et al., 2021; Murray et al., 2021; Wolf and Tseng 2012). While most of the work in this area has been conducted with cocaine, the role of the mPFC in the drug craving and relapse appears to be similar for other drug classes, including opioids, alcohol, and methamphetamine (Schmidt et al., 2005; Bossert et al., 2006; Kuntz et al., 2008; Lalumiere and Kalivas, 2008; Rogers et al., 2008; Kuntz-Melcavage et al., 2009; See, 2009; Shen et al., 2011; Bauer et al., 2013; Doherty et al., 2013; Mishra et al., 2017; Hearing et al., 2018; Rubio et al., 2019). Results from both clinical and preclinical studies also similarly show an association between heightened drug-craving/relapse and activation of the mPFC to NAc pathway [Grüsser et al., 2004 (alcohol); LaLumiere and Kalivas, 2008; See, 2009; Goldstein and Volkow, 2011; Bauer et al., 2013 (alcohol); Shin et al., 2018; Szumlinski and Shin, 2018; Rubio et al., 2019].
One major caveat is that the evidence implicating glutamatergic signaling in SUD is based almost entirely on findings in men and male animals. Data obtained from women and female animals are beginning to accumulate, and they concur with the results from men and male laboratory animals indicating a critical role for glutamate in SUD. However, as detailed in the following discussion, there is also preliminary evidence indicating that there are sex differences in corticomesolimbic glutamate signaling that may contribute to sex differences in vulnerability to drug use and the faster course to addiction in females.
1. Human Studies: Glutamate and Substance Use
Very few clinical studies have examined sex differences in glutamatergic signaling. In healthy controls, women had higher levels of glutamate (as assessed using magnetic resonance spectroscopy) than men in the striatum, which includes the NAc and dorsal striatum (Zahr et al., 2013). Sex differences were also seen among recreational drinkers in the activation of the corticomesolimbic regions, presumably due to glutamatergic signaling. Specifically, Seo et al. (2011) showed that exposure to alcohol‐related cues increased activity in corticomesolimbic regions in both men and women, but women showed greater activation than men in the frontal gyrus (middle and superior; Seo et al., 2011).
2. Human Studies: Glutamate and SUD
To our knowledge, no studies have examined sex differences in glutamatergic signaling in individuals with a SUD. However, several studies have compared the effects of stress- or cue-induced craving on activity within corticomesolimbic regions in abstinent men and women with a SUD, typically CUD. These findings have been mixed but generally show that this circuit, presumed to be glutamatergic, is activated in both men and women (Grusser et al., 2004; Joseph et al., 2019) although the regions activated and degree of activation vary by sex between studies. For example, Kilts et al. (2014) showed that corticomesolimbic activity increased (as measured using regional cerebral blow flow) in both men and women following exposure to cocaine-associated cues. In women, increased activation was observed in the precentral, middle frontal, and posterior cingulate gyri whereas in men, increased activation occurred in the caudate, right postcentral gyrus, and left insula. Li et al. (2005) showed that both men and women show activation of the mPFC in response to stress-induced craving (using stress imagery), but under these conditions, activation was greater in women than men. Similarly, Potenza et al. (2012) showed that subjective reports of craving positively correlated with corticomesolimbic activation in both men and women with a CUD (Potenza et al., 2012), but in women, corticomesolimbic activation occurred in response to stress whereas in men, activation occurred in response to drug-associated cues. It is notable that in each of these studies, sex differences were apparent in brain regions activated in response to craving, yet subjective ratings of craving were similar between men and women. These findings add to a growing body of evidence indicating that, even in the absence of behavioral differences, the mechanisms underlying SUD in men and women may differ.
3. Animal Studies: Glutamate and Substance Use
Sex differences in mGlu signaling have been reported in drug-naïve laboratory animals in several brain regions, and differences in the NAc are thought to underlie the enhanced vulnerability observed in females to initial drug use. Specifically, mGlu5 appears to be required for estradiol-evoked dopamine release in the NAc in females (Song et al., 2019). In OVX rats, either an mGlu5 antagonist (MPEP) or an estrogen receptor (ICI-182 780) antagonist can block estradiol’s ability to enhance amphetamine-induced dopamine release in the NAc. Thus, mGlu5 likely contributes to sex differences in the reinforcing effects of psychostimulants and possibly other addictive drugs through an estradiol-dependent manner, which could translate to greater vulnerability to initial drug use.
4. Animal Studies: Glutamate and SUD
In OVX rats, mGlu5 activation is also necessary for estradiol-induced increases in extended-access cocaine self-administration (Martinez et al., 2016). In contrast to effects of estradiol–mGlu5 on dopamine release, which are likely mediated through rapid effects of membrane-associated estrogen receptors on neuronal excitability, effects of estradiol-mGlu5 on extended-access intake require repeated estradiol treatments over time indicating that changes are mediated through nuclear estrogen receptors that lead to altered synaptic plasticity. This idea is also in line with findings showing that estradiol mediates dendritic spine plasticity in the NAc through activation of mGlu5 in drug-naïve control females (Peterson et al., 2015). Females also have greater increases in spine density of medium spiny neurons following chronic drug exposure (Wissman et al., 2011; Strong et al., 2017), an effect also believed to be mediated via estradiol–mGlu5 interactions (for a review, see Tonn Eisinger et al., 2018). Differences are most apparent in the NAc core which is significant considering that dendritic spines on medium spiny neurons in this area integrate dopamine and glutamate signaling to mediate the reinforcing and motivational properties of addictive drugs. Thus, sex differences in mGlu5 signaling may contribute to sex differences during both initial exposure and the transition from use to addiction.
Notably, we showed that following the development of an addiction-like phenotype (i.e., an enhanced motivation for cocaine), the molecular mechanisms underlying drug use shifts from NAc dopamine to AMPA receptors in both males and females (Doyle et al., 2014). We further showed that estradiol is required in females for both the mechanistic shift to a diminished role of NAc dopamine and the development of an addiction-like phenotype (Ramôa et al., 2014). Considering that this mechanistic shift appears to accompany the development of an addiction-like phenotype, and considering that this phenotype develops sooner during abstinence in females than males, it is likely that estradiol is both necessary and accelerates the behavioral and molecular shifts (Ramôa et al., 2013). This idea is also supported by findings in drug-naïve rats showing that females have enhanced glutamatergic input in the NAc compared with males (Forlano and Woolley 2010); as such, they may be “primed” for the recruitment of the glutamate system.
Enhanced NAc AMPA signaling also appears to underlie the development of drug craving/vulnerability to relapse in both males and females. However, in females, these mechanisms may be ovarian hormone-dependent. Specifically, Bechard et al. (2018) showed that daily treatment with ceftriaxone, which offsets cocaine-induced deficits in the cystine-glutamate exchanger and the Na+-dependent glial glutamate transporter (GLT-1), effectively decreases cue-induced reinstatement in both male and female rats. However, in female rats, ceftriaxone was only effective in reducing craving during nonestrus phases possibly because during estrus, the protective effects of ceftriaxone were countered by estradiol-induced increases in synaptic Ca2+-permeable-AMPA receptors (as reflected by an increase in surface expression of GluA1 in the NAc). One caveat is that these effects were observed following short-access cocaine self-administration (2 h/d) and extinction training (2–3 weeks), which may cause different molecular adaptations than those observed following abstinence from extended-access self-administration accompanied by development of an addiction-like phenotype. However, as females are more vulnerable than males to developing addiction-like features following short-access self-administration and we observed similar results using extended-access conditions, these findings support the idea AMPA signaling is enhanced during estrus and with the development of an addiction-like phenotype.
There are also sex differences in glutamatergic signaling within mesocortical regions in drug-naïve controls and following the development of an addiction-like phenotype. In drug-naïve controls, females have less basal glutamatergic excitatory strength in the prelimbic region of the mPFC compared with males, but higher GluN1 subunit expression (which are ubiquitous to the NMDA receptor; Wange and Arnsten, 2015). Additionally, we recently showed that there are marked sex differences in molecular adaptations associated with the incubation of cocaine craving. This study focused on effects in the dmPFC a region known to mediate the incubation of cocaine craving in males. As with previous reports, in males, expression of brain-derived neurotrophic factor exon IV promoter, Bdnf-IV, a marker of epigenetic regulation, and NMDA receptor subunits, Grin2a, Grin2b, and Grin1, changed in response to abstinence and relapse testing; however, in females, only Grin1 expression was impacted. The timeline for the change in Grin1, the gene that encodes the GluN1 subunit of the NMDA receptor, also differed between males and females. In males, as with previous studies, Grin1 expression was increased following relapse testing during protracted abstinence (following 14 days) whereas in females, Grin1 expression was increased following relapse testing during intermediate abstinence (following 7 days). These effects also corresponded to differences in cocaine craving in response to drug-associated cues, which peaked during protracted abstinence in males and during intermediate abstinence in females (i.e., following 7 vs. 14 days; E.B. Towers et al., manuscript in preparation) suggesting that glutamatergic signaling in the dmPFC is recruited earlier during abstinence in females compared with males. Similar sex differences have also been reported for the effects of extended-access methamphetamine self-administration on NMDA signaling in the dmPFC (Mishra et al., 2017; Pena-Bravo et al., 2019). Effects were first characterized in males only and showed that NMDA receptor currents were increased following abstinence (8–14 days) from extended-access self-administration and were associated with an increased GluN2B surface expression (Mishra et al., 2017). The effect was confirmed in females in a more recent study that included both males and females (Pena-Bravo et al., 2019); however, this study used a shorter period of extended-access self-administration, and under these “threshold” conditions, NMDA receptor currents were increased in females but not males, providing further support for the idea that this molecular shift develops more rapidly in females. The authors also showed that the increase in NMDA receptor currents in females was not affected by GluN2B antagonism in the dmPFC indicating that, in contrast to effects with males, this molecular shift is independent of GluN2B NMDA receptors in females (Pena-Bravo et al., 2019). These findings are similar to our observations with cocaine showing that Grin2b, the gene that encodes GluN2B subunit of the NMDA receptor, was changed in males, but not females, in response to abstinence and relapse testing. These findings are intriguing and suggest that some of the molecular changes associated with the development of an addiction-like phenotype are accelerated in females versus males (Grin1/GluN1), while others are qualitatively different between females and males (Grin2b/GluN2B).
There is also evidence indicating that sex differences in the molecular adaptations induced by substance use and SUD impact the efficacy of treatments for SUD. For example, the sex differences we recently observed for relapse-associated changes in NMDA receptor gene expression in the dmPFC likely explain findings of sex difference in the efficacy of exercise as an anti-relapse intervention. Specifically, in males, dmPFC expression levels of Grin2a and Grin2b, the genes encoding the GluN2a and GluN2b subunits of NMDA receptors, were decreased during early abstinence (day 2) after extended-access cocaine self-administration. In contrast, NMDA receptor-related mRNA levels (Grin2a and Grin2b) were not impacted by extended-access cocaine self-administration (vs. saline) or abstinence in females. We have also shown that when exercise is available during early abstinence (days 1–7), it provides long-lasting protection against relapse during protracted abstinence (on abstinence day 15) but only in males (Beiter et al., 2016). In males, the efficacy of exercise appears to be mediated by upregulating NMDA signaling in the dmPFC during early withdrawal thereby preventing a cascade of molecular events that underlie the incubation drug craving (Abel et al., 2019). In contrast, exercise restricted to early abstinence is not effective at reducing craving during protracted abstinence in females possibly because females do not have cocaine-induced deficits in dmPFC NMDA receptor signaling during early withdrawal, and thus, there is not a deficit for exercise to offset. These findings highlight a need for further research on sex differences in both the neuroadaptations underlying addiction and the efficacy of potential interventions for addiction. This information is necessary to guide the development of prevention and treatment efforts for SUD in women and will also help shed light on the mechanisms underlying the telescoping effect.
C. Summary and Integration of Preclinical and Clinical Findings
A telescoping effect in females is supported by clinical and preclinical neurobiological evidence, which indicates that in females, interactions of estradiol with dopamine and glutamate lead to an enhanced sensitivity in females to the reinforcing effects of addictive drugs and the faster course to addiction in females versus males (Fig. 1). Enhanced reinforcing effects are evident in both women (among healthy controls) and female animals. Estradiol enhances mesolimbic dopamine signaling on its own and through interactions with mGlu5, which lead to greater dopamine release in response to addictive drugs in females versus males. This enhanced signaling may lead to a faster shift toward a diminished role for mesolimbic dopamine. This idea is supported by findings in humans showing a blunted dopaminergic response in women versus men in heavy drinkers and smokers and results showing that in women but not men, dopamine depletion biases women toward habitual responding. Preclinical studies similarly show that in females, a shift toward a diminished role of dopamine accompanies the development of an addiction-like phenotype and requires estradiol. An addiction-like phenotype is also accompanied by a shift toward enhanced corticomesolimbic glutamatergic signaling in both males and females. AMPA signaling in the NAc is similarly enhanced in male and female animals, but one key difference is that this shift likely occurs sooner in females than males and underlies the faster course to addiction in females. This idea is supported by findings in both humans and animals indicating that women (healthy controls and recreational drinkers) and female rats (drug naïve) have enhanced glutamatergic input to the striatum and are thus “primed” for the recruitment of the glutamate system.
Finally, it is important to note that sex differences in vulnerability to drug use and addiction likely involved many more brain regions (e.g., amygdala, hippocampus) and neurotransmitter signaling pathways (opioids, norepinephrine, serotonin, GABA, and endocannabinoids). To take an illustrative example, clinical and preclinical studies have shown acute stress potentiates dopamine function in the striatum, similar to acute drug use (Imperato et al., 1989; Wand et al., 2007; Bloomfield et al., 2019). This effect appears to be mediated by glucocorticoid in the mesolimbic dopamine reward pathway since adrenalectomy, which depletes glucocorticoid hormone levels, decreases dopamine release in the NAc following stress and glucocorticoid replacement prevents attenuation of this dopamine response (Piazza and Le Moal, 1996; Barrot et al., 2000). Glucocorticoids have also been shown to potentiate the reinforcing properties of addictive drugs (Piazza et al., 1993; for reviews, see Piazza and Le Moal, 1997; Berry et al., 2016), and this effect is likely magnified in females considering psychostimulants, such as cocaine and methamphetamine, produced an even greater increase in brain glucocorticoid levels in females than in males (Kuhn and Francis, 1997; Zuloaga et al., 2014). Therefore, acute stress may prime the brain reward circuit for subsequent action of addictive drugs or act synergistically with addictive drugs to accelerate sensitization of the reward pathway.
Additionally, in contrast to acute stress, chronic stress and/or exposure to glucocorticoids has been shown to lead to anhedonia and blunting of striatal dopamine function and receptor availability (Gresch et al., 1994; Chrapusta et al., 1997; Mangiavacchi et al., 2001; Meaney et al., 2002; Pacak et al., 2002; Brake et al., 2004; Bloomfield et al., 2019), similar to the neurobiological changes induced by chronic substance use (as discussed in the dopamine section). Women may be biologically more vulnerable to this stress-induced neuroadaptation, considering Oswald et al. (2014) showed that childhood trauma is negatively associated with D2 receptor availability in striatum of women whereas a positive relationship was observed for men. Additionally, women often initiate drug use as a form of self-medication to reduce stress or alleviate anxiety whereas, men are more likely to initiate drug use for their rewarding effects in social settings (for reviews, see Sinha, 2001; Sinha, 2008). Thus, the stress driving initial substance use likely disrupted the reward pathway prior to drug use and enhances vulnerability to transition to SUD. All of these effects could also contribute to the faster progression to addiction observed in females.
V. Conclusions and Future Directions
The data reviewed from human, animal, and neurobiological studies support a telescoping effect in females. The evidence is particularly strong for CoUD considering that it has been consistently observed in both treatment and nontreatment populations (McCance-Katz et al., 1999; Griffin et al., 1989; White et al., 1996; Haas and Peters, 2000; Sofuoglu et al., 1999; O’Brien and Anthony, 2005; but see Lewis et al., 2014); preclinical studies with cocaine also similarly indicate an accelerated course to addiction in females (Lynch and Taylor, 2004; Kerstetter et al., 2012; Perry et al., 2013, 2015; Kawa and Robinson, 2019; Towers et al., 2021). The neurobiological data, which has focused almost exclusively on cocaine and other psychostimulants, also support its biologic basis with findings from both human and animal studies indicating that in females, estradiol “primes” both the dopamine reward pathway and the corticomesolimbic glutamatergic pathway, thereby enhancing risk of addiction. The evidence for a telescoping effect with cannabis is also strong considering that it is observed in both treatment- and nontreatment-seeking populations although its biologic basis has not yet been established in preclinical studies. Preclinical findings with cannabinoids do suggest that females have an enhanced sensitivity to their reinforcing effects although it is not yet clear whether these differences would translate to a faster course to addiction. Future research is also necessary to determine sex differences in the neurobiological effects of cannabis/cannabinoids since these effects are virtually unexplored in women and female animals.
A telescoping effect is also evident with other addictive drugs including alcohol, opioids, methamphetamine, and tobacco, but in these cases, effects may be restricted treatment populations (e.g., vulnerable individuals that develop a severe SUD). This appears to contrast with effects in preclinical studies with these compounds, which indicate an enhanced vulnerability in females for both use and the development of addiction-like features (excessive drug use and a loss of control over drug use under extended-access drug self-administration conditions). Neurobiological differences between males and females would also be presumed to impact psychostimulants and many of these drugs similarly; however, much less is known about sex differences in the neurobiological effects of alcohol, opioids, nicotine, and methamphetamine. Additionally, to date, no studies have examined sex differences in the time course for the development of addiction-like phenotype with alcohol, opioids, methamphetamine, or tobacco. Such studies are necessary since they will determine whether females are biologically biased to have an accelerated course to addiction with these drugs. Future epidemiologic studies are also needed to determine gender differences in trajectories to addiction using models that control for known differences between men and women with regard to probabilities of drug use, SUD, and seeking treatment of SUD.
Future studies are necessary to identify intervention strategies for women to prevent the development of a SUD. In addition to the obvious need for additional research on hormone-based strategies, medications that target mGlu5 may have therapeutic potential in women considering that mGlu5 likely enhances both initial vulnerability to drug use and the development of addiction in females. mGlu5 is being considered as a therapeutic target for several disorders (addiction, bulimia nervosa, schizophrenia), and compounds are available for use in both humans and animals (e.g., Mihov et al., 2020). mGlu5 was recently shown to be dysregulated in the striatum of individuals, mainly men (13 of 16), with SUD; normalization of these receptors over a period of protracted abstinence was also associated with decreased craving (Ceccarini et al., 2019). Preclinical studies have also noted sex differences in the effects of Glu5 manipulations on drug-related behaviors, including findings showing that Glu5 antagonism is more effective at decreasing binge alcohol drinking in females than males (Cozzoli et al., 2014). A better understanding of sex differences in the time course for the disease progression and the underlying mechanisms is critical for the development of sex-specific personalized medicine approaches for the prevention and treatment of SUDs.
Acknowledgments
The authors would like to thank Katriel E. Cho for creating the graphical abstract.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AUD
alcohol use disorder
- CaUD
cannabis use disorder
- CoUD
cocaine use disorder
- Bdnf-IV
brain-derived neurotrophic factor exon IV promoter
- CB1 receptor
cannabinoid receptor type 1
- D1 receptor
dopamine receptor D1 and D5
- D2 receptor
dopamine receptor D2, D3, D4
- DA
dopamine
- dmPFC
dorsal medial prefrontal cortex
- GLT-1
Na+-dependent glial glutamate transporter
- Grin1
glutamate ionotropic receptor NMDA type subunit 1
- Grin2a
glutamate ionotropic receptor NMDA type subunit 2a
- Grin2b
glutamate ionotropic receptor NMDA type subunit 2b
- LH
luteinizing hormone
- FCG
four core genotype
- FSH
follicle-stimulating hormone
- mGlu
metabotropic glutamate receptor
- MPEP
2-methyl-6-(phenylethynyl)pyridine)
- mPFC
medial prefrontal cortex
- NAc
nucleus accumbens
- NMDA
N-methyl-D-aspartate
- OUD
opioid use disorder
- OVX
ovariectomized
- PFC
prefrontal cortex
- SUD
substance use disorder
- TUD
tobacco use disorder
- VTA
ventral tegmental area
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Towers, Williams, Qillawala, Rissman, Lynch.
Footnotes
This work was supported by National Institutes of Health National Institute on Drug Abuse [Grants R01-DA024716, R01-DA052893, and R21-DA049992] (to W.J.L.), [Grant R01-DA048638] (to W.J.L. and E.F.R.), a National Institute of General Medical Sciences Pharmacological Sciences Training Grant [Grant 5T32-GM007055-47] (to E.B.T.), and an MSTP Training Grant [Grant T32-GM007267] (to E.B.T.).
No author has an actual or perceived conflict of interest with the contents of this article.
References
- Abel JM, Nesil T, Bakhti-Suroosh A, Grant PA, Lynch WJ (2019) Mechanisms underlying the efficacy of exercise as an intervention for cocaine relapse: a focus on mGlu5 in the dorsal medial prefrontal cortex. Psychopharmacology (Berl) 236:2155–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelson M, Linzy S, Peles E (2018) Characteristics and outcome of male and female methadone maintenance patients: MMT in Tel Aviv and Las Vegas. Subst Use Misuse 53:230–238. [DOI] [PubMed] [Google Scholar]
- Agabio R, Campesi I, Pisanu C, Gessa GL, Franconi F (2016) Sex differences in substance use disorders: focus on side effects. Addict Biol 21:1030–1042. [DOI] [PubMed] [Google Scholar]
- Aganoff JA, Boyle GJ (1994) Aerobic exercise, mood states and menstrual cycle symptoms. J Psychosom Res 38:183–192. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300. [DOI] [PubMed] [Google Scholar]
- Albrecht DS, Kareken DA, Yoder KK (2013a) Effects of smoking on D2/D3 striatal receptor availability in alcoholics and social drinkers. Brain imaging and behavior 7:326-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht DS, Skosnik PD, Vollmer JM, Brumbaugh MS, Perry KM, Mock BH, Zheng QH, Federici LA, Patton EA, Herring CM, Yoder KK (2013b) Striatal D(2)/D(3) receptor availability is inversely correlated with cannabis consumption in chronic marijuana users. Drug Alcohol Depend 128:52-7 DOI: 10.1016/j.drugalcdep.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain F, Minogianis EA, Roberts DC, Samaha AN (2015) How fast and how often: The pharmacokinetics of drug use are decisive in addiction. Neurosci Biobehav Rev 56:166–179. [DOI] [PubMed] [Google Scholar]
- Alvanzo AAH, Storr CL, La Flair L, Green KM, Wagner FA, Crum RM (2011) Race/ethnicity and sex differences in progression from drinking initiation to the development of alcohol dependence. Drug Alcohol Depend 118:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, Ed. 5th. American Psychiatric Association, Washington, DC. [Google Scholar]
- Andersen SL, Teicher MH (2000) Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev 24:137–141. [DOI] [PubMed] [Google Scholar]
- Anglin MD, Hser YI, McGlothlin WH (1987) Sex differences in addict careers. 2. Becoming addicted. Am J Drug Alcohol Abuse 13:59–71. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Larson EB, Gliddon LA, Carroll ME (2007) Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol 15:472–480. [DOI] [PubMed] [Google Scholar]
- Antony T, Alzaharani SY, El-Ghaiesh SH (2020) Opioid-induced hypogonadism: Pathophysiology, clinical and therapeutics review. Clin Exp Pharmacol Physiol 47:741–750. [DOI] [PubMed] [Google Scholar]
- Arfken CL, Klein C, di Menza S, Schuster CR (2001) Gender differences in problem severity at assessment and treatment retention. J Subst Abuse Treat 20:53–57. [DOI] [PubMed] [Google Scholar]
- Arnold AP (2020) Four Core Genotypes and XY* mouse models: update on impact on SABV research. Neurosci Biobehav Rev 119:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley MJ, Olin JS, le Riche WH, Kornaczewski A, Schmidt W, Rankin JG (1977) Morbidity in alcoholics: evidence for accelerated development of physical disease in women. Arch Intern Med 137:883–887. [DOI] [PubMed] [Google Scholar]
- Bach AClausen BHKristensen LKAndersen MGEllman DGHansen PBLHasseldam HHeitz MÖzcelik DTuck EJ, et al. (2019) Selectivity, efficacy and toxicity studies of UCCB01-144, a dimeric neuroprotective PSD-95 inhibitor. Neuropharmacology 150:100–111. [DOI] [PubMed] [Google Scholar]
- Back SE, Lawson KM, Singleton LM, Brady KT (2011) Characteristics and correlates of men and women with prescription opioid dependence. Addict Behav 36:829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backes E, Hemby SE (2003) Discrete cell gene profiling of ventral tegmental dopamine neurons after acute and chronic cocaine self-administration. J Pharmacol Exp Ther 307:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhti-Suroosh A, Nesil T, Lynch WJ (2019) Tamoxifen Blocks the Development of Motivational Features of an Addiction-Like Phenotype in Female Rats. Front Behav Neurosci 13:253 DOI: 10.3389/fnbeh.2019.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhti-Suroosh A, Towers EB, Lynch WJ (2021) A buprenorphine-validated rat model of opioid use disorder optimized to study sex differences in vulnerability to relapse. Psychopharmacology (Berl) 238:1029–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaton BP, Dixon-McDougall T, Peeters SB, Brown CJ (2018) The eXceptional nature of the X chromosome. Hum Mol Genet 27 (R2):R242–R249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balster RL, Woolverton WL (1982) Intravenous buspirone self-administration in rhesus monkeys. J Clin Psychiatry 43:34–39. [PubMed] [Google Scholar]
- Barker JM, Torregrossa MM, Arnold AP, Taylor JR (2010) Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. J Neurosci 30:9140–9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Marinelli M, Abrous DN, Rougé-Pont F, Le Moal M, Piazza PV (2000) The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci 12:973–979. [DOI] [PubMed] [Google Scholar]
- Barry SM, McGinty JF (2017) Role of Src family kinases in BDNF-mediated suppression of cocaine-seeking and prevention of cocaine-induced ERK, GluN2A, and GluN2B dephosphorylation in the prelimbic cortex. Neuropsychopharmacology 42:1972–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Pedersen A, Scherbaum N, Bening J, Patschke J, Kugel H, Heindel W, Arolt V, Ohrmann P (2013) Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology 38:1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechard AR, Hamor PU, Schwendt M, Knackstedt LA (2018) The effects of ceftriaxone on cue-primed reinstatement of cocaine-seeking in male and female rats: estrous cycle effects on behavior and protein expression in the nucleus accumbens. Psychopharmacology (Berl) 235:837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Anton RF, De Trana C, Randall CL (1985) Sensitivity to ethanol in female mice: effects of ovariectomy and strain. Life Sci 37:1293–1300. [DOI] [PubMed] [Google Scholar]
- Becker JB (1990) Direct effect of 17 β-estradiol on striatum: sex differences in dopamine release. Synapse 5:157–164. [DOI] [PubMed] [Google Scholar]
- Becker JB, Beer ME, Robinson TE (1984) Striatal dopamine release stimulated by amphetamine or potassium: influence of ovarian hormones and the light-dark cycle. Brain Res 311:157-60 DOI: 10.1016/0006-8993(84)91410-0. [DOI] [PubMed] [Google Scholar]
- Becker JB, Cha JH (1989) Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res 35:117–125. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M (2008) Sex differences in drug abuse. Front Neuroendocrinol 29:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF (2016) Sex differences in animal models: focus on addiction. Pharmacol Rev 68:242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Ramirez VD (1981) Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res 204:361–372. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN (1999) Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav 64:53–57. [DOI] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H (2011) Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry 69:708–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiter RM, Peterson AB, Abel J, Lynch WJ (2016) Exercise during early, but not late abstinence, attenuates subsequent relapse vulnerability in a rat model. Transl Psychiatry 6:e792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Deroche-Gamonet V (2012) Responses to novelty and vulnerability to cocaine addiction: contribution of a multi-symptomatic animal model. Cold Spring Harb Perspect Med 2:a011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, Szumlinski KK (2009) Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse 63:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A, Raggi C, Borgi M, Cirulli F (2016) Sex-driven vulnerability in stress and drug abuse. Ann Ist Super Sanita 52:167–175. [DOI] [PubMed] [Google Scholar]
- Bloomfield MAP, McCutcheon RA, Kempton M, Freeman TP, Howes O (2019) The effects of psychosocial stress on dopaminergic function and the acute stress response. eLife 8:e46797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y (2006) Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology 31:2197–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A (2004) Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci 19:1863–1874. [DOI] [PubMed] [Google Scholar]
- Brecht ML, O’Brien A, von Mayrhauser C, Anglin MD (2004) Methamphetamine use behaviors and gender differences. Addict Behav 29:89–106. [DOI] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE,, London ED, Farahi J, Meyer JH, Grossman P, Lee GS, Huang J,, Hahn EL, Mandelkern MA (2004) Smoking-induced ventral striatum dopamine release. American Journal of Psychiatry 161:1211–8. [DOI] [PubMed] [Google Scholar]
- Brown AK, Mandelkern MA, Farahi J, Robertson C, Ghahremani DG, Sumerel B, Moallem N, London ED (2012) Sex differences in striatal dopamine D2/D3 receptor availability in smokers and non-smokers. Int J Neuropsychopharmacol 15:989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busto UE, Redden L, Mayberg H, Kapur S, Houle S, Zawertailo LA (2009) Dopaminergic activity in depressed smokers: a positron emission tomography study. Synapse 63:681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffino L, Moro F, Mottarlini F, Targa G, Di Clemente A, Toia M, Orrù A, Giannotti G, Fumagalli F, Cervo L (2021) Repeated exposure to cocaine during adolescence enhances the rewarding threshold for cocaine-conditioned place preference in adulthood. Addict Biol 26:e13012. [DOI] [PubMed] [Google Scholar]
- Caffino L, Verheij MMM, Roversi K, Targa G, Mottarlini F, Popik P, Nikiforuk A, Golebiowska J, Fumagalli F, Homberg JR (2020) Hypersensitivity to amphetamine’s psychomotor and reinforcing effects in serotonin transporter knockout rats: Glutamate in the nucleus accumbens. Br J Pharmacol 177:4532–4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailhol S, Mormède P (2002) Conditioned taste aversion and alcohol drinking: strain and gender differences. J Stud Alcohol 63:91–99. [PubMed] [Google Scholar]
- Calakos KC, Hillmer AT, Angarita GA, Baldassarri SR, Najafzadeh S, Emery PR, Matuskey D, Huang Y, Cosgrove KP (2022) Recently abstinent smokers exhibit mood-associated dopamine dysfunction in the ventral striatum compared to nonsmokers: a [11C]-(+)-PHNO PET Study. Nicotine Tob Res 24:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ESJuarez BMorel CWalker DMCahill MERibeiro ERoman-Ortiz CRamakrishnan CDeisseroth KHan MH, et al. (2017) Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun 8:13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Batulis DK, Landry KL, Morgan AD (2005) Sex differences in the escalation of oral phencyclidine (PCP) self-administration under FR and PR schedules in rhesus monkeys. Psychopharmacology (Berl) 180:414–426. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP (2004) Sex and estrogen influence drug abuse. Trends Pharmacol Sci 25:273–279. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK (2002) Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 161:304–313. [DOI] [PubMed] [Google Scholar]
- Casey KF, Benkelfat C, Cherkasova MV, Baker GB, Dagher A, Leyton M (2014) Reduced dopamine response to amphetamine in subjects at ultra-high risk for addiction. Biol Psychiatry 76:23–30. [DOI] [PubMed] [Google Scholar]
- Castner SA, Xiao L, Becker JB (1993)Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res 610:127–134. [DOI] [PubMed] [Google Scholar]
- Ceccarini J, Leurquin-Sterk G, Crunelle CL, de Laat B, Bormans G, Peuskens H, Van Laere K (2019) Recovery of decreased metabotropic glutamate receptor 5 availability in abstinent alcohol-dependent patients. J Nucl Med 61:256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality (2015) Treatment episode data set (TEDS): 2004–2014. State admissions to substance abuse treatment services, SAMHSA, Rockville, MD. [Google Scholar]
- Chang G (2020) Maternal substance use: consequences, identification, and interventions. Alcohol Res 40:06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Yang YK, Yeh TL, Cherng CFG, Hsu HC, Hsiao SY, Yu L (2003) Methamphetamine-induced conditioned place preference is facilitated by estradiol pretreatment in female mice. Chin J Physiol 46:169–174. [PubMed] [Google Scholar]
- Chen YW, Barson JR, Chen A, Hoebel BG, Leibowitz SF (2013) Glutamatergic input to the lateral hypothalamus stimulates ethanol intake: role of orexin and melanin-concentrating hormone. Alcohol Clin Exp Res 37:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrapusta SJ, Wyatt RJ, Masserano JM (1997) Effects of single and repeated footshock on dopamine release and metabolism in the brains of Fischer rats. J Neurochem 68:2024–2031. [DOI] [PubMed] [Google Scholar]
- Cisternas CD, Garcia-Segura LM, Cambiasso MJ (2018) Hormonal and genetic factors interact to control aromatase expression in the developing brain. J Neuroendocrinol 30:12535. [DOI] [PubMed] [Google Scholar]
- Collins A, Eneroth P, Landgren BM (1985) Psychoneuroendocrine stress responses and mood as related to the menstrual cycle. Psychosom Med 47:512–527. [DOI] [PubMed] [Google Scholar]
- Committee on Understanding the Biology of Sex and Gender Differences. Exploring the biological contributions to human health: does sex matter? (Wizemann TM, Pardue ML, eds.), National Academies Press, Washington, DC. https://www.ncbi.nlm.nih.gov/books/NBK222293/ [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME (2008) Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DB, Patel P, Mahdy H (2022) Oral contraceptive pills, StatPearls, Treasure Island, FL. [PubMed] [Google Scholar]
- Cooper ZD, Foltin RW, Evans SM (2013) Effects of menstrual cycle phase on cocaine self-administration in rhesus macaques. Horm Behav 63:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett CM, Dunn E, Loweth JA (2021) Effects of sex and estrous cycle on the time course of incubation of cue-induced craving following extended-access cocaine self-administration. eNeuro 8:ENEURO.0054-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Wang S, Kim SJ, McGovern E, Nabulsi N, Gao H, Labaree D, Tagare HD, Sullivan JM, Morris ED (2014) Sex differences in the brain’s dopamine signature of cigarette smoking. J Neurosci 34:16851–16855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotto JH, Davis E, Dowling GJ, Elcano JC, Staton AB, Weiss SRB (2010) Gender effects on drug use, abuse, and dependence: a special analysis of results from the National Survey on Drug Use and Health. Gend Med 7:402–413. [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Strong-Kaufman MN, Tanchuck MA, Hashimoto JG, Wiren KM, Finn DA (2014) The effect of mGluR5 antagonism during binge drinking on subsequent ethanol intake in C57BL/6J mice: sex- and age-induced differences. Alcohol Clin Exp Res 38:730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Jagannathan L, Jackson LR, Becker JB (2014) Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: neurochemistry and behavior. Drug Alcohol Depend 135:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Riddick NV, Gage HD, Sandridge M, Nader SH, Garg S, Bounds M, Garg PK, Nader MA (2009) Effect of menstrual cycle phase on dopamine D2 receptor availability in female cynomolgus monkeys. Neuropsychopharmacology 34:548–554. [DOI] [PubMed] [Google Scholar]
- Daniels K, Abma JC (2020) Current contraceptive status among women aged 15-49: United States, 2017–2019. NCHS Data Brief 388:1–8. [PubMed] [Google Scholar]
- Daniels K, Mosher WD (2013)Contraceptive methods women have ever used: United States, 1982–2010. Natl Health Stat Rep 62:1–15. [PubMed] [Google Scholar]
- de la Fuente L, Molist G, Espelt A, Barrio G, Guitart A, Bravo MJ, Brugal MT; Spanish Working Group for the Study of Mortality among Drug Users (2014) Mortality risk factors and excess mortality in a cohort of cocaine users admitted to drug treatment in Spain. J Subst Abuse Treat 46:219–226. [DOI] [PubMed] [Google Scholar]
- Des Jarlais DC, Feelemyer JP, Modi SN, Arasteh K, Hagan H (2012)Are females who inject drugs at higher risk for HIV infection than males who inject drugs: an international systematic review of high seroprevalence areas. Drug Alcohol Depend 124:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Babuscio TA, Nich C, Ball SA, Carroll KM (2014) Gender differences in clinical outcomes for cocaine dependence: randomized clinical trials of behavioral therapy and disulfiram. Drug Alcohol Depend 145:156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP (2002) A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci 22:9005–9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit S, Standing HR, Devito EE, Robinson OJ, Ridderinkhof KR, Robbins TW, Sahakian BJ (2012) Reliance on habits at the expense of goal-directed control following dopamine precursor depletion. Psychopharmacology (Berl) 219:621-31 DOI: 10.1007/s00213-011-2563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickmann PJ, Mooney ME, Allen SS, Hanson K, Hatsukami DK (2009) Nicotine withdrawal and craving in adolescents: effects of sex and hormonal contraceptive use. Addict Behav 34:620–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl A, Croissant B, Batra A, Mundle G, Nakovics H, Mann K (2007) Alcoholism in women: is it different in onset and outcome compared to men? Eur Arch Psychiatry Clin Neurosci 257:344–351. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Rigotti NA, Fletcher K, Ockene JK, McNeill AD, Coleman M, Wood C (2002) Development of symptoms of tobacco dependence in youths: 30 month follow up data from the DANDY study. Tob Control 11:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JRSavageau JAFletcher KO’Loughlin JPbert LOckene JKMcNeill ADHazelton JFriedman KDussault G, et al. (2007) Symptoms of tobacco dependence after brief intermittent use: the Development and Assessment of Nicotine Dependence in Youth-2 study. Arch Pediatr Adolesc Med 161:704–710. [DOI] [PubMed] [Google Scholar]
- Disteche CM, Berletch JB (2015) X-chromosome inactivation and escape. J Genet 94:591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, Ramirez VD (1984) Bimodal effect of progesterone on in vitro dopamine function of the rat corpus striatum. Neuroendocrinology 39:149–155. [DOI] [PubMed] [Google Scholar]
- Dobson H, Smith RF (1998) Stress and subfertility. Reprod Domest Anim 33:107–111. [Google Scholar]
- Doherty JM, Cooke BM, Frantz KJ (2013) A role for the prefrontal cortex in heroin-seeking after forced abstinence by adult male rats but not adolescents. Neuropsychopharmacology 38:446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S (2000) Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 151:392–405. [DOI] [PubMed] [Google Scholar]
- Doyle SE, Ramôa C, Garber G, Newman J, Toor Z, Lynch WJ (2014) A shift in the role of glutamatergic signaling in the nucleus accumbens core with the development of an addicted phenotype. Biol Psychiatry 76:810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutertre M, Smith CL (2000) Molecular mechanisms of selective estrogen receptor modulator (SERM) action. J Pharmacol Exp Ther 295:431–437. [PubMed] [Google Scholar]
- Ehlers CL, Gizer IR, Vieten C, Gilder DA, Stouffer GM, Lau P, Wilhelmsen KC (2010) Cannabis dependence in the San Francisco Family Study: age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addict Behav 35:102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Rosser AA, Zhou Z, Mash DC, Yuan Q, Goldman D (2014) Expression of glutamatergic genes in healthy humans across 16 brain regions; altered expression in the hippocampus after chronic exposure to alcohol or cocaine. Genes Brain Behav 13:758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier AR, McKinney TL, Tottenham LS, Gordon JL (2021) The effect of reproductive hormones on women’s daily smoking across the menstrual cycle. Biol Sex Differ 12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Foltin RW (2006) Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology 31:659–674. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW (2002) The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 159:397–406. [DOI] [PubMed] [Google Scholar]
- Evans SM, Levin FR (2011) Response to alcohol in women: role of the menstrual cycle and a family history of alcoholism. Drug Alcohol Depend 114:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW (2008) Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci 363:3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Fadda P, Fratta W (2009) Sex differences in the self-administration of cannabinoids and other drugs of abuse. Psychoneuroendocrinology 34 (Suppl 1):S227–S236. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Altea S, Fadda P, Fratta W (2010) Drug- and cue-induced reinstatement of cannabinoid-seeking behaviour in male and female rats: influence of ovarian hormones. Br J Pharmacol 160:724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, Eberhardt A, Klager M, Smolka MN, Scheurich A, Dielentheis T, Schmidt LG, Rosch F (2008) Association of low striatal dopamine D2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry 165:507–514. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Byrd EA, Henderson AR, See RE (2009) Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology 34:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE (2007) Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend 89:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Montalvo J, Lopez Goni JJ, Azanza P, Cacho R (2014) Gender differences in drug-addicted patients in treatment. Am J Addict 23:399–406. [DOI] [PubMed] [Google Scholar]
- Fernández-Solà J, Estruch R, Nicolás JM, Paré JC, Sacanella E, Antúnez E, Urbano-Márquez A (1997) Comparison of alcoholic cardiomyopathy in women versus men. Am J Cardiol 80:481–485. [DOI] [PubMed] [Google Scholar]
- Festa ED, Jenab S, Weiner J, Nazarian A, Niyomchai T, Russo SJ, Kemen LM, Akhavan A, Wu HBK, Quinones-Jenab V (2006) Cocaine-induced sex differences in D1 receptor activation and binding levels after acute cocaine administration. Brain Res Bull 68:277–284. [DOI] [PubMed] [Google Scholar]
- Fiad TM, Cunningham SK, McKenna TJ (1996) Role of progesterone deficiency in the development of luteinizing hormone and androgen abnormalities in polycystic ovary syndrome. Eur J Endocrinol 135:335–339. [DOI] [PubMed] [Google Scholar]
- Fischer KD, Houston ACW, Rebec GV (2013) Role of the major glutamate transporter GLT1 in nucleus accumbens core versus shell in cue-induced cocaine-seeking behavior. J Neurosci 33:9319–9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch TE, Roberts DC (1993) The effects of dose and access restrictions on the periodicity of cocaine self-administration in the rat. Drug Alcohol Depend 33:119–128. [DOI] [PubMed] [Google Scholar]
- Flores RJ, Pipkin JA, Uribe KP, Perez A, O’Dell LE (2016) Estradiol promotes the rewarding effects of nicotine in female rats. Behav Brain Res 307:258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel JS, Kelly TH, Westgate PM, Lile JA (2017) Sex differences in the subjective effects of oral Δ9-THC in cannabis users. Pharmacol Biochem Behav 152:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH (2002) Ethanol consumption in the female Long-Evans rat: a modulatory role of estradiol. Alcohol 26:103–113. [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH (2004) Determination of an estradiol dose-response relationship in the modulation of ethanol intake. Alcohol Clin Exp Res 28:20–28. [DOI] [PubMed] [Google Scholar]
- Forger NG, Morin LP (1982) Reproductive state modulates ethanol intake in rats: effects of ovariectomy, ethanol concentration, estrous cycle and pregnancy. Pharmacol Biochem Behav 17:323–331. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Woolley CS (2010) Quantitative analysis of pre- and postsynaptic sex differences in the nucleus accumbens. J Comp Neurol 518:1330–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Hong KA, Paliwal P, Morgan PT, Sinha R (2008) Altered levels of sex and stress steroid hormones assessed daily over a 28-day cycle in early abstinent cocaine-dependent females. Psychopharmacology (Berl) 195:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sofuoglu M, Morgan PT, Tuit KL, Sinha R (2013) The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type. Psychoneuroendocrinology 38:1532–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME (2006) Administration of estrogen to ovariectomized rats promotes conditioned place preference and produces moderate levels of estrogen in the nucleus accumbens. Brain Res 1067:209–215. [DOI] [PubMed] [Google Scholar]
- Funk D, Coen K, Tamadon S, Hope BT, Shaham Y, Lê AD (2016) Role of central amygdala neuronal ensembles in incubation of nicotine craving. J Neurosci 36:8612–8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo MA, Smith SS (1993) Progesterone withdrawal decreases latency to and increases duration of electrified prod burial: a possible rat model of PMS anxiety. Pharmacol Biochem Behav 46:897–904. [DOI] [PubMed] [Google Scholar]
- Gancarz-Kausch AM, Adank DN, Dietz DM (2014) Prolonged withdrawal following cocaine self-administration increases resistance to punishment in a cocaine binge. Sci Rep 4:6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF (2006) Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci 26:2335–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George BE, Barth SH, Kuiper LB, Holleran KM, Lacy RT, Raab-Graham KF, Jones SR (2021) Enhanced heroin self-administration and distinct dopamine adaptations in female rats. Neuropsychopharmacology 46:1724–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser JH, Rubin BS, Barfield RJ (1983) Onset of the receptive and proceptive components of feminine sexual behavior in rats following the intravenous administration of progesterone. Horm Behav 17:18–27. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goletiani NV, Siegel AJ, Lukas SE, Hudson JI (2015) The effects of smoked nicotine on measures of subjective states and hypothalamic-pituitary-adrenal axis hormones in women during the follicular and luteal phases of the menstrual cycle. J Addict Med 9:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Mooney ME (2012) Telescoping phenomenon in pathological gambling: association with gender and comorbidities. J Nerv Ment Dis 200:996–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT (2010) Substance abuse in women. Psychiatr Clin North Am 33:339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, Lincoln M, Hien D, Miele GM (2007) Substance abuse treatment entry, retention, and outcome in women: a review of the literature. Drug Alcohol Depend 86:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM (1994) Stress-induced sensitization of dopamine and norepinephrine efflux in medial prefrontal cortex of the rat. J Neurochem 63:575–583. [DOI] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U (1989) A comparison of male and female cocaine abusers. Arch Gen Psychiatry 46:122–126. [DOI] [PubMed] [Google Scholar]
- Grüsser SMWrase JKlein SHermann DSmolka MNRuf MWeber-Fahr WFlor HMann KBraus DF, et al. (2004) Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 175:296–302. [DOI] [PubMed] [Google Scholar]
- Haas AL, Peters RH (2000) Development of substance abuse problems among drug-involved offenders. Evidence for the telescoping effect. J Subst Abuse 12:241–253. [DOI] [PubMed] [Google Scholar]
- Hafenbreidel M, Rafa Todd C, Mueller D (2017) Infralimbic GluN2A-containing NMDA receptors modulate reconsolidation of cocaine self-administration memory. Neuropsychopharmacology 42:1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MS, Licaj I, Braaten T, Langhammer A, Le Marchand L, Gram IT (2018) Sex differences in risk of smoking-associated lung cancer: results from a cohort of 600,000 Norwegians. Am J Epidemiol 187:971–981. [DOI] [PubMed] [Google Scholar]
- Hanzal N, Joyce KM, Tibbo PG, Stewart SH (2019) A pilot daily diary study of changes in stress and cannabis use quantity across the menstrual cycle. Cannabis 2:120–134. [Google Scholar]
- Harrison PA, Sidebottom AC (2009) Alcohol and drug use before and during pregnancy: an examination of use patterns and predictors of cessation. Maternal and child health journal 13:386–94. [DOI] [PubMed] [Google Scholar]
- Hearing M, Graziane N, Dong Y, Thomas MJ (2018) Opioid and psychostimulant plasticity: targeting overlap in nucleus accumbens glutamate signaling. Trends Pharmacol Sci 39:276–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht GS, Spear NE, Spear LP (1999) Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Dev Psychobiol 35:136–145. [PubMed] [Google Scholar]
- Hemby SE, Tang W, Muly EC, Kuhar MJ, Howell L, Mash DC (2005) Cocaine-induced alterations in nucleus accumbens ionotropic glutamate receptor subunits in human and non-human primates. J Neurochem 95:1785–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR (2004) Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend 74:265–272. [DOI] [PubMed] [Google Scholar]
- Herzog AG (1995) Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology 45:1660–1662. [DOI] [PubMed] [Google Scholar]
- Hesselbrock MN, Meyer RE, Keener JJ (1985) Psychopathology in hospitalized alcoholics. Arch Gen Psychiatry 42:1050–1055. [DOI] [PubMed] [Google Scholar]
- Hilderbrand ER, Lasek AW (2018) Estradiol enhances ethanol reward in female mice through activation of ERα and ERβ. Horm Behav 98:159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinderaker K, Allen AM, Tosun N, al’Absi M, Hatsukami D, Allen SS (2015) The effect of combination oral contraceptives on smoking-related symptomatology during short-term smoking abstinence. Addict Behav 41:148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Kaiser E, Rawlings R (2001) Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry 158:198–204. [DOI] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Rawlings R, Ragan P, Williams W, Rio D, Eckardt M (1996) Decreased corpus callosum size among alcoholic women. Arch Neurol 53:359–363. [DOI] [PubMed] [Google Scholar]
- Hser YI, Anglin MD, Booth MW (1987) Sex differences in addict careers. 3. Addiction. Am J Drug Alcohol Abuse 13:231–251. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB (2003) Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci 23:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Becker JB (2008) Acquisition of cocaine self-administration in ovariectomized female rats: effect of estradiol dose or chronic estradiol administration. Drug Alcohol Depend 94:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggett SB, Hatoum AS, Hewitt JK, Stallings MC (2018) The speed of progression to tobacco and alcohol dependence: A twin study. Behavior genetics 48:109–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues JN, Coste T, Perret G, Jayle MF, Sebaoun J, Modigliani E (1980) Hypothalamo-pituitary ovarian function in thirty-one women with chronic alcoholism. Clin Endocrinol (Oxf) 12:543–551. [DOI] [PubMed] [Google Scholar]
- Ibáñez A, Blanco C, Moreryra P, Sáiz-Ruiz J (2003) Gender differences in pathological gambling. J Clin Psychiatry 64:295–301. [DOI] [PubMed] [Google Scholar]
- Imperato A, Puglisi-Allegra S, Casolini P, Zocchi A, Angelucci L (1989) Stress-induced enhancement of dopamine and acetylcholine release in limbic structures: role of corticosterone. Eur J Pharmacol 165:337–338. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Mackie R, Kampf K, Domadia S, Brown JD, O’Neill R, Arnold AP (2015) Four core genotypes mouse model: localization of the Sry transgene and bioassay for testicular hormone levels. BMC Res Notes 8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen J, Wand H, Gonnermann A, Maher L; Collaboration of Australian Needle and Syringe Programs (2010) Gender differences in hepatitis C antibody prevalence and risk behaviours amongst people who inject drugs in Australia 1998-2008. Int J Drug Policy 21:471–476. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB (2006) Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology 31:129–138. [DOI] [PubMed] [Google Scholar]
- Jandíková H, Dušková M, Stárka L (2017) The influence of smoking and cessation on the human reproductive hormonal balance. Physiol Res 66 (Suppl 3):S323–S331. [DOI] [PubMed] [Google Scholar]
- Johnson AR, Thibeault KC, Lopez AJ, Peck EG, Sands LP, Sanders CM, Kutlu MG, Calipari ES (2019) Cues play a critical role in estrous cycle-dependent enhancement of cocaine reinforcement. Neuropsychopharmacology 44:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PB, Richter L, Kleber HD, McLellan AT, Carise D (2005) Telescoping of drinking-related behaviors: gender, racial/ethnic, and age comparisons. Subst Use Misuse 40:1139–1151. [DOI] [PubMed] [Google Scholar]
- Joseph JE, McRae-Clark A, Sherman BJ, Baker NL, Moran-Santa Maria M, Brady KT (2019) Neural correlates of oxytocin and cue reactivity in cocaine-dependent men and women with and without childhood trauma. Psychopharmacology (Berl) DOI: 10.1007/s00213-019-05360-7 [published ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce KM, Good KP, Tibbo P, Brown J, Stewart SH (2021) Addictive behaviors across the menstrual cycle: a systematic review. Arch Women Ment Health 24:529–542. [DOI] [PubMed] [Google Scholar]
- Joyce KM, Hudson A, O’Connor R, Thompson K, Hodgin M, Perrot T, Stewart SH (2018) Changes in coping and social motives for drinking and alcohol consumption across the menstrual cycle. Depress Anxiety 35:313–320. [DOI] [PubMed] [Google Scholar]
- Justice AJH, De Wit H (2000) Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacol Biochem Behav 66:509–515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K (2003) Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 168:44–56. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND (2005)The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162:1403–1413. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND (2011) New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry 16:974–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, O’Brien CP (2004) A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend 75:233–240. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB (2004) Ovarian dysfunction, stress, and disease: a primate continuum. ILAR J 45:89–115. [DOI] [PubMed] [Google Scholar]
- Kawa AB, Robinson TE (2019) Sex differences in incentive-sensitization produced by intermittent access cocaine self-administration. Psychopharmacology (Berl) 236:625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Svikis DS, Sundquist K, Sundquist J (2017) The protective effect of pregnancy on risk for drug abuse: a population, co-relative, co-spouse, and within-individual analysis. Am J Psychiatry 174:954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE (2008) Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology (Berl) 198:63–75. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE (2012) Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology 37:2605–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Martins SS, Blanco C, Hasin DS (2010) Telescoping and gender differences in alcohol dependence: new evidence from two national surveys. Am J Psychiatry 167:969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SS, Secades-Villa R, Okuda M, Wang S, Pérez-Fuentes G, Kerridge BT, Blanco C (2013) Gender differences in cannabis use disorders: results from the National Epidemiologic Survey of Alcohol and Related Conditions. Drug Alcohol Depend 130:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesner J (2012) Affective response to the menstrual cycle as a predictor of self-reported affective response to alcohol and alcohol use. Arch Women Ment Health 15:423–432. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Kennedy A, Elton AL, Tripathi SP, Young J, Cisler JM, James GA (2014) Individual differences in attentional bias associated with cocaine dependence are related to varying engagement of neural processing networks. Neuropsychopharmacology 39:1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara C, Ohno Y (2010) Sex differences in lung cancer susceptibility: a review. Gend Med 7:381–401. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW (2009) Glutamate and reinstatement. Curr Opin Pharmacol 9:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokane SS, Perrotti LI (2020) Sex differences and the role of estradiol in mesolimbic reward circuits and vulnerability to cocaine and opiate addiction. Front Behav Neurosci 14:74 10.3389/fnbeh.2020.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2021) Drug addiction: hyperkatifeia/negative reinforcement as a framework for medications development. Pharmacol Rev 73:163–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucerova J, Vrskova D, Sulcova A (2009) Impact of repeated methamphetamine pretreatment on intravenous self-administration of the drug in males and estrogenized or non- estrogenized ovariectomized female rats. Neuroendocrinol Lett 30:663–670. [PubMed] [Google Scholar]
- Kuhn C, Francis R (1997) Gender difference in cocaine-induced HPA axis activation. Neuropsychopharmacology 16:399–407. [DOI] [PubMed] [Google Scholar]
- Kuntz KL, Patel KM, Grigson PS, Freeman WM, Vrana KE (2008) Heroin self-administration: II. CNS gene expression following withdrawal and cue-induced drug-seeking behavior. Pharmacol Biochem Behav 90:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz-Melcavage KL, Brucklacher RM, Grigson PS, Freeman WM, Vrana KE (2009) Gene expression changes following extinction testing in a heroin behavioral incubation model. BMC Neurosci 10:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy RT, Strickland JC, Feinstein MA, Robinson AM, Smith MA (2016) The effects of sex, estrous cycle, and social contact on cocaine and heroin self-administration in rats. Psychopharmacology (Berl) 233:3201–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd GT, Petry NM (2002) Gender differences among pathological gamblers seeking treatment. Exp Clin Psychopharmacol 10:302–309. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW (2008) Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci 28:3170–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large M, Sharma S, Compton MT, Slade T, Nielssen O (2011) Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry 68:555–561. [DOI] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME (2007) Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol 15:461–471. [DOI] [PubMed] [Google Scholar]
- Lee JY, Ko YJ, Park SM (2013) Factors associated with current smoking and heavy alcohol consumption among women of reproductive age: the Fourth Korean National Health and Nutrition Examination Survey 2007-2009. Public Health 127:473–481. [DOI] [PubMed] [Google Scholar]
- Lesage MG, Keyler DE, Burroughs D, Pentel PR (2007) Effects of pregnancy on nicotine self-administration and nicotine pharmacokinetics in rats. Psychopharmacology (Berl) 194:413–421. [DOI] [PubMed] [Google Scholar]
- Lewis B, Nixon SJ (2014) Characterizing gender differences in treatment seekers. Alcohol Clin Exp Res 38:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B, Hoffman LA, Nixon SJ (2014) Sex differences in drug use among polysubstance users. Drug Alcohol Depend 145:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Dang J, Zhang X, Zhang Q, Guo J (2014) Internet addiction among Chinese adolescents: The effect of parental behavior and self-control. Comput Human Behav 41:1–7. [Google Scholar]
- Li CSR, Kosten TR, Sinha R (2005) Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study. Biol Psychiatry 57:487–494. [DOI] [PubMed] [Google Scholar]
- Li X, Caprioli D, Marchant NJ (2015) Recent updates on incubation of drug craving: a mini-review. Addict Biol 20:872–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Gamma A, Vollenweider FX (2001) Gender differences in the subjective effects of MDMA. Psychopharmacology (Berl) 154:161–168. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kendall SL, Babalonis S, Martin CA, Kelly TH (2007) Evaluation of estradiol administration on the discriminative-stimulus and subject-rated effects of d-amphetamine in healthy pre-menopausal women. Pharmacol Biochem Behav 87:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loft S, Olesen KL, Døssing M (1987) Increased susceptibility to liver disease in relation to alcohol consumption in women. Scand J Gastroenterol 22:1251–1256. [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, Okuda M, Wang S, Grant BF, Blanco C (2011) Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend 115:120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell-Badge R, Robertson E (1990) XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development 109:635–646. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, Kragie L, Mendelson JH (1996) Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology (Berl) 125:346–354. [DOI] [PubMed] [Google Scholar]
- Lund E, Jacobsen BK (1990) Use of oral contraceptives in relation to dietary habits and alcohol consumption. Contraception 42:171–177. [DOI] [PubMed] [Google Scholar]
- Lynch WJ (2006) Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol 14:34–41. [DOI] [PubMed] [Google Scholar]
- Lynch WJ (2008) Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 197:237–246. [DOI] [PubMed] [Google Scholar]
- Lynch WJ (2018) Modeling the development of drug addiction in male and female animals. Pharmacol Biochem Behav 164:50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Bakhti-Suroosh A, Abel JM, Davis C (2021) Shifts in the neurobiological mechanisms motivating cocaine use with the development of an addiction-like phenotype in male rats. Psychopharmacology (Berl) 238:811–823 DOI: 10.1007/s00213-020-05732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME (2001) Regulation of drug intake. Exp Clin Psychopharmacol 9:131–143. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR (2004) Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology 29:943–951. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR (2005) Decreased motivation following cocaine self-administration under extended access conditions: effects of sex and ovarian hormones. Neuropsychopharmacology 30:927–935. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Nicholson KL, Dance ME, Morgan RW, Foley PL (2010) Animal models of substance abuse and addiction: implications for science, animal welfare, and society. Comp Med 60:177–188. [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ (2008) Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 197:237–246. [DOI] [PubMed] [Google Scholar]
- Lynch WJ (2009) Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav 94:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME (2000) Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology (Berl) 152:132–139. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Kalayasiri R, Sughondhabirom A, Pittman B, Coric V, Morgan PT, Malison RT (2008) Subjective responses and cardiovascular effects of self-administered cocaine in cocaine-abusing men and women. Addict Biol 13:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Kiraly DD, Caldarone BJ, Picciotto MR, Taylor JR (2007) Effect of cocaine self-administration on striatal PKA-regulated signaling in male and female rats. Psychopharmacology (Berl) 191:263–271. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME (2002) Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 164:121–137. [DOI] [PubMed] [Google Scholar]
- Mackay L, Ickowicz S, Hayashi K, Abrahams R (2020) Rooming-in and loss of child custody: key factors in maternal overdose risk. Addiction 115:1786–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean RR, DeVito EE,, Eid T, Parida S, Gueorguieva R, Sofuoglu M (2021) Threshold dose for intravenous nicotine self-administration in young adult non-dependent smokers. Psychopharmacology 238:2083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevaiah SKOdorisio TElliott DJRattigan ASzot MLaval SHWashburn LLMcCarrey JRCattanach BMLovell-Badge R, et al. (1998) Mouse homologues of the human AZF candidate gene RBM are expressed in spermatogonia and spermatids, and map to a Y chromosome deletion interval associated with a high incidence of sperm abnormalities. Hum Mol Genet 7:715–727. [DOI] [PubMed] [Google Scholar]
- Maher EE, Kipp ZA, Leyrer-Jackson JM, Khatri S, Bondy E, Martinez GJ, Beckmann JS, Hinds TD Jr, Bimonte-Nelson HA, Gipson CD (2022) Ovarian hormones regulate nicotine consumption and accumbens glutamatergic plasticity in female rats. eNeuro 9:ENEURO.0286-21.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiavacchi S, Masi F, Scheggi S, Leggio B, De Montis MG, Gambarana C (2001) Long-term behavioral and neurochemical effects of chronic stress exposure in rats. J Neurochem 79:1113–1121. [DOI] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A (2005) Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol Clin Exp Res 29:896–901. [DOI] [PubMed] [Google Scholar]
- Mann K, Batra A, Günthner A, Schroth G (1992) Do women develop alcoholic brain damage more readily than men? Alcohol Clin Exp Res 16:1052–1056. [DOI] [PubMed] [Google Scholar]
- Manza PShokri-Kojori EWiers CEKroll DFeldman DMcPherson KBiesecker EDennis EJohnson AKelleher A, et al. (2022) Sex differences in methylphenidate-induced dopamine increases in ventral striatum. Mol Psychiatry 27:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CA, Mainous AG 3rd, Curry T, Martin D (1999) Alcohol use in adolescent females: correlates with estradiol and testosterone. Am J Addict 8:9–14. [DOI] [PubMed] [Google Scholar]
- Martinez DBroft AFoltin RWSlifstein MHwang DRHuang YPerez AFrankle WGCooper TKleber HD, et al. (2004) Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology 29:1190–1202. [DOI] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, Kumar D, Van Heertum R, Kleber HD, Nunes E (2011) Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry 168:634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez DGil RSlifstein MHwang DRHuang YPerez AKegeles LTalbot PEvans SKrystal J, et al. (2005) Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry 58:779–786. [DOI] [PubMed] [Google Scholar]
- Martinez D, Saccone PA, Liu F, Slifstein M, Orlowska D, Grassetti A, Cook S, Broft A, Van Heertum R, Comer SD (2012) Deficits in dopamine D(2) receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction. Biol Psychiatry 71:192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Gross KS, Himmler BT, Emmitt NL, Peterson BM, Zlebnik NE, Foster Olive M, Carroll ME, Meisel RL, Mermelstein PG (2016) Estradiol facilitation of cocaine self-administration in female rats requires activation of mGluR5. eNeuro 3:ENEURO.0140-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini M, Irvin JW, Lee CG, Lynch WJ, Rissman EF (2020) Sex chromosome complement influences vulnerability to cocaine in mice. Horm Behav 125:104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson J, Sproule B, Di Ciano P, Fares A, Le Foll B, Mann RE, Brands B (2020)Sex differences in the acute effects of smoked cannabis: evidence from a human laboratory study of young adults. Psychopharmacology (Berl) 237:305–316. [DOI] [PubMed] [Google Scholar]
- Mayo LM, Paul E, DeArcangelis J, Van Hedger K, de Wit H (2019) Gender differences in the behavioral and subjective effects of methamphetamine in healthy humans. Psychopharmacology (Berl) 236:2413–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Carroll KM, Rounsaville BJ (1999) Gender differences in treatment-seeking cocaine abusers—implications for treatment and prognosis. Am J Addict 8:300–311. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Hart CL, Boyarsky B, Kosten T, Jatlow P. Gender effects following repeated administration of cocaine and alcohol in humans.. Subst Use Misuse. 2005;40(4):511–28. doi: 10.1081/ja-200030693. P MID: 15830733. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Brake W, Gratton A (2002) Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology 27:127–138. [DOI] [PubMed] [Google Scholar]
- Mello NK, Bree MP, Skupny AS, Mendelson JH (1984) Blood alcohol levels as a function of menstrual cycle phase in female macaque monkeys. Alcohol 1:27–31. [DOI] [PubMed] [Google Scholar]
- Mello NK, Knudson IM, Kelly M, Fivel PA, Mendelson JH (2011) Effects of progesterone and testosterone on cocaine self-administration and cocaine discrimination by female rhesus monkeys. Neuropsychopharmacology 36:2187–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Knudson IM, Mendelson JH (2007) Sex and menstrual cycle effects on progressive ratio measures of cocaine self-administration in cynomolgus monkeys. Neuropsychopharmacology 32:1956–1966. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Lex BW (1990) Alcohol use and premenstrual symptoms in social drinkers. Psychopharmacology (Berl) 101:448–455. [DOI] [PubMed] [Google Scholar]
- Melón LC, Wray KN, Moore EM, Boehm SL 2nd (2013) Sex and age differences in heavy binge drinking and its effects on alcohol responsivity following abstinence. Pharmacol Biochem Behav 104:177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM (1999)Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology 21:294–303. [DOI] [PubMed] [Google Scholar]
- Mihov Y, Treyer V, Akkus F, Toman E, Milos G, Ametamey SM, Johayem A, Hasler G (2020) Metabotropic glutamate receptor 5 in bulimia nervosa. Sci Rep 10:6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Weafer J, Fillmore MT (2009) Gender differences in alcohol impairment of simulated driving performance and driving-related skills. Alcohol Alcohol 44:586–93 DOI: 10.1093/alcalc/agp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbaha H, Tabaeizadeh M, Shaterian-Mohammadi H, Tahsili-Fahadan P, Dehpour AR (2009) Estrogen pretreatment modulates morphine-induced conditioned place preference in ovariectomized mice. Pharmacol Biochem Behav 92:399–403. [DOI] [PubMed] [Google Scholar]
- Mishra D, Pena-Bravo JI, Leong KC, Lavin A, Reichel CM (2017) Methamphetamine self-administration modulates glutamate neurophysiology. Brain Struct Funct 222:2031–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CF, Lynch WJ (2015) Alcohol preferring (P) rats as a model for examining sex differences in alcohol use disorder and its treatment. Pharmacol Biochem Behav 132:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MH, Goldberg M, Smith SS (1998) Progesterone withdrawal. II: insensitivity to the sedative effects of a benzodiazepine. Brain Res 807:91–100. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA (2002) Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci 5:169–174. [DOI] [PubMed] [Google Scholar]
- Munro CAMcCaul MEWong DFOswald LMZhou YBrasic JKuwabara HKumar AAlexander MYe W, et al. (2006) Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry 59:966–974. [DOI] [PubMed] [Google Scholar]
- Murray CH, Christian DT, Milovanovic M, Loweth JA, Hwang EK, Caccamise AJ, Funke JR, Wolf ME (2021) mGlu5 function in the nucleus accumbens core during the incubation of methamphetamine craving. Neuropharmacology 186:108452 10.1016/j.neuropharm.2021.108452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MANader SHCzoty PWRiddick NVGage HDGould RWBlaylock BLKaplan JRGarg PKDavies HM, et al. (2012) Social dominance in female monkeys: dopamine receptor function and cocaine reinforcement. Biol Psychiatry 72:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides BM (1996) The state’s “sharp line between the sexes”: women, alcohol and the law in the United States, 1850–1980. Addiction 91:1211–1229. [DOI] [PubMed] [Google Scholar]
- Nicolas C, Russell TI, Pierce AF, Maldera S, Holley A, You ZB, McCarthy MM, Shaham Y, Ikemoto S (2019) Incubation of cocaine craving after intermittent-access self-administration: sex differences and estrous cycle. Biol Psychiatry 85:915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien MS, Anthony JC (2005) Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000-2001. Neuropsychopharmacology 30:1006–1018. [DOI] [PubMed] [Google Scholar]
- Okita K, Petersen N, Robertson CL, Dean AC, Mandelkern MA, London ED (2016) Sex differences in midbrain dopamine D2-type receptor availability and association with nicotine dependence. Neuropsychopharmacology 41:2913–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Origer A, Le Bihan E, Baumann M (2014) Social and economic inequalities in fatal opioid and cocaine related overdoses in Luxembourg: a case-control study. Int J Drug Policy 25:911–915. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wand GS, Kuwabara H, Wong DF, Zhu S, Brasic JR (2014) History of childhood adversity is positively associated with ventral striatal dopamine responses to amphetamine. Psychopharmacology (Berl) 231:2417–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald LM, Wand GS, Wong DF, Brown CH, Kuwabara H, Brašić JR (2015) Risky decision-making and ventral striatal dopamine responses to amphetamine: a positron emission tomography [(11)C]raclopride study in healthy adults. Neuroimage 113:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K, Tjurmina O, Palkovits M, Goldstein DS, Koch CA, Hoff T, Chrousos GP (2002) Chronic hypercortisolemia inhibits dopamine synthesis and turnover in the nucleus accumbens: an in vivo microdialysis study. Neuroendocrinology 76:148–157. [DOI] [PubMed] [Google Scholar]
- Pacchioni AM, Gabriele A, See RE (2011) Dorsal striatum mediation of cocaine-seeking after withdrawal from short or long daily access cocaine self-administration in rats. Behav Brain Res 218:296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrat C, Ouimette JF, Rougeulle C (2020) X chromosome inactivation in human development. Development 147:147. [DOI] [PubMed] [Google Scholar]
- Peltier MR, Sofuoglu M (2018) The role of exogenous progesterone in the treatment of males and females with substance use disorders: a narrative review. CNS Drugs 32:421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier MR, Sofuoglu M, Petrakis IL, Stefanovics E, Rosenheck RA (2021) Sex differences in opioid use disorder prevalence and multimorbidity nationally in the Veterans Health Administration. J Dual Diagn 17:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Bravo JI, Penrod R, Reichel CM, Lavin A (2019) Methamphetamine self-administration elicits sex-related changes in postsynaptic glutamate transmission in the prefrontal cortex. eNeuro 6:ENEURO.0401-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA (1999) Nicotine discrimination in men and women. Pharmacol Biochem Behav 64:295–299. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Levine M, Marcus M, Shiffman S, D’Amico D, Miller A, Keins A, Ashcom J, Broge M (2000) Tobacco withdrawal in women and menstrual cycle phase. J Consult Clin Psychol 68:176–180. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Scott J (2008) Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res 10:1245-50 DOI: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB (2013) Impact of pubertal and adult estradiol treatments on cocaine self-administration. Horm Behav 64:573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Jagannathan L, Becker JB (2015) The roles of dopamine and α1-adrenergic receptors in cocaine preferences in female and male rats. Neuropsychopharmacology 40:2696–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW (2008) Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci 28:6046–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BM, Mermelstein PG, Meisel RL (2015) Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct 220:2415–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza NJ, Vrbka JL, Yeager RD (1989) Telescoping of alcoholism in women alcoholics. Int J Addict 24:19–28. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Deminière JM, Maccari S, Le Moal M, Simon H (1993) Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci USA 90:11738–11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML (1996) Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol 36:359–378. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M (1997) Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Res Brain Res Rev 25:359–372. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y (2011) Neurobiology of the incubation of drug craving. Trends Neurosci 34:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V (2006) The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev 30:215–238. [DOI] [PubMed] [Google Scholar]
- Poole N, Isaac B (2001) Barriers to treatment for substance-using mothers, British Columbia Centre of Excellence for Women’s Health, British Columbia. [Google Scholar]
- Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R (2012) Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry 169:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priddy BM, Carmack SA, Thomas LC, Vendruscolo JCM, Koob GF, Vendruscolo LF (2017) Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav 152:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puetz VB, McCrory E (2015) Exploring the relationship between childhood maltreatment and addiction: a review of the neurocognitive evidence. Curr Addict Rep 2:318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purgianto A, Scheyer AF, Loweth JA, Ford KA, Tseng KY, Wolf ME (2013) Different adaptations in AMPA receptor transmission in the nucleus accumbens after short vs long access cocaine self-administration regimens. Neuropsychopharmacology 38:1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR (2007) Sex chromosome complement regulates habit formation. Nat Neurosci 10:1398–1400. [DOI] [PubMed] [Google Scholar]
- Quintero GC (2013) Role of nucleus accumbens glutamatergic plasticity in drug addiction. Neuropsychiatr Dis Treat 9:1499–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasingh JBord EQin GIi MSilver MHamada HAhluwalia DGoukassian DZhu YLosordo DW, et al. (2007) Enhanced voluntary alcohol consumption after estrogen supplementation negates estrogen-mediated vascular repair in ovariectomized mice. Endocrinology 148:3618–3624. [DOI] [PubMed] [Google Scholar]
- Ramôa CP, Doyle SE, Lycas MD, Chernau AK, Lynch WJ (2014) Diminished role of dopamine D1-receptor signaling with the development of an addicted phenotype in rats. Biol Psychiatry 76:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramôa CP, Doyle SE, Naim DW, Lynch WJ (2013) Estradiol as a mechanism for sex differences in the development of an addicted phenotype following extended access cocaine self-administration. Neuropsychopharmacology 38:1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME (1999) Telescoping of landmark events associated with drinking: a gender comparison. J Stud Alcohol 60:252–260. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Chan CH, Ghee SM, See RE (2012) Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology (Berl) 223:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Dasilva MC, Shinal RM, Glover T, Williams RS, Staud R, Riley JL 3rd, Fillingim RB (2011) Evaluation of menstrual cycle effects on morphine and pentazocine analgesia. Pain 152:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi PLi RAnsari MSZald DPark SDawant BAnderson SDoop MWoodward NSchoenberg E, et al. (2006) Amphetamine-induced displacement of [18F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacology 31:1016–1026. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF (1998) Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res 22:1564–1569. [PubMed] [Google Scholar]
- Roberts DC, Morgan D, Liu Y (2007) How to make a rat addicted to cocaine. Prog Neuropsychopharmacol Biol Psychiatry 31:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DCS, Bennett SAL, Vickers GJ (1989) The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 98:408–411. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE (2008) The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience 151:579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M, Carroll M (2004) Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav 78:199–207. [DOI] [PubMed] [Google Scholar]
- Roth ME, Casimir AG, Carroll ME (2002) Influence of estrogen in the acquisition of intravenously self-administered heroin in female rats. Pharmacol Biochem Behav 72:313–318. [DOI] [PubMed] [Google Scholar]
- Roura-Martínez D, Díaz-Bejarano P, Ucha M, Paiva RR, Ambrosio E, Higuera-Matas A (2020) Comparative analysis of the modulation of perineuronal nets in the prefrontal cortex of rats during protracted withdrawal from cocaine, heroin and sucrose self-administration. Neuropharmacology 180:108290. [DOI] [PubMed] [Google Scholar]
- Rubio FJQuintana-Feliciano RWarren BLLi XWitonsky KFRValle FSDSelvam PVCaprioli DVenniro MBossert JM, et al. (2019) Prelimbic cortex is a common brain area activated during cue-induced reinstatement of cocaine and heroin seeking in a polydrug self-administration rat model. Eur J Neurosci 49:165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJSun WLMinerly ACEWeierstall KNazarian AFesta EDNiyomchai TAkhavan ALuine VJenab S, et al. (2008) Progesterone attenuates cocaine-induced conditioned place preference in female rats. Brain Res 1189:229–235. [DOI] [PubMed] [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH, Lynch WJ (2014) Sex differences in the effect of wheel running on subsequent nicotine-seeking in a rat adolescent-onset self-administration model. Psychopharmacology (Berl) 231:1753–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC (2005) Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol 526:65–76. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC (2010) Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci 1187:35–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KT, Sharp JL, Ethridge SB, Pearson T, Ballard S, Potter KM, Smith MA (2021) The effects of strain and estrous cycle on heroin- and sugar-maintained responding in female rats. Behav Brain Res 409:113329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schückher F, Sellin T, Fahlke C, Engström I (2018) The impact of childhood maltreatment on age of onset of alcohol use disorder in women. Eur Addict Res 24:278–285. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Anthenelli RM, Bucholz KK, Hesselbrock VM, Tipp J (1995) The time course of development of alcohol-related problems in men and women. J Stud Alcohol 56:218–225. [DOI] [PubMed] [Google Scholar]
- Scragg R, Wellman RJ, Laugesen M, DiFranza JR (2008) Diminished autonomy over tobacco can appear with the first cigarettes. Addict Behav 33:689–698. [DOI] [PubMed] [Google Scholar]
- See RE (2009) Dopamine D1 receptor antagonism in the prelimbic cortex blocks the reinstatement of heroin-seeking in an animal model of relapse. Int J Neuropsychopharmacol 12:431–6 DOI: 10.1017/S1461145709000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Jia Z, Lacadie CM, Tsou KA, Bergquist K, Sinha R (2011) Sex differences in neural responses to stress and alcohol context cues. Hum Brain Mapp 32:1998–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevy S, Smith GS, Ma Y, Dhawan V, Chaly T, Kingsley PB, Kumra S, Abdelmessih S, Eidelberg D (2008) Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology (Berl) 197:549–56 DOI: 10.1007/s00213-008-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams WM, Cossette MP, Shizgal P, Brake WG (2018) 17β-estradiol locally increases phasic dopamine release in the dorsal striatum. Neurosci Lett 665:29–32. [DOI] [PubMed] [Google Scholar]
- Shams WM, Sanio C, Quinlan MG, Brake WG (2016) 17β-Estradiol infusions into the dorsal striatum rapidly increase dorsal striatal dopamine release in vivo. Neuroscience 330:162–170. [DOI] [PubMed] [Google Scholar]
- Sharp JL, Ethridge SB, Ballard SL, Potter KM, Schmidt KT, Smith MA (2021) The effects of chronic estradiol treatment on opioid self-administration in intact female rats. Drug Alcohol Depend 225:108816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW (2011) Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci USA 108:19407–19412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BJ, Caruso MA, McRae-Clark AL (2019) Exogenous progesterone for cannabis withdrawal in women: Feasibility trial of a novel multimodal methodology. Pharmacol Biochem Behav 179:22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin CB, Templeton TJ, Chiu AS, Kim J, Gable ES, Vieira PA, Kippin TE, Szumlinski KK (2018) Endogenous glutamate within the prelimbic and infralimbic cortices regulates the incubation of cocaine-seeking in rats. Neuropharmacology 128:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemsen BM, Giannotti G, McFaddin JA, Scofield MD, McGinty JF (2019) Biphasic effect of abstinence duration following cocaine self-administration on spine morphology and plasticity-related proteins in prelimbic cortical neurons projecting to the nucleus accumbens core. Brain Struct Funct 224:741–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Koenig JI (2007) Evidence for the involvement of ERbeta and RGS9-2 in 17-β estradiol enhancement of amphetamine-induced place preference behavior. Horm Behav 52:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 158:343–359. [DOI] [PubMed] [Google Scholar]
- Sinha R (2008) Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141:105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox H, Hong KI, Sofuoglu M, Morgan PT, Bergquist KT (2007) Sex steroid hormones, stress response, and drug craving in cocaine-dependent women: implications for relapse susceptibility. Exp Clin Psychopharmacol 15:445–452. [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ (2006) Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 63:324–331. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Piasecki TM, Deutsch AR, Statham DJ, Martin NG (2015) Telescoping and gender differences in the time course of disordered gambling: evidence from a general population sample. Addiction 110:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small CM, Manatunga AK, Marcus M (2007) Validity of self-reported menstrual cycle length. Ann Epidemiol 17:163–170. [DOI] [PubMed] [Google Scholar]
- Smethells JR, Swalve NL, Eberly LE, Carroll ME (2016) Sex differences in the reduction of impulsive choice (delay discounting) for cocaine in rats with atomoxetine and progesterone. Psychopharmacology (Berl) 233:2999–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CTDang LCBurgess LLPerkins SFSan Juan MDSmith DKCowan RLLe NTKessler RMSamanez-Larkin GR, et al. (2019) Lack of consistent sex differences in D-amphetamine-induced dopamine release measured with [18F]fallypride PET. Psychopharmacology (Berl) 236:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Arma, s SP, Schmidt KT (2022) Modulation of morphine physical dependence and discriminative stimulus effects by ovarian hormones: Role of estradiol. Pharmacol Biochem Behav 218:173431 DOI: 10.1016/j.pbb.2022.173431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ethridge SB, Pearson T, Zhang H, Marcus MM, Ballard SL, Casimir AT, Potter KM, Schmidt KT, Sharp JL, et al. (2021) Modulation of heroin intake by ovarian hormones in gonadectomized and intact female rats. Psychopharmacology (Berl) 238:969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Walker KL, Cole KT, Lang KC (2011) The effects of aerobic exercise on cocaine self-administration in male and female rats. Psychopharmacology (Berl) 218:357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X (1998) GABA(A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 392:926–930. [DOI] [PubMed] [Google Scholar]
- Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, King JK, Arnold AP, Singh RR, Voskuhl RR (2008) A role for sex chromosome complement in the female bias in autoimmune disease. J Exp Med 205:1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon A (2019) Contributions of D1 vs. D2 Receptor-Expressing Neurons in the Nucleus Accumbens Core to Compulsive-Like Alcohol Consumption. M.Sc. thesis, Miami University, Miami, FL. [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK (2002) Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav 72:431–435. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK (1999) Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol 7:274–283. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR (2004) Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav 78:699–705. [DOI] [PubMed] [Google Scholar]
- Song Z, Yang H, Peckham EM, Becker JB (2019) Estradiol-induced potentiation of dopamine release in dorsal striatum following amphetamine administration requires estradiol receptors and mGlu5. eNeuro 6:ENEURO.0446-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, Woodside B, Shaham Y (1996) Ovarian hormones do not affect the initiation and maintenance of intravenous self-administration of heroin in the female rat. Psychobiology (Austin Tex) 24:154–159. [Google Scholar]
- Stokes PR, Egerton A, Watson B, Reid A, Lappin J, Howes OD, Nutt DJ, Lingford-Hughes AR (2011) History of cannabis use is not associated with alterations in striatal dopamine D2/D3 receptor availability. J Psychopharmacol 26:144–9 DOI: 10.1177/0269881111414090. [DOI] [PubMed] [Google Scholar]
- Stoltman JJK, Woodcock EA, Lister JJ, Greenwald MK, Lundahl LH (2015) Exploration of the telescoping effect among not-in-treatment, intensive heroin-using research volunteers. Drug Alcohol Depend 148:217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong CE, Schoepfer KJ, Dossat AM, Saland SK, Wright KN, Kabbaj M (2017) Locomotor sensitization to intermittent ketamine administration is associated with nucleus accumbens plasticity in male and female rats. Neuropharmacology 121:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Shchepakin D, Kalachev LV, Kavanaugh MP (2014) Glutamate transporter control of ambient glutamate levels. Neurochem Int 73:146–151. [DOI] [PubMed] [Google Scholar]
- Svikis DS, Miles DR, Haug NA, Perry B, Hoehn-Saric R, McLeod D (2006) Premenstrual symptomatology, alcohol consumption, and family history of alcoholism in women with premenstrual syndrome. J Stud Alcohol 67:833–836. [DOI] [PubMed] [Google Scholar]
- Sylvestre MP, Chagnon M, Wellman RJ, Dugas EN, O’Loughlin J (2018) Sex differences in attaining cigarette smoking and nicotine dependence milestones among novice smokers. Am J Epidemiol 187:1670–1677. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Shin CB (2018) Kinase interest you in treating incubated cocaine-craving? A hypothetical model for treatment intervention during protracted withdrawal from cocaine. Genes Brain Behav 17:e12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Wesley M, Freeman WM, Liang B, Hemby SE (2004) Alterations in ionotropic glutamate receptor subunits during binge cocaine self-administration and withdrawal in rats. J Neurochem 89:1021–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares H, Martins SS, Lobo DS, Silveira CM, Gentil V, Hodgins DC (2003) Factors at play in faster progression for female pathological gamblers: an exploratory analysis. J Clin Psychiatry 64:433–438. [DOI] [PubMed] [Google Scholar]
- Terner JM, de Wit H (2006) Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend 84:1–13. [DOI] [PubMed] [Google Scholar]
- Thorner ED, Jaszyna-Gasior M, Epstein DH, Moolchan ET (2007) Progression to daily smoking: is there a gender difference among cessation treatment seekers? Subst Use Misuse 42:829–835. [DOI] [PubMed] [Google Scholar]
- Tonn Eisinger KR, Gross KS, Head BP, Mermelstein PG (2018) Interactions between estrogen receptors and metabotropic glutamate receptors and their impact on drug addiction in females. Horm Behav 104:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O’Dell LE (2009) Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology (Berl) 206:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers EB, Bakhti-Suroosh A, Lynch WJ (2021) Females develop features of an addiction-like phenotype sooner during withdrawal than males. Psychopharmacology (Berl) 238:2213–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers EB, Setaro B, Lynch WJ (2022)Sex- and Dose-Dependent Differences in the Development of an Addiction-Like Phenotype Following Extended-Access Fentanyl Self-Administration. Front Pharmacol 13:841873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers EB, Tunstall BJ, McCracken ML, Vendruscolo LF, Koob GF (2019) Male and female mice develop escalation of heroin intake and dependence following extended access. Neuropharmacology 151:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Kim RK, Robinson HL, Marsh SA, Banks ML, Hamilton PJ (2021) Opioid withdrawal produces sex-specific effects on fentanyl-vs.-food choice and mesolimbic transcription. Biol Psychiatry Glob Open Sci 1:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Ducrocq F, Van Der Veldt S, Martinez D (2017) Blunted dopamine transmission in addiction: potential mechanisms and implications for behavior, in Seminars in Nuclear Medicine ; 2017. Jan 1. Vol 47, No 1, pp 64–74, WB Saunders. [DOI] [PubMed] [Google Scholar]
- Urban NBKegeles LSSlifstein MXu XMartinez DSakr ECastillo FMoadel TO’Malley SSKrystal JH, et al. (2010) Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [11C]raclopride. Biol Psychiatry 68:689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano-Márquez A, Estruch R, Fernández-Solá J, Nicolás JM, Paré JC, Rubin E (1995) The greater risk of alcoholic cardiomyopathy and myopathy in women compared with men. JAMA 274:149–154. [DOI] [PubMed] [Google Scholar]
- Urban NB, Slifstein M, Thompson JL, Xu X, Girgis RR, Raheja S, Haney M, Abi-Dargham A (2012) Dopamine release in chronic cannabis users: a [11c]raclopride positron emission tomography study. Biol Psychiatry 71:677–83 DOI: 10.1016/j.biopsych.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Giessen E, Weinstein JJ, Cassidy CM, Haney M, Dong Z, Ghazzaoui R, Ojeil N, Kegeles LS, Xu X, Vadhan NP, et al. (2017) Deficits in striatal dopamine release in cannabis dependence. Mol Psychiatry 22:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegrift BJHilderbrand ERSatta RTai RHe DYou CChen HXu PColes CBrodie MS, et al. (2020) Estrogen receptor α regulates ethanol excitation of ventral tegmental area neurons and binge drinking in female mice. J Neurosci 40:5196–5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegrift BJ, You C, Satta R, Brodie MS, Lasek AW (2017) Estradiol increases the sensitivity of ventral tegmental area dopamine neurons to dopamine and ethanol. PLoS One 12:e0187698 DOI: 10.1371/journal.pone.0187698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderziel A, Parker MA, Alshaarawy O (2020) Trends in heroin use among women of reproductive age in the United States, 2004-2017. Addict Behav 110:106518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Stoops WW, Rush CR (2010) Human sex differences in d-amphetamine self-administration. Addiction 105:727–31 DOI: 10.1111/j.1360-0443.2009.02858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Oranges ML, Toorie AM, Byrnes EM (2018) Oxycodone self-administration during pregnancy disrupts the maternal-infant dyad and decreases midbrain OPRM1 expression during early postnatal development in rats. Pharmacol Biochem Behav 173:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Moore KE, Pittman BP, Roberts W, Oberleitner LM, Smith PH, Cosgrove KP, McKee SA (2018) Intersection of stress and gender in association with transitions in past year DSM-5 substance use disorder diagnoses in the United States. Chronic Stress (Thousand Oaks) DOI: 10.1177/2470547017752637 [published ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Morales M (2015) The brain on drugs: from reward to addiction. Cell 162:712–25. [DOI] [PubMed] [Google Scholar]
- Volkow NDChang LWang GJFowler JSLeonido-Yee MFranceschi DSedler MJGatley SJHitzemann RDing YS, et al. (2001) Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 158:377–382 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ding YS, Fowler JS, Wang GJ (1996) Cocaine addiction: hypothesis derived from imaging studies with PET. J Addict Dis 15:55–71. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP (1993) Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14:169–177. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F (2007) Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol 64:1575–1579. [DOI] [PubMed] [Google Scholar]
- Volkow NDFowler JSWolf APSchlyer DShiue CYAlpert RDewey SLLogan JBendriem BChristman D, et al. (1990) Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry 147:719–724. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Han B, Compton WM, McCance-Katz EF (2019) Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA 322:167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Logan J, Alexoff DL, Jayne M, Fowler JS, Wong C, Yin P, Du C (2014) Stimulant-induced dopamine increases are markedly blunted in active cocaine abusers. Mol Psychiatry 19:1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow NDWang GJFischman MWFoltin RWFowler JSAbumrad NNVitkun SLogan JGatley SJPappas N, et al. (1997) Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature 386:827–830. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C, Hitzemann R, Pappas NR (1999) Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther 291:409–415. [PubMed] [Google Scholar]
- Wagner FA, Anthony JC (2007) Male–female differences in the risk of progression from first use to dependence upon cannabis, cocaine, and alcohol. Drug Alcohol Depend 86:191–198. [DOI] [PubMed] [Google Scholar]
- Wand GS, Oswald LM, McCaul ME, Wong DF, Johnson E, Zhou Y, Kuwabara H, Kumar A (2007) Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology 32:2310–2320. [DOI] [PubMed] [Google Scholar]
- Wang C, Zheng D, Xu J, Lam W, Yew DT (2013) Brain damages in ketamine addicts as revealed by magnetic resonance imaging. Front Neuroanat 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Arnsten AFT (2015) Contribution of NMDA receptors to dorsolateral prefrontal cortical networks in primates. Neurosci Bull 31:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JG, Fallon VM, Goodwin L, Gage SH, Rose AK (2021) Menstrual cycle phase, hormonal contraception, and alcohol consumption in premenopausal females: a systematic review. Front Glob Womens Health 2:745263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinland C, Mühle C, Kornhuber J, Lenz B (2021) Progesterone serum levels correlate negatively with craving in female postmenopausal in-patients with alcohol use disorder: A sex- and menopausal status-separated study. Prog Neuropsychopharmacol Biol Psychiatry 110:110278. [DOI] [PubMed] [Google Scholar]
- White KA, Brady KT, Sonne S (1996) Gender differences in patterns of cocaine use. Am J Addict 5:259–261. [Google Scholar]
- White TL, Justice AJH, de Wit H (2002) Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav 73:729–741. [DOI] [PubMed] [Google Scholar]
- Wiers CE, Cabrera EA, Tomasi D, Wong CT, Demiral SB, Kim SW, Wang GJ, Volkow ND (2017) Striatal dopamine D2/D3 receptor availability varies across smoking status. Neuropsychopharmacology 42:2325–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers CE, Shokri-Kojori E, Wong CT, Abi-Dargham A, Demiral SB, Tomasi D, Wang GJ, Volkow ND (2016) Cannabis abusers show hypofrontality and blunted brain responses to a stimulant challenge in females but not in males. Neuropsychopharmacology 41:2596–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissman AM, McCollum AF, Huang GZ, Nikrodhanond AA, Woolley CS (2011) Sex differences and effects of cocaine on excitatory synapses in the nucleus accumbens. Neuropharmacology 61:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Tseng KY (2012) Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci 5:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock EA, Zakiniaeiz Y, Morris ED, Cosgrove KP (2020) Sex and the dopaminergic system: Insights from addiction studies. Handb Clin Neurol 175:141–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worhunsky PD, Matuskey D, Gallezot JD, Gaiser EC, Nabulsi N, Angarita GA, Calhoun VD, Malison RT, Potenza MN, Carson RE (2017) Regional and source-based patterns of [11C]-(+)-PHNO binding potential reveal concurrent alterations in dopamine D2 and D3 receptor availability in cocaine-use disorder. Neuroimage 148:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M, Wickens CM, Di Ciano P, Sproule B, Fares A, Matheson J, Mann RE, Rehm J, Shuper PA, George TP, Huestis MA (2021) Sex differences in the acute pharmacological and subjective effects of smoked cannabis combined with alcohol in young adults. Psychology of Addictive Behaviors 35:536. [DOI] [PubMed] [Google Scholar]
- Yoest KE, Quigley JA, Becker JB (2018) Rapid effects of ovarian hormones in dorsal striatum and nucleus accumbens. Horm Behav 104:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis JS, Iskander R, Fauser BCJM, Izhaki I (2020) Does an association exist between menstrual cycle length within the normal range and ovarian reserve biomarkers during the reproductive years? A systematic review and meta-analysis. Hum Reprod Update 26:904–928. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Rohlfing T, Chanraud S, Gu M, Sullivan EV, Pfefferbaum A (2013) In vivo glutamate measured with magnetic resonance spectroscopy: behavioral correlates in aging. Neurobiol Aging 34:1265–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakiniaeiz YHillmer ATMatuskey DNabulsi NRopchan JMazure CMPicciotto MRHuang YMcKee SAMorris ED, et al. (2019) Sex differences in amphetamine-induced dopamine release in the dorsolateral prefrontal cortex of tobacco smokers. Neuropsychopharmacology 44:2205–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Yang S, Yang C, Jin G, Zhen X (2008) Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology (Berl) 199:625–635. [DOI] [PubMed] [Google Scholar]
- Zhao W, Becker JB (2010) Sensitization enhances acquisition of cocaine self-administration in female rats: estradiol further enhances cocaine intake after acquisition. Horm Behav 58:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Oleson EB, Roberts DC (2012) The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology 37:1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Johnson LA, Agam M, Raber J (2014) Sex differences in activation of the hypothalamic-pituitary-adrenal axis by methamphetamine. J Neurochem 129:495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]