Abstract
Objective.
Electrocochleography (ECochG) is increasingly being used during cochlear implant (CI) surgery to detect and mitigate insertion-related intracochlear trauma, where a drop in ECochG signal has been shown to correlate with a decline in hearing outcomes. In this study, an ECochG-guided robotics-assisted CI insertion system was developed and characterized that provides controlled and consistent electrode array insertions while monitoring and adapting to real-time ECochG signals.
Study Design.
Experimental research.
Setting.
A research laboratory and animal testing facility.
Methods.
A proof-of-concept benchtop study evaluated the ability of the system to detect simulated ECochG signal changes and robotically adapt the insertion. Additionally, the ECochG-guided insertion system was evaluated in a pilot in vivo sheep study to characterize the signal-to-noise ratio and amplitude of ECochG recordings during robotics-assisted insertions. The system comprises an electrode array insertion drive unit, an extracochlear recording electrode module, and a control console that interfaces with both components and the surgeon.
Results.
The system exhibited a microvolt signal resolution and a response time <100 milliseconds after signal change detection, indicating that the system can detect changes and respond faster than a human. Additionally, animal results demonstrated that the system was capable of recording ECochG signals with a high signal-to-noise ratio and sufficient amplitude.
Conclusion.
An ECochG-guided robotics-assisted CI insertion system can detect real-time drops in ECochG signals during electrode array insertions and immediately alter the insertion motion. The system may provide a surgeon the means to monitor and reduce CI insertion–related trauma beyond manual insertion techniques for improved CI hearing outcomes.
Keywords: electrocochleography, cochlear implant insertion, robotics assisted
Preservation of the delicate intracochlear structures is a central tenet of current cochlear implant (CI) surgery, especially for patients with significant residual acoustic hearing. Whether through direct damage to cochlear tissues during the electrode array (EA) insertion1–6 or from postoperative fibrosis,7,8 the loss of residual hearing following CI surgery significantly impairs speech recognition in noisy environments and can reduce the quality of life for CI patients.9–11 Current efforts to preserve residual hearing during CI surgery include the use of intraoperative steroids, the design of slimmer and more flexible EAs, and ‘‘atraumatic’’ techniques and tools designed to reduce insertional forces during EA insertion. Importantly, intraoperative electrocochleography (ECochG) has recently emerged as a tool to measure cochlear hair cell/neural responses and to aid the surgeon in detecting and avoiding insertion trauma.
ECochG was originally used as an alternative hearing test for children but has since evolved clinically to become a diagnostic tool for a variety of auditory pathologies and an intraoperative monitoring tool during cochlear implantation.12–20 ECochG recordings can be taken intracochlearly by utilizing the CI electrodes themselves as the recording electrodes or via a separate stationary extracochlear recording electrode during CI insertion. Intracochlear recordings are advantageous because response signals are generally larger than extracochlear recordings due to proximity of the recording electrodes to the response-generating hair cells and neurons.19,21 However, because the recording electrode is constantly moving as the array is inserted into the cochlea, the ECochG signal fluctuates independently of cellular output, making it difficult to attribute changes in ECochG response to electrode-induced trauma or to changes in electrode position relative to the location of the response generators.
Extracochlear ECochG recordings are advantageous because the recording electrode is stationary; therefore, signal fluctuations are more directly linked to physiologic changes of the cochlea. Nevertheless, the greater distance between the signal-generating cells and the electrode presents a challenge in acquiring a strong low-noise signal. The signal quality can be affected by factors including inherent system properties such as electrode impedance and system noise floor, as well as surgical factors including anatomic location of the recording electrode or operating room environment electrical noise.
Currently available technologies from the major CI companies provide the surgeon (or researcher) a convenient intracochlear method for ECochG monitoring during surgery. Intraoperative intracochlear ECochG monitoring has been accomplished through clinical or research software in CI users implanted with Cochlear Ltd, Advanced Bionics, and MED-EL EAs.22–24 In theory, if cochlear damage was detected via ECochG monitoring during insertion, movement of the EA should be altered to mitigate further trauma and loss of residual hearing. However, to prevent trauma, real-time ECochG monitoring is useful only if the electrode insertion can be altered in real time in response to a clinically significant signal change—a micromechanical task difficult for any surgeon to perform by hand—especially given that the limit of human responsiveness to auditory or visual stimulation is up to 500 milliseconds.25 Furthermore, no currently available ECochG-related technologies have the ability to interface with the movement of a CI EA.
Therefore, an ECochG-guided robotics-assisted CI insertion system was developed to provide controlled and consistent EA insertions while monitoring and adapting to real-time extracochlear ECochG signals. The objective of this study was to evaluate the factors that affect recording signal quality and response time and to demonstrate the feasibility of a prototype system in a pilot large animal model.
Methods
ECochG-Guided Robotics-Assisted CI Insertion System
A custom prototype ECochG-guided robotics-assisted CI insertion system (iotaMotion Inc) was fabricated, consisting of a wireless extracochlear ECochG recording module (Figure 1A), a robotic drive unit for EA insertion, and a surgeon interface panel with foot pedal control. A custom coated silver wire electrode with 1-mm ball tip was utilized for recordings (Figure 1B) with standard subdermal needle ground electrodes (Neuroguard). The robotic-assistive insertion drive unit was coupled to the CI lead as previously described with the recording electrode.26 The surgeon controlled the movement of the CI EA insertion via a foot pedal while guiding the trajectory with standard manual instrumentation. Manual instrumentation was used by the surgeon to guide the advancing EA into the round window and, rarely, momentarily maintain the trajectory of the EA during insertion (no grasping or advancement, just guidance). Specifically, the surgeon would place a ‘‘claw’’ instrument on the side the EA tip to direct it to the opening in the round window membrane. Because manual instrumentation was maintained in a fixed position near the round window while the drive unit controlled movement of the EA, the effect of any hand tremor was minimized with this technique, especially when compared with a fully manual insertion. A user computer interface provided a real-time graphical indicator of the ECochG signal status.
Figure 1.
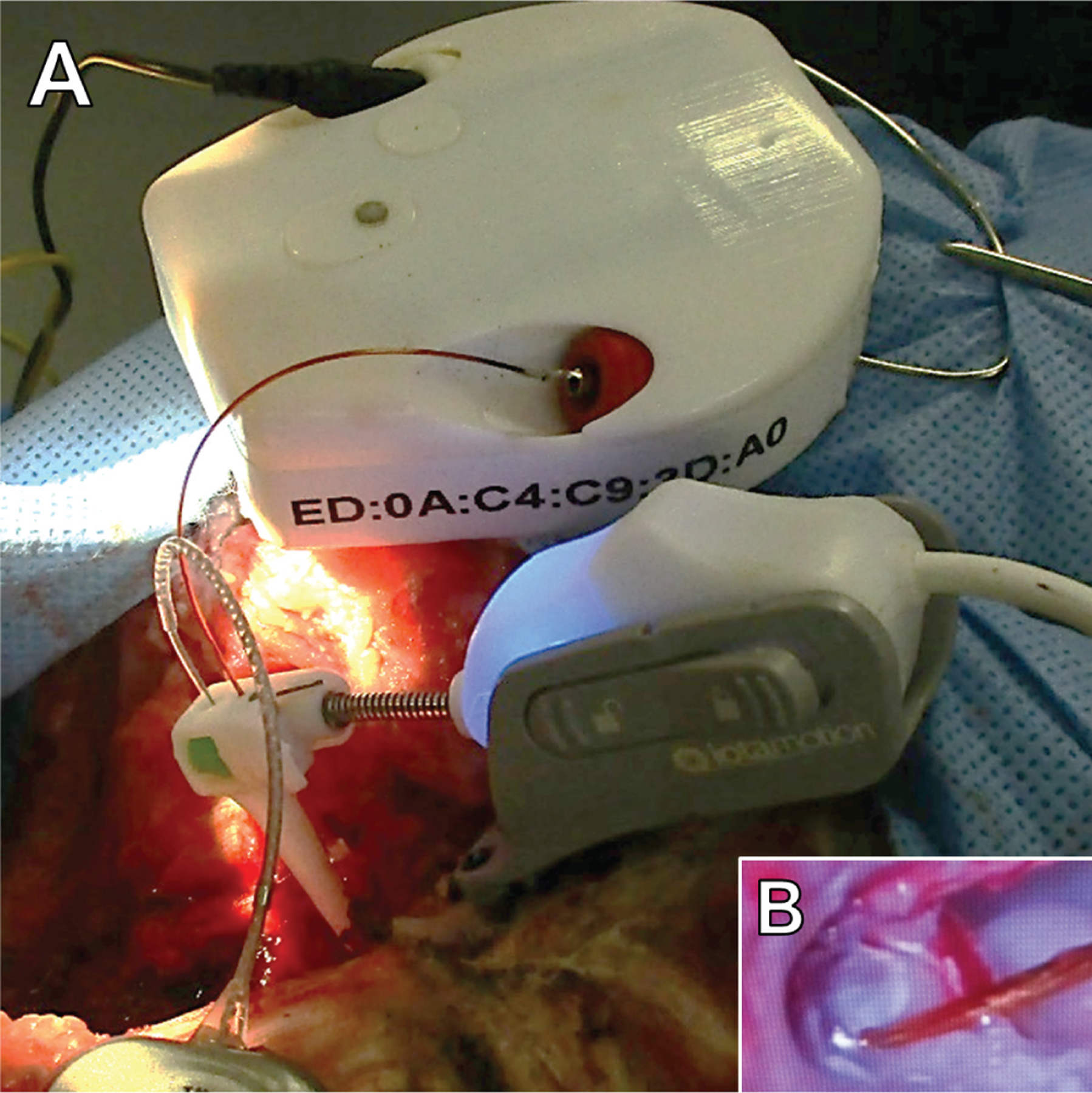
(A) Robotic drive (bottom) and electrocochleography recording module (top) during sheep study. Robotic drive holds recording electrode and drives the electrode array into the cochlea. (B) Recording electrode near round window during electrode array insertion.
Pilot In Vivo Evaluation in Sheep
The prototype ECochG-guided insertion system was used to demonstrate the feasibility of ECochG signal acquisition and the potential to detect and mitigate insertion trauma events in a large animal model. Two adult female Suffolk/Dorset crossbred sheep (Iowa State University) were used in this non-survival pilot evaluation study. Care was reviewed and performed in accordance with approved protocols of the University of Iowa Institutional Animal Care and Use Committee. Briefly and as previously described,27 sheep underwent a bilateral retrofacial mastoidectomy to expose the bulla, base of the cochlea, and round window bilaterally. Following exposure of the round window, impedance was measured in triplicate with an impedance meter (Grass Technologies EZM) at each anatomic location (facial recess, promontory, round window).
Following impedance measures, a baseline ECochG recording (only stimulus, no electrode insertion) was obtained with an acoustic stimulus delivered via an insert earphone (Etymotic ER-3C) secured in the ear canal. The stimulus was a continuous 500-Hz tone, and signal recording was achieved by continuously streaming data from the ECochG module to the control console. The received signal was then processed to assess the cochlear microphonic component in real time. After being filtered, the data were displayed in the graphical user interface in the time and frequency domains, achieved per the fast Fourier transform (FFT) and stored locally for post hoc analysis. ECochG signals were analyzed by FFT magnitude (in microvolts) and signal-to-noise ratio (SNR). The SNR was calculated by dividing the average of the root mean square of the waveform by the standard deviation. Baseline recordings were obtained sequentially and in triplicate by varying recording electrode anatomic location (round window membrane, promontory, and facial recess) to determine the optimal position for maximized signal amplitude and quality. Based on the baseline recording signal amplitude, recordings were taken from the round window (periphery) location during the actual electrode insertion.
Following all impedance and baseline ECochG recordings, the round window membrane was opened with a fine pick, and a lateral wall electrode was inserted manually or with the robotics-assisted insertion tool. Manual insertions were performed with forceps via ‘‘soft techniques’’ over 30 seconds until resistance or a significant ECochG drop was detected. On the contralateral side, the electrode was inserted with the robotics-assisted insertion tool at 0.5 mm/s with real-time ECochG guidance. The recording electrode was positioned at the periphery of the round window membrane within the niche (Figure 1B). There was ample space around the round window to ensure that the recording electrode and EA never touched during insertion. If a drop in the cochlear microphonic signal was detected (10%-20% drop in amplitude from peak), the insertion was halted, and the surgeon partially withdrew the electrode by reversing the advancement and monitoring for any change or recovery of signal.
X-ray Imaging
Following euthanasia, EAs were fixed in place with methacrylate glue just external to round window niche. The temporal bone immediately surrounding the cochlea was carefully excised and fixed in formalin. Explanted cochleae were imaged with the EA in situ via high-resolution x-ray microscopy (Xradia 520 Versa; Zeiss) as previously described.26,28,29 The EA was then removed while maintaining sample position and the cochlea reimaged.
Results
ECochG Recording Characterization and System Response Time
Table 1 presents a summary of the ECochG FFT magnitude, filtered SNR, and impedance values by location. Regarding observed impedances, all recording locations remained <25 kΩ. FFT magnitude was significantly greater for recordings taken near the round window versus the other locations. However, location did not significantly affect impedance or SNR.
Table 1.
Electrocochleography FFT Magnitude, Filtered SNR, and Recording Electrode Impedance at Each Anatomic Location.a
| Recording electrode location | FFT magnitude, μV | Filtered SNR | Impedance, kΩ |
|---|---|---|---|
| Round window | 592.0 ± 628.0b | 1.00 ± 0.32 | 17.9 ± 7.0 |
| Promontory | 91.0 ± 93.1 | 0.79 ± 0.16 | 14.4 ± 8.2 |
| Facial recess | 24.3 ± 36.7 | 0.71 ± 0.05 | 17.2 ± 5.0 |
Abbreviations: FFT, fast Fourier transform; SNR, signal-to-noise ratio.
Results are averaged across animals and individual recordings.
P <.05 vs other locations (analysis of variance).
Last, by using the robotic system in this study, a benchtop characterization with a simulated signal and waveform generator showed that typical motion adjustment could be made in <100 milliseconds. This is the overall time from when an event is detected to when the electrode insertion is halted.
In Vivo ECochG
Representative FFT amplitude and ECochG voltage graphs during the electrode insertion are shown in Figure 2. Each ECochG voltage recording shows the baseline recording with minimal noise and a large peak at 500 Hz that reflects the stimulus frequency. Images were analyzed in the time and frequency domains. In the frequency domain, the large peaks at 500 Hz reflect the frequency at which the acoustic stimulus was presented. The baseline recording looks as expected (‘‘NoStim’’ in Figure 2), with no notable peaks beyond the remaining low-frequency noise present in all recordings.
Figure 2.

Representative electrocochleography fast Fourier transform recording taken during electrode insertion in sheep (top). Representative electrocochleography recordings show a drop and recovery (bottom left) and sustained drop (bottom right) during electrode array insertions.
Figure 2 shows representative plots of ECochG amplitude (cochlear microphonic component) over time during electrode insertions. When a significant drop was noted, the surgeon stopped advancing and slightly reversed the electrode, and signal amplitude recovered in 3 of 4 insertions. Table 2 summarizes the ECochG events for each insertion. No electrodes were fully removed and reinserted during testing.
Table 2.
Pilot In Vivo Insertion Data.
| Insertion |
|||||
|---|---|---|---|---|---|
| Animal: Side | Method | Depth, mm | Angle,º | Intracochlear trauma event | Electrocochleography notes |
| 1 | |||||
| Left | Device | 10.4 | 220 | EA touching BM, no elevation | 45% drop, signal recovered |
| Right | Manual | 10.0 | 235 | BM elevation | 60% drop, signal recovered and increased |
| 2 | |||||
| Left | Device | 9.9 | 201 | BM elevation | 50% drop, no signal recovery |
| Right | Manual | 9.4 | 219 | EA touching BM, no elevation | 55% drop, signal recovered and increased |
Abbreviations: BM, basilar membrane; EA, electrode array.
X-ray Microscopy
The cochleae of each sheep were explanted and imaged via 3-dimensional x-ray microscopy. Figure 3 shows a representative 3-dimensional reconstruction and 2-dimensional slices. As shown in a representative image in panel C, EAs were observed within the cochlea at insertion depths of approximately (Table 2). In postnecropsy imaging, the EA was observed touching or elevating the basilar membrane in all insertions (Figure 3C and 3D, respectively). More severe trauma was not observed, such as osseous spiral lamina fracture or electrode translocations.
Figure 3.
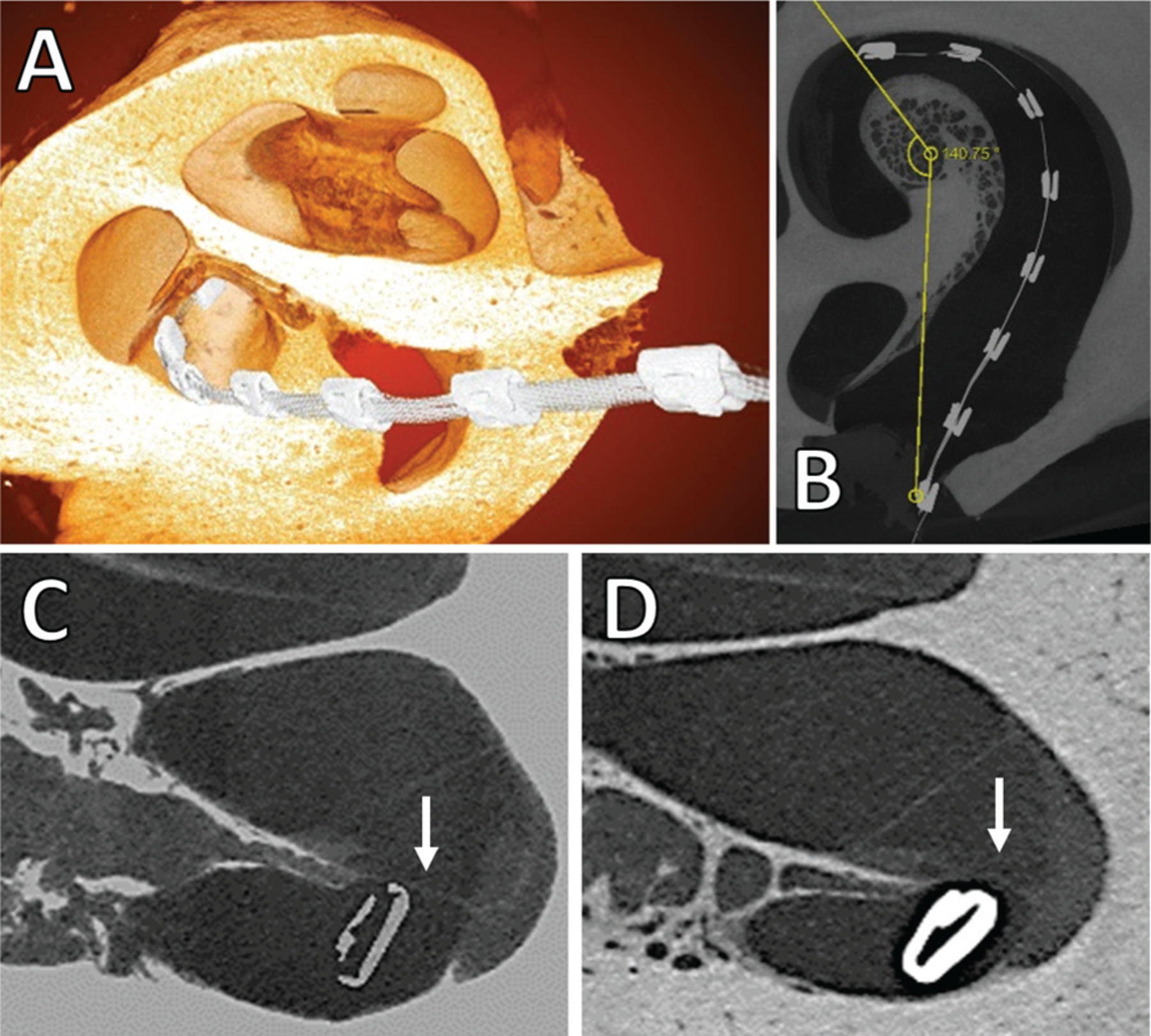
Representative x-ray microscopy images: (A) 3-dimensional overlay of electrode array within cochlea, (B) 2-dimensional angle of insertion calculation, (C) 2-dimensional image showing electrode array near basilar membrane (indicated by white arrow) with no elevation, (D) 2-dimensional slice showing basilar membrane elevation by electrode array.
Table 2 presents a summary of electrode insertion depth, intracochlear trauma observations, and ECochG notes. Drops in ECochG amplitude were observed in every insertion, and recovery of drop was observed in 3 of 4 insertions with reversal or stopping further insertion of the EA.
Discussion
Here we present early feasibility data on a system designed to monitor and rapidly respond to changes in ECochG amplitude during CI EA insertion. Several design requirements were considered, including sensitivity to ECochG responses, ability to detect physiologic changes in cochlear function, and integration with a robotics assist system. This integration enables automated and rapid halting of electrode insertion to limit intracochlear trauma.
The effect of high impedances on recording electrode functionality is known to reduce signal magnitude and increase noise.30–32 In the current study, relatively low impedances (<25 kΩ) were observed for the system at all electrode locations tested (Table 1). This finding is advantageous for 2 reasons. First, an inherently minimal impedance electrode can presumably detect minute changes in the response signal of cells deep within the cochlea, thus improving the overall accuracy of the system without the need for intracochlear recording electrodes. Second, the consistently low impedances allow for greater flexibility during potentially variable clinical applications and the operating room environment. For example, variable surgical approaches and mastoidectomy sizes may enhance or limit access to potential recording locations around the cochlea. Reducing the impedance of the system with design and electrode material choices allows for recording electrode placement in a wider range of locations near the cochlea. This helps mitigate problems associated with variable exposure of the cochlea and round window due to anatomic or surgical constraints. Future clinical studies are needed to determine the extent to which ECochG signals can be detected with the current recording module in humans undergoing cochlear implantation.
Widespread clinical adoption of an ECochG recording device depends on many factors, perhaps most importantly the ability of the system to acquire meaningful data. In the current study, the decision to use extracochlear recording electrodes was made to reduce variations in the ECochG amplitude associated with movement of recording electrodes within the cochlea. Theoretically, changes in ECochG amplitudes detected from a stationary recording electrode reflect physiologic changes in cochlear function, making it easier to interpret the significance of amplitude variations. Although not analyzed here, ECochG phase variations from stationary recording electrodes are easier to interpret than those that occur with recording electrodes that are moving within the cochlea. However, a potential limitation of extracochlear recordings is the inherently lower signal strength as compared with intracochlear recordings due to signal attenuation that occurs between the signaling cells (within or very near the cochlea) and the recording electrode outside the cochlea. Nevertheless, clinical studies utilizing extracochlear ECochG recording during CI surgery have shown promise in illuminating signal differences between presumed ‘‘atraumatic’’ and traumatic insertions33 and even improving outcomes when used by surgeons to monitor the insertion. For example, Mandalà et al found that hearing was preserved in 85% (11/13) of patients when intraoperative extracochlear ECochG feedback was used, as opposed to 33% (4/12) of control patients with no feedback.14 In the current study, the system was designed to go beyond these previous efforts by reducing the impedance and increasing the SNR through the overall system design. As evidenced in recordings, extracochlear electrodes were able to detect changes in the cochlear microphonic response in various anatomic locations near the cochlea.
One of the most important aspects in any ECochG-guided electrode insertion is the speed at which the system can respond to any detected changes. In a study evaluating surgeon perception during cochlear implantation, surgeons reported the perception of insertion resistance 5.9 ± 4.0 seconds after a CAP amplitude change occurred, suggesting that perception of resistance is not a reliable indicator of intracochlear changes and highlighting the need for a more rapid response.34 Currently available ECochG systems rely on the reaction time of a technician to recognize a signal change and the surgeon to halt the insertion in response. A study evaluating human reaction times found that the limit of human response time to visual or auditory stimulation was >500 milliseconds.25 In a study evaluating the human kinematics of electrode insertions, Kesler et al found that the average speed that a surgeon could steadily insert the electrode (not stop-and-go) was approximately 0.87 mm/s.35 Therefore, from the time that a change in ECochG response occurs, over a second and nearly a millimeter of additional electrode insertion would also occur until the insertion is halted, presumably causing unnecessary trauma to sensitive tissues. Therefore, reaction time is critical and is one of the primary advantages in using robotics-assisted devices. Because the current system can detect and respond to ECochG changes in <0.1 second and steadily insert electrodes at speeds as low as 0.1 mm/s, the insertion could theoretically be halted with as little as 0.01 mm of additional electrode insertion once a drop or event is detected, potentially precluding significant intracochlear damage and improving hearing in noise for the CI recipient.
The use of x-ray microscopy in the current study provides a powerful tool to qualify trauma to the cochlea. As shown in representative images in Figure 3, the resolution of this technique is sufficient to illuminate even slight elevations to the basilar membrane by the EA. As described in Table 2, more serious traumatic events were not observed, such as disruptions to the basilar membrane or scalar translocations. This fact, in conjunction with the small sample size of the study, made it difficult to determine any correlation between ECochG signals and any cochlear pathology, especially in the nascent model of sheep cochlear implantation.36 While previous studies have evaluated ECochG and cochlear trauma using animal models of cochlear implantation, they utilized small animals needing customized intracochlear electrodes.37 This is the first study to associate intraoperative ECochG changes and drops with intracochlear events of basilar membrane elevation in a large animal model with a commercially available CI EA. The large animal sheep enables future evaluation of how differences in the EA insertion method may affect the immediate observation of cochlear trauma. Future studies involving larger sample sizes and variable insertion algorithms may give nuance to the relationship among the EA insertion, the behavior of the intraoperative ECochG profile, and the resulting functionality of the cochlea.
Conclusion
The current study is an important step toward what some consider the ultimate goal of intraoperative ECochG utilization—a semiautomated system that detects and responds to cochlear trauma responses instantaneously. Toward this goal, a wireless module was shown to be effective at obtaining a low-noise ECochG signal and transmitting the filtered data in real time to a robotic-assisted insertion tool. The insertion system described here integrating closed-loop ECochG monitoring overcomes the current limitations of manual CI insertion techniques and has the potential to decrease insertion trauma for improved patient hearing outcomes.
Funding source:
Small Business Innovation Research Phase I (FAIN R43DC017640), National Institute on Deafness and Other Communication Disorders, National Institutes of Health.
Footnotes
Disclosures
Competing interests: iotaMotion, Inc: Allan Henslee, employee; Chris Kaufmann, cofounder; Matt Andrick, employee; Parker Reineke, employee; Viral Tejani, consultant; Marlan Hansen, cofounder.
Sponsorships: None.
This article was presented for the AAO-HNSF 2020 Virtual Annual Meeting & OTO Experience; September 13–October 25, 2020.
References
- 1.De Seta D, Torres R, Russo FY, et al. Damage to inner ear structure during cochlear implantation: correlation between insertion force and radio-histological findings in temporal bone specimens. Hear Res 2017;344:90–97. [DOI] [PubMed] [Google Scholar]
- 2.Adunka O, Gstoettner W, Hambek M, Unkelbach MH, Radeloff A, Kiefer J. Preservation of basal inner ear structures in cochlear implantation. ORL 2004;66(6):306–312. [DOI] [PubMed] [Google Scholar]
- 3.Adunka OF, Pillsbury HC, Buchman CA. Minimizing intracochlear trauma during cochlear implantation. Adv Otorhinolaryngol 2010;67:96–107. [DOI] [PubMed] [Google Scholar]
- 4.Eshraghi AA, Van De Water TR. Cochlear implantation trauma and noise-induced hearing loss: apoptosis and therapeutic strategies. Anat Rec PartA Discov Mol Cell Evol Biol 2006;288(4):473–481. [DOI] [PubMed] [Google Scholar]
- 5.Pfingst BE, Hughes AP, Colesa DJ, Watts MM, Strahl SB, Raphael Y. Insertion trauma and recovery of function after cochlear implantation: evidence from objective functional measures. Hear Res 2015;330(pt A):98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roland PS, Wright CG. Surgical aspects of cochlear implantation: mechanisms of insertional trauma. Adv Otorhinolaryngol 2006;64:11–30. [DOI] [PubMed] [Google Scholar]
- 7.Foggia MJ, Quevedo RV, Hansen MR. Intracochlear fibrosis and the foreign body response to cochlear implant biomaterials. Laryngoscope Investig Otolaryngol 2019;4(6):678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benatti A, Castiglione A, Trevisi P, et al. Endocochlear inflammation in cochlear implant users: case report and literature review. Int J Pediatr Otorhinolaryngol 2013;77(6):885–893. [DOI] [PubMed] [Google Scholar]
- 9.Carlson ML, Driscoll CLW, Gifford RH, et al. Implications of minimizing trauma during conventional cochlear implantation. Otol Neurotol 2011;32(6):962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gantz BJ, Turner C, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope 2005;115(5):796–802. [DOI] [PubMed] [Google Scholar]
- 11.Büchner A, Schüssler M, Battmer RD, Stöver T, Lesinski-Schiedat A, Lenarz T. Impact of low-frequency hearing. Audiol Neurotol 2009;14(suppl 1):8–13. [DOI] [PubMed] [Google Scholar]
- 12.Gibson WP. The clinical uses of electrocochleography. Front Neurosci 2017;11:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eggermont JJ. Ups and downs in 75 years of electrocochleography. Front Syst Neurosci 2017;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandalá M, Colletti L, Tonoli G, Colletti V. Electrocochleography during cochlear implantation for hearing preservation. Otolaryngol Neck Surg 2012;146(5):774–781. [DOI] [PubMed] [Google Scholar]
- 15.Ramos-Macias A, O’Leary S, Ramos-deMiguel A, Bester C, Falcon-González JC. Intraoperative intracochlear electrocochleography and residual hearing preservation outcomes when using two types of slim electrode arrays in cochlear implantation. Otol Neurotol 2019;40(5S, suppl 1):S29–S37. [DOI] [PubMed] [Google Scholar]
- 16.Calloway NH, Fitzpatrick DC, Campbell AP, et al. Intracochlear electrocochleography during cochlear implantation. Otol Neurotol 2014;35(8):1451–1457. [DOI] [PubMed] [Google Scholar]
- 17.Harris MS, Riggs WJ, Giardina CK, et al. Patterns seen during electrode insertion using intracochlear electrocochleography obtained directly through a cochlear implant. Otol Neurotol 2017;38(10):1415–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorens A, Walkowiak A, Polak M, et al. Cochlear microphonics in hearing preservation cochlear implantees. J Int Adv Otol 2019;15(3):345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giardina CK, Brown KD, Adunka OF, et al. Intracochlear electrocochleography: response patterns during cochlear implantation and hearing preservation. Ear Hear 2019;40(4):833–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haumann S, Imsiecke M, Bauernfeind G, et al. Monitoring of the inner ear function during and after cochlear implant insertion using electrocochleography. Trends Hear 2019;23:23312165 19833567–2331216519833567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalbert A, Sijgers L, Grosse J, et al. Simultaneous intra- and extracochlear electrocochleography during electrode insertion. Ear Hear 2020;42(2):414–424. [DOI] [PubMed] [Google Scholar]
- 22.Acharya AN, Dayse T-V, Rajan GP. Using the implant electrode array to conduct real-time intraoperative hearing monitoring during pediatric cochlear implantation: preliminary experiences. Otol Neurotol 2016;37(2):e148–e153. [DOI] [PubMed] [Google Scholar]
- 23.Campbell L, Kaicer A, Sly D, et al. Intraoperative real-time cochlear response telemetry predicts hearing preservation in cochlear implantation. Otol Neurotol 2016;37(4):332–338. [DOI] [PubMed] [Google Scholar]
- 24.Dalbert A, Pfiffner F, Hoesli M, et al. Assessment of cochlear function during cochlear implantation by extra- and intracochlear electrocochleography. Front Neurosci 2018;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan AHS, Ng AWY. Finger response times to visual, auditory and tactile modality stimuli. Lect Notes Eng Comput Sci 2012; 2196:1449–1454. [Google Scholar]
- 26.Kaufmann CR, Henslee AM, Claussen A, Hansen MR. Evaluation of insertion forces and cochlea trauma following robotics-assisted cochlear implant electrode array insertion. Otol Neurotol 2020;41(5):631–638. [DOI] [PubMed] [Google Scholar]
- 27.Kaufmann C, Tejani V, Sun D, Fredericks D, Abbas P, Hansen M. Pilot evaluation of cochlear implant electrode position control system in a sheep model. Presented at: Association for Research in Otolaryngology MidWinter Meeting; February 10–14, 2018; San Diego, CA. [Google Scholar]
- 28.Claussen AD, Vielman Quevedo R, Mostaert B, Kirk JR, Dueck WF, Hansen MR. A mouse model of cochlear implantation with chronic electric stimulation. PLoS One 2019;14(4):e0215407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banakis Hartl RM, Kaufmann C, Hansen MR, Tollin DJ. Intracochlear pressure transients during cochlear implant electrode insertion: effect of micro-mechanical control on limiting pressure trauma. Otol Neurotol 2019;40(6):736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loeb GE, Peck RA, Martyniuk J. Toward the ultimate metal microelectrode. J Neurosci Methods 1995;63(1):175–183. [DOI] [PubMed] [Google Scholar]
- 31.Chung T, Wang JQ, Wang J, Cao B, Li Y, Pang SW. Electrode modifications to lower electrode impedance and improve neural signal recording sensitivity. J Neural Eng 2015;12(5):56018. [DOI] [PubMed] [Google Scholar]
- 32.Kappenman ES, Luck SJ. The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology 2010;47(5):888–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giardina CK, Khan TE, Pulver SH, et al. Response changes during insertion of a cochlear implant using extracochlear electrocochleography. Ear Hear 2018;39(6):1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pile J, Simaan N. Modeling, design, and evaluation of a parallel robot for cochlear implant surgery. IEEE/ASME Transactions on Mechatronics 2014;19(6):1746–1755. [Google Scholar]
- 35.Kesler K, Dillon NP, Fichera L, Labadie RF. Human kinematics of cochlear implant surgery: an investigation of insertion micro-motions and speed limitations. Otolaryngol Neck Surg 2017; 157(3):493–498. [DOI] [PubMed] [Google Scholar]
- 36.Kaufmann CR, Tejani VD, Fredericks DC, et al. Pilot evaluation of sheep as in vivo model for cochlear implantation. Otol Neurotol 2020;41(5):596–604. [DOI] [PubMed] [Google Scholar]
- 37.DeMason C, Choudhury B, Ahmad F, et al. Electrophysiological properties of cochlear implantation in the gerbil using a flexible array. Ear Hear 2012;33:534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]


