Abstract
Background
Prosthetic joint infection (PJI) caused by Pseudomonas aeruginosa represents a severe complication in orthopedic surgery. We report the case of a patient with chronic PJI from P. aeruginosa successfully treated with personalized phage therapy (PT) in combination with meropenem.
Methods
A 62-year-old woman was affected by a chronic right hip prosthesis infection caused by P. aeruginosa since 2016 . The patient was treated with phage Pa53 (I day 10 mL q8h, then 5 mL q8h via joint drainage for 2 weeks) in association with meropenem (2gr q12h iv) after a surgical procedure. A 2-year clinical follow up was performed. An in vitro bactericidal assay of the phage alone and in combination with meropenem against a 24-hour-old biofilm of bacterial isolate was also carried out.
Results
No severe adverse events were observed during PT. Two years after suspension, there were no clinical signs of infection relapse, and a marked leukocyte scan showed no pathological uptake areas. In vitro studies showed that the minimum biofilm eradicating concentration of meropenem was 8 µg/mL. No biofilm eradication was observed at 24 hours incubation with phages alone (108 plaque-forming units [PFU]/mL). However, the addition of meropenem at suberadicating concentration (1 µg/mL) to phages at lower titer (103 PFU/mL) resulted in a synergistic eradication after 24 hours of incubation.
Conclusions
Personalized PT, in combination with meropenem, was found to be safe and effective in eradicating P. aeruginosa infection. These data encourage the development of personalized clinical studies aimed at evaluating the efficacy of PT as an adjunct to antibiotic therapy for chronic persistent infections.
Keywords: biofilms, meropenem, phage therapy, prosthetic joint infections, Pseudomonas aeruginosa
Prosthetic joint infections caused by Pseudomonas aeruginosa are challenging infections. Phage therapy is an effective alternative antibacterial strategy. Combination of DAIR, personalized phage therapy, and meropenem was successful in the eradication of P aeruginosa from a prosthetic joint.
Prosthetic joint infection (PJI) is a highly undesirable complication after a total arthroplasty because of its significant impact on the patient's quality of life and on health system costs [1]. The incidence of PJI ranges between 1% and 3% depending on the arthroplasty site, and it is estimated that patients with PJI require 7 times longer hospital stays than patients without PJI [2]. Although Gram-negative bacteria are less common in PJI, Pseudomonas aeruginosa is considered the most difficult-to-treat pathogen, mainly because of its ability to adhere and colonize both osteoarticular structures and prostheses [3]. Moreover, few antibiotic molecules are available against P. aeruginosa biofilm-associated infections. In general, the management of PJI often requires surgical intervention [4]. In delayed and late-stage bacterial infections associated with biofilm formation, the best surgical approach is the 1- or 2-stage exchange, with the removal of the old prosthesis, followed by a new implant and a prolonged antibiotic therapy [5]. Unfortunately, many patients with PJI caused by P. aeruginosa experience a failure of multiple surgeries and recalcitrance of infection [3]. In the last 10 years, phage therapy (PT), mainly based on the use of strictly lytic phages to kill the bacteria, has re-emerged as a potentially effective solution to fight the difficult-to-treat infections [6–10]. Moreover, phage in vitro ability to kill clinical-relevant bacteria embedded in biofilm has been also reported [11, 12]. It is interesting to note that bacteriophages have shown a wider effect on eradication of infections when administered in combination with antibiotic therapy [13, 14]. Thus, PT is considered in many cases a promising complementary strategy to treat bone and joint infections [15]. Different case reports on osteoarticular and prosthetic infections by P. aeruginosa treated with PT are described [10, 16]. In most cases, the PT is applied as compassionate use and its application occurs during hospitalization, where patients receive continuous clinical monitoring of their conditions. Unfortunately, the regulatory framework for the application of experimental therapy is even more stringent in Italy, where only good manufacturing practice (GMP)-produced drugs, already in clinical trial, can be administered in the hospital [17]. Therefore, currently, PT cannot be prescribed in Italy, leaving phage self-administration as the only option available to patients. In this study, we describe the clinical case of a female patient affected by a chronic hip-PJI due to P. aeruginosa, which was successfully treated with a combination of a self-administered customized phage preparation and meropenem after a debridement, antibiotics, and implant retention (DAIR) procedure. Moreover, a microbiological analysis of in vitro phage activity alone and in combination with antibiotic against P. aeruginosa biofilm is also reported.
METHODS
Bacteriophage Supply and Genome Sequencing
In January 2020, P. aeruginosa isolate (referred to here as Pa_AR1 and identified by standard microbiology procedure [see Supplementary material]) was sent to the Eliava Institute, which provided the patient with a final clinical phage preparation custom-made, named Pa53 (titer: 108 plaque-forming units (PFU)/mL) in the end of July 2020, for self-administration. An informed consent was signed to the Eliava Institute.
Phages were shipped in July 2020 and they were stopped at the Italian border for routine controls. When phage formulations arrived in Italy, in vitro tests of Pa53 versus the patient's strain Pa_AR1 by plaque assay showed a titer of 103 PFU/mL. Although the phage titer seemed rather low, phage therapy was still administered.
Then phages were amplified by using Pa_AR1 as host and used for genome extraction and antibiofilm activity tests. The phage genome was sequenced as reported in Supplementary material. The deoxyribonucleic acid (DNA) data were deposited to National Center for Biotechnology Information and are available under BioProject PRJNA870273.
Surgical Treatment Protocol
At the beginning of August 2020, the patient (1) underwent debridement of all the tissues adherent to the total hip arthroplasty components that showed signs of infection and (2) replacement with new components of both the polyethylene acetabular liner and the ceramic head of the femoral stem. A hemovac wound suction drain of 15 Fr. Diameter (Zimmer, Warsaw, IN) was positioned around the neck of the femoral stem, and the wound was closed. The suction drain container was removed 24 hours later, and only the surgical drain cable was left. After 48 hours of medical observation, the patient was discharged. During surgery, tissue samples and swabs were collected from the periarticular space.
Lytic Activity of Pa53 Alone and in Combination With Meropenem Versus Pa_AR1 Biofilm
The lytic activity of Pa53 was assessed against preformed Pa_AR1 biofilm as previously reported [18]. Phage titer was determined by collecting medium of phage-infected biofilm samples at different time points and centrifugation to collect supernatant. Then, samples were filtered (0.22 µm) and used for phage titration by a plaque assay. Twenty-four-hour-old biofilms of Pa_AR1 were incubated at 37°C with both Pa53 (ranging from 103 to 105 PFU/mL) and meropenem (ranging from 0 to 2 µg/mL). Samples treated with either phages or antibiotic alone and an untreated control were also included. After 24-hour incubation, beads were washed 3 times with phosphate-buffered saline and sonicated for plating and colony counting [18].
The minimum biofilm-eradication concentration (MBEC) and the synergistic effect (fractional bactericidal concentration index [FBCIphages]) of meropenem and Pa53 was evaluated as previously reported [11, 19]. If the FBCIphages is ≤0.26, the concentrations of antibiotic and phage used in the combination had a synergistic effect that resulted in the complete eradication of the biofilm [11].
Patient Consent Statement
The patient’s written consent was obtained and any information, including illustrations, have been anonymized as much as possible. The work is conforming to standards currently applied in Georgia, where the patient's consent was obtained, for phage therapy and in Italy for orthopedic surgery and antibiotic treatment.
RESULTS
Case Presentation
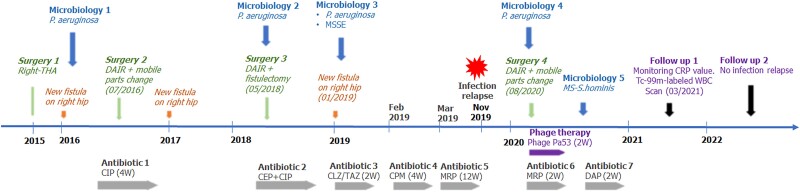
The patient is a 62-years-old woman affected by a right congenital hip subluxation treated at the age of 6 by proximal femoral osteotomy. In 2005, she underwent a total right hip arthroplasty (THA) complicated by a greater trochanter nonunion that was surgically fixed in in 2007. In 2010, she began to develop a strong pain on her right quadriceps. Several cycles of steroids and anti-inflammatory therapy were undertaken with partial benefits on symptoms. In 2013, a magnetic resonance scan showed the presence of a periprosthetic fluid collection suspected for infection. Therefore, she underwent debridement of the periprosthetic space and methicillin-susceptible Staphylococcus hominis was isolated from tissue biopsies. Since then, she developed recurrent swelling and pain in her right hip with evidence of a fluid collection (by ultrasound exam) for which periodical surgical debridement procedures and washouts of the prosthesis were required. All the surgical procedures were complicated by the isolation of methicillin-susceptible Staphylococcus epidermidis and methicillin-susceptible Staphylococcus capitis, followed by antibiotic therapy. Finally, in 2015, she underwent a 1-stage THA exchange (Figure 1). After an initial period without symptoms, in January 2016 the patient complained of pain and swelling to the right hip, where a new periprosthetic fluid collection was detected. The microbiological analysis of this periprosthetic fluid revealed the presence of P. aeruginosa, and the patient began oral therapy with ciprofloxacin 500 mg q8h (Figure 1). Then, she underwent acetabular line exchange and drainage of a large haematoma, debridement, and fistulectomy (2016). On collected samples, P. aeruginosa was always detected, and from 2018 on, several cycles of antibiotics were started via iv (ceftolozane/tazobactam 2.5 gr q8h for 2 weeks, cefepime 2 gr q8h for 2 weeks, meropenem 2 gr q12h for 1 month) and a chronic oral suppressive therapy with ciprofloxacin 750 mg q12h, without any resolution (Figure 1).
Figure 1.
The graphic shows the timeline. A summary of patient's history from 2015 to 2022, reporting information on isolated pathogens ("Microbiology"), relapse of infection ("New Fistula"), surgical procedures ("Surgery"), antibiotic ("Antibiotic") and phage therapy ("Phage Therapy"). CEP, cephalosporin (dosage unknown); CIP, ciprofloxacin (500 mg q8h); CLZ/TAZ, ceftolozane/tazobactam (1.5 gr q8h); CPM, cefepime (2 gr q8h); CRP, C-reactive protein; DAIR, debridement, antibiotics and implant retention; DAP, daptomycin (500 mg q24h); MRP, meropenem (2 gr q12h); MSSE, methicillin-susceptible Staphylococcus epidermidis; MS-S hominis, methicillin-susceptible Staphylococcus hominis; THA, total hip arthroplasty; W, weeks; WBC, white blood cell.
Successful Treatment of Right Total Right Hip Arthroplasty Infected by Pseudomonas aeruginosa Using Custom Phages
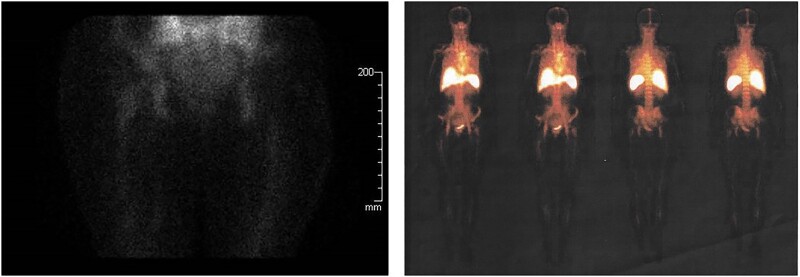
During the DAIR procedure performed on August 1, 2020 (Surgical Treatment Protocol), P. aeruginosa was isolated from all samples collected. The patient began phage therapy on August 5th together with meropenem 2 gr q12h iv (chosen based on the antibiotic susceptibility profile of the Pa_AR1 isolate; minimum inhibitory concentration, 2 µg/mL), after the debridement surgery. Through the surgical drain catheter, 10 mL phage Pa53 preparation was injected 3 times a day on the first day [10]. Before each phage's application, 5 mL sodium bicarbonate (1.4%) was administered as previously described [10, 20, 21]. After the first PT administration, an onset of high-grade fever (39°C) and chills was observed, and thus we suggested to the patient to reduce the dosage of phage formulation to 5 mL 3 times per day from the 2nd to the 15th day of phage treatment. No further adverse reactions in the next administrations were reported. Blood examinations did not reveal any modification in creatinine and transaminase values. On the 14th day of phage application and antibiotic therapy, a scarce production of serous liquid was observed from a little edge of the surgery wound, in absence of redness, swelling, or pain. A deep swab was made into the wound and methicillin-resistant S. hominis was detected. Being aware of the previous microbiological results of the patient, and on suspicion of the emergence of underlying pathogens during the targeted therapy against P. aeruginosa, on August 28th the antibiotic therapy was completed with intravenous daptomycin 500 mg every 24 hours for a period of 4 weeks. After the end of PT on August 20th, the drain cable was surgically removed. From September 2020 a clinical and laboratory data follow up was started. The value of C-reactive protein (CRP) gradually reduced until persistent negative results (last control on October 13, 2021, CRP 3.15 mg/L). During medical examinations the patient did not complain of any pain or symptoms of infection to the right hip and showed no signs of local inflammation. On March 24, 2021, a whole body 99m-Tc-labeled white blood cell scintigraphy was performed, and the result was confirmed the absence of enhancement at the right hip (Figure 2). By September 2022, the patient was still in clinical good conditions and no local signs of infection relapse were present.
Figure 2.
Images from Tc-99m-labeled white blood cell scan performed 7 months after phage therapy. No leucocytic activity was detected in proximity of the right-hip prosthetic joint.
In Vitro Synergistic Effect of Phages in Combination With Meropenem Against Pseudomonas aeruginosa Biofilm
The genome of phage Pa53 was sequenced (BioProject PRJNA870273). After visualization of the de novo assembly of the phage sequencing reads (Supplementary Figure 1), 12 short regions between 235 base pairs (bp) and 1726 bp, showing genomic variation, could be observed. These regions encode several proteins: 10 hypothetical proteins, a tail protein, an amidoligase, an aminotransferase, and the DNA metabolism-associated ligase, primase, and DNA polymerase. The applied phage preparation was therefore actually a mixture of very closely related phages of approximately 46.3 kb. A BLASTn analysis revealed this phage belongs to the Bruynoghevirus genus, which contains strictly lytic phages that are regularly used in therapeutic settings [22, 23]. No known toxins, virulence factors, or antibiotic resistance genes could be identified.
To test the antibiofilm activity of phage, the ability of patient’s clinical isolate to form biofilms was evaluated in vitro. Pseudomonas aeruginosa Pa_AR1 was a strong biofilm producer able to grow as a sessile microorganism on different materials, including porous glass beads used later on to test phage/antibiotic activity (Supplementary Figure 2A and B).
Then, a phenotypic characterization of phages was performed. Pa53 showed clear, small plaques (data not shown) and a latent period of 20 minutes with a burst size of 18 PFU released phage particles per infected-host versus Pa_AR1 (Supplementary Figure 3).
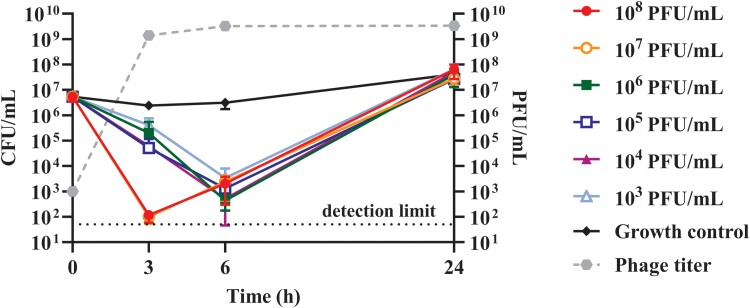
As shown in Figure 3, when biofilms were treated with higher phage titers (107–108 PFU/mL), a stronger reduction of over 4 log10 colony-forming units (CFU)/mL was observed within 3 hours of incubation, compared to the untreated control. Although the PFU/mL number of phages released by bacterial lysis remained high at different time points, the CFU/mL number of samples treated with phages increased up to ≈ 5 × 107 CFU/mL after 24 hours of incubation (similar to the untreated control), suggesting that the development of phage resistance might have occurred.
Figure 3.
Lytic activity at 3, 6, and 24 hours of Pa53 versus Pa_RA1 biofilm. Phage application conditions are shown as follow: filled circle (108 plaque-forming units [PFU]/mL), empty circle (107 PFU/mL), filled square (106 PFU/mL), empty square (105 PFU/mL), filled triangle (104 PFU/mL), empty triangle (103 PFU/mL) and diamond (no phage, Growth control). Dashed line represents the phage titer over the time. The 2 higher phage titers showed the greater biofilm reduction after 3 hours, whereas from 106 to 103 PFU/mL the peak of reduction occurred at 6 hours, then, both trends increased like the untreated control at 24 hours. Each point of the graph represents the mean ± standard deviations (error bars) of 3 independent replicates (N = 3). Detection limit (y = 50) for the colony-forming units (CFU)/mL count corresponds to the dotted line.
To investigate the in vitro appearance of resistant clones of Pa_AR1 during phages-biofilm incubation, 16 and 8 bacterial colonies were collected from bacterial samples exposed for 24 hours to Pa53 and from an untreated control, respectively, and rechallenged (in their planktonic form) with phages. Supplementary Figure 4 showed that all 8 of the clones from untreated control colonies were still susceptible to phages, indicating that no spontaneous resistant mutant appeared. By contrast, 11 of 16 clones from phage preincubated samples developed resistance, whereas 5 of 16 clones were still susceptible.
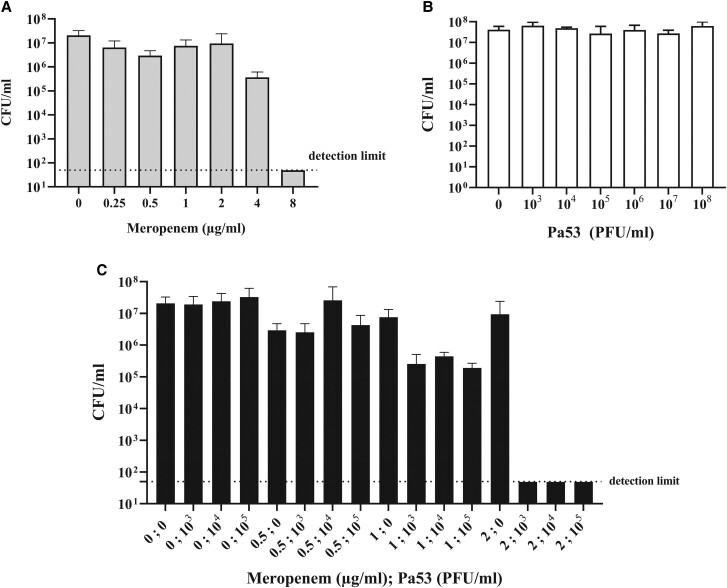
Given the in vitro results of killing kinetics of Pa53 versus Pa_AR1 and the risk of a likely resistance development in vivo, a potential synergistic effect phage-antibiotic was investigated. When meropenem was tested individually for 24 hours against biofilm embedded Pa_AR1 cells, its MBEC was 8 µg/mL (Figure 4A). As shown in Figure 4B and previously observed in the killing kinetics, after 24 hours of incubation, biofilm samples treated with different phage titers alone (up to 108 PFU/mL) were not able to eradicate the Pa_AR1 biofilm. However, biofilm eradication was observed when samples were coincubated with both antibiotic and phage (Figure 4C) at MBEC values of 2 µg/mL and 103 PFU/mL, respectively. Here, the values were at least 4-fold lower compared with MBEC obtained with single treatments and the calculated FBCIphages was 0.25, suggesting that the eradicating effect of Pa53 and meropenem versus Pa_AR1 biofilm was synergic.
Figure 4.
In vitro antimicrobial activity of meropenem (A), Pa53 (B), and combinations of both (C) versus biofilm-embedded Pa_AR1 after 24-hour incubation. Histogram represents the mean of colony-forming units (CFU) number ± standard deviations of Pa_AR1 biofilm treated/untreated with meropenem (A), phage (B), and phage/meropenem (C), obtained in 3 independent experiments. The detection limit is 50 CFU/mL (horizontal dotted line). PFU, plaque-forming units.
DISCUSSION
The interest in utilizing bacteriophages to treat PJIs has increased in recent years due to their ability to selectively attack bacteria, including those that are either antibiotic-resistant or biofilm-embedded [24]. To the best of our knowledge, we report the first Italian case of a patient with hip implant-associated infection due to P. aeruginosa treated with phages and meropenem after surgery, demonstrating both in real-life and in vitro the efficacy of phage therapy as adjunct to antibiotic treatment.
Guidelines from the Infectious Diseases Society of America suggest managing recalcitrance PJIs with 2-stage surgery [4]. However, in our case, a total hip arthroplasty would have permanently endangered the patient's lower limb. The best option for the patient was debridement and removal of mobile parts, followed by self-administration of phages in combination with antibiotic therapy. A similar approach, called “PhagoDAIR”, was previously reported by Ferry et al [16, 25] in 2 cases to treat relapsing PJIs due to both Staphylococcus aureus and P. aeruginosa. In both cases, the previous antibiotics treatments failed, and the general clinical conditions did not allow the standard surgery approach; therefore, a personalized bacteriophage cocktail was applied.
In this study, we decided to rinse the site with sodium bicarbonate to re-equilibrate the pH of the environment within biofilm-associated infection (acid) before phage application, as already reported in previously described cases [10, 20, 21]. The lysis of Gram-negative bacteria due to phages might release high level of endotoxins [26], which represent one of the most potent inducers of the inflammatory response [27]. We registered the onset of high-grade fever after the first administration of phages to the patient, and this effect might be due the rapid accumulation of pyrogenic toxins. The onset of fever after the administration of phages in humans has previously been reported by Ujmajuridze et al [28] in one patient during the treatment of urinary tract infections caused by P. aeruginosa. In our case, the slight reduction of the phage dosage from 10 to 5 mL resulted in the absence of this adverse event. However, because no endotoxin evaluation was performed on phage preparation at this time, we cannot exclude that the inflammatory response has been due to some residual endotoxin present in the formulation.
The phage provided by the Eliava Institute and used by the patient for the therapy belonged to Bruynoghevirus, a genus containing several viruses for which in vitro tests and characterization within a phage therapy context have been reported previously [22]. As indicated by genome analysis, Pa53 is a strictly virulent phage that does not harbor known toxins, virulence factors, or antibiotic resistance genes. Therefore, it was considered to be a safe therapeutic candidate from a molecular microbiology perspective.
Another critical point was the low titer of the applied phage product, because a reduction from 108 to 103 PFUs/mL was observed after being shipped and temporarily retained at the Italian border for routine controls. Although the titer was rather low, a decision to administer phage therapy was made. It is notable that the application of low-titer phages together with meropenem resulted in a successful outcome. This effect was consistent with the observed in vitro synergistic activity between the phage Pa53 and the meropenem.
The in vitro analysis showed the development of P. aeruginosa clones resistant to Pa53 (tested alone) within 24 hours of incubation. However, the combination of phages with meropenem prevented the selection of phage-resistant subpopulations versus P. aeruginosa biofilm. It is noteworthy that an in vitro synergistic activity between the phage Pa53 and the meropenem was also observed.
The synergistic effect phage-antibiotic was reported by different in vitro studies versus biofilm [11, 29, 30], and it might occur also in an in vivo context. This experience suggests that the potential role of bacteriophages is not to substitute the standard of care for bacterial infections but to increase its efficacy.
In several reported cases, PT is the last resort treatment for infections due to multidrug-resistant pathogens, despite the authorization of expanded access use [31–33]. As reported in our case, biofilm-associated infections are recalcitrant antibiotics and surgery, even when antibiotic-resistance pathogens do not sustain them. Thus, the phage-antibiotic combination approach for persistent infections could avoid invasive surgery procedures in high-risk patients and still allow a resolution of the infection. Nevertheless, more clinical evidence from clinical trials is required to validate this hypothesis.
Finally, we described that the patient had to self-administer the bacteriophage preparation for legal reasons. The self-administration of bacteriophages in 10 outpatients to prevent a long hospitalization has been already reported by Aslam et al [34], who developed a dedicated protocol for phage administration at home, after a first phage injection in the hospital, and providing the patients with all required instructions. Moreover, telemedicine video visits to monitor the patient were also used. In our case, we recognize several drawbacks associated with self-administration of phages, where patients might not be completely assisted in managing their phage treatment, most notably a potential lack of a correct therapeutic protocol and a medical follow up. In fact, PT has not yet been approved for clinical use in Italy, and it cannot be administered even for compassionate use (as it is used in Germany, France, United Kingdom, and United States, among others) if it is not produced in GMP and tested in clinical trials [17]. The GMP-based therapy would be incompatible with the personalized approach used in this case. At present, there is no standard regulatory framework to support the use of personalized phage-based medicine for human infections in the European Union. However, a recent publication reported that the EDQM (European Directorate for the Quality of Medicine and Healthcare) is aiming to draft an appropriate document for the definition of the indications to produce custom-made therapeutic formulations for human and veterinary use based on bacteriophages [35]. Hopefully, this might facilitate the approval of PT also in Italy.
CONCLUSIONS
In conclusion, we believe that to validate the use of bacteriophages as an efficient strategy to fight antimicrobial resistance and difficult-to-treat infections, it will be necessary to design specific clinical trials focused on a personalized approach. In the United States, 2 US Food and Drug Administration-approved Phase I/II clinical studies are already ongoing, in which each enrolled patient receives a personalized phage formulation adapted for its bacterial strain [36, 37].
Supplementary Material
Acknowledgments
We thank the exceptional patient for her trust.
Financial support . This work is supported by the Università di Pisa under the “PRA –Progetti di Ricerca di Ateneo ” (Institutional Research Grants) - Project no. PRA_2020_32 “I batteriofagi: un’alternativa agli antibiotici contro batteri multi-resistenti in comunità sessili” (to MDL) and the KU Leuven PHAGEFORCE IDN/20/024 to (RL and JW).
Contributor Information
Novella Cesta, Microbiology, Immunology, Infectious Diseases, and Transplants (MIMIT), University of Rome Tor Vergata, Rome, Italy.
Marco Pini, Department of Biology, University of Pisa, Pisa, Italy.
Tiziana Mulas, Infectious Diseases Clinic, Policlinic of Tor Vergata, Rome, Italy.
Alessandro Materazzi, Department of Biology, University of Pisa, Pisa, Italy.
Ernesto Ippolito, Department of Orthopaedic Surgery, University of Tor Vergata, Rome, Italy.
Jeroen Wagemans, Laboratory of Gene Technology, Department of Biosystems, KU Leuven, Leuven, Belgium.
Mzia Kutateladze, Eliava Institute of Bacteriophages, Microbiology and Virology, Tbilisi, Georgia.
Carla Fontana, Department of Experimental Medicine and Surgery, Unit of Microbiology and Virology, Policlinic of Tor Vergata, Rome, Italy.
Loredana Sarmati, Infectious Diseases Clinic, Policlinic of Tor Vergata, Rome, Italy; Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy.
Arianna Tavanti, Department of Biology, University of Pisa, Pisa, Italy.
Rob Lavigne, Laboratory of Gene Technology, Department of Biosystems, KU Leuven, Leuven, Belgium.
Massimo Andreoni, Infectious Diseases Clinic, Policlinic of Tor Vergata, Rome, Italy; Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy.
Mariagrazia Di Luca, Department of Biology, University of Pisa, Pisa, Italy.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Zardi EM, Franceschi F. Prosthetic joint infection. A relevant public health issue. J Infect Public Health 2020; 13:1888–91. [DOI] [PubMed] [Google Scholar]
- 2. Alp E, Cevahir F, Ersoy S, Guney A. Incidence and economic burden of prosthetic joint infections in a university hospital: a report from a middle-income country. J Infect Public Health 2016; 9:494–8. [DOI] [PubMed] [Google Scholar]
- 3. Shah NB, Osmon DR, Steckelberg JM, et al. Pseudomonas prosthetic joint infections: a review of 102 episodes. J Bone Jt Infect 2016; 1:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56:e1–e25. [DOI] [PubMed] [Google Scholar]
- 5. Li C, Renz N, Trampuz A. Management of periprosthetic joint infection. Hip Pelvis 2018; 30:138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clarke AL, De Soir S, Jones JD. The safety and efficacy of phage therapy for bone and joint infections: a systematic review. Antibiotics (Basel) 2020; 9:795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aslam S, Schooley RT. What's old is new again: bacteriophage therapy in the 21st century. Antimicrob Agents Chemother 2019; 64:e01987–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gordillo Altamirano FL, Barr JJ. Phage therapy in the postantibiotic era. Clin Microbiol Rev 2019; 32:e00066–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petrovic Fabijan A, Khalid A, Maddocks S, et al. Phage therapy for severe bacterial infections: a narrative review. Med J Aust 2020; 212:279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Onsea J, Soentjens P, Djebara S, et al. Bacteriophage application for difficult-to-treat musculoskeletal infections: development of a standardized multidisciplinary treatment protocol. Viruses 2019; 11:891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tkhilaishvili T, Lombardi L, Klatt A-B, Trampuz A, Di Luca M. Bacteriophage Sb-1 enhances antibiotic activity against biofilm, degrades exopolysaccharide matrix and targets persisters of Staphylococcus aureus. Int J Antimicrob Agents 2018; 52:842–53. [DOI] [PubMed] [Google Scholar]
- 12. Fong SA, Drilling A, Morales S, et al. Activity of bacteriophages in removing biofilms of Pseudomonas aeruginosa isolates from chronic rhinosinusitis patients. Front Cell Infect Microbiol 2017; 7:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nir-Paz R, Gelman D, Khouri A, et al. Successful treatment of antibiotic-resistant, poly-microbial bone infection with bacteriophages and antibiotics combination. Clin Infect Dis 2019; 69:2015–8. [DOI] [PubMed] [Google Scholar]
- 14. Chan BK, Turner PE, Kim S, Mojibian HR, Elefteriades JA, Narayan D. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol Med Public Health 2018; 2018:60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Onsea J, Wagemans J, Pirnay JP, et al. Bacteriophage therapy as a treatment strategy for orthopaedic-device-related infections: where do we stand? Eur Cell Mater 2020; 39:193–210. [DOI] [PubMed] [Google Scholar]
- 16. Ferry T, Kolenda C, Batailler C, et al. Case report: arthroscopic “debridement antibiotics and implant retention” with local injection of personalized phage therapy to salvage a relapsing Pseudomonas aeruginosa prosthetic knee infection. Front Med 2021; 8:569159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cesta N, Di Luca M, Corbellino M, Tavio M, Galli M, Andreoni M. Bacteriophage therapy: an overview and the position of Italian society of infectious and tropical diseases. Infez Med 2020; 28:322–31. [PubMed] [Google Scholar]
- 18. Tkhilaishvili T, Wang L, Tavanti A, Trampuz A, Luca MD. Antibacterial efficacy of two commercially available bacteriophage formulations, staphylococcal bacteriophage and PYO bacteriophage, against methicillin-resistant Staphylococcus aureus: prevention and eradication of biofilm formation and control of a systemic infection of galleria mellonella larvae. Front Microbiol 2020; 11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Macià MD, Rojo-Molinero E, Oliver A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin Microbiol Infect 2014; 20:981–90. [DOI] [PubMed] [Google Scholar]
- 20. Vogt D, Sperling S, Tkhilaishvili T, Trampuz A, Pirnay J-P, Willy C. [Beyond antibiotic therapy—future antiinfective strategies—update 2017]. Unfallchirurg 2017; 120:573–84. [DOI] [PubMed] [Google Scholar]
- 21. Tkhilaishvili T, Winkler T, Müller M, Perka C, Trampuz A. Bacteriophages as adjuvant to antibiotics for the treatment of periprosthetic joint infection caused by multidrug-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 2019; 64:e00924–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knezevic P, Fabijan AP, Gavric D, Pejic J, Doffkay Z, Rakhely G. Phages from genus bruynoghevirus and phage therapy: pseudomonas phage Delta case. Viruses 2021; 13:1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ceyssens P-J, Hertveldt K, Ackermann H-W, et al. The intron-containing genome of the lytic Pseudomonas phage LUZ24 resembles the temperate phage PaP3. Virology 2008; 377:233–8. [DOI] [PubMed] [Google Scholar]
- 24. Morris JL, Letson HL, Elliott L, et al. Evaluation of bacteriophage as an adjunct therapy for treatment of peri-prosthetic joint infection caused by Staphylococcus aureus. PLoS One 2019; 14:e0226574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferry T, Leboucher G, Fevre C, et al. Salvage debridement, antibiotics and implant retention (“DAIR”) with local injection of a selected cocktail of bacteriophages: is it an option for an elderly patient with relapsing Staphylococcus aureus prosthetic-joint infection? Open Forum Infect Dis 2018; 5:ofy269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu D, Van Belleghem JD, de Vries CR, et al. The safety and toxicity of phage therapy: a review of animal and clinical studies. Viruses 2021; 13:1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang X, Quinn PJ. Endotoxins: lipopolysaccharides of Gram-negative bacteria. Subcell Biochem 2010; 53:3–25. [DOI] [PubMed] [Google Scholar]
- 28. Ujmajuridze A, Chanishvili N, Goderdzishvili M, et al. Adapted bacteriophages for treating urinary tract infections. Front Microbiol 2018; 9:1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oechslin F, Piccardi P, Mancini S, et al. Synergistic interaction between phage therapy and antibiotics clears Pseudomonas aeruginosa infection in endocarditis and reduces virulence. J Infect Dis 2017; 215:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akturk E, Oliveira H, Santos SB, et al. Synergistic action of phage and antibiotics: parameters to enhance the killing efficacy against mono and dual-Species biofilms. Antibiotics 2019; 8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Corbellino M, Kieffer N, Kutateladze M, et al. Eradication of a multidrug-resistant, carbapenemase-producing Klebsiella pneumoniae isolate following oral and intra-rectal therapy with a custom made, lytic bacteriophage preparation. Clin Infect Dis 2020; 70:1998–2001. [DOI] [PubMed] [Google Scholar]
- 32. Dedrick RM, Guerrero-Bustamante CA, Garlena RA, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med 2019; 25:730–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schooley RT, Biswas B, Gill JJ, et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 2017; 61:e00954–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aslam S, Lampley E, Wooten D, et al. Lessons learned from the first 10 consecutive cases of intravenous bacteriophage therapy to treat multidrug-resistant bacterial infections at a single center in the United States. Open Forum Infect Dis 2020; 7:ofaa389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Verbeken G, Pirnay J-P. European Regulatory aspects of phage therapy: magistral phage preparations. Curr Opin Virol 2022; 52:24–9. [DOI] [PubMed] [Google Scholar]
- 36. Adaptive Phage Therapeutics, Inc. A Phase I/II Study of Bacteriophage Therapy to Evaluate Safety, Tolerability, and Efficacy of Targeted ‘Personalized’ Bacteriophage Treatments in Patients With Bacterial Infection of the Urinary Tract. clinicaltrials.gov, 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT04287478. Accessed 5 May 2022.
- 37. Adaptive Phage Therapeutics, Inc . A Phase IIa Randomized, Parallel Group, Double-blind, Repeat Dose, Investigating the Safety, Tolerability, and Efficacy of Phage Treatment and Standard of Care Antimicrobials for Patients With Diabetic Foot Osteomyelitis. clinicaltrials.gov, 2022. Available at: https://clinicaltrials.gov/ct2/show/NCT05177107. Accessed 5 May 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.